- 1Intensive Care and Department of Pediatric Surgery Erasmus MC–Sophia Children's Hospital, University Medical Center Rotterdam, Rotterdam, Netherlands
- 2Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
- 3Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, NICU, Milan, Italy
- 4Department of Development and Regeneration and Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium
- 5Hospital Pharmacy, Erasmus MC, Rotterdam, Netherlands
Background: Bacterial and fungal infections are common and often contribute to death in patients undergoing extracorporeal membrane oxygenation (ECMO). Drug disposition is altered during ECMO, and adsorption in the circuit is an established causative factor. Vancomycin and voriconazole are widely used, despite the lack of evidence-based prescription guidelines.
Objective: The objective of this study was to determine the extraction of voriconazole and vancomycin by the Xenios/Novalung ECMO circuits.
Methods: We have set up nine closed-loop ECMO circuits, consisting of four different iLAActivve® kits for neonatal, pediatric, and adult support: three iLA-ActivveMiniLung® petite kits, two iLA-ActivveMiniLung® kits, two iLA-ActivveiLA® kits, and two iLA-Activve X-lung® kits. The circuits were primed with whole blood and maintained at physiologic conditions for 24 h. Voriconazole and vancomycin were injected as a single-bolus age-related dose into the circuits. Pre-membrane (P2) blood samples were obtained at baseline and after drug injection at 2, 10, 30, 180, 360 min, and 24 h. A control sample at 2 min was collected for spontaneous drug degradation testing at 24 h.
Results: Seventy-two samples were analyzed in triplicate. The mean percentage of drug recovery at 24 h was 20% for voriconazole and 62% for vancomycin.
Conclusions: The extraction of voriconazole and vancomycin by contemporary ECMO circuits is clinically relevant across all age-related circuit sizes and may result in reduced drug exposure in vivo.
Introduction
Critically ill patients on extracorporeal membrane oxygenation (ECMO) are at high risk of serious infections, with rates of 15.4 per 1,000 ECMO days across all age groups (1). As the mortality of ECMO patients facing an infective event increases up to 68%, a timely and adequate antimicrobial therapy is essential to improve outcomes (1–3).
Vancomycin is widely used as the first-line treatment for both the empiric and targeted treatment of blood stream infections caused by methicillin-resistant Staphylococcus aureus (MRSA), coagulase-negative staphylococci (CONS), and ampicillin-resistant enterococci (1, 4–6). Although bacteria remain the most common causative agents, fungi contribute to the healthcare burden being the second cause of nosocomial infection during ECMO (1), with a prevalence ranging from 0.04 to 5%, and differences based upon age and timing of infection (2, 5). Voriconazole is a second-generation triazole and a synthetic derivative of fluconazole, acting against Candida and Aspergillus spp., which are the most frequently isolated fungi in ICU (7). Indeed, the azole class is pivotal in the prevention and treatment of invasive fungal infections in critically ill patients (7, 8). While the pharmacology of fluconazole, which is the recommended first-line treatment against Candida spp., has been extensively addressed in the pediatric ECMO population (9–11), scanty evidence is available for voriconazole, a first-line treatment for invasive aspergillosis (7, 12, 13).
Besides the choice of the proper agent, the selection of the right dose to attain target exposure is crucial for therapeutic success (14). Drug dosing is known to be complex in critically ill patients, and it is further complicated in specific populations, in which pathology-related factors sum up to physiologic age-specific PK changes (15). The need for an extracorporeal support adds interfering mechanisms, which are responsible for further PK variability (16).
ECMO is increasingly spreading, especially across adult intensive care settings, to support potentially reversible cardiac or respiratory failure, unresponsive to conventional treatment (17). This procedure requires the addition of fluids and exogenous blood products to prime the circuit, thereby increasing the circulating volume. As a consequence of hemodilution, priming compositions, and organ dysfunction, both the volume of distribution (Vd) and clearance (Cl) of drugs are altered (18). A certain degree of sequestration into the circuit may be expected based on the physicochemical characteristics of a drug, such as molecular size, plasma protein binding, degree of ionization at physiological pH, and lipophilicity, as all are based on the characteristics of the circuits and oxygenators (18–21). Moreover, the exposure of blood to the exogenous surface triggers a systemic inflammatory response, further contributing to altered antimicrobial disposition and overall therapeutic efficacy (16, 20, 22).
