
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 03 April 2020
Sec. Pediatric Critical Care
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.00140
 En-Pei Lee1,2†
En-Pei Lee1,2† Lu-Lu Zhao3,4†
Lu-Lu Zhao3,4† Shao-Hsuan Hsia1,2†
Shao-Hsuan Hsia1,2† Jung Lee2,5
Jung Lee2,5 Oi-Wa Chan1,2
Oi-Wa Chan1,2 Chia-Ying Lin1,2
Chia-Ying Lin1,2 Ya-Ting Su1,2
Ya-Ting Su1,2 Jainn-Jim Lin1,2*
Jainn-Jim Lin1,2* Han-Ping Wu5,6,7,8*
Han-Ping Wu5,6,7,8*Traumatic brain injury (TBI) is a leading cause of pediatric morbidity and mortality and is categorized as abusive head trauma (AHT) and accidental head injury. A retrospective chart review of 124 children aged <1 year diagnosed with TBI were analyzed. Outcomes were evaluated at discharge and 6 months later by using the Pediatric Cerebral Performance Category (PCPC) Scale. The receiver operating characteristic (ROC) curve was applied to determine the cutoff values for hemoglobin (HB) levels. In the study, 50 infants (40.3%) achieved a favorable neurologic outcome (PCPC ≦ 2) and 74 (59.7%) had poor neurologic outcomes (PCPC ≧ 3). Infants with poor neurologic outcomes had lower HB on admission and nadir HB (p < 0.05). Based on multivariate logistic regression analysis, the nadir HB was a predictor of poor neurologic outcomes at discharge and 6 months later in both AHT and accidental head injury. Nadir HB had the largest area under the ROC curve for predicting poor neurologic outcomes. We determined the appropriate cutoff value of nadir HB as 9.35 g/dl for predicting neurologic outcomes in infants with TBI. Furthermore, the cutoff value of nadir HB in predicting poor neurologic outcomes in infants caused by AHT and accidental head injury were taken as 9.36 and 8.75 g/dl, respectively.
Physical abuse and neglect are the major types of child maltreatment and affect an increasing number of children in the world (1). Despite the efforts of child protection medical services, physical abuse and neglect still remain important and serious problems. Based on the data of the National Child Abuse and Neglect Data System (NCANDS) in the United States of America, the number of fatalities fluctuated in the past 5 years. Younger children are the most frequent victims of child maltreatment because of their dependency and inability to protect themselves. Children aged <3 years old accounted for about three-quarters of all fatalities, especially infants (<1-year-old) who accounted for half of all fatalities in the USA in 2015 due to child maltreatment (2). In the infant group suffering from child maltreatment, the primary cause of death is traumatic brain injury (TBI). In general TBI can be classified as abusive head trauma (AHT) and accidental head injury (3–5). Intracranial hemorrhage is frequent after TBI in infants due to the highly vascularized nature of the brain and is associated with poor outcomes. About two thirds of AHT and quarter of accidental head injury have the feature of subdural hemorrhage (SDH). And epidural hemorrhages (EDH) were significantly associated with accidental head injury rather than AHT (17 vs. 4 percent, respectively) (6). Therefore, it is important to analyze the risk factors associated with morbidity and mortality in infants with traumatic intracranial hemorrhage to develop guidelines for adequate treatment to prevent further complications such as higher brain dysfunction.
In adults with intracranial hemorrhage caused by TBI or stroke, several risk factors such as the volume of intracranial hemorrhage, the score on the Glasgow Coma Scale (GCS) on admission, or older age are predictors of poor outcomes. In particular, the common laboratory examination of HB can be used as a predictor for outcome, with low HB indicating poor functional outcomes after non-traumatic brain hemorrhage at discharge and at 3 months (7, 8). The pathophysiology of anemia in TBI or stroke is evidence of primary blood loss. The severe impact of anemia on brain function in various pathological conditions is somewhat unclear. It may be that lower HB impaired the function of brain oxygenation (9). However, evidence about the utility of HB in pediatric intracranial hemorrhage is limited, and its use is not endorsed in predicting outcomes of pediatric intracranial hemorrhage. The main objective of this study was to determine whether the HB level in traumatic intracranial hemorrhagic infants is associated with poor neurologic outcomes. Our hypothesis was that low HB is associated with increased mortality or poor neurologic outcomes.
From January 2006 to 2017, we retrospectively collected data from infants aged <1 years old with intracranial hemorrhage (ICH) caused by TBI who were admitted to the pediatric intensive care unit (PICU) via the emergency department (ED) or outpatient department (OPD). A total of 124 infants with ICH confirmed by brain computed tomography (CT) at admission were included in this study. The study was approved by the Institutional Review Board of the Chang Gung Memorial Hospital. All methods were performed in accordance with the relevant guidelines and regulations.
