
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 10 September 2021
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.635176
Background: Colic is a common condition in infants <4 months of age. Attempts to treat infantile colic with probiotics have shown variable efficacy and overall low evidence of success. In this work, we tested the hypothesis that oral administration of Bifidobacterium longum CECT7894 (KABP042) and Pediococcus pentosaceus CECT8330 (KABP041) mix (1 × 109 colony forming units) would improve the symptoms of infantile colic.
Methods: A total of 112 exclusively breastfed or mixed fed infants aged <2 months and meeting the ROME IV criteria for infantile colic were recruited. The infants were randomized in a double-blind, placebo-controlled trial to receive orally administered probiotics (intervention group, IG, n = 48) or placebo (placebo group, PG, n = 42) daily for 21 days.
Results: Infants in the IG had significantly shorter crying time (p < 0.001) on day 7 [IG vs. PG, median (25−75th percentile): 38 (3.5–40.5) vs. 62 (40–108) min/day], day 14 [IG vs. PG: 20 (0–40) vs. 50 (30–75) min/day], and day 21 [IG vs. PG: 14 (0–33) vs. 40 (28–62) min/day]. Higher responder ratio and fewer crying/fussing episodes on days 7, 14, and 21 and better stool consistency on day 21 were observed in the IG (p < 0.01) as compared to the PG. Conversely, no significant effects on stool frequency or quality of life were observed.
Conclusions: In summary, daily oral administration of B. longum CECT7894 (KABP042) and P. pentosaceus CECT8330 (KABP041) was an effective treatment for shortening crying time due to infantile colic and for improving fecal consistency. This trial was registered retrospectively in December 2019 with a trial number of ISRCTN92431452.
Infantile colic is a behavioral condition in early infancy that involves long crying bouts and hard-to-soothe behavior for no apparent cause. This distressing condition affects up to 20% of infants under 3 months of age (1). Although infantile colic spontaneously resolves after the first 3–4 months after birth, it is associated with maternal depression, early breastfeeding cessation, and shaken baby syndrome (2). As a common problem in pediatric and family medicine practice, infantile colic imposes significant costs to healthcare sectors (3). The etiology of infantile colic remains unclear; however, various theories have been proposed such as overproduction of intestinal gas, hypersensitivity to cow's milk proteins, transient lactase deficiency, and gut inflammation (4–6). The parents and caretakers often seek medical care for colic including the use of drugs, vegetable fiber, lactase, sucrose solution, hypoallergenic diet, and herbal tea. Nevertheless, no single effective and safe intervention for infantile colic exists (7, 8).
The interest of using probiotics as a potential treatment to reduce crying in colicky infants has increased lately. Recent studies have reported that gut microbiota in colicky infants is characterized by lower proportions of Lactobacilli and Bifidobacteria and higher proportions of opportunistic proteobacteria (such as Escherichia coli, Enterobacter aerogenes, and Klebsiella spp.) (9) as compared with that in the control infants. Thus, several researchers have suggested that probiotics may be useful for treating breastfed colicky infants and reducing their crying time (10, 11).
Pediococcus pentosaceus CECT8330 (KABP041) can induce anti-inflammatory interleukin 10 (IL-10) production. Its combination with Bifidobacterium longum CECT7894 (KABP042) has shown a broad spectrum of inhibitory activities against gas-producing enteropathogens. Both strains have been isolated from fecal samples of healthy infants (12). In this randomized, double-blind, placebo-controlled trial, we aim to assess whether intervention with the two-combined probiotic strains, B. longum CECT7894 (KABP042) and P. pentosaceus CECT8330 (KABP041), as found in a currently marketed product, would reduce daily crying or fussing time (primary outcome), decrease daily episodes of crying or fussing, and improve parental quality of life (secondary outcomes).
This randomized, double-blinded, placebo-controlled parallel-group study was performed in Qingbaijiang, Jinniu, and Wuhou Districts of Chengdu City and Huili County of Xichang City, Sichuan Province, China from June 1st, 2018 to June 1st, 2019. Infants diagnosed with colic by pediatricians in the outpatient care department at Qingbaijiang Maternal and Child Health Hospital, Caojiaxiang Community Healthcare Center, Huili Maternity and Child Care Center, and Angel Children's Hospital Chengdu were recruited. Nursing advices were provided to caregivers of the infants by nurses in each hospital. The enrollment and research plan were reviewed and approved by the institutional ethics committee of Angel Children's Hospital Chengdu (acting as the centralized ethics committee). Written informed consent was obtained from parents of each infant. The present study complied with the code of ethics of the World Medical Association (Declaration of Helsinki). No important changes to methodology was made after trial commencement and all planned outcomes were reported. This trial was registered retrospectively in December 2019 with a trial number of ISRCTN92431452 (http://www.isrctn.com/ISRCTN92431452).