Despite the wide use of antimicrobial agents during ECMO (23), PK data related to newer systems are limited and heterogeneous, resulting in a poor level of evidence-based pharmacotherapy (22, 24). Previous pharmacological knowledge (25–27) needs to be updated according to the recent advances of extracorporeal technology to prevent altered antimicrobial exposure during ECMO. Furthermore, the optimization of pharmacotherapy is required to limit the burden of microbial resistance (28, 29).
The aim of this study was to provide new insights into the disposition of voriconazole and vancomycin in contemporary neonatal, pediatric, and adult ECMO circuits.
Materials and Methods
The study was carried out at the Intensive Care Unit of the Sophia Children's Hospital—ECMO Center, Erasmus MC in Rotterdam, the Netherlands. The need for the institutional review board approval was waived as ethical approval was not required, according to the Dutch Law of research on human subjects. In particular, the fact that no patients were used in this in vitro study determined this decision. Study circuits were provided by the Xenios® company as part of an unrestricted research grant.
In vitro ECMO Model
The in vitro ECMO model has been previously published (28). Here, we present a brief overview of the setting. We have set up nine different in vitro ECMO circuits, consisting of four iLAActivve® kits: iLA-ActivveMiniLung® petite kits (n = 3), iLA-ActivveMiniLung® kits (n = 2), iLA-ActivveiLA® kits (n = 2), and iLA-Activve X-lung® kits (n = 2). They were made up of hollow fiber oxygenators with a diffusion membrane for neonatal, pediatric, and adult patients. All circuits were whole blood primed, connected to a 100-ml reservoir bag and run for 24 h at a flow rate of 500 ml/min (neonatal), 700 ml/min (infant), 2.5 L/min (pediatric), and 3.5 L/min (adult) with an estimated circuit volume of 225, 280, 360, and 400 ml, respectively. Anticoagulation was provided by administering a bolus of heparin 500 UI to maintain activated clotting times over 200 s. Experimental conditions (temperature, pH, hematocrit) have been maintained stable throughout the study period, as previously described (30).
Drug Selection and Administration
We selected voriconazole and vancomycin for their clinical relevance in the Intensive Care Unit (ICU) practice and as illustrative examples of different chemical properties and their drug classes. Dosing for neonatal circuits was based on a standardized neonatal weight (3.5 kg), while for the other systems, we have increased the dose in proportion to the rise of volume to achieve similar theoretical concentrations. We injected voriconazole 35 mg/40 mg/52.5 mg/68 mg (neonatal/infant/pediatric/adult) followed by vancomycin 45 mg/54 mg/67.5 mg/91.8 mg through a line connected to the tubing right after the reservoir bag (P1), with 5-s intervals in-between injections. We flushed the line with 1 ml of physiological saline solution (0.9%) after voriconazole administration to avoid crystallization or pooling effects.
Samples
A line attached to the tubing prior to the reservoir bag was used as a pre-membrane (P2) sample-drawing site. For each system, we collected samples before a single-bolus drug administration (T0) and during the circuit run at the following time intervals: 2 (T2), 10 (T10), 30 (T30), 180 (T180), 360 (T360) min, and 24 h (T24) after injection. At T2, a control sample was collected and analyzed at 24 h (T2-24) to determine spontaneous drug degradation. The T2–24 samples were stored in ethylenediaminetetraacetate (EDTA) sample tubes, which were maintained at the same temperature as the tested systems until 24 h. For all other samples (T0–T24), whole blood was collected in polypropylene EDTA tubes and stored at 4°C for a maximum of 12 h until further processing. Afterward, all blood samples were centrifuged (3,000 rounds/min for 6 min), and the plasma supernatant was transferred to labeled polypropylene cryogenic vials with polyethylene screw caps (Nalgene Labware, Rochester, NY, USA). Plasma samples were maintained at −80°C until measurement.
Quantification of Drugs in Plasma Samples
Voriconazole and vancomycin were analyzed by means of ultrafast liquid chromatography–mass spectrometry (LC-MS/MS) in the pharmacy laboratory of Erasmus MC, as previously described (30) with regard to voriconazole or a validated immune-assay (Architect, Abbott) for vancomycin. Methods were validated according to the US Food and Drug Administration guidelines for bioanalytical method validation (31).
