
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 26 February 2021
Sec. Neonatology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.644462
This article is part of the Research Topic Organ Perfusion and Oxygenation in the Sick Infant View all 15 articles
Background: Anemia remains a common comorbidity of preterm infants in the neonatal intensive care unit (NICU). Left untreated, severe anemia may adversely affect organ function due to inadequate oxygen supply to meet oxygen requirements, resulting in hypoxic tissue injury, including cerebral tissue. To prevent hypoxic tissue injury, anemia is generally treated with packed red blood cell (RBC) transfusions. Previously published data raise concerns about the impact of anemia on cerebral oxygen delivery and, therefore, on neurodevelopmental outcome (NDO).
Objective: To provide a systematic overview of the impact of anemia and RBC transfusions during NICU admission on cerebral oxygenation, measured using near-infrared spectroscopy (NIRS), brain injury and development, and NDO in preterm infants.
Data Sources: PubMed, Embase, reference lists.
Study Selection: We conducted 3 different searches for English literature between 2000 and 2020; 1 for anemia, RBC transfusions, and cerebral oxygenation, 1 for anemia, RBC transfusions, and brain injury and development, and 1 for anemia, RBC transfusions, and NDO.
Data Extraction: Two authors independently screened sources and extracted data. Quality of case-control studies or cohort studies, and RCTs was assessed using either the Newcastle-Ottawa Quality Assessment Scale or the Van Tulder Scale, respectively.
Results: Anemia results in decreased oxygen-carrying capacity, worsening the burden of cerebral hypoxia in preterm infants. RBC transfusions increase cerebral oxygenation. Improved brain development may be supported by avoidance of cerebral hypoxia, although restrictive RBC transfusion strategies were associated with better long-term neurodevelopmental outcomes.
Conclusions: This review demonstrated that anemia and RBC transfusions were associated with cerebral oxygenation, brain injury and development and NDO in preterm infants. Individualized care regarding RBC transfusions during NICU admission, with attention to cerebral tissue oxygen saturation, seems reasonable and needs further investigation to improve both short-term effects and long-term neurodevelopment of preterm infants.
Anemia, described as low hemoglobin (Hb) or hematocrit (Ht) levels, is a common comorbidity in preterm infants in the neonatal intensive care unit (NICU) (1). The causes are multifactorial and include an immature hematopoietic system resulting in poor iron stores, decreased red blood cell (RBC) lifespan, low erythropoietin levels, and frequent blood sampling (2–4). Anemia is often poorly tolerated, resulting in tachycardia, apneic events, and poor feeding, and growth. Furthermore, it has been described that apparently stable anemic preterm infants increase their cardiac output up to 48 h after a transfusion. Though uncommon, this increases the risk of the development of left ventricular dysfunction (5).
When untreated, severe anemia may adversely affect organ function due to inadequate oxygen supply, possibly resulting in anemic tissue hypoxia and injury (6). Anemia may also result in alterations in cerebral oxygenation (7) and an increased risk for cerebral injury (8, 9). Existing data raise concerns about the impact of anemia on both short- and long-term neurodevelopmental outcome (NDO). The underlying mechanisms for neurodevelopmental sequelae are multifactorial and incompletely understood, but known causative factors include cerebral hypoxia, ischemia, oxidative injury, and fluctuations in cerebral perfusion (10–12).
Adequate neurologic development requires optimal oxygen supply to the central nervous system (13, 14). Anemia is usually treated with RBC transfusions to improve both short-term symptoms and long-term neurodevelopment. RBC transfusions increase red cell mass and oxygen-carrying capacity, although transfused adult RBCs have lower affinity for oxygen than fetal Hb, and thus lower the relative concentration of fetal Hb which may disrupt preterm homeostasis causing a potential decrease in cerebral blood flow (CBF) (15).
It has been estimated that more than 90% of extremely low-birth-weight infants receive one or more RBC transfusions during their NICU stay (3, 16). Transfusion thresholds remain controversial as RBC transfusions are associated with increased risk for ischemia-reperfusion damage or oxidative injury potentially resulting in transfusion-associated necrotizing enterocolitis, bronchopulmonary dysplasia and retinopathy of prematurity (1, 16). Several studies comparing high (liberal) and low (restrictive) Hb or Ht thresholds for RBC transfusion have been published (17–20), but controversies about when to transfuse anemic preterm infants still remain (21–23).
Near-infrared spectroscopy (NIRS) allows continuous, non-invasive monitoring of regional tissue oxygen saturation (rSO2) reflecting oxygen supply and metabolism (24, 25). The fractional tissue oxygen extraction (FTOE) reflects the balance between oxygen supply and consumption in the measured organ, taking the arterial oxygen saturation into account. It has been suggested that NIRS monitoring can provide relevant real-time data to assist in bedside decision-making regarding the hemodynamic status of an individual patient and to monitor the effect of therapeutic interventions such as RBC transfusions (26, 27).
This article provides a systematic review on the impact of anemia and RBC transfusions during NICU admission on neonatal cerebral oxygenation, measured using NIRS, and its association with brain injury and development and with neurodevelopmental outcomes in preterm-born children. In this systematic review, we present the literature published on this topic from the past 20 years.
This systematic review was performed according to the PRISMA guidelines for systematic reviews (28). To include all relevant original research articles for this review, we performed three separate PUBMED/EMBASE database searches independently by 2 authors (WSK and EMWK). Publications from January 1, 2000 to December 31, 2020 containing data on the impact of anemia and RBC transfusions on NIRS-based cerebral oxygenation, and/or brain injury and development, and/or NDO were selected. The complete search string of all three searches is available in the Supplementary Material.
