
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 16 November 2020
Sec. Pediatric Neurology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.570708
Background: Patients with chronic kidney disease (CKD) on maintenance hemodialysis frequently present with neurological complications. These complications include peripheral neuropathy, encephalopathy, and stroke.
Objectives: To detect the prevalence of neurological manifestations and complications in children with CKD through neurophysiological and neuro-radiological findings.
Methods: The study included 50 patients with CKD admitted to a pediatric nephrology unit. Their history and complete physical and neurological examination findings had been recorded. All patients underwent nerve conduction, electromyography, electroencephalography, and magnetic resonance imaging of the brain.
Results: Fifty children of both sexes (23 males and 27 females) with a mean age of (12.08 ± 3.46 year) were studied. Eleven (22%) patients with CKD developed polyneuropathy, mostly of an axonal polyneuropathy pattern, while 39 (78%) of them showed normal electrophysiological studies. No myopathy was detected. Abnormal electroencephalography findings were detected in 18% of patients, mostly generalized and focal (temporal, occipital, and frontal) epileptogenic activity. Abnormal MRI brain findings were detected in 16% of patients, mostly of encephalomalacia.
Conclusion: Uremic neuropathy was highly prevalent in children with CKD on maintenance hemodialysis. They developed polyneuropathy, mostly of an axonal polyneuropathy pattern. EEG is a useful method for early recognition of subclinical uremic encephalopathy and/or epileptogenic activity. Early demonstration and management of uremic neurological conditions may decrease the physical disability of CKD patients.
The National Kidney Foundation's Kidney Disease and Outcome Quality Initiative (KDOQI) Group defined chronic kidney disease (CKD) as when the glomerular filtration rate (GFR) is <60 mL/min per 1.73 m2 for ≥ 3 months or when the GFR is ≥ 60 mL/min per 1.73 m2 if other evidence of kidney damage exists, which is manifested by pathologic abnormalities reported by renal biopsy or abnormalities detected by urine, blood tests, and imaging procedures (1). CKD may be caused by a primary renal disorder or a multisystem disorder complication, such as diabetes. Neurological features and changes in children with CKD are highly prevalent whatever the cause (2). Encephalopathy detected in patients with chronic kidney disease results from their exposure to several factors, such as uremia, hypertension, and fluid, and electrolyte disturbances (3). Uremic encephalopathy features include alterations of mental status (alertness and awareness alterations, poor concentration, psychosis, and hallucinations, without treatment of which stupor, and coma may develop) and motor system abnormalities, such as clouding of the sensorium as an early feature and delirium, seizures, and coma as late features (4). Dialysis disequilibrium syndrome's neurological manifestations include headache, blurred vision, muscle cramps, tremors, disturbed consciousness, and convulsions, which are caused by the rapid removal of urea during hemodialysis. Rapid removal of urea from blood due to rapid hemodialysis resulting in osmotic gradients between the plasma and the brain that leads to cerebral edema which leads to these neurological manifestations (5). Peripheral neuropathy or uremic neuropathy affects 60–90% of CKD dialysis patients, so it is considered the most common neurological complication (6). Early clinical features of uremic neuropathy are numbness and paresthesia, resulting from large myelinated sensory fibers' affection. Distal sensory loss in the lower limbs and decreased or absent deep tendon reflexes are detected by clinical examination. Motor involvement showing weakness and muscle atrophy is associated with more severe conditions (7). When GFRs levels decrease below 12 ml/min, clinical manifestations of uremic neuropathy become clear (8). The uremic myopathy mechanism in CKD is unclear but may occur due to the role of oxidative stress (9). If the GFR decreases below 25 mL/min with an impairment of kidney function, uremic myopathy clinical features will appear (10) as proximal muscle weakness in the muscles of the lower limbs, leading to limited function of these muscles (11). Stroke displays a highly prevalent pattern in patients with CKD. Its prevalence is 17% for long term hemodialysis CKD patients, and 10% for mild to moderate CKD patients. Silent brain infarcts on magnetic resonance imaging (MRI) of the brain have been found in up to 50% of progressive stages of CKD (12). Hypertension, hypercholesterolemia, and disorders of mineral and bone metabolism increase the risk of stroke (13). So, we aimed to detect the prevalence of neurological manifestations and complications in children with chronic kidney disease through neurological examination and neurophysiological and neuro-radiological findings.