Infants meeting the ROME IV diagnostic criteria for infantile colic (i.e., colicky full force crying or fussing episodes lasting at least 3 h per day and occurring at least 3 days per week within 7 days prior to enrollment, confirmed with a prospectively-kept diary between baseline visit and day 1 visit) (13) were recruited. Additional inclusion criteria were: <3 months (12 weeks) of age, ≥37 weeks of gestation at birth, vaginal delivery, birth weight >2,500 g, and parents providing written informed consent. Exclusion criteria were: average weight gain <100 grams/week from birth to the last recorded weight, major medical problems (e.g., immunocompromised disease, major developmental or genetic abnormality), gastrointestinal disorder, taking antibiotics 4 weeks prior to enrollment or during the intervention, using probiotic supplements 2 weeks prior to enrollment. Participation in the study was voluntary. The parents had the right to withdraw their child from the study without providing a reason and with no loss of benefits to which the child was entitled. If parents chose to withdraw their child, the study personnel was informed immediately. The investigators had the right to terminate participation of any child at any time if they deemed it the child's best interest. Possible reasons for withdrawal of a study subject included: child's parents withdrew consent for personal reasons; child's general condition contraindicated continuing the study as judged by the study personnel or the medical expert, significant non-compliance with study protocol or lack of cooperation, serious adverse event, and loss to follow-up. To exclude organic causes of crying, infants were examined by the study pediatrician at recruitment and during the intervention.
Based on a previous study (14), a sample size of 40 infants per group was estimated as sufficient to detect a significant difference in crying time between the intervention and placebo groups using a two-sided Z test. The significant level of the test was targeted at 0.05 with a power of 80%. Statistical power was calculated using the G*Power software (15). The sample size was rounded up 40% to account for potential attrition. As a result, a total of 112 subjects were recruited into this study.
The study included five visits to the clinics: (i) a baseline visit, which corresponded to the day when parents were referred to the pediatrician with the main complaint of the baby's crying and/or fussing without a definite reason; (ii) day 1 visit, when the ROME IV colic diagnostic criteria were verified and when participants were randomized for intervention; and (iii) day 7, 14, and 21 visits, that corresponded to the weekly assessments during the 3-week intervention phase from day 1. Caregivers were reminded of the study instructions on each visit.
A completely randomized schedule that maintains balance between treatment arms in a 1:1 proportion was prepared by an independent statistician, who was not directly involved in the analysis of the study results. The RAND function of Excel (Microsoft, Redmond, WA, USA) was used to generate randomly permutated codes. The physicians responsible for enrollment of patients allocated the next available number to the subject. At enrollment, the parents of the patient received a closed envelope containing a written usage guidance for the oil drops. To minimize potential biases, the study was double-blinded whereby treatment allocation was concealed from all study investigators and participants. Drug containers prepared by an independent pharmacy were sequentially numbered and opened sequentially. Containers were of identical appearance, tamper-proof, and equal in weight. All the caregivers have been well-trained by using a standardized protocol.
All eligible colicky infants were randomized to receive either the probiotics (intervention group, IG) or a reference product without the probiotics (placebo group, PG). There was no delay between randomization and the initiation of the intervention. The probiotic formula comprised a pure sunflower oil suspension (not an emulsion) of strains B. longum CECT7894 (KABP042) and P. pentosaceus CECT8330 (KABP041). Each five drops of the formula contained one billion total colony-forming units (CFU) in a 1:1 proportion for the two strains, as stated by the manufacturer under ICH zone-II stability conditions. However, the exact counts in the clinical batch were not independently verified in this study. The reference product (placebo) contained an identical sunflower oil, without the probiotics. Both the probiotic and the placebo were manufactured by PROCEMSA srl (Italy) for AB-Biotics SA (Spain), the company owning IP rights of the strains. PROCEMSA has GMP and ISO9001 certifications and tests the quality of each batch using the European pharmacopeia-compliant methods for total yeasts and molds, total Gram-negative bile-tolerant bacteria, E. coli, Salmonella, Staphylococcus aureus, Listeria monocytogenes, and peroxides. Additionally, PROCEMSA tests for heavy metals using ICP-OES. Products are routinely shipped from Spain to China in containers controlled for temperature with data loggers. Shelf life of the product is 2 years from manufacturing when stored at room temperature (25°C) or below. Expiry date is noted on boxes. The products were used before reaching the expiry date.
