- 1Department of Neonatology, Children's Hospital of Soochow University, Suzhou, China
- 2Department of Intervention, The First Affiliated Hospital of Soochow University, Suzhou, China
Purpose: To explore the lung function of bronchopulmonary dysplasia (BPD) in premature infants to guide clinical prevention, early diagnosis and treatment.
Methods: Thirty infants with BPD at 4–36 months of corrected gestational age were enrolled and divided into mild BPD and moderate and severe BPD groups. Thirty full-term healthy infants, and 30 non-BPD infants at 4–36 months of corrected gestational age were included as controls. Clinical information, including respiratory infections and re-hospitalization, was compared among these groups. Furthermore, lung function analysis was performed in the infants.
Results: The upper respiratory tract infection rate and re-hospitalization rate were significantly higher in the infants with BPD than in the non-BPD infants. The tidal volume/kg, proportion of time to reach peak tidal expiratory flow/total expiratory time, tidal volume exhaled at peak tidal expiratory flow/total tidal volume in BPD group were significantly lower in the BPD group than those in non-BPD group. These values gradually decreased as the severity of BPD increased. The respiratory rate (RR) in BPD group was significantly higher than that in non-BPD group. As the severity of the BPD increased, slope of the descending branch of expiration of tidal breathing flow capacity ring (TBFVL) increased.
Conclusion: There is a correlation between the severity of BPD and a poor prognosis of respiratory system. TBFVL can directly reflect the characteristics of Tidal Pulmonary Function in children with different degrees of BPD.
Introduction
Bronchopulmonary dysplasia (BPD) is the most serious respiratory disorder that is observed in premature infants. The “old” and classic definition of BPD was first proposed by Northway et al. (1). According to this report, BPD is usually observed secondary to severe respiratory distress syndrome, and is characterized by oxygen poisoning, barotrauma, volumetric injury, and sustained oxygen for at least 28 days. Following the implementation of lung protective ventilation strategies, including prenatal glucocorticoids and postnatal pulmonary surfactants, the incidence of classic BPD has significantly reduced. Currently, the term BPD more commonly refers to light BPD (also known as “new” BPD). This definition was adopted by the National Institute of Child Health and Human Development in June of 2001 (2). Light BPD mainly occurs in the immature lung of extremely premature babies without symptoms of respiratory distress syndrome (RDS) or with mild symptoms after birth. In these cases, oxygen delivery is not usually required, or there is only a need for a low concentration of oxygen and oxygen dependence gradually appears during hospitalization. The period of continuous oxygen supply usually exceeds 36 weeks of corrected gestational age (GA). The foremost features of new BPD are delays in lung development and simplified alveolar structure. Following the development of perinatal medicine and technologies of neonatal intensive care, the incidence and survival rate of very low birth weight infants (VLBWI) and extremely low birth weight infants (ELBWI) have increased. However, although there has been a decrease in the rate that other adverse outcomes are observed in preterm infants, the rate of respiratory diseases that are associated with preterm birth has not declined.
Many studies investigating the risk factors of BPD have been reported worldwide. However, few studies have investigated lung function in BPD. In this study, the clinical characteristics, follow-up data, and lung function of premature infants with BPD, as well as non-BPD premature infants and normal lungs of health babies, who were hospitalized in the neonatology department of the Children's Hospital of Soochow University over the same period, were compared and analyzed to explore the respiratory system prognoses. The purpose of this study is to enable early detection of respiratory diseases and pulmonary dysfunction, in order to enable early intervention and treatment, helping to reduce the use of medical resources, improve the disease treatment, and the quality of life of children.
Methods
Subjects
Data of premature infants who were diagnosed with BPD and hospitalized in the Neonatology Department of The Children's Hospital of Soochow University between October 1st, 2013 and October 1st, 2017 were collected in this study. The long-term situation of these patients was then recorded between October 1, 2014 and October 2017. Some data of premature infants without BPD and full-term health babies, who were hospitalized over the same period, were collected to serve as controls. This study was approved by The Medical Ethics Committee of The Children's Hospital of Soochow University (ethical examination approval number: 2013LW001).
