
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 03 July 2020
Sec. Neonatology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.00335
 Cuie Chen1†
Cuie Chen1† Huachun Weng2†
Huachun Weng2† Xixi Zhang3
Xixi Zhang3 Shi Wang4
Shi Wang4 Chaosheng Lu5
Chaosheng Lu5 Hongxing Jin1
Hongxing Jin1 Shujun Chen1
Shujun Chen1 Yuanyuan Liu5
Yuanyuan Liu5 Anqun Sheng5
Anqun Sheng5 Yuanyuan Sun5*
Yuanyuan Sun5*Background and Objective: As bronchopulmonary dysplasia (BPD) can lead to considerable mortality and morbidity, this disease is the focus of attention in neonatology. Vitamin D (VD), which has anti-inflammatory properties and promotes lung growth, may have a therapeutic effect on BPD. The overexpression of neutrophil extracellular traps (NETs) has been demonstrated to be involved in the pathogenesis of BPD in our previous study. This study aimed to elucidate the effect of VD on BPD and the role of NETs in this process.
Methods: Newborn rats were exposed to 90% oxygen continuously for 7 days to mimic BPD, and rats under hyperoxia were injected with 1,25(OH)2D3 at different doses (0.5 ng/g, 3 ng/g). Alveolarization, pulmonary vascular development, inflammatory cytokines and NETs were assessed.
Results: Hyperoxia increased mortality, decreased body weight, impaired alveolarization with a decrease in radial alveolar count (RAC) and an increase in mean linear intercept (MLI), and impaired vascular development with low vascular endothelial growth factor (VEGF) expression. Meanwhile, hyperoxia enhanced expression of the proinflammatory factors TNF-α, IL-1β, and IL-6, and elevated NETs in lung tissues and plasma. Low-dose VD (0.5 ng/g) administration increased the survival rate, attenuated developmental retardation, improved alveolarization, and pulmonary vascular development in hyperoxia-induced BPD, and reduced the expression of proinflammatory factors and NETs. However, high-dose VD (3 ng/g) treatment did not attenuate lung injury or NETs significantly, and even led to more severe developmental retardation and a higher mortality rate.
Conclusions: Low-dose VD increased the survival rate, attenuated developmental retardation, and improved alveolarization and pulmonary vascularization arrest in hyperoxia-induced BPD partially by inhibiting NETs.
Bronchopulmonary dysplasia (BPD) is a severe complication of extreme preterm birth that may affect pulmonary function, leading to pulmonary hypertension, mental retardation and growth retardation, or even cause considerable mortality, leading to a large health care burden (1, 2). The incidence of BPD increases annually due to improvement in the survival of extreme premature infants, but strategies to protect the lung are limited (1, 3). BPD is characterized by the arrest of alveolar and pulmonary vascular development. Chorioamnionitis, sepsis, hyperoxia, and invasive mechanical ventilation may contribute to this disease (1). Activation, infiltration and delayed clearance of polymorphonuclear neutrophils (PMNs), the most crucial innate immune cells, play important roles in the pathogenesis of BPD (4). Similarly, we found that neutrophil counts and the neutrophil-to-lymphocyte ratio (NLR) of patients with BPD were higher than those in individuals without BPD at birth and at 72 h. The neutrophil counts and NLR at 72 h of the group with severe BPD were higher than those in the group with mild BPD, and our previous study showed that an increase in the NLR at 72 h could be an early predictor of BPD, especially severe BPD (5).
Neutrophil extracellular traps (NETs) are extracellular, web-like structures composed of DNA, histones, myeloperoxidase (MPO), neutrophil elastase (NE), calprotectin, cathelicidins, defensins, and actin (6). NETs are stimulated by activated PMNs and can trap and kill invading pathogens under normal conditions. However, excess NETs formation and delayed NETs clearance lead to tissue and organ injury. NETs contribute to the pathogenesis of a variety of immune- and inflammation- related diseases, such as rheumatoid arthritis (7), acute lung injury (8), chronic obstructive pulmonary disease (9), and sepsis (10). In our previous study, we provided evidence that NETs were increased in hyperoxia-induced BPD rats, and anti-histone antibodies and heparin could attenuate lung injury by inhibiting NETs formation (11). Therefore, inhibition of NETs production may be a potential therapeutic approach in BPD.
Vitamin D (VD) is a secosteroid hormone, and its active form is 1,25(OH)2D3. Recently, VD was discovered to be an important modulatory molecule in immunity and inflammation in many organs (12–14). VD has also been shown to have a critical role in lung development, as it can increase surfactant synthesis and promote alveolar epithelial-mesenchymal interactions (15, 16). VD deficiency at an early stage of life is involved in BPD in very preterm infants. A low VD level at 24 h of life was shown to be a risk factor for the development of BPD (17), and a lower VD level was associated with the increased severity of BPD (18). Studies have demonstrated that VD could attenuate lung injury in an animal model of BPD through the suppression of interferon-gamma (IFN-γ) production (19) or downregulation of Toll-like receptor 4 (TLR4) (20); however, the mechanism of VD in BPD remains poorly understood and uncertain. Because VD can decrease NETs activity (21), we hypothesized that VD plays a protective role against BPD by regulating NETs.
In this study, we report alveolar and pulmonary vascular development and the inflammatory response after VD supplementation in hyperoxia-induced BPD and the effect of NETs on this process.
Sprague Dawley rats (12 weeks old, 200–250 g, male:female=1:2 mating) were provided by the Shanghai Laboratory Animal Center. After natural pregnancy, each female rat was put into a separate cage. We used an established animal model of BPD that has been described previously (11). Briefly, after spontaneous delivery, the neonatal rats were exposed to hyperoxia (90% oxygen) with a 12-h light/dark cycle for 7 days to mimic BPD. The oxygen concentration was continuously monitored using an electronic recorder (EnviteC, Wismar, Germany). The CO2 in the environment was absorbed using soda lime. Dams were rotated between the hyperoxia and normoxia groups every 24 h. Cages were regularly opened for 30 min every day to replace the padding and provide clean drinking water and food, to which the rats had ad libitum access. The hyperoxia group was randomly divided into three subgroups, and the rats in these subgroups were treated with 1,25(OH)2D3 (Roche Pharma Ltd., Schweiz) at 0.5 ng/g or 3 ng/g i.p. once a day for 7 days or administered an equivalent volume of normal saline. The study was terminated on postnatal day 14 [P14]. The experimental protocol was approved by the Experimental Animal Ethics Committee of Wenzhou Medical University (wydw2019-0957).
