
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 18 January 2021
Sec. Pediatric Rheumatology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.635080
 Alessandra Meneghel1*
Alessandra Meneghel1* Giorgia Martini1
Giorgia Martini1 Angela Amigoni2
Angela Amigoni2 Andrea Pettenazzo2
Andrea Pettenazzo2 Massimo Padalino3
Massimo Padalino3 Francesco Zulian1
Francesco Zulian1Macrophage activation syndrome (MAS) is a rare, potentially life-threatening, condition triggered by infections or flares in rheumatologic and neoplastic diseases. The mainstay of treatment includes high dose corticosteroids, intravenous immunoglobulins and immunosuppressive drugs although, more recently, a more targeted approach, based on the use of selective cytokines inhibitors, has been reported. We present the case of a two-year-old boy with 1-month history of high degree fever associated with limping gait, cervical lymphadenopathy and skin rash. Laboratory tests showed elevation of inflammatory markers and ferritin. By exclusion criteria, systemic onset Juvenile Idiopathic Arthritis (sJIA) was diagnosed and steroid therapy started. A couple of weeks later, fever relapsed and laboratory tests were consistent with MAS. He was promptly treated with high doses intravenous methylprednisolone pulses and oral cyclosporin A. One day later, he developed an acute myocarditis and a systemic capillary leak syndrome needing intensive care. Intravenous Immunoglobulin and subcutaneous IL-1-antagonists Anakinra were added. On day 4, after an episode of cardiac arrest, venous-arterial extracorporeal membrane oxygenation (VA-ECMO) was started. Considering the severe refractory clinical picture, we tried high dose intravenous Anakinra (HDIV-ANA, 2 mg/Kg q6h). This treatment brought immediate benefit: serial echocardiography showed progressive resolution of myocarditis, VA-ECMO was gradually decreased and definitively weaned off in 6 days and MAS laboratory markers improved. Our case underscores the importance of an early aggressive treatment in refractory life-threatening sJIA-related MAS and adds evidence on safety and efficacy of HDIV-ANA particularly in acute myocarditis needing VA-ECMO support.
Macrophage activation syndrome (MAS) is a rare, potentially life-threatening complication of some rheumatologic diseases, such as systemic onset Juvenile Idiopathic Arthritis (sJIA), Kawasaki Disease (KD) and Systemic Lupus Erythematous (SLE), or a condition triggered by viral and or bacterial infections in predisposed individuals or associated with neoplastic disease (1). Early diagnosis is challenging as an appropriate therapy can significantly improve the outcome. The mainstay of treatment is the use of high dose corticosteroids (1, 2) but, in the last few years, the better understanding of the pathological pathways of the disease opened up new perspectives on the use of selective cytokines inhibitors (1). Recently Anakinra (ANA), a recombinant IL-1 receptor antagonist, has been successfully used for the treatment of sJIA-related MAS, pointing out the need for high doses in those refractory to conventional treatment (3–5). Acute myocarditis is a rare and potentially fatal complication of sJIA (6) and, given the rarity of this condition, experience on treatment is very limited. In adult-onset Still disease, single case reports suggested that ANA may play a role also for this complication (7–9). Taken together, these experiences suggest that ANA represents a potential effective treatment for MAS and myocarditis complicating sJIA.
We describe the case of a child with sJIA complicated by severe MAS, Systemic Capillary Leak Syndrome (SCLS) and acute myocarditis, leading to distributive and cardiogenic shock, cardiac arrest needing cardiopulmonary resuscitation and veno-arterial Extracorporeal Membrane Oxygenation (VA-ECMO), who was successfully treated with high dose intravenous ANA (HDIV-ANA).