The data were collected, reviewed, de-identified, and anonymously analyzed by the authors, and the Ethics Committee waived the requirement for informed consent because of the anonymized nature of the data, and scientific purpose of the study.
The setting in our study was a tertiary medical center receiving cases transferred from local clinics, regional hospitals, and social and politic institutions (child protection agency subordinated by the government). The PICU of our hospital was a tertiary ICU with 29 beds for hospitalized patients aged <18 years. In our study, all the victims with ICH caused by child maltreatment by the definition of World Health Organization (WHO) (10) were admitted to the PICU. Victims with accompanying internal bleeding from other organs (except intracranial bleeding) were excluded. After review of the clinical history, physical examination findings, and past medical charts, TBI was categorized as AHT and accidental head injury according to predefined criteria (Table 1) (3–5). All the victims of AHT and accidental head injury were viewed by the child protection team. Our hospital was a Child Protection Medical Service Demonstration Center which consisting of well-trained medical doctors, nursing staff, and social workers for identifying abused and neglected victims.
Information related to the cases of AHT and accidental head injury, including age (months), gender, initial presentations, Glasgow Coma Score (GCS), injury severity score (ISS), imaging examinations, length of stay in hospitals, and ICUs, laboratory data such as hemoglobin (HB) levels, neurologic outcomes, and mortality were obtained from the social welfare reporting system and medical records. Admission of victims to the PICU may indicate an urgent clinical condition requiring critical care following the guidelines of the Society of Critical Care Medicine (11), meeting at least one of its defined criteria. Rotterdam scoring system was used in the study to grade acute TBI on the basis of CT findings. It included four independently scored elements such as degree of basal cistern compression, degree of midline shift, traumatic subarachnoid, or intraventricular hemorrhage, and epidural hematoma (12). Neurologic outcome at discharge was evaluated by pediatric neurologists using the system of Pediatric Cerebral Performance Category (PCPC) Scale. Neurologic outcomes at 6 months after discharge were evaluated by pediatric neurologists in the OPD, using the PCPC scale. Outcome scores were divided into favorable (PCPC 1–2) and poor neurologic outcomes (PCPC 3–6 at discharge; PCPC 3–5, 6 months later). The patients with favorable neurologic outcomes (PCPC 1–2) could go to the regular school, whereas poor neurologic outcomes could only go to the special school or respiratory care ward.
The exposures of concern were admission HB, nadir HB (the lowest HB value during hospital stay), and mean HB (calculated from all values within 1 month). Daily or more frequent rechecks of HB were performed if the initial HB was <9 g/dl or in the event of any hypotension episode. All infants with a nadir of 9 g/dl or less received red blood cell (RBC) transfusions.
The chi-square test, Fisher's exact test, Student's t-test, Mann–Whitney U-test, and multivariate logistic regression analysis were used where appropriate. In the descriptive analysis, values are presented as mean ± standard deviation (SD) or median (interquartile range). The difference between groups is presented as 95% confidence intervals (CIs). For comparison of dichotomous variables between groups, the chi-square test or Fisher's exact test was used. Comparisons of continuous variables between two groups were made with the Mann–Whitney U-test. Predicted probabilities of a poor neurologic outcome (PCPC scale 3–6 at discharge and PCPC scale 3–5 at 6 months) and 95% confidence intervals were calculated by using a logistic regression model.
Finally, the receiver operating characteristic (ROC) curve was applied to determine the ideal cut-off values of HB for poor neurologic outcomes. The test characteristics of the different cut-off values, including sensitivity, specificity, area under the curve (AUC), positive likelihood ratio (LR+), and negative likelihood ratio (LR−) were also examined. Statistical significance was defined at the p < 0.05 level and all statistical analyses were conducted using IBM SPSS Statistics software (version 22.0; SPSS Inc., Chicago, IL, USA).
In the study, among the 124 infants, fifty (40.3%) infants achieved a favorable neurologic outcome and 74 (59.7%) had poor neurologic outcomes. The comparison of good neurologic outcomes and poor neurologic outcomes in both groups are listed Table 2. In the cohort, 78 (62.9%) were classified as suffering from AHT and 46 (37.1%) were classified as suffering from accidental head injury. At initial presentation, only the initial GCS score was significantly lower in infants with poor neurologic outcomes in both groups (p < 0.05). The brain image findings showed no significant difference between good and poor neurologic outcomes in both groups. But the Rotterdam CT score were significant higher in the poor neurologic group in both group (p < 0.05). The admission HB and nadir HB were both significantly lower but the numbers for RBC transfusion were significantly higher in the poor neurologic group (all p < 0.05).
