
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 24 November 2020
Sec. Pediatric Pulmonology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.574014
This article is part of the Research TopicEmerging Pneumonia in ChildrenView all 11 articles
 Ti-An Tsai1
Ti-An Tsai1 Chang-Ku Tsai1
Chang-Ku Tsai1 Yao-Hsu Yang2
Yao-Hsu Yang2 Zon-Min Lee3†
Zon-Min Lee3† Jiunn-Ming Sheen1
Jiunn-Ming Sheen1 Yi-Chen Lee1
Yi-Chen Lee1 Chih-Min Tsai1
Chih-Min Tsai1 Chih-Cheng Chen1
Chih-Cheng Chen1 Chih-Hao Chang4
Chih-Hao Chang4 Chen-Kuang Niu1
Chen-Kuang Niu1 Hong-Ren Yu1,5*†
Hong-Ren Yu1,5*†Few studies have addressed the risk of infection in transfusion-naïve thalassemia patients. We aimed to investigate whether transfusion-naïve thalassemia population has higher hospitalization rates for lower airway infection-related diseases than non-thalassemia population in children. A nationwide population-based retrospective cohort study was conducted using detailed medical records of the Taiwan National Health Insurance Research Database. Transfusion-naïve thalassemia patients were compared with a matched cohort at a ratio of 1:4. Data of the selected patients were adjusted for age, sex, and related comorbidities. We recorded the frequency of admissions or outpatient clinic visits for patients with a diagnosis of pneumonia or acute bronchitis/bronchiolitis. Based on our results, the hospitalization rates and incidence rate ratios of bronchitis/bronchiolitis and pneumonia for transfusion-naïve thalassemia children were all higher than those for non-thalassemia controls. Therefore, we conclude that transfusion-naïve thalassemia children are more likely to experience lower airway infections and have a higher probability of hospitalization for these conditions.
Thalassemia is a common autosomal-recessive hereditary hemoglobinopathy, mainly characterized by a point mutation on globin gene expression (1). In adults, hemoglobin comprises four protein chains (two α and two β globin chains) that are assembled into a heterotetramer. In α-thalassemia and β-thalassemia, the production of the α and β globin chains is affected, respectively. Thalassemia is commonest among people of Italian, Greek, Middle Eastern, South Asian, and African descent (2). In China, the prevalence of α-thalassemia, β-thalassemia, and α + β-thalassemia were estimated at 7.88, 2.21, and 0.48%, respectively (3). In a previous study, the prevalence of α-thalassemia traits in Taiwan was 3.4% (4).
Clinical manifestations of thalassemia may range from no symptoms to severe lethal complications depending on how many of the four genes for α globin or two genes for β globin are involved. People with thalassemia may have iron overload (owing to the disease itself or frequent blood transfusions), infection, splenomegaly, slowed growth rates, or heart disease. Infection is a major cause of morbidity and mortality in patients with thalassemia major and is assumed to result from splenectomy, blood transfusion, and immunological changes with iron overload (2, 5). Patients with splenectomy are more susceptible to gram-negative microorganisms and pneumococcus than other organisms (6, 7), and patients who receive regular blood transfusions are at a risk of developing transfusion-transmitted infections such as Toxoplasma gondii, hepatitis C virus, and hepatitis B virus (8–10).
Unlike thalassemia major, patients with non-transfusion-dependent thalassemia (NTDT) (including those with various phenotypes) do not require lifelong regular transfusion therapy for survival. NTDT patients can be at risk of ineffective erythropoiesis, peripheral hemolysis, and iron overload that contribute to a number of clinical morbidities (11, 12), and these patients with iron overload may have higher risk for severe bacterial infection (13).
Intracellular iron overload has been proven to lead to DNA damage of lymphocytes and immune dysfunction in thalassemia major patients who receive blood transfusions (14). However, whether the immune function in NTDT patients is impaired is still unclear. Some reports suggested the presence of decreased CD4+/CD8+ ratios and increased Treg cells, which might suppress immune activation status, in β-thalassemia major patients, but not in those with β-thalassemia traits, compared to controls (15, 16). Another report stated that reduced CXCR2 expression and neutrophil migration were observed in NTDT patients (17). Therefore, this study aimed to examine whether NTDT children without history of blood transfusion have a higher risk of infection, especially lower respiratory tract infections.
Nearly the entire Taiwanese population, i.e., over 23 million people, have been enrolled in National Health Insurance since 1995. Details of the medical records of each person are stored in the National Health Insurance Research Database (NHIRD), which was created by the National Health Research Institutes (NHRI). In this retrospective cohort study, we used the Longitudinal Health Insurance Database 2010 (LHID 2010), released by NHRI, as our data source. The LHID 2010 includes one million people randomly selected from the 2010 Registry for Beneficiaries. The database contains detailed medical records of each insurant from 1997 to 2013. Among these one million people, we selected people whose birth year was from 1998 to 2007, so all people we analyzed were children and adolescents. International Classification of Diseases, ninth revision (ICD-9-CM) coding was used for disease identification. The study protocol was approved by the institutional review board of the study hospital.