A previously healthy two-year-old boy presented with 1 month history of fever associated with limping gait, cervical lymphadenopathy and evanescent skin rash. He was evaluated at a regional hospital where laboratory tests showed: WBC 25990/mm3 (N 18740/mm3); CRP 65 mg/L; ESR 68 mm/h; ferritin 1,259 ug/L; tryglicerides 1.5 mmol/L; AST 61 U/L, ALT 45 U/L. Echocardiography was normal. Bone marrow aspiration was negative for blasts. A short course of oral prednisone (1 mg/kg/day) was started with benefit on fever. However, upon steroid tapering, fever and limping reappeared. MRI showed synovial membrane hypertrophy and effusion in both hips. Therefore, according with ILAR criteria (10), sJIA was diagnosed and high dose pulse intravenous (IV) methylprednisolone (MPDN, 30 mg/Kg/day) was given for 2 days then followed by prednisone maintenance dose (2 mg/Kg/day). Two days later, due to a methicillin-resistant staphylococcus aureus cellulitis of the right hand, IV teicoplanin was started and the patient was referred to our Pediatric Rheumatology Unit.
On admission, physical examination revealed unremitting high-grade fever, erythematous skin rash on face and limbs and mild hepato-splenomegaly. No arthritis was detected.
Laboratory tests showed: Hb 8.5 g/dl, PLT 44,000/mm3; FDP 1522 ug/L, fibrinogen 1.6 g/L, CRP 100 mg/L, AST 57 U/L, ALT 52 U/L; ferritin 2,200 ug/L; triglycerides 2.86 mmol/L. Suspecting an incipient MAS, high doses IV MDPN and oral Cyclosporin A (CSA, 2 mg/Kg/day) were started (Figure 1). 24 h later (day 2) he presented a systemic capillary leak syndrome (SCLS) with rapidly increasing weight (+ 5 Kg, ~30% BW), persistent hypotension (85/45 mmHg), tachycardia (160/min) and oliguria. He was urgently admitted into the Pediatric Intensive Care Unit (PICU) where inotropic and vasoactive support was maximized and conventional mechanical ventilation soon after started. Echocardiography revealed significantly increased myocardial thickness and echoic appearance, consistent with acute myocarditis. As summarized in Figure 1, IV Immunoglobulin (IVIG 400 mg/Kg/day for 5 days) and subcutaneous ANA (2 mg/Kg/day) were added. Indeed, multiple blood and platelet transfusions were needed to treat severe anemia and thrombocytopenia. Unfortunately, 48 h later (day 4) an episode of acute hypotension, bradycardia and oxygen desaturation led to cardiac arrest needing cardiopulmonary resuscitation and then VA-ECMO because cardiac function did not improve (Figure 2). On hemodynamic stability, upon informed consent by the parents, we started high dose intravenous ANA treatment (HDIV-ANA, 2 mg/Kg q6h). Starting from 72 h later (day 7), serial echocardiography revealed progressive resolution of myocarditis therefore VA-ECMO support was gradually decreased and definitively weaned off on day 10. Laboratory test showed improvement of MAS markers (Figure 1) but also the presence of a severe neutropenia (PMNs 0–100/mm3). Bone marrow aspirate confirmed poor PMNs representation, mainly consisting of pro-myelocytes with rare residual aspects of hemophagocytosis. Suspecting iatrogenic neutropenia, HDIV-ANA was gradually reduced to the maintenance subcutaneous dose of 2 mg/kg/day without resolution of neutropenia. Therefore, on day 22, ANA was stopped and neutropenia gradually resolved. The patient was discharged from the PICU 16 days after admission (Figure 2). Genetic analysis for familial hemophagocytic lymphohistiocytosis revealed a mutation in the PRF1 gene [c.(272C>T) p.(Ala91Val)] in heterozygosis, reported in sJIA-related MAS (11). 49 days after admission the patient was discharged on oral PDN (1 mg/Kg/day) and CSA (2 mg/Kg/day). Neither neurological signs or symptoms nor other internal organ consequences related to MAS were reported. Interestingly, a few months later, on tapering down of therapy, he relapsed with fever and increased ferritin and CRP. Subcutaneous ANA (2 mg/Kg/day) was restarted with rapid clinical and laboratory improvement and no side effects. During the follow up visits, corticosteroid and CSA therapy were gradually tapered down until stopping 4 and 6 months after discharge, respectively. Indeed, since parents reported difficulty maintaining adherence to ANA daily injections, 6 months after discharge, we switched to Canakinumab, a long-acting human monoclonal antibody targeting IL-1β, at the dose of 4 mg/Kg q4wk and then gradually tapered down. Currently, after 24 months, the disease is in clinical remission on medication (Canakinumab 4 mg/Kg q6wk).