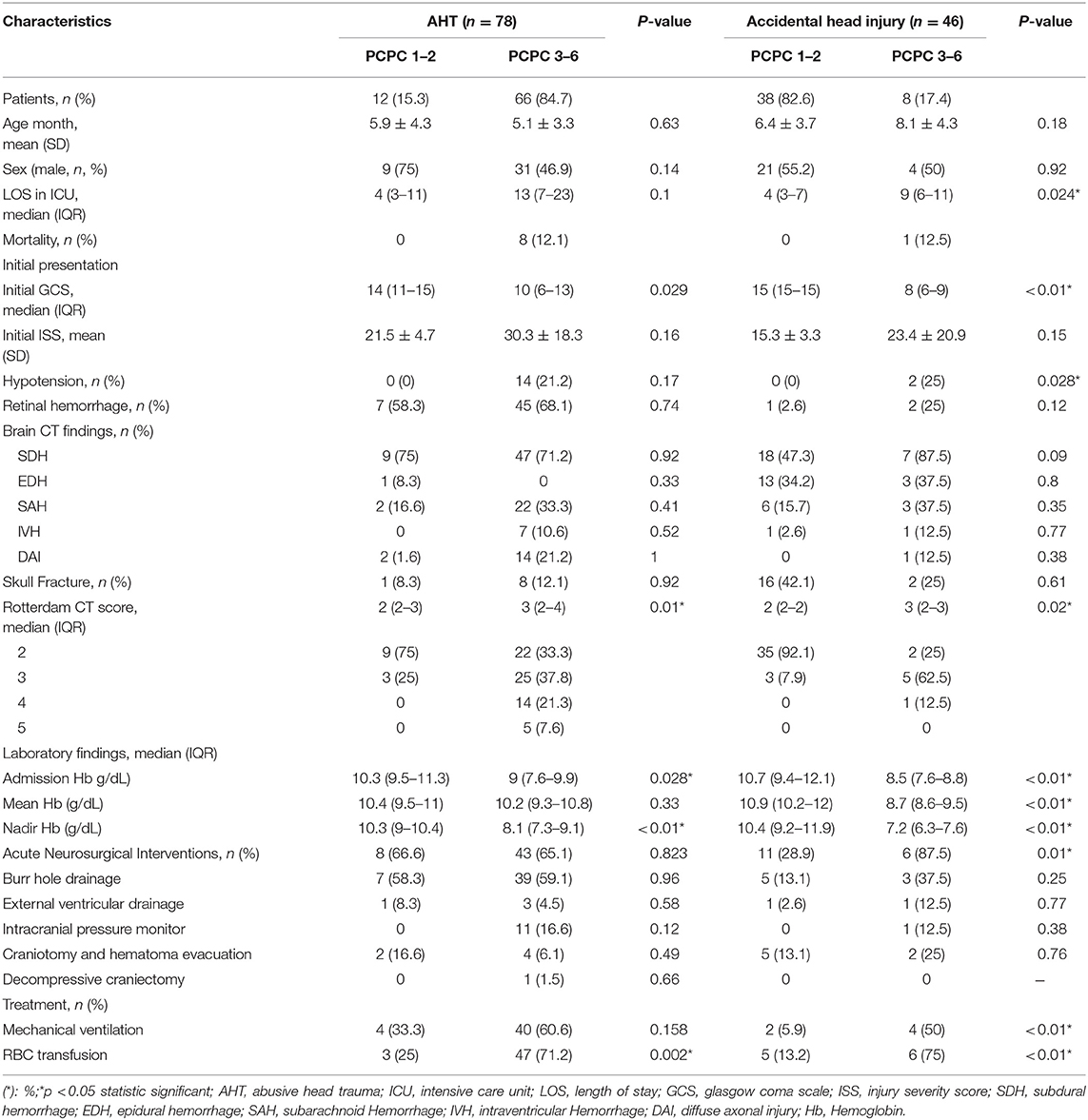
Table 2. Correlation between poor outcome and characteristics in the group with AHT and accidental head injury.