On P7 and P14, 6 newborn rats from each group were anesthetized by i.p. injection of 5% chloral hydrate (10 g in 0.05 ml), and blood samples then were aseptically collected by arteria carotis communis puncture and placed into tubes with sodium citrate (1:10) on ice. The samples were centrifuged at 3,000 rpm for 30 min at 4°C, and the plasma was then stored at −80°C. The whole lungs were aseptically collected by an open-chest procedure. Samples of the right lung were kept at −80°C. The left lung was perfused with 4% polyformaldehyde at a pressure of 20 cm H2O and then fixed in a 4% paraformaldehyde solution at 4°C.
Lungs were sectioned at a thickness of 4 μm and stained with hematoxylin and eosin (H&E) to reveal the lung morphometry. Three random non-overlapping fields in one distal lung section per rat were utilized for morphometric examinations. The radial alveolar counts (RAC) were measured according to Cooney and Thurlbeck, who proposed drawing a perpendicular line from the center of the most peripheral bronchiole to the pleura or the nearest interlobular septum and counting the number of alveoli transected by this line (22). The mean linear intercept (MLI) was also determined to evaluate alveolar size (14, 23).
Vascular endothelial growth factor (VEGF) expression in the lung tissue was detected by immunohistochemistry. First, the sections were deparaffinized in xylene, rehydrated, and rinsed in deionized water. Then, antigen retrieval was performed by microwave treatment in EDTA buffer (pH 9.0) for 8 min. The slides were cooled and held at room temperature for 15 min. Sections were washed with phosphate-buffered saline (PBS) (pH 7.4), and endogenous peroxidase activity was blocked in 3% H2O2 for 25 min. Next, the sections were incubated with anti-VEGF antibody (Ab-1, goat polyclonal antibody, Abcam, USA) diluted 1:100 in antibody diluent buffer overnight at 4°C. After washing with PBS, sections were incubated with biotinylated HRP-labeled secondary antibodies (GB23301, Servicebio, China). The sections were visualized using a 3,3′-diaminobenzidine tetrahydrochloride chromogen kit (K5007, DAKO, Danish), and the cell nuclei were stained again with hematoxylin. To remove water, an increasing alcohol series and xylene were used. Finally, the sections were covered with a slide with neutral gum. From each of the sections, four different fields were selected under the microscope (200×), and the average optical density (AOD) of stained VEGF was determined by Image-Pro Plus 6.0 image analysis software.
Lung samples were kept at −80°C until use. The samples were homogenized on ice and centrifuged at 12,000 rpm for 30 min, and the supernatants were collected for analyses. The tissue levels of tumor necrosis factor-alpha (TNF-α), interleukin-1Beta (IL-1β) and interleukin-6 (IL-6) were measured with an ELISA kit (Ray Biotech, Inc., Guangzhou) following the manufacturer's instructions.
The sections were fixed, stained and imaged to detect NETs. The sections were incubated with the specific primary antibodies anti-citrullinated histone 3 (Cit-H3) (1:100; SC51716, Santa Cruz) and anti-MPO (1:500; GB11224, Google biological) overnight at 4°C. Then, the sections were incubated with Alexa Fluor 488-conjugated goat anti-rabbit (1:400; GB21303, Google biological) and Alexa Fluor Cy3-conjugated goat anti-mouse (1:300; GB21301, Google biological) secondary antibodies for 1 h. 4′,6-Diamidino-2-phenylindole (DAPI, G1012, Google biological) was used to detect DNA. All the slides were visualized using an Olympus FluoView 500 confocal scanning laser microscope.
Plasma NETs were detected by the NET-specific markers cell-free DNA and Cit-H3. The plasma cell-free DNA was quantified with a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Canada) following the manufacturer's protocol. A 50-μl dilution of a standard solution was added to 96-well plates, and then 100 μl of PicoGreen fluorescent dye was added before incubation at room temperature away from light for 5 min. Fluorescence intensity was detected by a fluorescence plate reader, and the cell-free DNA/NETs were quantified. Cit-H3 was measured with a Cell Death Detection ELISA PLUS kit (Roche, Switzerland). An appropriately diluted sample (1:500) was added to the plates and incubated at room temperature for 2 h. Then, the substrate was added and incubated for 30 min, and a chromogenic agent was added and incubated for 15 min. The absorbance at 490 nm and 405 nm was captured, and the difference in OD at 405 nm and 490 nm was calculated as the relative quantitative value of Cit-H3.
The results are presented as the mean ± standard deviation. The data were analyzed by SPSS 25.0 (SPSS, Inc., Chicago, IL, USA). Two-group comparisons were carried out using Student's t-test. Comparisons of more than two groups were carried out using one-way ANOVA. A P < 0.05 indicated statistical significance.
In vivo treatment of rats with low-dose VD (LVD) (0.5 ng/g) significantly reduced the mortality caused by hyperoxia, which was not different from that in the normoxia group. Only 83.3% of the rats survived hyperoxia, while LVD increased the rate to 96.7%, but the survival rate of rats treated with high-dose VD (HVD) (3 ng/g) was 73.3% (Figure 1A). The rats in the hyperoxia group presented with developmental retardation, and a decreased body weight compared to that of the control groups was observed from P3. Treatment of the rats with LVD significantly attenuated this retardation, but treatment of the rats with HVD led to more severe developmental retardation (Figure 1B).