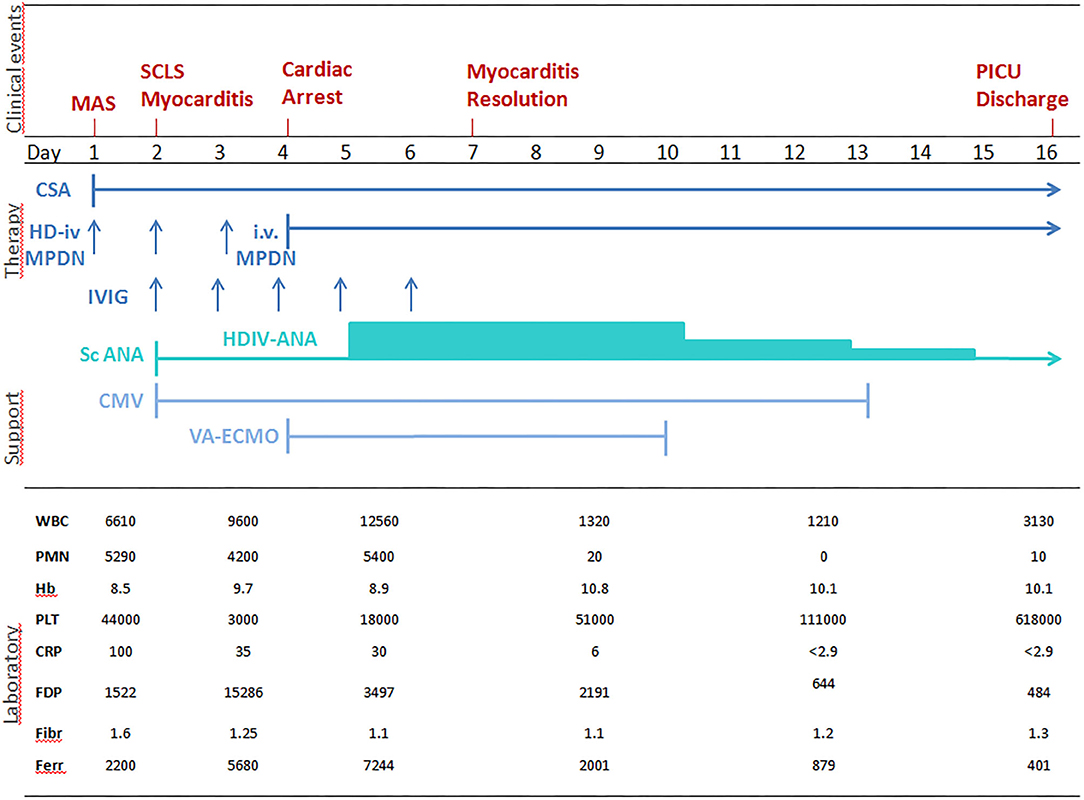
Figure 1. Correlation between clinical aspects, laboratory tests and treatment of sJIA-related MAS. WBC, white blood cells n./mm3; PMNs, polymorphonuclear cells n./mm3; Hb, hemoglobin gr/dl; PLT, platelets × 103/mm3; CRP, C-reactive protein mg/l (n.v. <5); FDP, fibrinogen degradation products, μg/l (n.v. <250); FBG, serum fibrinogen g/l, (n.v.1.5 – 4.5); FER, ferritin μg/l (n.v. 20 – 250); IV HD-MPDN, intravenous methylprednisolone 30 mg/Kg/die iv; CSA, cyclosporine-A 2 mg/Kg/day; IVIG, intravenous immunoglobulin 400 mg/Kg/day; scANA, subcutaneous Anakinra 2 mg/Kg/day; HDIV-ANA, high dose intravenous Anakinra 2 mg/Kg/6 hours; CMV, Conventional mechanical ventilation; VA-ECMO, veno-arterial extracorporeal membrane oxygenation); SCLS, systemic capillary leak syndrome; MAS: macrophage activation syndrome.