Reference values are reported [including lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ)]. Intra- and inter-assay means were within 15% of the target range value, as requested by the FDA. For voriconazole, the linear calibration range was 0.1–10 ng/ml. For vancomycin, the linear calibration range was 0.6–50 mg/L.
Spontaneous Drug Degradation and Expected Drug Levels
Spontaneous drug degradation was defined as the amount of drug left after 24 h of spontaneous decay, and it was calculated as a difference in drug recovery between T2 and T2–24. Expected blood drug levels were calculated, accounting for the amount of drug administered and the total circulating volume. Mean recovery of each circuit category at 24 h was corrected by the average drug spontaneous degradation.
Data Analysis
We performed a comparative evaluation of drug disposition between four different ECMO circuits (MiniLung® petite: MLP, MiniLung®: ML, iLAActivve®: iLA, Xlung®). We calculated the average recovery corrected by spontaneous drug degradation at 2, 10, 30 min and 3, 6, and 24 h across all circuit categories. The trend over time is presented for the entire study period for each drug (see Figures 1, 2). Data are expressed as mean (±SD) or percentage. All analyses were performed using R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
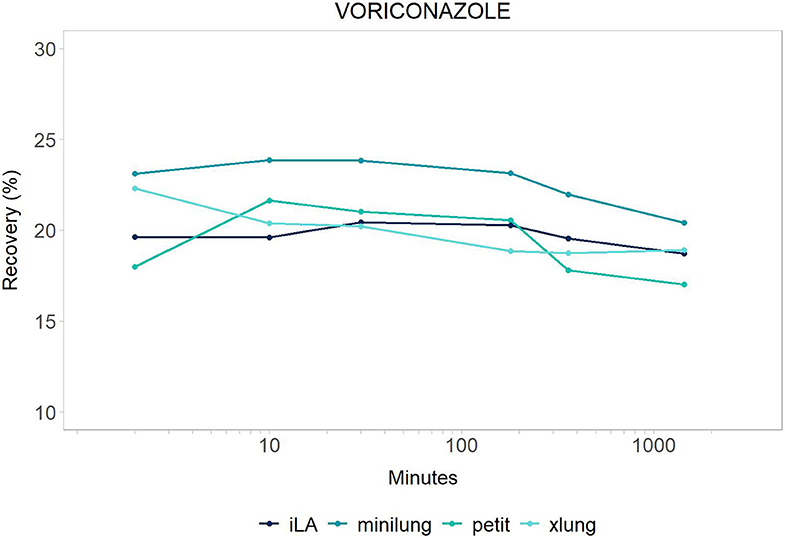
Figure 1. Voriconazole: mean drug recovery (%) plotted vs. time (min) over 24 h for antimicrobial across the four different categories of systems tested: Minilung petite (green), MiniLung (blue), iLaActivve (dark blue), and XLung (light blue).
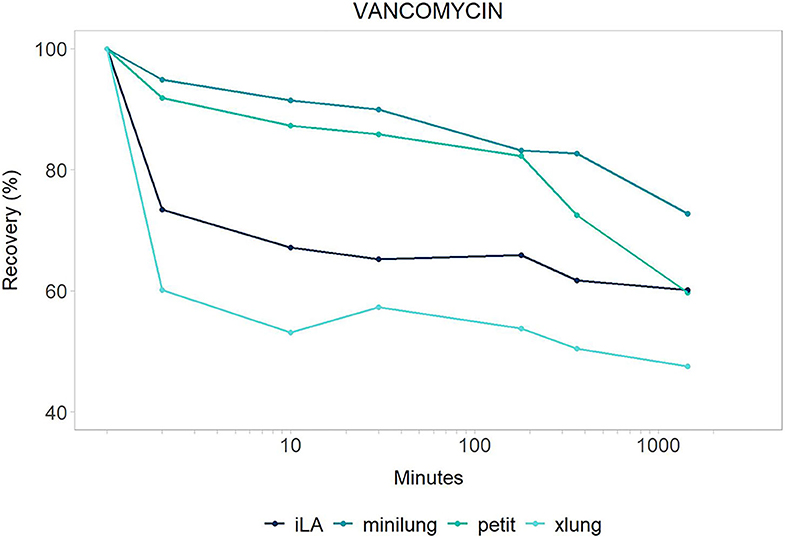
Figure 2. Vancomycin: mean drug recovery (%) plotted vs. time (min) over 24 h for antimicrobial across the four different categories of systems tested: Minilung petite (green), MiniLung (blue), iLaActivve (dark blue), and XLung (light blue).
