- Division of Pediatric Urology, Children's Hospital of Philadelphia, Philadelphia, PA, United States
Management of vesicoureteral reflux (VUR) has evolved over the past several decades, with a trend toward a decrease in surgical management. In spite of this, ureteral reimplantation remains a commonly performed procedure by pediatric urologists in selected cases. Although the basic tenets of the ureteral reimplant procedure remain the same, the extra- vs. intravesical approach, and the traditional open vs. minimally invasive approach remain the primary options to correct reflux. Considering the advantages conferred by the robotic surgery platform, many leading centers have preferentially adopted robot-assisted laparoscopic extravesical anti-reflux surgery, or in common surgical parlance, the robot-assisted laparoscopic ureteral reimplantation (RALUR), over pure laparoscopic or open approaches. Predicated on our experience of performing over 170 cases of RALUR, we have made technical modifications which we posit reduce the morbidity of the procedure while offering acceptable outcomes. This review highlights the evolution and establishment of RALUR as a standardization of care in the surgical management of VUR at our institution. In particular, we emphasize the technical nuances and specific challenges encountered through the learning curve in hopes of facilitating this process for others.
Introduction
Over the past two decades, both the evaluation and therapeutic interventions for vesicoureteral reflux (VUR) have undergone extensive evolutions, with a clear trend toward non-operative management and a focus on voiding dysfunction as the major risk factor for urinary tract infection (UTI) (1–3).
In spite of significant investigations aimed at identifying risk factors for the development of recurrent urinary tract infections and predicting the potential for VUR resolution and/or the development of renal scarring, our ability to do so remains limited. Still, when conservative management fails, and febrile UTIs or significant renal scarring occurs, children do require surgical management.
The surgical principles for the correction of VUR remain consistent, several years after its initial description (4). In order to prevent VUR the length of the anti-refluxing tunnel is extended, traditionally in a 5:1 ratio of tunnel length to width of the ureter. While the open ureteral reimplant has been the traditional gold standard repair for VUR, the minimally invasive approaches, such as sub-ureteral injections or laparoscopic and robotic assisted laparoscopic ureteral reimplant (RALUR), have been established as viable alternatives (3).
Although initial enthusiasm for RALUR has waned for some due to concerns about a steep learning curve which can potentially increase the risk of patient morbidity or persistent VUR (5, 6), the well-established benefits of the robotic approach encourage its use (7, 8). Benefits of the robotic vs. the open approach include an improved field of vision via magnification intraperitoneal visualization of the ureters and bladder, improved cosmesis for the patient, and a more rapid recovery in the immediate postoperative period due to an extravesical approach. In our experience several technical modifications mitigate the aforementioned risk of RALUR while maximizing patient outcomes.
In this review, we seek to explore the evolution of extravesical RALUR–as it is the most widely adapted robotic approach–and describe technical modifications that render this procedure reproducible across surgeons and lessen the learning curve trajectory.
