- Division of Pediatric Nephrology, Department of Pediatrics, Seattle Children's Hospital, University of Washington School of Medicine, Seattle, WA, United States
With the advent of the electronic medical record, automated alerts have allowed for improved recognition of patients with acute kidney injury (AKI). Pediatric patients have the opportunity to benefit from such alerts, as those with a diagnosis of AKI are at risk of developing long-term consequences including reduced renal function and hypertension. Despite extensive studies on the implementation of electronic alerts, their overall impact on clinical outcomes have been unclear. Understanding the results of these studies have helped define best practices in developing electronic alerts with the aim of improving their impact on patient care. As electronic alerts for AKI are applied to pediatric patients, identifying their strengths and limitations will allow for continued improvement in its use and efficacy.
Introduction
Acute kidney injury (AKI) is commonly seen in hospitalized children, particularly those who are critically ill, and/or have underlying medical conditions (1, 2). It is independently associated with prolonged hospital stay and an increased risk of mortality (3). Post-hospital discharge, patients with AKI have higher healthcare utilization and are at risk of developing long-term consequences such as proteinuria, reduced renal function, and hypertension (4). Thus, the ability to detect these episodes of AKI can improve the management of these patients to provide the best care possible. However, AKI is often under-recognized and under-documented (5, 6). With the widespread use of electronic health records (EHR), it has become possible to use a variety of clinical decision support systems (CDSS) to improve patient care (7). These include automated alerts that allow providers to receive real-time notifications when triggered by a particular threshold. AKI is an ideal clinical condition for the use of electronic alerts because it has a consensus definition and it can be diagnosed from data available in EHR (8). In this review, we discuss the best practices for AKI alerts, special considerations when developing such tools for children, and its impact on patient outcomes.
Best Practices for AKI Alerts
The 15th Acute Dialysis Quality Initiative (ADQI) consensus conference focused on utilizing EHR to predict AKI risk and outcomes (8–11). They recognized AKI alerts as an opportunity to prompt earlier evaluation and intervention, and provided guidelines around the development of electronic alert systems. One of the recommendations of the 15th ADQI conference was to further refine the structure of AKI alerts and to link them to actionable interventions for AKI care. More recently, the 22nd ADQI consensus conference provided quality improvement (QI) initiatives around the identification and care of patients with AKI. They recognized the role of bundled interventions in preventing AKI or reducing the severity in patients at risk for AKI. The key recommendations from these consensus conferences are summarized here, highlighting particular challenges when applying these guidelines to pediatric patients (8, 12).
Purpose of Alert
Alerts associated with AKI serve different purposes depending on the data used to inform the notification and the criteria used to trigger the alert. In particular, some AKI alerts have been designed to identify patients at risk for AKI while others are designed to detect patients currently with AKI (13–15).
Identification of Patients at Risk of AKI
The 22nd ADQI emphasized identifying populations at risk of AKI, which can include a baseline set of risk factors including age, medications, baseline creatinine, and a problem list (12). As most pediatric patients inherently have few of these risk factors, efforts in detecting children at risk for AKI have focused on the initiation of nephrotoxic medications. Nephrotoxic Injury Negated by Just-in-Time Action (NINJA) is an ongoing prospective quality improvement project that works to reduce nephrotoxic medication-associated AKI among non-critically ill hospitalized children (12). It involves systematic EHR screening and a decision support process (trigger report). This trigger report is reviewed by pharmacists who recommend daily serum creatinine monitoring in the exposed patients. Upon implementation, it was successful in reducing the number of AKI days per 100 days by 42% in its first year (13), and has since been shown to maintain a 23.8% decrease in AKI rates when incorporated across nine pediatric institutions (16). Risk prediction models have been specifically designed for pediatric patients which incorporate data beyond nephrotoxic agents. Implementation of an AKI risk prediction tool by Driest el al. resulted in increased screening for AKI via measurement of serum creatinine in a pediatric intensive care unit (17).
More recently, artificial intelligence and machine learning methods have made it possible to design algorithms to predict future episodes of AKI. Tomašev et al. developed a model for the prediction of AKI using a dataset of more than 700,000 patients from the United States Department of Veterans Affairs (18). Their model was able to predict 55.8% of all inpatient episodes of AKI and 90.2% of all AKI that required subsequent dialysis up to 48 h before they occurred. Sandokji et al. developed a machine learning algorithm that selected 10 factors to predict AKI within a 48-h window specifically in pediatric patients as well as the neonatal population (19). More data on these predictive algorithms and their effect on patient outcomes will be seen in the coming years (20).