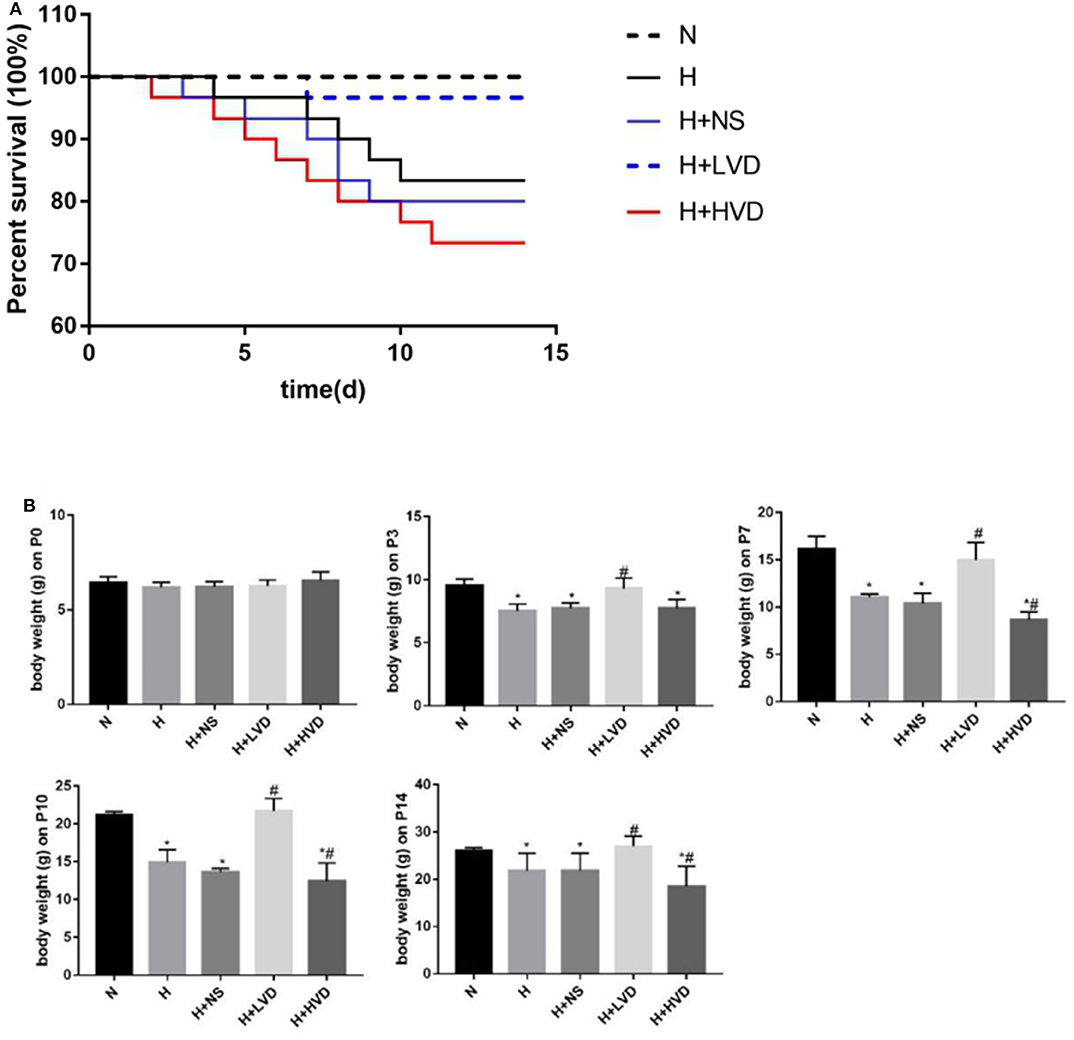
Figure 1. LVD (0.5 ng/g) treatment increased survival and attenuated developmental retardation in hyperoxia. (A) Kaplan-Meier survival analysis (P < 0.05). (B) The body weight in different groups. *P < 0.05, compared to normoxia group (N); #P < 0.05, compared to hyperoxia group (H).
The lung structures of rats in the hyperoxia group had characteristics typical of alveolar simplification, as indicated by enlarged alveoli with decreased terminal alveoli and secondary septa, as well as irregular alveolar shape and thickening of the alveolar wall (Figure 2A). In the rescue experiment, the rats treated with LVD (0.5 ng/g) exhibited improved alveolarization after hyperoxia exposure, but the rats treated with high-dose HVD (3 ng/g) did not exhibit attenuated lung injury (Figure 2A). These effects were achieved by an increase in RAC and a decrease in MLI (Figure 2B).
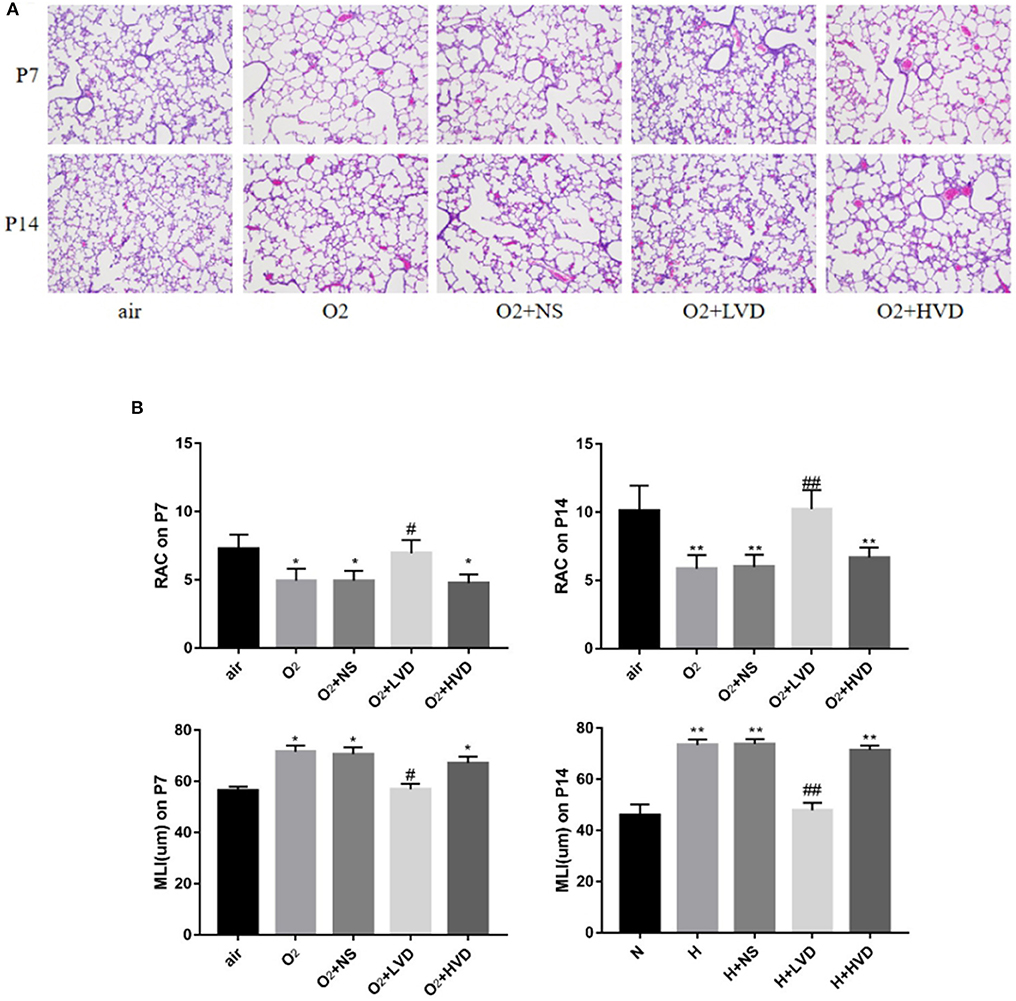
Figure 2. LVD (0.5 ng/g) treatment improved alveolarization in hyperoxia-induced BPD. (A) The H&E assessment of lung tissues. Magnification, ×200. (B) The RAC and MLI value. *P < 0.05, **P < 0.01, compared to normoxia group (N); #P < 0.05,##P < 0.01, compared to hyperoxia group (H). n = 6.