Observation
This study is a retrospective analysis. The infants were divided into four groups: a full-term healthy control group, a non-BPD premature infant group, a mild BPD infant group, and a moderate-to-severe BPD infant group. All of the clinical data from the infants [including the conditions of their mothers during pregnancy, the birth situation of the children, the clinical information (manifestation, symptoms, and examinations, diagnosis, and complications), and treatment measures during hospitalization] were collected, compared, and analyzed. Lung function tests and questionnaires were performed at 4–36 months of GA in the premature infant group. These tests were performed at 4–36 months in the full-term infant groups. The lung function and prognosis of the respiratory system of the infants with BPD were analyzed.
Diagnosis of BPD and Clinical Grading
The diagnosis of BPD was based on the standard of the National Institute of Child Health and Human Development (NICHD). The diagnostic criteria according to the last post-menstrual GA (PMA) (2) were as follows: (1) preterm low birth weight infants, with or without history of mechanical ventilation therapy, oxygen therapy time ≥28 days (PMA ≥ 32 weeks) or 36 weeks of corrected GA (PMA < 32 weeks) who still require oxygen therapy; (2) persistent or progressive respiratory insufficiency; (3) typical X-ray or CT findings of the lungs (such as, both lungs with enhanced texture, reduced permeability, ground glass-like, localized emphysema, or cystic changes); (4) exclusion of congenital heart disease, pneumothorax, pleural effusion and sputum. The clinical grading (2) is based on the aerobic degree of the infants whose GA <32 weeks, the corrected GA of 36 weeks or at discharge, and whose GA ≥ 32 weeks, 56 days of post birth or at the time of discharge were as follows: (i) mild: no oxygen necessary; (ii) moderate: fraction of inspired oxygen (FiO2) <30%; iii) severe: FiO2 ≥ 30% and/or continuous positive pressure ventilation or mechanical ventilation.
Exclusion Criteria
Infants with the following criteria were excluded from the study: (i) a hospitalization time <28 days, (ii) patients with central nervous system and respiratory system malformation, paralysis, or severe complex congenital heart disease (except atrial septal defect, ventricular septal defect, and patent ductus arteriosus), (iii) patients who were transferred to another hospital, died, or showed chromosomal abnormalities or hereditary metabolic diseases within 28 days after birth, due to surgical diseases or other reasons or (iv) patients whose clinical data was incomplete.
Pulmonary Function Measurement
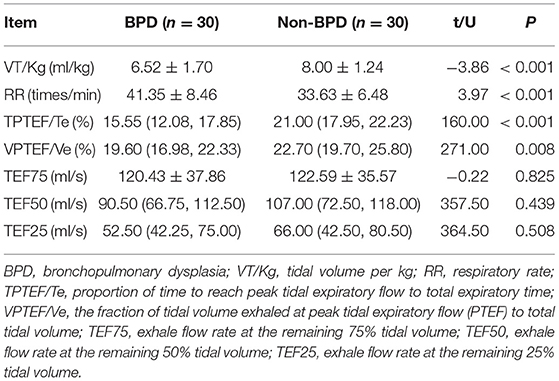
The measurement was performed by a MasterScreen PAED Lung Function Analyzer (CareFusion, Hoechberg, German) to obtain the flow signals by flow sensors, which were then integrated to obtain the volume data, thus depicting the flow-volume curve under the tidal breathing state. The indices of tidal volume per kg (VT/Kg), respiratory rate (RR), proportion of time to reach peak tidal expiratory flow to total expiratory time (TPTEF/Te), peak volume ratio (VPTEF/Ve), exhalation flow rate (TEF) at the remaining 75% tidal volume (TEF75), TEF50, and TEF25 were recorded.
