
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr. , 24 October 2019
Sec. Pediatric Immunology
Volume 7 - 2019 | https://doi.org/10.3389/fped.2019.00432
This article is part of the Research Topic Pediatric Inflammatory Bowel Diseases: Looking to the Future View all 13 articles
Inflammatory bowel disease (IBD) is an idiopathic inflammatory disease characterized by chronic and relapsing manifestations. It is noteworthy that the prevalence of IBD is gradually increasing in both children and adults. Currently, the pathogenesis of IBD remains to be completely elucidated. IBD is believed to occur through interactions among genetics, environmental factors, and the gut microbiota. However, the relapsing and remitting course of IBD underlines the importance of other modifiers, such as psychological stress. Growing evidence from clinical and experimental studies suggests that stress acts as a promoting or relapsing factor for IBD. Importantly, recent studies have reported an increasing incidence of anxiety or depression in both children and adults with IBD. In this article, we review the mechanisms by which stress affects IBD, such as via impaired intestinal barrier function, disturbance of the gut microbiota, intestinal dysmotility, and immune and neuroendocrine dysfunction. With regard to both children and adults, we provide recent evidence to describe how stress can affect IBD at various stages. Furthermore, we emphasize the importance of mental healing and discuss the value of approaches targeting stress in clinical management to develop enhanced strategies for the prevention and treatment of IBD.
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC), and Crohn's disease (CD), is a chronic, relapsing, and remittent intestinal inflammatory disorder (1) affecting millions of people worldwide (2). Notably, IBD is gradually becoming a global disease with rapidly increasing incidence in emerging industrial countries in the twenty-first century (3). Although IBD can occur at any age, ~25% of patients are diagnosed with IBD before 20 years of age (4). The incidence of IBD in children varies among different countries, but the overall trend is increasing globally. The incidence is about 0.5–23/1,00,000 for IBD, 0.1 to 13.9/1,00,000 for CD, 0.3 to 15.0/1,00,000 for UC (3, 5–7). In addition to the common gastrointestinal symptoms (abdominal pain, diarrhea, hematochezia, and weight loss) similar to those of adults, children may present with unique manifestations, including poor growth and delayed puberty (8). IBD is considered to be an immune-mediated intestinal disorder, resulting from complex interactions among genetics, environmental factors, and gut microbiota (9). Various factors, such as genetic transmission, intestinal immune disruption, gut microbiota disturbance, diet, infection, lifestyle, psychological stress, sleep disorders, smoking, and early life exposure to antibiotics, have been found to influence the progress of IBD on the basis of studies in recent decades (10, 11). However, the exact pathophysiological mechanism of IBD remains to be inadequately understood. Its complex and multifactorial pathogenesis, severity of symptoms, uncertainty of the condition and prognosis, and adverse reactions to medication and cancer risk bring multiple challenges to the cure of IBD. Owing to these challenges, patients' quality of life may be significantly affected, particularly by increasing psychosocial burdens and inducing psychological disorders. For children and adolescents, IBD can even threaten the healthy psychosocial development.
Stress may cause abnormalities of behavior and/or mentality, such as anxiety and depression, and also influence the function of visceral organs, especially the digestive system. Psychological comorbidities, especially depression, have similar pathophysiological mechanisms to IBD. Pro-inflammatory cytokines and plasma acute phase protein C increased in depression patients (12). Elevated levels of malondialdehyde, a fatty acid peroxide, in the serum of depressed patients suggests that mental disorders may be associated with oxidation and oxidative stress (13). Meanwhile, autoimmune changes and bacterial translocations have also been observed in depression (14, 15). Thus, depression and IBD share a common pathway that seems to explain the interaction between the two diseases. In recent years, a growing number of studies have indicated that the prevalence of mental disorders in both children and adults with IBD is higher than that of healthy people (16). Significant progress has been made in elucidating the pathophysiological mechanisms of IBD, which indicates that stress is closely correlated with IBD. Accumulating evidence suggests that there is a bidirectional influence between IBD and stress. The underlying mechanisms consist of immune dysfunction, intestinal microbiota disturbance, impaired intestinal barrier function, and neuroendocrine system alterations (17). In addition, whenever stress occurs, whether in early life or adulthood, the development of IBD can be affected to some extent.
This paper provides an overview of recent literature focusing on the connection between stress and IBD in children and adults. Both experimental and clinical evidence illustrating the importance of stress in the pathogenesis of IBD is presented in this review. Finally, insights into comprehensive approaches for managing IBD and potential therapeutic implications of psychological interventions are provided.
More than 80 years ago, Hungarian endocrinologist Hans Hugo Bruno Selye first defined the medical term “stress” as the physiological adaptive responses of organisms to adverse threats (stressors), which are endogenous or exogenous, psychological or physical, real or perceived (18). To maintain homeostasis under threat, organisms have evolved an extremely complex system, called the stress system, which involves physiological and behavioral adaptations via appropriate central and peripheral neuroendocrine responses. When exposed to long-term or severe stress, the organisms may reach a state called cacostasis, in which many vital physiological functions are impaired, and may develop many acute and chronic diseases (19). Stress-induced disorders occur in multiple systems throughout the body, among which the gastrointestinal tract is a sensitive system.
When the brain receives stress input, multiple pathways containing the autonomic nervous system and hypothalamic-pituitary-adrenal axis (HPA axis) are activated (20). Stress from different sources results in modifications of the brain-gut axis, which eventually leads to the progression of a wide range of gastrointestinal disorders. The frequently involved diseases include IBD, irritable bowel syndrome (IBS), peptic ulcers, food antigen allergic reactions, and gastroesophageal reflux disease. The potential mechanisms are summarized in the following sections (Figure 1).
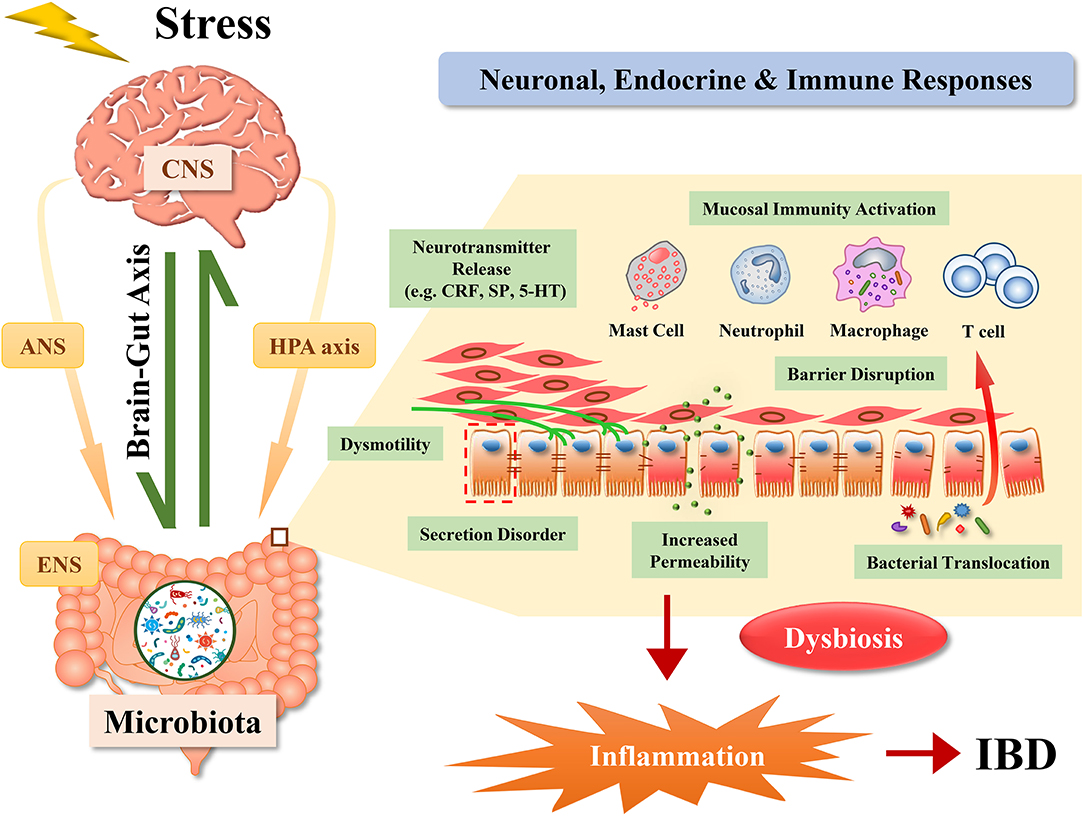
Figure 1. Pathways by which stress affects inflammatory bowel disease. Effects of stress on the gut involve comprehensive reactions among neuronal, endocrine, and immune systems. Stress induces the activation of the brain-gut axis, the hypothalamic-pituitary-adrenal axis (HPA axis), the autonomic nervous system (ANS) and the enteric nervous system (ENS), contributing to the development of inflammatory bowel disease (IBD) via dysbiosis, alterations of secretion and mobility, disruption of the intestinal barrier and the release of inflammatory mediators. CNS, central nervous system; CRF, corticotrophin releasing factor; SP, substance P; 5-HT, 5-hydroxytryptamine.