Compared to rats in the normoxia group, rats in the hyperoxia group exhibited decreased lung VEGF expression, as shown by immunohistochemistry (Figure 3A). The rats treated with LVD exhibited significantly improved pulmonary vascular development due to significantly increased protein expression of VEGF (Figure 3B). In contrast, the rats treated with HVD did not exhibit increased expression of VEGF under hyperoxia.
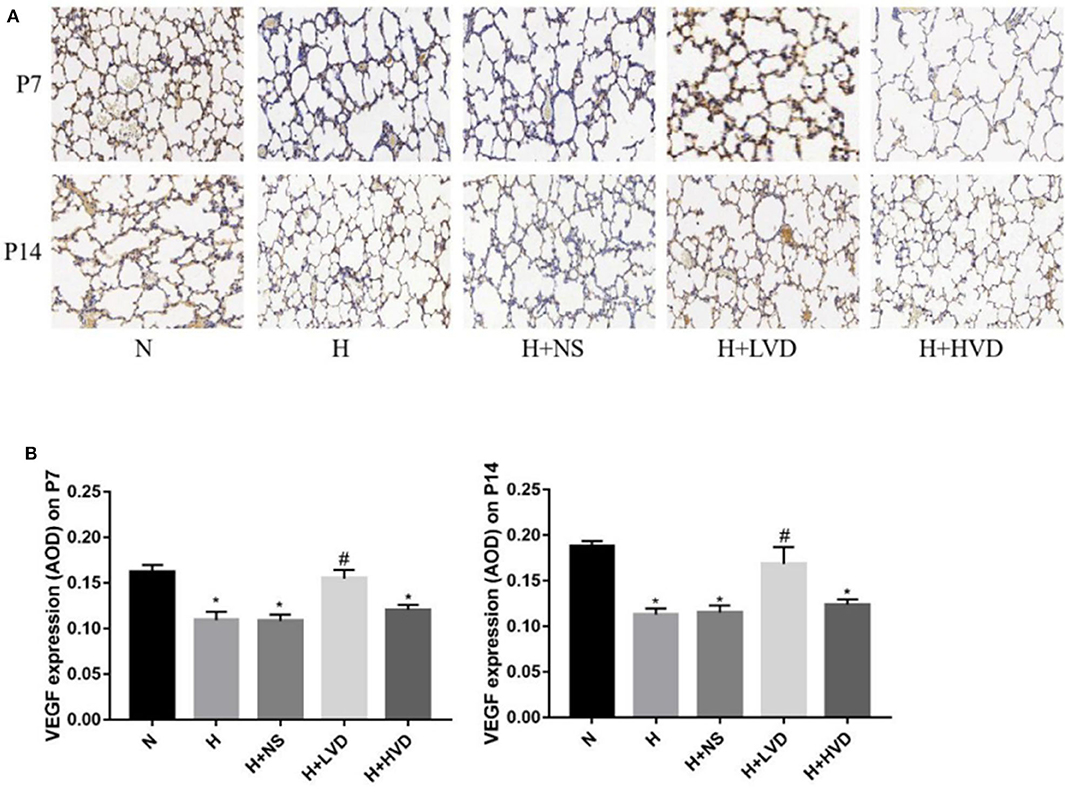
Figure 3. LVD (0.5 ng/g) treatment improved lung angiogenesis. (A) The immunohistochemical assessment for VEGF protein. Magnification, ×200. (B) The AOD value of VEGF. *P < 0.05, compared to normoxia group (N); #P < 0.05, compared to hyperoxia group (H) n = 6.
Exposure of newborn rats from birth to P7 to hyperoxia (90%) resulted in increased expression of the inflammatory cytokines TNF-α, IL-1β, and IL-6 in the lung tissues, and LVD treatment significantly decreased the levels of these inflammatory cytokines, but the rats administered HVD i.p. did not exhibit an inhibited inflammation response (Figure 4).