We defined patients as having thalassemia if they were diagnosed with thalassemia (ICD-9-CM: 282.4) once during hospitalization or at least twice when examined at the outpatient department in 1 year. We excluded patients with a history of catastrophic illness certificates, blood transfusion (ICD-9 procedure code 94005C or 990), partial or total splenectomy (ICD-9 procedure code 70001B, 70003B, and 70006B), immunodeficiency (ICD-9-CM: 279) and other hematological disorders, including iron deficiency, and other deficiency anemias (ICD-9-CM: 280~281), sickle-cell anemia and other hemoglobinopathies, hereditary/acquired hemolytic anemia, aplastic anemia, and chronic disease-related anemia (ICD-9-CM: 282.6~285.8), myelodysplastic syndrome (ICD-9-CM: 238.7), hyperferritinemia and primary or secondary hemochromatosis (ICD-9-CM: 275.0), and hematological malignancies (ICD-9-CM: 200~208) diagnosed once during hospitalization or more than once in the outpatient department in 1 year. We also excluded patients for whom information on age or sex was missing. The catastrophic illness certificates in Taiwan include cancer, hematologic abnormality, renal failure with hemodialysis required, generalized autoimmune diseases with life-long treatment required, chronic mental disorders, congenital metabolic disorders, major organs and genes abnormality (e.g., congenital anomalies of heart), massive burns, major organs transplantation, complicated nervous, or musculoskeletal disorders (e.g., cerebral palsy), injury severity scores more than 16, chronic respiratory failure with ventilator required, uncorrected malnutrition status, Myasthenia gravis, spinal cord injuries, occupational diseases, acute stage of cerebrovascular diseases, multiple sclerosis, leprosy, liver cirrhosis with complication, complications related to prematurity, toxic effect of arsenic and its compounds, Creutzfeldt-Jakob disease, and other rare diseases (e.g., cystic fibrosis).
One thalassemia patient was matched with four control patients without thalassemia (1:4 matching) according to birth year, sex with frequency match. The comorbidities included were asthma (ICD-9-CM: 493), diabetes mellitus (DM) (ICD-9-CM: 450), chronic lung disease (ICD-9-CM: 770.7), heart failure (ICD-9-CM: 428), epilepsy (ICD-9-CM: 345), and chronic kidney disease (CKD) (ICD-9-CM: 580~589), diagnosed once at hospitalization or at least thrice in the outpatient department in 1 year. These medical conditions were proved to be risk factors of pneumonia (18, 19). The propensity score was calculated using the Statistical Analysis System 9.4 program (SAS Institute, Cary, North Carolina, USA).
The patients in this cohort study were followed up from their birthday to death or 2013/12/31. The outcome was defined as having records of lower airway infections such as acute bronchitis/bronchiolitis (ICD-9-CM: 466) and pneumonia (ICD-9-CM: 480~486), diagnosed at hospitalization or at least thrice in the outpatient department in 1 year. Each incident of hospitalization due to lower respiratory tract infection was included for analysis. The effects of baseline comorbidities, which may be related to lower airway infection, were eliminated via the propensity score model.
Differences between the demographic characteristics and comorbidities and the events of hospitalization due to lower airway infections in patients with thalassemia and matched non-thalassemia controls were analyzed using the chi-square test. The incidence rate ratios (IRRs) and 95% confidence intervals (CIs) were estimated using Poisson regression. A P < 0.05 in 2-tailed tests was considered statistically significant.
One million people were randomly selected from LHID 2010, and those born between 1998 and 2007 were selected for further analysis. Subjects who matched the inclusion and exclusion criteria were separated into a group with thalassemia and a group with non-thalassemia. Details of these processes are shown in Figure 1. Finally, 393 patients were identified in the thalassemia group and 1,572 controls in the non-thalassemia group (the control group), with 1:4 matching according to birth year, sex, and propensity score.
