- 1Pediatric Unit, S. Chiara Hospital, Trento, Italy
- 2Section of Pediatrics, Department of Translational Medical Science, Regional Center of Pediatric Diabetes, Federico II University of Naples, Naples, Italy
- 3Humanitas Clinical and Research Center, Rozzano, Italy
Aim: To explore the impact of real-time continuous glucose monitoring (rtCGMs) or intermittently scanned/viewed CGM (isCGM) on psychological outcomes in children and caregivers, and to grade the level of evidence.
Method: Systematic review of the literature from PubMed, Embase, Cochrane Library, Web of Science, CINAHL, Nursing reference center, Up to date, Google Scholar, and PsycINFO databases. The studies selected used validated questionnaires for investigating the psychological outcomes. We applied GRADE (Grading of Recommendations Assessment, Development and Evaluation) to rank the quality of a body of evidence.
Results: A total of 192 studies were identified in the initial search and after the process of evaluation 25 studies were selected as appropriate to be included in this systematic review. We found in moderate quality studies that isCGM in adolescents can improve diabetes related distress, family conflicts, fear of hypoglycemia, and quality of life, while depression, anxiety, and quality of sleep have not yet been evaluated by validated questionnaires. In moderate—high quality studies, rtCGM technology does not impact on diabetes burden, diabetes specific family conflict, and depressive symptoms. The effect on fear of hypoglycemia, sleep quality, and anxiety is still debated and RCT studies powered to find significant results in psychological outcomes are lacking. RtCGM increases satisfaction and quality of life in parents and patients wearing rtCGM.
Conclusion: these data present an interesting point to consider when families are deciding whether or not to start CGM use, choosing between rtCGM to reach a tighter metabolic control, or isCGM which allows greater benefits on psychological outcomes.
Introduction
The advent of real-time continuous glucose monitoring systems (rtCGMs) or intermittently scanned/viewed CGM (isCGM) is one of the major technological innovation for the treatment of Type I Diabetes (T1D). Real-time CGM allows individuals with diabetes to follow their glucose concentration simultaneously, and to obtain information on glucose trends and trajectories. Moreover, the systems can provide warnings on upcoming hypoglycemia or hyperglycemia as well as alarms for rapid glycemic excursions (1). Meta-analyses provided evidence for real-time CGM to lower hemoglobin A1c (HbA1C) levels without increasing hypoglycemic events (1).
Importantly, recent studies confirmed that the use of isCGM has a positive impact on glucose control, by limiting glucose variability, reducing hypoglycemia, and improving long-term glucose control (2).
In addition to the stand-alone rtCGM systems, the integrated combination of pump therapy with rtCGMs allows to automatically suspend insulin delivery in the case of upcoming hypoglycemia, thus reducing or avoiding nocturnal hypoglycemia (3).
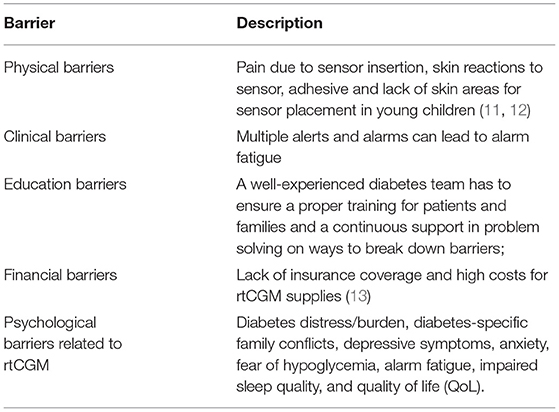
Although a clear evidence that the benefits associated with the use of rtCGMs are strictly related to a near daily use (1, 4, 5), a constant rtCGM use remains problematic for many patients in the pediatric age group (6, 7). Indeed, a better glycemic control is achieved by patients who use rtCGM for the majority of time, generally considered to be 70% or more (1, 8). Nevertheless, recent data from the Type 1 Diabetes Exchange Clinic Registry still reports that only one third of T1D-affected youth regularly wears rtCGM, although there has been an increase of use from 2013 (4% of T1D youth) to 2015 (14%) and 2017 (31%) (9). Furthermore, rtCGM wearing declines significantly over-time among T1D users (10). Barriers to a regular rtCGM use in pediatrics are reported in the following Table:
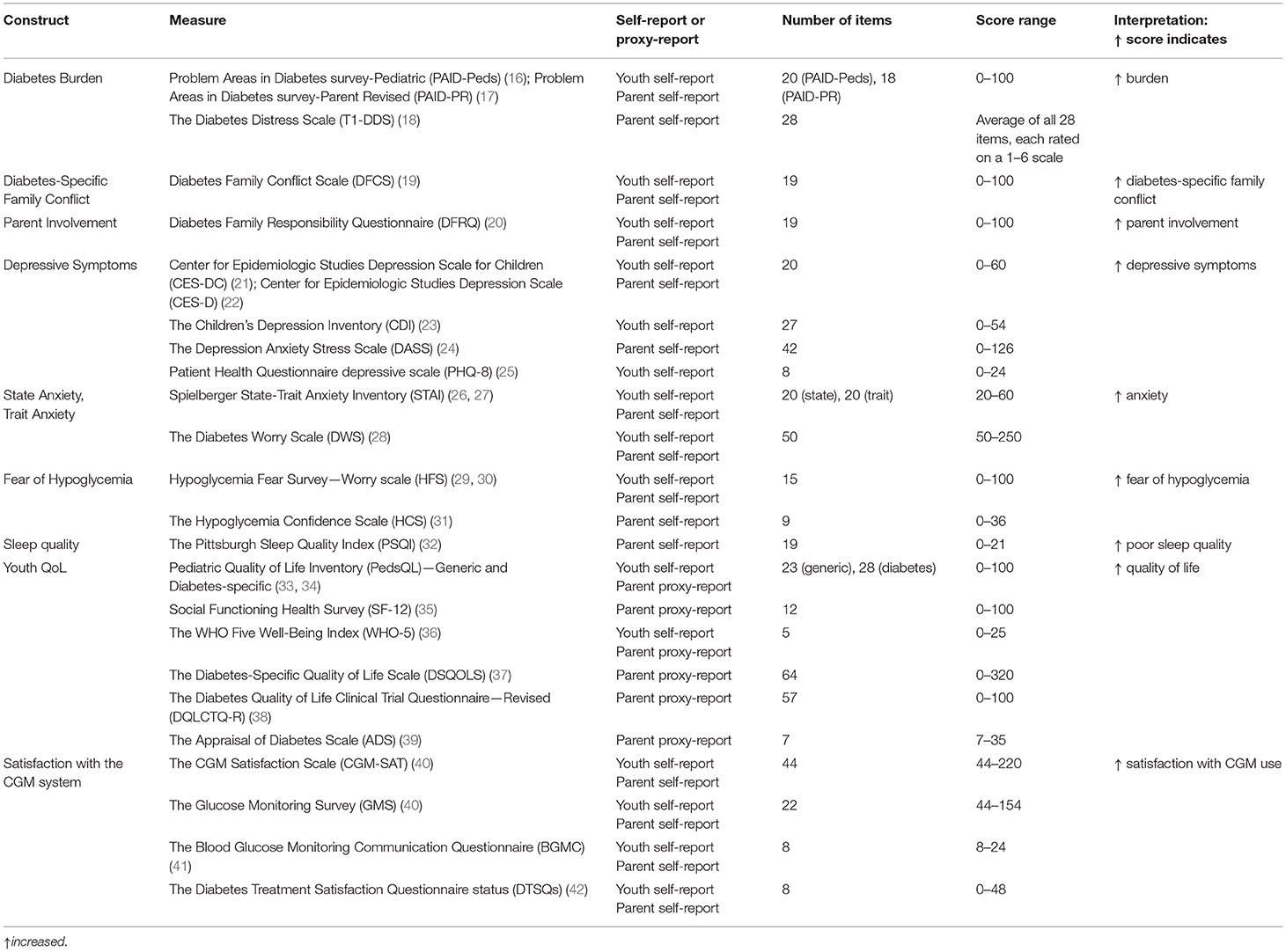
A deeper understanding of the factors related to technologies uptake and adherence remains a crucial topic of investigation. In particular, studies on psychological factors that may predict sensor success or interruption are still limited. On the contrary, identifying psychological issues related to the sensor use would support both diabetologists in tailoring the best treatment for each patient, and youth and families in setting realistic expectations. The impact of rtCGM and isCGM on psychological outcomes in children and caregivers remains controversial (6, 14, 15). This may be due to the fact that psychological measures are usually considered as secondary outcomes in trials involving CGMs (Laffel LM 2020 JAMA, Massa GG 2019, JDRF-CGM Study Group, Diabetes Care 2010), compared to the metabolic control (HbA1c, hypoglycemia, CGM glucose metrics). Moreover, different questionnaires are used to assess the outcomes in the published studies. Also, each area of investigation (depression, fear of hypoglycemia, QoL) could be explored by different validated measures, self-reported or administered by health care providers, as summarized in Table 1 (16–42).