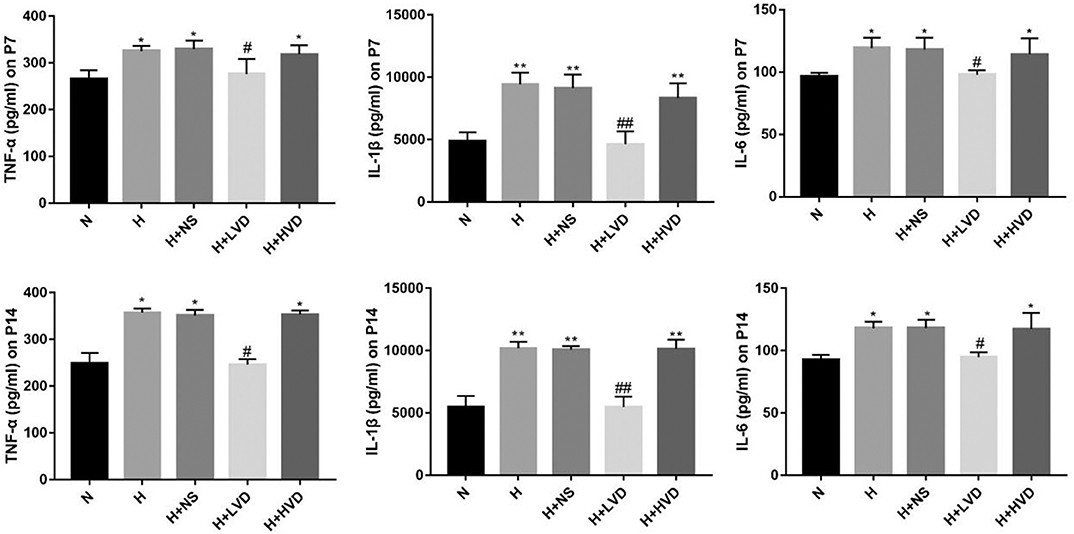
Figure 4. LVD (0.5 ng/g) treatment reduced proinflammation factors in hyperoxia-induced BPD rats on P7 and P14. *P < 0.05, **P < 0.01, compared to normoxia group (N); #P < 0.05, ##P < 0.01, compared to hyperoxia group (H) n = 6.
To investigate the NETs formation and changes, Cit-H3, MPO, and DNA, which are known markers of NETs formation, in lung tissues were identified by immunofluorescence staining. Marked staining for Cit-H3, MPO, and DNA was observed in rats under hyperoxia but not rats under normoxia (Figures 5A,B). The treatment of rats with LVD inhibited NETs formation, as manifested by decreased staining. Plasma cell-free DNA and Cit-H3 were markedly elevated in the hyperoxia group compared with the normoxia group (Figures 5C,D). LVD treatment significantly decreased the level of cell-free DNA and Cit-H3 expression in rats exposed to hyperoxia, but HVD treatment did not obviously reduce NETs formation.
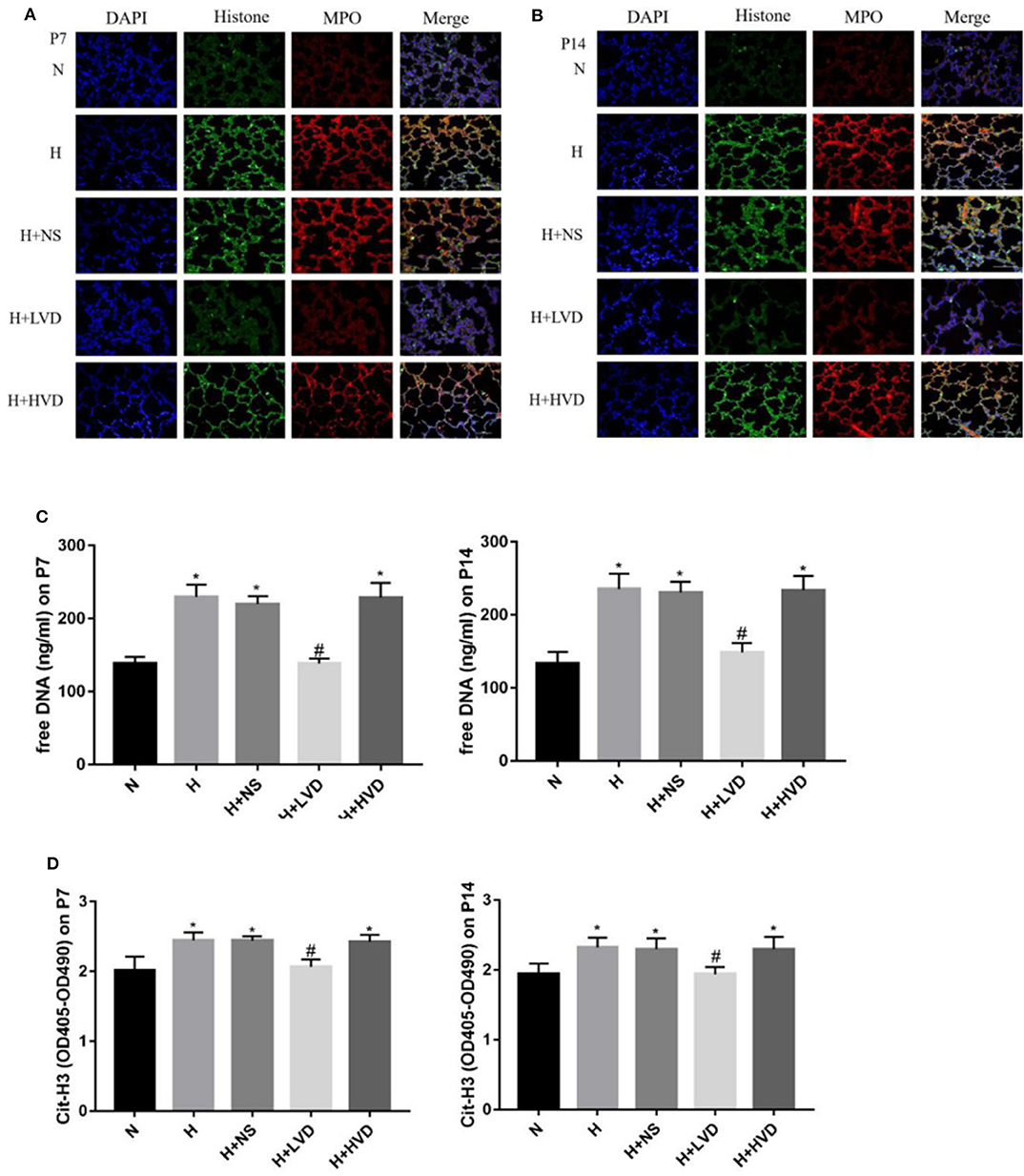
Figure 5. LVD (0.5 ng/g) treatment reduced NETs formation in hyperoxia-induced BPD rats (A,B). The NETs formation identified by immunofluorescence staining. Magnification, ×200. (C) The expressions of free DNA in plasma. (D) The expressions of circulating histones. *P < 0.05, compared to normoxia group (N); #P < 0.05, compared to hyperoxia group (H) n = 6.