Results
In vitro ECMO models were maintained under physiological conditions for 24 h. Throughout the experimental time, we did not experience any circuit complications. A total of 72 samples (eight for each circuit) were analyzed, in triplicate, for each drug. All baseline plasma samples were free of study drugs.
The recovery of drugs after a 24-h exposure to the extracorporeal circuit has been corrected for average drug spontaneous degradation, and it is graphically reported in Figures 1, 2. The mean percentage of drug recovery at 24 h was 20% for voriconazole and 62% for vancomycin. While the loss of voriconazole was similar across all age circuits, vancomycin levels decreased by 38% in the miniLung petite, by 25% in the minilung, by 38% in the ILA active, and by 50% in the XLung circuits (Table 1). Drug physicochemical properties, namely, lipophilicity and protein binding, were derived from the Drug Bank online database (32) and are summarized in Table 1.
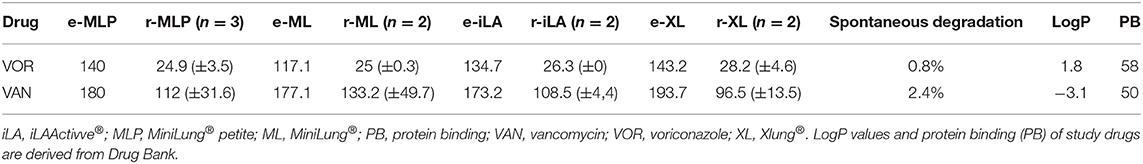
Table 1. Mean drug recovery (mg/L; ±SD) after 24 h of circulation in the study testing circuits (r), corrected for spontaneous drug degradation compared to expected drug levels (e).
Discussion
Suboptimal antimicrobial exposure may be associated with therapeutic failure, toxicity, antimicrobial resistance, and, ultimately, worsened patient outcomes (2, 3). In this in vitro study, we provide the equipment-related disposition of voriconazole and vancomycin in contemporary ECMO circuitry with iLAActivve, contributing insights into circuit- and drug-specific determinants of PK changes during ECMO. Voriconazole was largely sequestered in the circuits, with no distinction across the different circuit sizes. Vancomycin was absorbed slightly more in the neonatal (miniLung petite), pediatric (ILA active), and adult (XLung) circuits than in the infant ones (miniLung). Spontaneous drug decay over 24 h was limited for both study drugs.
We consider these drugs as representative for the pharmacological classes they belong to, thus extending the relevance of our findings.
As expected, based on its lipophilicity and high protein binding (32), voriconazole was largely sequestered in the systems, uniformly across all size groups. Our data confirm those by Mehta et al., who reported a dramatic loss of 60% of voriconazole within 3 h from administration, in an ex vivo silicone membrane-based model of blood primed circuit (33). Similarly, the altered PK profile of voriconazole has been observed in pediatric and adult case series (34, 35), showing a drop of voriconazole plasma levels immediately after ECMO initiation or membrane change (36). Higher initial loading and daily doses have been suggested, with an intense therapeutic drug monitoring (TDM) to allow the attainment of therapeutic serum concentrations (37).