A cross-sectional study with a cluster sample technique was carried out at a nephrology unit on 50 children aged <18 years old who presented with chronic kidney disease. Children included in this study had been on regular hemodialysis for more than 6 months. There were 23 males and 27 females with a mean age of (12.08 ± 3.46 year). Patients with central or peripheral nervous system disease from congenital or other causes other than CKD, and who had previous polyneuropathy and myopathy caused by thyroid dysfunction or diabetes mellitus, were excluded.
All patients were subjected to history taking and complete physical and neurological clinical examination.
- Polyneuropathy was assessed through a number of factors. Clinical factors included numbness, tingling, insomnia, pain, or paresthesia. Patients were also examined for the detection of distal motor wasting, weakness, hypotonia, and hyporeflexia. Nerve conduction and electromyography (EMG) were also used to confirm the diagnosis and detect the type of polyneuropathy (axonal or demyelinating). Myopathy was assessed clinically through the detection of proximal weakness and wasting, and electro-physiologically by nerve conduction and (EMG) studies that confirm the diagnosis and determine the muscle affected. All studied patients underwent electroencephalography (EEG) and magnetic resonance imaging of the brain. A nerve conduction study was carried out on 50 patients with CKD and 50 healthy children matched for age, gender, residence, and socioeconomic state as a control group.
- Serum creatinine, blood urea nitrogen, and albumin were measured for all subjected patients. Calculation of GFR according to the modified Schwartz formula was done for all patients: GFR (mL/min/1.73 m2) = height (cm) × 0.413/serum creatinine (mg/dL). Normal value of eGFR was ≥ 90 ml/min/1.73 m2 and decreased value of eGFR was <90 ml/min/1.73 m2 (14).
A. Nerve conduction and electromyography (EMG): All patients in the current study underwent a motor nerve conduction study of both tibial and peroneal nerves on both sides and a sensory nerve conduction study of both sural nerves. Eleven patients diagnosed as having polyneuropathy underwent a motor and sensory nerve conduction study of both median and ulnar nerves on both sides, which showed normal nerve conduction study. An EMG study of the gluteus maximus, tibialis anterior, and gastrocnemius (medial head) muscles on both sides had been performed.
B. Diagnostic criteria of polyneuropathy and myopathy: Myopathy is detected through normal nerve conduction studies, and is characterized by the presence of small, polyphasic motor units with short durations and early recruitment patterns in both gluteus maximus muscles (15). Axonal polyneuropathy is characterized by decreased compound muscle action potential and sensory nerve action potential to <80% of normal in two or more nerves with no conduction block and normal conduction velocities with an EMG study that illustrates denervation potential, large polyphasic motor units, and decreased interference patterns in both the tibialis anterior and gastrocnemius muscle. Acute demyelination polyneuropathy is characterized by a prolonged distal motor latency to more than 115% of the upper limit of normal and low conduction velocity to <90 % of the lower level of normal with the presence of conduction block (16). Diagnosis of acute demyelination polyneuropathy by EMG study demonstrates no denervation potential at rest in the distal muscle, normal motor unit, and decreased recruitment pattern.
C. Electroencephalogram: An electroencephalogram was carried out with a recording time of 20 min under standard conditions using Galileo Sirius 9231134, from 19 scalp electrodes, with average reference, according to the International 10–20 System (17).
Data were analyzed using IBM SPSS statistics version 22.0 (SPSS Inc., Chicago, IL, USA). Percentage (%), mean, and standard deviation (SD) were used for the descriptive statistics. For quantitative data, comparison between two means was done using Student t-test. For comparison of not normally distributed variables, Mann-Whitney was used. Fisher's exact test was used for qualitative data when the expected value is < 5. P < 0.05 is considered significant. Receiver operating characteristic (ROC curve) is a graphical plot of the sensitivity, vs. false positive rate (one minus the specificity). Sensitivity or true positive rate (TPR) = True +ve/(True +ve + False –ve). Specificity (SPC) or True Negative Rate = True –ve/(True –ve + False +ve) = 1- False +ve rate. Accuracy (ACC) = (True +ve) + (True –ve)/(Positive + Negative). Positive predictive value (PPV): True +ve/(True +ve + False +ve). Negative predictive value (NPV): True –ve/(True –ve + False –ve). Positive Likelihood Ratio (+LR) = sensitivity/(100–specificity). Negative Likelihood Ratio (–LR) = (100–sensitivity)/specificity.