Lung development in extremely preterm infants occurs in the transition from the canalicular to saccular stages, which results in a gas-exchange barrier and leaves extremely preterm infants at high risk of neonatal respiratory distress syndrome (NRDS). To improve the survival rates of extremely preterm infants, it is necessary to use supplemental oxygen or mechanical ventilation for respiratory failure. However, these measures induce further lung injury and interrupt pulmonary alveolar and vascular development, eventually contributing to BPD. The incidence of BPD, as demonstrated by major cohort studies, is 11–50% and increases with decreased gestational age and birth weight (1). Due to the increase of in vitro fertilization (IVF), multiple births and preterm infants in recent years, BPD is associated with high morbidity and mortality due to its limited effective therapeutics and thus represents a major public health concern, drawing attention worldwide.
The pathogenesis of BPD is multifactorial; however, inflammation is generally believed to be the primary mediator of lung injury in preterm infants (24). Inflammatory signaling pathways, such as the TLR4 and NF-κB pathways, were indicated to be associated with BPD (21, 25). The levels of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-8, are increased, and the levels of anti-inflammatory cytokines, such as IL-4, IL-10, IL-12, IL-13, and IL-18, are decreased in BPD (26, 27). Pulmonary inflammation in BPD is characterized by the presence of inflammatory cells, PMNs, monocytes, proinflammatory cytokines, and soluble adhesion molecules (25). Neutrophil influx into the airways and pulmonary edema formation occur after exposure to hyperoxia or mechanical ventilation, increasing the risk of developing BPD. PMNs are activated and adhere to epithelial cells of alveoli and the endothelium of the pulmonary vasculature during the inflammatory process, initiating a series of pulmonary injuries. Activated PMN exosomes play an important role in chronic inflammatory diseases in the lung and have been demonstrated as the pathogenic entities causing BPD (4). Furthermore, neutrophils undergo apoptosis at a slower pace and cannot be cleared in a timely manner in preterm neonates, prolonging neutrophil survival and aggravating lung inflammation and BPD (26).
Activated PMNs expel decondensed chromatin with embedded inflammatory proteins, known as NETs, which have been shown to damage tissues and organs in infection and sterile inflammatory disease upon dysregulation (28). NETs and histones can directly induce the release of several proinflammatory cytokines, including TNF-α, IL-1β, and IL-6 (29). NETs release occurs primarily through a cell death process termed NETosis (30). Furthermore, excessive NETosis can compromise cell membrane integrity, disrupt cell junctions, and kill epithelial and endothelial cells, effects that have been described in pulmonary diseases such as pulmonary infection, acute lung injury and COPD (28, 31, 32). Studies demonstrated that the disruption of NETs by DNase I administration resulted in decreased disease severity in mouse models of transplantation-related lung injury (33), and anti-histone antibodies could improve lung injury and survival in a mouse model of a lipopolysaccharide-induced sepsis-like syndrome (34). We previously investigated NETs in an animal model of BPD and showed that inhibition of NETs with anti-histone antibodies or heparin reduced lung injury (11), which also implicated antagonizing the activation of NETs as a potential therapeutic approach in BPD.
In recent decades, VD has been shown to be involved in the modulation of immunity, inflammation, infection, fibrosis and cancer, in addition to its traditional role in regulating calcium and phosphorus homeostasis (12, 13, 35). VD can suppress neutrophil activation, induce antimicrobial peptides, and reduce the production of inflammatory mediators and reactive oxygen intermediates in PMNs (36, 37). VD also plays a crucial role in cellular growth and differentiation, including the regulation of lung maturation (15). Moreover, the impact of VD on early lung development and BPD has become an emerging field of research in recent years (18, 20). Yao et al. (20) suggested that VD attenuated hyperoxia-induced lung injury by protecting the integrity of the lung structure, decreasing extracellular matrix deposition, inhibiting inflammation, and antagonizing the activation of TLR4. Kose et al. (14) demonstrated that treatment with VD protected against hyperoxia-induced lung injury by decreasing MLI and apoptosis and increasing the proliferating cell nuclear antigen (PCNA) index. Liu et al. (19) suggested that supplementation with VD could enhance alveolar development in an LPS-induced BPD rat model through the suppression of IFN-γ production. However, whether other regulatory mechanisms are involved in VD as a treatment in hyperoxia-induced lung injury remains unknown. Moreover, VD was proven to have the capacity to inhibit the formation of phagocytic oxidase (Phox), which is a key enzyme in the formation of NETs (38). Handono et al. (21) demonstrated that VD treatment could decrease NETs activity in patients with systemic lupus erythematosus (SLE) to prevent endothelial damage.
In our study, we found that newborn rats exposed to hyperoxia had increased mortality, decreased body weight, and restricted lung development, resulting in fewer enlarged alveolar air spaces, the loss of lung capillaries, and increased inflammatory factors. We began our investigation with an evaluation of lung tissue and plasma samples for the NETs markers MPO, Cit-H3, and cell-free DNA and found that their levels were significantly elevated in hyperoxia-induced BPD rats. At the same time, treatment with LVD increased the survival rate, attenuated developmental retardation, improved alveolar and lung vascular growth by increasing the RAC, decreasing MLI, upregulating VEGF expression, reducing the production of inflammatory cytokines, and inhibiting the formation of NETs. However, treatment with HVD did not significantly attenuate lung injury or NETs and even led to more severe developmental retardation and a higher mortality rate. Interestingly, Kose et al. (14) found that 1,25(OH)2D3 has more therapeutic potential at higher dosages (3 ng/g/day and 5 ng/g/day) than at a dosage of 1 ng/g/day. In contrast, we observed that LVD, but not HVD, had therapeutic effects. Furthermore, we demonstrated that LVD protected against hyperoxia-induced lung injury by inhibiting the formation of NETs, which has not been reported in previous studies. We believe that LVD inhibit NETs and further inhibit the release of proinflammatory factors, thus improving lung injury caused by hyperoxia, which is part of the mechanism of the treatment of BPD with LVD. We also observed weight loss as a side effect of HVD. Abbas (39) suggested that VD regulates adipogenesis at various stages of the differentiation process and certain molecular factors. Therefore, we speculate that VD affects the key genes in adipose metabolism and lung development, which will be confirmed in our future studies.