Identification of Patients With AKI
Most alerts are designed to trigger based on the definition of AKI by the Kidney Disease: Improving Global Outcomes (KDIGO) criteria (21). AKI is defined in stages of increasing severity by a rising serum creatinine from baseline or declining urine output (UOP). Urine output is a simple and sensitive measure of kidney function (22). In the neonatal population, defining AKI by urine output captures more diagnoses than using serum creatinine alone (23). Inconsistent intake and output documentation in the electronic medical record, however, limits the use of UOP as a trigger for alerts. While serum creatinine is a reasonable biomarker to represent a patient's glomerular filtration rate in most cases, creatinine-based alerts alone can produce a false positive rate as high as 30% (24). The recent 23rd ADQI (25) emphasized the utility of incorporating additional injury biomarkers to these systems to select patients most likely to benefit from AKI-specific interventions, but recognize that many of these biomarkers still require clinical validation before widespread use.
Components of the Alert
The 15th Acute Dialysis Quality Initiative workgroup summary discusses the characteristics of an optimal alert, considering the technological as well as human factors impacting the implementation and efficacy of an electronic alert (8–10). At minimum, the content of the alert should include patient identification, the data used to trigger the alert, and the stage of AKI if available. The alert should occur as close to the time of AKI onset as possible. Some alerts target the primary contact for the patient directly whereas other alerts are displayed in the EHR for any provider with access. Alerts may be passive with no acknowledgment of receipt, or interruptive where a series of actions are required to dismiss the alert or proceed with additional orders.
Responses to the Alert
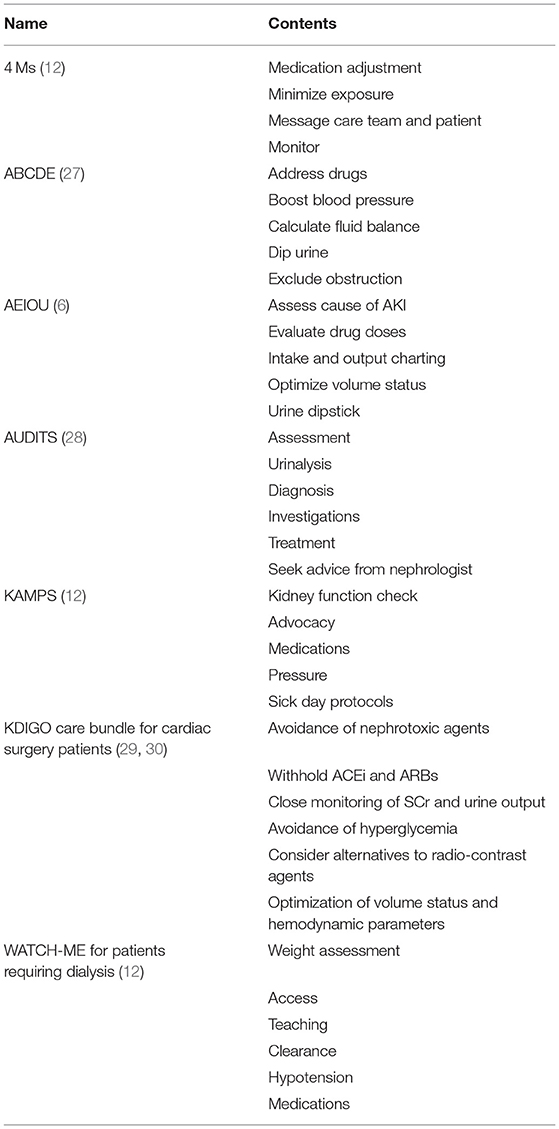
While e-alerts have been shown to improve the recognition of AKI, they have not consistently translated into improved outcomes of patients with AKI (15, 26). It has been proposed that alerts should be accompanied by a bundle of diagnostic and therapeutic interventions to prevent further injury. The 22nd ADQI emphasized that every exposure to risk factors associated with AKI, as well as episodes of AKI itself, should be followed by a kidney health response, also called a care bundle (12). Many of these care bundles incorporate the best practices in response to kidney injury in a simple and easy to remember mnemonic (Table 1). While they vary slightly depending on the context of their use, most include similar themes of fluid and blood pressure management, medication review, and the evaluation of urine (31).
