
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr., 18 March 2021
Sec. Pediatric Rheumatology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.625377
This article is part of the Research TopicClinical Heterogeneity, Immunological Features, and Health Impact of the COVID-19 Pandemic in ChildrenView all 30 articles
 Parham Mardi1
Parham Mardi1 Marzieh Esmaeili2
Marzieh Esmaeili2 Parisa Iravani3,4
Parisa Iravani3,4 Mohammad Esmail Abdar5
Mohammad Esmail Abdar5 Kumars Pourrostami6*
Kumars Pourrostami6* Mostafa Qorbani7,8*
Mostafa Qorbani7,8*Recent studies have shown that several children diagnosed with COVID-19 have developed Kawasaki Disease (KD)-like symptoms. This systematic review aims to assess the demographic, laboratory, and clinical characteristics of children with KD-like syndrome during the COVID-19 pandemic and evaluate efficacy of treatments and patients' outcome. A comprehensive search was carried out systematically through PubMed, Scopus, and Web of Science (WoS), medRxiv, and bioRxiv by two reviewers independently for all studies or preprints data on the demographic, laboratory, and clinical characteristics of children with K.D-like signs during the COVID-19 outbreak. Overall, 378 studies were identified by the systematic search, of which 25 studies were included in the study. The included studies involved 599 patients in total. Thirteen studies (52%) were case reports or case series, and the rest (48%) were cohort studies. In 19 studies, patients were diagnosed with Multisystem inflammatory syndrome in children (MIS-C). In 16 studies COVID-19 was diagnosed in all patients based on their polymerase chain reaction result, serological findings, and computed tomography results. Higher C-reactive protein and erythrocyte sedimentation rate level were the most prevalent laboratory findings. In most studies, patients had leucopenia with marked lymphopenia, hypoalbuminemia, and increased ferritin, as well as hyponatremia. Abnormal echocardiography and respiratory outcomes were the most common clinical outcomes. In 11 studies, all patients required intensive care unit admission. Findings of the present systematic review show that the incidence of KD-like syndrome in the COVID-19 pandemic increased significantly. Moreover, this study offers new insights in the KD-like syndrome pathogenesis and clinical spectrum during COVID-19 pandemic.
Coronavirus Disease 2019 (COVID-19), caused by SARS-CoV-2, has created a global pandemic. Millions of people have been infected, and thousands have lost their lives (1). The main clinical manifestations of this disease are fever, coughing, shortness of breath, fatigue, and malaise (2).
At the beginning of the crisis, children were rarely reported as infected. These reports led to the assumption that children are immune to this virus. With the continuation of the pandemic and the quarantines that caused many families to stay close in their houses, more children with COVID-19 were identified (3). The children infected with the novel coronavirus can be asymptomatic or present with fever, dry coughs, fatigue, and a few upper respiratory symptoms, including nasal congestion and runny nose (4, 5). Even though the disease is not as severe as in adults, yet some case reports and case series have reported more severe symptoms in some cases, compared to the other symptomatic children (6).
Kawasaki disease (KD) is one of the vasculitis of medium-sized vessels, that only affects children. Although no identified etiology can clearly explain this disease, some assumptions consider viral infections such as coronavirus family act as a trigger in genetically predisposed children (7, 8).
Recent studies have shown that several children diagnosed with COVID-19 have developed symptoms such as prolonged fever, bilateral conjunctival injections, changes in the lips and oral cavity, cervical lymphadenopathy, extremity changes, and polymorphous rash that are similar to those of KD or Hyper inflammatory syndrome (9, 10). On the other hand, there are some differences between KD-like syndrome and KD including a faster advancement of symptoms, especially fever and multisystem organ dysfunction such as cardiac and respiratory dysfunction along with a current or recent COVID-19 diagnosis in KD-like syndrome patients. Also, KD-like syndrome patients suffer a tachycardia and are at higher risk of cardiac or respiratory arrest. Moreover, Primary studies pointed out that KD-like syndrome is associated with a poorer prognosis for the patients (8, 11). On the ground of lack of information on KD-like syndrome, the objective of this study is to assess the demographic, laboratory, and clinical characteristics of children with KD-like syndrome during the COVID-19 pandemic and evaluate efficacy of treatments and patients' outcome.
This study is reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12, 13).