This study has several limitations. BPD is a complex, multifactorial disease that may only be partially explained by the effects of hyperoxia exposure, and antenatal infection also needs to be considered in a future study. In addition, further work is needed to determine the most effective dosage of VD in an animal model or in a clinical setting. Finally, future studies are needed to address the effects of VD exposure on metabolic functions, which may play an important role during lung development.
In conclusion, administration of LVD (0.5 ng/g) increased the survival rate, attenuated developmental retardation, improved alveolarization and pulmonary vascular development, and alleviated inflammation in hyperoxia-induced BPD partially by inhibiting NETs.
Bronchopulmonary dysplasia (BPD) is a severe complication in extreme preterm infants. Vitamin D (VD) has anti-inflammatory properties, promotes lung growth, and may have a therapeutic effect on BPD. In our previous study, neutrophil extracellular traps (NETs) overexpression was demonstrated to be involved in the pathogenesis of BPD.
In the present study, we found that low-dose VD increased the survival rate, attenuated developmental retardation, and improved alveolarization and pulmonary vascularization arrest in hyperoxia-induced BPD partially by inhibiting NETs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
The experimental protocol was approved by the ethics committee of Wenzhou Medical University (wydw 2019-055).
CC provided the idea, managed the experiments, analyzed the results, involved in manuscript preparation, and provided the fund. HW designed experiment, managed the experiments, analyzed the results and involved in manuscript preparation. XZ and SW was involved in animal experiments and samples test. CL and HJ was involved in data collection and analysis. SC analyzed the results, and was involved in manuscript preparation. YL was involved in animal experiments. AS was involved in samples test. YS provided the idea of this experiments and managed it. All authors contributed to the article and approved the submitted version.
This study were supported by Yiwu Municipal Science and Technology Bureau, China (19-3-100), Zhejiang Province Medical Science and Technology Project, China (2020366886), and Jinhua Science and Technology Research Project, China (2020-4-156).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.00335/full#supplementary-material
BPD, bronchopulmonary dysplasia; VD, Vitamin D/1,25(OH)2D3; NETs, neutrophil extracellular traps; RAC, radial alveolar count; MLI, mean linear intercept; VEGF, vascular endothelial growth factor; PMNs, polymorphonuclear neutrophils; NLR, neutrophil-to-lymphocyte ratio; VLBWI, very low birth weight infants; MPO, myeloperoxidase; NE, neutrophil elastase; H&E, hematoxylin and eosin; PBS, phosphate-buffered saline; AOD, average optical density; IFN-γ, interferon-gamma; TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1Beta; IL-6, interleukin-6; Cit-H3, anti-citrullinated-histone 3; DAPI 4′, 6-diamidino-2- phenylindole; LVD, low-dose VD; HVD, high-dose VD; NRDS, neonatal respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; Phox, phagocytic oxidase; PCNA, proliferating cell nuclear antigen; SLE, systemic lupus erythematosus.
1. Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. (2019) 5:78. doi: 10.1038/s41572-019-0127-7
2. Mowitz ME, Ayyagari R, Gao W, Zhao J, Mangili A, Sarda SP. Health care burden of bronchopulmonary dysplasia among extremely preterm infants. Front Pediatr. (2019) 7:510. doi: 10.3389/fped.2019.00510
3. Principi N, Di Pietro GM, Esposito S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J Transl Med. (2018) 16:36. doi: 10.1186/s12967-018-1417-7
4. Genschmer KR, Russell DW, Lal C, Szul T, Bratcher PE, Noerager BD, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. (2019) 176:113–26.e15. doi: 10.1016/j.cell.2018.12.002
5. Sun Y, Chen C, Zhang X, Weng X, Sheng A, Zhu Y, et al. High neutrophil-to-lymphocyte ratio is an early predictor of bronchopulmonary dysplasia. Front Pediatr. (2019) 7:464. doi: 10.3389/fped.2019.00464
6. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. (2004) 303:1532–5. doi: 10.1126/science.1092385
7. Ribon M, Seninet S, Mussard J, Sebbag M, Clavel C, Serre G, et al. Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1q and LL-37. J Autoimmun. (2019) 98:122–31. doi: 10.1016/j.jaut.2019.01.003
8. Li H, Pan P, Su X, Liu S, Zhang L, Wu D, et al. Neutrophil extracellular traps are pathogenic in ventilator-induced lung injury and partially dependent on TLR4. Biomed Res Int. (2017) 2017:8272504. doi: 10.1155/2017/8272504
9. Dicker AJ, Crichton ML, Pumphrey EG, Cassidy AJ, Suarez-Cuartin G, Sibila O, et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. (2018) 141:117–27. doi: 10.1016/j.jaci.2017.04.022
10. Li RHL, Tablin F. A comparative review of neutrophil extracellular traps in sepsis. Front Vet Sci. (2018) 28:291 doi: 10.3389/fvets.2018.00291
11. Sun Y, Chen C, Zhang X, Wang S, Zhu R, Zhou A, et al. Heparin improves alveolarization and vascular development in hyperoxia-induced bronchopulmonary dysplasia by inhibiting neutrophil extracellular traps. Biochem Biophys Res Commun. (2020) 522:33–9. doi: 10.1016/j.bbrc.2019.11.041
12. Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, Heydari ST, et al. The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. (2018) 50:271–9. doi: 10.1055/s-0044-101355
13. Lee JW, Kim SC, Ko YS, Lee HY, Cho E, Kim MG, et al. Renoprotective effect of paricalcitol via amodulation of the TLR4-NF-κB pathway in ischemia/reperfusion-induced acute kidney in jury. Biochem Biophys Res Commun. (2014) 444:121–7. doi: 10.1016/j.bbrc.2014.01.005
14. Kose M, Bastug O, Sonmez MF, Per S, Ozdemir A, Kaymak E, et al. Protective effect of vitamin D against hyperoxia-induced lung injury in newborn rats. Pediatr Pulmonol. (2017) 52:69–76. doi: 10.1002/ppul.23500
15. Lykkedegn S, Sorensen GL, Beck-Nielsen SS, Christesen HT. The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. Am J Physiol Lung Cell Mol Physiol. (2015) 308:L587–602. doi: 10.1152/ajplung.00117.2014
16. Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1alpha,25-dihydroxyvitamin D3. Am J Physiol Lung Cell Mol Physiol. (2005) 289:L617–26. doi: 10.1152/ajplung.00129.2004
17. Mao X, Qiu J, Zhao L, Xu J, Yin J, Yang Y, et al. Vitamin D and IL-10 deficiency in preterm neonates with bronchopulmonary dysplasia. Front Pediatr. (2018) 6:246. doi: 10.3389/fped.2018.00246
18. Kazzi SNJ, Karnati S, Puthuraya S, Thomas R. Vitamin D deficiency and respiratory morbidity among African American very low birth weightinfants. Early Hum Dev. (2018) 119:19–24. doi: 10.1016/j.earlhumdev.2018.02.013
19. Liu C, Chen Z, Li W, Huang L, Zhang Y. Vitamin D enhances alveolar development in antenatal lipopolysaccharide-treated rats through the suppression of interferon-gamma production. Front Immunol. (2018) 8:1923. doi: 10.3389/fimmu.2017.01923
20. Yao L, Shi Y, Zhao X, Hou A, Xing Y, Fu J, et al. Vitamin D attenuates hyperoxia-induced lung injury through downregulation of Toll-like receptor 4. Int J Mol Med. (2017) 39:1403–8. doi: 10.3892/ijmm.2017.2961
21. Handono K, Sidarta YO, Pradana BA, Nugroho RA, Hartono IA, Kalim H, et al. Vitamin D prevents endothelial damage induced by increased neutrophil extracellular traps formation in patients with systemic lupus erythematosus. Acta Med Indones. (2014) 46:189–98.