Pulmonary Function Analysis
The pulmonary function test was based on the guidelines for tidal breathing and lung function produced by the Respiratory Group Pulmonary Function Cooperative Group of the Chinese Medical Association Pediatrics Branch (3). The test was performed by a designated, trained, and experienced respiratory nurse in a designated and relatively quiet lung function room. The children that were tested did not have respiratory infections in the 2 weeks prior to the analysis, were not taking oral bronchodilator drugs in the week prior to the analysis, and did not show significant abdominal distension 1–2 h after eating. Briefly, the children were sedated with 0.5 ml/kg chloral hydrate, administered orally (if necessary, an enema was used), and placed in the supine position. The necks of the children were then slightly stretched. An appropriate mask was selected to fit tightly around the nose and mouth, to ensure that there was no air leakage. The mask was then connected to the flow sensor. The flow and volume signals of the tidal breathing of the subjects were recorded in real time. Once the breath was stable, 5 consecutive recordings were made. Each recording included at least 20 tidal breathing flow-volume loops. The average value was automatically calculated as the final result by the instrument.
Statistical Analysis
Collected data were analyzed using SPSS (version 19.0) statistical software (SPSS, Chicago, IL, USA). The measurement data that met the normal distribution are presented as mean ± standard deviation (±s). Comparisons between two groups were examined using t-tests. Comparison among groups was analyzed by variance. Non-normal distribution data are expressed as 50% saturation (P50), P25, or P75. The rank sum test was employed for comparison between the two groups. The K test was applied for comparison between groups. Count data are presented as (%). Chi-square test or Fisher's exact probability method was used for comparison between the two groups (4). P < 0.05 was considered statistically significant.
Results
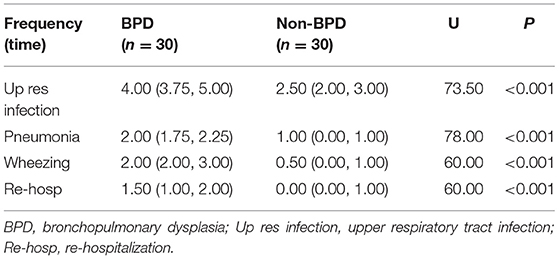
Respiratory Infections and Re-hospitalization
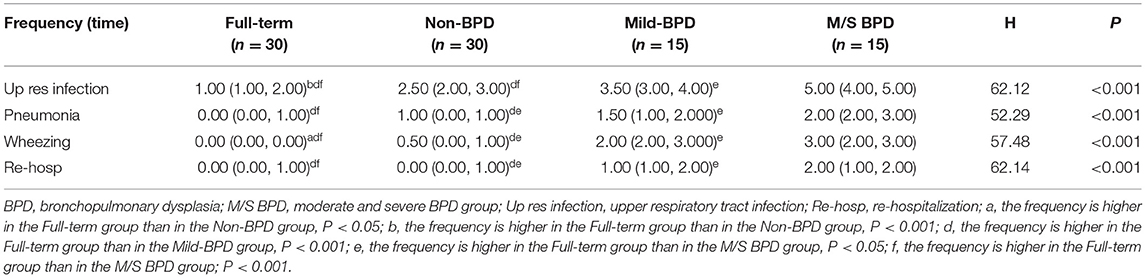
The rate of respiratory infection and re-hospitalization in infants under 1 year of age were compared the between the BPD and non-BPD groups. The frequencies of upper respiratory tract infection, pneumonia, wheezing, and re-hospitalization were significantly higher in the BPD group than those in the non-BPD group (p < 0.01; Table 1). In addition, the frequency of upper respiratory tract infection, pneumonia, and wheezing were significantly higher in the moderate/severe BPD group than in the mild BPD group. Similarly, these rates were higher in the mild BPD group than in the non-BPD group. Furthermore, the frequency of these conditions was higher in the non-BPD group than in the full term healthy controls (Table 2).