Vancomycin is a hydrophilic and moderately protein-bound agent and, given the narrow therapeutic window and the risk of nephrotoxicity, its PK profile has been extensively evaluated both in in vitro and in vivo settings (25–27). Indeed, targeting the ratio of the area under the vancomycin concentration–time curve over a 24-h period to the minimum inhibitory concentration of the bacteria is crucial, especially among critically ill patients (1, 38). Based on previous neonatal studies, vancomycin has shown an increased volume of distribution (Vd) and a decreased clearance (Cl) during ECMO, resulting in prolonged half-life (25–27). However, these findings were related to older roller pump-based systems, whereas data on centrifugal pump-based circuits are limited. In vitro studies on adult contemporary circuits have revealed either steady vancomycin levels or minimal loss over 24 and 48 h (22, 39). In contrast, we have observed a substantial decay of vancomycin, especially within the first 30 min. These results are in line with our previous in vitro findings on neonatal centrifugal-based circuits (18) (Table 2). The discrepancy among these results may reflect the heterogeneity of experimental settings and highlights the need for a better understanding of drug–circuit interactions, as new materials and coatings become available. A matched-cohort study conducted in adult critically ill patients on ECMO receiving continuous infusion of vancomycin showed comparable drug concentrations between ECMO and non-ECMO patients (40). Similarly, other clinical studies showed no PK differences of vancomycin between ECMO adults and critically ill patients not on ECMO (41, 42). The inconsistency between neonatal, pediatric, and adult findings may originate from either ECMO circuit-related factors or (patho-) physiological determinants, as the priming fluid impacts more on neonatal circulating blood volume compared to adults. Pending new data from pediatric clinical PK of vancomycin, it is currently recommended to increase the loading dose and to perform TDM, with target trough plasma levels between 15 and 20 μg/ml (24, 37, 43–45).
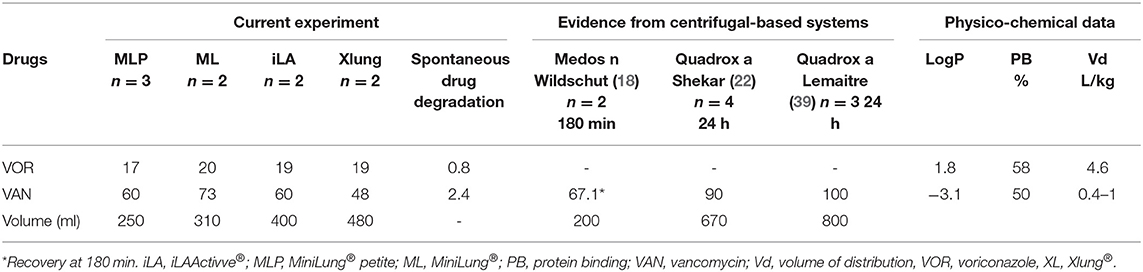
Table 2. Comparison of mean drug recovery at 24 h (%) between centrifugal pump-based in vitro studies.
Although spontaneous drug degradation was negligible for both voriconazole and vancomycin, it is methodologically rigorous to collect a dedicated sample on purpose, as the degradation may be relevant for certain drugs undergoing high spontaneous decay over time, such as midazolam (30). Moreover, according to standard practice, we have measured the total concentration of drugs, while in ECMO patients, the detection of free drug concentration could be worth exploring.
We acknowledge that our findings are not directly transferable to the bedside for a number of reasons. The number of circuits tested, which is limited by the high costs of the equipment required, calls for caution when interpreting the results to adapt the in vitro PK knowledge to clinical practice. Owing to the ex vivo setting, patient- and disease-related variables of drug disposition have not been taken into account. However, exploring the impact of circuit-related non-maturational covariates on antimicrobial PK variability is suitable to inform the ECMO compartment within the physiologically based PK modeling to develop a targeted dosing regimen during ECMO (24). Given the dearth of data related to antimicrobial drug disposition into contemporary ECMO circuits, our findings might be valuable to fill this knowledge gap in pharmacotherapy. Further efforts should be made to integrate both preclinical and clinical evidence to optimize pharmacotherapy in this vulnerable patient population by taking into account different ECMO circuit types provided by various manufacturers.
Conclusions
These results highlight the specific impact of contemporary extracorporeal technology on antimicrobial therapy in critically ill ECMO patients. Sequestration of voriconazole in newer circuits was significant and similar to older silicone-based ones. Although to a lesser extent than voriconazole, adsorption was relevant for vancomycin. As suboptimal antibiotic therapy may lead to treatment failure, maturational toxicity, and antimicrobial resistance, TDM should be performed where available.
Meanwhile, further research is required to improve dosing accuracy and move toward tailored pharmacotherapy with the aim to deliver safe and effective ECMO management, across all age groups.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The need for the institutional review board approval was waived as ethical approval was not required, according to the Dutch Law of research on human subjects. In particular, the fact that no patients were used in this in vitro study determined this decision.