Evolution of RALUR
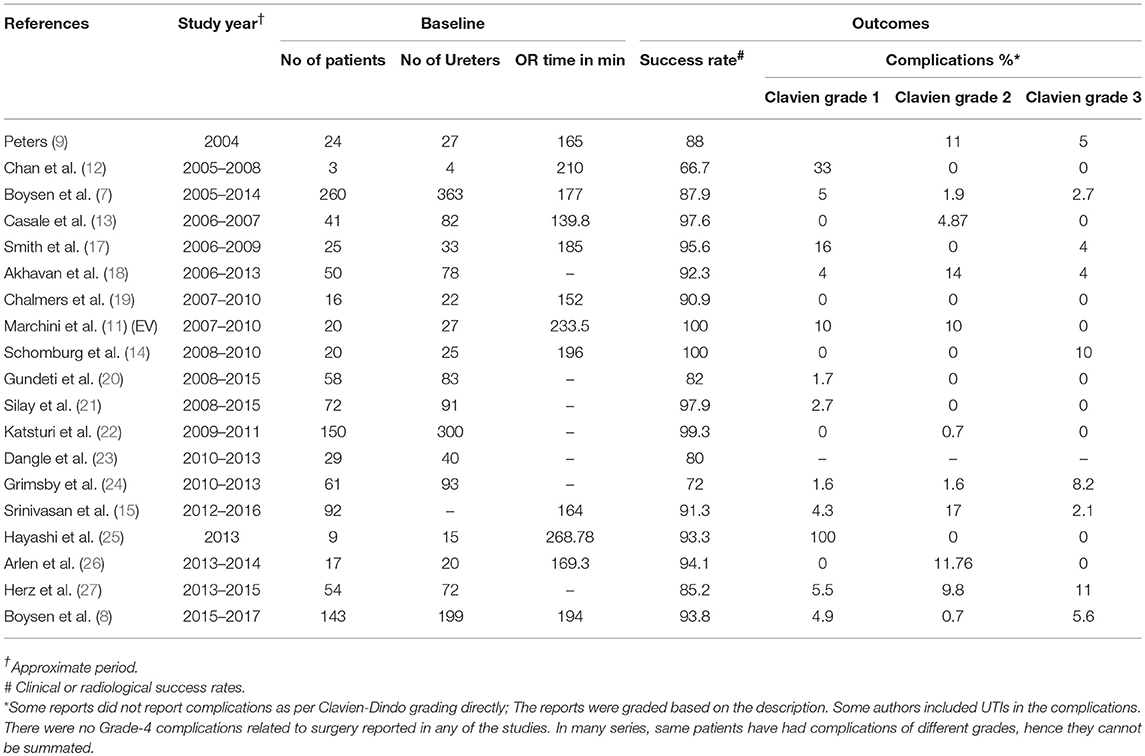
Since its first description by Peters (9), the technical aspects of the RALUR have undergone several modifications. Along with the initial descriptions of robotic assisted pediatric urologic procedures, Peters highlighted the advantages of the robotic system, the need for evolution and evaluation (9). The surgical principles illustrated for both the robotic-assisted intravesical (RAIVUR) and extravesical techniques were adapted to the new platform while adhering to the principles of contemporary open surgical procedures. The intravesical techniques were favored in bilateral cases due the concerns of urinary retention with bilateral extravesical reimplantation (9).
Since then, while the RAIVUR confers advantages of decreased hematuria and bladder spasms compared to the open approach, it failed to gain widespread adaptation, largely due to technical challenges such as insufflation leaks through the larger trocar hiatus and limited working space of a small bladder (10, 11). Only three groups have reported their experience with RAIVUR, with modest success rates (83, 92, and 100% reflux resolution in 6, 19, and 3 patients, respectively) and highly variable complication rates (17, 52, and 0%) (10–12).
Meanwhile, the Lich-Gregoir technique has become popular for the robotic approach due to its ready adaptability to the technology. This trans-abdominal approach provides excellent visualization of the retrovesical space, particularly when compared to the open approach. The magnification of the robotic camera facilitates meticulous detrusor dissection, which combined with judicious use of energy devices limits the potential for collateral damage to the nerve bundles of the bladder (13).
As with other minimally invasive approaches, RALUR confers the significant benefits of minimally invasive surgery including a shorter convalescence, reduced hospital stay, and improved cosmesis. The realization of the potential improved experience for patients has led to more widespread acceptance for utilization of RALUR. This had led to more centers having a higher number of RALUR over open ureteral reimplants, including ours, and this is reflected in publication trends as well (Figure 1).