We performed a logistic regression model to identify indicators for predicting poor neurologic outcome at discharge and 6 months after discharge, and found that initial GCS score, ISS, nadir HB and Rotterdam CT score were all significant factors (Table 3). In the final model, only nadir HB remained as an independent predictor of poor neurologic outcome at discharge, but nadir HB and the initial GCS score were predictors of poor neurologic outcome at 6 months after discharge in the abused group. In the group with accidental head injury, only nadir HB appeared as an independent predictor for poor neurologic outcome at 6 months after discharge. And the detected mean time of nadir HB was 3.6 ± 3.5 h after admission. In-hospital mortality was 9.7% (12 infants) (Table 4). Based on the results of univariate analysis, the initial GCS score, nadir HB and Rotterdam CT score were associated with in-hospital mortality. In the multivariate logistic regression model, only the initial GCS score (OR 0.528, 95% CI 0.389–0.716, p < 0.001) remained an independent predictor for in-hospital mortality (Table 5). Outcome at 6 months was available for 112 (90.3%) infants; and 12 infants died during admission. The nadir HB levels showed significant differences between groups with favorable and poor neurologic outcomes in the group with AHT (Figure 1) and accidental head injury (Figure 2) at discharge and 6 months after discharge (both p < 0.05).
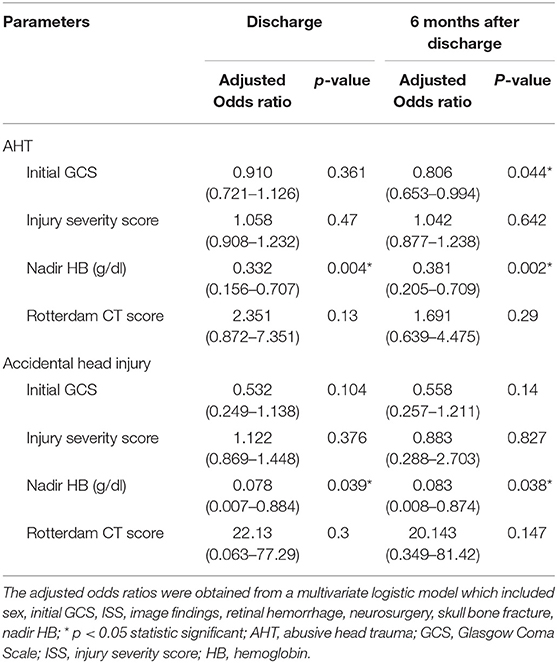
Table 3. Multivariate Logistic regression model to predict poor neurologic outcome (PCPC ≧ 3) at discharge and 6 months.
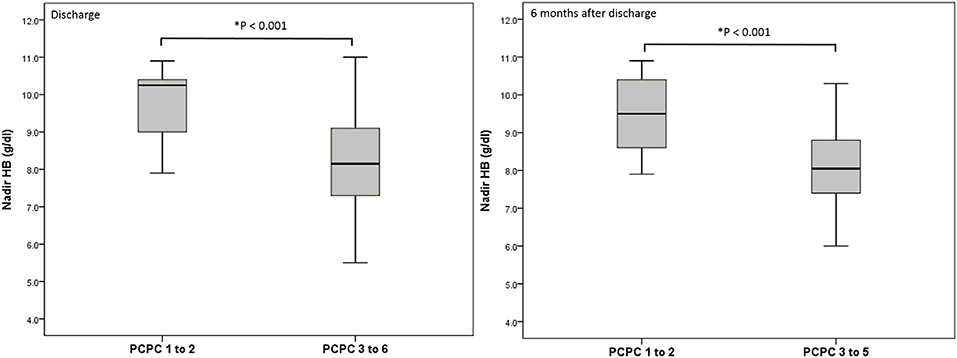
Figure 1. Nadir HB (g/dl) and outcome at discharge and 6 months after discharge in the group with AHT.
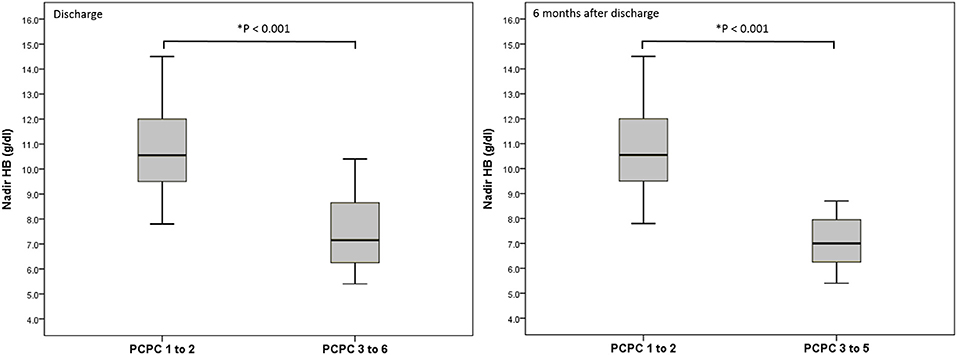
Figure 2. Nadir HB (g/dl) and outcome at discharge and 6 months after discharge in the group with accidental head injury.