Aim
The aim of this systematic literature review is to explore the impact of rtCGM or isCGM on psychological outcomes (diabetes distress/burden, diabetes-specific family conflicts, depressive symptoms, anxiety, fear of hypoglycemia, alarm fatigue, impaired sleep quality, quality of life, and satisfaction with the CGM system) in children and caregivers and to grade the level of evidence.
Methods
Criteria for Study Selection
Types of Studies
We included RCTs, observational studies, prospective studies, cross-sectional studies, exploratory studies, mix of qualitative, and quantitative studies. We included only published studies.
Types of Participants
We included patients with T1D aged between 0 and 18 years and their caregivers.
Types of Interventions
We included the following comparisons:
Comparison 1: rtCGM on psychological outcomes (diabetes distress/burden, diabetes-specific family conflicts, depressive symptoms, anxiety, fear of hypoglycemia, alarm fatigue, impaired sleep quality and quality of life, satisfaction) vs. capillary glucose testing for glycemic assessment in children and caregivers;
Comparison 2: isCGM on psychological outcomes (diabetes distress/burden, diabetes-specific family conflicts, depressive symptoms, anxiety, fear of hypoglycemia, alarm fatigue, impaired sleep quality and quality of life, satisfaction) vs. capillary glucose testing for glycemic assessment in children and caregivers.
Comparison 3: rtCGM vs. isCGM on psychological outcomes (diabetes distress/burden, diabetes-specific family conflicts, depressive symptoms, anxiety, fear of hypoglycemia, alarm fatigue, impaired sleep quality and quality of life, satisfaction) in children and caregivers.
Outcomes
Psychological outcomes in children and caregivers included: diabetes distress/burden, diabetes-specific family conflicts, depressive symptoms, anxiety, fear of hypoglycemia, alarm fatigue, impaired sleep quality, quality of life, satisfaction.
A detailed description of outcomes and related measures is reported in Table 1 (16–42).
Search Methods
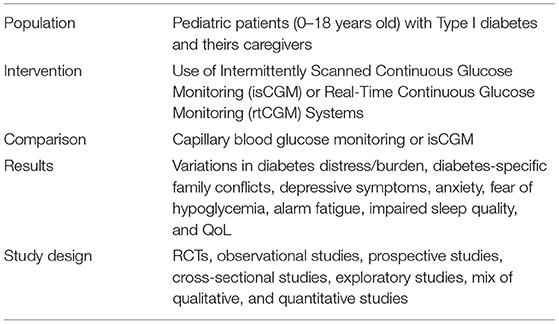
We conducted a systematic search of the literature according to the PICOS model (Population, Intervention, Comparison, Results, Study design).
The study exclusion criteria were:
- patients >18 years; patients with Type II Diabetes;
- studies not meeting the established primary and secondary outcomes;
- animal research studies;
- devices: use of closed loop systems;
- reviews, conference abstracts, full texts not available.
We did not apply language restrictions.
Sources used for literature review included: PubMed, Embase, Cochrane Library, Web of Science, CINAHL, Nursing reference center, Up to date, Google Scholar, and PsycINFO.
Articles published from 1/01/2006 to 31/12/2020 were considered for the current review. Search terms, or “mesh” (MEdical Subject Headings) for this systematic review included: “CGM AND distress,” “CGM AND sleep quality,” “CGM AND psychological variables,” “Glucose monitoring AND distress,” “Glucose monitoring AND sleep quality,” “Glucose monitoring AND psychological variables,” “Flash glucose monitoring AND distress,” “Flash glucose monitoring AND sleep quality,” “Flash glucose monitoring AND psychological variables.”
According to the PICOS detailed above, filters for participants' age (0–18 years), and study characteristics were activated.
Data Extraction and Management
Two review authors independently extracted data by using the forms integrated in the sources' systems.
The following characteristics were reviewed for each included study:
• reference aspects: authorship(s); published or unpublished; year of publication; year in which study was conducted; other relevant papers cited;
• study characteristics: study design; type, duration; informed consent; ethics approval;
• population characteristics: age, number of participants;
• intervention characteristics: type, duration, mode of use of rtCGM and isCGM;
• evaluation of the outcomes as reported in Table 1 (16–42).