Initial record titles were screened for relevance and abstracts of those records of potential relevance were reviewed. The third selection was based on the full-text of selected articles. Articles were included if they were written in English, contained original research in human subjects, focused on preterm neonates, and if at least part of the study group had anemia and/or received an RBC transfusion. Furthermore, cerebral oxygenation had to be assessed utilizing NIRS. We excluded articles that focused on fetal anemia or fetal transfusions. Articles focusing on exchange transfusions, erythropoietin and specific iron-deficiency anemia were also excluded. In addition to the database search, we reviewed the reference lists of the selected articles for additional relevant studies.
The quality of all selected cohort and case-control studies was assessed using the Newcastle-Ottawa Quality Assessment Scale. This assessment scale consists of 3 parts: selection, comparability, and outcome. Ratings of these 3 factors generate a score, ranging from 0–9 points, with 9 points for the highest quality. In addition, the quality of selected randomized controlled trials (RCTs) was assessed using the Van Tulder Scale for randomized controlled trials. This scale consists of 11 items for which 1 point can be acquired per item. Therefore, the total score ranges from 0 to 11, with 11 representing highest quality. The Van Tulder Scale is a scale tool that has been recommended by the Cochrane Collaboration Back Review Group for the methodological assessment of RCTs (29).
Our first search for anemia, RBC transfusions, and cerebral oxygenation resulted in 433 articles. The second search for anemia, RBC transfusions, and brain injury and development resulted in 514 articles. Our third search for anemia, RBC transfusions and NDO produced 2,370 articles. After removing duplicates, a total of 2,645 articles remained. We excluded 2,550 articles based on titles alone. Reasons for exclusion were pre-clinical/non-human studies or studies focusing on fetal anemia or anemia resulting from iron-deficiency.
Abstracts or full-text articles were assessed within the remaining 96 articles. By analyzing the reference lists of the remaining articles, we included one additional article. Fifty-nine articles were additionally excluded due to the following: no data on cerebral oxygenation, not based on preterm infants, being a review article, or no full-text publication available. Four articles were eligible for both outcome two and three. Finally, 38 studies were included in our systematic review (Figure 1): 22 studies on cerebral oxygenation (7, 15, 30–49), 10 on brain injury and development (17–20, 50–55), and 10 on neurodevelopmental outcome (17, 18, 50, 53, 56–61). Characteristics of these articles are presented in Tables 1–3. Quality assessment scores are presented in Supplementary Tables 1–3.
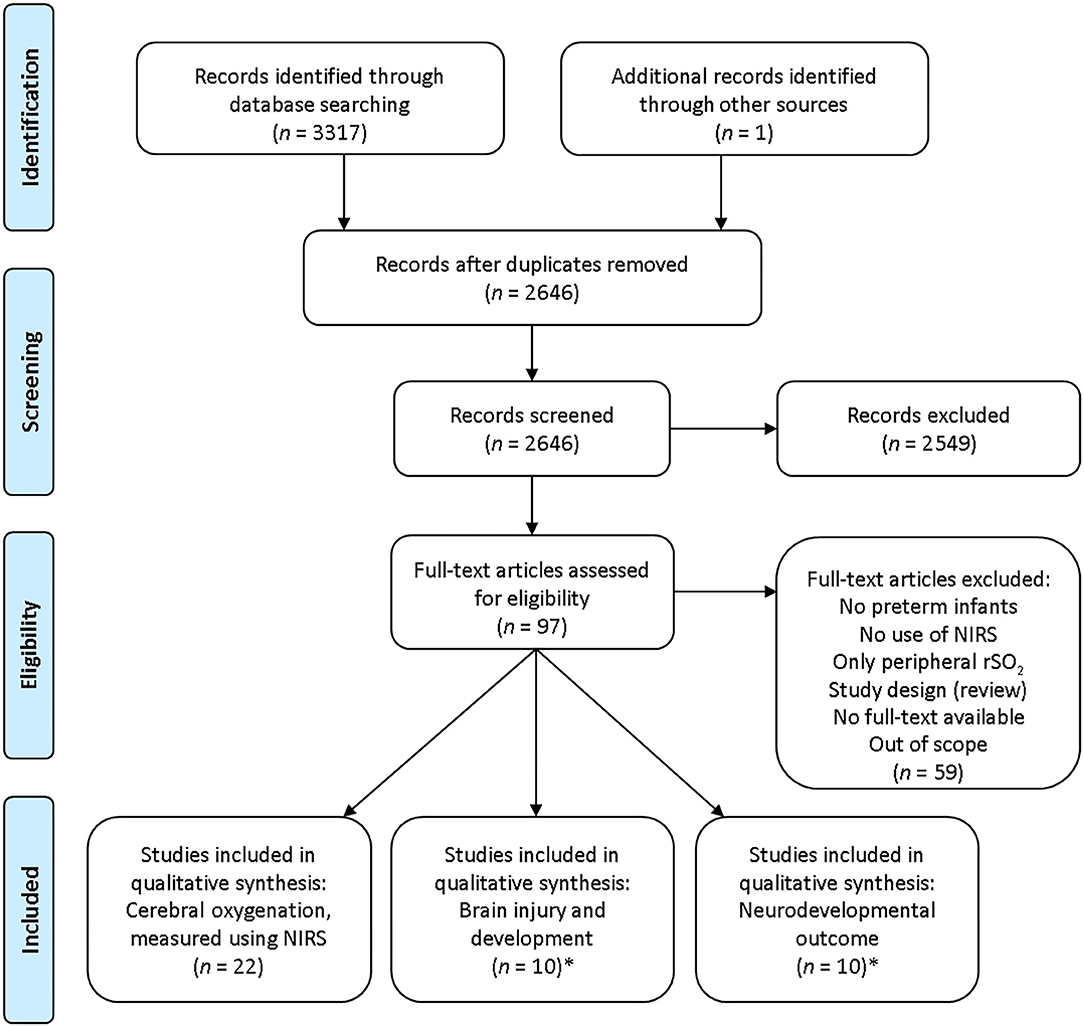
Figure 1. Flow diagram search strategy. NIRS, near-infrared spectroscopy; rSO2, regional tissue oxygen saturation.*Four articles were eligible for both outcome 2 and 3.