Nadir HB had the highest area under the ROC curve (AUC) for predicting poor neurologic outcomes in all victims (Figure 3A). When divided into group with AHT (Figure 3B) and accidental head injury (Figure 3C), the nadir HB also had the largest AUC for predicting poor neurologic outcomes in each group. The AUCs of nadir HB, mean HB, and admission HB for predicting good or poor neurologic outcome in the all victims, victims with AHT and accidental head injury were calculated. The AUC of nadir HB was 0.884 (95% CI, 0.827–0.941) in the all group; 0.839 (95% CI, 0.718–0.96) in the group with AHT; and 0.919 (95% CI, 0.808–1) in the group with accidental head injury which were all significantly higher than both admission HB and mean HB in all groups (all p < 0.001) (Table 6). The best cutoff values of nadir HB in predicting poor neurologic outcomes are shown in Table 7. We identified nadir HB levels of 9.35 g/dl in the cohort, 9.36 g/dl in the group with AHT and 8.75 g/dl in the group with accidental head injury to predict poor neurologic outcomes at discharge.
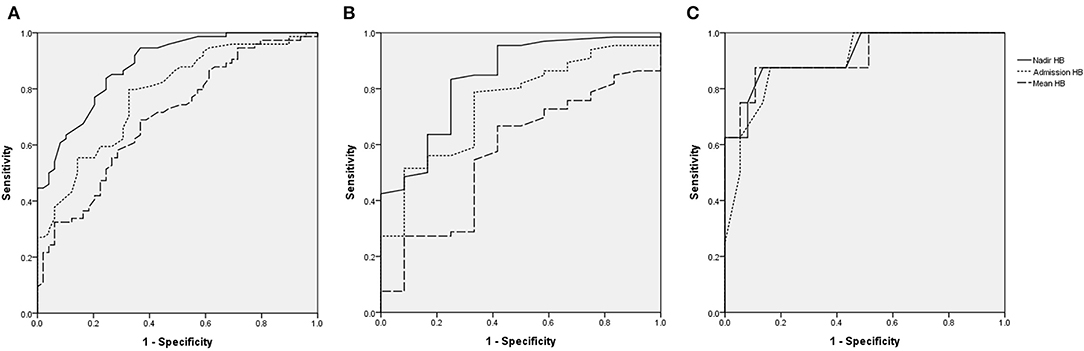
Figure 3. Receiving operating characteristic (ROC) curves for assessing the predictive accuracy of HB for poor functional outcomes. (A), All victims. (B), AHT. (C), Accidental head injury.
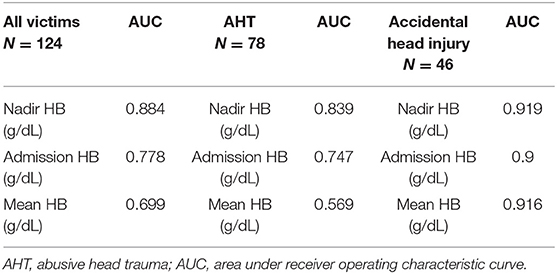
Table 6. The victims and ROC analysis at the parameters of HB between good and poor neurologic outcome.
In addition, we compared RBC transfused and non-transfused patients in Table 8 which reporting that the RBC transfused group had more critical initial presentations and worse outcomes including lower initial GCS, higher ISS, more episodes of hypotension, lower admission HB, nadir HB, and higher percentage of poor neurologic outcomes.
Low HB is a common complication in patients with intracranial hemorrhage (13). In adults with ICH, low HB levels and initial levels of consciousness may be associated with poor functional outcome (7, 14, 15). Initial GCS scores related to poor functional outcome have been reported in some studies (16, 17), but few related studies focused on this field in pediatric cases. We believe that our study is the first to analyze and report the role of nadir HB in predicting neurologic outcomes in infants with traumatic ICH, and to identify how low HB levels may be used as a predictor for poor neurologic outcome in infants. In this study, we found that nadir HB was a more powerful predictor for predicting poor neurologic outcomes at discharge and 6 months after discharge than both the initial GCS score and other forms of HB. The pathophysiology of anemia-related poor neurologic outcomes in patients with ICH is mainly caused by two factors: first, anemia can reflect the volume of primary blood loss, which is a powerful predictor for poor prognosis (9, 18, 19); second, anemia may cause secondary brain damage resulting from cerebral hypoxia. The cerebral oxygen delivery (DO2) is equal to cerebral blood flow (CBF) multiplied by arterial oxygen content (CaO2). More oxygenation is needed in the acute phase of an injured brain, but impaired global cerebrovascular autoregulation develops in patients with ICH causing compensatory mechanisms to fail (9, 13, 20). The results indicate that although the CBF increases in patients with an injured brain, it is still insufficient to conquer the reduced CaO2 induced by the low HB. Consequently, decreased levels of cerebral DO2 develop and result in hypoxic damage to the brain.