Caregivers, who received the standardized operation training from nurses at the time of recruitment, administered five drops of the study product orally to each infant, daily for 21 days. Caregivers were instructed to give five more drops if regurgitation occurred right after the first administration. The dose was not required to be given at a fixed time or given with feeds. However, for compliance and ease of administration, each family has been recommended to give the dose with the same feed each day. When the administration was completed, the empty bottle was retrieved to assess the adherence.
The probiotics and the placebo products were indistinguishable in smell, taste, and appearance. Both products were only labeled with the randomization number, batch number, expiry date, and the statement “For clinical trial use only.” The random allocation sequence was generated by an independent statistician. Caregivers of each participant received a numbered treatment bottle based on the sequence. Parents of the subjects, the clinical team, statisticians, and representatives from AB-Biotics S.A were blinded during the entire study until the database was unlocked.
The primary outcome (daily crying or fussing time) and secondary outcome (number of crying/fussing episodes, fecal consistency, and parental quality of life) assessments were adapted from Sung et al. with minor modifications (16) and were measured in all study visits. The proportion of responders [defined as subjects who had a reduction in colicky full force crying/fussing time of ≥50% from baseline according to a previous publication (10)] were calculated on the 7th, 14th, and 21st days of the intervention. The validated Barr diary (17) was used to record the infant colicky full force crying/fussing time (mins/day), number of episodes of colicky full force crying/fussing/day, stool consistency, and stool frequency. Stool consistency on diapers was scored as 0 for watery stool, 1 for loose stool, 2 for formed stool, and 3 for hard stool as per Amsterdam consistency subscale (18). PedsQLTM, a 15-item validated questionnaire, was used to assess family functioning (19), each item being scored based on its frequency (0: never happened, 1: almost never happened, 2: happened sometimes, 3: happened often, 4: almost always happened, and 5: always happened). The nurses explained to the children's caregivers how to properly record all the information. There were no changes to trial outcome measures after the trial commenced. In addition, demographic information was collected by a questionnaire at baseline. Potential confounders were also recorded at baseline (family history of atopy, antenatal or current probiotic/antibiotic use, and smoking during pregnancy) and during the intervention [infant feeding method (breast vs. formula); mother's intake of dairy products, probiotics, and medications; infant's intake of dairy products, probiotics, solids, and medications; concurrent illnesses/immunizations]. Compliance was measured by the number of days over the preceding week when the study drops were not administered. Occurrence of side effects was monitored throughout the study.
The distribution of the data was tested for normality prior to analysis using the Shapiro-Wilks normality test. Baseline characteristics and study outcomes were presented as mean and standard deviation (SD) for normally distributed variables or median and 25th and 75th percentiles (P25, P75) for variables with a skewed distribution. All tests of significance were two-tailed with an α = 0.05. The Student's t test was used to compare the mean values of continuous variables approximating a normal distribution. For non-normally distributed variables, the Kruskal-Wallis or chi-square test was used. Relative risk based on the proportion of responders on the 7th, 14th, and 21st days during the intervention and its 95% confidence interval were calculated. Data were analyzed using the SAS for Windows statistical software package (SAS Institute Inc., Cary, NC, USA).
A total of 112 infants met the inclusion criteria (56 for each group). Five infants were excluded due to parental rejection. Of the remaining 107 infants, 17 (15.9%) dropped out during the study. Three of these infants were withdrawn for using other probiotics, seven for loss to follow-up, three for formula-related allergy (formulas used in the study were of the partially hydrolyzed type), and four for diarrhea. Of note, antacid medication is normally not used in colicky infants in China. No infant in the study was taking antacid medication. Thus, the primary and secondary outcome measures were obtained from 90 infants (48 for the IG and 42 for the PG) (Figure 1). Despite the drop-outs, the study sample size (n = 48 for the IG and n = 42 for the PG) retained 80.9% power for crying time, 99.1% power for number of episodes, and 81.6% power for responder rate on day 21, when the differences between the IG and the PG were the smallest during the 7–21 day period. Even higher statistical powers were reached on days 7 and 14.
There were no significant differences in gender, allergy history, age, birth weight, passive smoking exposure, living environment, parents' educational levels (all above illiteracy, primary school, and middle school levels), and feeding method during the intervention between the two groups (p > 0.05) (Table 1). Most infants were exclusively breastfed during the study period, few had mixed feeding (all of them being >50% breastfed), and none received water-based products or fruit juices. No treatment-related side effects occurred during the study period.