The following inclusion criteria were used: (I) Published in the English language; (II) Full-text available; (III) observational studies (including case reports and case series); (IV) studies reporting the characteristics and outcome of children with KD-like syndrome during the COVID-19 outbreak; (V) patients in the study fulfill the classical Kawasaki criteria according to the American Heart Association indications (2017) (fever for ≥5 days plus four or more clinical criteria, including bilateral bulbar non-exudative conjunctivitis, changes of the lips or oral cavity, non-suppurative laterocervical lymphadenopathy, polymorphic rash, erythema of the palms and soles, firm induration of the hands or feet, or both) or KD-like syndrome criteria, based on CDC recommendation [An individual under 21 years presenting with fever, laboratory evidence of inflammation, and evidence of clinically severe illness requiring hospitalization, with multi-system (two or more) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurological); AND No alternative plausible diagnoses; AND Positive for current or recent SARS-CoV-2 (COVID-19) infection by reverse-transcriptase polymerase chain reaction (RT-PCR), serology or antigen test; or COVID-19 exposure within the 4 weeks prior to the onset of symptoms].
A comprehensive search was carried out systematically through PubMed, Scopus, and Web of Science (WoS), databases (from their inception until July 1, 2020), and medRxiv, bioRxiv (between January 1, 2020, and July 12, 2020) by two reviewers independently.
A search study was designed comprising of three concepts, the 2019 novel coronavirus disease, KD disease, and child. The following algorithm was used for screening the title and abstract (Supplementary Table 1); (“covid 19” OR “covid-19” OR “*covid-19*” OR “*covid*” OR “*SARS-CoV-2*” OR “*2019-nCoV*” OR “*novel coronavirus*” OR “*new coronavirus*” OR “*coronavirus*”) AND (Kawasaki OR KD OR Kawasaki-like OR multi-system OR multi-system) AND inflammatory AND (syndrome* OR disease) OR MISC OR MIS-COR PIMS OR [inflammatory AND (multi-system OR multi-system) AND (syndrome* OR disease*) OR Systemic Inflammatory Response Syndrome OR hyperinflammatory] AND (child* OR pediatric).
The study selection process was done in multiple phases via the EndNote reference management software to manage the acquired articles. At first, duplicate articles were identified through the software and manually. Then, in the screening phase, the title and abstract of the studies were examined based on the including criteria. Afterward, the full texts were screened in detail if needed. The selection process was done by two authors independently (M.E. & M.Q.). They came to an agreement regarding the conflicting results.
Two independent researchers filled data extraction forms containing age, gender, journal, study type, sample size, clinical characteristics, laboratory findings, and outcomes. Another researcher resolved conflicts.
Quality assessment (QA) of case-reports/case-series studies was assessed using the case-report (CARE) 13-item guideline (14). This guideline comprises 30 sub-items. Each item ratings are yes, or no, and the final QA score was the sum of sub-items. For QA of cohort studies, the Newcastle-Ottawa scale was used (maximum: nine stars) (15). This tool consists of three domains; comparability (maximum: two stars), selection (maximum: four stars), and outcome (maximum: three stars). The QA was carried out independently by two investigators (MQ and PM) addressing the items reported in the guidelines.
Our searches yielded 215 studies from PubMed, 310 studies from Scopus, and 156 study from Web of science. After removal of duplicates, we assess 378 studies for eligibility. We excluded 270 studies based on their title and abstract. Finally, 25 studies included in the qualitative synthesis. The detailed flow diagram is shown in Figure 1.
Table 1 shows the characteristics of the 25 included studies. Ten were conducted in the USA, 12 in Europe, 2 in India and 1 in Brazil. The included studies involved 599 patients in total; 347 (57.92%) were males. Thirteen studies (52%) were case reports or case series, and the rest (48%) were cohort studies. The minimum and maximum age for the patients ranged from 6 months to 16.6 years in the individual studies, respectively. In 19 studies, patients were diagnosed based on the multi-system inflammatory syndrome in children criteria (MIS-C) (KD-like syndrome) and in 6 studies patients fulfilled Kawasaki disease criteria.
In 16 studies COVID-19 was diagnosed in all patients based on their PCR result, serological findings, or computed tomography (CT) results. Fourhundred-fifty-five patients had positive serological indices (IgG, IgA, and IgM) for SARS-CoV-2 infection, while 175 patients were tested positive for COVID-19 using PCR (Table 2).