Previous studies have demonstrated that admission HB, mean HB, and nadir HB are all the predictors for poor prognosis in adults with ICH (7, 8, 21, 22), but they did not report which one was the most useful. In our study, according to the results of the final logistic mode, nadir HB appeared to be the best predictor of poor neurologic outcomes in infants with ICH. We hypothesize nadir HB may reflect the proportion of primary blood loss and the lowest CaO2 level indicating the severity of cerebral hypoxia. Therefore, once nadir HB develops within the 1st day after admission, it may indicate that nadir HB could play a feasible and practical role in predicting poor neurologic outcome. However, in infants with traumatic brain injury, we noticed that no related studies mentioned the issue in detail, so we determined the cutoff value of nadir HB as 9.35 g/dl to predict poor neurologic outcomes in infants with ICH, based on the ROC analysis, which may provide an indicator for primary clinicians to perform early interventions for infants with ICH. Moreover, we determined the cutoff value of nadir HB in predicting poor neurologic outcomes in infants with traumatic ICH caused by AHT and accidental head injury as 9.36 and 8.75 g/dl, respectively.
In previous studies, the initial GCS score and ISS were identified as risk factors for poor prognosis in pediatric cases, (23, 24). However, in our retrospective study, we found that nadir HB, initial GCS, and ISS were all related factors, and nadir HB may be more effective than the initial GCS score and ISS for predicting poor prognosis. The present study found that all of the nadir HB were detected within 12 h after admission (3.6 ± 3.5 h). Therefore, the nadir HB also provided other information: if the initial brain CT and admission HB were not critical to indicate aggressive surgical intervention, the lower nadir HB could be a parameter for monitoring disease progression and the need for further surgical intervention. In our study, we surveyed the related factors for in-hospital mortality, and found that the initial GCS score remained the independent predictor for in-hospital mortality in the multivariate mode rather than nadir HB. This may be because 66.6% (8 of 12) of mortalities presented with out-of-hospital cardiac arrest and a GCS score of 3. Considering the brain lesions, 91.7% (11 of 12) of mortalities had diffuse axonal injuries with hypoxia and hypotension, a potential risk for in-hospital mortality (25). In consequence, initial GCS scores are a powerful predictive value for in-hospital mortality in infants with traumatic ICH.
In our study, we also found why accidental head injury and AHT showed different cutoff value of nadir HB in predicting outcomes and different blood transfusion strategies. We think that the reason may be due to different trauma mechanisms. As we know, the trauma mechanisms are quietly different between the group with accidental head injury and AHT. In the group with accidental head injury, EDH resulting from injured meningeal artery is more common causing by one trauma episode. Repeated shaking head at different time points resulting in acute on chronic SDH, diffuse axonal injury may be common in the group with AHT. EDH may cause more blood loss than SDH potentially. The different blood transfusion strategies and different outcomes may be based on the different trauma mechanisms.
Until now, there is no golden standard for blood transfusion in adult and pediatric TBI (26, 27). Previous studies demonstrated that HB lower than 7 g/dl will cause anemia-induced brain injury. But the optimal transfusion threshold is still controversial. The transfusion threshold from 7 to 10 g/dl is the common transfusion strategy in the clinical practice. There is still no conclusion whether blood transfusion is associated with better or worse outcomes. Some studies demonstrated RBCT is benefit (28, 29) or harmful (30, 31) or no impact (32, 33) for neurologic outcome in patients with TBI. Our multivariate logistic regression analysis was not powered to detect an association between RBC transfusion and poor prognosis. Further randomized controlled trials based on different transfusion thresholds settings are required.
We demonstrated the association between nadir HB and poor neurologic outcome in infants with traumatic ICH. We determined the best cutoff value of nadir HB to be 9.35 g/dl for predicting poor neurologic outcomes in infants with traumatic ICH. Furthermore, the cutoff value of nadir HB in predicting poor neurologic outcomes in infants with ICH caused by AHT and accidental head injury were taken as 9.36 and 8.75 g/dl, respectively.
All datasets generated for this study are included in the article/supplementary material.