Use of AKI Alerts in Adults
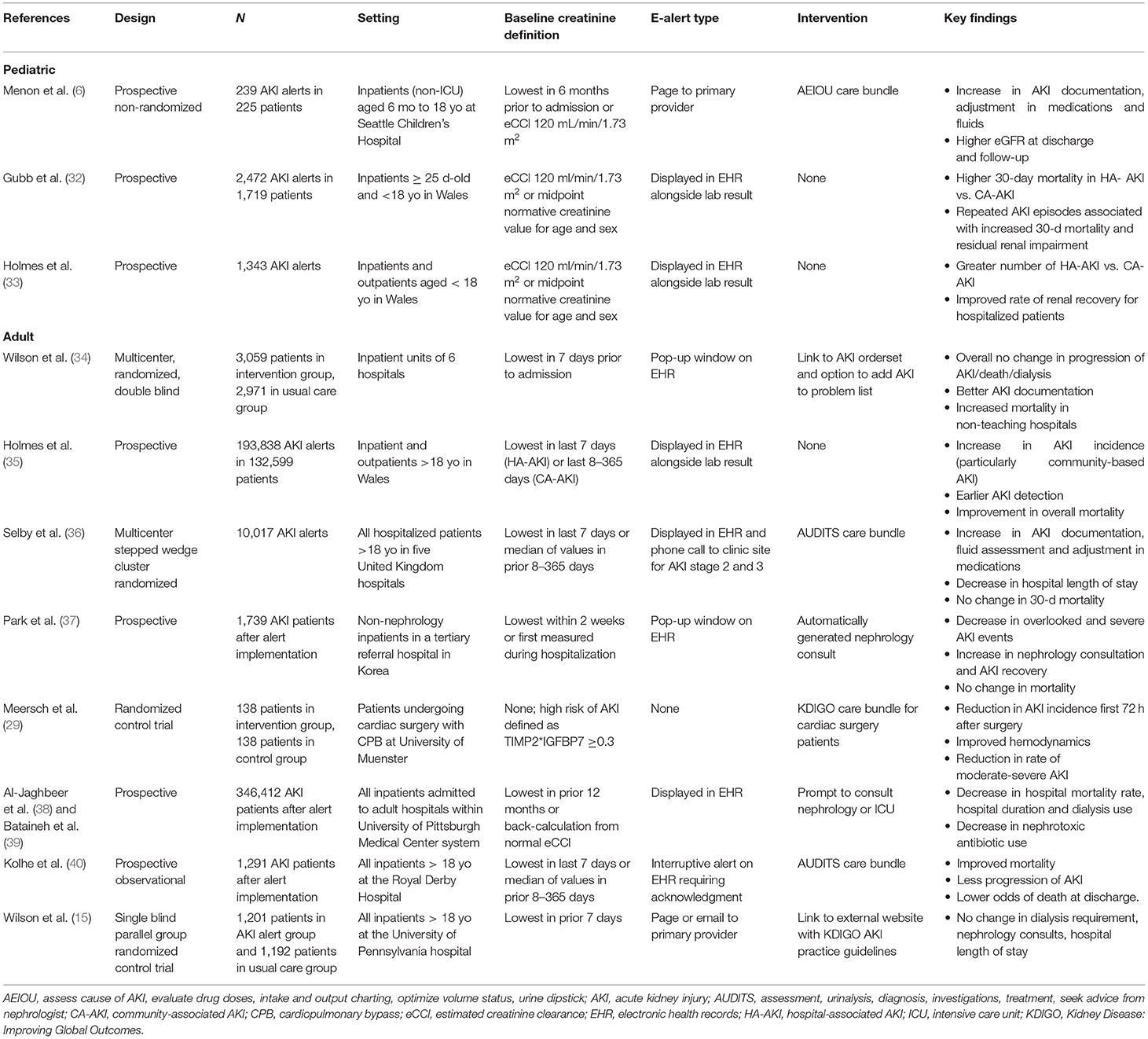
The utilization of AKI alerts and its impact on clinical outcomes has been studied in a number of settings throughout the years. Table 2 provides a summary of key publications studying the implementation of AKI alerts, selected from a literature review using the key words “AKI,” “electronic alert,” and “clinical decision support system.” Early studies looked at the impact on e-alert implementation in the recognition of AKI and were helpful in understanding the epidemiology of AKI within the hospital population (41, 42). A study out of Ghent University Hospital by Colpaert et al. (43) was one of the first to report positive clinical outcomes following an automated AKI alert, reporting a significant increase in fluid intervention and a higher proportion of patients returning to baseline kidney function within 8 h of the alert.