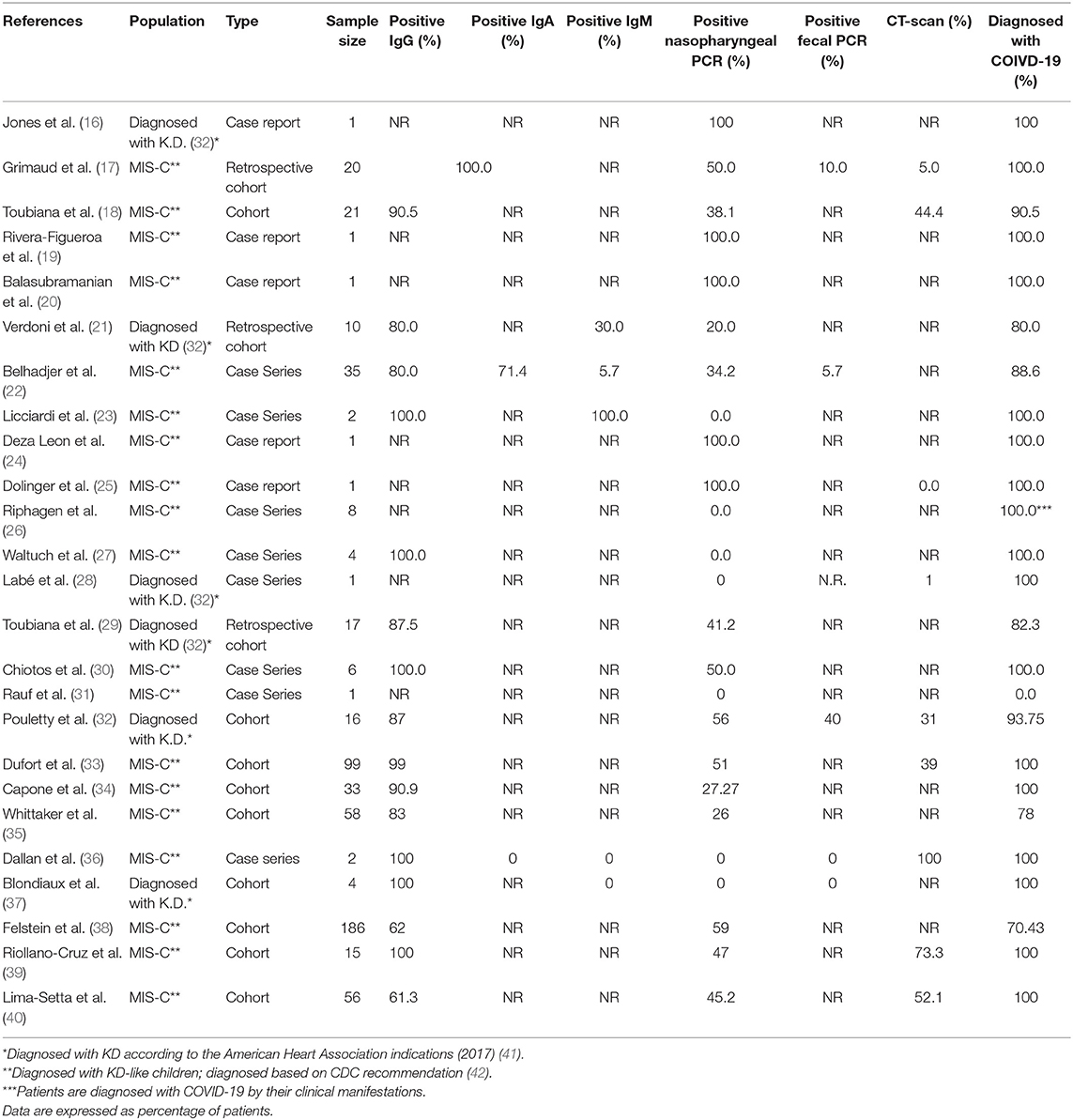
Table 2. COVID-19 related features of KD-like syndrome patients, including serological, PCR, and imaging findings.
Twenty one studies showed the CRP level; CRP was increased in all patients who participated in all studies. Highest CRP was reported in Deza Leon et al. study (450 mg/L) (24). A higher ESR level was also reported in 9 studies. In most studies, patients had leukopenia with marked lymphopenia, hypoalbuminemia, and increased ferritin, as well as hyponatremia. Laboratory Characteristics of the patients are shown in Table 3.