Disagreements were solved by discussion.
Assessment of the Certainty of the Evidence
We used the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) to rank the quality of a body of evidence (www.gradeworkinggroup.org) for the following outcomes: diabetes distress/burden, diabetes-specific family conflicts, depressive symptoms, anxiety, fear of hypoglycemia, alarm fatigue, impaired sleep quality, quality of life, and satisfaction with the rtCGM and the isCGM systems.
Two review authors independently assessed the certainty of the evidence for each of the outcomes above. In the case of risk of bias in the study design, imprecision of estimates, inconsistency across studies, indirectness of the evidence, and publication bias, we had the option of decreasing the level of certainty by one or two levels according the GRADE guidelines (43).
The GRADE approach results in an assessment of the certainty of a body of evidence and allocation to one of four grades:
Results
A total of 192 studies were identified following the literature review. After screening, we excluded 20 records as they were duplicates. When we reviewed titles and abstracts we excluded 112 records: 9 studies were published only in abstract form, 100 studies did not investigate the outcomes of interest (Table 1), 3 studies were not available in the full text form.
A total of 60 full-text manuscripts were assessed for eligibility: 27 studies were excluded as no data were available for the analysis, besides the ones reported in the abstracts; 4 studies were excluded as they reported data from the same cohort of patients; 4 studies were excluded as they resulted to be literature reviews when the full-texts were analyzed. A final number of 25 studies, 6 on isCGM, 19 on rtCGM, were included in this systematic review.
The PRISMA flow diagram in Figure 1 shows the process of study evaluation.
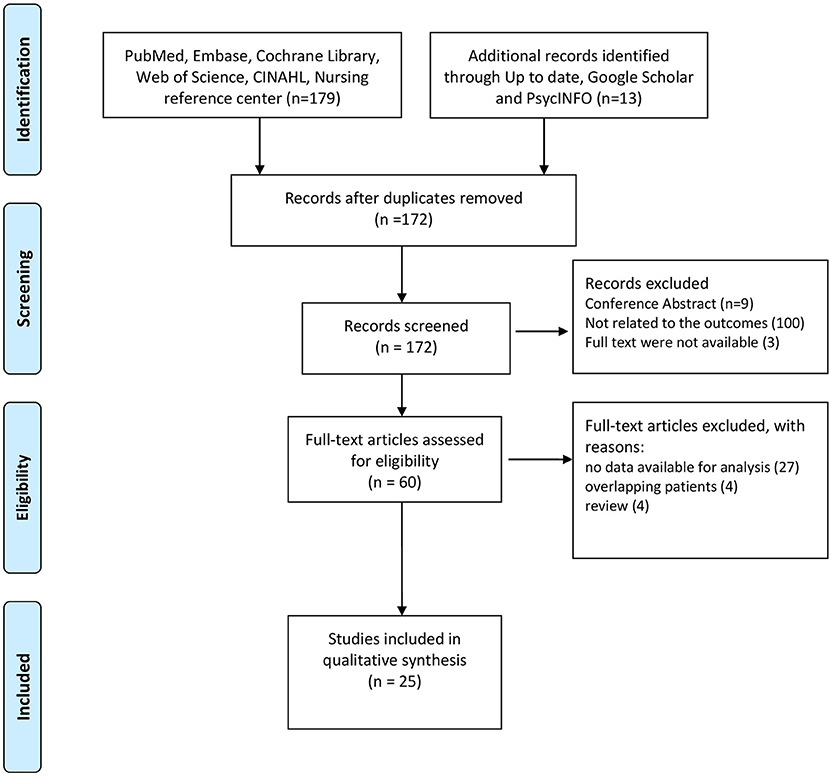
Figure 1. Preferred Reporting Items for Systematic Reviews (PRISMA) flow diagram showing the progress of studies through the review.
A summary of results from the studies included in this systematic review is reported in Tables 2, 3.
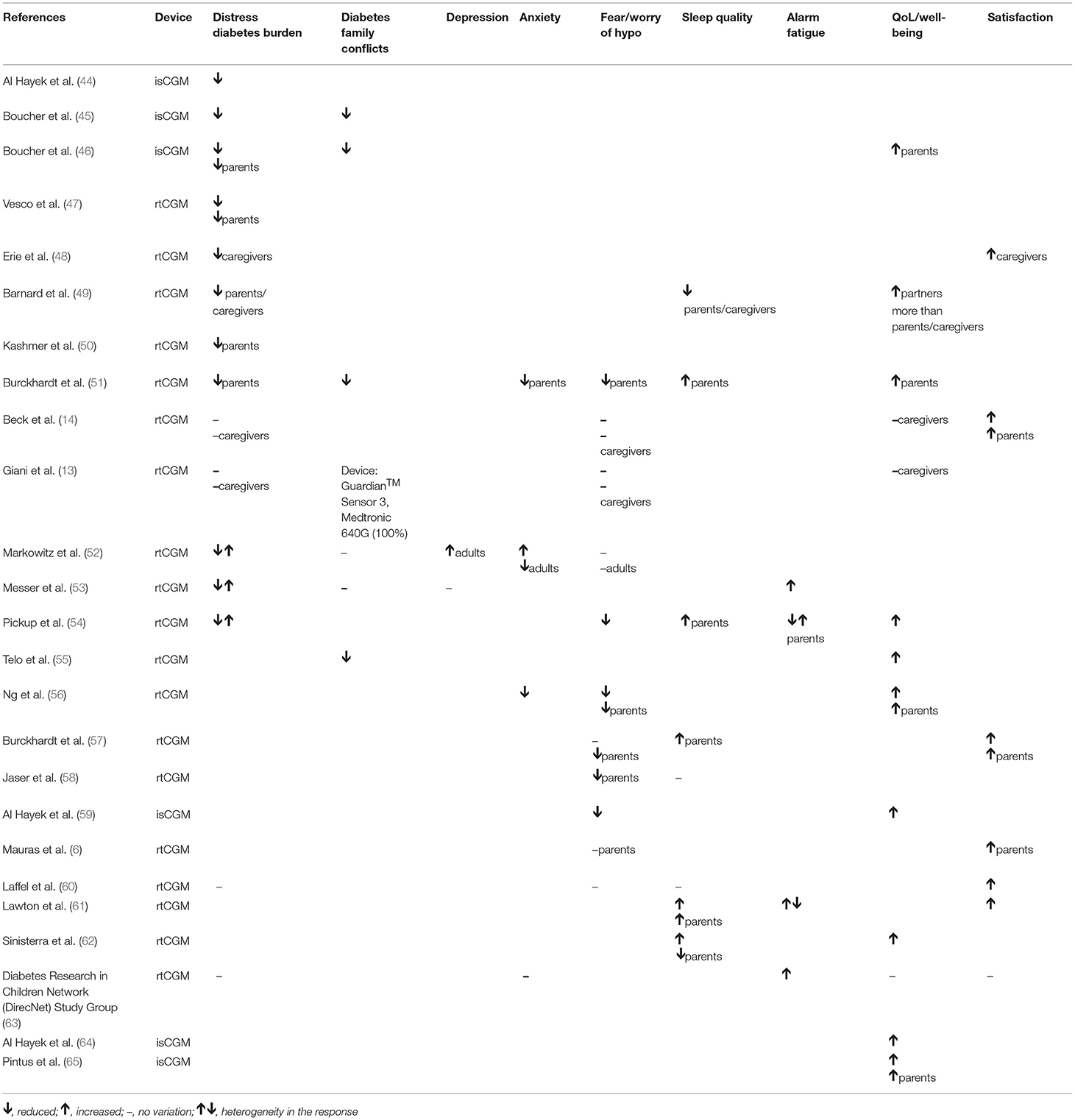
Table 3. Summary of the evidence: rtCGM and isCGM impact on psychological outcomes in children (and parents/caregivers where specified).