22. Cooney TP, Thurlbeck WM. The radial alveolar count method of emery and mithal: a reappraisal 2–intrauterine and early postnatal lung growth. Thorax. (1982) 37:580–3. doi: 10.1136/thx.37.8.580
23. Dunnill MS. Quantitative methods in the study of pulmonary pathology. Thorax. (1962) 17:320–8. doi: 10.1136/thx.17.4.320
24. Balany J, Bhandari V. Understanding the impact of infection, inflammation, and their persistence in the pathogenesis of bronchopulmonary dysplasia. Front Med (Lausanne). (2015) 2:90. doi: 10.3389/fmed.2015.00090
25. McKenna S, Butler B, Jatana L, Ghosh S, Wright CJ. Inhibition of IkappaBbeta/NFkappaB signaling prevents LPS-induced IL1beta expression without increasing apoptosis in the developing mouse lung. Pediatr Res. (2017) 82:1064–72. doi: 10.1038/pr.2017.182
26. Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. (2006) 11:354–62. doi: 10.1016/j.siny.2006.03.004
27. Bhandari A, Bhandari V. Biomarkers in bronchopulmonary dysplasia. Paediatr Respir Rev. (2013) 14:173–9. doi: 10.1016/j.prrv.2013.02.008
28. Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. (2017) 23:279–87. doi: 10.1038/nm.4294
29. Alhamdi Y, Toh CH. Recent advances in pathophysiology of disseminated intravascular coagulation: the role of circulating histones and neutrophil extracellular traps. F1000Res. (2017) 18:2143. doi: 10.12688/f1000research.12498.1
30. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. (2007) 176:231–41. doi: 10.1083/jcb.200606027
31. Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. (2012) 119:6335–43. doi: 10.1182/blood-2012-01-405183
32. Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary diseases: too much of a goodthing? Front Immunol. (2016) 7:311. doi: 10.3389/fimmu.2016.00311
33. Sayah DM, Mallavia B, Liu F, Ortiz-Muñoz G, Caudrillier A, DerHovanessian A, et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. (2015) 191:455–63. doi: 10.1164/rccm.201406-1086OC
34. Toru K, Kuei-Chen C, Masafumi I, Yayoi S, Naoya O, Takeshi G, et al. A novel anti-histone H1 monoclonal antibody, SSV monoclonal antibody, improves lung injury and survival in a mouse model of lipopolysaccharide-induced sepsis-like syndrome. Biomed Res Int. (2015) 2015:491649. doi: 10.1155/2015/491649
35. Chen L, Yang R, Qiao W, Yuan X, Wang S, Goltzman D, et al. 1,25-Dihydroxy vitamin D prevents tumorigenesis by inhibiting oxidative stress and inducing tumor cellular senescence in mice. Int J Cancer. (2018) 143:368–82. doi: 10.1002/ijc.31317
36. Lisakovska O, Shymanskyy I, Mazanova A, Khomenko A, Veliky M. Vitamin D3 protects against prednisolone-induced liver injury associated with the impairment of the hepatic NF-kappaB/iNOS/NO pathway. Biochem Cell Biol. (2017) 95:213–22. doi: 10.1139/bcb-2016-0070
37. Hirsch D, Archer FE, Joshi-Kale M, Vetrano AM, Weinberger B, Weinberger B. decreased anti-inflammatory responses to vitamin D in neonatal neutrophils. Mediators Inflamm. (2011) 2011:598345. doi: 10.1155/2011/598345
38. Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J.Cell Biol. (2012) 198:773–83. doi: 10.1083/jcb.201203170
Keywords: bronchopulmonary dysplasia, vitamin D, neutrophil extracellular traps, hyperoxia, histone
Citation: Chen C, Weng H, Zhang X, Wang S, Lu C, Jin H, Chen S, Liu Y, Sheng A and Sun Y (2020) Low-Dose Vitamin D Protects Hyperoxia-Induced Bronchopulmonary Dysplasia by Inhibiting Neutrophil Extracellular Traps. Front. Pediatr. 8:335. doi: 10.3389/fped.2020.00335
Received: 08 March 2020; Accepted: 21 May 2020;
Published: 03 July 2020.
Edited by:
Heber C. Nielsen, Tufts Medical Center, United StatesReviewed by:
Hercília Guimarães, University of Porto, PortugalCopyright © 2020 Chen, Weng, Zhang, Wang, Lu, Jin, Chen, Liu, Sheng and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Sun, NjExMzIzMThAcXEuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.