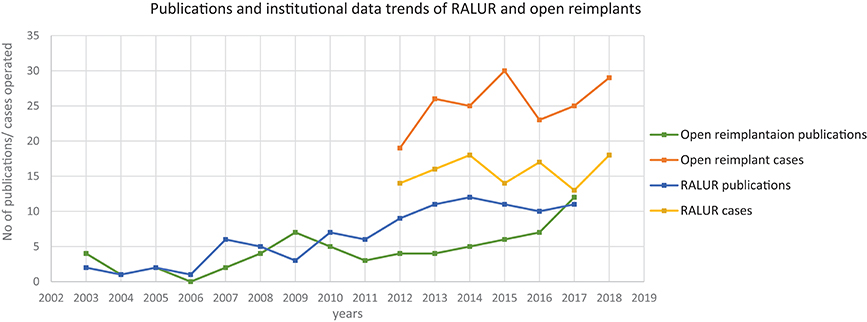
Figure 1. Trends in publications and number of RALURs performed at our institute. Source: PubMed search report of terms “Open ureteral reimplantation” and “Robot-assisted ureteral reimplantation” as on 23 July 2018.
A negative postoperative voiding cystourethrogram (VCUG) remains the gold standard to declare surgical success after a ureteral reimplantation. But, traditionally high success rates of the open approach obviated a post-operative VCUG in practice. Similarly, with accumulating experience with the RALUR, and pilot studies have demonstrated that our technique delivers reliable VCUG proven success (14). Our institution now defines surgical success as a lack of postoperative febrile UTI and a negative VCUG, if obtained in the postoperative period (15). Potential short term complications reported after RALUR include minor self-limiting adverse events such as bladder spasms, hematuria, and GI disturbances. Other reported complications include urinary extravasation, UTIs, incisional hernia, ureteral obstruction resulting in anuria, and the rare complication of ureteral strictures.
Early reports for RALUR are typical for most new techniques: they are comprised of single institution experiences with small patient numbers. To compound this, there is a lack of uniformity in data reporting, including the age at the time of repair, the indication for ureteral reimplantation (VUR vs. obstruction), and the degree of reflux at the time of surgery. Bladder and bowel dysfunction has proven to be a significant risk factor for surgical failure yet remains under reported in the literature (16). Overview of the literature review (Table 1) suggests, in the first decade of utilization of RALUR, there were inconsistencies in reported success (66.7–100%) and complication (0–100%) rates (7–9, 11–15, 17–28). It is notable that higher Clavien grade complications occurred in reports following smaller patient cohorts. However, recent growing evidence in literature has been relatively consistent in proving the safety and efficacy of RALUR in prospective multi-institutional collaborative efforts (7, 8).
The Learning Curve
The learning curve refers to variations in the productivity of a new surgical procedure or surgeon over a specific time period that leads to achieving a consistent level of expertise to meet contemporary standards. Defining a learning curve is a challenging concept. Although some authors claim that proxies such as operative time, complication rates, and functional outcomes are inadequate measures to assess a true learning curve, most reports have defaulted to these as practical measures of surgical success (28, 29).
As outlined above, the heterogeneity of the published literature on RALUR on key variables such as patient demographics, grades of reflux, comorbidities, in addition to variable study catchment periods makes assessment of a true learning curve difficult.
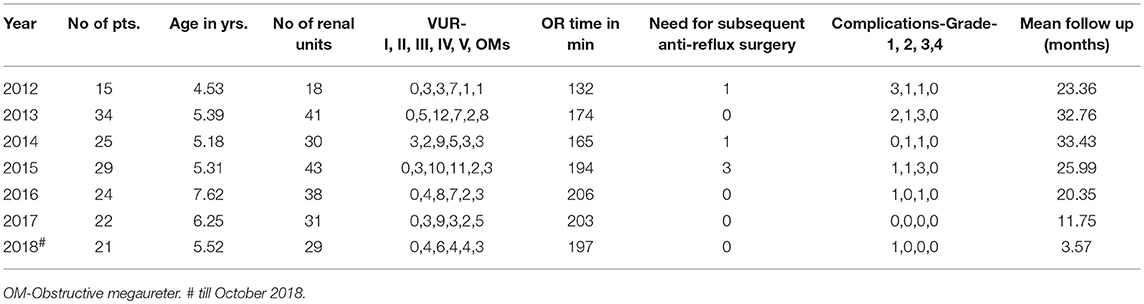
In order to trace the influence of a learning curve to surgical parameters and outcomes for RALUR at our institution, we have compared the failure rates (need for secondary anti-reflux surgery), radiographic reflux resolution rate, operative time, and/or complication rates over defined time frames (yearly trends) for a series of consecutive cases (Table 2). We feel as though a single institution center with a relatively high surgical volume as well as 5 different surgeons provides a unique opportunity to carry out this analysis.