Twenty-two studies reported echocardiography findings of their patients. In six studies, all patients had abnormal echocardiography. The lowest rate of abnormality in echocardiography is demonstrated in Whittaker et al. (35) study (31.03%). Slightly increased troponin level and decreased ejection fraction, as well as increased BNP on admission, were reported. In terms of respiratory outcomes, ventilation was conducted in all patients in six studies. The lowest rate of ventilation is 20 percent revealed in Feldstein et al. study (38). The most common types of ventilation are invasive, non-invasive, and nasal high oxygen flow, respectively. In 11 studies, all patients required intensive care unit admission. Pouletty et al. (32) reported the lowest rate of ICU admission. Patients outcomes and drugs used to treat patients are summarized in Table 4.
In this study, the highest and the lowest score of included case-reports/case-series based on CARE guidelines was 93 and 74%, respectively. The cohort studies included in this study were considered as high quality in seven articles and medium quality in five articles. This scale classifies papers as high quality (8–9 points), medium quality (6–7 points), and low quality (<6 points) (43).
KD is a vasculitis of early childhood, which is the most common reason for acquired heart disease in children in developed countries (41). Although more than 40 years of study has tried to explain it, the etiology of KD remains unidentified (44). So far, researches have been able to determine immune response to a stimulus as a significant factor in the pathogenesis of the disease; however, researchers have not been able to identify the stimulus yet (45, 46). One probable explanation is exposure to an infectious agent and subsequently triggering the immune system. This idea can explain the peak of KD cases in winter (47, 48).
In the Dean et al. study in a KD epidemic in Hawaii, 44 percent of the cases had a history of respiratory infection in the month before their visit. In their research, although an infectious agent is proved as one of the immune system triggers and an etiology for KD, yet the analysis failed to determine the microorganism responsible for it (49). Other studies suggested viral agents such as adenoviruses as possible etiologies (50–52).
Members of the coronaviridae family have also been suggested as triggers for KD (53). Some studies before the COVID-19 pandemic showed that 7 percent of the patients with KD symptoms had positive PCR for at least one of the coronaviridae family members (54). Among the family, the new Haven coronavirus (HCoV-NH), which is similar to HCoV NL-63, has drawn more attention (55–57). Esper et al. revealed in their study that 72.7 percent of KD patients tested positive for HCoV-NH by PCR (58). Even though other studies did not fully support Esper's findings (55, 59), yet it pointed out that the studies that searched for the virus traces via serological tests showed a higher rate of virus detection than the studies that utilized PCR (60).
With the incidence of the COVID-19 pandemic, the number of children who presented with KD symptoms increased dramatically. The Verdoni et al. study showed that the monthly incidence of KD in an Italian province had increased 30-folds (21). The significant inflammatory response of the body to the novel coronavirus alongside the epidemiological studies have been in favor of the theory that suggests COVID-19 as a trigger for the immune system and an etiology for KD's (61–63).
This review has gathered the findings of 25 articles that have presented patients with KD-like syndrome in the era of the COVID-19 pandemic. Most of the patients have exhibited traces of COVID-19 in their tests. Among these data, it seems that KD-like syndrome are more strongly associated with positive serology tests. To justify this finding, we can point out that PCR turns negative in a shorter period compared to serology (measuring immunoglobulin levels) tests (64). It seems that the immune response that will lead to KD-like syndrome needs an amount of time to develop. This time exceeds the time in which the PCR test will result positive. We should note that a small fraction of the patients with KD-like syndrome did not reveal any trace of the novel coronavirus in their tests. This result could be due to the limited sensitivity of serology and PCR tests (65–67), or it could simply be due to the normal incidence of KD because of other etiologies. The current study suggests that in these days of the pandemic, it is highly recommended that children who present with KD or KD-like syndrome in the regions affected with COVID-19, be tested for SARS-CoV-2 infection. It seems that serology tests are preferred to PCR; however, we recommend that both tests be conducted for the patients.
In most reviewed articles, leukopenia and especially lymphopenia decreased levels of serum albumin, and an increase in Ferritin, ESR, and CRP was noted. Impaired heart function and myocarditis are the unfortunate outcomes of KD-like syndrome (68, 69). The articles have measured troponin and BNP levels and also conducted echocardiography for patients. Despite having near-normal levels of troponin, most children had increased BNP and decreased ejection fraction that confirmed the previous studies on KD. It seems that the risk of developing heart complications is increased when KD symptoms has co-occurred with COVID-19 compared to when the sole problem is KD. Based on the studies, we strongly recommend heart function screening for patients who are suspected of having KD-like syndrome.