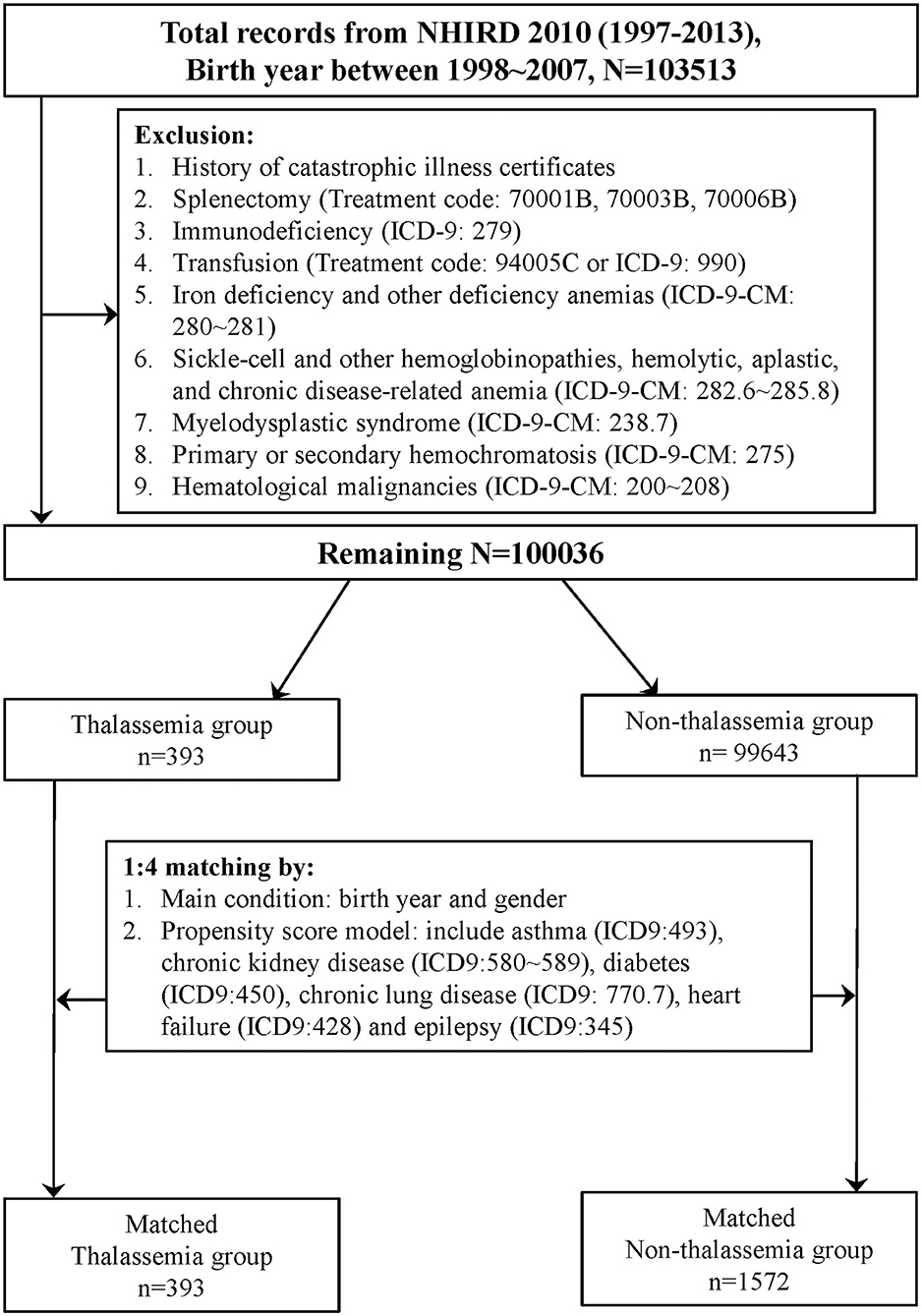
Figure 1. Flow chart of matched cohorts' selection. One million people were randomly selected from the Longitudinal Health Insurance Database 2010 (LHID 2010). After the screening process, 393 persons in the thalassemia group and 1,572 persons in the non-thalassemia group were analyzed.
Demographic characteristics and comorbidities in these matched cohorts are shown in Table 1. Similar distributions occurred with sex, age, place of residence, income, and comorbidities, including asthma, DM, CKD, chronic lung disease, heart failure, and epilepsy between the thalassemia and non-thalassemia groups because both cohorts were matched for factors. For lower respiratory diseases, the patients in the thalassemia group had a higher prevalence of acute bronchiolitis/bronchitis (92.88% vs. 87.60%, P = 0.003) and pneumonia (60.65 vs. 36.96%, P < 0.001) than those in the non-thalassemia group.
Thereafter, we focused on events related to hospitalization due to lower respiratory diseases (Table 2). The hospitalization rates for acute bronchitis/bronchiolitis (31.55 vs. 13.42%, P < 0.001) and pneumonia (49.11 vs. 23.22%, P < 0.001) were also higher in the thalassemia group than in the non-thalassemia group. The average number of admissions for inpatients and the average number of hospitalization days each time were also compared, and there was almost no difference between these two groups. Figures 2, 3 show the cumulative number of admissions or hospitalization days per person due to acute bronchitis/bronchiolitis and pneumonia across the 17 years, respectively. For both acute bronchitis/bronchiolitis and pneumonia, the cumulative rates for the thalassemia group were obviously higher than for the non-thalassemia group.