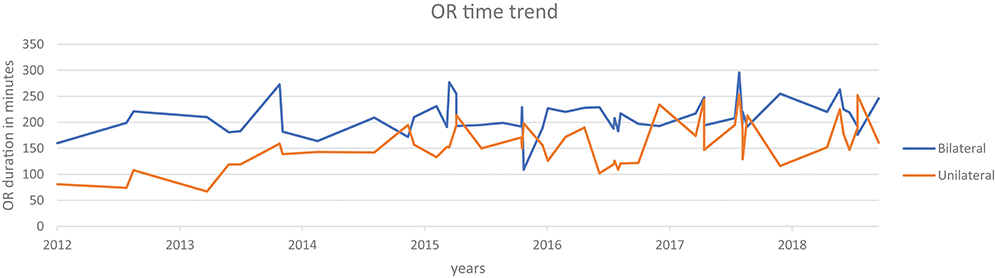
A review of data from a prospectively maintained database from 2012 till October 2018, includes six surgeons performing RALUR on 170 patients, of which 60 were bilateral. One hundred twenty-seven were female patients. In our series, the average age at surgery was 5.9 years with a general trend toward higher age at surgery each year, contrary to the literature on national data (3). A Majority of RALUR were done for dilating VUR (Grade 3 or higher) with breakthrough UTIs or renal scarring (100 cases), 36 cases had a duplex anomaly, 23 obstructive megaureter and 10 bladder diverticula. Additionally, 18 cases had a prior history of failed sub-ureteric injection. Among VUR cases, 40% had high grade VUR (IV and V). The operative time varied depending on the number of ureters, need for cystoscopy, retrograde pyelography, placement of a suprapubic tube, and other concomitant procedures depending on the associated pathology (nephrectomy, heminephrectomy, etc.). For unilateral procedures without any concomitant procedure the mean operative time was 161 min (49 cases), and it was 208 min for bilateral cases (48 cases). Operative time includes time of first incision or procedure start, to procedure end time as recorded by the nursing staff. The operative time did not vary significantly between the first and last quarters of consecutive case series (Figure 2). Blood loss was minimal in most of the cases from the beginning.
Over the follow up period of 1 to 75 months (mean 23, median 20 months), six cases had transient urinary retention and four cases needed surgery for port site hernia (we now meticulously close fascia even for 5 mm port sites under direct vision). Four cases of ureteral obstruction were noted based on increased dilation of calyces on renal ultrasound with symptoms of flank pain with nausea; of which three cases resolved with cystoscopy and ureteral stenting for 6 weeks and, in another case, required open ureteral reimplantation. We previously reported our surgical outcomes on an initial cohort and found that postoperative febrile UTIs occurred in 15.8% of unilateral cases compared with 20% of bilateral cases (p = 0.61) (15). Surgical failure, denoted by postoperative febrile UTI and a positive VCUG was noted in five (8.7%) of the unilateral cases vs. three (8.6%) of the bilateral RALUR cases (p = 0.98). The updated demographic and clinical information is summarized in Table 2.
Although the intraoperative surgical time has remained consistent over the years, we note that the number and complexity of complications are decreasing over subsequent years, concomitant to a reduced need for secondary interventions. Indeed, these results encouraged increasing use of RALUR at our institution with the increase in expertise and confidence level. Backed by comparable outcomes there has been an increase in the proportion of RALUR cases as compared to open ureteral reimplants (Figure 1). We expect, also, that being an academic teaching institute with many surgeons and trainees, inherent variations in the proficiency levels with atypical learning curve patterns would affect continued improvement in specific parameters.