KD is rare disease, which was mainly managed by pediatric rheumatologists, but as a result of COVID-19 the prevalence of this disease and KD-like syndrome has increased notably and these disease spectrums are now considered as a health issue. Although most of the articles reviewed in this study have similar findings, this systematic review assists clinicians to determine the best therapeutic approach for their patients based on their demographic, clinical, and laboratory findings to achieve the best outcome.
Although the clinical findings of KD is very similar to KD-like syndrome, there are some differences including the older age of KD-like Syndrome patients, which could be justified due to the higher exposure of school-aged children and adolescents to the virus compared to infants and toddlers. In addition, gastrointestinal Symptoms, lymphopenia, thrombocytopenia, and hypertriglyceridemia are more prevalent in KD-like syndrome compared to KD. KD-like syndrome patients are at higher risk of complications compared to KD patients. Moreover, the inflammatory factors have had more noticeable changes in these new patients, which may be related to primary infection with SARS-CoV-2 rather than KD itself (70, 71) (Table 5).
It should be noted that there are some diagnostic limitations due to the relatively low sensitivity of serological test compared to PCR. Moreover, most of the articles that were reviewed in this study were case reports and had relatively low evidence. We found only a few articles concerning our subject (Even with lower evidence level). It is necessary to perform pathophysiological assessments in addition to conducting case-control studies so that we would be able to understand the relationship between the novel coronavirus and KD better.
Incidence of children presenting with a severe inflammatory syndrome with KD-like features are increased during the COVID-19 pandemic. These children present with more severe symptoms which is attributed to worse clinical outcome and require intensive treatment and close monitoring. This study indicates that serological indices for SARS-CoV-2 have stronger correlation with the KD-like disease incidence compared to PCR. Moreover, CRP could also be helpful in terms of diagnosis. Although only one patient in included studies died of the complications the disease, most of the patients required ICU admission.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
MQ and KP: conceptualization. ME and MQ: search. PM and MA: data extraction. PM, ME, and MQ: writing—original draft. PI and KP: writing—review & editing. All authors read and approved final version of the manuscript.
This study was supported by the Alborz University of Medical Sciences (ABZUMS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.625377/full#supplementary-material
COVID-19, coronavirus disease 2019; KD, Kawasaki disease; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; MIS-C, Multisystem inflammatory syndrome in children; CARE, guidelines for Case Reports; NOS, The Newcastle-Ottawa Scale; ICU, intensive care unit; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; N.R., not reported; BNP, brain natriuretic peptide; IVIG, intravenous immunoglobulin.
1. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019. (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:343–6. doi: 10.15585/mmwr.mm6912e2
2. Hasani H, Mardi S, Shakerian S, Taherzadeh-Ghahfarokhi N, Mardi P. The Novel Coronavirus Disease (COVID-19): a PRISMA systematic review and meta-analysis of clinical and paraclinical characteristics. BioMed Res Int. (2020) 2020:3149020. doi: 10.1101/2020.04.19.20071456
3. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. (2020) 145:e20200702. doi: 10.1542/peds.2020-0702
4. Cruz AT, Zeichner SL. COVID-19 in children: initial characterization of the pediatric disease. Pediatrics. (2020) 145:e20200834. doi: 10.1542/peds.2020-0834