Corticotrophin-releasing factor (CRF) is considered to be a pivotal component in the HPA axis. It is produced by central and peripheral tissues in response to stress. CRF plays an important role in translating stimuli into physical responses in the brain (21). Stress directly activates the hypothalamus (mainly paraventricular nucleus of the hypothalamus system) to release CRF, inducing the anterior pituitary gland to secrete adrenocorticotrophic hormone, which further stimulates the adrenal cortex to secrete cortisol. Ultimately, cortisol acts on all tissues in the body via the circulation of blood. CRF receptors, as well as their ligands, which can be modulated by stress, are expressed in the gut as well as in the brain (22).
CRF acts on enteric peristalsis, secretion, and the mucosal barrier, playing a role in functional and organic disorders, such as IBS and IBD (23). In experimental animal studies, CRF has the opposite effect on upper and lower digestive transit, such as gastric emptying inhibition, reduction of small intestinal transit and increase of colonic transit and defecation (24, 25). In addition, CRF can induce mast cell degranulation and increase mucosal permeability (26), which is the key mechanism of intestinal disorders. Blocking CRF restrains the development of IBD by inhibiting mast cell degranulation and reducing tumor necrosis factor-alpha (TNF-α) and protease production (27).
The results from clinical studies are consistent with the above findings. In one study, healthy volunteers were asked to give a public speech to induce psychological pressure. The pressure induced by public speaking enhanced the permeability of the gut only in subjects with significantly elevated cortisol levels, suggesting that HPA axis activation is affected. Furthermore, peripheral injections of CRF were administered to reproduce stress-induced disorder. The results showed that exogenous CRF, as well as psychological stress, can increase the ratio of urine excretion rates of milk fructose and mannitol, showing signs of increased permeability in the small intestine. Furthermore, the mechanism appears to rely on intestinal mast cells because it can be abrogated by a mast cell stabilizer (28).
During the stress-induced changes of the gut, effectors cells, including mast cells (28), neutrophils (29) and lymphocytes, as well as pro-inflammatory cytokines, are placed in a pivotal position. Mast cells play an important role in the transmission of stress signals to the gut. Animal experiments have demonstrated that stress can damage the gut barrier function in a mast cell-dependent manner, which may facilitate the development of IBD (30, 31). In wild-type rats, chronic stress can induce intestinal barrier dysfunction, inflammatory cell infiltration, ultrastructural changes in epithelial cells, and mast cell proliferation and activation. In contrast, the intestinal epithelial function and morphology are not damaged in mast cell-deficient rats, and there is no evidence of inflammatory cell infiltration, which highlights the regulatory role played by mast cells (32).
The sympathetic and parasympathetic autonomic nervous systems serve the entire gastrointestinal tract and are closely connected with the enteric nervous system (ENS). Together, these systems govern secretion, motility, sphincter control, and microcirculation in the gut (33). Under stress conditions, the ENS produces large neuropeptides, which in turn affect intestinal immunity and inflammation. Geboes et al. found that there are mixed abnormalities in all CD and UC patients for different cell types of the ENS (34). Another study showed that patients with UC have markedly lower autonomic functions in comparison to those with CD and healthy controls (35).
Stress can activate the sympathetic autonomic system, leading to increased production of major adrenal medulla hormones, mainly catecholamines, such as epinephrine and norepinephrine. Catecholamines mediate increases of central and peripheral inflammatory cytokines and activation of the inflammatory nuclear factor κB signaling pathway in response to stress (36). In addition, the vagus nerve, which has anti-inflammatory effects, is inhibited by stress, leading to an increased systemic inflammatory response to endotoxin and intestinal inflammation (37, 38).
In addition, changes in tissue levels of neurotransmitters have been demonstrated in patients with IBD. Stress can also affect the follicle-associated epithelial barrier via vasoactive intestinal polypeptide (VIP) and its receptor on mucosal mast cells. These findings highlight an important effect of VIP-bacterial-epithelial interactions on regulating intestinal barrier function (39). In a mouse model of chronic restraint stress, substance P (SP) and its receptors enhanced CRH expression and release in eosinophils, resulting in epithelial barrier dysfunction mediated by mast cells (40). Another study revealed that water avoidance stress (WAS)-induced colonic hypermotility is probably dependent on the upregulation of the neurokinin-1 receptor (NK1R) in the colon and increased serum SP levels, suggesting a potential mechanism for diarrhea in IBD patients with anxiety or depression (41).
The effects of the gut microbiota on IBD have attracted much attention in the last decade. Microbiota communicates with the brain-gut axis through mucosal cells, immune cell, and neural endings (42). Published data from animal and clinical studies indicate that stress causes dysbiosis. Stress-induced dysbiosis is characterized by a decrease in the abundance of Lactobacillus and aggravated bacterial translocation. Notably, reduced Lactobacillus abundance contributes to opportunistic infections of Campylobacter jejuni and Shigella flexneri in monkeys (43). The gut microbiota of male mice exposed to chronic social defeat is characterized by reduced richness and diversity. The predicted functional profile shows reduced functional diversity. In particular, the lower prevalence of pathways involved in the synthesis and metabolism of short-chain fatty acids and neurotransmitter precursors has been described (44). A study showed that exposure to stress inhibits the NOD-like receptor, pyrin domain containing (NLRP)-6 inflammasome, altering the constitution of the gut flora, thus leading to inflammation of the intestine. Interestingly, transmissible intestinal inflammation, accompanied by upregulated CRF and reduced NLRP6, was observed after the mice were cohoused (45).
Stress can also break the established tolerance and augments immune responses in chronic intestinal inflammation. Increased intestinal permeability caused by stress allows microbiota to cross the gut epithelial barrier to trigger the mucosal immune reactions (42) and then transfer to secondary lymphoid organs (46) to activate the innate immune system. A recent study based on a dextran sulfate sodium (DSS)-induced colitis model provided evidence that chronic stress increases sensitivity to colitis via dysbiosis and immune system dysfunction. Under chronic stress conditions, the colonic lamina propria showed B cell, neutrophil, and pro-inflammatory ly6Chi macrophage infiltration. Mesenteric lymph node (MLN) changes were also discovered with a significant change in the proportion of MLN-associated immune cells. The results of this study further showed marked activation of IL-6/STAT3 signaling in response to stress. Interestingly, the detrimental effects of stress were not terminated in IL-6−/− mice, indicating that the hyperinflammatory response is not the real culprit. In contrast, when the intestinal microbiota was shared by cohousing or was destroyed by antibiotics, the severity of DSS-induced colitis was indistinguishable between the stressed and control groups, unequivocally suggesting that the gut microbiota is responsible for the deleterious effects of stress. In general, stress disturbs the gut microbiota, triggers immune system dysfunction and facilitates DSS-induced colitis (47). A novel phenomenon has been revealed, showing that stress restrains the suppressive action of intestinal regulatory T cells (Tregs), instead of changing their quantity. It was found that prolactin, a stress-related mediator, can transform the phenotypes of intestinal Tregs, thus contributing to intestinal inflammation (48).
It has been shown that stress-induced flora disturbance has a vital impact on IBD by influencing host-microbiota crosstalk and regulating the neuro-immune-endocrine system (49–51). There is a complex network among the gut microbial landscape, immune system and nervous system. Microbiota-targeted therapies have been highlighted as a novel approach to treat systemic inflammation diseases, such as IBD, multiple sclerosis, systemic inflammatory arthritis, and asthma (52). Generalized microbiota-targeted therapies include antibiotics, antibacterial conjugate vaccines, probiotics, fecal microbiota transplants (FMTs) and other interventions that alter the community composition (53). In conclusion, these therapies might be beneficial to both physical and psychological recovery in IBD patients.
In the 1950s, IBD was considered a psychosomatic disorder (54), and previous studies have demonstrated a close association between IBD and stress. Specifically, IBD patients are often exposed to stress, which induces mood swings or even leads to mental complications. Meanwhile, increased emotional disorders can exacerbate symptoms such as abdominal pain, and can enhance the severity of IBD in turn.