The studies involving human participants were reviewed and approved by the Institution Review Board and ethics committee of Chang-Gung Memorial hospital. IRB No.: 104-8307B. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
E-PL, L-LZ, and S-HH conceived and designed the study. L-LZ and S-HH participated in data analysis. O-WC and C-YL gathered the data. E-PL, Y-TS, and JL drafted the manuscript. H-PW and J-JL designed and oversaw the study, interpreted the data. H-PW revised the manuscript. All authors have read and approved the final manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the statistician in Chang-Gung Memorial hospital for completing the statistical analysis. The study was supported in part by the China Medical University Hospital (Grant number: DMR-108–162).
1. Sedlak AJ, Mettenburg J, Basena M. Fourth National Incidence Study of Child Abuse and Neglect (NIS−4): Report to Congress, Executive Summary. Washington, DC: U.S. Department of Health and Human Services, Administration for Children and Families (2010). doi: 10.1037/e565022012-001
2. Child Welfare Information Gateway. Child Abuse Neglect Fatalities 2015: Statistics Interventions. Washington, DC: U.S. Department of Health and Human Services, Children's Bureau (2017).
3. Reece RM, Sege R. Childhood head injuries: accidental or inflicted? Arch Pediatr Adolesc Med. (2000) 154:11–5.
4. Bechtel K, Stoessel K, Leventhal JM, Ogle E, Teague B, Lavietes S, et al. Characteristics that distinguish accidental from abusive injury in hospitalized young children with head trauma. Pediatrics. (2004) 114:165–8. doi: 10.1542/peds.114.1.165
5. Kelly P, John S, Vincent AL, Reed P. Abusive head trauma and accidental head injury: a 20-years comparative study of referrals to a hospital child protection team. Arch Dis Child. (2015) 100:1123–30. doi: 10.1136/archdischild-2014-306960
6. Kemp AM, Jaspan T, Griffiths J, Stoodley N, Mann MK, Tempest V, et al. Neuroimaging: what neuroradiological features distinguish abusive from non-abusive head trauma? a systematic review. Arch Dis Child. 96:1103–12. doi: 10.1136/archdischild-2011-300630
7. Diedler J, Sykora M, Hahn P, Heerlein K, Schölzke MN, Kellert L, et al. Low hemoglobin is associated with poor functional outcome after non-traumatic, supratentorial intracerebral hemorrhage. Crit Care. (2010) 14:R63. doi: 10.1186/cc8961
8. Naidech AM, Jovanovic B, Wartenberg KE, Parra A, Ostapkovich N, Connolly ES, et al. Higher hemoglobin is associated with improved outcome after subarachnoid hemorrhage. Crit Care Med. (2007) 35:2383–9. doi: 10.1097/01.CCM.0000284516.17580.2C
9. Lelubre C, Bouzat P, Crippa IA, Taccone FS. Anemia management after acute brain injury. Crit Care. (2016) 20:152. doi: 10.1186/s13054-016-1321-6
11. Guidelines for developing admission and discharge policies for the pediatric intensive care unit. pediatric section task force on admission and discharge criteria, society of critical care medicine in conjunction with the American college of critical care medicine and the committee on hospital care of the American academy of pediatrics. Crit Care Med. (1999) 27:843–5. doi: 10.1097/00003246-199904000-00051
12. Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. (2005) 57:1173–82. doi: 10.1227/01.NEU.0000186013.63046.6B
13. Utter GH, Shahlaie K, Zwienenberg-Lee M, Muizelaar JP. Anemia in the setting of traumatic brain injury: the arguments for and against liberal transfusion. J Neurotrauma. (2011) 28:155–65. doi: 10.1089/neu.2010.1451
14. Rathor MY, Rani MF, Jamalludin AR, Amran M, Shahrin TC, Shah A. Prediction of functional outcome in patients with primary intracerebral hemorrhage by clinical-computed tomographic correlations. J Res Med Sci. (2012) 17:1056–62.
15. Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American heart association/american stroke association stroke council, high blood pressure research council, and the quality of care and outcomes in research interdisciplinary working group. Circulation. (2007) 116:e391–413. doi: 10.3410/f.1087891.540830
16. Chiaretti A, Piastra M, Pulitanò S, Pietrini D, De Rosa G, Barbaro R, et al. Prognostic factors and outcome of children with severe head injury: an 8-years experience. Childs Nerv Syst. (2002) 18:129–36. doi: 10.1007/s00381-002-0558-3
17. Fanconi M, Lips U. Shaken baby syndrome in Switzerland: results of a prospective follow-up study, 2002–2007. Eur J Pediatr. (2010) 169:1023–8. doi: 10.1007/s00431-010-1175-x
18. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-days mortality. Stroke. (1993) 24:987–93. doi: 10.1161/01.STR.24.7.987
19. Salihović D, Smajlović D, Ibrahimagić OC. Does the volume and localization of intracerebral hematoma affect short-term prognosis of patients with intracerebral hemorrhage? ISRN Neurosci. (2013) 2013:327968. doi: 10.1155/2013/327968
20. Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care. (2009) 13:R89. doi: 10.1186/cc7916
21. Litofsky NS, Martin S, Diaz J, Ge B, Petroski G, Miller DC, et al. The negative impact of anemia in outcome from traumatic brain injury. World Neurosurg. (2016) 90:82–90. doi: 10.1016/j.wneu.2016.02.076
22. Chang TR, Boehme AK, Aysenne A, Albright KC, Burns C, Beasley TM, et al. Nadir hemoglobin is associated with poor outcome from intracerebral hemorrhage. Springerplus. (2013) 2:379. doi: 10.1186/2193-1801-2-379
23. Patregnani JT, Borgman MA, Maegele M, Wade CE, Blackbourne LH, Spinella PC. Coagulopathy and shock on admission is associated with mortality for children with traumatic injuries at combat support hospital. Pediatr Crit Care Med. (2012) 13:273–7. doi: 10.1097/PCC.0b013e31822f1727
24. Ducrocq SC, Meyer PG, Orliaguet GA, Blanot S, Laurent-Vannier A, Renier D, et al. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: experience of a French pediatric trauma center. Pediatr Crit Care Med. (2006) 7:461–7. doi: 10.1097/01.PCC.0000235245.49129.27
25. Vieira RC, Paiva WS, de Oliveira DV, Teixeira MJ, de Andrade AF, de Sousa RM. Diffuse axonal injury: epidemiology, outcome and associated risk factors. Front Neurol. (2016) 7:178. doi: 10.3389/fneur.2016.00178
26. Lacroix J, Demaret P, Tucci M. Red blood cell transfusion: decision making in pediatric intensive care units. Semin Perinatol. (2012) 36:225–31. doi: 10.1053/j.semperi.2012.04.002
27. Retter A, Wyncoll D, Pearse R, Carson D, McKechnie S, Stanworth S, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol. (2013) 160:445–64. doi: 10.1111/bjh.12143
28. Figaji AA, Zwane E, Kogels M, Fieggen AG, Argent AC, Le Roux PD, et al. The effect of blood transfusion on brain oxygenation in children with severe traumatic traumatic brain injury. Pediatr Crit Care Med. (2010) 11:325–32. doi: 10.1097/PCC.0b013e3181b80a8e
29. Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK, et al. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med. (2009) 37:1074–8. doi: 10.1097/CCM.0b013e318194ad22
30. Carlson AP, Schermer CR, Lu SW. Retrospective evaluation of anemia and transfusion in traumatic brain injury. J Trauma. (2006) 61:567–71. doi: 10.1097/01.ta.0000231768.44727.a2
31. Salim A, Hadjizacharia P, DuBose J, Brown C, Inaba K, Chan L, et al. Role of anemia in traumatic brain injury. J Am Coll Surg. (2008) 207:398–406. doi: 10.1016/j.jamcollsurg.2008.03.013
32. Boutin A, Chassé M, Shemilt M, Lauzier F, Moore L, Zarychanski R, et al. Red blood cell transfusion in patients with traumatic brain injury: a systematic review and meta-analysis. Transfus Med Rev. (2016) 30:15–24. doi: 10.1016/j.tmrv.2015.08.004
33. Elterman J, Brasel K, Brown S, Bulger E, Christenson J, Kerby JD, et al. Transfusion of red blood cells in patients with a prehospital glasgow coma scale score of 8 or less and no evidence of shock is associated with worse outcomes. J Trauma Acute Care Surg. (2013) 75:8–14. doi: 10.1097/TA.0b013e318298492e
Keywords: brain injury, trauma, intracranial hemorrhage, nadir, hemoglobin, infant
Citation: Lee E-P, Zhao L-L, Hsia S-H, Lee J, Chan O-W, Lin C-Y, Su Y-T, Lin J-J and Wu H-P (2020) Clinical Significance of Nadir Hemoglobin in Predicting Neurologic Outcome in Infants With Abused Head Trauma. Front. Pediatr. 8:140. doi: 10.3389/fped.2020.00140
Received: 22 August 2019; Accepted: 11 March 2020;
Published: 03 April 2020.
Edited by:
Melania M. Bembea, Johns Hopkins University, United StatesReviewed by:
Oliver Karam, Children's Hospital of Richmond at VCU, United StatesCopyright © 2020 Lee, Zhao, Hsia, Lee, Chan, Lin, Su, Lin and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jainn-Jim Lin, bGluMDIyN0BjZ21oLm9yZy50dw==; Han-Ping Wu, YXJ0aHVyMTIyNkBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.