The first randomized controlled trial on AKI alerts was conducted by Wilson et al. (15) at the University of Pennsylvania. In this single blind, parallel group trial, 1,201 adult patients were assigned to receive an AKI alert and 1,192 were assigned to the usual care group (21). There was no difference between the alert and usual care group in composite relative maximum change in creatinine, dialysis, and death at 7 days. There was no difference in the number of renal consults, number of nephrotoxic agents prescribed, no change in length of stay, and no change in number of creatinine lab measurements within 7 days (15, 44). Interestingly, a secondary analysis of this clinical trial using uplift modeling identified patients who might benefit most from AKI alerts. These were patients at risk of a more slowly developing AKI, including older patients, women, and those with a lower baseline creatinine (45). While pediatric patients were not included in this study, they certainly fall into this category.
Among the largest studies on AKI alerts has been the work of Holmes et al. and the Welsh AKI Steering Group (25, 26, 31, 41, 42). They employed a national AKI alert in a population of more than 3 million people in the hospital as well as the primary care setting. The Wales Laboratory Information Management System tracks creatinine values on patients in real time and an alert is issued according to the KDIGO AKI criteria. Over the course of 4 years, they found that the majority of adult AKI alerts were community acquired (53.5%) vs. hospital acquired (29.3%) and the rest (17.2%) were undetermined (25). They were also able to provide nationwide characterization of AKI in various clinical settings and report the true incidence of AKI in Wales.
Subsequent studies have looked at the impact of pairing the AKI alert with an actionable intervention on clinical outcomes. In the PrevAKI study, Meersch et al. (29) assessed the efficacy of a care bundle based on KDIGO guidelines to prevent cardiac surgery-associated AKI in high-risk patients. Patients were randomized to receive usual care or the KDIGO bundle, which included guidelines for managing volume status, hemodynamics, nephrotoxic agents, and hyperglycemia prevention (Table 1). They found a significantly reduced rate of AKI within 72 h after surgery, as well as improvement in hemodynamic parameters and severity of AKI. There were no changes to dialysis requirements, hospital length of stay, or adverse events related to the kidney.
Kolhe et al. (28) implemented an interruptive EHR alert at the Royal Derby Hospital which forced the provider to override or acknowledge the AKI care bundle. The care bundle required completion before a new blood test or medication could be ordered. The care bundle was completed in 25% patients within 24 h, and case fatality was higher when the care bundle was not completed. With their alert, they found a significant improvement in mortality, less progression to higher AKI stages, and lower odds of death at discharge (40). This study later expanded to the Tackling AKI study, a large multi-center pragmatic stepped wedge cluster randomized trial (36). The interruptive alert was no longer used; instead an alert with the care bundle was displayed in the EHR and a phone call was made to the clinical site for patients with AKI stages 2 and 3. Across five UK hospitals, results were significant for an increase in documentation of AKI. While there were no changes in 30-day mortality, there were improvements in medication optimization, fluid assessment, hospital length of stay, and quality of care (37). One of the reasons cited for the lackluster performance of clinical decision support systems (CDSS) in AKI is the difficulty in achieving effect sizes. Al-Jaghbeer et al. implemented a CDSS for AKI in a large regional health care system (38). They looked at > 500,000 total patients, 12 months before (n = 181,696) and 24 months after (n = 346,412) alert implementation. The system alerted clinicians on “possible AKI” based on the KDIGO SCr criteria. It also provided information on the reference creatinine used, stage of AKI, and a prompt to consult nephrology or intensive care. In comparing pre- vs. post-alert implementation, they found that mortality rate decreased from 10.2 to 9.4% after alert implementation, and there was a decrease in length of stay from 7.2 to 6.0 days for patients with AKI. A 2-year follow-up study on an additional 337,433 patients demonstrated sustained decrease in mortality rate and length of stay, as well as a significant decrease in the use of nephrotoxic agents (39).