Most clinical studies have shown that mood disorders are associated with an increased risk of a variety of chronic diseases, such as IBD, arthritis, asthma, and diabetes mellitus (55, 56). In an IBD cohort, patients exhibited a high incidence of psychological distress and comorbidities, including depression, anxiety disorders, and bipolar disorder (57). Research from Canada examined the prevalence of depression in two typical surveys in a large sample. Statistical data indicated that the 12-month depression incidence rates of people with IBD and similar intestinal disorders in the survey mentioned above were 14.7 and 16.3%, respectively. Furthermore, IBD patients showed a three-fold higher incidence of depression than healthy people (58). In the National Health and Nutrition Examination Survey (NHANES) of Americans, the relationship between IBD and depression was examined. In this big data study, IBD hallmarked by chronic and recurrent disease, was found to act as an independent risk factor for depression (59). The Canadian Community Health Survey in 2012 reported that IBD is strongly associated with generalized anxiety disorder. Generalized anxiety disorder was identified by the WHO-CIDI lifetime criteria. The results revealed that IBD patients were prone to generalized anxiety with a two-fold increased incidence (60). Neuendorf et al. screened 171 articles, including a total of 158,371 participants, to conduct a comprehensive systematic review. The findings showed that 35% of IBD patients develop anxiety symptoms and 21% develop anxiety disorders; 22% of IBD patients develop depression symptoms; and 15% develop a depressive disorder. Furthermore, this study pointed out that this condition is more prevalent during the active period of the disease (61).
With the objective of exploring the bidirectional relationship, Sexton et al. assessed symptom activity, intestinal inflammation, and perceived stress using the Manitoba IBD Index, fecal calprotectin in the stool, and Cohen's Perceived Stress Scale at months 0, 3, and 6. Perceived stress at month 0 was found to be positively correlated with disease activity at months 3 and 6 in both UC and CD. Nevertheless, no correlation between intestinal inflammation, evaluated by fecal calprotectin and perceived stress, was found (62).
A total of 403,665 patients with depression and 5323,986 people without a history of depression were followed up for an average of 6.7 years. A total of 0.05% of the depression cohort developed CD, while 0.03% of individuals in the non-depression cohort developed CD. Furthermore, 0.13% of patients in the depression cohort developed UC, and only 0.09% of individuals in the non-depression cohort developed UC. Compared with the non-depression cohort, the unadjusted hazards of CD and UC in the depression cohort increased by 67 and 41%, respectively. After adjusting for various confounding factors, the risk of developing IBD remained significantly increased in the depression cohort (63).
Despite the limitation of inclusion age criteria for adolescent subjects, the phenomenon that adolescents with IBD have a higher prevalence of anxiety and depression symptoms can still be concluded (64, 65). According to parental reports, emotional problems, including anxious/depressed mood and withdrawn/depressed mood, appear to be more common in adolescents with IBD than National Health and Nutrition Examination in population-based controls. Both parental and self-reported psychosocial symptoms are related to the increased severity of self-perceived IBD symptoms (66). The incidence of mental illnesses, especially depression, among young people with IBD is increasing (67). In a prospective study of 121 patients with IBD aged 16–21 years, 55% reported increased anxiety/depression symptoms and 83% had a reduced quality of life compared with the baseline (68). A study including 374 IBD patients from the Netherlands found elevated symptoms of psychological comorbidities in both adolescents (10–17 years) and young adults (18–25 years), but there was no difference (64). A Swedish study found that IBD children who were identified with younger than 18 years had a three-fold increased hazard ratio for death in adulthood compared to children in the general population. The highest estimated risk of overall mortality was higher in UC patients than in CD patients (69).
Similar phenomena have been observed in animal experiments. Depressive- and anxiety-like behaviors were found in mice with dinitrobenzene sulfonic acid (DNBS)-induced colitis. Upregulated expression of inflammatory genes and mitochondrial dysfunction in the hippocampus might be responsible for the abnormal mouse behaviors (70). Recent studies have shown that mice with chronic colitis exhibit increased anxiety-related behaviors in open-field and acoustic stress tests, accompanied by visceral hypersensitivity and low levels of intestinal inflammation (71).
Overall, IBD patients are more prone to developing emotional disorders than the general population. In addition, depression and anxiety have adverse effects on the course of the disease. Psychological comorbidity and IBD seem to fall into a vicious circle.
Life always includes stresses which change over time. In adulthood, stress mainly originates from family, work, economic status, and major life-threatening events. Early life and childhood exposure to antibiotics, vaccination, diet, smoke, and psychosocial stress seems to lead to a long-term adverse influence throughout life. The stressors of the above different periods may increase adulthood susceptibility to diabetes, cardiovascular disease, autoimmune disease, stroke, and certain cancers (72–74).
A Manitoba IBD cohort study in Canada ascertained the first onset of psychotic symptoms via a structured diagnostic interview. The report showed that approximately two-thirds of patients who had both anxiety disorder and IBD actually developed psychiatric symptoms predating the IBD diagnosis by over 2 years. This more than 2-year time interval for diagnosis was also present in more than half of IBD patients with mood disorders. Moreover, IBD patients with lifelong anxiety or mood disorders displayed an earlier onset of IBD symptoms than those without the above disorders, and there was a tendency for an early diagnosis of IBD (75). These results reminded us of the potential interactions between IBD and psychiatric diseases. It is possible that the existence of these psychiatric illnesses may increase the susceptibility of individuals to IBD.
Recent studies have focused on the long-term effects of early life adversity on the immune system, including impaired cellular immunity, increased inflammation, and accelerated immunosenescence (76, 77). An animal experiment showed that early- life stress results in an altered microbiota and increased visceral sensation and psychiatric illnesses (78). A recent study found that nerve growth factor (NGF)-mediated tropomyosin receptor kinase A (TrkA) signaling mediates bowel dysfunctions that resemble IBS induced by neonatal maternal separation (79). Moreover, there are sex differences in the effects of early life adversity on gut microbiota and emotional behaviors (80). Parental separation in childhood can lead to psychological distress in adulthood to varying degrees. The adverse impact caused by this abnormal family pattern contributes to the development of IBD in adulthood (81). Researchers analyzed the relationship between the annual rhythm of IBD symptom onset and academic semesters in children. The results showed that academic stress may facilitate disease onset in pediatric IBD (82).
In animal models, there seem to be different views as to whether early stress increases the incidence of IBD, which may be related to different patterns and periods of stress (83–85).
Animals exposed to WAS developed acute small intestinal inflammation as evaluated by histological scores in an experimental study. Leukocytic infiltration, intestinal hyperpermeability, increased serum TNF-α, and upregulated IL-17 and IL-6 expression in mucosa have also been discovered during stress (45). In addition, acoustic stress has been found to cause severe enteritis in the healthy intestinal tract (86). Chronic stress can cause excessive growth of pro-inflammatory bacteria and thus induce increased susceptibility to colitis in subjects after fecal microbiota transplant. Stress is known to cause low-grade intestinal inflammation via increased bacterial translocation and the production of poisons (87).
Stress causes wide-ranging effects on patients with IBD, especially the recurrence and aggravation of the disease. Some studies have shown that high perceived stress has a bearing on the frequency of symptomatic flares (88). A systematic review of 15 high-quality studies arrived at the conclusion that emotions are associated with abdominal pain symptoms in IBD patients. Among IBD patients, depression, anxiety, and perceived stress are common emotional disorders (89). Although the symptoms become distinctly intensified, IBD activity evaluated by the fecal calprotectin level may not be apparent during perceived stress (90). In contrast, a German cross-sectional study including 1,032 IBD patients, revealed that relevant reported depressive symptoms correlate with increased rates of disease activity (91). In addition to exacerbating symptoms, stress can also lead to relapse in IBD patients (92). Moreover, in a prospective longitudinal study, 60 patients with quiescent IBD were followed-up for up to 18 months. The baseline depression score was found to be connected with the first recrudescence time. In particular, patients with anxiety appeared to have an increased recurrence frequency (93). A multicenter cohort study in Germany found that patients with CD were more likely to be affected by psychological disorders than those with UC or controls. Compared with the healthy controls, both UC and CD patients scored higher on psychological disorders and maladaptive stress coping tests during the active phase. Interestingly, UC patients in remission were minimally affected by psychological disorders, while CD patients in remission showed insecurity and paranoid ideation. The neuroticism score of CD patients was found to be higher than that of the healthy controls, while that of UC patients was not (94). Another study also showed that CD patients with depression were more likely to deteriorate than UC patients with depression (95). In addition, a prospective study found that depression increased the risk of CD, rather than UC, in women (96). A study in Switzerland recruited 468 adults with CD, who were followed-up for 18 months. The results of the study showed that, among those who were under perceived stress, patients with anxiety and depression were more likely to develop worsening of the disease, indicating the significance of emotional elements (97).
Several recent studies have identified factors beyond disease activity associated with pain burden at birth. Family stressors, such as divorce and loss of family members, were found to increase pain-related distress in children by influencing coping and depression symptoms (98). Thirteen percent of children with CD still suffer from abdominal pain despite clinical remission (99).
Constraint stress has been reported to aggravate spontaneous colitis in IL-10−/− mice compared to those without stress (100). Another study demonstrated that neonatal maternal separation induces disruption of the colon barrier and exacerbates colitis symptoms in adult IL-10−/− mice (83). Moreover, a recent study revealed that chronic stress damages the gut microbiota and increases susceptibility to DSS-induced colitis in mice. The downregulated expression of mucin-2 and lysozyme caused by stress is implicated in the disturbance of the microbiota (47). Additionally, 12 weeks of WAS significantly increased the relative abundance of the Clostridium genus, which produces the toxin phospholipase C in C57BL/6 mice. WAS has also been proven to change the concentration of luminal secreted immunoglobulin A, which is probably connected to gut microbiota alterations and colitis-associated deterioration (51).