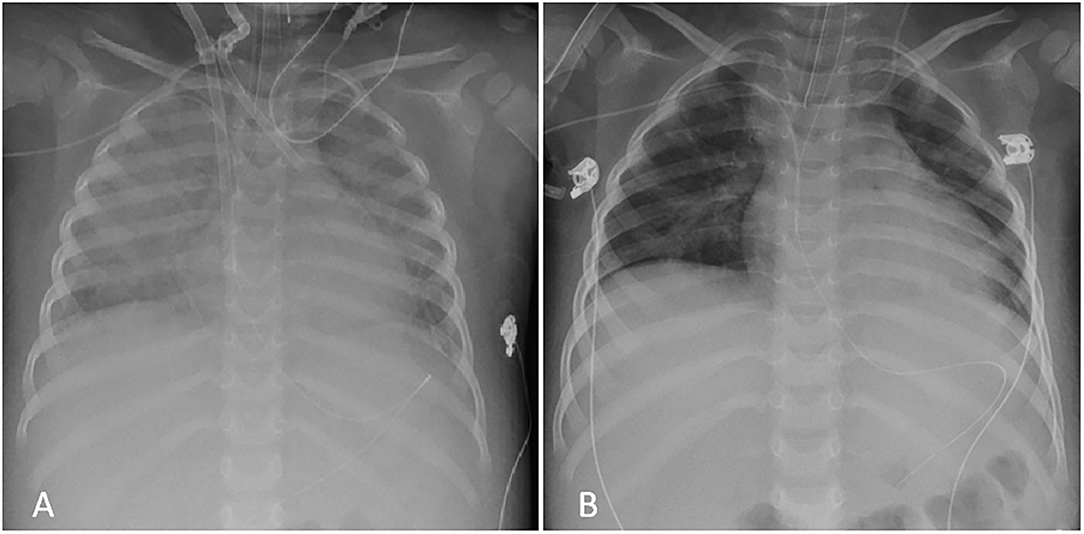
Figure 2. Chest X-Ray during PICU admission. (A) Severe bilateral interstitial thickening and right perihilar consolidation soon after VA-ECMO placement (day 4); (B) Complete resolution of the pattern after HDIV-ANA treatment at PICU discharge (day 16).
MAS is a life-threatening condition, most commonly reported as a complication of sJIA and triggered by infections in up to one-third of the patients (2). It is the result of a cytokine storm that lead to a dysregulated inflammatory activation of the immune system, with rapid progression to multiorgan failure if not promptly diagnosed and properly treated. Early diagnosis is still a clinical challenge since there is no diagnostic test able to differentiate MAS from a flare of the underlying systemic inflammatory disease (12). In 2016, Classification Criteria of MAS in sJIA were proposed providing a sensitive and specific tool for the recognition of MAS in this subset of patients (13). The main clinical and laboratory features of MAS include unremitting high-degree fever, hyperferritinemia, pancytopenia, fibrinolytic consumptive coagulopathy and liver dysfunction (12). The goal of therapy is controlling and, possibly, stopping the immune hyper-activation and cytokine related overproduction as quickly as possible. Treatment usually includes high dose corticosteroids and immunosuppressive agents in refractory cases. Recently, the better understanding of the disease pathogenesis, in particular the crucial role of IL-1, suggested adopting a more targeted approach based upon the use of selective cytokine inhibitors. Although no standardized guidelines are available to date, the use of ANA, a recombinant IL-1 receptor antagonist, has been reported in adult-onset Still Disease, SLE and Undifferentiated Connective Tissue Disease (UCTD) complicated by MAS, sometimes with cardiac involvement (7, 8, 14). In pediatric MAS, treatment with ANA has been also proposed, pointing out the need for a higher doses regimen in severe refractory cases (3–5, 15). Although supportive evidence is still limited, intravenous ANA has also been used in patients with cytokine storm syndromes (5, 14) probably because it enables higher and faster maximal plasma concentration as compared with subcutaneous administration (5, 16). Moreover, pharmacokinetic studies have shown an increasing ANA half-life according with Body Mass Index, if administered subcutaneously, meaning that patients with greater adipose tissue have slower drug's transport and absorption (5, 16). Therefore, it is reasonable that in patients with subcutaneous edema or anasarca the intravenous route might be preferable.