More recently, Wilson et al. looked at e-alerts for AKI in 6,030 patients in a double blinded, parallel, randomized controlled trial across six centers (34). In the electronic health record alerts for acute kidney injury (ELAIA-1) study, they found that patients randomized to alerts were more likely to receive intravenous fluids, get a urinalysis or repeat SCr measured, and have documentation of AKI. There was however no difference in their primary outcome, which was a composite of AKI progression, receipt of dialysis, or death. Interestingly, there was a heterogeneity of treatment effect across the different hospitals. In the non-teaching hospitals in the study, patients in the alert arm were more likely to have met the primary outcome [relative risk (RR) 1.49, 95%CI 1.12–1.98].
Pediatric AKI Alert Studies
The studies discussed above focused on the adult population. Only a few studies have looked at implementing AKI alerts in pediatric patients (Table 2). The Welsh AKI group studied AKI alerts in pediatric patients in both the hospital and community setting (33). Over a period of 30 months, they reported a total of 2,087 alerts, corresponding to 1,343 incident episodes of AKI, of which 468 occurred in neonates. Hospital-acquired AKI accounted for 40.1%, community-acquired AKI accounted for 29.4%, and the rest was unclassified. They reported an incidence rate of pediatric AKI at 1.37 cases per 1,000 person-years.
A prospective study at Seattle Children's Hospital by Menon et al. (6) aimed to determine whether an AKI alert paired with a standardized care pathway would improve AKI detection and renal outcomes. This study included 239 unique AKI alerts with most being stage 1 AKI (68.6%) and 47% were defined as hospital-acquired AKI. With the alert intervention, this study found a significant increase in AKI documentation, intake and output charting as well as adjustments to fluid and medications. While there was a trend toward decreases in AKI stage, this finding was not statistically significant. Larger multi-center studies with greater longitude will be necessary to better understand the impact of AKI alerts on pediatric patients.
Limitations
There are limitations in the implementation of AKI alerts, some of which are unique to pediatric patients. Addressing these alerts in future studies may improve their efficacy and interpretability.
Accuracy of the Alert
The definition of AKI is highly dependent on the reliability and accuracy of information presented in the EHR. Unfortunately, urine output is not documented frequently or accurately enough to use for AKI alerts and a patient's baseline creatinine often does not exist in the medical record. Studies have used different methods to ascertain the baseline serum creatinine (SCr), including using the admission SCr, a pre-admission outpatient creatinine, or nadir inpatient SCr. There are concerns with all methods. For example, if a patient has community-acquired AKI, the admission SCr is likely to be higher than the patient's true baseline resulting in underdiagnosis of AKI (46). An additional issue in pediatrics is that the baseline kidney function evolves as a child grows. This is particularly challenging in neonates as their creatinine at birth is reflective of their mother's kidney function. Using the KDIGO definitions overestimated neonatal AKI in the study done by Holmes et al. (33), and the authors recommend using a serum creatinine >0.5 mg/dl as a threshold for AKI. While imperfect, the most common solutions to calculating baseline creatinine in pediatrics are to estimate baseline SCr including back-calculation based on eGFR of 120 ml/min per 1.73 m2 or use a normative midpoint value for age (33, 47).
Type of Alert
For an alert to work, it must be noticed. Much research has been done on the balance between intrusive and passive alerts and their relative efficacy (7). Providers are more likely to act on an interrupting alert that forces an action. However, if these intrusive alerts are too frequent or disproportionately associated with false positives, all alerts of the same type are more likely to be dismissed without action (48, 49). Improperly implemented alerts can lead to alert fatigue, which may further affect the efficacy of the alert. When considering how to deliver an alert to maximize patient benefit while also reducing alert fatigue, applying alerts only to patients at high risk who may gain most from intervention would be a potential solution (49). Alerts could also be targeted at providers working directly with the patient in question at the time of potential error, such as when nephrotoxic agents are ordered.
Interventions Associated With Alert
Care bundles have been recommended and used with e-alerts as an attempt to improve the outcomes associated with AKI (6, 28, 29, 38, 40). Currently, care bundles include general common sense measures such as optimal fluid management, medication review, and urinalyses (Table 1). However, as seen in the ELAIA-1 study (34), care bundles that do not provide patient specific recommendations may not be helpful, and have the potential to cause more harm. Additional research is needed on this aspect of CDSS.