The effect of anemia and/or RBC transfusions on cerebral oxygenation was described in 22 articles (Table 1), representing a total of 854 preterm infants. These studies were observational case-control studies or cohort studies that compared cerebral oxygenation in preterm infants either before and after RBC transfusion or at subsequent times during NICU admission.
Five studies described anemia of prematurity and cerebral oxygenation. In general, during the first weeks after birth an increasing degree of anemia with progressive decrease in cerebral rSO2 (rcSO2) or increase in cerebral FTOE (cFTOE) was reported (31, 38, 40, 41). Mintzer et al. found no changes in cerebral oxygen saturation and extraction in 9 non-transfused neonates during the first week after birth (43). In a further report, they reported Hb to be inversely correlated with cFTOE, with increasing cFTOE hypothesized as a potential early marker of nascent anemia during the first 10 days after birth (37). Similar correlations between Hb and cerebral rSO2 or cerebral FTOE were described in 5 other articles (38–40, 44, 49). Conversely, Seidel et al. (46) and Bailey et al. (47) found no correlation between rcSO2 and Hb-levels.
In preterm infants receiving RBC transfusions according to local protocols, anemia was associated with lower rcSO2 in most cases (30, 39, 40, 44), but Wardle et al. found similar cFTOE between anemic infants and controls (49). In the latter study, however, many babies were transfused based on physician discretion, rather than on the cFTOE cut-off levels mentioned in their study protocol. Whitehead et al. reported a critical Hb threshold of 9.5 g/dL before cerebral oxygen saturation declined (31, 38). Similar results were demonstrated by Van Hoften et al. who described diminished cerebral oxygen saturation and increased cFTOE with a Hb-level below 9.7 g/dL (7).
The majority (83%) of the 18 studies that reported on cerebral oxygenation during and after RBC transfusion found rcSO2 to be higher during and after RBC transfusion compared to pre-transfusion levels in anemic preterm infants (7, 15, 30, 34, 35, 39, 41–49). Non-significant changes in cerebral oxygen saturation during and after RBC transfusions were observed in 3 studies (32, 33, 36).
The effect of RBC transfusion on cerebral oxygenation parameters was mostly short-lasting. Increased rcSO2 remained elevated until 12 or 24-h following transfusion in several studies (7, 32, 46, 47). Twenty-four hours following RBC transfusion, an even greater difference was measured compared with pre-transfusion cerebral oxygenation (7, 32), especially in infants with the lowest pre-transfusion Hb (7). Saito-Benz et al. described an immediate increase in rcSO2, followed by an attenuated rcSO2 back to pre-transfusion levels, during the 5 days after the RBC transfusion (35).
In eight studies, the effect of pre-transfusion anemia severity on cerebral oxygenation was taken into account when assessing rcSO2 and cFTOE after RBC transfusion (7, 30, 34, 39, 42, 43, 45, 46). Goldstein et al. (30) and Mintzer et al. (43) found an increased rcSO2 and decreased cFTOE irrespective of the pre-transfusion Hb or Ht. All others described a correlation with anemia severity. In particular, Van Hoften et al. reported that infants with a lower Hb-level before RBC transfusion demonstrated a more pronounced effect on cerebral oxygenation parameters (7). Andersen et al. only observed lowered cFTOE following RBC transfusion in infants with higher pre-transfusion cFTOE (42), and Seidel et al. described a more pronounced rcSO2 increase following RBC transfusion when infants had lower pre-transfusion rcSO2 values (46).
The main findings regarding the effects of neonatal anemia and RBC transfusions on brain injury and development were reported in 10 studies, most typically consisting of preterm infants being followed-up after participation in liberal vs. restrictive RBC transfusion threshold randomized trials. Table 2 provides an overview of these studies. Brain injury during NICU admission was described in 6 studies (n = 3,602 infants). In four other studies, brain development was described either at school age (n = 95 children) or at 34–37 weeks postmenstrual age (PMA) (n = 21 infants).
Brain injury during NICU admission was assessed using brain ultrasound (17–20, 50, 55). Both Kirpalani et al. (17), Franz et al. (18), and Chen et al. (55) showed no differences in percentage of infants with moderate IVH, severe IVH, or PVL between infants assigned to liberal vs. restrictive RBC transfusion thresholds. Non-significantly less abnormalities were shown on brain ultrasound in the low threshold group (19). Interestingly, more infants with severe IVH and PVL were reported in the group of infants that received less RBC transfusions during the IOWA randomized controlled trial (20). A retrospective study observed a higher incidence of severe brain injury in transfused preterm infants vs. non-transfused infants (50).
Concerning brain development, regional brain measures assessed on brain MRI were mostly smallest in female study participants, and were inversely related to average Ht-level: those children with the highest neonatal average Ht-level were the ones with the lowest volumes of white matter and thalamic volume at 12 years (51, 53, 54). Liberal RBC transfusion practices were associated with reduced cerebral white matter at school age, especially within the temporal lobe and subcortical nuclei (51, 53, 54).
Brain MRI at near-term age (PMA range 34.0–36.9 weeks) showed increased fractional oxygen extraction in brain tissue in infants with lower Ht-levels, suggesting ongoing hemodynamic compensation for anemia (52).
Ten studies (both RCTs and observational) described a relationship between anemia and RBC transfusions during NICU admission and NDO (Table 3).