Author Contributions
GR was involved in the performance of experiments, acquisition, analysis, interpretation of data, writing of the first draft, and critical revision of the manuscript. GC and FM contributed to analysis, interpretation of data, and critical revision of the article. KA contributed to planning of the study, interpretation of the data, and critical revision of the article. BK performed the pharmacological analysis and critically revised the article. DT was involved in the conception and planning of the study, supervision of data collection, interpretation of the data, and critical revision of the article. EW was involved in the conception and planning of the study, performance of experiments, acquisition, analysis, interpretation of data, and critical revision of the manuscript. All authors revised the manuscript, gave final approval of the version to be submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Xenios Germany for providing the circuits and Griffini Miglierina Foundation for supporting this study, without being involved in study design or interpretation of results. We would also like to thank Dr. Nicola Pesenti for graphical support.
References
1. Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P, Extracorporeal Life Support Organization Task Force on Infections Extracorporeal Membrane Oxygenation. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. (2011) 12:277–81. doi: 10.1097/PCC.0b013e3181e28894
2. Pluim T, Halasa N, Phillips SE, Fleming G. The morbidity and mortality of patients with fungal infections before and during extracorporeal membrane oxygenation support. Pediatr Crit Care Med. (2012) 13:e288–93. doi: 10.1097/PCC.0b013e31824fbaf7
3. Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. (2006) 34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9
4. MacLaren G, Schlapbach LJ, Aiken AM. Nosocomial infections during extracorporeal membrane oxygenation in neonatal, pediatric, and adult patients: a comprehensive narrative review. Pediatr Crit Care Med. (2020) 21:283–90. doi: 10.1097/PCC.0000000000002190
5. Grasselli G, Scaravilli V, Di Bella S, Biffi S, Bombino M, Patroniti, et al. Nosocomial infections during extracorporeal membrane oxygenation: incidence, etiology, and impact on patients' outcome. Crit Care Med. (2017) 45:1726–33. doi: 10.1097/CCM.0000000000002652
6. Rybak MJ, Lomaestro BM, Rotscahfer JC, Moellering RC Jr, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. (2009) 49:325–7. doi: 10.1086/600877
7. Chatelon J, Cortegiani A, Hammad E, Cassir N, Leone M. Choosing the right antifungal agent in ICU patients. Adv Ther. (2019). 36:3308–20 doi: 10.1007/s12325-019-01115-0
8. Watt K, Manzoni P, Cohen-Wolkowiez M, Rizzollo S, Boano E, Jacqz-Aigrain, et al. Triazole use in the nursery: fluconazole, voriconazole, posaconazole, and ravuconazole. Curr Drug Metab. (2013) 14:193–202. doi: 10.2174/138920013804870583
9. Watt KM, Benjamin DK Jr, Cheifetz IM, Moorthy G, Wade KC, Smith PB, et al. Pharmacokinetics and safety of fluconazole in young infants supported with extracorporeal membrane oxygenation. Pediatr Infect Dis J. (2012) 31:1042–7. doi: 10.1097/INF.0b013e31825d3091
10. Watt KM, Gonzalez D, Benjamin DK, Brouwer KL, Wade KC, Capparelli E, et al. Fluconazole population pharmacokinetics and dosing for prevention and treatment of invasive Candidiasis in children supported with extracorporeal membrane oxygenation. Antimicrob Agents Chemother. (2015) 59:3935–43. doi: 10.1128/AAC.00102-15
11. Watt KM, Cohen-Wolkowiez M, Barrett JS, Sevestre M, Zhao P, Brouwer KL, et al. Physiologically based pharmacokinetic approach to determine dosing on extracorporeal life support: fluconazole in children on ECMO. CPT Pharmacometrics Syst Pharmacol. (2018) 7:629–37. doi: 10.1002/psp4.12338