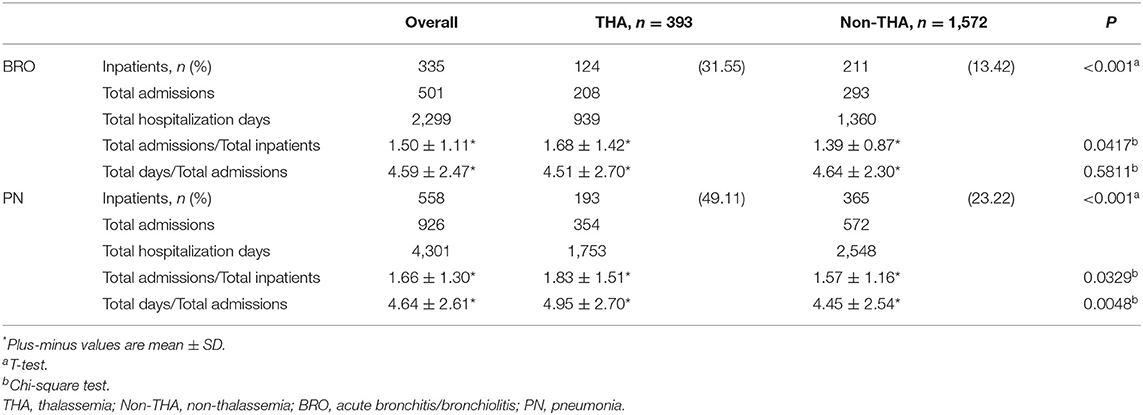
Table 2. Number of inpatients, number of admissions and hospitalization days for lower airway disease (acute bronchitis/bronchiolitis and pneumonia) from matched cohorts.
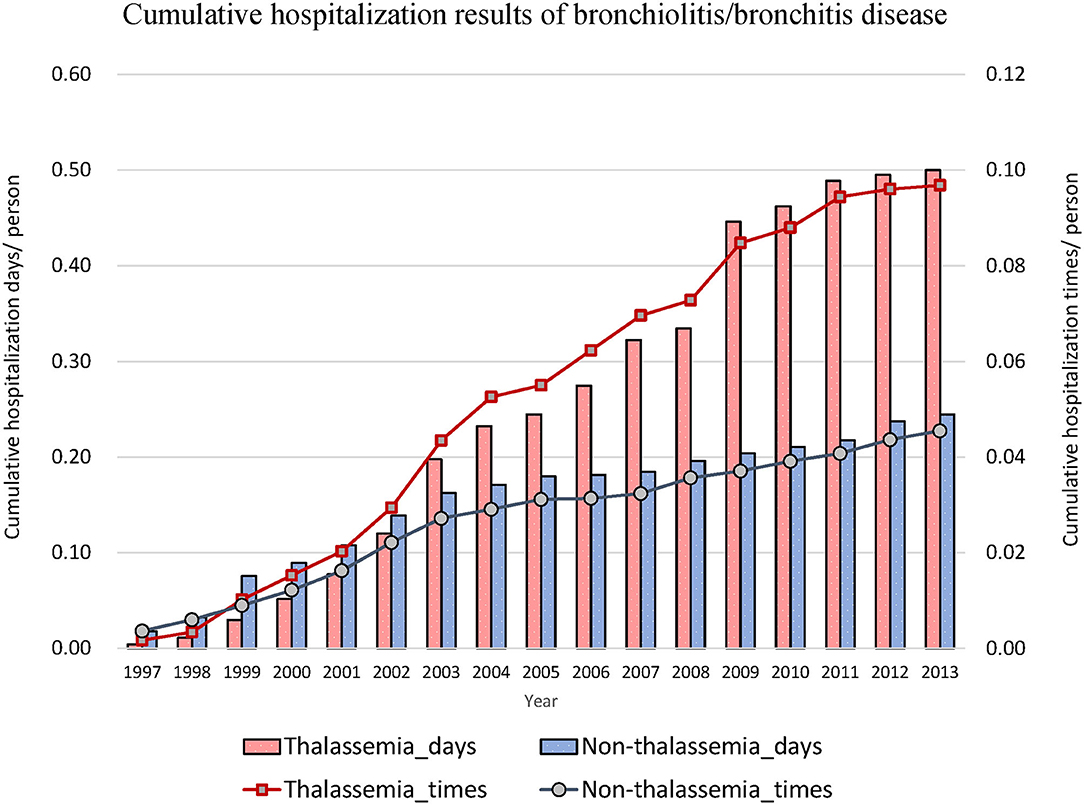
Figure 2. Cumulative number of admissions due to acute bronchitis/bronchiolitis for patients with (dashed line) or without (solid line) thalassemia, and cumulative hospitalization days for patients with (white bar) or without (black bar) thalassemia. The x-axis is the cumulated years from 1997 to 2013, 17 years in total. The cumulative rate for the thalassemia group was higher than that for the non-thalassemia group.