Lung Function Analysis
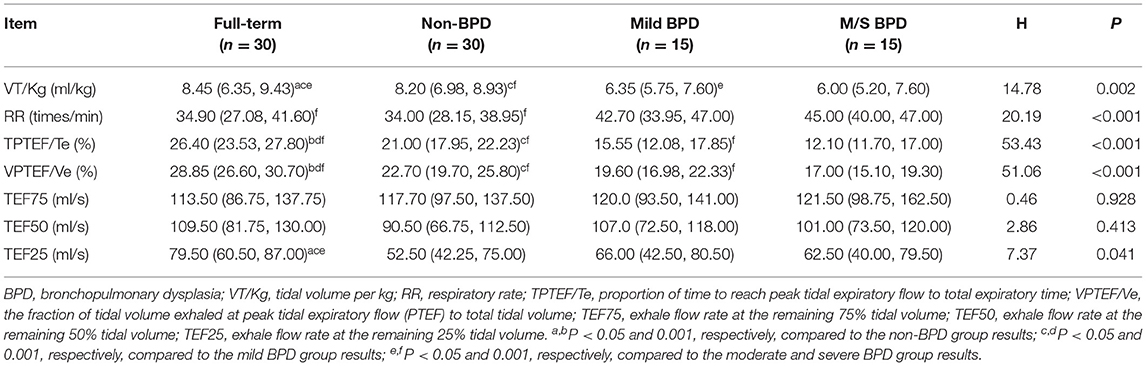
Compared with the results in the non-BPD group, the lung function parameters, including VT/Kg, TPTEF/Te, and the fraction of tidal volume exhaled at peak tidal expiratory flow to total tidal volume (VPTEF/Ve), were lower in the BPD group (p < 0.05). However, RR was higher in the non-BPD group than in the BPD group (p < 0.05). There was no significant difference in the TEF75, TEF50, or TEF25 between the two groups (p > 0.05; Table 3). The lung function analysis among the four groups indicated that the TPTEF/Te, VPTEF/Ve, and VT/Kg in the moderate and severe BPD group were lower than those in the mild BPD group (p < 0.05). These same parameters were lower in the mild BPD group than in the non-BPD group (p < 0.05). Furthermore, these parameters were lower in the non-BPD group than in the full-term healthy controls (p < 0.05; Table 4).
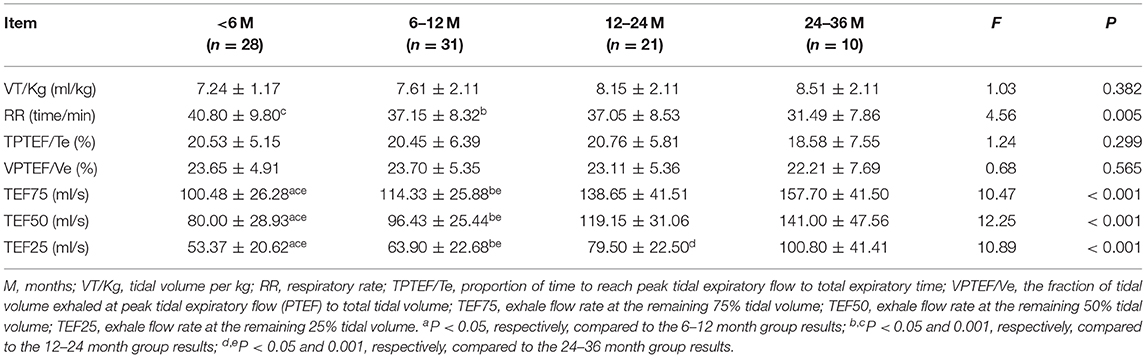
Lung Function Analysis in Different Age Groups
The lung function analysis in the different age groups indicated that the TEF75 and TEF50 in the 12–24 and 24–36 month groups were higher than those in the 6–12 and < 6 month groups (p < 0.05). The TEF75 and TEF50 in the 6–12 months below group were higher than those of the < 6-month group (P < 0.05). The TEF25 gradually increased with age (Table 5).
Tidal Breathing Flow Capacity Ring (TBFVL) Status
Taking the 4 months of corrected gestational age as an example, the inspiratory phase was relatively smooth, the peak of exhalation was advanced, and the expiratory phase was steeper in the BPD group than in the Non-BPD groups. The more severe the BPD, the steeper the descending branch and the more severe of the slope (Figure 1).