Focusing only on the RCTs, there were 4 clinical trials comparing liberal and restrictive RBC transfusion strategies in which a total of 2919 children participated. The first by Kirpalani et al. was the TOP trial in which they found no differences in NDO at 22–26 months corrected age between preterm infants randomized to either liberal or restrictive transfusion thresholds (17). Another recently published RCT was the ETTNO trial by Franz et al. in which NDO was determined at 24 months corrected age in ELBW neonates (18). No significant differences in NDO were observed between the liberal and restrictive transfusion groups. Whyte et al. assessed NDO at 18–21 months corrected age in ELBW infants who originally participated in the PINT study (60). At follow-up, they observed a lower cognitive outcome in preterm-born children treated with a restrictive transfusion strategy. McCoy et al. reported NDO at 8 to 15 years of age in preterm-born children (58). Children transfused under the liberal strategy performed less on associative verbal fluency, visual memory and reading compared to children treated under the restrictive transfusion strategy. Furthermore, in a follow-up analysis, they found lower verbal fluency in preterm born female children at an average age of 13 years compared to preterm born male children (53).
Three observational studies demonstrated that the number of RBC transfusions was correlated with lower NDO scores at both 2 and 5 years corrected age (50), with lower cognitive, language and motor scores at 12 months adjusted age (57), and with a lower performance IQ than verbal IQ at 8–11 years (61). In the fourth observational study by Wang et al. they observed a higher mental developmental index score at 18 and 24 months corrected age in 62 ELBW infants who received RBC transfusions within 7 days after birth (56). There was one study reporting a lack of effect of transfusion volume on NDO at 24 months' corrected age (59).
In this systematic review, we aimed to increase understanding of the impact of anemia and RBC transfusions on the developing brain of the preterm infant. This systematic review demonstrated that anemia of varying severity may reduce oxygen supply to the brain of preterm infants. RBC transfusions, on the other hand, improve oxygen supply to the brain. Infants with more severe anemia demonstrated a more pronounced short-term effect of an RBC transfusion, which is likely important for long-term outcomes by avoiding anemic hypoxic injury. Severe anemia during NICU admission seems to be associated with disturbances of brain development, even though findings on long-term outcome suggest potential neuroprotective benefits from a restrictive RBC transfusion threshold.
Cerebral oxygenation continues to demonstrate promise for predicting outcome in preterm infants (11, 62), as this measure reflects the integration of multiple parameters including oxygen delivery and oxygen demand and consumption (63).
In general, decreasing Hb-level correlated with either decreasing rcSO2 or increasing cFTOE (30, 37–40, 44, 49) A few studies described a critical Hb-threshold around 9.5 g/dL before cerebral oxygen saturation and extraction undergo noticeable changes (7, 31, 38). Furthermore, increased PNA was associated with lower Hb-levels and a progressive decrease in rcSO2 or increase in cFTOE (31, 38, 40, 41). In other studies, varying PMA might have prevented demonstration of a correlation between Hb-level and cerebral oxygenation (46, 47). Additionally, the duration of measuring cerebral oxygenation seems to be important. Wardle et al. did not find a difference in cFTOE between anemic infants and controls (49). However, cFTOE was measured for only 10 min in this study, as compared with measurements taken over hours by subsequent researchers.
As pre-transfusion baseline cerebral oxygen saturation decreases with increasing chronological age, it is likely that CBF and oximetry responses to RBC transfusion are dependent on chronological age in preterm infants. As expected, cerebral oxygen saturation and extraction in most cases were significantly affected by RBC transfusion. RcSO2 was higher during and up to 24 h after the RBC transfusion when compared to rcSO2 pre-transfusion levels (7, 15, 30, 34, 35, 39, 41–49). Cerebral oxygen saturation, however, attenuated to pre-transfusion values during subsequent days, questioning the clinical relevance of the briefly improved cerebral oxygenation. A possible explanation is the RBC transfusion leading to an increased preload, cardiac output and CBF. Over subsequent days, this enhanced CBF response may diminish with oxygenation parameters returning to pre-transfusion values (35). Another explanation is the increased fraction of adult Hb in comparison with before RBC transfusion, thus reducing the fraction of fetal Hb with a shift in dissociation curve (15). Possible explanations for not finding a significant difference in cerebral oxygenation during and after RBC transfusion in several reports, may relate to liberal transfusion thresholds (32), missing rcSO2 data before RBC transfusions (36), or adequate cerebral autoregulation providing a constant CBF (33).
The effect of the RBC transfusion on cerebral oxygenation was more pronounced in infants with lower pre-transfusion Hb- or Ht-levels (7, 34, 39, 42, 45, 46). Increased oxygen extraction under baseline conditions leaves little reserve to meet the demands of brain tissue during oxygen desaturations. An explanation for not finding differences between pre-transfusion anemia severity might be the fact that peripheral tissues demonstrate a more robust response than the brain, possibly as a result of the neuroprotective maintenance of cerebral oxygen delivery (37, 48). This regulation of oxygen-carrying capacity to the brain might explain the findings of increased rcSO2 and decreased cFTOE after RBC transfusion irrespective of pre-transfusion anemia severity (30). Another possibility for these findings may be related to the effects of other RBC transfusion strategies, i.e., “booster” transfusions (43).
Concerning brain injury, anemia has previously been associated with a significant increase in CBF (64), which has been posited as a risk factor for developing IVH (8). Conversely, if this compensatory mechanism fails, there could be an increased risk for hypoxic brain injury. The association between RBC transfusion strategy and brain injury during NICU admission is still under debate. Most studies observed no difference in presence of brain injury between RBC transfusion strategies (17–19, 55). Conversely, Bell et al. (20) reported more infants with severe IVH and PVL following restrictive RBC transfusion thresholds, possibly because of rather low Hb- and Ht-levels in their restrictive RBC transfusion threshold infants compared to mean Hb- and Ht-levels in other study participants (17–19).
Regarding brain development, this systematic review demonstrated more available evidence for brain structure abnormalities at school age among neonates transfused under liberal transfusion thresholds (51, 53, 54) Children with highest average Ht-levels had lowest brain volumes at 12-years of age, supporting the notion that the abnormalities are indeed related to Ht-level (and thus to transfusion status) (51, 53, 54). Of note, all three follow-up studies describing brain MRI at school age included a sample of children that were initially enrolled in the same randomized controlled trial (20).