5. Hong H, Wang Y, Chung HT, Chen CJ. Clinical characteristics of novel coronavirus disease 2019. (COVID-19) in newborns, infants and children. Pediatrics Neonatol. (2020) 61:131–2. doi: 10.1016/j.pedneo.2020.03.001
6. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatrica. (2020) 109:1088–95. doi: 10.1111/apa.15270
7. Hedrich CM, Schnabel A, Hospach T. Kawasaki disease. Front Pediatrics. (2018) 6:198. doi: 10.3389/fped.2018.00198
8. Son MB, Sundel RP. Chapter 35 - Kawasaki disease. In: Petty RE, Laxer RM, Lindsley CB, Wedderburn LR, editors. Textbook of Pediatric Rheumatology. 7th ed. Philadelphia, PA: W.B. Saunders (2016). p. 467–83.e6. doi: 10.1016/B978-0-323-24145-8.00035-1
9. Rowley AH. Kawasaki disease: AHA statement and recommendations. Contemp Pediatrics. (2018) 35:15–21. Available online at: https://www.contemporarypediatrics.com/view/kawasaki-disease-aha-statement-and-recommendations
10. Singh S, Jindal AK, Pilania RK. Diagnosis of Kawasaki disease. Int J Rheumatic Dis. (2018) 21:36–44. doi: 10.1111/1756-185X.13224
11. Ebina-Shibuya R, Namkoong H, Shibuya Y, Horita N. Multisystem Inflammatory Syndrome in Children (MIS-C) with COVID-19: insights from simultaneous familial Kawasaki disease cases. Int J Infect Dis. (2020) 97:371–3. doi: 10.1016/j.ijid.2020.06.014
12. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
13. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
14. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Europ J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatrics. (2020) 10:537–40. doi: 10.1542/hpeds.2020-0123
17. Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. (2020) 10:69. doi: 10.1186/s13613-020-00690-8
18. Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. (2020) 369:m2094. doi: 10.1136/bmj.m2094
19. Rivera-Figueroa EI, Santos R, Simpson S, Garg P. Incomplete Kawasaki disease in a child with Covid-19. Indian Pediatrics. (2020) 57:680–1. doi: 10.1007/s13312-020-1900-0
20. Balasubramanian S, Nagendran TM, Ramachandran B, Ramanan AV. Hyper-inflammatory syndrome in a child with COVID-19 treated successfully with intravenous immunoglobulin and tocilizumab. Indian Pediatrics. (2020) 57:681–3. doi: 10.1007/s13312-020-1901-z
21. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. (2020) 395:1771–8. doi: 10.1016/S0140-6736(20)31103-X
22. Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. (2020) 142:429–36. doi: 10.1161/CIRCULATIONAHA.120.048360
23. Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, et al. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. (2020) 146:e20201711. doi: 10.1542/peds.2020-1711
24. Deza Leon MP, Redzepi A, McGrath E, Abdel-Haq N, Shawaqfeh A, Sethuraman U, et al. COVID-19-associated pediatric multisystem inflammatory syndrome. J Pediatric Infect Dis Soc. (2020) 9:407–8. doi: 10.1093/jpids/piaa061
25. Dolinger MT, Person H, Smith R, Jarchin L, Pittman N, Dubinsky MC, et al. Pediatric crohn disease and Multisystem Inflammatory Syndrome in Children (MIS-C) and COVID-19 treated with infliximab. J Pediatric Gastroenterol Nutrit. (2020) 71:153–5. doi: 10.1097/MPG.0000000000002809
26. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. (2020) 395:1607–8. doi: 10.1016/S0140-6736(20)31094-1
27. Waltuch T, Gill P, Zinns LE, Whitney R, Tokarski J, Tsung JW, et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. (2020) 38:2246.e3-.e6. doi: 10.1016/j.ajem.2020.05.058
28. Labé P, Ly A, Sin C, Nasser M, Chapelon-Fromont E, Ben Saïd P, et al. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J Europ Acad Dermatol Venereol. (2020) 34:e539–e41. doi: 10.1111/jdv.16666
29. Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Outbreak of Kawasaki disease in children during COVID-19 pandemic: a prospective observational study in Paris, France. MedRxiv. [Preprint]. (2020) doi: 10.1101/2020.05.10.20097394
30. Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, et al. Multisystem inflammatory syndrome in children during the coronavirus 2019. pandemic: a case series. J Pediatric Infect Dis Soc. (2020) 9:393–8. doi: 10.1093/jpids/piaa069