Demographic features and clinical and neurological examinations are illustrated in (Table 1). Seizures were reported in 22% (n = 11), headache in 24% (n = 12), numbness in 18%(n = 9), dizziness in 10 % (n = 5), insomnia in 14% (n = 7), memory disturbance in 6% (n = 3), delayed speech in 8 % (n = 4), hypotonia in 22% (n = 11), hyporeflexia in 22% (n = 11), and hyperreflexia in 10% (n = 5) of children with CKD. The prevalence of peripheral neuropathy among the studied patients detected through electrophysiological study was 22% (n = 11 of 50), axonal motor and sensory neuropathy was observed in 81.8% (n = 9), and demyelinating motor neuropathy was reported in 18.2% (n = 2). By the analysis of the nerve conduction study, the results illustrated a high significant difference between both groups regarding amplitude. There was marked reduction of the amplitude of tibial, peroneal, and sural nerves in the patient group. Also, there was marked reduction in conduction velocity in the patient group in comparison to the control group of tibial, peroneal nerves, and right sural nerve. There was a high significant difference concerning the distal motor latencies in both groups (Table 2).
When analyzing the EMG study, the results revealed normal motor units in 41 patients (82%), while nine patients (18%) revealed large polyphasic motor units (neuropathic) as regards the tibialis anterior muscle and medial head of the gastrocnemius muscle, while all studied patients (100%) revealed normal motor units as regards the gluteus maximus muscle. There was a decreased interference pattern in 11 patients (22%) and denervation potential in the tibialis anterior and medial head of the gastrocnemius muscles in nine patients (18%). Abnormal EEG findings were demonstrated in 18% (n = 9) of studied subjects; 44.4% (n = 4) had generalized epileptogenic activity and 55.6% (n = 5) had focal epileptogenic activity (40% temporal, 40% occipital, and 20% frontal). EEG background was normal in 80% (n = 40) and diffuse slowing (of high voltage theta and delta waves) in 20% (n = 10) of studied patients with elevated serum creatinine. 60% (n = 6) of studied patients who had diffuse slowing presented with peripheral polyneuropathy. Abnormal brain MRI findings were reported in eight patients (16%), including mild brain atrophy in 37.5% (n = 3) and encephalomalacia in 62.5% (n = 5) (Table 3).
Numbness, decreased eGFR, and increased serum creatinine had significant relation to peripheral neuropathy in patients with CKD (Table 4).
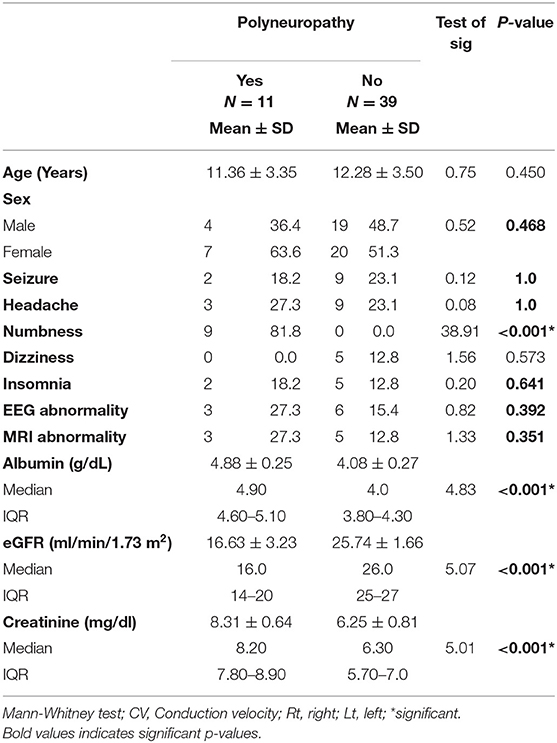
Table 4. Distribution of demographic, clinical, and neurological examination in patients with CKD with and without peripheral neuropathy.
Sensitivity and specificity of eGFR, serum creatinine, and serum albumin were illustrated (Table 5, Figure 1).