The Shapiro-Wilks normality test showed that crying/fussing time and frequency were both non-normally distributed variables. Therefore, the Kruskal-Wallis test was used to compare the difference between the two groups at different time points. There were no significant differences in crying/fussing time and frequency between the IG and the PG at baseline (p > 0.05, Figure 2). However, crying/fussing time in the IG was significantly shorter than that in the PG on the 7th, 14th, and 21st days of the intervention (p < 0.001, Figure 2).
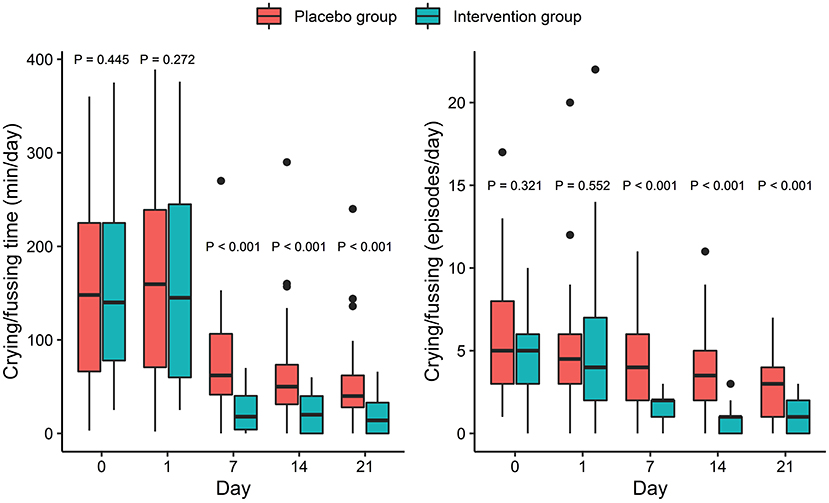
Figure 2. Effect of intervention on crying/fussing time (left) and number of crying/fussing episodes (right). P-values correspond to Kruskal-Wallis test performed at each timepoint. Box and whiskers denote quartiles and 95% percentiles.
The crying/fussing frequency, fecal frequency, and fecal consistency scores were all non-normally distributed variables and thus the Kruskal-Wallis test was used to assess the differences between the groups. There were no significant differences in crying/fussing frequency between the IG and the PG at baseline (p > 0.05, Figure 2). Nevertheless, the frequency of the crying/fussing episodes in the IG was lower than that in the PG on the 7th, 14th, and 21st days of the intervention (p < 0.001, Figure 2). Also, a higher proportion of responders was observed in the IG as compared with the PG on the 7th (83.3 vs. 35.7%, p < 0.001), 14th (95.8 vs. 42.9%, p < 0.001), and 21st (89.5 vs. 64.3%, p = 0.004) days (Table 2).
As shown in Table 3, there were no significant differences in fecal frequency and consistency score between the IG and the PG before the intervention (p > 0.05). Infants in the IG had lower fecal frequency/day than infants in the PG. The differences were already significant on the 1st day and further increased on the 7th, 14th, and 21st days (p < 0.001, Table 3). Conversely, the fecal consistency score of infants in the IG was significantly higher than that of infants in the PG on the 21st day of intervention only (p < 0.001, Table 3).
There were no significant differences in the PedsQL™ Family Impact Subscales between the IG and the PG at baseline (p > 0.05, Table 4). The Physical Function Subscale in the IG was marginally improved without reaching significance when compared with infants in the PG on the 21st day (p = 0.065) and a similar result was found for the Social Function Subscale on the 14th day (p = 0.070, Table 4). Finally, no significant side effects occurred during the study period.
Administration of B. longum CECT7894 (KABP042) and P. pentosaceus CECT8330 (KABP041) at a dose of 109 CFU per day to exclusively breastfed or mixed fed infants was superior to the placebo for the management of infantile colic. The use of the two strains significantly reduced the crying/fussing time and the frequency of episodes. No differences were observed on stool consistency until day 21, when a small but significant increase in consistency was observed in the IG group. Conversely, differences in the stool frequency were already apparent on day 1 and became more significant during the study. However, the fact those differences were already present on day 1 precludes attributing the effect to the probiotic intervention. Besides, no significant effects were observed on family quality of life. No adverse events and unintended effects were recorded during the intervention. Pediococcus strains have been used as probiotics in other clinical trials to treat several diseases, including obesity (20), diarrhea (21), trauma (22), and Helicobacter pylori infection (23). Strains belonging to Lactobacillus and Bifidobacterium groups have been previously trialed in colicky infants, while evidence for Pediococcus is scant. However, one must consider that Pediococcus are not only phylogenetically close to the Lactobacillus genus (24), but are also found in breast milk (25).
