- 1Department of Obstetrics and Gynecology, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Hubei Clinical Research Center for Prenatal Diagnosis and Birth Health, Wuhan, China
- 3Wuhan Clinical Research Center for Reproductive Science and Birth Health, Wuhan, China
- 4Department of Ultrasound Imaging, Zhongnan Hospital of Wuhan University, Wuhan, China
- 5Department of Radiology, Zhongnan Hospital of Wuhan University, Wuhan, China
Background: A fetal bronchogenic cyst (BC) is a rare congenital anomaly with an incidence of 0.147–0.238‰. The coronavirus disease 2019 (COVID-19) pandemic, as a particular situation, hindered pregnant women from receiving periodic prenatal checkups.
Case Description: Until 34+6 weeks of gestation, a fetal case of the intrathoracic cyst was found by ultrasound examination. Further, MRI examination confirmed the diagnosis of the congenital mediastinal cystic lesion, probably a BC. Genetic testing was not conducted due to the COVID-19 pandemic. At 38+5 weeks of gestation with maternal COVID-19 testing negative, a live girl was delivered by cesarean section. Five months later, the child underwent bronchocystectomy, and the postoperative pathological lesions confirmed a (right upper mediastinum) BC.
Conclusion: Herein, we reported the prenatal and postnatal management for a rare case of the congenital BC by multidisciplinary approaches during the COVID-19 pandemic. Fetal MRI and screening for fetal chromosomal abnormalities are especially recommended. This case contributes to the awareness that the COVID-19 pandemic interferes with regular follow-up schedules during pregnancy and may interfere with timely performed additional tests; which leads to more accurate genetic counseling. A combination of multidisciplinary approaches, including radiology, infection control, genetic counseling, obstetrics, and pediatric surgery, is pivotal for managing fetal BC during the COVID-19 pandemic.
Introduction
A fetal bronchogenic cyst (BC) is a benign but rare congenital anomaly and generally detected after 20 weeks of gestation by periodic prenatal checkups. Chromosomal abnormalities are considered to be associated with 1.6% of fetal pulmonary malformations. For example, there was a fetal congenital pulmonary airway malformation (CPAM) associated with mosaic Klinefelter (1). Therefore, invasive prenatal diagnosis is recommended for suspected fetal BC. However, the coronavirus disease 2019 (COVID-19) pandemic disrupted the regular schedules of most pregnant women. A case of the congenital BC was not found until 34+6 weeks of gestation. To improve the understanding of this rare disease, especially during the COVID-19 pandemic, we report observations and treatment by multidisciplinary approaches. This paper presents a systematic prenatal and postnatal management for fetal BCs.
Case Description
A 31-year-old woman with a singleton pregnancy missed the regular prenatal checkups (including her second trimester ultrasound) due to the COVID-19 pandemic and came to our hospital for a fetal ultrasound examination at 34+6 weeks of gestation. A 1.3 × 1.4 cm cyst was found on the right side of the fetal thorax (Figure 1). Further, a fetal MRI was performed at 35+3 weeks of gestation. The MRI showed a 1.5 × 1.2 cm × 1.5 cm cystic lesion in the right thoracic cavity of the fetus, which was considered as a congenital BC (Figure 2). The woman was not able to undergo prenatal genetic diagnosis because of the late gestational age. A multidisciplinary consultation was implemented by the pediatric surgery, radiology, and obstetrics departments, and a regular follow-up was recommended. There was no significant change in cyst size during the follow-up. A planned cesarean section (C-section) was performed at 38+5 weeks of gestation due to a previous C-section and rejection of vaginal delivery. The reaction of the baby girl was good, with Apgar score 9 at 1 min and 10 at 5 min after birth.