Distress/Diabetes Burden
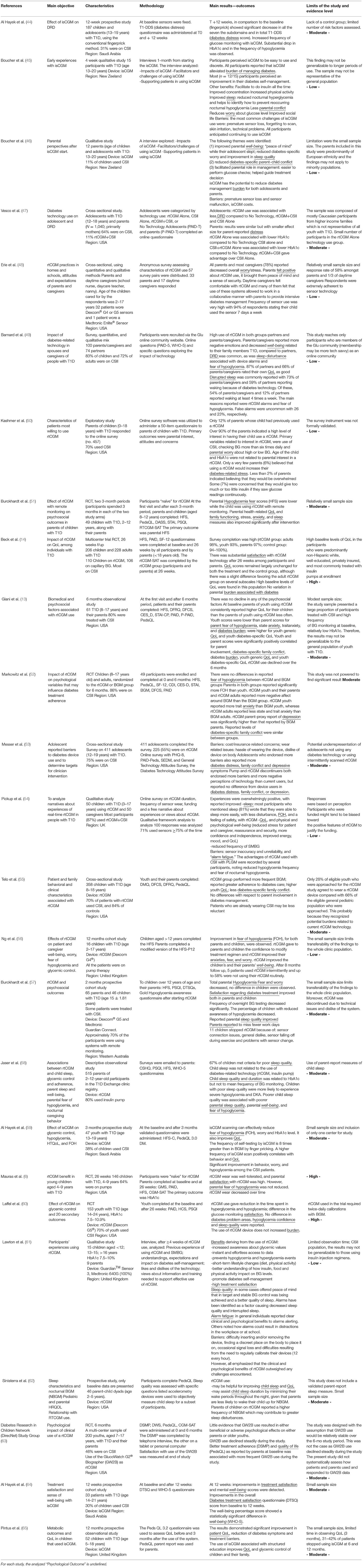
This outcome is analyzed in 3 studies on isCGM use and in 12 studies on rtCGM use in youth and their caregivers.
In pediatric patients isCGM reduced psychological distress for all the domains analyzed during a 12-weeks prospective study in children/adolescents [(44), Moderate] and in a 4-weeks qualitative study in adolescents/young adults [(45), Low]. This effect was reported also in parents of children and adolescents in a qualitative study [(46), Low].
RtCGM reduced diabetes burden in adolescent patients according to a cross-sectional study [(47), Moderate]. A similar effect was described for caregivers in five studies [(48–50), Low, (47, 51), Moderate]. In two studies no variation in diabetes burden was found both in children and caregivers [(13, 14), High-Moderate]. Broad effects were highlighted in three studies [(52–54), Moderate-Low].
Family Conflict in the Management of Diabetes
This outcome is measured in 2 studies on isCGM use and in 5 studies on rtCGM use in youth and their caregivers.
IsCGM use was associated with a reduction in diabetes specific parent-child conflict and parental conflict in patients aged 13–20 years in 2 qualitative studies [(45, 46), Low].
RtCGM use was associated with both a reduction in family conflicts and an improvement in rtCGMs related family functioning in 2 studies included in the review [(51, 55), Moderate]. These benefits were related to a decrease in the workload associated to blood glucose monitoring (BGM) and to an increased sense of safety [(51), Moderate]. In a RCT very similar levels of family conflict between the intervention group (rtCGM) and the control group (BGM) were found [(52), Moderate]. In other two studies no differences in family conflict were reported after the initiation of rtCGM use [(13, 53), Moderate]. The perception of a high number of obstacles and barriers related to the use of rtCGM sensors is related to a greater number of family conflicts and difficulties in managing the disease [(53), Moderate].
Depression
Depression in youth using rtCGM is evaluated in two studies. In a cross-sectional study on rtCGM use in adolescents, more depressive symptoms were reported by those who faced more barriers [(53), Moderate]. In a RCT in children 8–17 years old, rtCGM parent-proxy report of depression was significantly higher than that reported by BGM parents [(52), Moderate]. Data on depression in youths using isCGM are lacking.
Anxiety
This outcome is measured in 3 studies on rtCGM use in youth. In a RCT evaluating children in the age 2–12 years and their parents, parental stress level was lower in the arm using rtCGM compared to the control group (51, Moderate). In another study including 16 children aged 2–17 years, rtCGM use was associated with an improvement in children and parents' anxieties [(56), Low].
In a RCT study, the group of youth with rtCGM reported more trait anxiety than BGM youth, whereas rtCGM adults reported less state and trait anxiety than BGM adults [(52), Moderate].
Data on anxiety in youths using isCGM are lacking.
Fear/Worry of Hypoglycemia
This outcome is measured in 1 study on isCGM use and in 14 studies on rtCGM use in youth.
Fear of hypoglycemia (FOH) was reduced by isCGM use in adolescents older than 12 years in a 3-month prospective study [(59), Moderate]. Similarly, rtCGM use reduced FOH in 16 children aged 2–12 years in a 12-month cohort study [(56), Low]. Likewise, fear associated with hypoglycemic events resulted significantly lower in parents of youth using rtCGM in several studies [(51, 57, 58), Moderate, (56), Low]. RtCGM reduced the fear of nocturnal hypoglycemia in youth when integrated with a pump that automatically suspend insulin delivery in case of hypoglycemia [(54), Low].