Our results were in agreement with previous studies that used probiotics to treat infantile colic. The administration of Lactobacillus reuteri DSM 17938 improved colic symptoms, although the effectiveness has only been seen in breastfed infants and not in formula-fed infants (11, 26). Another study showed that treatment with a combination of L. casei, L. rhamnosus, Streptococcus thermophilus, B. breve, L. acidophilus, B. infantis, L. bulgaricus, and fructooligosaccharides (FOS) reduced the duration of crying by almost 35 min compared to the placebo (27). Moreover, Saavedra et al. and Ivakhnenko et al. reported reduced incidence of caregiver-reported colic when infants were supplemented with a combination of B. animalis subsp. lactis BB-12 and an unidentified S. thermophilus strain, although colic was not formally diagnosed by a physician which reduced the strength of the studies (28, 29). A recent study on the same BB-12 strain overcame this shortcoming by formally diagnosing colic using the Rome-III criteria (30). However, this study showed that the response rate was not significantly improved against placebo until day 21, while in our study a significant improvement was observed from day 7. Conversely, a study reported that the use of L. rhamnosus GG (ATCC53103) had no significant effect on crying of colicky infants (31). In another study, no significant differences in crying and irritability were found between the probiotics and placebo groups when supplemented with either L. reuteri ATCC55730 or B. lactis BB-12 (32).
Possible placebo effect should be recognized (33). Previous studies have shown that placebo response rates in trials on infantile colic could range from 5 to 83% (10, 34, 35). In the present study, 27 of 42 (64.3%) infants responded to the placebo at day 21. Moreover, very short crying times were reported toward the end of the study in both probiotic and control groups, further indicating the existence of a placebo effect. Although a direct placebo effect in young infants is unlikely, an indirect placebo effect (for example, the different degree of tolerance, attention and/or care skills of caregivers to the crying infant, or subjectivity in the recall of exact crying time) may be possible. Another factor contributing to the placebo effect is the natural regression to mean (subjects are enrolled when most symptomatic and inevitably improve with time owing to the natural variation in symptom severity and irrespective of trial participation) (36, 37).
The exact mechanisms by which B. longum CECT7894 (KABP042) and P. pentosaceus CECT8330 (KABP041) might exert this action have yet to be elucidated. Intestinal inflammation has been discussed as one of the possible causes of infantile colic, together with dysbiosis, production of gas (such as H2), and hypersensitivity to some nutrients (4–6). In this regard, P. pentosaceus CECT8330 has been reported to induce the anti-inflammatory cytokine IL-10 (12, 38). In addition, P. pentosaceus CECT8330 (KABP041) and especially B. longum CECT7894 (KABP042) were able to inhibit the growth of a wide spectrum of opportunistic gas-producing enterobacteria of the Escherichia and Klebsiella genera, known to be abnormally abundant in colicky infants (39, 40). This is consistent with the reported capacity of Bifidobacterium strains to modulate the intestinal microbiota (41) and the antimicrobial activity of P. pentosaceus strains (42, 43). Additionally, B. longum CECT7894 (KABP042) is effective in inhibiting the growth of Ent. aerogenes. Future studies should aim at elucidating this effect by exploring microbiome, metabolome, and inflammation markers (e.g., fecal calprotectin).
The strengths of our study include adequate sample size for the predefined outcomes, proper blinding maintained throughout the treatment, data management, and analyses. In addition, high retention and reported adherence rates allowed the achievement of the predetermined statistical power and significance. Moreover, a generally accepted definition of colic was used for diagnosis (ROME-IV criteria), and infants were recruited at a similar, early age. Similar to previous studies mentioned above, a potential limitation of this study is the assessment of the duration and frequency of colicky full force crying and fussing in infants with colic relied solely on the caregivers' report. Thus, we used a placebo-controlled, blinded design to minimize this potential shortcoming. Moreover, the design of our study did not allow for a better description of the crying (e.g., no difference for the food-related crying and typical colicky full force afternoon crying). Another limitation was that only a non-significant effect could be observed in parental quality of life despite a significant effect on crying/fussing. This could be due to an inadequate sample size for this outcome or other factors having a stronger effect on parental quality of life than the baby's crying (e.g., impact on jobs or family pressure). Another limitation of this study is that the compliance with the study products was not objectively assessed. A potential approach to assessing compliance is to weigh the study bottles both before and after dispensing; however, this method has reportedly produced highly variable results (44). Finally, although sufficient for the desired statistical power, the sample size used in this study is too small to derive a definitive conclusion about the universal usefulness of this probiotic formula for teating infantile colic. Therefore, this study should be replicated with larger sample sizes, and preferably with a sufficient representation of all feeding types.