In our case, the intravenous ANA administration was a forced choice, partly due to the prominent generalized edema related to the SCLS and partly to the severe risk of bleeding, related both to MAS and ECMO. We now recognize that this choice has been successful. Along with the unexpected favorable result, no major adverse events were noticed, except for a transient neutropenia, already reported (17).
Based on our experience, HDIV-ANA is a safe and effective treatment for refractory life-threatening sJIA-related MAS, even if complicated by acute fulminant myocarditis. Based on our positive experience, this therapeutic approach may be also considered in the current pandemic COVID-19 emergency where myocardial injury has been recently reported and where recent evidence showed interleukin-β-driven MAS-like complication, triggered by SARS-CoV-2 virus, as predictor of bad outcome (18–20).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
AM, GM, and FZ decided the personalized treatment, reviewed the literature about Anakinra use in MAS and received final approval by the hospital pharmacy, and general management department for its use. AM collected all data, conceptualized, and wrote the manuscript. FZ supervised the study team and critically reviewed the manuscript for important intellectual aspect of the work. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. All authors took care of the patient during hospitalization, reviewed, and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank the Pediatric Rheumatology European Society (PReS) for accepting an abstract regarding this case as e-poster viewing at the last 26th PReS e-Congress held in September 2020 (21).
ALT, alanine aminotransferase; ANA, anakinra; AST, aspartate aminotransferase; CRP, C-reactive protein; CSA, cyclosporin A; ESR, erythrocyte sedimentation rate; FDP, fibronogen degradation products; Hb, hemoglobin; HDIV-ANA, high dose intravenous anakinra; IV, intravenous; IVIG, intravenous immunoglobulin; MAS, macrophage activation syndrome; PDN, prednisone; PICU, pediatric intensive care unit; PLT, platelets; PMNs, polymorphonuclear leukocytes; SCLS, systemic capillary leak syndrome; sJIA, systemic juvenile idiopathic arthritis; VA-ECMO, veno-arterial extracorporeal membrane oxygenation; WBC, white blood cells.
1. Boom V, Anton J, Lahdenne P, Quartier P, Ravelli A, Wulffraat NM, et al. Evidence-based diagnosis and treatment of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Pediatric Rheumatol. (2015) 13:55. doi: 10.1186/s12969-015-0055-3
2. Minoia F, Davì S, Horne A, Demirkaya E, Bovis F, Li C, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. (2014) 66:3160–9. doi: 10.1002/art.38802
3. Bruck N, Suttorp M, Kabus M, Heubner G, Gahr M, Pessler F. Rapid and sustained remission of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome through treatment with anakinra and corticosteroids. J Clin Rheumatol. (2011) 17:23–7. doi: 10.1097/RHU.0b013e318205092d
4. Sönmez HE, Demir S, Bilginer Y, Özen S. Anakinra treatment in macrophage activation syndrome: a single center experience and systemic review of literature. Clin Rheumatol. (2018) 37:3329–35. doi: 10.1007/s10067-018-4095-1
5. Mehta P, Cron RQ, Hartwell J, Manson JJ, Tattersall RS. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. (2020) 2:e358–67. doi: 10.1016/S2665-9913(20)30096-5
6. Goldenberg J, Ferraz MB, Pessoa AP, Fonseca AS, Carvalho AC, Hilario MO, et al. Symptomatic cardiac involvement in juvenile rheumatoid arthritis. Int J Cardiol. (1992) 34:57–62. doi: 10.1016/0167-5273(92)90082-e
7. Parisi F, Paglionico A, Varriano V, Ferraccioli G, Gremese E. Refractory adult-onset still disease complicated by macrophage activation syndrome and acute myocarditis. A case report treated with high doses (8mg/Kg/d) of anakinra. Medicine. (2017) 96:24. doi: 10.1097/MD.0000000000006656
8. Reffeiner B, Botsios C, Dinarello C, Ometto F, Punzi L, Ramonda R. Adult-onset still's disease with myocarditis successfully treated with the interleukin-1 receptor antagonist anakinra. Joint Bone Spine. (2011) 78:98–103. doi: 10.1016/j.jbspin.2010.09.014
9. Movva R, Brown BS, Morris DL, Figueredo VM. Anakinra for myocarditis in juvenile idiopathic arthritis. Tex Heart Inst J. (2013) 40:623–5.
10. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. (2004) 31:390–2.
11. Vastert SJ, van Wijk R, D'Urbano LE, de Vooght KMK, de Jager W, Ravelli A, et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology. (2010) 49:441–9. doi: 10.1093/rheumatology/kep418
12. Crayne CB, Albeituni S, Nichols KE, Cron RQ. The immunology of macrophage activation syndrome. Front Immunol. (2019) 10:119. doi: 10.3389/fimmu.2019.00119
13. Ravelli A, Minoia F, Davi S, Horne A, Bovis F, Pistorio A et al. 2016 Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis. (2016) 75:481–9. doi: 10.1136/annrheumdis-2015-208982
14. Monteagudo LA, Boothby A, Gertner E. Continuous intravenous Anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatology. (2020) 2:1–7. doi: 10.1002/acr2.11135
15. Rajasekaran S, Kruse K, Kovey K, Davis AT, Hassan NE, Ndika AN, et al. Therapeutic role of anakinra, an interleukin-1 receptorantagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. (2014) 15:401–8. doi: 10.1097/PCC.0000000000000078
16. Yang BB, Gozzi P, Sullivan S. Pharmacokinetics of anakinra in subjects of heavier vs. lighter body weights. Clin Transl Sci. (2019) 12:371–78. doi: 10.1111/cts.12622
17. Perrin F, Néel A, Graveleau J, Ruellan AL, Masseau A, Hamidou M. Two cases of anakinra-induced neutropenia during autoinflammatory diseases: drug reintroduction can be successful. Presse Med. (2014) 43:319–31. doi: 10.1016/j.lpm.2013.06.028
18. Wolfler A, Mannarino S, Giacomet V, Camporesi A, Zuccotti G. Acute myocardial injury: a novel clinical pattern in children with COVID-19. Lancet Child Adolesc Health. (2020) 4:e26–7. doi: 10.1016/S2352-4642(20)30168-1
19. Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. (2020) 19:102567. doi: 10.1016/j.autrev.2020102567
20. Trpkov C, MacMullan P, Feuchter P, Kachra R, Heydari B, Merchant N, et al. White, rapid response to cytokine storm inhibition using anakinra in a patient with COVID-19 myocarditis. CJC Open. (2020). doi: 10.1016/j.cjco.2020.10.003
Keywords: macrophage activation syndrome, anakinra, systemic juvenile idiopathic arthritis, myocarditis, ECMO
Citation: Meneghel A, Martini G, Amigoni A, Pettenazzo A, Padalino M and Zulian F (2021) Case Report: Life-Threatening Macrophage Activation Syndrome With Fulminant Myocarditis Successfully Rescued by High Dose Intravenous Anakinra. Front. Pediatr. 8:635080. doi: 10.3389/fped.2020.635080
Received: 29 November 2020; Accepted: 22 December 2020;
Published: 18 January 2021.
Edited by:
Jordi Anton, Hospital Sant Joan de Déu Barcelona, SpainReviewed by:
Marija Jelusic, University of Zagreb, CroatiaCopyright © 2021 Meneghel, Martini, Amigoni, Pettenazzo, Padalino and Zulian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandra Meneghel, YWxlc3NhbmRyYS5tZW5lZ2hlbEBhb3BkLnZlbmV0by5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.