Conclusions
As a tool that is able to detect patients with AKI, electronic alerts meet the need for identifying patients at high risk for poor outcomes. Criticism of existing studies on AKI alerts note that little impact on overall mortality has been seen with the implementation of alerts. However, a higher level of care is consistently provided to patients after AKI alerts were triggered, particularly when bundled with resources of a care plan. Patients with AKI alerts also benefited from detailed documentation of AKI diagnoses, closer attention to fluid and medication management, and the involvement of nephrology providers. This comprehensive level of care that occurs with an automatic real-time notification has few downsides. For pediatric patients in particular, these simple interventions can be an effective resource to reduce the burden of AKI on our communities and hospitals.
Author Contributions
EN and SM contributed equally to the conception and design of the manuscript, drafted the article and made critical revisions related to the intellectual content of the manuscript, and approved the final version of the article to be published. All authors contributed to the article and approved the submitted version.
Funding
EN was supported by an Institutional Training grant funded by the NIH/NIDDK, Grant Number T32 DK007662.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A. Epidemiology of acute kidney injury in critically ill children and young adults. New Engl J Med. (2017) 376:11–20. doi: 10.1056/nejmoa1611391
2. Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Heal. (2017) 1:184–94. doi: 10.1016/s2352-4642(17)30069-x
3. Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, et al. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephro. (2013) 8:1661–9. doi: 10.2215/cjn.00270113
4. Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. (2006) 69:184–9. doi: 10.1038/sj.ki.5000032
5. McGregor TL, Jones DP, Wang L, Danciu I, Bridges BC, Fleming GM, et al. Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: a retrospective observational study. Am J Kidney Dis. (2016) 67:384–90. doi: 10.1053/j.ajkd.2015.07.019
6. Menon S, Tarrago R, Carlin K, Wu H, Yonekawa K. Impact of integrated clinical decision support systems in the management of pediatric acute kidney injury: a pilot study. Pediatr Res. (2020). doi: 10.1038/s41390-020-1046-8. [Epub ahead of print].
7. Kashani K. Computer decision support for acute kidney injury. Curr Opin Crit Care. (2016) 22:520–6. doi: 10.1097/mcc.0000000000000353
8. Hoste EAJ, Kashani K, Gibney N, Wilson FP, Ronco C, Goldstein SL, et al. Impact of electronic-alerting of acute kidney injury: workgroup statements from the 15th ADQI Consensus Conference. Can J Kidney Heal Dis. (2016) 3:10. doi: 10.1186/s40697-016-0101-1
9. James MT, Hobson CE, Darmon M, Mohan S, Hudson D, Goldstein SL, et al. Applications for detection of acute kidney injury using electronic medical records and clinical information systems: workgroup statements from the 15(th) ADQI Consensus Conference. Can J Kidney Heal Dis. (2016) 3:9. doi: 10.1186/s40697-016-0100-2
10. Mehta R, Bihorac A, Selby NM, Quan H, Goldstein SL, Kellum JA, et al. Establishing a continuum of acute kidney injury—tracing AKI using data source linkage and long-term follow-up: workgroup statements from the 15th ADQI consensus conference. Can J Kidney Heal Dis. (2016) 3:13. doi: 10.1186/s40697-016-0102-0
11. Sutherland SM, Chawla LS, Kane-Gill SL, Hsu RK, Kramer AA, Goldstein SL, et al. Utilizing electronic health records to predict acute kidney injury risk and outcomes: workgroup statements from the 15th ADQI Consensus Conference. Can J Kidney Heal Dis. (2016) 3:11. doi: 10.1186/s40697-016-0099-4
12. Kashani K, Rosner MH, Haase M, Lewington AJP, O'Donoghue DJ, Wilson FP, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephro. (2019) 14:941–53. doi: 10.2215/cjn.01250119
13. Goldstein SL, Kirkendall E, Nguyen H, Schaffzin JK, Bucuvalas J, Bracke T, et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. (2013) 132:e756–67. doi: 10.1542/peds.2013-0794