In summary, exclusively breastfed or mixed fed infants with colic benefited from the treatment with B. longum CECT7894 (KABP042) and P. pentosaceus CECT8330 (KABP041) mix in comparison to the placebo. We recommend these probiotics for reducing crying times of colicky infants. Future studies should clarify the mechanism of the mix of probiotics in the management of infantile colic.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional ethics committee of Angel Children's Hospital Chengdu. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
KC and FS designed the research. KC, HL, YL, CZ, SX, and JL were responsible for the collection and assembly of the data. KC and CL analyzed the data. KC, FS, and CL wrote the first draft of the manuscript. All authors critically reviewed the manuscript, contributed to its revision, and approved the final manuscript.
This work was supported by the Maternal and Infant Health and Care Science Laboratory, Shanghai, China (MIHC/2017/10/AKO2018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. AB-Biotics SA (Barcelona, Spain) is the company owning worldwide IP rights of the probiotic strains used in this research and PROCEMSA Spa (Nichelino, Italy) is a toll manufacturer employed by AB-BIOTICS SA. These two companies did not participate in study funding, design, data collection, analysis, and interpretation, or in writing the manuscript. Maternal and Infant Health and Care Science Laboratory (Shanghai, China) holds an interest in the distribution of the probiotic used in this study in China, but its contribution was limited to funding the study and publication, and did not participate in study design, data collection, analysis, and interpretation, or in writing the manuscript. The authors are not employed by any of the aforementioned companies nor hold their shares. The companies did not compensate for time and efforts of the authors in cash or kind for conducting this study.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the parents or main caregivers and their children for their participation in the study and thank the health care workers in the field trial whose names are not mentioned for their diligent assistance.
1. Wake M, Morton-Allen E, Poulakis Z, Hiscock H, Gallagher S, Oberklaid F. Prevalence, stability, and outcomes of cry-fuss and sleep problems in the first 2 years of life: prospective community-based study. Pediatrics. (2006) 117:836–42. doi: 10.1542/peds.2005-0775
2. Landgren K, Lundqvist A, Hallström I. Remembering the chaos-but life went on and the wound healed. A four year follow up with parents having had a baby with infantile colic. Open Nurs J. (2012) 6:53–61. doi: 10.2174/1874434601206010053
3. Akman I, Kuşçu K, Özdemir N, Yurdakul Z, Solakoglu M, Orhan L, et al. Mothers' postpartum psychological adjustment and infantile colic. Arch Dis Child. (2006) 91:417–9. doi: 10.1136/adc.2005.083790
4. Shenassa ED, Brown M-J. Maternal smoking and infantile gastrointestinal dysregulation: the case of colic. Pediatrics. (2004) 114:e497–505. doi: 10.1542/peds.2004-1036
5. Eutamène H, Garcia-Rodenas C, Yvon S, d'Aldebert E, Foata F, Berger B, et al. Luminal contents from the gut of colicky infants induce visceral hypersensitivity in mice. Neurogastroenterol Motil. (2017) 29:e12994. doi: 10.1111/nmo.12994
6. Savino F, Garro M, Montanari P, Galliano I, Bergallo M. Crying time and RORγ/FOXP3 expression in Lactobacillus reuteri DSM17938-treated infants with colic: a randomized trial. The J Pediatr. (2018) 192:171–7.e1. doi: 10.1016/j.jpeds.2017.08.062
7. Biagioli E, Tarasco V, Lingua C, Moja L, Savino F. Pain-relieving agents for infantile colic. Cochrane Database Syst Rev. (2016) 9:CD009999. doi: 10.1002/14651858.CD009999.pub2
8. Gordon M, Biagioli E, Sorrenti M, Lingua C, Moja L, Banks SS, et al. Dietary modifications for infantile colic. Cochrane Database Syst Rev. (2018) 10:CD011029. doi: 10.1002/14651858.CD011029.pub2
9. Rhoads JM, Collins J, Fatheree NY, Hashmi SS, Taylor CM, Luo M, et al. Infant colic represents gut inflammation and dysbiosis. J Pediatr. (2018) 203:55–61.e3. doi: 10.1016/j.jpeds.2018.07.042
10. Savino F, Cordisco L, Tarasco V, Palumeri E, Calabrese R, Oggero R, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. (2010) 126:e526–33. doi: 10.1542/peds.2010-0433
11. Sung V, D'Amico F, Cabana MD, Chau K, Koren G, Savino F, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. (2018) 141:e20171811. doi: 10.1542/peds.2017-1811
12. Santas J, Fuentes MC, Tormo R, Guayta-Escolies R, Lázaro E, Cuñé J. Pediococcus pentosaceus CECT 8330 and Bifidobacterium longum CECT 7894 show a trend towards lowering infantile excessive crying syndrome in a pilot clinical trial. Int J Pharm Bio Sci. (2015) 6:458–66.