12. Ruiz S, Papy E, Da Silva D, Nataf P, Massias L, Wolff M, et al. Potential voriconazole and caspofungin sequestration during extracorporeal membrane oxygenation. Intensive Care Med. (2009) 35:183. doi: 10.1007/s00134-008-1269-3
13. Koch BC, Wildschut ED, de Goede AL, de Hoog M, Brüggemann RJ. Insufficient serum caspofungin levels in a paediatric patient on ECMO. Med Mycol Case Rep. (2013) 2:23–4. doi: 10.1016/j.mmcr.2012.12.006
14. Roberts JA, Kumar A, Lipman J. Right dose, right now: customized drug dosing in the critically ill. Crit Care Med. (2017) 45:331–6. doi: 10.1097/CCM.0000000000002210
15. Allegaert K, van den Anker JN, Naulaers G, de Hoon J. Determinants of drug metabolism in early neonatal life. Curr Clin Pharmacol. (2007) 2:23–9. doi: 10.2174/157488407779422294
16. Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet. (2003) 42:403–17. doi: 10.2165/00003088-200342050-00001
17. Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. (2015). 61:31–6. doi: 10.1097/MAT.0000000000000160
18. Wildschut ED, Ahsman MJ, Allegaert K, Mathot RAA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. (2010) 36:2109–16. doi: 10.1007/s00134-010-2041-z
19. Mulla H, Lawson G, von Anrep C, Burke MD, Upton DU, Firmin RK, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. (2000) 15:21–6. doi: 10.1177/026765910001500104
20. Wildschut ED, Ahsman M, Houmes RJ, Pokorna P, de Wildt SN, Mathot RAA, et al. Pharmacotherapy in neonatal and pediatric extracorporeal membrane oxygenation (ECMO). Curr Drug Metab. (2012) 13:767–77. doi: 10.2174/138920012800840383
21. Bhatt-Mehta V, Annich G. Sedative clearance during extracorporeal membrane oxygenation. Perfusion. (2005) 20:309–15. doi: 10.1191/0267659105pf827oa
22. Shekar K, Roberts JA, Mcdonald CI, Fisquet S, Barnett AG, Mullany DV, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. (2012) 16:R194. doi: 10.1186/cc11679
23. Thibault C, Collier H, Naim MY, Heichel J, Schwartz E, Zuppa AF. Patterns of medication exposure in children on extracorporeal membrane oxygenation: a step in prioritizing future pharmacologic studies. Crit Care Explor. (2019) 1:e0045. doi: 10.1097/CCE.0000000000000045
24. Raffaeli G, Pokorna P, Allegaert K, Mosca F, Cavallaro G, Wildschut ED, et al. Drug disposition and pharmacotherapy in neonatal ECMO: from fragmented data to integrated knowledge. Front Pediatrics. (2019) 7:360. doi: 10.3389/fped.2019.00360
25. Hoie E, Swigart SA, Leuschen MP, Willett LD, Bolam DL. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm. (1990) 9:711–5.
26. Amaker RD, Dipiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. (1996) 40:1139–42. doi: 10.1128/AAC.40.5.1139
27. Buck ML. Vancomycin pharmacokinetics in neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. (1998) 18:1082–6.
28. Roberts JA, Kruger P, Paterson DL, Lipman J. Antibiotic resistance—what's dosing got to do with it? Crit Care Med. (2008) 36:2433–40. doi: 10.1097/CCM.0b013e318180fe62
29. Dryden M, Johnson AP, Ashiru-Oredope D, Sharland M. Using antibiotics responsibly: right drug, right time, right dose, right duration. J Antimicrob Chemother. (2011) 66:2441–3. doi: 10.1093/jac/dkr370
30. Raffaeli G, Allegaert K, Koch B, Cavallaro G, Mosca F, Tibboel D, et al. In vitro adsorption of analgosedative drugs in new extracorporeal membrane oxygenation circuits. Pediatr Crit Care Med. (2018) 19:e251–8. doi: 10.1097/PCC.0000000000001484
31. U.S. Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM). Guidance for Industry Bioanalytical Method Validation. (2001). Available online at: https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf (accessed October 15, 2017).
32. Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. (2008) 36 (Suppl. 1):D901–6. doi: 10.1093/nar/gkm958
33. Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. (2007) 33:1018–24. doi: 10.1007/s00134-007-0606-2
34. Spriet I, Annaert P, Meersseman P, Hermans G, Meersseman W, Verbesselt R, et al. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J Antimicrob Chemother. (2009) 63:767–70. doi: 10.1093/jac/dkp026
35. Brüggemann RJ, Antonius T, van Heijst A, Hoogerbrugge PM, Burger DM, Warris A. Therapeutic drug monitoring of voriconazole in a child with invasive aspergillosis requiring extracorporeal membrane oxygenation. Ther Drug Monit. (2008) 30:643–6. doi: 10.1097/FTD.0b013e3181898b0c
36. Winiszewski H, Rougny AC, Lagoutte-Renosi J, Millon L, Capellier G, Navellou JC, et al. The pharmacokinetic challenge of treating invasive aspergillosis complicating severe influenzae assisted by extracorporeal membrane oxygenation. Crit Care. (2018) 22:355. doi: 10.1186/s13054-018-2285-5
37. Cheng V, Abdul-Aziz MH, Roberts JA, Shekar K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J Thorac Dis. (2018) 10 (Suppl. 5):S629–41. doi: 10.21037/jtd.2017.09.154
38. Varghese JM, Roberts JA, Lipman J. Antimicrobial pharmacokinetic and pharmacodynamic issues in the critically ill with severe sepsis and septic shock. Crit Care Clin. (2011) 27:19–34. doi: 10.1016/j.ccc.2010.09.006
39. Lemaitre F, Hasni N, Leprince P, Corvol E, Belhabib G, Fillâtre P, et al. Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood. Crit Care. (2015) 19:1. doi: 10.1186/s13054-015-0772-5
40. Donadello K, Roberts JA, Cristallini S, Beumier M, Shekar K, Jacobs F, et al. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study. Crit Care. (2014) 18:632. doi: 10.1186/s13054-014-0632-8
41. Wu C.-C., Shen LJ, Hsu LF, Ko WJ, Wu, et al. Pharmacokinetics of vancomycin in adults receiving extracorporeal membrane oxygenation. J Formos Med Assoc. (2016) 115:560–70. doi: 10.1016/j.jfma.2015.05.017
42. Park SJ, Yang JH, Park HJ, In YW, Lee YM, Cho H, et al. Trough concentrations of vancomycin in patients undergoing extracorporeal membrane oxygenation. PLoS ONE. (2015) 10:e0141016. doi: 10.1371/journal.pone.0141016
43. Sherwin J, Heath T, Watt K. Pharmacokinetics and dosing of anti-infective drugs in patients on extracorporeal membrane oxygenation: a review of the current literature. Clin Ther. (2016) 38:1976–94. doi: 10.1016/j.clinthera.2016.07.169
44. Hahn J, Choi J, Chang M. Pharmacokinetic changes of antibiotic, antiviral, antituberculosis and antifungal agents during extracorporeal membrane oxygenation in critically ill adult patients. J Clin Pharm Ther. (2017) 42:661–71. doi: 10.1111/jcpt.12636
Keywords: extracorporeal membrane oxygenation, pharmacokinetics, pharmacology, antibiotics, infection, antifungals, drug disposition
Citation: Raffaeli G, Cavallaro G, Allegaert K, Koch BCP, Mosca F, Tibboel D and Wildschut ED (2020) Sequestration of Voriconazole and Vancomycin Into Contemporary Extracorporeal Membrane Oxygenation Circuits: An in vitro Study. Front. Pediatr. 8:468. doi: 10.3389/fped.2020.00468
Received: 12 May 2020; Accepted: 03 July 2020;
Published: 27 August 2020.
Edited by:
John McGuire, University of Washington, United StatesReviewed by:
Uri Pollak, Hadassah-Hebrew University Medical Center, IsraelArun Saini, Texas Children's Hospital, United States
Copyright © 2020 Raffaeli, Cavallaro, Allegaert, Koch, Mosca, Tibboel and Wildschut. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Genny Raffaeli, Z2VubnkucmFmZmFlbGlAdW5pbWkuaXQ=
 Genny Raffaeli
Genny Raffaeli Giacomo Cavallaro
Giacomo Cavallaro Karel Allegaert
Karel Allegaert Birgit C. P. Koch5
Birgit C. P. Koch5 Dick Tibboel
Dick Tibboel Enno D. Wildschut
Enno D. Wildschut