Psychosocial dysfunction has a negative effect on the treatment of IBD in adults. Psychosocial dysfunction causes adverse effects on the quality of life in IBD patients. IBD patients with psychological comorbidities seem to have more hospitalizations than those without (101). The Inflammatory Bowel Disease Questionnaire (IBDQ), which involves bowel symptoms (bowel movements and abdominal pain), systemic symptoms, and emotional and social factors, was applied to assess the health-related quality of life (HRQOL) of IBD patients. The data showed that an increased level of perceived stress, as judged by a 10-item Perceived Stress Scale, is one of the most predictive factors of reduced HRQOL (102). It has been confirmed that psychological symptoms, perceived stress, and disease severity can have a deleterious effect on HRQOL (103, 104). An IBD cohort study in Boston, including 5,405 CD and 5,429 UC patients, found that CD patients with emotional or anxiety disorders had a 28% increase in the risk of surgery compared to those without psychosocial disorders (105).
A recent study found that stress can inhibit endogenous opioids and can switch their signaling in dorsal root ganglion neurons from inhibition to excitation during chronic colitis, causing exacerbated pain and requiring increased doses of opioid analgesics in IBD patients (106). For adolescent patients with IBD, medication non-adherence is regarded as a major health care problem. A systematic review suggested that psychosocial factors, including poor child-coping strategies, family dysfunction, anxiety, and depressive symptoms, are relevant to medication non-adherence, which may lead to an unnecessary escalation in treatment and can jeopardize IBD therapy outcomes (107).
Due to the bidirectional effect between stress and IBD, patients may fall into a vicious cycle, leading to a poor prognosis. Therefore, attention should be paid to the role of stress therapy in the management of IBD.
According to the present clinical practice guidelines for IBD, therapeutic interventions mainly involve 5-aminosalicylic acid (ASA), corticosteroids, immunomodulators, antibiotics, probiotics, and anti-TNF agents. These traditional treatments can effectively relieve symptoms and promote mucosal healing (108). However, with the in-depth study of the adverse effects of stress on IBD, mental healing seems to be the ultimate treatment goal of IBD and is expected to surpass mucosal healing. Relieving psychological stress is of great benefit to improving symptoms and increasing quality of life. The emerging field of psychogastroenterology focuses on the application of brain-gut psychotherapies, which are considered to be an integral part of the management of digestive diseases (109). Although psychotherapy has limitations, it should be considered to be one of the therapeutic strategies for IBD.
Pain symptoms in IBD patients appear to be associated with inflammation of enteric neurons (110). Enteric neurological abnormalities, including glial cell hyperplasia, and hypertrophy, were more pronounced in CD patients than UC patients (111), and appear to be associated with worsening symptoms and prognosis in CD patients. Intestinal inflammation may induce visceral hypersensitivity through the peripheral and central systems (112). Antidepressants are beneficial for relieving chronic pain, especially in patients with emotional disorders (113). Studies have demonstrated that a high percentage of IBD patients are treated with psychotropic drugs (114), with ~30% of patients taking antidepressants (115). Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) are the most common drugs used for the treatment of IBD patients with emotional complications, especially anxiety and depression (116). TCAs has been proven to have anti-inflammatory effects in animal intestines. Meanwhile, TCAs seem to relieve severe pain in IBD patients, even at low doses. However, TCAs can also cause side effects, such as dry mouth, blurred vision, and constipation, especially at high doses. These side effects usually recede after a few weeks. Tetracyclic antidepressants are beneficial for patients affected by sleep disorders and pain, but they have not been trialed in IBD patients (117). Moreover, propranolol, a β1-adrenoreceptor/β2-adrenoreceptor inhibitor, has been proven to suppress neutrophil infiltration in the colon and to attenuate tissue injuries caused by chronic stress, suggesting a potential therapeutic value of neuroprotectants, which guard against the recurrence of IBD by inhibiting immune activation (29).
Nondrug psychological interventions for IBD include cognitive behavioral therapy (CBT), medical hypnosis, and mindfulness meditation. Some studies have shown that these methods reduce gastrointestinal symptoms in IBD patients (117, 118).
IBD-specific CBT is helpful in promoting a higher quality of life and reducing anxiety and depression in IBD patients who have a low level of HRQOL (119). In addition, a benchmark study found that CBT may help IBD patients with moderate to severe mood disorders (120). Moser G used gut-directed hypnotherapy (GHT) in the treatment of IBD. The results showed that GHT may prolong the remission duration of patients with inactive UC (121). Clinical hypnosis has been used to guide adolescents to cope with various diseases. Hypnosis can effectively relieve chronic abdominal pain in adolescents with IBD (122). Current mindfulness therapies include mindfulness stress reduction and mindfulness behavioral cognitive therapy, most of which are used in adults and show effectiveness in patients suffering from IBD. Furthermore, the physical and mental intervention of the Breath-Body-Mind Workshop (BBMW) were found to be beneficial for IBD patients for the alleviation of symptoms and emotional disorders (123). As an accepted decompression method, yoga appears to be a safe and efficacious method for the treatment of UC patients (124). In a study of adolescent IBD patients, yoga was found to be an effective complementary therapy. Unfortunately, this was a short survey with a small sample size (31).
A randomized controlled trial showed that psychotherapy (psychoeducation, problem-solving, and relaxation) for patients with IBD did not inhibit disease progression or relapse but enhanced the quality of life (125). A parallel group, randomized and controlled trial evaluated the effectiveness of a disease-specific CBT protocol on anxiety, depressive symptoms and HRQOL in adolescents and young adults with IBD. The preliminary results showed that IBD-specific CBT added to standard medical care did not perform better than standard medical care alone in improving psychological symptoms or HRQOL in youths with IBD (126).
With the clear evidence of gut dysbiosis in IBD, novel treatments will doubtless require a microbiota-modulating approach (127). This has been an active field of research, with mixed results.
Although exposure to antibiotics is considered to be a potential risk factor for IBD (11, 128, 129), several meta-analyses have revealed that antibiotics are effective in inducing remission and treating flares in patients with IBD (130, 131). Antibiotic therapy remains controversial, especially considering the current mixed results and the potential risks of systemic adverse events and bacterial antibiotic resistance (132, 133). Rifaximin, a non-systemic bactericidal antibiotic, may be therapeutically beneficial for IBD (134). A study found that Lactobacillus species were significantly enriched after oral administration of rifaximin. Moreover, rifaximin treatment protected against the intestinal inflammation, barrier damage, and visceral hypersensitivity caused by chronic water avoidance and repeat restraint stressors in Wistar rats (135).
Supplementation with prebiotics and probiotics is favorable for reducing stress-related behavior and HPA activation. Probiotics such as Bifidobacterium and Lactobacillus can alleviate anxiety and depression (136). Arase et al. studied microbiota-targeted therapies and found that a probiotic Lactobacillus strain can assist in protecting against enteritis aggravated by stress (137). Animal studies have found that Bifidobacterium P122, Lactobacillus LA804, and Lactobacillus Switzerland are beneficial in colitis (138). B. longum 536 alleviates the symptoms of patients with mild to moderately active UC (139). However, a recent study found that the B. breve strain in Yakult did not delay relapse time, compared to a matched placebo in patients with inactive UC. This result may be related to a deficiency in the amount of B. breve (140). Most studies have suggested that probiotics are beneficial for IBD patients. The effectiveness and safety of probiotics for alleviating intestinal inflammation in patients with IBD needs more exploration (141).
FMT has become a helpful and increasingly available therapy because of stool banks (142). Randomized controlled studies have indicated that FMT appears to be somewhat effective in the treatment of UC (143, 144). In a prospective trial, 21 children with a median age of 12 years, with IBD refractory to medical treatment, were subjected to a single FMT. Clinical responses were observed in 57 and 28% of the patients at 1 and 6 months after FMT, respectively (145). Paramsothy et al. performed a systematic review and meta-analysis to assess the effectiveness and safety of FMT in IBD. A total of 53 studies (41 in UC, 11 in CD, 4 in pouchitis) published before January 2017 were included. The results showed that the rates of clinical remission in UC, CD, and pouchitis were 36, 50.5, and 21.5%, respectively. Sub-analyses suggested that remission in UC improved with lower gastrointestinal tract administration and an increased number of FMT infusions (146). However, some researchers did not find significant differences in the efficacy of FMT, which may be related to the number and cycle of enemas, preparation process, and limited numbers (147, 148). More large-scale studies evaluating the safety and efficacy of FMT for IBD patients would be a further promising direction.