Similarly, available evidence supports a restrictive RBC transfusion strategy, showing a favorable NDO at school age among preterm infants randomized to lower RBC transfusion thresholds during NICU admission (17, 18, 53, 57, 58, 61). Apart from one study (60), this also holds true for NDO at 2 years corrected age.
There seems to be a discrepancy between short-term outcomes, NDO at 2 years of age, and long-term NDO at school age. A restrictive RBC transfusion strategy was associated with poorer short-term outcomes and with poorer NDO at 2 years' corrected age (20, 60), while longer-term outcomes may be adversely affected by liberal RBC transfusion strategies (53, 54, 58). In light of the beneficial effect of a more restrictive strategy, the liberal transfusion group expectantly demonstrated the greatest abnormality in brain structure with significant decrements in intracranial volume (53, 54). However, data on the relationship between brain structure in school-aged children originally assigned to the restrictive transfusion strategy are lacking. These authors speculate that a lack of endogenous erythropoietin in the liberal group may be associated with worse outcome. Endogenous erythropoietin is essential for the production of erythrocytes. Several studies have reported substantial neuroprotective properties of erythropoietin, functioning in the brain as both an important growth factor and a neuroprotective agent (65–68). RBC transfusions during NICU admission may result in less endogenous erythropoietin production. This suppression of erythropoietin may translate into “loss” of a growth factor known to promote brain growth and recovery from brain injury (66).
The results of this review suggest that a restrictive transfusion strategy is associated with better gain in Hb-level, oxygen delivery, and cerebral oxygen saturation following RBC transfusion. The preterm brain, however, is particularly vulnerable to hypoxic injury (69). Cerebral oxygenation may be at risk when Hb-levels decrease below 9.5 g/dL (7, 31, 38). Existing reference data on rcSO2 suggest reference values between 65 and 75% using an INVOS monitor in combination with neonatal sensors during the first week after birth (70–73). Furthermore, Verhagen et al. showed cerebral oxygenation between 72 and 83% to be associated with a favorable NDO (11). More recently, Alderliesten et al. also observed low cerebral oxygenation to be associated with poorer cognitive outcome, suggesting a threshold of approximately 65% using neonatal sensors (74). An increasing cFTOE may also indicate an early pathophysiological response to anemia (37, 52) and may serve as a potential biomarker for cerebral injury and long-term NDO in premature infants. Identification of the vulnerable subgroup of preterm infants with low cerebral oxygen saturation may be clinically important to administer RBC transfusions in a timely manner leading to better clinical outcomes. We confirm previous implications that RBC transfusions improve tissue oxygenation and that tissue oxygenation itself may play an important role in identifying the trigger for RBC transfusion (7, 15, 34, 44, 46, 47). Suboptimal precision of current NIRS measurements, however, preclude us from determining absolute thresholds (75).
This systematic review has several limitations. First, many included studies were observational in nature. These are associated with a risk of bias of either under- or overestimating outcome measures. Furthermore, inclusion of mainly observational studies makes it difficult to draw definite conclusions. Second, unless studied prospectively, infants who were assigned in both observational studies and RCTs investigating RBC transfusion strategies form a biased group. Almost all studies, however, were of reasonable to good quality according to the quality assessments. Finally, preterm infants requiring RBC transfusions were younger, smaller, sicker, and had more frequent inotropic treatments. Therefore, they already have a higher risk for morbidity and adverse NDO. A relatively large number of infants who had otherwise similar neonatal clinical conditions, however, were enrolled in all included publications.
This systematic review suggests that anemia and RBC transfusions during NICU admission contributed significantly to brain development and NDO in preterm infants, possibly by its association with cerebral oxygenation. An individualized approach regarding RBC transfusion strategy using NIRS-based cerebral tissue oxygen saturation assessments in order to support brain growth and development and to prevent neurodevelopmental delay in anemic preterm infants seems reasonable. When combining the results of the aims for this review, one might suggest that when cerebral oxygen saturation drops below the levels associated with poorer NDO, i.e., below 65 or 70%, this insinuates the need for further evaluation to determine whether anemia is present. If Hb-level is low, this would warrant considering an RBC transfusion. Whether using a lower threshold of cerebral oxygen saturation to trigger RBC transfusion needs further prospective investigation.