Sensorimotor axonal polyneuropathy caused by axonal degradation and loss of myelin sheath is common and affects the lower limbs (18, 19). The accumulation of uremic toxins attributed to oxidative stress leads to motor, sensory, and autonomic nerve damage, resulting in uremic neuropathy (20, 21). Chronic uremic nerve depolarization contributes to uremic neuropathy (UN) and is caused by hyperphosphatemia and hyperkalemia. Axonal death results from potassium disruption of the normal ionic gradient (22).
Seizures were reported in 11 patients (22%) due to epilepsy and uncontrolled hypertension and headache in 12 patients (24%) due to uncontrolled hypertension; both were the major central neurological manifestation in patients with CKD. This is in accordance with both Scorza et al. (23) who reported five cases with seizures and Afsharkhas et al. (24) who reported that 12 (40%) patients had neurologic findings including seizures in seven (23.4%) patients due to epileptic syndromes, hypertension with posterior reversible encephalopathy syndrome, febrile convulsion, and increased intracranial tension.
In the current study, neurological presentation of uremic neuropathy varies from numbness, insomnia, weakness, hypotonia, and hyporeflexia. Early symptoms of UN are paresthesia, increased pain sensation, and cramps. Long-term symptoms of UN are weakness, numbness, impaired deep tendon reflexes, and lower limb atrophy (25, 26).
The prevalence of peripheral neuropathy [uremic neuropathy (UN)] in the current study was 22%. Axonal motor and sensory neuropathy represented 81.8%, while demyelinating motor neuropathy represented 18.2%. Some studies reported UN prevalence ranging from 0 to 59% (27). Yoganathan et al. (28) demonstrated that uremic neuropathy prevalence was 52%, with axonal neuropathy (80.8%) and demyelinating neuropathy (11.5%) with predominant motor neuropathy. Ackil et al. (20) showed that 59% of children had sural nerve conduction abnormalities, and motor conduction abnormality was found in 29% of children on maintenance dialysis. The most common affected nerves were the tibial and common peroneal nerves as there was reduced conduction velocity and amplitude in our patients; this is in agreement with Yoganathan et al. (28). Another study reported that low peroneal nerve conduction velocity was considered as a useful tool for uremic neuropathy detection (29). De Camargo et al. (30) demonstrated that children with mild renal failure presented with a significant decrease of peroneal motor nerve conduction velocity.
The current study illustrated normal motor and sensory nerve conduction of both the ulnar and median nerves on both sides in our polyneuropathy patients; this is in agreement with Yoganathan et al. (28). Analytical interpretation of EMG results of the current study revealed normal motor units in 41 patients (82%), while nine patients (18%) revealed large polyphasic motor units (neuropathic). All studied patients (100%) revealed normal motor units as regards the gluteus maximus muscle. There was a decreased interference pattern in 11 patients (22%) and denervation potential in the tibialis anterior and medial head of the gastrocnemius muscles in nine patients (18%). Fahal and Bell reported normal electromyography (EMG) findings in chronic kidney disease patients (11). Berretta et al. (31) illustrated lower extremities proximal muscle weakness, particularly pelvic girdle proximal musculature, and EMG findings illustrated high-voltage polyphasic potentials in a boy with poor kidney function with elevated BUN (blood urea nitrogen) and creatinine. Abnormal EEG findings were demonstrated in 18% (n = 9) of the studied subjects, which were distributed as 44.4% (n = 4) with generalized epileptogenic activity and 55.6% (n = 5) with focal epileptogenic activity (40% temporal, 40% occipital, and 20% frontal). EEG background was normal in 80% (n = 40) and diffuse slowing (of high voltage theta and delta waves) in 20% (n = 10) of studied patients with elevated serum creatinine. Six patients (60%) of those who had diffuse slowing (n = 10) were presented by peripheral polyneuropathy. Gadewar et al. (32) showed that, with progression of CKD stage, frontal sharp wave transients were reported in 71.43% of patients at stage 