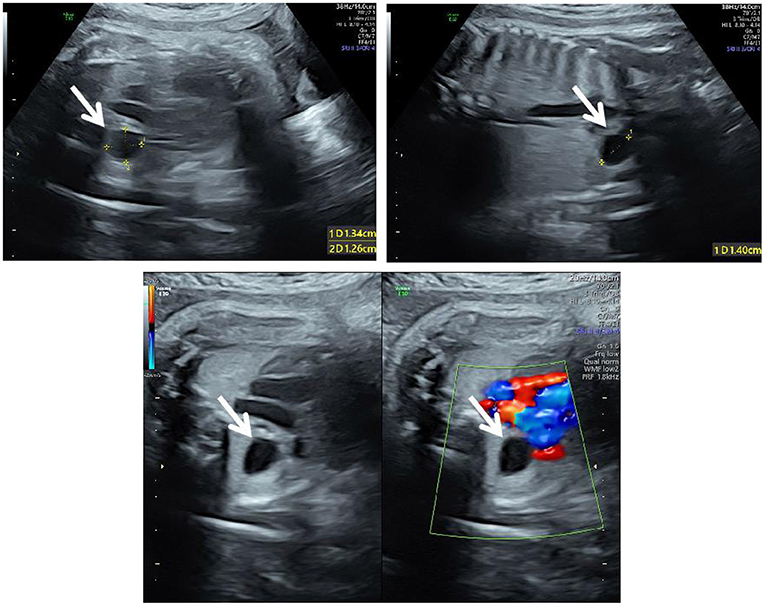
Figure 1. Ultrasound at 34+6 weeks of gestation. The three-vessel trachea section of the fetus showed an anechoic area of 1.3 × 1.4 × 1.3 cm (white arrows) on the right side of the thorax, with clear boundaries and without vascular supply; the trachea was compressed and flattened.
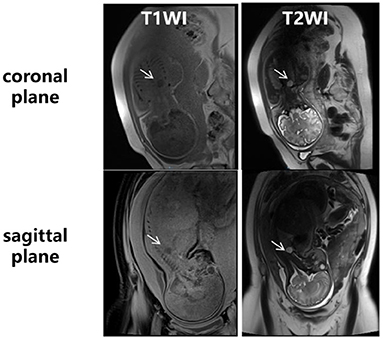
Figure 2. Fetal MRI at 35+3 weeks of gestation. An ovular-like area with long T1 and long T2 signal (white arrows) was found in the upper right thoracic cavity, on the right side of the lower trachea, above the ridge of the trachea. The boundary was clear, and the size of the lesion was about 1.5 × 1.2 × 1.5 cm.
A postnatal chest CT scan confirmed a cystic lesion of about 1.6 cm on the right upper mediastinum (Figure 3A). The child was breastfed and had a normal growth curve during the follow-up. A reexamination of chest enhanced CT scan was performed at 4 months of age. A 2.4 × 2.3 × 2.1 cm low-density shadow was shown on the right upper mediastinum, without enhancement after injection of a contrast agent. Considering the gradual enlargement of the cyst, the multidisciplinary team proposed a surgical treatment plan. With COVID-19 testing negative, doctors for infectious disease evaluated the risk of infection and took preventative measures during the COVID-19 pandemic. Pediatric surgeons carried out a video-assisted thoracoscopic surgery with closed thoracic drainage for the 5-month old girl. Subsequent pathological findings (Figure 3B) confirmed the diagnosis of BC. The patient's recovery was smooth, and she was discharged after 6 days. The girl recovered well with normal growth and development at present.
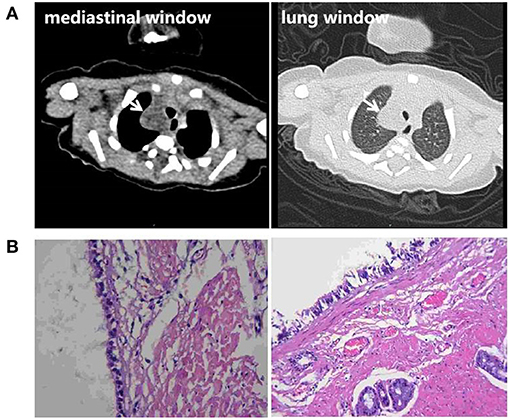
Figure 3. (A) Chest CT scan of the newborn 3 days after birth. An irregular low-density lesion with a diameter of about 16 mm was seen in the upper right mediastinum, with a slightly unclear boundary. The CT value was about 8 Hu. No abnormal thickening of the pleura was observed, and no signs of effusion were observed in the pleural cavity. (B) Postoperative pathological findings show a bronchogenic cyst.