Rooks et al. demonstrated that genetic inactivation of quorum-sensing Escherichia coli regulator C (QseC) can reduce the virulence and colonization capacity in a pathogenic, IBD-associated E. coli strain. Further results indicated that biochemical inhibition of QseC can reduce intestinal inflammation in a variety of preclinical IBD models, and provides a new approach for the treatment of colitis (149). Additionally, dietary interventions that modulate the interaction between the immune system and microbiota can also be an option for the treatment of IBD (150).
Studies of the efficacies of psychotherapy and psychopharmacological treatments in patients with IBD are controversial and limited. A systematic literature review has shown that 1/3 of the included 43 studies supported the effectiveness of psychotherapy on the quality of life and disease activity (113). More research is needed to validate this result. As an adjuvant therapy, stress management cannot completely replace drugs. In addition, the study of the gut microbiome and dietary therapy may be future directions for the treatment of IBD.
Sufficient evidence has indicated that the incidence of psychiatric comorbidities in patients with IBD is higher than that in healthy controls. In turn, these comorbidities exacerbate IBD symptoms and promote intestinal inflammation. The underlying mechanisms may involve alterations of the neuroendocrine system and the brain-gut-microbiota axis. However, the exact mechanisms underlying mucosal immune activation remain to be explored. The effects of psychological stress on IBD should be emphasized, especially in children and adolescents, because of the unique psychological problems encountered in the pediatric population. Thus far, some clinical data support the view that stress management, such as through relaxation exercises, is beneficial to IBD patients, particularly those who are refractory. The psychological score may act as a new measure to evaluate the severity and prognosis of IBD. In the course of future IBD treatment, emotional management, stress release, the use of psychotropic drugs, and care from family needs to be emphasized. Additionally, it should be highlighted that the combination of mucosal and psychological healing as the ultimate goal of therapy will improve the prognosis. The management of IBD goes far beyond traditional drugs and surgical treatment. What is more important is the practice of psychogastroenterology, which appears to be promising.
YS and LL wrote the manuscript. HC, RX, KJ, and BW designed the review. YS, LL, and RX participated in the literature search. YS and RX designed and created the figure. HC made critical revisions. All authors read the manuscript and ultimately approved the article.
This study was supported by grants 81741075 and 81570478 from the National Natural Science Foundation of China and the grant 17JCYBJC24900 and Tianjin Research Program of Application Foundation and Advanced Technology of China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. (2007) 369:1627–40. doi: 10.1016/S0140-6736(07)60750-8
2. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. (2017) 152:313–21.e2. doi: 10.1053/j.gastro.2016.10.020
3. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2018) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
4. Baldassano RN, Piccoli DA. Inflammatory bowel disease in pediatric and adolescent patients. Gastroenterol Clin North Am. (1999) 28:445–58. doi: 10.1016/S0889-8553(05)70064–9
5. Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. (2018) 24:2741–63. doi: 10.3748/wjg.v24.i25.2741
6. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. (2011) 17:423–39. doi: 10.1002/ibd.21349
7. Abraham BP, Mehta S, El-Serag HB. Natural history of pediatric-onset inflammatory bowel disease: a systematic review. J Clin Gastroenterol. (2012) 46:581–9. doi: 10.1097/MCG.0b013e318247c32f
8. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. (2015) 169:1053–60. doi: 10.1001/jamapediatrics.2015.1982
9. de Souza HSP, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. (2017) 14:739–49. doi: 10.1038/nrgastro.2017.110
10. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. (2015) 12:205–17. doi: 10.1038/nrgastro.2015.34
11. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ai RAR, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. (2018) 15:39–49. doi: 10.1038/nrgastro.2017.136
12. Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. (2012) 36:764–85. doi: 10.1016/j.neubiorev.2011.12.005
13. Maes M, Kubera M, Mihaylova I, Geffard M, Galecki P, Leunis JC, et al. Increased autoimmune responses against auto-epitopes modified by oxidative and nitrosative damage in depression: implications for the pathways to chronic depression and neuroprogression. J Affect Disord. (2013) 149:23–9. doi: 10.1016/j.jad.2012.06.039
14. Ohlsson L, Gustafsson A, Lavant E, Suneson K, Brundin L, Westrin Å, et al. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr Scand. (2019) 139:185–93. doi: 10.1111/acps.12978
15. Kéri S, Szabó C, Kelemen O. Expression of toll-like receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain Behav Immun. (2014) 40:235–43. doi: 10.1016/j.bbi.2014.03.020
16. Mikocka-Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. (2016) 22:752–62. doi: 10.1097/MIB.0000000000000620
17. Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. (2011) 62:591–9.
18. Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. (1992) 267:1244–52. doi: 10.1001/jama.1992.03480090092034
19. Nicolaides NC, Kyratzi E, Lamprokostopoulou A, Chrousos GP, Charmandari E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation. (2015) 22:6–19. doi: 10.1159/000362736
20. Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. (2003) 463:235–72. doi: 10.1016/S0014-2999(03)01285-8
21. Stengel A, Taché Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp Biol Med. (2010) 235:1168–78. doi: 10.1258/ebm.2010.009347
22. Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. (2009) 60(Suppl. 7):33–46.
23. Tache Y, Larauche M, Yuan PQ, Million M. Brain and gut CRF signaling: biological actions and role in the gastrointestinal tract. Curr Mol Pharmacol. (2018) 11:51–71. doi: 10.2174/1874467210666170224095741
24. Chatoo M, Li Y, Ma Z, Coote J, Du J, Chen X. Involvement of corticotropin-releasing factor and receptors in immune cells in irritable bowel syndrome. Front Endocrinol. (2018) 9:21. doi: 10.3389/fendo.2018.00021
25. Czimmer J, Tache Y. Peripheral corticotropin releasing factor signaling inhibits gastric emptying: mechanisms of action and role in stress-related gastric alterations of motor function. Curr Pharm Des. (2017) 23:4042–7. doi: 10.2174/1381612823666170228142428
26. Hill LT, Kidson SH, Michell WL. Corticotropin-releasing factor: a possible key to gut dysfunction in the critically ill. Nutrition. (2013) 29:948–52. doi: 10.1016/j.nut.2012.12.023
27. Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS ONE. (2012) 7:e39935. doi: 10.1371/journal.pone.0039935
28. Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. (2014) 63:1293–9. doi: 10.1136/gutjnl-2013-305690
29. Deng Q, Chen H, Liu Y, Xiao F, Guo L, Liu D, et al. Psychological stress promotes neutrophil infiltration in colon tissue through adrenergic signaling in DSS-induced colitis model. Brain Behav Immun. (2016) 57:243–54. doi: 10.1016/j.bbi.2016.04.017
30. Vicario M, Guilarte M, Alonso C, Yang P, Martínez C, Ramos L, et al. Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav Immun. (2010) 24:1166–75. doi: 10.1016/j.bbi.2010.06.002
31. Arruda JM, Bogetz AL, Vellanki S, Wren A, Yeh AM. Yoga as adjunct therapy for adolescents with inflammatory bowel disease: A pilot clinical trial. Complement Ther Med. (2018) 41:99–104. doi: 10.1016/j.ctim.2018.09.007
32. Söderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. (2002) 123:1099–108. doi: 10.1053/gast.2002.36019
33. Million M, Larauche M. Stress, sex, and the enteric nervous system. Neurogastroenterol Motil. (2016) 28:1283–9. doi: 10.1111/nmo.12937
34. Villanacci V, Bassotti G, Nascimbeni R, Antonelli E, Cadei M, Fisogni S, et al. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol Motil. (2008) 20:1009–16. doi: 10.1111/j.1365-2982.2008.01146.x
35. Sharma P, Makharia GK, Ahuja V, Dwivedi SN, Deepak KK. Autonomic dysfunctions in patients with inflammatory bowel disease in clinical remission. Dig Dis Sci. (2009) 54:853–61. doi: 10.1007/s10620-008-0424-6
36. Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. (2005) 135:1295–307. doi: 10.1016/j.neuroscience.2005.06.090