The original contributions generated for the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
WK conceptualized and designed the study, developed the search strategy, screened databases for eligible studies, assessed full-text articles for eligibility, conducted the quality assessment, drafted the initial manuscript, and revised the manuscript after feedback from coauthors. EV conceptualized and designed the study, drafted part of the initial manuscript, and critically reviewed and revised the manuscript. JM and AB conceptualized and designed the study, and critically reviewed and revised the manuscript. EK conceptualized and designed the study, provided support with the search strategy, screened databases for eligible studies, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
WK was financially supported by the Junior Scientific Masterclass of the University of Groningen.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Ms S. van der Werf, medical information specialist of the University Medical Center Groningen, for her assistance in compiling the search strategy for this systematic review. This study was part of the research program of the Graduate School of Medical Sciences, Research Institute SHARE, University of Groningen.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.644462/full#supplementary-material
1. Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. (2016) 315:889–97. doi: 10.1001/jama.2016.1204
2. Colombatti R, Sainati L, Trevisanuto D. Anemia and transfusion in the neonate. Semin Fetal Neonatal Med. (2016) 21:2–9. doi: 10.1016/j.siny.2015.12.001
3. Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. (2009) 155:331–7.e1. doi: 10.1016/j.jpeds.2009.02.026
4. Aher S, Melwatkar K, Kadam S. Neonatal anemia. Semin Fetal Neonatal Med. (2008) 13:239–47. doi: 10.1016/j.siny.2008.02.009
5. Quante M, Pulzer F, Blaser A, Gebauer C, Kluge J, Robel-Tillig E. Effects of anaemia on haemodynamic and clinical parameters in apparently stable preterm infants. Blood Transfus. (2013) 11:227–32. doi: 10.2450/2012.0171-11
6. Alkalay AL, Galvis S, Ferry DA, Simmons CF, Kruger Jr RC. Hemodynamic changes in anemic premature infants: are we allowing the hematocrits to fall to low? Pediatrics. (2003) 112:838–45. doi: 10.1542/peds.112.4.838
7. Van Hoften JCR, Verhagen EA, Keating P, ter Horst HJ, Bos AF. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch Dis Child Fetal Neonatal Ed. (2010) 95:F352–8. doi: 10.1136/adc.2009.163592
8. Balegar KK, Stark MJ, Briggs N, Andersen CC. Early cerebral oxygen extraction and the risk of death or sonographic brain injury in very preterm infants. J Pediatr. (2014) 164:475–80.e1. doi: 10.1016/j.jpeds.2013.10.041
9. Andersen CC, Collins CL. Poor circulation, early brain injury, and the potential role of red cell transfusion in premature newborns. Pediatrics. (2006) 117:1464–6. doi: 10.1542/peds.2005-3197
10. Serenius F, Ewald U, Farooqi A, Fellman V, Hafstrom M, Hellgren K, et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatr. (2016) 170:954–63. doi: 10.1001/jamapediatrics.2016.1210
11. Verhagen EA, Van Braeckel KNJA, van der Veere CN, Groen H, Dijk PH, Hulzebos CV, et al. Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Dev Med Child Neurol. (2015) 57:449–55. doi: 10.1111/dmcn.12622
12. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. (2008) 371:261–9. doi: 10.1016/S0140-6736(08)60136-1
13. Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA. (2018) 319:2190–201. doi: 10.1001/jama.2018.5725
14. Andersen CC, Hodyl NA, Kirpalani HM, Stark MJ. A theoretical and practical approach to defining Adequate oxygenation in the preterm newborn. Pediatrics. (2017) 139:e20161117. doi: 10.1542/peds.2016-1117
15. Dani C, Pezzati M, Martelli E, Prussi C, Bertini G, Rubaltelli FF. Effect of blood transfusions on cerebral haemodynamics in preterm infants. Acta Paediatr. (2002) 91:938–41. doi: 10.1111/j.1651-2227.2002.tb02881.x
16. Nunes dos Santos AM, Guinsburg R, Branco de Almeida MF, Procianoy RS, Rodrigues Leone C, Martins Marba ST, et al. Red blood cell transfusions are independently associated with intra-hospital mortality in very low birth weight preterm infants. J Pediatr. (2011) 159:371–6.e1–3. doi: 10.1016/j.jpeds.2011.02.040
17. Kirpalani H, Bell EF, Hintz SR, Tan S, Schmidt B, Chaudhary AS, et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N Engl J Med. (2020) 383:2639–51. doi: 10.1056/NEJMoa2020248
18. Franz AR, Engel C, Bassler D, Rudiger M, Thome UH, Maier RF, et al. Effects of liberal vs restrictive transfusion thresholds on survival and neurocognitive outcomes in extremely low-birth-weight infants: the ETTNO randomized clinical trial. JAMA. (2020) 324:560–70. doi: 10.1001/jama.2020.10690
19. Kirpalani H, Whyte RW, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The premature infants in need of transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. (2006) 149:301–7. doi: 10.1016/j.jpeds.2006.05.011
20. Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. (2005) 115:1685–91. doi: 10.1542/peds.2004-1884
21. Kirpalani H, Whyte RK. What is new about transfusions for preterm infants? An update. Neonatology. (2019) 115:406–10. doi: 10.1159/000499048
22. Howarth C, Banerjee J, Aladangady N. Red blood cell transfusion in preterm infants: current evidence and controversies. Neonatology. (2018) 114:7–16. doi: 10.1159/000486584
23. Askie LM, Darlow BA, Davis PG, Finer N, Stenson B, Vento M, et al. Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants. Cochrane Database Syst Rev. (2017) 4:CD011190. doi: 10.1002/14651858.CD011190.pub2