Discussion
The incidence of congenital BCs was about 0.147–0.238‰; 20–30% of them are congenital anterior intestinal cysts (2, 3). By reviewing the literature, we list fetuses with BCs in Table 1. The abnormal development of the buds of the primitive foregut and tracheobronchial tree leads to the formation of BCs (8). BCs can be divided into three types according to their original location: intrapulmonary, mediastinal, and ectopic. The timing of the formation of the cyst during fetal development affects its location: If a cyst was formed in the early stage, it would more likely be mediastinal; if formed in the later stage, the cyst would more likely be intrapulmonary; if migrating farther away, the abnormal buds would be an ectopic cyst (8, 9). Most bronchial cysts are located in the mediastinum, while the ectopic cyst is exceedingly rare (10). Our reported case was a typical mediastinal BC on the right superior mediastinum.
Generally, BCs have no symptoms. With the development of prenatal ultrasound and MRI, BCs can be detected prenatally after 20 weeks of gestation. The ultrasonographic characteristics of BCs are described as follows: a single hypoechoic or medium–high echoic circular or quasi-circular lesion. However, the misdiagnostic incidence of ultrasonic examination is about 25% in fetal intrathoracic malformations (11). Therefore, a fetal MRI is indispensable for further investigation of fetal pulmonary malformations. The fetal pulmonary tissue usually is a T2-hyperintense structure in MRI after 24 weeks of gestation (3). The manifestations of BCs in MRI were mainly round, well-defined cystic lesions, but a few were irregular or lobulated. Like in our case, MRI indicated classical homogeneous long T1 and long T2 signals. In 15% of cases, the BCs are parenchymatous. A differential diagnosis of CPAM, pleural pulmonary blastoma (PPB), and macrocytic lymphatic anomaly should be considered.
Chromosomal abnormalities are associated with 1.6% of fetal pulmonary malformations, especially those with pulmonary hypoplasia and bronchial cyst (12, 13). Therefore, invasive prenatal diagnosis such as amniocentesis is recommended for fetuses with BCs to exclude potential chromosomal abnormalities. Genetic counseling and multidisciplinary evaluation should be carried out. In our case, the prenatal diagnosis has not been performed because of the late gestational age. Even during the COVID-19 pandemic, a planned prenatal and postnatal follow-up is necessary.
For fetuses with BCs in prenatal MRI, according to the level of tracheal compression and pleural effusion, a multidisciplinary consultation with pediatric surgeons, radiologists, and obstetricians should be organized to discuss the fetal prognosis. The follow-up of congenital BCs may be performed every 2 weeks, depending on its location and size (3). For the fetus with a sign of compression, transfer to a fetal medicine center for further estimations and possible treatment is recommended. The mode and timing of delivery depend on the compression signs, such as fetal edema and tracheal compression. If a tracheal compression is suspected, the patient should be transferred to a tertiary hospital with a neonatal surgical team. Ex utero intrapartum therapy (EXIT) and extracorporeal membrane oxygenation (ECMO) should be prepared (7). In our case, the BC was relatively small with slight tracheal compression, without a sign of deterioration. A C-section was performed at term because of previous C-section and refusal of vaginal delivery.
After birth, the general condition of the neonate should be monitored, and a CT scan should be performed to estimate the size and location of the cyst, especially if there is any tracheal compression. Mediastinal bronchial cysts could occur anywhere along the mediastinum. Most mediastinal BCs are located next to the trachea and below the carina (14). The BCs mostly developed close to the trachea or bronchus but rarely communicated with the bronchus. One of the characteristic manifestations of BCs is flat borderlines with a “D” shape. The BCs are almost single, elliptic, or quasi-round, with smooth borderline and uniform foliation or soft tissue density in CT. In an enhanced CT scan, the cystic wall but not the contents would be partially enhanced. The size of the BC may gradually increase because of its secretion function. The complications include compression, infection, and hemorrhage (15). Despite the low malignancy risk, early management such as thoracoscopy for resection is recommended (4, 16, 17). For the asymptomatic patient, the timing of surgery is generally at 3–6 months after birth due to the low risk of complications (4, 18). For those with prenatal pleural effusion, a transabdominal thoracentesis or pleural amniotic shunt (TAS) could be selected to relieve the fetal symptoms after a comprehensive benefit–risk balance evaluation (3). The typical histological findings are as follows: the cyst wall is lined with pseudostratified ciliated columnar epithelium, containing airway cell components such as cartilage, glandular cells, smooth muscle, and ciliated cells (19, 20).