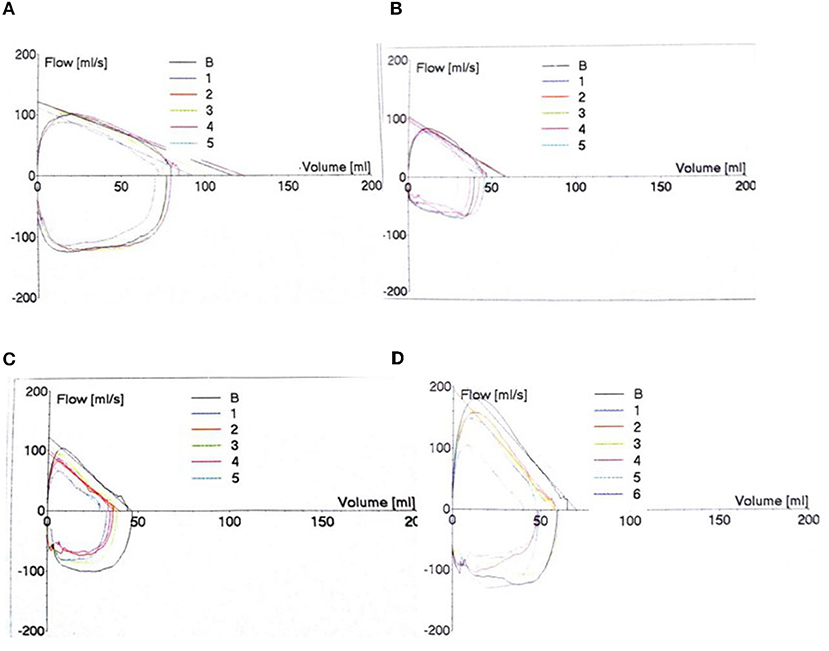
Figure 1. Tidal Breathing Flow Capacity Ring (TBFVL) Status. The changes in TBFVL were recorded in the non-BPD (A), mild (B), moderate (C) and severe (D) BPD groups.
Discussion
BPD is the most common pulmonary complication in preterm infants. This condition is associated with a variety of risk factors and multiple long-term effects (5). BPD can reduce lung function (6), physical activity, and cardiovascular function (7). These effects can be sustained into adulthood (8). Therefore, BPD has a serious impact on the public health system, due to the burden it places on medical resources. In the past decade, researchers have described the long-term sequelae of premature survivors. These studies have mainly focused on the lungs and the nervous system. However, in this field, few studies have been performed in China. Previous studies have indicated that infants with BPD have an increased risk of respiratory infections, higher rates of re-hospitalization (9) and are more prone to wheezing and recurrent wheezing (10). Abnormalities in persistent lung function are more common in infancy, and the extent of the abnormalities depends on the severity of BPD (11). The data in this study suggest that the infants with BPD had more respiratory infections, pneumonia, wheezing and a higher frequency of re-hospitalization than those in the non-BPD premature group. There was a correlation between the BPD severity and the number of infections, which is consistent with the above findings.
BPD-induced pulmonary dysfunction is a restrictive or obstructive disorder syndrome that changes over time. The restrictive dysfunction is more severe clinically. However, the obstructive dysfunction is more common (12). Due to alveolar and pulmonary microvascular developmental delay in BPD infants (13), the number of alveoli was lower than in non-BPD infants. Furthermore, BPD infants showed simplified alveolar structure, mild fibrosis of airway and surrounding tissue, and microvascular dysplasia, leading to increased pulmonary vascular resistance and pulmonary vascular remodeling (14). Colin et al. (15) reported that the high elasticity of the chest wall of the newborn resulted in a decrease in transpulmonary pressure and a reduction in the lung capacity during expiration and closed airway, resulting in a decreased flow rate. Because the elasticity of the chest wall was higher in premature infants, the decreased expiratory flow rate was more pronounced in this group. Friedrich et al. (16) believed that, due to the immature lung development in premature infants, even if the lung capacity is normal after birth, the respiratory flow rate would still be decreased. This suggests that the long-term development of the airway is delayed. Our study found that premature infants with BPD had small airway obstructions. The more severe the BPD disease, the more obvious the small airway obstruction was. Thus, in severe cases of BPD, oxygen supply would be more reliant on the increased respiratory rate for compensation to maintain normal ventilation function. The lung volume flow rate increased significantly after 1 year of age. However, the small airway obstruction was still observed. This suggests that the development of premature infants with lung dysplasia is dominated by the lung capacity development, while their airway development is delayed. All of these results are consistent with the above findings.