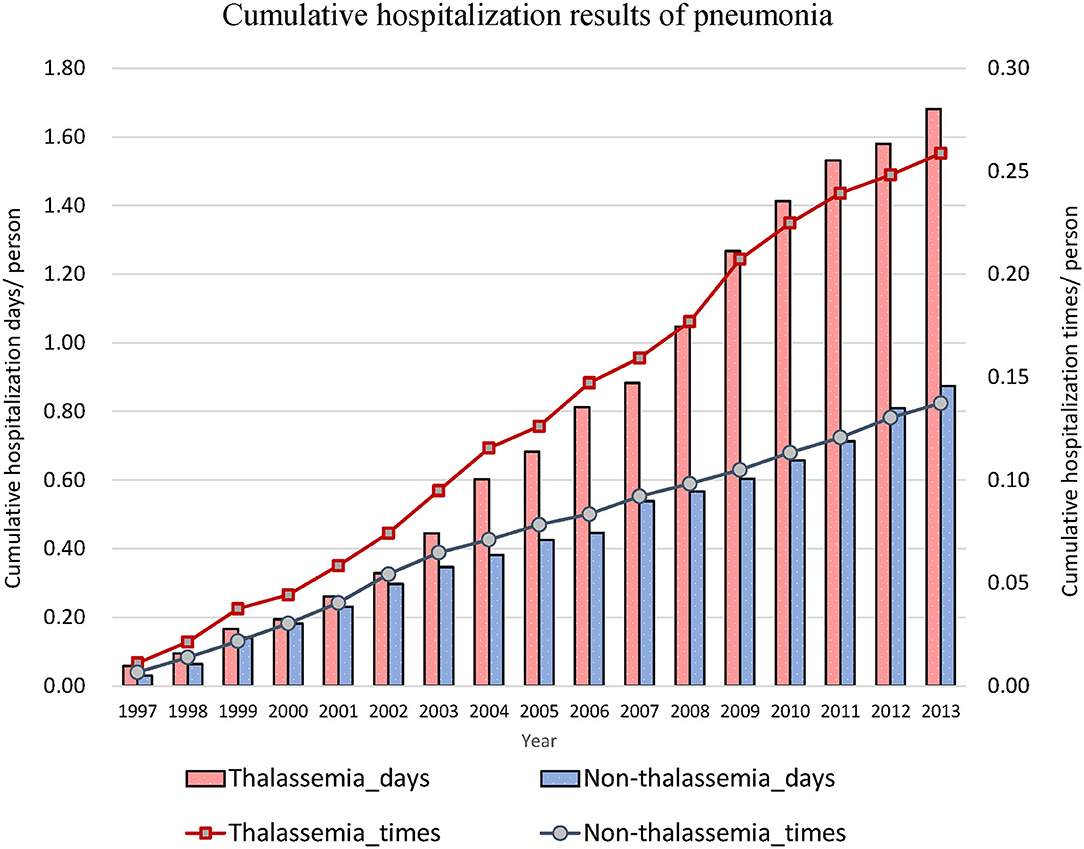
Figure 3. Cumulative number of admissions due to pneumonia for patients with (dashed line) or without (solid line) thalassemia, and cumulative hospitalization days for patients with (white bar) or without (black bar) thalassemia. The x-axis is the cumulated years from 1997 to 2013, 17 years in total. The cumulative rate for the thalassemia group was higher than that for the non-thalassemia group.
The IRR of admissions due to acute bronchitis/bronchiolitis and pneumonia was also analyzed (Tables 3, 4, respectively). Age of the study patients was classified into three groups: preschooler (<6 years); grade schooler (≥6 to <12 years); and teenager (≥12 to <16 years). In Tables 3, 4, “Age (year old)” implied the age of the patient at the date of admission, and “Times” implied the number of admissions. Person-years (PY) was calculated as:
For acute bronchitis/bronchiolitis, those with transfusion-naïve thalassemia had a higher incidence rate of admission than the non-thalassemia controls, irrespective of sex, age, or whether there was comorbidity or not. The only exception was patients with epilepsy or chronic kidney diseases, but these were not statistically significant because only few data were included for analysis. Comparing the age at admission, grade schoolers (≥6 to <12 years) had higher IRR (IRR 4.48, 95% CI 2.64–7.61) than preschoolers. The IRR of teenagers could not be calculated because no non-thalassemia teenagers were admitted for acute bronchiolitis/bronchitis during our study period. The admissions due to pneumonia showed the same trend. Those with transfusion-naïve thalassemia had higher incidence rates of admissions than non-thalassemia controls, irrespective of sex, age, or presence of comorbidity, except for the patients with chronic lung diseases or epilepsy. The highest IRR according to age of admission was observed in the teenager group (IRR 23.98, 95% CI 2.89–199.22). Most inpatients who were admitted for acute bronchitis/bronchiolitis or pneumonia were aged below 6-year-old.