14. Holmes J, Roberts G, Meran S, Williams JD, Phillips AO, Group WAS. Understanding electronic AKI alerts: characterization by definitional rules. Kidney Int Rep. (2017) 2:342–9. doi: 10.1016/j.ekir.2016.12.001
15. Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet. (2015) 385:1966–74. doi: 10.1016/s0140-6736(15)60266-5
16. Goldstein SL, Dahale D, Kirkendall ES, Mottes T, Kaplan H, Muething S, et al. A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int. (2019) 97:580–8. doi: 10.1016/j.kint.2019.10.015
17. Driest SLV, Wang L, McLemore MF, Bridges BC, Fleming GM, McGregor TL, et al. Acute kidney injury risk-based screening in pediatric inpatients: a pragmatic randomized trial. Pediatr Res. (2020) 87:118–24. doi: 10.1038/s41390-019-0550-1
18. Tomašev N, Glorot X, Rae JW, Zielinski M, Askham H, Saraiva A, et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature. (2019) 572:116–9. doi: 10.1038/s41586-019-1390-1
19. Sandokji I, Yamamoto Y, Biswas A, Arora T, Ugwuowo U, Simonov M, et al. A time-updated, parsimonious model to predict AKI in hospitalized children. J Am Soc Nephrol. (2020) 31:1348–57. doi: 10.1681/asn.2019070745
20. Rashidi P, Bihorac A. Artificial intelligence approaches to improve kidney care. Nat Rev Nephrol. (2020) 16:71–2. doi: 10.1038/s41581-019-0243-3
21. Workgroup K. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int. (2012) 120:c179–84. doi: 10.1159/000339789
22. Goldstein SL. Urine output assessment in acute kidney injury: the cheapest and most impactful biomarker. Front Pediatr. (2020) 7:565. doi: 10.3389/fped.2019.00565
23. Roy J-P, Goldstein S, Schuh M. Under-recognition of neonatal acute kidney injury and lack of follow-up. Am J Perinat. (2020). doi: 10.1055/s-0040-1716841. [Epub ahead of print].
24. Lin J, Fernandez H, Shashaty MGS, Negoianu D, Testani JM, Berns JS, et al. False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol Cjasn. (2015) 10:1723–31. doi: 10.2215/cjn.02430315
25. Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference. JAMA Netw Open. (2020) 3:e2019209. doi: 10.1001/jamanetworkopen.2020.19209
26. Meijers B, Moor BD, Bosch BVD. The acute kidney injury e-alert and clinical care bundles: the road to success is always under construction. Nephrol Dial Transpl. (2016) 31:1761–3. doi: 10.1093/ndt/gfw213
27. Forde C, McCaughan J, Leonard N. Acute kidney injury: it's as easy as ABCDE. Bmj Qual Improv Rep. (2012) 1:u200370.w326. doi: 10.1136/bmjquality.u200370.w326
28. Kolhe NV, Reilly T, Leung J, Fluck RJ, Swinscoe KE, Selby NM, et al. A simple care bundle for use in acute kidney injury: a propensity score-matched cohort study. Nephrol Dial Transpl. (2016) 31:1846–54. doi: 10.1093/ndt/gfw087
29. Meersch M, Schmidt C, Hoffmeier A, Aken HV, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intens Care Med. (2017) 43:1551–61. doi: 10.1007/s00134-016-4670-3
30. Kellum JA. AKI: the myth of inevitability is finally shattered. Nat Rev Nephrol. (2017) 13:140–1. doi: 10.1038/nrneph.2017.11
31. Bagshaw SM. Acute kidney injury care bundles. Nephron. (2016) 131:247–51. doi: 10.1159/000437152
32. Gubb S, Holmes J, Smith G, Geen J, Williams J, Donovan K, et al. Acute kidney injury in children based on electronic alerts. J Pediatr. (2020) 220:14–20.e4. doi: 10.1016/j.jpeds.2019.11.019
33. Holmes J, Roberts G, May K, Tyerman K, Geen J, Williams JD, et al. The incidence of pediatric acute kidney injury is increased when identified by a change in a creatinine-based electronic alert. Kidney Int. (2017) 92:432–9. doi: 10.1016/j.kint.2017.03.009
34. Wilson FP, Martin M, Yamamoto Y, Partridge C, Moreira E, Arora T, et al. Electronic health record alerts for acute kidney injury: multicenter, randomized clinical trial. BMJ. (2021) 372:m4786. doi: 10.1136/bmj.m4786
35. Holmes J, Donovan K, Geen J, Williams J, Phillips AO. Acute kidney injury demographics and outcomes: changes following introduction of electronic acute kidney injury alerts-an analysis of a national dataset. Nephrol Dialysis Transplant. (2020). doi: 10.1093/ndt/gfaa071. [Epub ahead of print].