Technical Modifications to Achieve a Successful RALUR
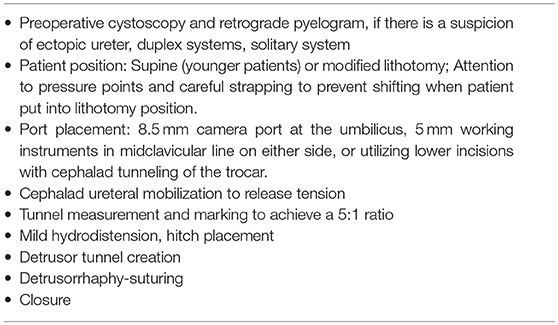
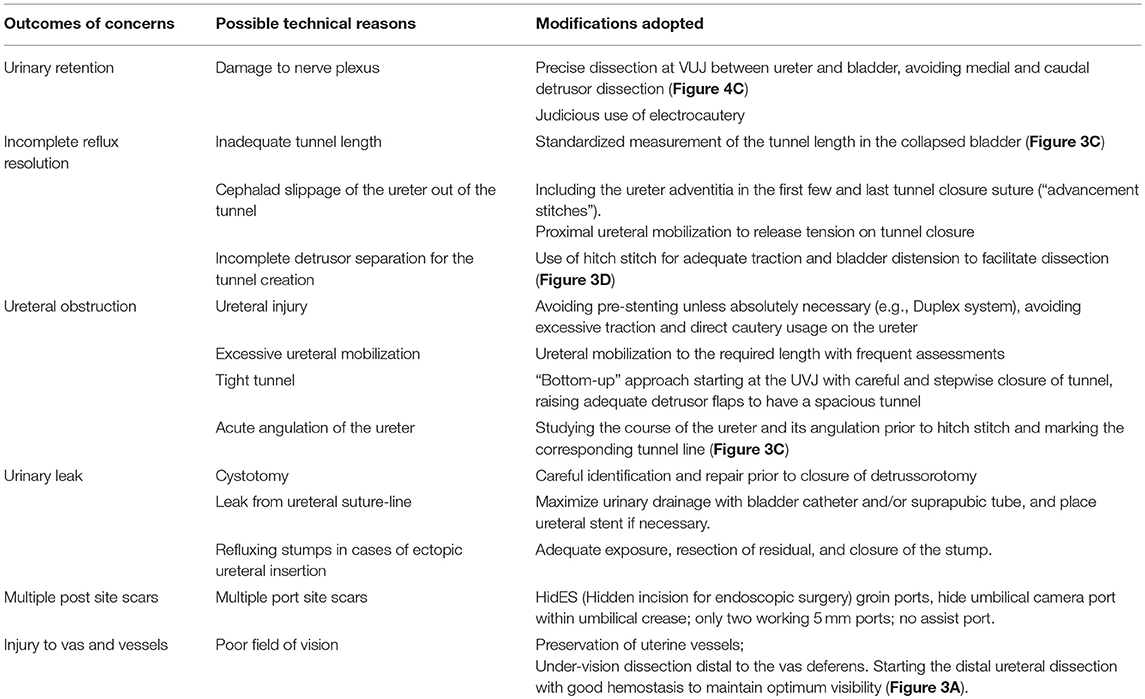
Based on our experiences with RALUR, in addition to standard steps (Table 3), we have adopted several key technical modifications that we believe have improved our institutional outcomes (Table 4). Proper case selection is vital and must consider patient age, toilet training status, and the presence or absence of dysfunctional elimination. If there is a concern for secondary reflux due to a neurogenic bladder, this must be worked up prior to intervention. If significant bowel and/or bladder dysfunction persists in spite of adequate therapy, a suprapubic tube should be considered in order to ensure proper, low-pressure post-operative voiding prior to removing within a week.