Thalassemia is a common hematologic disorder, and its complications include bone dysmorphism, hemochromatosis, splenomegaly, infection, endocrine disorders, and heart failure. Some of these complications result from long-term, repeated blood transfusions (20). Infection is a major issue for patients with the severe form of thalassemia, and the proposed predisposing factors are anemia, iron overload, splenectomy, transfusion-associated viral infections, and a range of immune abnormalities (2). For thalassemia patients without a history of blood transfusion, although there may be no symptoms of anemia or it may be mild, such as thalassemia minor patients, they also have a higher risk of coronary artery disease, erectile dysfunction, and fractures (21–23). Their hematopoiesis may be less effective, and the abnormal erythrocyte cell membrane structure leads to peripheral hemolysis and subsequent iron overload, which are risk factors for susceptibility to infection (11, 12). However, no strong evidence to this effect has been provided before our study.
The risk of infection for thalassemia major patients has been demonstrated in previous studies; our study, a nationwide, population-based cohort study, revealed that transfusion-naïve thalassemia children are also susceptible to lower respiratory tract infection. This present study showed a higher prevalence of acute bronchitis/bronchiolitis and pneumonia, common diseases related to lower respiratory infection, in the transfusion-naïve thalassemia population compared to the non-thalassemia controls. Previous studies had shown that non-transfusion-dependent thalassemia (NTDT) patients may have altered immune function and increased susceptibility to severe bacterial infections, especially for patients with iron overload or post splenectomy (13, 17). The NTDT patients do not need lifelong regular blood transfusion but occasional blood transfusion may be required for some stressful conditions. Patients who had ever received blood transfusion, splenectomy, or had been diagnosed as having other hematologic disorders were excluded based on the ICD-9-CM codes in this study. Thus, our study group is in a milder status than NTDT. We assume that the thalassemia patients that included are silent carriers and have α- or β- thalassemia minor or α- or β-thalassemia intermedia without a history of blood transfusion.
The effects of confounding factors, i.e., age, sex, and comorbidities of asthma, heart failure, DM, chronic lung disease, epilepsy, and CKD, were adjusted for when matching the thalassemia and control groups. Focusing on those who were hospitalized with diseases related to lower respiratory infection, the thalassemia group had a higher percentage of admissions for both acute bronchitis/bronchiolitis and pneumonia compared to the control group. However, there was almost no difference between the two groups with respect to average number of admissions and average days of hospitalization of inpatients. It seemed that although transfusion-naïve thalassemia patients had a higher possibility of hospitalization than the non-thalassemia controls, irrespective of sex, age, or presence of comorbidity, the severity of the diseases was similar. In this study, most inpatients were aged below 6 years, which may be because the immune system of preschoolers is relatively immature (24, 25), and to some extent, the criterion of admission maybe loose. Among different age groups, the highest IRR fell into the teenager (≥12 to <16 years).
Similar to previous similar studies, this study has several limitations (21–23). First, there are several genotypes of thalassemia, in which the structure of hemoglobin is different. Thus, thalassemia patients have varying hemoglobin and ferritin levels. These data may affect the risk of infection and they could not be obtained from the NHIRD. However, the proportion of thalassemia intermedia is just about 1.5 percent among the NHIRD (26). We had also excluded cases that received blood transfusion and splenectomy, which account for near 50% and 30 of thalassemia intermedia (27). Thus, the effect of thalassemia intermedia in NHIRD is too small and can be neglected. Second, although we used ICD-9 codes to screen out thalassemia patients, some asymptomatic patients were missed because the physicians did not include thalassemia in the diagnostic lists for this population. There might have been some thalassemia patients in the control group. Thus, the hospitalization rate for lower airway infection in the control group may be overestimated. In other words, in real condition, the NHIRD may have higher hospitalization rates for lower airway infection than control. The incidence of pneumococcus disease decreased dramatically after the introduction of pneumococcal vaccine (28). In Taiwan, pneumococcal vaccine has been provided for free since 2015 to all young children (29), which was beyond our study period (1997–2013). The introduction of pneumococcal vaccine should have no influence on our data.
Nowadays, the number of cases of severe thalassemia in Taiwan is decreasing because of the policy of prenatal screening. Most thalassemia patients are silent carriers or have thalassemia minor. Previously the prevention of infections was usually focused on thalassemia patients with history of blood transfusion. The main contribution of our study is the elucidation of the risk of lower airway infection in transfusion-naïve thalassemia patients. It is therefore important to identify the nearly asymptomatic thalassemia populations and educate them regarding the importance of preventing themselves from acquiring airborne or droplet-transmitted infections.