37. de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. (2005) 6:844–51. doi: 10.1038/ni1229
38. Meregnani J, Clarençon D, Vivier M, Peinnequin A, Mouret C, Sinniger V, et al. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci. (2011) 160:82–9. doi: 10.1016/j.autneu.2010.10.007
39. Keita AV, Carlsson AH, Cigéhn M, Ericson AC, McKay DM, Söderholm JD. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle-associated epithelium and during stress in rats. Neurogastroenterol Motil. (2013) 25 e406–17. doi: 10.1111/nmo.12127
40. Zheng PY, Feng BS, Oluwole C, Struiksma S, Chen X, Li P, et al. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut. (2009) 58:1473–9. doi: 10.1136/gut.2009.181701
41. Lu P, Luo H, Quan X, Fan H, Tang Q, Yu G, et al. The role of substance P in the maintenance of colonic hypermotility induced by repeated stress in rats. Neuropeptides. (2016) 56:75–82. doi: 10.1016/j.npep.2016.01.006
42. Kiliaan AJ, Saunders PR, Bijlsma PB, Berin MC, Taminiau JA, Groot JA, et al. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol. (1998) 275 G1037–44. doi: 10.1152/ajpgi.1998.275.5.G1037
43. Bailey MT. The contributing role of the intestinal microbiota in stressor-induced increases in susceptibility to enteric infection and systemic immunomodulation. Horm Behav. (2012) 62:286–94. doi: 10.1016/j.yhbeh.2012.02.006
44. Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. (2016) 63:217–27. doi: 10.1016/j.psyneuen.2015.10.001
45. Sun Y, Zhang M, Chen CC, Gillilland M, Sun X, El-Zaatari M, et al. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology. (2013) 144:1478–87, 1487.e1–8. doi: 10.1053/j.gastro.2013.02.038
46. Bailey MT, Engler H, Sheridan JF. Stress induces the translocation of cutaneous and gastrointestinal microflora to secondary lymphoid organs of C57BL/6 mice. J Neuroimmunol. (2006) 171:29–37. doi: 10.1016/j.jneuroim.2005.09.008
47. Gao X, Cao Q, Cheng Y, Zhao D, Wang Z, Yang H, et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci USA. (2018) 115:E2960–9. doi: 10.1073/pnas.1720696115
48. Wu W, Sun M, Zhang HP, Chen T, Wu R, Liu C, et al. Prolactin mediates psychological stress-induced dysfunction of regulatory T cells to facilitate intestinal inflammation. Gut. (2014) 63:1883–92. doi: 10.1136/gutjnl-2013-306083
49. Galley JD, Bailey MT. Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes. (2014) 5:390–6. doi: 10.4161/gmic.28683
50. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. (2012) 13:701–12. doi: 10.1038/nrn3346
51. Watanabe Y, Arase S, Nagaoka N, Kawai M, Matsumoto S. Chronic psychological stress disrupted the composition of the murine colonic microbiota and accelerated a murine model of inflammatory bowel disease. PLoS ONE. (2016) 11:e0150559. doi: 10.1371/journal.pone.0150559
52. Uchiyama K, Naito Y, Takagi T. Intestinal microbiome as a novel therapeutic target for local and systemic inflammation. Pharmacol Ther. (2019) 199:164–72. doi: 10.1016/j.pharmthera.2019.03.006
53. Lemon KP, Armitage GC, Relman DA, Fischbach MA. Microbiota-targeted therapies: an ecological perspective. Sci Transl Med. (2012) 4:137rv5. doi: 10.1126/scitranslmed.3004183
54. Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. (2005) 54:1481–91. doi: 10.1136/gut.2005.064261
55. Filipčić I, Šimunović FI, Grošić V, Bakija I, Šago D, Benjak T, et al. Patterns of chronic physical multimorbidity in psychiatric and general population. J Psychosom Res. (2018) 114:72–80. doi: 10.1016/j.jpsychores.2018.09.011
56. Scott KM, Lim C, Al-Hamzawi A, Alonso J, Bruffaerts R, Caldas-de-Almeida JM, et al. Association of mental disorders with subsequent chronic physical conditions: world mental health surveys from 17 countries. JAMA Psychiatry. (2016) 73:150–8. doi: 10.1001/jamapsychiatry.2015.2688
57. Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, et al. Physical comorbidities increase the risk of psychiatric comorbidity in immune-mediated inflammatory disease. Gen Hosp Psychiatry. (2018) 51:71–8. doi: 10.1016/j.genhosppsych.2018.01.003
58. Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflamm Bowel Dis. (2006) 12:697–707. doi: 10.1097/00054725-200608000-00005
59. Bhandari S, Larson ME, Kumar N, Stein D. Association of Inflammatory Bowel Disease (IBD) with depressive symptoms in the united states population and independent predictors of depressive symptoms in an IBD population: a NHANES study. Gut Liver. (2017) 11:512–9. doi: 10.5009/gnl16347
60. Fuller-Thomson E, Lateef R, Sulman J. Robust association between inflammatory bowel disease and generalized anxiety disorder: findings from a nationally representative Canadian study. Inflamm Bowel Dis. (2015) 21:2341–8. doi: 10.1097/MIB.0000000000000518
61. Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J Psychosom Res. (2016) 87:70–80. doi: 10.1016/j.jpsychores.2016.06.001
62. Sexton KA, Walker JR, Graff LA, Bernstein MT, Beatie B, Miller N, et al. Evidence of bidirectional associations between perceived stress and symptom activity: a prospective longitudinal investigation in inflammatory bowel disease. Inflamm Bowel Dis. (2017) 23:473–83. doi: 10.1097/MIB.0000000000001040
63. Frolkis AD, Vallerand IA, Shaheen AA, Lowerison MW, Swain MG, Barnabe C, et al. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut. (2018) 68:1606–12. doi: 10.1136/gutjnl-2018-317182
64. van den Brink G, Stapersma L, Vlug LE, Rizopolous D, Bodelier AG, van Wering H, et al. Clinical disease activity is associated with anxiety and depressive symptoms in adolescents and young adults with inflammatory bowel disease. Aliment Pharmacol Ther. (2018) 48:358–69. doi: 10.1111/apt.14832
65. Reigada LC, Hoogendoorn CJ, Walsh LC, Lai J, Szigethy E, Cohen BH, et al. Anxiety symptoms and disease severity in children and adolescents with Crohn disease. J Pediatr Gastroenterol Nutr. (2015) 60:30–5. doi: 10.1097/MPG.0000000000000552
66. Väistö T, Aronen ET, Simola P, Ashorn M, Kolho KL. Psychosocial symptoms and competence among adolescents with inflammatory bowel disease and their peers. Inflamm Bowel Dis. (2010) 16:27–35. doi: 10.1002/ibd.21002
67. Brooks AJ, Rowse G, Ryder A, Peach EJ, Corfe BM, Lobo AJ. Systematic review: psychological morbidity in young people with inflammatory bowel disease - risk factors and impacts. Aliment Pharmacol Ther. (2016) 44:3–15. doi: 10.1111/apt.13645
68. Brooks AJ, Norman P, Peach EJ, Ryder AH, Scott AJ, Narula P, et al. Prospective study of psychological morbidity and illness perceptions in young people with inflammatory bowel disease. J Crohns Colitis. (2019) 13:1003–11. doi: 10.1093/ecco-jcc/jjz028
69. Olén O, Askling J, Sachs MC, Frumento P, Neovius M, Smedby KE, et al. Increased mortality of patients with childhood-onset inflammatory bowel diseases, compared with the general population. Gastroenterology. (2019) 156:614–22. doi: 10.1053/j.gastro.2018.10.028
70. Haj-Mirzaian A, Amiri S, Amini-Khoei H, Hosseini MJ, Haj-Mirzaian A, Momeny M, et al. Anxiety- and depressive-like behaviors are associated with altered hippocampal energy and inflammatory status in a mouse model of Crohn's disease. Neuroscience. (2017) 366:124–37. doi: 10.1016/j.neuroscience.2017.10.023
71. Salameh E, Meleine M, Gourcerol G, do RJC, do RJL, Legrand R, et al. Chronic colitis-induced visceral pain is associated with increased anxiety during quiescent phase. Am J Physiol Gastrointest Liver Physiol. (2019) 316:G692–700. doi: 10.1152/ajpgi.00248.2018
72. Olvera AHA, Kubzansky LD, Campen MJ, Slavich GM. Early life stress, air pollution, inflammation, and disease: an integrative review and immunologic model of social-environmental adversity and lifespan health. Neurosci Biobehav Rev. (2018) 92:226–42. doi: 10.1016/j.neubiorev.2018.06.002
73. Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain Behav Immun. (2012) 26:239–50. doi: 10.1016/j.bbi.2011.11.003
74. Bernstein CN. Review article: changes in the epidemiology of inflammatory bowel disease-clues for aetiology. Aliment Pharmacol Ther. (2017) 46:911–9. doi: 10.1111/apt.14338
75. Walker JR, Ediger JP, Graff LA, Greenfeld JM, Clara I, Lix L, et al. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. (2008) 103:1989–97. doi: 10.1111/j.1572-0241.2008.01980.x
76. MMC E, Kuehn A, Muller CP, Turner JD. The effects of early life adversity on the immune system. Psychoneuroendocrinology. (2017) 82:140–54. doi: 10.1016/j.psyneuen.2017.05.012
77. Avitsur R, Levy S, Goren N, Grinshpahet R. Early adversity, immunity and infectious disease. Stress. (2015) 18:289–96. doi: 10.3109/10253890.2015.1017464
78. O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. (2009) 65:263–7. doi: 10.1016/j.biopsych.2008.06.026