The tidal breathing flow volume loop can reflect the presence or absence of an obstruction in the airway, the degree and location of the obstruction, and the ventilatory function. This measurement assists in diagnosing the of nature of the pulmonary disease and the degree of lung damage, providing an objective indicator for clinical evaluation. Tidal pulmonary function is a new technology that reflects the lung function of infants and young children. The advantage of this method is that it is a safe, non-invasive, sensitive, and accurate reflection of changes in lung capacity and ventilation function. Our analysis indicates that the inspiratory phase of the BPD premature infants was relatively smooth and nearly a half elliptical shape. The exhalation curve, however, was not smooth. The peak of exhalation occurred sooner in BPD premature infants. The expiratory decline was steep. The more severe the BPD, the larger the slope of the descending branch. These findings support the work of Wei et al. (17).
This study was a small-sample study in a single center and external cohort verification was not performed. Because of time constraints, some parents did not aware the importance of early intervention, some patients with severe BPD died after they were discharged or transferred to other hospitals, resulting in fewer follow-up cases than expected. The larger the follow-up age span, the more discrete the observation indicators. In addition, we did not carry out dynamic follow-up assessment, thus, the results do not reflect the trend of dynamic alteration of the indices along with the age increase. Therefore, it would be valuable to perform further studies with a larger sample size, longer follow-up period, dynamic follow-up, and fully internal and external cohort verification.
In summary, the etiology of BPD is complex and diverse. BPD can cause abnormal lung function and seriously affect the prognosis. Therefore, clinicians should develop a preventive strategy for BPD, provide prenatal guidance, avoid the risk factors for BPD as much as possible, and establish follow-up management after discharge, systemically improving the long-term prognosis of children with BPD.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of The Children's Hospital of Soochow University (ethical examination approval number: 2013LW001). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
DC participated in study design and protocol development and writing of the manuscript. JC carried out the data analysis and interpretation of data. NC, MC, and XC participated in clinical data collection. XuZ participated in data analysis, interpretation of data and writing of the manuscript. XiZ participated in the design of the study and coordination. All authors read and approved the final manuscript.
Funding
This work was supported by the General Program of the National Natural Science Foundation of China (81771626; 81971423), Jiangsu Provincial Key Talent Program for Women's and Children's Health (FRC201731), Suzhou Science and Technology Development Program (SS201428), Diagnosis and Treatment Technology Project of Clinical Key Diseases in Suzhou Province (LCZX201612), and Minsheng Technology - Key Technology Application Research Project (SS201644).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AUC, area under the curve; BPD, bronchopulmonary dysplasia; BTT, blood transfusion times; BW, body weight; CI, confidence interval; ELBWI, extremely low birth weight infants; FiO2, fraction of inspired oxygen; GA, gestational age; nCPAP, nasal continuous positive airway pressure; NEC, necrotizing enterocolitis; NRDS, neonatal respiratory distress syndrome; OIT, oxygen inhalation time; P50, 50% saturation; PCO2, pressure carbon dioxide; PDA, patent ductus arteriosus; PEEP, positive end expiratory pressure; PIP, peak inspiratory pressure; PMA, post-menstrual gestational age; ROC, receiver operating characteristic; RF-II, type II respiratory failure; RR, respiratory rate; sd, standard deviation; TBFVL, tidal breathing flow capacity ring; TEF75, exhale flow rate at the remaining 75% tidal volume; TPTEF/Te, proportion of time to reach peak tidal expiratory flow to total expiratory time; VLBWI, very low birth weight infants; VPTEF/Ve, the fraction of tidal volume exhaled at peak tidal expiratory flow (PTEF) to total tidal volume; VT/Kg, tidal volume per kg.