13. Zeevenhooven J, Koppen IJ, Benninga MA. The new Rome IV criteria for functional gastrointestinal disorders in infants and toddlers. Pediatr Gastroenterol Hepatol Nutr. (2017) 20:1–13. doi: 10.5223/pghn.2017.20.1.1
14. Chau K, Lau E, Greenberg S, Jacobson S, Yazdani-Brojeni P, Verma N, et al. Probiotics for infantile colic: a randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. J Pediatr. (2015) 166:74–8.e1. doi: 10.1016/j.jpeds.2014.09.020
15. Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G* Power 3.1: tests for correlation and regression analyses. Behav Res Methods. (2009) 41:1149–60. doi: 10.3758/BRM.41.4.1149
16. Sung V, Hiscock H, Tang M, Mensah FK, Heine RG, Stock A, et al. Probiotics to improve outcomes of colic in the community: protocol for the Baby Biotics randomised controlled trial. BMC Pediatr. (2012) 12:135. doi: 10.1186/1471-2431-12-135
17. Barr R, Kramer M, Boisjoly C, McVey-White L, Pless I. Parental diary of infant cry and fuss behaviour. Arch Dis Child. (1988) 63:380–7. doi: 10.1136/adc.63.4.380
18. Bekkali N, Hamers SL, Reitsma JB, Van Toledo L, Benninga MA. Infant stool form scale: development and results. J Pediatr. (2009) 154:521–6.e1. doi: 10.1016/j.jpeds.2008.10.010
19. Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL™ family impact module: preliminary reliability and validity. Health Qual Life Outcomes. (2004) 2:55. doi: 10.1186/1477-7525-2-55
20. Higashikawa F, Noda M, Awaya T, Danshiitsoodol N, Matoba Y, Kumagai T, et al. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: a randomized, double-blind, placebo-controlled clinical trial. Eur J Clin Nutr. (2016) 70:582–7. doi: 10.1038/ejcn.2016.17
21. Sirichokchatchawan W, Temeeyasen G, Nilubol D, Prapasarakul N. Protective effects of cell-free supernatant and live lactic acid bacteria isolated from Thai pigs against a pandemic strain of porcine epidemic diarrhea virus. Probiotics Antimicro Proteins. (2018) 10:383–90. doi: 10.1007/s12602-017-9281-y
22. Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte®) in critically ill trauma patients: Early results of a randomized controlled trial. World J Surg. (2006) 30:1848–55. doi: 10.1007/s00268-005-0653-1
23. McNicholl AG, Molina-Infante J, Lucendo AJ, Calleja JL, Pérez-Aisa Á, Modolell I, et al. Probiotic supplementation with Lactobacillus plantarum and Pediococcus acidilactici for Helicobacter pylori therapy: a randomized, double-blind, placebo-controlled trial. Helicobacter. (2018) 23:e12529. doi: 10.1111/hel.12529
24. Zheng J, Ruan L, Sun M, Gänzle M. A genomic view of lactobacilli and pediococci demonstrates that phylogeny matches ecology and physiology. Appl Environ Microbiol. (2015) 81:7233–43. doi: 10.1128/AEM.02116-15
25. Quigley L, O'Sullivan O, Stanton C, Beresford TP, Ross RP, Fitzgerald GF, et al. The complex microbiota of raw milk. FEMS Microbiol Rev. (2013) 37:664–98. doi: 10.1111/1574-6976.12030
26. Schreck Bird A, Gregory PJ, Jalloh MA, Risoldi Cochrane Z, Hein DJ. Probiotics for the treatment of infantile colic: a systematic review. J Pharm Pract. (2017) 30:366–74. doi: 10.1177/0897190016634516
27. Kianifar H, Ahanchian H, Grover Z, Jafari S, Noorbakhsh Z, Khakshour A, et al. Synbiotic in the management of infantile colic: a randomised controlled trial. J Paediatr Child Health. (2014) 50:801–5. doi: 10.1111/jpc.12640
28. Saavedra JM, Abi-Hanna A, Moore N, Yolken RH. Long-term consumption of infant formulas containing live probiotic bacteria: Tolerance and safety. Am J Clin Nutr. (2004) 79:261–7. doi: 10.1093/ajcn/79.2.261
29. Ivakhnenko E, Nian'kovskii S. Effect of probiotics on the dynamics of gastrointestinal symptoms of food allergy to cow's milk protein in infants. Georgian Med News. (2013) 219:46–52.