24. Mintzer JP, Moore JE. Regional tissue oxygenation monitoring in the neonatal intensive care unit: evidence for clinical strategies and future directions. Pediatr Res. (2019) 86:296–304. doi: 10.1038/s41390-019-0466-9
25. Garvey AA, Kooi EMW, Smith A, Dempsey EM. Interpretation of cerebral oxygenation changes in the preterm infant. Children. (2018) 5:94. doi: 10.3390/children5070094
26. Van Bel F, Mintzer JP. Monitoring cerebral oxygenation of the immature brain: a neuroprotective strategy? Pediatr Res. (2018) 84:159–64. doi: 10.1038/s41390-018-0026-8
27. Banerjee J, Aladangady N. Biomarkers to decide red blood cell transfusion in newborn infants. Transfusion. (2014) 54:2574–82. doi: 10.1111/trf.12670
28. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
29. van Tulder M, Furlan A, Bombardier C, Bouter L; Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine. (2003) 28:1290–9. doi: 10.1097/01.BRS.0000065484.95996.AF
30. Goldstein GP, Rao A, Ling AY, Ding VY, Chang IJ, Chock VY. Influence of enteral feeding and anemia on tissue oxygen extraction after red blood cell transfusion in preterm infants. Transfusion. (2020) 60:466–72. doi: 10.1111/trf.15680
31. Whitehead HV, Vesoulis ZA, Maheshwari A, Rambhia A, Mathur AM. Progressive anemia of prematurity is associated with a critical increase in cerebral oxygen extraction. Early Hum Dev. (2019) 140:104891. doi: 10.1016/j.earlhumdev.2019.104891
32. Jani P, Lowe K, Hinder M, Galea C, D'Cruz D, Badawi N, et al. Liberal hemoglobin threshold affects cerebral arterial pulsed doppler and cardiac output, not cerebral tissue oxygenation: a prospective cohort study in anemic preterm infants. Transfusion. (2019) 59:3093–101. doi: 10.1111/trf.15452
33. Aktas S, Ergenekon E, Ozcan E, Aksu M, Unal S, Hirfanoglu IM, et al. Effects of blood transfusion on regional tissue oxygenation in preterm newborns are dependent on the degree of anemia. J Paediatr Child Health. (2019) 55:1209–13. doi: 10.1111/jpc.14378
34. Jain D, D'Ugard C, Bancalari E, Claure N. Cerebral oxygenation in preterm infants receiving transfusion. Pediatr Res. (2019) 85:786–9. doi: 10.1038/s41390-018-0266-7
35. Saito-Benz M, Gray C, Tzeng Y-C, Atkinson G, Berry MJ. Cerebral oxygenation and cardiorespiratory stability following liberal transfusion in preterm neonates. Acta Paediatr. (2019) 108:559–61. doi: 10.1111/apa.14631
36. Kalteren WS, Kuik SJ, Van Braeckel KNJA, Hulscher JBF, Bos AF, Kooi EMW, et al. Red blood cell transfusions affect intestinal and cerebral oxygenation differently in preterm infants with and without subsequent necrotizing enterocolitis. Am J Perinatol. (2018) 35:1031–7. doi: 10.1055/s-0038-1636532
37. Mintzer JP, Parvez B, La Gamma EF. Regional tissue oxygen extraction and severity of anemia in very low birth weight neonates: a pilot NIRS analysis. Am J Perinatol. (2018) 35:1411–8. doi: 10.1055/s-0038-1660458
38. Whitehead HV, Vesoulis ZA, Maheshwari A, Rao R, Mathur AM. Anemia of prematurity and cerebral near-infrared spectroscopy: should transfusion thresholds in preterm infants be revised? J Perinatol. (2018) 38:1022–9. doi: 10.1038/s41372-018-0120-0
39. Li L, Wu R, Kong X, Huang L, Wang Z, Hao J, et al. Effect of anemia and blood transfusion on tissue oxygen saturation and blood pressure in very preterm infants. Int J Clin Exp Med. (2017) 10:2974–9.
40. El-Dib M, Aly S, Govindan R, Mohamed M, du Plessis A, Aly H. Brain maturity and variation of oxygen extraction in premature infants. Am J Perinatol. (2016) 33:814–20. doi: 10.1055/s-0036-1572542
41. Banerjee J, Leung TS, Aladangady N. Cerebral blood flow and oximetry response to blood transfusion in relation to chronological age in preterm infants. Early Hum Dev. (2016) 97:1–8. doi: 10.1016/j.earlhumdev.2015.10.017
42. Andersen CC, Karayil SM, Hodyl NA, Stark MJ. Early red cell transfusion favourably alters cerebral oxygen extraction in very preterm newborns. Arch Dis Child Fetal Neonatal Ed. (2015) 100:F433–5. doi: 10.1136/archdischild-2014-307565
43. Mintzer JP, Parvez B, Chelala M, Alpan G, La Gamma EF. Monitoring regional tissue oxygen extraction in neonates <1250 g helps identify transfusion thresholds independent of hematocrit. J Neonatal Perinatal Med. (2014) 7:89–100. doi: 10.3233/NPM-1477213
44. Sandal G, Oguz SS, Erdeve O, Akar M, Uras N, Dilmen U. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion. (2014) 54:1100–5. doi: 10.1111/trf.12359
45. Koyano K, Kusaka T, Nakamura S, Nakamura M, Konishi Y, Miki T, et al. The effect of blood transfusion on cerebral hemodynamics in preterm infants. Transfusion. (2013) 53:1459–67. doi: 10.1111/j.1537-2995.2012.03953.x
46. Seidel D, Blaser A, Gebauer C, Pulzer F, Thome U, Knupfer M. Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants. J Perinatol. (2013) 33:282–7. doi: 10.1038/jp.2012.108
47. Bailey SM, Hendricks-Munoz KD, Wells JT, Mally P. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am J Perinatol. (2010) 27:445–53. doi: 10.1055/s-0030-1247598
48. Dani C, Pratesi S, Fontanelli G, Barp J, Bertini G. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion. (2010) 50:1220–6. doi: 10.1111/j.1537-2995.2009.02575.x
49. Wardle SP, Yoxall CW, Weindling AM. Determinants of cerebral fractional oxygen extraction using near infrared spectroscopy in preterm neonates. J Cereb Blood Flow Metab. (2000) 20:272–9. doi: 10.1097/00004647-200002000-00008
50. Fontana C, Raffaeli G, Pesenti N, Boggini T, Cortesi V, Manzoni F, et al. Red blood cell transfusions in preterm newborns and neurodevelopmental outcomes at 2 and 5 years of age. Blood Transfus. (2020). doi: 10.2450/2020.0207-20. [Epub ahead print].