The phenomenon that transfusion-naïve thalassemia patients are susceptible to lower respiratory tract infection was observed in our study, and this observation proved that besides of blood transfusion, there are other mechanisms contributes to higher infection risk, like ineffective erythropoiesis and increased intestinal iron absorption (30), although the exact pathologic mechanism remains unclear. Further studies should be designed to obtain more details on the pathophysiology concerned. In addition, it would be valuable to study whether these transfusion-naïve thalassemia patients have a higher risk of other types of infection, such as urinary tract infection and acute gastroenteritis.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving human participants were reviewed and approved by Chang Gung Memorial Hospital-Kaohsiung Medical Center. Written informed consent for participation was not provided by the participants' legal guardians/next of kin because: big data (Taiwan National Health Insurance Research Database).
H-RY, Z-ML, T-AT, and C-KT contributed to design of the work. H-RY, Y-HY, Y-CL, and T-AT contributed to data acquisition. H-RY, C-KT, Y-CL, C-CC, and C-MT performed data analysis and interpretation. H-RY, C-MT, C-HC, and C-KN drafted the manuscript. H-RY, Z-ML, T-AT, C-KT, and C-KN finalized the article. All authors have read and approved the final manuscript and agreed to be accountable for all aspects of the work.
This study was supported in part by grants CFRPG8H0251 (H-RY) from Chang Gung Memorial Hospital, Taiwan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, for their assistance with the statistics.
2. Vento S, Cainelli F, Cesario F. Infections and thalassaemia. Lancet Infect Dis. (2006) 6:226–33. doi: 10.1016/S1473-3099(06)70437-6
3. Lai K, Huang G, Su L, He Y. The prevalence of thalassemia in mainland China: evidence from epidemiological surveys. Sci Rep. (2017) 7:920. doi: 10.1038/s41598-017-00967-2
4. Lin CK, Lee SH, Wang CC, Jiang ML, Hsu HC. Alpha-thalassemic traits are common in the Taiwanese population: usefulness of a modified hemoglobin H preparation for prevalence studies. J Lab Clin Med. (1991) 118:599–603.
5. Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect. (2001) 43:182–6. doi: 10.1053/jinf.2001.0904
6. Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. Br J Surg. (1991) 78:1031–8. doi: 10.1002/bjs.1800780904
7. Sakran W, Levin C, Kenes Y, Colodner R, Koren A. Clinical spectrum of serious bacterial infections among splenectomized patients with hemoglobinopathies in Israel: a 37-year follow-up study. Infection. (2012) 40:35–9. doi: 10.1007/s15010-011-0178-5
8. Choudhury N, Saraswat S, Naveed M. Serological monitoring of thalassaemia major patients for transfusion associated viral infections. Indian J Med Res. (1998) 107:263–8.
9. Hassanshahi G, Arababadi MK, Assar S, Hakimi H, Karimabad MN, Abedinzadeh M, et al. Post-transfusion-transmitted hepatitis C virus infection: a study on thalassemia and hemodialysis patients in southeastern Iran. Arch Virol. (2011) 156:1111–5. doi: 10.1007/s00705-011-0950-y
10. Atwa ZT, Abdel Wahed WY. Transfusion transmitted infections in frequently transfused thalassemic children living in Fayoum Governorate, Egypt: Current prevalence and risk factors. J Infect Public Health. (2017) 10:870–4. doi: 10.1016/j.jiph.2017.02.012
11. Musallam KM, Rivella S, Vichinsky E, Rachmilewitz EA. Non-transfusion-dependent thalassemias. Haematologica. (2013) 98:833–44. doi: 10.3324/haematol.2012.066845
12. Musallam KM, Cappellini MD, Wood JC, Taher AT. Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood Rev. (2012) 26(Suppl 1):S16–9. doi: 10.1016/S0268-960X(12)70006-1
13. Teawtrakul N, Jetsrisuparb A, Sirijerachai C, Chansung K, Wanitpongpun C. Severe bacterial infections in patients with non-transfusion-dependent thalassemia: prevalence and clinical risk factors. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. (2015) 39:53–6. doi: 10.1016/j.ijid.2015.09.001
14. Hagag AA, Elgamsy MA, El-Asy HM, Gamal RM, Elshahaby WN, Abd Elbar ES. Immune status 'in children with beta thalassemia' in correlation 'with iron overload': single center Egyptian study. Endocr Metab Immune Disord Drug Targets. (2016) 16:181–8. doi: 10.2174/1871530317666161107160213