On the contrary, in several studies no differences were found in FOH in both youth using rtCGM/isCGM [(13, 14, 52, 57), Moderate-High] and their caregivers [(6, 13, 14, 52, 60), Moderate-High]. The fear of hypoglycemic events resulted higher in parents than in children [(52), Moderate] although the sensor use. This is probably related to the fact that not all parents have full confidence in rtCGM systems: some parents are worried that the sensor may not work properly and it does not intercept hypoglycemic events [(53), Moderate].
Sleep Quality
This outcome is measured in 7 studies on rtCGM use in youth. In an observational study, overall 67% of children with T1D met the criteria for poor sleep quality; a worse child sleep quality was associated with worse metabolic control and poorer parental sleep quality. Child sleep was not related to the use of diabetes-related technology (rtCGM, insulin pump) [(58), Moderate]. About caregivers, most experimented better sleep patterns with rtCGM [(51, 54), Low-Moderate], while others reported disturbed sleep due to the presence of alarms and to the fear of hypoglycemia [(49), Low].
In a qualitative study, 9 pairs of children and parents reported improved sleep quality with the sensor use [(61), Low]. A prospective study on 46 children and their parents found that kids who used rtCGM experienced fewer sleep disturbances than those who did not, but their parents had greater sleep disturbances related to a higher frequency of nocturnal blood glucose monitoring (NBGM) [(62), Moderate]. A RCT on youth aged 14–24 years using rtCGM, reported there were no differences in sleep quality between sensors users and non-users [(60), High]. Data on sleep quality in youths using isCGM are lacking.
Alarm Fatigue
This outcome is measured in 5 studies on rtCGM use in youth. Parents of children aged 3–17 years using rtCGM reported both positive and negative responses for alarms: helpful when signaling hypoglycemia but annoying when repeatedly sounding during the night; thus, most parents reported they would like to louder alarms [(54), Low]. In a qualitative study, most parents reported clear clinical and psychological benefits associated with alarms alerting, but others noted that alarms could interfer with daily activities in the workplace or at school [(61), Low]. While alarms could reinforce a sense of hypoglycemic safety, some individuals expressed ambivalent views, especially those who perceived alarms as signaling personal failure to achieve optimal glycemic control [(61), Low]. Two additional studies included in the review highlighted that alarms can often cause annoyance and discomfort [(53, 63), Moderate].
Day caregivers, teachers or school nurses, generally appreciate alarm systems and these are not perceived as a source of distraction or disturbance but as a tool that simplifies the management of the disease [(48), Low].
Quality of Life/Well-Being
Four studies reported on this outcome in patients with isCGM, as well as 9 studies in patients with rtCGM. The use of isCGM has been reported to improve QoL in children and adolescents [(59, 64), Moderate] as well as in their parents [(46, 65), Moderate-Low].
RtCGM systems has been reported to improve QoL in children, for easier management of insulin dosages, diet, physical activity and in school and extra-home management [(54, 55, 62), Moderate-Low]. Similarly, in parents of youths, rtCGM has been reported to improve QoL and well-being [(51, 56), Moderate-Low].
In 3 studies included in this review no variations in QoL were found after rtCGM intervention [(13, 14, 63), Moderate-High] in youths and their parents.
Parents scores regarding the QoL are significantly higher (indicative of a less favorable QoL) than the youth's one, confirming that the perception of parents regarding the QoL of their children is less favorable than the prospects of youth regarding their QoL [(63), Moderate]. Moreover, parents/caregivers compared to partners, reported more negative emotions and decreased well-being related to their family members with T1D [(49), Low].
Satisfaction
This outcome is measured in 7 studies on rtCGM use in youth. Most patients using rtCGM and their parents reported high treatment-related satisfaction [(49, 57, 61), Low-Moderate].
Three RCTs of high quality confirmed the satisfaction with rtCGM use (6, 14, 60). In the first RCT, 90% of parents of 4–9 years old children, reported a high degree of satisfaction with rtCGM: the use of rtCGM makes adjusting insulin easier, shows patterns in blood glucose not seen before, and makes them feel safer knowing that they will be warned about low blood glucose before it happens [(6), High]. In the second RCT, patients aged 14–24 years using rtCGM, reported higher glucose monitoring satisfaction compared to the BGM group over a 26-weeks study period [(60), High]. In the third RCT, in patients aged 7–17 years, satisfaction scores at 26 weeks were higher for both, youths and parents, with higher scores associated with a more frequent use of rtCGM [(14), High].
In a cross-sectional study using qualitative and quantitative methods, parents and caregivers of children aged 2–17 years, felt positive about rtCGM use [(48), Low].
Data on satisfaction in youths using isCGM are lacking.
Discussion And Conclusions
A large percentage of pediatric patients with T1D experiences negative emotions, including state of anxiety, fear, discouragement, and frustration for the burden of the disease management. The use of CGM systems improves glycemic control (60) but demands for extra efforts from patients and their parents. Therefore, it is important to assess if the use of rtCGM and isCGM systems is related to psychological issues (52).
Studies on how isCGM and rtCGM impact the psychological outcomes in children and their caregivers were evaluated in this systematic review. Some limitations of the revised studies need to be addressed (Table 2):
(i) the sample size resulted small or not representative of the general population is some studies; (ii) psychological measures were included as secondary outcomes in most of the studies; thus, in some cases, the study design was not adequate to support significant results; (iii) some of the questionnaires used to measure the psychological outcomes were not previously validated. Also, questionnaires varied from one study to another.
Data on psychological outcomes in the pediatric population using isCGM systems are still limited, probably due to their recent availability on the market. The use of isCGM in adolescents can reduce psychological distress, family conflicts and fear of hypoglycemia (44, 59) and improves QoL (59, 65) as reported by a Saudi Arabia group (44) in moderate quality studies. Currently, there is no evidence of a negative impact of the isCGM system on the psychological outcomes evaluated in this review. However, results from our literature review highlighted the lack of data on depression, anxiety, and quality of sleep in pediatric patients using isCGM.
Most of the studies reported that the use of rtCGM did not increase diabetes burden in adolescents and their parents/caregivers with a moderate-high quality of evidence and using the PAID-T and P-PAID-T questionnaires (6, 13, 14, 52, 60). Likewise, rtCGM did not impact the diabetes specific family conflict, as measured by DFRQ and DFCS questionnaires in a moderate quality study (13, 52). Furthermore, rtCGM did not change depressive symptoms assessed with CDI, CES-D (13), and PHQ8 questionnaires (53).