51. Benavides A, Conrad AL, Brumbaugh JE, Magnotta V, Bell EF, Nopoulos P. Long-term outcome of brain structure in female preterm infants: possible associations of liberal versus restrictive red blood cell transfusions. J Matern Fetal Neonatal Med. (2019) 13:1–8. doi: 10.1080/14767058.2019.1683157
52. Morris EA, Juttukonda MR, Lee CA, Patel NJ, Pruthi S, Donahue MJ, et al. Elevated brain oxygen extraction fraction in preterm newborns with anemia measured using noninvasive MRI. J Perinatol. (2018) 38:1636–43. doi: 10.1038/s41372-018-0229-1
53. McCoy TE, Conrad Al, Richman LC, Brumbaugh JE, Magnotta VA, Bell EF, et al. The relationship between brain structure and cognition in transfused preterm children at school age. Dev Neuropsychol. (2014) 39:226–32. doi: 10.1080/87565641.2013.874428
54. Nopoulos PC, Conrad AL, Bell EF, Strauss RG, Widness JA, Magnotta VA, et al. Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch Pediatr Adolesc Med. (2011) 165:443–50. doi: 10.1001/archpediatrics.2010.269
55. Chen HL, Tseng HI, Lu CC, Yang SN, Fan HC, Yang RC. Effect of blood transfusions on the outcome of very low birth weight preterm infants under two different transfusion criteria. Pediatr Neonatol. (2009) 50:110–6. doi: 10.1016/S1875-9572(09)60045-0
56. Wang Y-C, Chan O-W, Chiang M-C, Yang P-H, Chu S-M, Hsu J-F, et al. Red blood cell transfusion and clinical outcomes in extremely low birth weight preterm infants. Pediatr Neonatol. (2017) 58:216–22. doi: 10.1016/j.pedneo.2016.03.009
57. Velikos K, Soubasi V, Michalettou I, Sarafidis K, Nakas C, Papadopoulou V, et al. Bayley-III scales at 12 months of corrected age in preterm infants: patterns of developmental performance and correlations to environmental and biological influences. Res Dev Disabil. (2015) 45–6:110–9. doi: 10.1016/j.ridd.2015.07.014
58. McCoy TE, Conrad AL, Richman LC, Lindgren SD, Nopoulos PC, Bell EF. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol. (2011) 17:347–67. doi: 10.1080/09297049.2010.544647
59. Von Lindern JS, Khodabux CM, Hack KEA, van Haastert IC, Koopman-Esseboom C, van Zwieten PHT, et al. Long-term outcome in relationship to neonatal transfusion volume in extremely premature infants: a comparative cohort study. BMC Pediatr. (2011) 11:48. doi: 10.1186/1471-2431-11-48
60. Whyte RK, Kirpalani H, Asztalos EV, Andersen C, Blajchman M, Heddle N, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. (2009) 123:207–13. doi: 10.1542/peds.2008-0338
61. Gabrielson J, Hard AL, Ek U, Svensson E, Carlsson G, Hellstrom A. Large variability in performance IQ associated with postnatal morbidity, and reduced verbal IQ among school-aged children born preterm. Acta Paediatr. (2002) 91:1371–8. doi: 10.1111/j.1651-2227.2002.tb02836.x
62. Hyttel-Sorensen S, Greisen G, Als-Nielsen B, Gluud C. Cerebral near-infrared spectroscopy monitoring for prevention of brain injury in very preterm infants. Cochrane Database Syst Rev. (2017) 9:CD011506. doi: 10.1002/14651858.CD011506.pub2
63. Naulaers G, Morren G, van Huffel S, Casaer P, Devlieger H. Measurements of tissue oxygenation index during the first three days in premature born infants. Adv Exp Med Biol. (2003) 510:379–83. doi: 10.1007/978-1-4615-0205-0_63
64. Pryds O, Greisen G. Effect of PaCO2 and haemoglobin concentration on day to day variation of CBF in preterm neonates. Acta Paediatr Scand Suppl. (1989) 360:33–6. doi: 10.1111/j.1651-2227.1989.tb11279.x
65. Juul SE, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, Robinson T, et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N Engl J Med. (2020) 382:233–43. doi: 10.1056/NEJMoa1907423
66. Juul SE, Vu PT, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, et al. Effect of high-dose erythropoietin on blood transfusions in extremely low gestational age neonates: post hoc analysis of a randomized clinical trial. JAMA Pediatr. (2020) 174:933–43. doi: 10.1001/jamapediatrics.2020.2271
67. Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev. (2020) 2:CD004863. doi: 10.1002/14651858.CD004863.pub6
68. Aher SM, Ohlsson A. Late erythropoiesis-stimulating agents to prevent red blood cell transfusion in preterm or low birth weight infants. Cochrane Database Syst Rev. (2020) 1:CD004868. doi: 10.1002/14651858.CD004868.pub6
69. Altman DI, Perlman JM, Volpe JJ, Powers WJ. Cerebral oxygen metabolism in newborns. Pediatrics. (1993) 92:99–104.
70. Alderliesten T, Dix L, Baerts W, Caicedo A, van Huffel S, Naulaers G, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res. (2016) 79:55–64. doi: 10.1038/pr.2015.186
71. Dix LML, van Bel F, Baerts W, Lemmers PMA. Comparing near-infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr Res. (2013) 74:557–63. doi: 10.1038/pr.2013.133
72. Hyttel-Sorensen S, Austin T, van Bel F, Benders M, Claris O, Dempsey EM, et al. Clinical use of cerebral oximetry in extremely preterm infants is feasible. Dan Med J. (2013) 60:A4533.
73. McNeill S, Gatenby JC, McElroy S, Engelhardt B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J Perinatol. (2011) 31:51–7. doi: 10.1038/jp.2010.71
74. Alderliesten T, van Bel F, van der Aa NE, Steendijk P, van Haastert IC, de Vries LS, et al. Low cerebral oxygenation in preterm infants is associated with adverse neurodevelopmental outcome. J Pediatr. (2019) 207:109–16e2. doi: 10.1016/j.jpeds.2018.11.038
Keywords: anemia, prematurity, cerebral oxygenation, neuroimaging, neurodevelopmental outcome
Citation: Kalteren WS, Verhagen EA, Mintzer JP, Bos AF and Kooi EMW (2021) Anemia and Red Blood Cell Transfusions, Cerebral Oxygenation, Brain Injury and Development, and Neurodevelopmental Outcome in Preterm Infants: A Systematic Review. Front. Pediatr. 9:644462. doi: 10.3389/fped.2021.644462
Received: 21 December 2020; Accepted: 08 February 2021;
Published: 26 February 2021.
Edited by:
Eugene Dempsey, University College Cork, IrelandReviewed by:
Frank Van Bel, University Medical Center Utrecht, NetherlandsCopyright © 2021 Kalteren, Verhagen, Mintzer, Bos and Kooi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Willemien S. Kalteren, dy5zLmthbHRlcmVuQHVtY2cubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.