31. Rauf A, Vijayan A, John ST, Krishnan R, Latheef A. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 pandemic. Indian J Pediatrics. (2020) 87:745–7. doi: 10.1007/s12098-020-03357-1
32. Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. (2020) 79:999–1006. doi: 10.1136/annrheumdis-2020-217960
33. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. (2020) 383:347–58. doi: 10.1056/NEJMoa2021756
34. Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 infection. J Pediatrics. (2020) 224:141–5. doi: 10.1016/j.jpeds.2020.06.044
35. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324:259–69. doi: 10.1001/jama.2020.10369
36. Dallan C, Romano F, Siebert J, Politi S, Lacroix L, Sahyoun C. Septic shock presentation in adolescents with COVID-19. Lancet Child Adolescent Health. (2020) 4:e21–e3. doi: 10.1016/S2352-4642(20)30164-4
37. Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. (2020) 297:E283–e8. doi: 10.1148/radiol.2020202288
38. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. Children and Adolescents. N Engl J Med. (2020) 383:334–46. doi: 10.1056/NEJMoa2021680
39. Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Reed J, Posada R, et al. Multisystem inflammatory syndrome in children related to COVID-19: a New York City experience. J Med Virol. (2020) 93:424–33. doi: 10.1002/jmv.26224
40. Lima-Setta F, Magalhães-Barbosa MC, Rodrigues-Santos G, Figueiredo E, Jacques ML, Zeitel RS, et al. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J Pediatria. (in press). doi: 10.1016/j.jped.2020.10.008
41. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–e99. doi: 10.1161/CIR.0000000000000484
42. Belay E, Cheung E, Oster M, Tremoulet A. Clinical Management of Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Atlanta, GA (2020). Available online at: https://stacks.cdc.gov/view/cdc/90767
43. Huang Y, Ouyang YQ, Redding SR. Previous breastfeeding experience and its influence on breastfeeding outcomes in subsequent births: a systematic review. Women Birth. (2019) 32:303–9. doi: 10.1016/j.wombi.2018.09.003
44. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. (2005) 9:185–94. doi: 10.1016/j.ijid.2005.03.002
45. Laupland KB, Dele Davies H. Epidemiology, etiology, and management of Kawasaki disease: state of the art. Pediatric Cardiol. (1999) 20:177–83. doi: 10.1007/s002469900436
46. Rowley AH. Kawasaki disease: novel insights into etiology and genetic susceptibility. Ann Rev Med. (2011) 62:69–77. doi: 10.1146/annurev-med-042409-151944
47. Burns JC, Cayan DR, Tong G, Bainto EV, Turner CL, Shike H, et al. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. (2005) 16:220–5. doi: 10.1097/01.ede.0000152901.06689.d4
48. Yanagawa H, Nakamura Y, Kawasaki T, Shigematsu I. Nationwide epidemic of Kawasaki disease in Japan during winter of 1985-86. Lancet. (1986) 2:1138–9. doi: 10.1016/S0140-6736(86)90541-6
49. Dean AG, Melish ME, Hicks R, Palumbo NE. An epidemic of Kawasaki syndrome in Hawaii. J Pediatrics. (1982) 100:552–7. doi: 10.1016/S0022-3476(82)80751-8
50. Jaggi P, Kajon AE, Mejias A, Ramilo O, Leber A. Human adenovirus infection in Kawasaki disease: a confounding bystander? Clin Infect Dis. (2013) 56:58–64. doi: 10.1093/cid/cis807
51. Okano M, Thiele GM, Sakiyama Y, Matsumoto S, Purtilo DT. Adenovirus infection in patients with Kawasaki disease. J Med Virol. (1990) 32:53–7. doi: 10.1002/jmv.1890320109
52. Shike H, Shimizu C, Kanegaye JT, Foley JL, Schnurr DP, Wold LJ, et al. Adenovirus, adeno-associated virus and Kawasaki disease. Pediatric Infect Dis J. (2005) 24:1011–4. doi: 10.1097/01.inf.0000183769.31951.1e
53. Belay ED, Erdman DD, Anderson LJ, Peret TC, Schrag SJ, Fields BS, et al. Kawasaki disease and human coronavirus. J Infect Dis. (2005) 192:352–3; author reply 3. doi: 10.1086/431609
54. Dominguez SR, Anderson MS, Glodé MP, Robinson CC, Holmes KV. Blinded case-control study of the relationship between human coronavirus NL63 and Kawasaki syndrome. J Infect Dis. (2006) 194:1697–701. doi: 10.1086/509509