79. HLX W, Qin HY, Tsang SW, Zuo X, Che S, CFW C, et al. Early life stress disrupts intestinal homeostasis via NGF-TrkA signaling. Nat Commun. (2019) 10:1745. doi: 10.1038/s41467-019-09744-3
80. Rincel M, Aubert P, Chevalier J, Grohard PA, Basso L, de Oliveira CM, et al. Multi-hit early life adversity affects gut microbiota, brain and behavior in a sex-dependent manner. Brain Behav Immun. (2019) 80:179–92. doi: 10.1016/j.bbi.2019.03.006
81. Włodarczyk M, Sobolewska-Włodarczyk A, Stec-Michalska K, Fichna J, Wiśniewska-Jarosinska M. The influence of family pattern abnormalities in the early stages of life on the course of inflammatory bowel diseases. Pharmacol Rep. (2016) 68:852–8. doi: 10.1016/j.pharep.2016.04.008
82. Krishna MZ, Barton KR, Perez CM, Walsh SM, Assa A, Kellermayer R. Academic stress may contribute to the onset of pediatric inflammatory bowel diseases. J Pediatr Gastroenterol Nutr. (2018) 67:e73–6. doi: 10.1097/MPG.0000000000002032
83. Lennon EM, Maharshak N, Elloumi H, Borst L, Plevy SE, Moeser AJ. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10-/- mice. Inflamm Bowel Dis. (2013) 19:712–9. doi: 10.1097/MIB.0b013e3182802a4e
84. Riba A, Olier M, Lacroix-Lamandé S, Lencina C, Bacquié V, Harkat C, et al. Early life stress in mice is a suitable model for Irritable Bowel Syndrome but does not predispose to colitis nor increase susceptibility to enteric infections. Brain Behav Immun. (2018) 73:403–15. doi: 10.1016/j.bbi.2018.05.024
85. Veenema AH, Reber SO, Selch S, Obermeier F, Neumann ID. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. (2008) 149:2727–36. doi: 10.1210/en.2007-1469
86. Miranda S, Roux ME. Acoustic stress induces long term severe intestinal inflammation in the mouse. Toxicol Lett. (2017) 280:1–9. doi: 10.1016/j.toxlet.2017.07.898
87. de Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. (2015) 6:223. doi: 10.3389/fimmu.2015.00223
88. Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, Cheang M. A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol. (2010) 105:1994–2002. doi: 10.1038/ajg.2010.140
89. Sweeney L, Moss-Morris R, Czuber-Dochan W, Meade L, Chumbley G, Norton C. Systematic review: psychosocial factors associated with pain in inflammatory bowel disease. Aliment Pharmacol Ther. (2018) 47:715–29. doi: 10.1111/apt.14493
90. Bernstein CN. Psychological stress and depression: risk factors for IBD. Dig Dis. (2016) 34:58–63. doi: 10.1159/000442929
91. Bokemeyer B, Hardt J, Hüppe D, Prenzler A, Conrad S, Düffelmeyer M, et al. Clinical status, psychosocial impairments, medical treatment and health care costs for patients with inflammatory bowel disease (IBD) in Germany: an online IBD registry. J Crohns Colitis. (2013) 7:355–68. doi: 10.1016/j.crohns.2012.02.014
92. Jaghult S, Saboonchi F, Moller J, Johansson UB, Wredling R, Kapraali M. Stress as a trigger for relapses in IBD: a case-crossover study. Gastroenterology Res. (2013) 6:10–16. doi: 10.4021/gr528e
93. Mittermaier C, Dejaco C, Waldhoer T, Oefferlbauer-Ernst A, Miehsler W, Beier M, et al. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med. (2004) 66:79–84. doi: 10.1097/01.psy.0000106907.24881.f2
94. Petruo VA, Krauss E, Kleist A, Hardt J, Hake K, Peirano J, et al. Perceived distress, personality characteristics, coping strategies and psychosocial impairments in a national German multicenter cohort of patients with Crohn's disease and ulcerative colitis. Z Gastroenterol. (2019) 57:473–83. doi: 10.1055/a-0838-6371
95. Alexakis C, Kumar S, Saxena S, Pollok R. Systematic review with meta-analysis: the impact of a depressive state on disease course in adult inflammatory bowel disease. Aliment Pharmacol Ther. (2017) 46:225–35. doi: 10.1111/apt.14171
96. Ananthakrishnan AN, Khalili H, Pan A, Higuchi LM, de Silva P, Richter JM, et al. Association between depressive symptoms and incidence of Crohn's disease and ulcerative colitis: results from the Nurses' Health Study. Clin Gastroenterol Hepatol. (2013) 11:57–62. doi: 10.1016/j.cgh.2012.08.032
97. Cámara RJ, Schoepfer AM, Pittet V, Begré S, von KR. Mood and nonmood components of perceived stress and exacerbation of Crohn's disease. Inflamm Bowel Dis. (2011) 17:2358–65. doi: 10.1002/ibd.21623
98. Reed-Knight B, van Tilburg MAL, Levy RL, Langer SL, Romano JM, Murphy TB, et al. Maladaptive coping and depressive symptoms partially explain the association between family stress and pain-related distress in youth with IBD. J Pediatr Psychol. (2018) 43:94–103. doi: 10.1093/jpepsy/jsx082
99. Zimmerman LA, Srinath AI, Goyal A, Bousvaros A, Ducharme P, Szigethy E, et al. The overlap of functional abdominal pain in pediatric Crohn's disease. Inflamm Bowel Dis. (2013) 19:826–31. doi: 10.1097/MIB.0b013e3182802a0a
100. Koh SJ, Kim JW, Kim BG, Lee KL, Kim JS. Restraint stress induces and exacerbates intestinal inflammation in interleukin-10 deficient mice. World J Gastroenterol. (2015) 21:8580–7. doi: 10.3748/wjg.v21.i28.8580
101. Mikocka-Walus AA, Turnbull D, Holtmann G, Andrews JM. An integrated model of care for inflammatory bowel disease sufferers in Australia: development and the effects of its implementation. Inflamm Bowel Dis. (2012) 18:1573–81. doi: 10.1002/ibd.22850
102. Tabibian A, Tabibian JH, Beckman LJ, Raffals LL, Papadakis KA, Kane SV. Predictors of health-related quality of life and adherence in Crohn's disease and ulcerative colitis: implications for clinical management. Dig Dis Sci. (2015) 60:1366–74. doi: 10.1007/s10620-014-3471-1
103. Guthrie E, Jackson J, Shaffer J, Thompson D, Tomenson B, Creed F. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn's disease. Am J Gastroenterol. (2002) 97:1994–9. doi: 10.1111/j.1572-0241.2002.05842.x
104. Engelmann G, Erhard D, Petersen M, Parzer P, Schlarb AA, Resch F, et al. Health-related quality of life in adolescents with inflammatory bowel disease depends on disease activity and psychiatric comorbidity. Child Psychiatry Hum Dev. (2015) 46:300–7. doi: 10.1007/s10578-014-0471-5
105. Ananthakrishnan AN, Gainer VS, Perez RG, Cai T, Cheng SC, Savova G, et al. Psychiatric co-morbidity is associated with increased risk of surgery in Crohn's disease. Aliment Pharmacol Ther. (2013) 37:445–54. doi: 10.1111/apt.12195
106. Guerrero-Alba R, Valdez-Morales EE, Jimenez-Vargas NN, Lopez-Lopez C, Jaramillo-Polanco J, Okamoto T, et al. Stress activates pronociceptive endogenous opioid signalling in DRG neurons during chronic colitis. Gut. (2017) 66:2121–31. doi: 10.1136/gutjnl-2016-311456
107. Spekhorst LM, Hummel TZ, Benninga MA, van Rheenen PF, Kindermann A. Adherence to oral maintenance treatment in adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2016) 62:264–70. doi: 10.1097/MPG.0000000000000924
108. Matsuoka K, Kobayashi T, Ueno F, Matsui T, Hirai F, Inoue N, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. (2018) 53:305–53. doi: 10.1007/s00535-018-1439-1
109. Keefer L, Palsson OS, Pandolfino JE. Best practice update: incorporating psychogastroenterology into management of digestive disorders. Gastroenterology. (2018) 154:1249–57. doi: 10.1053/j.gastro.2018.01.045
110. Beyak MJ, Vanner S. Inflammation-induced hyperexcitability of nociceptive gastrointestinal DRG neurones: the role of voltage-gated ion channels. Neurogastroenterol Motil. (2005) 17:175–186. doi: 10.1111/j.1365-2982.2004.00596.x
111. Lakhan SE, Kirchgessner A. Neuroinflammation in inflammatory bowel disease. J Neuroinflammation. (2010)7:37. doi: 10.1186/1742-2094-7-37
112. Srinath AI, Walter C, Newara MC, Szigethy EM. Pain management in patients with inflammatory bowel disease: insights for the clinician. Therap Adv Gastroenterol. (2012) 5:339–57. doi: 10.1177/1756283X12446158
113. Tarricone I, Regazzi MG, Bonucci G, Rizzello F, Carini G, Muratori R, et al. Prevalence and effectiveness of psychiatric treatments for patients with IBD: A systematic literature review. J Psychosom Res. (2017) 101:68–95. doi: 10.1016/j.jpsychores.2017.07.001
114. Mikocka-Walus AA, Gordon AL, Stewart BJ, Andrews JM. The role of antidepressants in the management of inflammatory bowel disease (IBD): a short report on a clinical case-note audit. J Psychosom Res. (2012) 72:165–7. doi: 10.1016/j.jpsychores.2011.06.006
115. Mikocka-Walus A, Prady SL, Pollok J, Esterman AJ, Gordon AL, Knowles S, et al. Adjuvant therapy with antidepressants for the management of inflammatory bowel disease. Cochrane Database Syst Rev. (2019) 4:CD012680. doi: 10.1002/14651858.CD012680.pub2
116. Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis. (2016) 22:1509–22. doi: 10.1097/MIB.0000000000000734