References
1. Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. (1967) 276:357–68. doi: 10.1056/NEJM196702162760701
2. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. NICHD/NHLBI/ORD workshop summary. Am J Respir Crit Care Med. (2001) 163:1723–29. doi: 10.1164/ajrccm.163.7.2011060
3. Respiratory Lung Function Collaboration Group SoP, Chinese Medical Association. Children's lung function series guidelines (4th): tidal respiratory lung function. Chin J Pract Pediatr. (2016) 31:1617–21. doi: 10.3760/cma.j.issn.2095-428X.2016.21.005
4. Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. (2016) 4:91. doi: 10.21037/atm.2016.02.11
5. Katz SL, Luu TM, Nuyt AM, Lacaze T, Adamo KB, Adatia I, et al. Long-term follow-up of cardiorespiratory outcomes in children born extremely preterm: recommendations from a Canadian consensus workshop. Paediatr Child Health. (2017) 22:75–9. doi: 10.1093/pch/pxx028
6. Kotecha SJ, Edwards MO, Watkins WJ, Henderson AJ, Paranjothy S, Dunstan FD, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax. (2013) 68:760–6. doi: 10.1136/thoraxjnl-2012-203079
7. Clemm H, Roksund O, Thorsen E, Eide GE, Markestad T, Halvorsen T. Aerobic capacity and exercise performance in young people born extremely preterm. Pediatrics. (2012) 129:e97–105. doi: 10.1542/peds.2011-0326
8. Landry JS, Chan T, Lands L, Menzies D. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J. (2011) 18:265–70. doi: 10.1155/2011/547948
9. Bhandari A, McGrath-Morrow S. Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia. Semin Perinatol. (2013) 37:132–7. doi: 10.1053/j.semperi.2013.01.010
10. Fauroux B, Gouyon JB, Roze JC, Guillermet-Fromentin C, Glorieux I, Adamon L, et al. Respiratory morbidity of preterm infants of less than 33 weeks gestation without bronchopulmonary dysplasia: a 12-month follow-up of the CASTOR study cohort. Epidemiol Infect. (2014) 142:1362–74. doi: 10.1017/S0950268813001738
11. Doyle LW, Anderson PJ. Long-term outcomes of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. (2009) 14:391–5. doi: 10.1016/j.siny.2009.08.004
12. Beverley R, Young-Jee K, Jaimee H, Jim K, Margaret T, Tepper RS, et al. Pulmonary function in bronchopulmonary dysplasia. Pediatr Pulmonol. (2010) 37:236–42. doi: 10.1002/ppul.10424
13. Copland I, Post M. Lung development and fetal lung growth. Paediatr Respir Rev. (2004) 5(Suppl. A):S259–64. doi: 10.1016/S1526-0542(04)90049-8
14. Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr. (2013) 25:329–37. doi: 10.1097/MOP.0b013e328360a3f6
15. Colin AA. Infant pulmonary testing – techniques, physiological perspectives and clinical applications. Paediatr Respir Rev. (2004) 5(Suppl. A):S73–6. doi: 10.1016/S1526-0542(04)90014-0
Keywords: bronchopulmonary dysplasia (BPD), prognosis, pulmonary function, tidal breathing flow volume loop, respiratory morbidity
Citation: Chen D, Chen J, Cui N, Cui M, Chen X, Zhu XP and Zhu XL (2020) Respiratory Morbidity and Lung Function Analysis During the First 36 Months of Life in Infants With Bronchopulmonary Dysplasia (BPD). Front. Pediatr. 7:540. doi: 10.3389/fped.2019.00540
Received: 01 August 2019; Accepted: 11 December 2019;
Published: 10 January 2020.
Edited by:
Mario Barreto, Sapienza University of Rome, ItalyReviewed by:
Sotirios Fouzas, University of Patras, GreeceKaren Mestan, Ann & Robert H. Lurie Children's Hospital of Chicago, United States
Hercília Guimarães, University of Porto, Portugal
Copyright © 2020 Chen, Chen, Cui, Cui, Chen, Zhu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueping Zhu, emh1eHVlcGluZzQ2MzdAaG90bWFpbC5jb20=; Xiaoli Zhu, emh1eGlhb2xpOTBAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Dandan Chen1†
Dandan Chen1† Xueping Zhu
Xueping Zhu