4 and in 50% of those at stage 5. They found a high theta pattern in both stages. Demir et al. (33) reported that bilateral spike wave activity was found in 14% of patients and decreased alpha activity in 8.5% of patients with uremic encephalopathy. Also, Koçer et al. (34) demonstrated that occipital sharp wave transients were found in stage 4 of CKD. While Demir et al. (33) and Burn and Bates (35) demonstrated generalized slowing and bilateral spike and wave complexes in 14% of patients, Arnold and Krishnan reported that the triphasic sharp waves were the typical features of EEG abnormalities found in uremic encephalopathy (36). EEG typical presentations in uremic encephalopathy are a slow alpha rhythm with excess delta and theta waves (10). Lai et al. (37) illustrated significant differences in EEG findings of the relative power of the theta band and absolute and relative power of the delta band. Arieff (38) showed the appearance of theta waves, disappearance of normal basic rhythms, and diminished reactivity of EEG to afferent stimulation and domination by generalized delta activity. Röhl et al. (39) showed prominent electrical power in theta, delta, and alpha frequencies in the temporal and central brain areas. Abnormal MRI findings were reported in 16% of studied subjects (n = 8), including mild brain atrophy in 37.5% (n = 3) and encephalomalacia in 62.5% (n = 5). This may be due to hypertensive encephalopathy, uremia, electrolytes disturbance, seizure, or hypoxia. Ishikura et al. (40) reported radiological abnormalities extending to 85% of gray matter, especially in the frontal and temporal lobes. Brain atrophy was estimated in three (23%) of 13 children who presented with chronic renal failure (27). The current study reported a significant association of eGFR, serum creatinine, and albumin with peripheral polyneuropathy with sensitivity (100% for all) and specificity (100, 97, and 85%, respectively). Yoganathan et al. (28) reported the sensitivity was 84.6% (95% CI 66.47, 93.85) and the specificity was 69.6% (95% CI 49.13, 84.4) for age, serum albumin, eGFR, serum copper and ferritin, and their relation to peripheral neuropathy prediction.
Autonomic neuropathy was not evaluated as any clinical manifestations, whether digestive, visual, urinary, body temperature, heart, or blood vessel symptoms, were not included in the studied patients. We recommend tilt-table, quantitative sudomotor axon reflex, and urodynamic tests and also the cutaneous sympathetic response as a rapid screening tool for the involvement of the autonomic nervous system.
Uremic neuropathy is highly prevalent in children with CKD on maintenance hemodialysis. They developed polyneuropathy was mostly of an axonal polyneuropathy pattern. EEG is a useful method for early recognition of subclinical uremic encephalopathy and/or epileptogenic activity. Early demonstration and management of uremic neurological conditions may decrease the physical disability CKD patients.
All datasets generated for this study are included in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by The Institutional Review Board (IRB) of the Menoufia Faculty of Medicine approved the study (ID: 190719Ped). Research work was performed in accordance with the Declaration of Helsinki. A written patient consent was taken from the parents and caregivers after explaining all aspects of the study, with the right to withdraw at any time. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
All authors co-operated in conceptualization, design of the work, data collection, resources detection, formal analysis, interpretation of data, the creation of new software used in the work, validation, methodology, and revision. SA, WB, and AM collected the data and wrote the manuscript. ZK analyzed the data, drafted, and revised the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All authors thank the participants and their guardians for their active participation in the study and the team who helped in data collection. This manuscript has been released as a pre-print at Research Square (41).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.570708/full#supplementary-material
UN, Uremic neuropathy; EMG, Electromyography; EEG, Electroencephalography; MRI, Magnetic Resonance imaging.