55. Chang LY, Chiang BL, Kao CL, Wu MH, Chen PJ, Berkhout B, et al. Lack of association between infection with a novel human coronavirus (HCoV), HCoV-NH, and Kawasaki disease in Taiwan. J Infect Dis. (2006) 193:283–6. doi: 10.1086/498875
56. Ebihara T, Endo R, Ma X, Ishiguro N, Kikuta H. Lack of association between New Haven coronavirus and Kawasaki disease. J Infect Dis. (2005) 192:351–2; author reply 3. doi: 10.1086/430797
57. Shimizu C, Shike H, Baker SC, Garcia F, Hoek Lvd, Kuijpers TW, et al. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease. J Infect Dis. (2005) 192:1767–71. doi: 10.1086/497170
58. Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. (2005) 191:499–502. doi: 10.1086/428291
59. Baker SC, Shimizu C, Shike H, Garcia F, van der Hoek L, Kuijper TW, et al. Human coronavirus-NL63 infection is not associated with acute Kawasaki disease. Adv Exp Med Biol. (2006) 581:523–6. doi: 10.1007/978-0-387-33012-9_94
60. Lehmann C, Klar R, Lindner J, Lindner P, Wolf H, Gerling S. Kawasaki disease lacks association with human coronavirus NL63 and human bocavirus. Pediatric Infect Dis J. (2009) 28:553–4. doi: 10.1097/INF.0b013e31819f41b6
61. Haslak FYM, Adrovic A, Sahin S, Barut K, Kasapçopur Ö. A recently explored aspect of the iceberg named COVID-19: multisystem inflammatory syndrome in children (MIS-C). Turk Arch Pediatr. (2021) 56:3–9. doi: 10.5152/TurkArchPediatr.2020.20245
62. Schvartz A, Belot A, Kone-Paut I. Pediatric inflammatory multisystem syndrome and rheumatic diseases during SARS-CoV-2 pandemic. Front Pediatrics. (2020) 8:605807. doi: 10.3389/fped.2020.605807
63. Mardi P, Shojaeian S, Taherzadeh-Ghahfarokhi N, Molaverdi G, Amiri Roudy M, Salahshour A, et al. SARS-CoV-2 and COVID-19, evidence from a literature review: review article. Tehran Univ Med J. (2021) 78:712–25. doi: 10.1016/j.jiph.2020.03.019
64. Xiang F, Wang X, He X, Peng Z, Yang B, Zhang J, et al. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin Infect Dis. (2020) 71:1930–4. doi: 10.1093/cid/ciaa461
65. Pan Y, Li X, Yang G, Fan J, Tang Y, Zhao J, et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. (2020) 81:e28–e32. doi: 10.1016/j.jinf.2020.03.051
66. Zainol Rashid Z, Othman SN, Abdul Samat MN, Ali UK, Wong KK. Diagnostic performance of COVID-19 serology assays. Malaysian J Pathol. (2020) 42:13–21. Available online at: http://www.mjpath.org.my/2020/v42n1/COVID-19-serology.pdf
67. Zhong L, Chuan J, Gong B, Shuai P, Zhou Y, Zhang Y, et al. Detection of serum IgM and IgG for COVID-19 diagnosis. Sci China Life Sci. (2020) 63:777–80. doi: 10.1007/s11427-020-1688-9
68. Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. (1978) 61:100–7.
69. Ronai C, Baker AL, Friedman KG, Fulton DR, Newburger JW, Lang P. Prevalence of undiagnosed structural heart disease in children undergoing echocardiography for Kawasaki disease. Clin Pediatrics. (2016) 55:557–9. doi: 10.1177/0009922815594588
70. Panupattanapong S, Brooks EB. New spectrum of COVID-19 manifestations in children: Kawasaki-like syndrome and hyperinflammatory response. Cleveland Clin J Med. (2020) 88:1–7. doi: 10.3949/ccjm.87a.ccc039
Keywords: COVID-19, Kawasaki, MIS-C, hyperinflammatory, children
Citation: Mardi P, Esmaeili M, Iravani P, Abdar ME, Pourrostami K and Qorbani M (2021) Characteristics of Children With Kawasaki Disease-Like Signs in COVID-19 Pandemic: A Systematic Review. Front. Pediatr. 9:625377. doi: 10.3389/fped.2021.625377
Received: 02 November 2020; Accepted: 11 February 2021;
Published: 18 March 2021.
Edited by:
Lorenzo D'Antiga, Papa Giovanni XXIII Hospital, ItalyReviewed by:
Ozgur Kasapcopur, Istanbul University-Cerrahpasa, TurkeyCopyright © 2021 Mardi, Esmaeili, Iravani, Abdar, Pourrostami and Qorbani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kumars Pourrostami, a2l1bWFyc3BvdXJyb3N0YW1pQHlhaG9vLmNvbQ==; Mostafa Qorbani, bXFvcmJhbmkxMzc5QHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.