Most studies have proven that pregnant women are not more prone to develop COVID-19 than the general population (21). Because of the limited data on COVID-19 in the first trimester and early pregnancy, there is no scientific evidence that COVID-19 increases the risk of fetal congenital malformations or causes miscarriage in early pregnancy (22). Previous studies have not shown any detrimental effect of COVID-19 during pregnancy on fetal growth (21). Therefore, since the pregnant woman in our report did not have a COVID-19 infection, the key was to take protective measures, such as wearing a mask and refraining from going outdoors.
Conclusions
In conclusion, a fetal MRI and planned follow-up is recommended for the fetus with a diagnosis of suspected BCs by prenatal ultrasound. Genetic counseling with fetal chromosomal analysis would be recommended for fetuses with BCs. A follow-up by a multidisciplinary team with an ultrasound assessment every 2–3 weeks will be needed. If any sign of compression shows up, an intrauterine treatment could be discussed. The mode and timing of delivery depend on the size, location, and complications of the BCs. The patient should be transferred to a tertiary hospital with a neonatal surgical team to ensure maternal–neonatal safety. For the patient with asymptomatic BCs, surgical treatment may be performed at 3–6 months after birth. The preventative measures against COVID-19 infection must be taken throughout the entire prenatal and postnatal management process.
A combination of multidisciplinary approaches, including radiology, infection, genetic counseling, obstetrics, and pediatric surgery, would provide a precise prenatal diagnosis and planned postnatal management, improving the neonatal survival rate with mediastinal masses during the COVID-19 pandemic.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LC and JD collated the data, reviewed the literature, and wrote the first draft of the manuscript. MW provided substantial contribution to the drafting and critical revisions of the manuscript. DL and HL performed imaging screening for the pregnant woman and provided the photographs. JM, JL, and CW performed the consultation and followed up the fetus. YZ carried out the concepts, design of the study, approval of the final version of the manuscript, and agrees to be accountable for all aspects of the work related to accuracy and integrity. All authors read and approved the final manuscript.
Funding
This study was supported by Science and Technology Program of Hubei Province (No. 2017ACB640) and Wuhan University Medical Development Plan (No. TFJC2018001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all physicians, nurses, and allied health professionals who contributed to the care and well-being of the described patient and her family.
References
1. Holubyeva A, Bracero LA, Feuerstein JL, Bush S. Congenital pulmonary airway malformation associated with mosaic Klinefelter syndrome. J Clin Ultrasound. (2020) 48:121–4. doi: 10.1002/jcu.22777
2. Ergin M, Yeginsu A, Celik A, Onuk Filiz N. A case of cervical bronchogenic cyst presenting with hoarseness in an adult. Tuberk Toraks. (2009) 57:73–6.
3. Adams NC, Victoria T, Oliver ER, Moldenhauer JS, Adzick NS, Colleran GC. Fetal ultrasound and magnetic resonance imaging: a primer on how to interpret prenatal lung lesions. Pediatr Radiol. (2020) 50:1839–54. doi: 10.1007/s00247-020-04806-x
4. Li XH, Zhang T, Wang S, He BC, Yang XN, Zhong WZ, et al. Minimally invasive, multi-disciplinary approach for surgical management of a mediastinal congenital bronchogenic cyst in a 6-month-old infant. J Thorac Dis. (2017) 9:E743–7. doi: 10.21037/jtd.2017.08.40
5. Venkatesh K, Pillarisetty K. Bronchogenic cyst presenting as content of omphalocele-a case report. Indian J Pathol Microbiol. (2020) 63:116–8. doi: 10.4103/IJPM.IJPM_841_18