1. KDOQI (Kidney Disease Outcome Quality Initiative): clinical practice guideline for nutrition in children with CKD: 2008 update. Executive summary. Am J Kidney Dis. (2009) 53:11–104. doi: 10.1053/j.ajkd.2008.11.017
2. National Kidney F. K/DOQI: clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. (2002) 39:S1–266. Available online at: https://pubmed.ncbi.nlm.nih.gov/11904577
3. Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg. (2004) 107:1–16. doi: 10.1016/j.clineuro.2004.07.012
4. Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, et al. Systematic review: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. (2010) 153:23–33. doi: 10.7326/0003-4819-153-1-201007060-00252
5. Patel N, Dalal P, Panesar M. Dialysis disequilibrium syndrome: a narrative review. Semin Dial. (2008) 21:493–8. doi: 10.1111/j.1525-139X.2008.00474.x
6. Krishnan AV, Kiernan MC. Neurological complications of chronic kidney disease. Nat Rev Neurol. (2009) 5:542–51. doi: 10.1038/nrneurol.2009.138
7. Krishnan AV, Pussell BA, Kiernan MC. Neuromuscular disease in the dialysis patient: an update for the nephrologist. Semin Dial. (2009) 22:267–78. doi: 10.1111/j.1525-139X.2008.00555.x
8. Arnold R, Kwai N, Pussell BA, Lin CSY, Kiernan MC, Krishnan AV. Effects of neuropathy on physical function and quality of life in moderate severity chronic kidney disease. Clin Neurophysiol. (2014). 125:e4. doi: 10.1016/j.clinph.2013.10.031
9. Kaltsatou A, Sakkas GK, Poulianiti KP, Koutedakis Y, Tepetes K, Christodoulidis G, et al. Uremic myopathy: is oxidative stress implicated in muscle dysfunction in uremia? Front Physiol. (2015) 6:102. doi: 10.3389/fphys.2015.00102
10. Arnold R, Krishnan A. Neuropathy and other neurological problems in chronic kidney disease. In: Arici M, editor. Management of Chronic Kidney Disease. Berlin: Springer (2014). p. 343–52. doi: 10.1007/978-3-642-54637-2_26
11. Fahal IH, Bell GM. Uraemic myopathy: fact or fiction. Int J Artif Organs. (1998) 21:185–7. doi: 10.1177/039139889802100401
12. Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidneybrain axis. J Am Soc Nephrol. (2013) 24:353–63. doi: 10.1681/ASN.2012050536
13. Arnold J, Sims D, Ferro CJ. Modulation of stroke risk in chronic kidney disease. Clin Kidney J. (2016) 9:29–38. doi: 10.1093/ckj/sfv136
14. Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. (2009) 20:629–37. doi: 10.1681/ASN.2008030287
15. Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. (2011) 10:931–41. doi: 10.1016/S1474-4422(11)70178-8
16. Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, de Jonghe B, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. (2009) 37:S299–308. doi: 10.1097/CCM.0b013e3181b6ef67
17. Stern JM, Engel J. An Atlas of EEG Patterns. Philadelphia: Lippincott Williiam & Wilkin (2004). p. 178–88.
18. Ouvrier RA, Wilmshurst JM. Overview of the neuropathies. In: Jones HR, DeVivo DC, Darras BT, Volpe JJ, editors. Neuromuscular Disorders of Infancy, Childhood, and Adolescence: a Clinician's Approach. Philadelphia, PA: Butterworth Heinemann Elsevier (2003). p. 339–59.
19. Preston DC, Shapiro BE. Electromyography And Neuromuscular Disorders, Clinical Electrophysiological Correlations, 2nd ed. Philadelphia, PA: Butterworth Heinemann Elsevier (2005). p. 25–45.
20. Ackil AA, Shahani BT, Young RR. Sural nerve conduction studies and late responses in children undergoing hemodialysis. Arch Phys Med Rehabil. (1981) 62:487–91.
21. de Beaufort CE, Andre JL, Heimans JJ, van der Eerden HA, van Diemen NG, Duc ML, et al. Peripheral nerve function in children with end-stage renal failure. Pediatr Nephrol. (1989) 3:175–8. doi: 10.1007/BF00852903
22. Fuglsang-Frederiksen A, Pugdahl K. Current status on electrodiagnostic standards and guidelines in neuromuscular disorders. Clin Neurophysiol. (2011) 122:440–5. doi: 10.1016/j.clinph.2010.06.025
23. Scorza FA, Albuquerque MD, Arida RM, Cysneiros RM, Henriques TMG, Scroza CA, et al. Seizure occurance in patients with chronic renal insufficiency in regular hemodialysis program. Arq Neuropsiquiatr. (2005) 63:757–60. doi: 10.1590/S0004-282X2005000500007
24. Afsharkhas L, Hoseininajad N, Kalbassi Z. Neurologic manifestations in children and adolescents with chronic kidney diseases. IJCA. (2015) 1:16–19. Available online at: http://ijca.iums.ac.ir/article-1-36-en.html
25. Chao CC, Wu VC, Tan CH, Wang YM, Tseng MT, Wu PC, et al. Skin denervation and its clinical significance in late-stage chronic kidney disease. Arch Neurol. (2011) 68:200–6. doi: 10.1001/archneurol.2010.372
26. Preston DC, Shapiro BE. Electromyography and Neuromuscular Disorders Clinical Electrophysiological Correlations, 2nd ed. Philadelphia, PA: Butterworth Heinemann Elsevier (2005). p. 47–53.