117. Szigethy E. Pain management in patients with inflammatory bowel disease. Gastroenterol Hepatol. (2018) 14:53–56.
118. Casellas JF, Vera MI, Barreiro-de AM, Vázquez MJM, López RJ, Júdez GJ. Managing iron deficiency and iron deficiency anemia in inflammatory bowel disease. The results of the “Gestiona hierro-EII” survey. Rev Esp Enferm Dig. (2018) 110:172–8. doi: 10.17235/reed.2018.5354/2017
119. Bennebroek EF, MAG S, Sitnikova K, PCF S, Ponsioen CY, Bartelsman JFWM B, et al. Effectiveness of cognitive-behavioral therapy on quality of life, anxiety, and depressive symptoms among patients with inflammatory bowel disease: a multicenter randomized controlled trial. J Consult Clin Psychol. (2017) 85:918–925. doi: 10.1037/ccp0000227
120. Jordan C, Hayee B, Chalder T. Cognitive behaviour therapy for distress in people with inflammatory bowel disease: a benchmarking study. Clin Psychol Psychother. (2019) 26:14–23. doi: 10.1002/cpp.2326
121. Moser G. The role of hypnotherapy for the treatment of inflammatory bowel diseases. Expert Rev Gastroenterol Hepatol. (2014) 8:601–6. doi: 10.1586/17474124.2014.917955
122. Sawni A, Breuner CC. Clinical hypnosis, an effective mind-body modality for adolescents with behavioral and physical complaints. Children. (2017) 4:E19. doi: 10.3390/children4040019
123. Gerbarg PL, Jacob VE, Stevens L, Bosworth BP, Chabouni F, DeFilippis EM, et al. The effect of breathing, movement, and meditation on psychological and physical symptoms and inflammatory biomarkers in inflammatory bowel disease: a randomized controlled trial. Inflamm Bowel Dis. (2015) 21:2886–96. doi: 10.1097/MIB.0000000000000568
124. Cramer H, Schäfer M, Schöls M, Köcke J, Elsenbruch S, Lauche R, et al. Randomised clinical trial: yoga vs written self-care advice for ulcerative colitis. Aliment Pharmacol Ther. (2017) 45:1379–89. doi: 10.1111/apt.14062
125. Boye B, Lundin KE, Jantschek G, Leganger S, Mokleby K, Tangen T, et al. INSPIRE study: does stress management improve the course of inflammatory bowel disease and disease-specific quality of life in distressed patients with ulcerative colitis or Crohn's disease? A randomized controlled trial. Inflamm Bowel Dis. (2011) 17:1863–73. doi: 10.1002/ibd.21575
126. Stapersma L, van den Brink G, van der Ende J, Szigethy EM, Beukers R, Korpershoek TA, et al. Effectiveness of disease-specific cognitive behavioral therapy on anxiety, depression, and quality of life in youth with inflammatory bowel disease: a randomized controlled trial. J Pediatr Psychol. (2018) 43:967–80. doi: 10.1093/jpepsy/jsy029
127. Knox NC, Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiome as a target for IBD treatment: are we there yet. Curr Treat Options Gastroenterol. (2019) 17:115–26. doi: 10.1007/s11938-019-00221-w
128. Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP, et al. Antibiotics associated with increased risk of new-onset Crohn's disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol. (2014) 109:1728–38. doi: 10.1038/ajg.2014.246
129. Theochari NA, Stefanopoulos A, Mylonas KS, Economopoulos KP. Antibiotics exposure and risk of inflammatory bowel disease: a systematic review. Scand J Gastroenterol. (2018) 53:1–7. doi: 10.1080/00365521.2017.1386711
130. Kerman DH, Deshpande AR. Gut microbiota and inflammatory bowel disease: the role of antibiotics in disease management. Postgrad Med. (2014) 126:7–19. doi: 10.3810/pgm.2014.07.2779
131. Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Abadir A, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. (2011) 106:661–73. doi: 10.1038/ajg.2011.72
132. Kale-Pradhan PB, Zhao JJ, Palmer JR, Wilhelm SM. The role of antimicrobials in Crohn's disease. Expert Rev Gastroenterol Hepatol. (2013) 7:281–8. doi: 10.1586/egh.13.6
133. Hansen JJ, Sartor RB. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr Treat Options Gastroenterol. (2015) 13:105–20. doi: 10.1007/s11938-014-0042-7
134. Sartor RB. Review article: the potential mechanisms of action of rifaximin in the management of inflammatory bowel diseases. Aliment Pharmacol Ther. (2016) 43(Suppl. 1):27–36. doi: 10.1111/apt.13436
135. Xu D, Gao J, Gillilland M III, Wu X, Song I, Kao JY, et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology. (2014) 146:484–96.e4. doi: 10.1053/j.gastro.2013.10.026
136. Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. (2017) 7:124–36. doi: 10.1016/j.ynstr.2017.03.001
137. Arase S, Watanabe Y, Setoyama H, Nagaoka N, Kawai M, Matsumoto S. Disturbance in the mucosa-associated commensal bacteria is associated with the exacerbation of chronic colitis by repeated psychological stress; is that the new target of probiotics. PLoS ONE. (2016) 11:e0160736. doi: 10.1371/journal.pone.0160736
138. Alard J, Peucelle V, Boutillier D, Breton J, Kuylle S, Pot B, et al. New probiotic strains for inflammatory bowel disease management identified by combining in vitro and in vivo approaches. Benef Microbes. (2018) 9:317–31. doi: 10.3920/BM2017.0097
139. Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: a randomized, double-blinded, placebo-controlled multicenter trial. Dig Endosc. (2016) 28:67–74. doi: 10.1111/den.12553
140. Matsuoka K, Uemura Y, Kanai T, Kunisaki R, Suzuki Y, Yokoyama K, et al. Efficacy of Bifidobacterium breve fermented milk in maintaining remission of ulcerative colitis. Dig Dis Sci. (2018) 63:1910–19. doi: 10.1007/s10620-018-4946-2
141. Bernstein CN. Treatment of IBD: where we are and where we are going. Am J Gastroenterol. (2015) 110:114–26. doi: 10.1038/ajg.2014.357
142. Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical application and potential of fecal microbiota transplantation. Annu Rev Med. (2019) 70:335–51. doi: 10.1146/annurev-med-111717-122956
143. Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. (2015) 149:102–9.e6. doi: 10.1053/j.gastro.2015.04.001
144. Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. (2017) 389:1218–28. doi: 10.1016/S0140-6736(17)30182-4
145. Goyal A, Yeh A, Bush BR, Firek BA, Siebold LM, Rogers MB, et al. Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm Bowel Dis. (2018) 24:410–21. doi: 10.1093/ibd/izx035
146. Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. (2017) 11:1180–99. doi: 10.1093/ecco-jcc/jjx063
147. Nishida A, Imaeda H, Ohno M, Inatomi O, Bamba S, Sugimoto M, et al. Efficacy and safety of single fecal microbiota transplantation for Japanese patients with mild to moderately active ulcerative colitis. J Gastroenterol. (2017) 52:476–82. doi: 10.1007/s00535-016-1271-4
148. Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. (2015) 149:110–8.e4. doi: 10.1053/j.gastro.2015.03.045
149. Rooks MG, Veiga P, Reeves AZ, Lavoie S, Yasuda K, Asano Y, et al. QseC inhibition as an antivirulence approach for colitis-associated bacteria. Proc Natl Acad Sci USA. (2017) 114:142–7. doi: 10.1073/pnas.1612836114
Keywords: inflammatory bowel disease, stress, pediatrics, gut microbiota, brain-gut axis, treatment
Citation: Sun Y, Li L, Xie R, Wang B, Jiang K and Cao H (2019) Stress Triggers Flare of Inflammatory Bowel Disease in Children and Adults. Front. Pediatr. 7:432. doi: 10.3389/fped.2019.00432
Received: 01 November 2018; Accepted: 07 October 2019;
Published: 24 October 2019.
Edited by:
Claudio Pignata, University of Naples Federico II, ItalyReviewed by:
Fatma Dedeoglu, Harvard Medical School, United StatesCopyright © 2019 Sun, Li, Xie, Wang, Jiang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kui Jiang, a2ppYW5nQHRtdS5lZHUuY24=; Hailong Cao, Y2FvaGFpbG9uZ0B0bXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.