15. Ezer U, Gülderen F, Culha VK, Akgül N, Gürbüz O. Immunological status of thalassemia syndrome. Pediatr Hematol Oncol. (2002) 19:51–8. doi: 10.1080/088800102753356194
16. Bozdogan G, Erdem E, Demirel GY, Yildirmak Y. The role of Treg cells and FoxP3 expression in immunity of β-thalassemia major AND β-thalassemia trait patients. Pediatr Hematol Oncol. (2010) 27:534–45. doi: 10.3109/08880018.2010.503334
17. Thiengtavor C, Siriworadetkun S, Paiboonsukwong K, Fucharoen S, Pattanapanyasat K, Vadolas J, et al. Increased ferritin levels in non-transfusion-dependent β°-thalassaemia/HbE are associated with reduced CXCR2 expression and neutrophil migration. Br J Haematol. (2020) 189:187–98. doi: 10.1111/bjh.16295
18. Pelton SI, Shea KM, Farkouh RA, Strutton DR, Braun S, Jacob C, et al. Rates of pneumonia among children and adults with chronic medical conditions in Germany. BMC Infect Dis. (2015) 15:470. doi: 10.1186/s12879-015-1162-y
19. Sterky E, Bennet R, Lindstrand A, Eriksson M, Nilsson A. The impact of pneumococcal conjugate vaccine on community-acquired pneumonia hospitalizations in children with comorbidity. Eur J Pediatr. (2017) 176:337. doi: 10.1007/s00431-016-2843-2
20. Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet Lond Engl. (2018) 391:155–67. doi: 10.1016/S0140-6736(17)31822-6
21. Chen Y-G, Lin C-L, Ho CL, Chen Y-C, Kao C-H. Risk of coronary artery disease in transfusion-naïve thalassemia populations: a nationwide population-based retrospective cohort study. Eur J Intern Med. (2015) 26:250–4. doi: 10.1016/j.ejim.2015.02.001
22. Chen Y-G, Lin T-Y, Lin C-L, Dai M-S, Ho C-L, Kao C-H. Risk of erectile dysfunction in transfusion-naive thalassemia men: a nationwide population-based retrospective cohort study. Medicine. (2015) 94:e700. doi: 10.1097/MD.0000000000000700
23. Chen Y-G, Lu C-S, Lin T-Y, Lin C-L, Tzeng H-E, Tsai C-H. Risk of fracture in transfusion-naïve thalassemia population: a nationwide population-based retrospective cohort study. Bone. (2018) 106:121–5. doi: 10.1016/j.bone.2017.10.016
24. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B Biol Sci. (2015) 282:20143085. doi: 10.1098/rspb.2014.3085
25. Georgountzou A, Papadopoulos NG. Postnatal innate immune development: from birth to adulthood. Front Immunol. (2017) 8:957. doi: 10.3389/fimmu.2017.00957
26. Ashtiani MTH, Monajemzadeh M, Sina AH, Berenji F, Abdollahi M, Said MG, et al. Prevalence of haemoglobinopathies in 34,030 healthy adults in Tehran, Iran. J Clin Pathol. (2009) 62:924–5. doi: 10.1136/jcp.2009.064568
27. Sripichai O, Makarasara W, Munkongdee T, Kumkhaek C, Nuchprayoon I, Chuansumrit A, et al. A scoring system for the classification of beta-thalassemia/Hb E disease severity. Am J Hematol. (2008) 83:482–4. doi: 10.1002/ajh.21130
28. Chen C-H, Su L-H, Li H-C, Hsu M-H, Wu T-L, Chen C-L, et al. Evaluation of the impact of 13-valent pneumococcal conjugate vaccine immunization in children by surveillance of culture-confirmed pneumococcal disease: a prospective clinical microbiological study. Vaccine. (2019) 37:5147–52. doi: 10.1016/j.vaccine.2019.07.073
29. Wei S-H, Chiang C-S, Chen C-L, Chiu C-H. Pneumococcal disease and use of pneumococcal vaccines in Taiwan. Clin Exp Vaccine Res. (2015) 4:121–9. doi: 10.7774/cevr.2015.4.2.121
30. Gardenghi S, Marongiu MF, Ramos P, Guy E, Breda L, Chadburn A, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. (2007) 109:5027–35. doi: 10.1182/blood-2006-09-048868
Keywords: thalassemia, transfusion-naïve, pneumonia, bronchitis, children
Citation: Tsai T-A, Tsai C-K, Yang Y-H, Lee Z-M, Sheen J-M, Lee Y-C, Tsai C-M, Chen C-C, Chang C-H, Niu C-K and Yu H-R (2020) Higher Hospitalization Rate for Lower Airway Infection in Transfusion-Naïve Thalassemia Children. Front. Pediatr. 8:574014. doi: 10.3389/fped.2020.574014
Received: 18 June 2020; Accepted: 02 November 2020;
Published: 24 November 2020.
Edited by:
Alan F. Isles, Children's Health Queensland, AustraliaReviewed by:
Yusei Ohshima, University of Fukui, JapanCopyright © 2020 Tsai, Tsai, Yang, Lee, Sheen, Lee, Tsai, Chen, Chang, Niu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Ren Yu, eXV1MjAwNHRhaXdhbkB5YWhvby5jb20udHc=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.