On the other hand, rtCGM resulted improving parental anxiety in a moderate quality RCT using the STAI questionnaire by Burckhardt et al. (51). However, these results were not confirmed in a moderate quality observational study using the same questionnaire, by Giani et al. (13).
Fear of hypoglycemia remains the most common diabetes-related issue among T1D, both for youth and their parents/caregivers. In a RCT (51), parental fear of hypoglycemia (FOH) evaluated by the HFS score resulted lower in the group using rtCGM. However, other moderate-high quality studies using the HFS and HCS questionnaire did not confirm this outcome (6, 13, 14, 60).
In a RCT, adolescents' sleep quality measured with the PSQI questionnaire was not different in youth using rtCGM (60). On the contrary, parental sleep quality improved with the use of rtCGM, both when measured with the PSQI questionnaire as well by accelerometry devices in parents of adolescents and of young children, respectively (62).
Alarm fatigue was broadly evaluated in patients using rtCGM by non-validated interviews. In most cases, individuals reported clear clinical and psychological benefits to alarms setting (61), but in some contexts alarms resulted annoying and intrusive (53).
In most of the studies the perceived QoL assessed by the PedsQL in patients and caregivers, resulted improved by the use of rtCGM (55, 62). In some other studies no variations in the PedsQL were reported (13, 14), probably due to the number of variables that may influence the perceived QoL in diabetes or due to the short-term follow-up. An increased satisfaction related with the rtCGM use was assessed in both parents and youth with the DTSQ, CGM-SAT, and GMS questionnaires in moderate-high quality studies (6, 14, 51, 60).
In conclusion, the benefits of isCGM and rtCGM use on glycemic control have been previously demonstrated (1, 2, 66, 67). Findings from the studies included in this systematic review suggest that: (i) the use of isCGM in adolescents can improve diabetes related distress, family conflicts, FOH and perceived QoL; depression, anxiety, and quality of sleep have not yet been evaluated with validated questionnaires; (ii) the use of rtCGM does not impact diabetes burden, diabetes specific family conflict and depressive symptoms. The effect of rtCGM use on the fear of hypoglycemia, the sleep quality and the anxiety is still debated. Further RCT studies specifically powered to investigate psychological outcomes are needed. The use of rtCGM increases both satisfaction and perceived QoL in youth and their parents, although alarm fatigue need to be prevented with alarm targeting.
Altogether, these findings represent an interesting overview to consider when families are in the process of deciding whether or not to start CGM use.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
RF and FM made a substantial contribution to the design of this literature review, in the acquisition of data, and their interpretation and analysis as well as in the writing of the manuscript. FM and RF selected the articles of this literary review. VC, MS, EM, and EG contributed to the critical revision of the manuscript for intellectual reasons and performed a thorough proofreading of the manuscript. All the authors have definitely approved the version to publish.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
T1D, type 1 diabetes; DRD, diabetes related distress; FOH, fear of hypoglycemia; QoL, quality of Life; HRQoL, health related quality of life; BG, blood glucose; BGM, blood glucose monitoring; isCGM, intermittently scanned/viewed CGM; CGM, continuous glucose monitoring; FGM, flash glucose monitoring; CSII, Continuous subcutaneous insulin infusion; PLGM, predictive low glucose management.
References
1. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. (2011) 343:d3805. doi: 10.1136/bmj.d3805
2. Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Ther. (2020) 11:83–95. doi: 10.1007/s13300-019-00720-0
3. Slover RH, Welsh JB, Criego A, Weinzimer SA, Willi SM, Wood MA, et al. Effectiveness of sensor-augmented pump therapy in children and adolescents with type 1 diabetes in the STAR 3 study. Pediatr Diabetes. (2012) 13:6–11. doi: 10.1111/j.1399-5448.2011.00793.x
4. Battelino T, Conget I, Olsen B, Schütz-Fuhrmann I, Hommel E, Hoogma R, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. (2012) 55:3155–62. doi: 10.1007/s00125-012-2708-9
5. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. (2010) 33:17–22. doi: 10.2337/dc09-1502
6. Mauras N, Beck R, Xing D, Ruedy K, Buckingham B, Tansey M. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care. (2012) 35:204–10. doi: 10.2337/dc11-1746
7. Tsalikian E, Fox L, Weinzimer S, Buckingham B, White NH, Beck R, et al. Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatr Diabetes. (2012) 13:301–7. doi: 10.1111/j.1399-5448.2011.00837.x
8. Chase HP, Beck RW, Xing D, Tamborlane WV, Coffey J, Fox LA, et al. Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther. (2010) 12:507–15. doi: 10.1089/dia.2010.0021
9. Miller KM, Hermann J, Foster N, Hofer SE, Rickels MR, Danne T, et al. Longitudinal changes in continuous glucose monitoring use among individuals with type 1 diabetes: international comparison in the German and Austrian DPV and U.S. T1D Exchange Registries. Diabetes Care. (2020) 43:e1–2. doi: 10.2337/dc19-1214
10. Beck RW, Buckingham B, Miller K, Wolpert H, Xing D, Block JM, et al. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. (2009) 32:1947–53. doi: 10.2337/dc09-0889