30. Nocerino R, De Filippis F, Cecere G, Marino A, Micillo M, Di Scala C, et al. The therapeutic efficacy of Bifidobacterium animalis subsp. lactis BB-12® in infant colic: a randomised, double blind, placebo-controlled trial. Aliment Pharmacol Ther. (2019) 51:110–20.
31. Pärtty A, Lehtonen L, Kalliomäki M, Salminen S, Isolauri E. Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: a randomized, controlled trial. Pediatr Res. (2015) 78:470–5. doi: 10.1038/pr.2015.127
32. Weizman Z, Alsheikh A. Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: a pilot study. J Am Coll Nutr. (2006) 25:415–9. doi: 10.1080/07315724.2006.10719554
33. Kirsch I. Role of placebo in irritable bowel syndrome. J Pediatr Gastroenterol Nutr. (2011) 53(Suppl 2):S42–3.
34. Savino F, Tarasco V. New treatments for infant colic. Curr Opin Pediatr. (2010) 22:791–7. doi: 10.1097/MOP.0b013e32833fac24
35. Szajewska H, Gyrczuk E, Horvath A. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. (2013) 162:257–62. doi: 10.1016/j.jpeds.2012.08.004
36. Bland JM, Altman DG. Statistics notes: some examples of regression towards the mean. BMJ. (1994) 309:780.
37. Musial F, Klosterhalfen S, Enck P. Placebo responses in patients with gastrointestinal disorders. World J Gastroenterol. (2007) 13:3425. doi: 10.3748/wjg.v13.i25.3425
38. Jonganurakkun B, Wang Q, Xu SH, Tada Y, Minamida K, Yasokawa D, et al. Pediococcus pentosaceus NB-17 for probiotic use. J Biosci Bioeng. (2008) 106:69–73. doi: 10.1263/jbb.106.69
39. Lehtonen L, Korvenranta H, Eerola E. Intestinal microflora in colicky and noncolicky infants: bacterial cultures and gas-liquid chromatography. J Pediatr Gastroenterol Nutr. (1994) 19:310–4. doi: 10.1097/00005176-199410000-00009
40. de Weerth C, Fuentes S, Puylaert P, de Vos WM. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics. (2013) 131:e550–8. doi: 10.1542/peds.2012-1449
41. Arboleya S, Salazar N, Solís G, Fernández N, Hernández-Barranco AM, Cuesta I, et al. Assessment of intestinal microbiota modulation ability of Bifidobacterium strains in in vitro fecal batch cultures from preterm neonates. Anaerobe. (2013) 19:9–16. doi: 10.1016/j.anaerobe.2012.11.001
42. Borrero J, Jiménez JJ, Gútiez L, Herranz C, Cintas LM, Hernández PE. Protein expression vector and secretion signal peptide optimization to drive the production, secretion, and functional expression of the bacteriocin enterocin A in lactic acid bacteria. J Biotechnol. (2011) 156:76–86. doi: 10.1016/j.jbiotec.2011.07.038
43. Tokatli M, Gülgör G, Bagder Elmaci S, Arslankoz Işleyen N, Özçelik F. In vitro properties of potential probiotic indigenous lactic acid bacteria originating from traditional pickles. BioMed Res Int. (2015) 2015:315819. doi: 10.1155/2015/315819
Keywords: Bifidobacterium longum CECT7894, Pediococcus pentosaceus CECT8330, infantile colic, infants, fecal consistency, probiotics
Citation: Chen K, Liu C, Li H, Lei Y, Zeng C, Xu S, Li J and Savino F (2021) Infantile Colic Treated With Bifidobacterium longum CECT7894 and Pediococcus pentosaceus CECT8330: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pediatr. 9:635176. doi: 10.3389/fped.2021.635176
Received: 01 February 2021; Accepted: 12 August 2021;
Published: 10 September 2021.
Edited by:
Steven Thomas Leach, University of New South Wales, AustraliaReviewed by:
Nicole Fatheree, University of Texas Health Science Center at Houston, United StatesCopyright © 2021 Chen, Liu, Li, Lei, Zeng, Xu, Li and Savino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Chen, MjYzNjYyMDg2QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.