6. Chatterjee D, Hawkins JL, Somme S, Galan HL, Prager JD, Crombleholme TM. Ex utero intrapartum treatment to resection of a bronchogenic cyst causing airway compression. Fetal Diagn Ther. (2014) 35:134–40. doi: 10.1159/000355661
7. Bayar UO, Numanoglu V, Bektaş S, Sade H, Tatlî D. Management of fetal bronchogenic lung cysts: a case report and short review of literature. Case Rep Med. (2010) 2010:751423. doi: 10.1155/2010/751423
8. Sumiyoshi K, Shimizu S, Enjoji M, Iwashita A, Kawakami K. Bronchogenic cyst in the abdomen. Virchows Arch A Pathol Anat Histopathol. (1985) 408:93–8. doi: 10.1007/BF00739965
9. Aydogdu K, Findik G, Kaya S, Koksal D, Demirag F. Schwannoma arising in a bronchogenic cyst wall. Asian Cardiovasc Thorac Ann. (2015) 23:328–31. doi: 10.1177/0218492314529292
10. Aktogu S, Yuncu G, Halilcolar H, Ermete S, Buduneli T. Bronchogenic cysts: clinicopathological presentation and treatment. Eur RespirJ. (1996) 9:2017–21. doi: 10.1183/09031936.96.09102017
11. Mon RA, Johnson KN, Ladino-Torres M, Heider A, Mychaliska GB, Treadwell MC, et al. Diagnostic accuracy of imaging studies in congenital lung malformations. Arch Dis Childhood Fetal Neonatal Ed. (2019) 104:372–7. doi: 10.1136/archdischild-2018-314979
12. Pressey TL, Wilson RD, Kasperski S, Bebbington MW, Adzick NS. Prenatal diagnosis of partial trisomy 1q and monosomy X in a fetus with a congenital lung lesion and hydrops fetalis. Am J Med Genet A. (2007) 143A:1104–7. doi: 10.1002/ajmg.a.31707
13. Hermelijn SM, Zwartjes RR, Tiddens HAWM, Cochius-den OSCM, Reiss IKM, Wijnen RMH, et al. Associated anomalies in congenital lung abnormalities: a 20-year experience. Neonatology. (2020) 25:1–7. doi: 10.1159/000509426
14. Syred K, Weissferdt A. Non-neoplastic mediastinal cysts. Adv Anat Pathol. (2020) 27:294–302. doi: 10.1097/PAP.0000000000000261
15. Dong B, Zhou HL, Zhang JJ, Wang YT, Fu YW. Diagnosis and treatment of retroperitoneal bronchogenic cysts: a case report. Oncol Lett. (2014) 7:2157–9. doi: 10.3892/ol.2014.1974
16. Durell J, Lakhoo K. Congenital cystic lesions of the lung. Early Hum Dev. (2014) 90:935–9. doi: 10.1016/j.earlhumdev.2014.09.014
17. Hall NJ, Stanton MP. Long term outcomes of congenital lung malformations. Semin Pediatr Surg. (2017) 26:311–6. doi: 10.1053/j.sempedsurg.2017.09.001
18. Palla J, Sockrider MM. Congenital lung malformations. Pediatr Ann. (2019) 48:e169–74. doi: 10.3928/19382359-20190326-02
19. Kawaguchi M, Kato H, Hara A, Suzui N, Tomita H, Miyazaki T, et al. CT and MRI characteristics for differentiating mediastinal mullerian cysts from bronchogenic cysts. Clin Radiol. (2019) 74:976.e19–25. doi: 10.1016/j.crad.2019.08.013
20. Cortes-Santiago N, Deutsch GH. Pediatric cystic lung lesions: where are we now? Surg Pathol Clin. (2020) 13:643–55. doi: 10.1016/j.path.2020.07.002
21. Sharma JB, Sharma E, Sharma S, Singh J. Recommendations for prenatal, intrapartum, and postpartum care during COVID-19 pandemic in India. Am J Reprod Immunol. (2020) 84:e13336. doi: 10.1111/aji.13336
Keywords: bronchogenic cyst, prenatal and postnatal management, MRI, multidisciplinary approaches, COVID-19 pandemic
Citation: Cheng L, Duan J, Wang M, Lu D, Li H, Ma J, Liu J, Wang C and Zhang Y (2021) Case Report: Prenatal and Postnatal Management for Fetal Bronchogenic Cysts During the COVID-19 Pandemic. Front. Pediatr. 9:675883. doi: 10.3389/fped.2021.675883
Received: 04 March 2021; Accepted: 08 June 2021;
Published: 07 July 2021.
Edited by:
Kostas N. Priftis, National and Kapodistrian University of Athens, GreeceReviewed by:
Aleksandar Sovtic, The Institute for Health Protection of Mother and Child Serbia, SerbiaHong-bo Qi, The First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2021 Cheng, Duan, Wang, Lu, Li, Ma, Liu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanzhen Zhang, emhhbmd5dWFuemhlbiYjeDAwMDQwO3dodS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Lin Cheng1,2,3†
Lin Cheng1,2,3† Jie Duan
Jie Duan Mei Wang
Mei Wang Yuanzhen Zhang
Yuanzhen Zhang