11. Englert K, Ruedy K, Coffey J, Caswell K, Steffen A, Levandoski, et al. Skin and adhesive issues with continuous glucose monitors: a sticky situation. J Diabetes Sci Technol. (2014) 8:745–51. doi: 10.1177/1932296814529893
12. Heinemann L, Kamann S. Adhesives used for diabetes medical devices: a neglected risk with serious consequences? J Diabetes Sci Technol. (2016) 10:1211–5. doi: 10.1177/1932296816662949
13. Giani E, Snelgrove R, Volkening LK, Laffel LM. Continuous glucose monitoring (CGM) adherence in youth with type 1 diabetes: associations with biomedical and psychosocial variables. J Diabetes Sci Technol. (2017) 11:476–83. doi: 10.1177/1932296816676280
14. Beck RW, Lawrence JM, Laffel L, Wysocki T, Xing D, Huang ES, et al. Quality-of-life measures in children and adults with type 1 diabetes: Juvenile Diabetes Research Foundation Continuous Glucose Monitoring randomized trial. Diabetes Care. (2010) 33:2175–7. doi: 10.2337/dc10-0331
15. Patton SR, Clements MA. Psychological reactions associated with continuous glucose monitoring in youth. J Diabetes Sci Technol. (2016) 10:656–61. doi: 10.1177/1932296816638109
16. Markowitz JT, Volkening LK, Butler DA, Laffel LM. Youth-perceived burden of type1diabetes: problem areas in diabetes survey-pediatric version (PAID-Peds). J Diabetes Sci Technol. (2015) 24:1080–5. doi: 10.1177/1932296815583506
17. Markowitz JT, Volkening LK, Butler DA, Antisdel-Lomaglio J, Anderson BJ, Laffel LM. Re-examining a measure of diabetes-related burden in parents of young people with type 1 diabetes: the Problem Areas in Diabetes Survey - Parent Revised version (PAID-PR). Diabet Med. (2012) 29:526–30. doi: 10.1111/j.1464-5491.2011.03434.x
18. Fisher L, Polonsky WH, Hessler DH, Masharani U, Blumer I, Peters AL, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Compl. (2015) 29:572–7. doi: 10.1016/j.jdiacomp.2015.01.012
19. Hood KK, Butler DA, Anderson BJ, Laffel LMB. Updated and revised diabetes family conflict scale. Diabetes Care. (2007) 30:1764–9. doi: 10.2337/dc06-2358
20. Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. J Pediatr Psychol. (1990) 15:477–92. doi: 10.1093/jpepsy/15.4.477
21. Fendrich M, Weissman MM, Warner V. Screening for depressive disorder in children and adolescents: validating the Center for Epidemiologic Studies Depression Scale for Children. Am J Epidemiol. (1990) 131:538–51. doi: 10.1093/oxfordjournals.aje.a115529
22. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Meas. (1977) 1:385–401. doi: 10.1177/014662167700100306
24. Brown TA, Chorpita BF, Korotitsch W, Barlow DH. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav Res Ther. (1997) 35:79–89. doi: 10.1016/S0005-7967(96)00068-X
25. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. (2009) 114:163–73. doi: 10.1016/j.jad.2008.06.026
26. Spielberger CD. Manual for the State-Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologists Press (1973).
27. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Test Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press (1983).
28. Wysocki T, Hough BS, Ward KM, Allen A, Murgai N. Use of blood glucose data by families of children and adolescents with IDDM. Diabetes Care. (1992) 15:1041–4. doi: 10.2337/diacare.15.8.1041
29. Green LB, Wysocki T, Reineck BM. Fear of hypoglycemia in children and adolescents with diabetes. J Pediatr Psychol. (1990) 15:633–41. doi: 10.1093/jpepsy/15.5.633
30. Clarke WL, Gonder-Frederick A, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab Suppl. (1998) 1:189–94. doi: 10.1515/JPEM.1998.11.S1.189
31. Polonsky WH, Fisher L, Hessler D, Edelman SV. Investigating hypoglycemic confidence in type 1 and type 2 diabetes. Diabetes Technol Ther. (2017) 19:131–6. doi: 10.1089/dia.2016.0366
32. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
33. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. (2001) 39:800–12. doi: 10.1097/00005650-200108000-00006
34. Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. (2003) 26:631–7. doi: 10.2337/diacare.26.3.631
35. Ware JE Jr. Identifying populations at risk: functional impairment and emotional distress. Manag Care. (2002) 11(10 Suppl.):15–7.
36. Topp CW, Ostergaard SD, Sondergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom. (2015) 84:167–76. doi: 10.1159/000376585
37. Bott U, Mühlhauser I, Overmann H, Berger M. Validation of a diabetes-specific quality-of-life scale for patients with type 1 diabetes. Diabetes Care. (1998) 21:757–69 doi: 10.2337/diacare.21.5.757
38. Shen W, Kotsanos JG, Huster WJ, Mathias SD, Andrejasich CM, Patrick DL. Development and validation of the Diabetes Quality of Life Clinical Trial Questionnaire. Med Care. (1999) 37(4 Suppl.):AS45–66. doi: 10.1097/00005650-199904001-00008
39. Carey MP, Jorgensen RS, Weinstock RS, Sprafkin RP, Lantinga LJ, Carnrike CL Jr, et al. Reliability and validity of the appraisal of diabetes scale. J Behav Med. (1991) 14:43–51. doi: 10.1007/BF00844767
40. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Validation of measures of satisfaction with and impact of continuous and conventional glucose monitoring. Diabetes Technol Ther. (2010) 12:679–84. doi: 10.1089/dia.2010.0015
41. Hood KK, Butler DA, Volkening LK, Anderson BJ, Laffel LM. The Blood Glucose Monitoring Communication questionnaire: an instrument to measure affect specific to blood glucose monitoring. Diabetes Care. (2004) 27:2610–5. doi: 10.2337/diacare.27.11.2610
42. Bradley C. The diabetes treatment satisfaction questionnaire: DTSQ. In: Bradley C, editor. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Chur: Harwood Academic Publishers (1994). p. 111–32.
43. Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. (2019) 111:105–14. doi: 10.1016/j.jclinepi.2018.01.012
44. Al Hayek AA, Robert AA, Al Dawish MA. Effectiveness of the freestyle libre flash glucose monitoring system on diabetes distress among individuals with type 1 diabetes: a prospective study. Diabetes Ther. (2020) 11:927–37. doi: 10.1007/s13300-020-00793-2
45. Boucher S, Blackwell M, Galland B, de Bock M, Crocket H, Wiltshire E, et al. Initial experiences of adolescents and young adults with type 1 diabetes and high-risk glycemic control after starting flash glucose monitoring - a qualitative study. J Diabetes Metab Disord. (2019) 19:37–46. doi: 10.1007/s40200-019-00472-5
46. Boucher SE, Aum SH, Crocket HR, Wiltshire EJ, Tomlinson PA, de Bock MI, et al. Exploring parental perspectives after commencement of flash glucose monitoring for type 1 diabetes in adolescents and young adults not meeting glycaemic targets: a qualitative study. Diabet Med. (2020) 37:657–64. doi: 10.1111/dme.14188
47. Vesco AT, Jedraszko AM, Garza KP, Weissberg-Benchell J. Continuous glucose monitoring associated with less diabetes-specific emotional distress and lower A1c among adolescents with type 1 diabetes. J Diabetes Sci Technol. (2018) 12:792–9. doi: 10.1177/1932296818766381
48. Erie C, Van Name MA, Weyman K, Weinzimer SA, Finnegan J, Sikes K, et al. Schooling diabetes: use of continuous glucose monitoring and remote monitors in the home and school settings. Pediatr Diabetes. (2018) 19:92–7. doi: 10.1111/pedi.12518
49. Barnard K, Crabtree V, Adolfsson P, Davies M, Kerr D, Kraus A, et al. Impact of type 1 diabetes technology on family members/significant others of people with diabetes. J Diabetes Sci Technol. (2016) 10:824–30. doi: 10.1177/1932296816645365
50. Kashmer L, Clarke W, Gurka M, Elchuri S, Nyer M, Gonder-Frederick L. Predictors of parental interest in continuous glucose monitoring for children with type 1 diabetes. Diabetes Technol Ther. (2009) 11:373–8. doi: 10.1089/dia.2008.0100
51. Burckhardt MA, Roberts A, Smith GJ, Abraham MB, Davis EA, Jones TW. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care. (2018) 41:2641–3. doi: 10.2337/dc18-0938
52. Markowitz JT, Pratt K, Aggarwal J, Volkening LK, Laffel LM. Psychosocial correlates of continuous glucose monitoring use in youth and adults with type 1 diabetes and parents of youth. Diabetes Technol Ther. (2012) 14:523–6. doi: 10.1089/dia.2011.0201
53. Messer LH, Tanenbaum ML, Cook PF, Wong JJ, Hanes SJ, Driscoll KA, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. (2020) 10:760–7. doi: 10.1089/dia.2019.0509
54. Pickup JC, Ford Holloway M, Samsi K. Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care. (2015) 38:544–50. doi: 10.2337/dc14-1855
55. Telo GH, Volkening LK, Butler DA, Laffel LM. Salient characteristics of youth with type 1 diabetes initiating continuous glucose monitoring. Diabetes Technol Ther. (2015) 17:373–8. doi: 10.1089/dia.2014.0290
56. Ng SM, Moore HS, Clemente MF, Pintus D, Soni A. Continuous glucose monitoring in children with type 1 diabetes improves well-being, alleviates worry and fear of hypoglycemia. Diabetes Technol Ther. (2019) 21:133–7. doi: 10.1089/dia.2018.0347
57. Burckhardt MA, Abraham MB, Mountain J, Coenen D, Paniora J, Clapin H, et al. Improvement in psychosocial outcomes in children with type 1 diabetes and their parents following subsidy for continuous glucose monitoring. Diabetes Technol Ther. (2019) 21:575–80. doi: 10.1089/dia.2019.0149
58. Jaser SS, Foster NC, Nelson BA, Kittelsrud JM, DiMeglio LA, Quinn M, et al. Sleep in children with type 1 diabetes and their parents in the T1D Exchange. Sleep Med. (2017) 39:108–15. doi: 10.1016/j.sleep.2017.07.005
59. Al Hayek AA, Robert AA, Al Dawish MA. Evaluation of FreeStyle Libre Flash Glucose Monitoring System on glycemic control, health-related quality of life, and fear of hypoglycemia in patients with type 1 diabetes. Clin Med Insights Endocrinol Diabetes. (2017) 10:10. doi: 10.1177/1179551417746957
60. Laffel LM, Kanapka LG, Beck RW, Bergamo K, Clements MA, Criego A, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. (2020) 323:2388–96. doi: 10.1001/jama.2020.6940
61. Lawton J, Blackburn M, Allen J, Campbell F, Elleri D, Leelarathna L, et al. Patients' and caregivers' experiences of using continuous glucose monitoring to support diabetes self-management: qualitative study. BMC Endocr Disord. (2018) 18:12. doi: 10.1186/s12902-018-0239-1
62. Sinisterra M, Hamburger S, Tully C, Hamburger E, Jaser S, Streisand R. Young children with type 1 diabetes: sleep, health-related quality of life, and continuous glucose monitor use. Diabetes Technol Ther. (2020) 22:639–42. doi: 10.1089/dia.2019.0437
63. Diabetes Research in Children Network (DirecNet) Study Group. Psychological aspects of continuous glucose monitoring in pediatric type 1 diabetes. Pediatr Diabetes. (2006) 7:32–8. doi: 10.1111/j.1399-543X.2006.00142.x
64. Al Hayek AA, Al Dawish MA. The potential impact of the freestyle libre flash glucose monitoring system on mental well-being and treatment satisfaction in patients with type 1 diabetes: a prospective study. Diabetes Ther. (2019) 10:1239–48. doi: 10.1007/s13300-019-0616-4
65. Pintus D, Ng SM. Freestyle libre flash glucose monitoring improves patient quality of life measures in children with Type 1 diabetes mellitus (T1DM) with appropriate provision of education and support by healthcare professionals. Diabetes Metab Syndr. (2019) 13:2923–6. doi: 10.1016/j.dsx.2019.07.054
66. Gruppo di studio SIEDP sul diabete in età pediatrica. Raccomandazioni sull'utilizzo della tecnologia in diabetologia pediatrica 2019. Acta Biomed. (2019) 90:5–88.
67. Massa GG, Gys I, Bevilacqua E, Wijnands A, Zeevaert R. Comparison of flash glucose monitoring with real time continuous glucose monitoring in children and adolescents with type 1 diabetes treated with continuous subcutaneous insulin infusion. Diabetes Res Clin Pract. (2019) 152:111–8. doi: 10.1016/j.diabres.2019.05.015
Keywords: psychological outcomes, isCGM, CGM, type 1 diabetes, child
Citation: Franceschi R, Micheli F, Mozzillo E, Cauvin V, Liguori A, Soffiati M and Giani E (2021) Intermittently Scanned and Continuous Glucose Monitor Systems: A Systematic Review on Psychological Outcomes in Pediatric Patients. Front. Pediatr. 9:660173. doi: 10.3389/fped.2021.660173
Received: 28 January 2021; Accepted: 06 April 2021;
Published: 05 May 2021.
Edited by:
Ronald Cohen, University of Chicago, United StatesReviewed by:
Ernesto Maddaloni, Sapienza University of Rome, ItalyGeorge Paltoglou, National and Kapodistrian University of Athens, Greece
Copyright © 2021 Franceschi, Micheli, Mozzillo, Cauvin, Liguori, Soffiati and Giani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Franceschi, cm9iZXJ0by5mcmFuY2VzY2hpQGFwc3MudG4uaXQ=; Francesca Micheli, bWljaGVsaS5mcmFuY2VzY2E5N0BnbWFpbC5jb20=
†These authors have contributed equally to this work
 Roberto Franceschi
Roberto Franceschi Francesca Micheli
Francesca Micheli Enza Mozzillo
Enza Mozzillo Vittoria Cauvin1
Vittoria Cauvin1 Massimo Soffiati
Massimo Soffiati Elisa Giani
Elisa Giani