27. Elzouki A, Carroll J, Butinar D, Moosa A. Improved neurological outcome in children with chronic renal disease from infancy. Pediatr Nephrol. (1994) 8:205–10. doi: 10.1007/BF00865479
28. Yoganathan S, Bagga A, Gulati S, Toteja GS, Hari P, Sinha A, et al. Prevalence and predictors of peripheral neuropathy in nondiabetic children with chronic kidney disease. Muscle Nerve. (2017) 57:792–8. doi: 10.1002/mus.26027
29. Van den Neucker K, Vanderstraeten G, Vanholder R. Peripheral motor and sensory nerve conduction studies in haemodialysis patients. A study of 54 patients. Electromyogr Clin Neurophysiol. (1998) 38:467–74.
30. De Camargo CR, Schoueri JHM, da Veiga GRL, Fonseca FLA, Bacci MR, da Costa Aguiar Alves B. Uremic neuropathy: an overview of the current literature. Rev Assoc Med Bras. (2019) 65:281–6. doi: 10.1590/1806-9282.65.2.281
31. Berretta JS, Holbrook CT III, Haller JS. Chronic renal failure presenting as proximal muscle weakness in a child. J Child Neurol. (1986) 1:50–2. doi: 10.1177/088307388600100108
32. Gadewar P, Acharya S, Khairkar P, Shukla S, Mahajan SN. Dynamics of electroencephalogram (EEG) in different stages of chronic kidney disease. J Clin Diagn Res. (2015) 9:25–7. doi: 10.7860/JCDR/2015/11257.5705
33. Demir AB, Bora I, Kaigili E, Ocakoglu G. Assessment of basic features of electroencephalography in metabolic encephalopathies. J Neurol Res. (2014) 4:101–9. doi: 10.14740/jnr285w
34. Koçer A, Yazgan H, Ýnce N. Evaluation of the electrical activity of the brain in children and adult uremic patients. Erciyes Med J. (2005) 27:115–21. Available online at: https://www.researchgate.net/publication/26437075
35. Burn DJ, Bates D. Neurology and the kidney. J Neurol Neurosurg Psychiatry. (1998). 65:810–21. doi: 10.1136/jnnp.65.6.810
36. Arnold R, Issar T, Krishnan A, Pussell BA. Neurological complications in chronic kidney disease. J R Soc Med Cardiovasc Dis. (2016) 5:1–13. doi: 10.1177/2048004016677687
37. Lai S, Mecarelli O, Pulitano P, Romanello R, Davi L, Zarabla A, et al. Neurological, psychological, and cognitive disordersin patients with chronic kidney disease on conservative and replacement therapy. Medicine. (2016) 95:5191. doi: 10.1097/MD.0000000000005191
39. Röhl JE, Harms L, Pommer W. Quantitative EEG findings in patients with chronic renal failure. Eur J Med Res. (2007) 12:173–8. Available online at: https://pubmed.ncbi.nlm.nih.gov/17509962
40. Ishikura K, Ikeda M, Hamasaki Y, Hataya H, Shishido S, Asanuma H, et al. Posterior reversible encephalopathy syndrome in children: its high prevalence and more extensive imaging findings. Am J Kidney Dis. (2006) 48:231–8. doi: 10.1053/j.ajkd.2006.04.076
Keywords: chronic kidney disease, uremic neuropathy, peripheral neuropathy, nerve conduction, electromyography, electroencephalography, magnetic resonance of brain
Citation: Abd El Naby SA, Bahbah WA, Kasemy ZA and Mahmoud AA (2020) Neurophysiological and Neuroradiological Changes in Children With Chronic Kidney Disease. Front. Pediatr. 8:570708. doi: 10.3389/fped.2020.570708
Received: 08 June 2020; Accepted: 07 September 2020;
Published: 16 November 2020.
Edited by:
Pasquale Striano, University of Genoa, ItalyReviewed by:
Paola Lanteri, Carlo Besta Neurological Institute (IRCCS), ItalyCopyright © 2020 Abd El Naby, Bahbah, Kasemy and Mahmoud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Asmaa A. Mahmoud, YXNtYWFzb2xpbWFuNTBAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.