36. Selby NM, Casula A, Lamming L, Stoves J, Samarasinghe Y, Lewington AJ, et al. An organizational-level program of intervention for AKI: a pragmatic stepped wedge cluster randomized trial. J Am Soc Nephrol. (2019) 30:505–15. doi: 10.1681/asn.2018090886
37. Park S, Baek SH, Ahn S, Lee K-H, Hwang H, Ryu J, et al. Impact of electronic acute kidney injury (AKI) alerts with automated nephrologist consultation on detection and severity of AKI: a quality improvement study. Am J Kidney Dis. (2018) 71:9–19. doi: 10.1053/j.ajkd.2017.06.008
38. Al-Jaghbeer M, Dealmeida D, Bilderback A, Ambrosino R, Kellum JA. Clinical decision support for in-hospital AKI. J Am Soc Nephrol. (2017) 29:654–60. doi: 10.1681/asn.2017070765
39. Bataineh A, Dealmeida D, Bilderback A, Ambrosino R, Al-Jaghbeer MJ, Fuhrman DY, et al. Sustained effects of a clinical decision support system for acute kidney injury. Nephrol Dial Transpl. (2020) 35:1819–21. doi: 10.1093/ndt/gfaa099
40. Kolhe NV, Staples D, Reilly T, Merrison D, Mcintyre CW, Fluck RJ, et al. Impact of compliance with a care bundle on acute kidney injury outcomes: a prospective observational study. PLoS ONE. (2015) 10:e0132279. doi: 10.1371/journal.pone.0132279
41. Selby NM, Crowley L, Fluck RJ, McIntyre CW, Monaghan J, Lawson N, et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephro. (2012) 7:533–40. doi: 10.2215/cjn.08970911
42. Sawhney S, Fluck N, Marks A, Prescott G, Simpson W, Tomlinson L, et al. Acute kidney injury—how does automated detection perform? Nephrol Dial Transpl. (2015) 30:1853–61. doi: 10.1093/ndt/gfv094
43. Colpaert K, Hoste EA, Steurbaut K, Benoit D, Hoecke SV, Turck FD, et al. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class&ast. Crit Care Med. (2012) 40:1164–70. doi: 10.1097/ccm.0b013e3182387a6b
44. Wilson FP, Reese PP, Shashaty MG, Ellenberg SS, Gitelman Y, Bansal AD, et al. A trial of in-hospital, electronic alerts for acute kidney injury: design and rationale. Clin Trials. (2014) 11:521–9. doi: 10.1177/1740774514542619
45. Biswas A, Parikh CR, Feldman HI, Garg AX, Latham S, Lin H, et al. Identification of patients expected to benefit from electronic alerts for acute kidney injury. Clin J Am Soc Nephro. (2018) 13:842–9. doi: 10.2215/cjn.13351217
46. Liu KD, Hsu C, Yang J, Tan TC, Zheng S, Ordonez JD, et al. Acute kidney injury ascertainment is affected by the use of first inpatient versus outpatient baseline serum creatinine. Kidney Int Rep. (2018) 3:211–5. doi: 10.1016/j.ekir.2017.08.011
47. Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephro. (2008) 3:948–54. doi: 10.2215/cjn.05431207
48. Martin M, Wilson FP. Utility of electronic medical record alerts to prevent drug nephrotoxicity. Clin J Am Soc Nephro. (2019) 14:115–23. doi: 10.2215/cjn.13841217
Keywords: acute kidney injury, electronic alerts, clinical decision support system, care bundles, electronic health record
Citation: Nguyen ED and Menon S (2021) For Whom the Bell Tolls: Acute Kidney Injury and Electronic Alerts for the Pediatric Nephrologist. Front. Pediatr. 9:628096. doi: 10.3389/fped.2021.628096
Received: 11 November 2020; Accepted: 16 March 2021;
Published: 12 April 2021.
Edited by:
Danielle Elise Soranno, University of Colorado, United StatesReviewed by:
Emily Lauren Joyce, Rainbow Babies and Children's Hospital, United StatesTennille N. Webb, University of Alabama at Birmingham, United States
Copyright © 2021 Nguyen and Menon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth D. Nguyen, ZWxpemFiZXRoLm5ndXllbkBzZWF0dGxlY2hpbGRyZW5zLm9yZw==
 Elizabeth D. Nguyen
Elizabeth D. Nguyen Shina Menon
Shina Menon