In order to avoid collateral damage to the detrusor muscle and the nerve plexus, meticulous dissection and judicious use of electrocautery is vital (Figure 3B). We recommend that estimating tunnel length is inaccurate–and overestimated–when the bladder is even slightly distended, hence we now delineate the tunnel length–ensuring a measurement of tunnel length five times the diameter of the distal ureter–while the bladder is completely drained with a foley catheter (Figure 3C). We regularly utilize a hitch stitch not only to aid detrusor dissection but also to mark the direction of proposed tunnel (Figure 3D). We use only the tip of the hook to cauterize the identified bleeding spots and spread the muscles bluntly, rather than cutting those layers (Figure 4B).
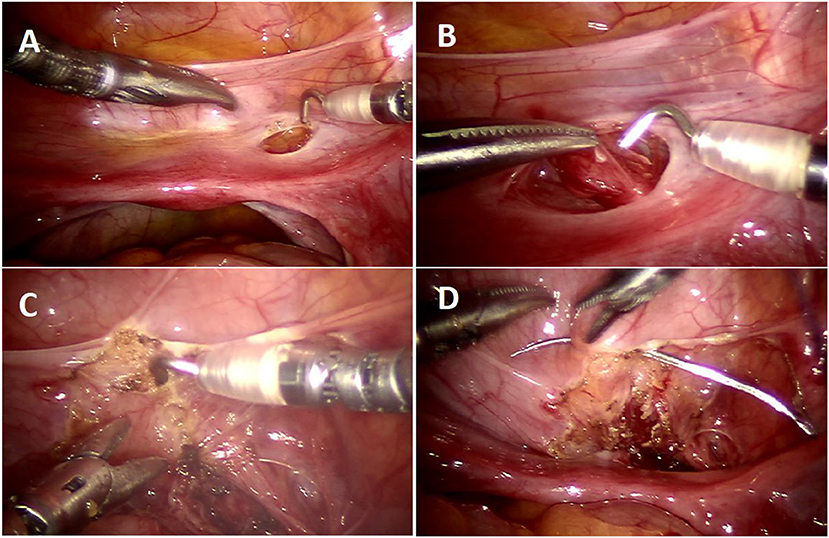
Figure 3. Operative steps of RALUR. (A) Small window created in the broad ligament to access the ureter directly. (B) Ureteral mobilization: gentle handling by grasping only the ureteral adventitia. (C) Marking the detrusor tunnel in a collapsed bladder in line with the ureter. (D) Hitch stitch at the distal end of tunnel marking.
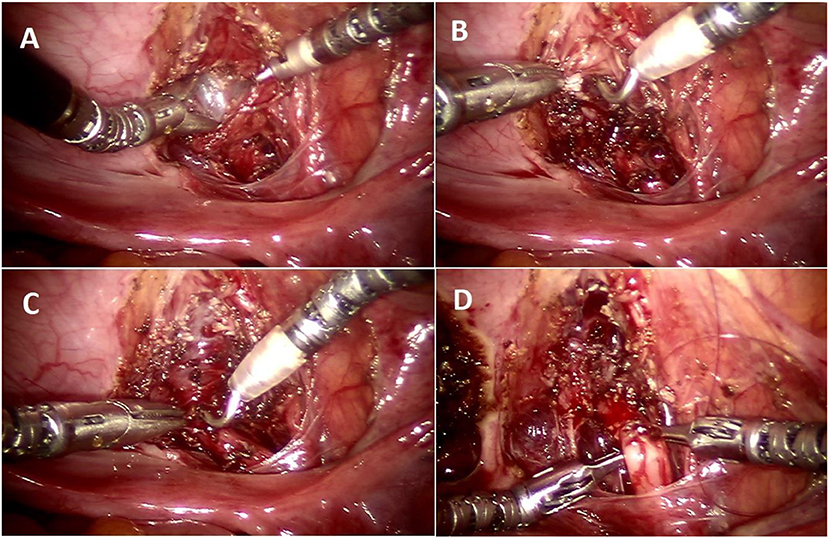
Figure 4. Operative steps of RALUR. (A) Making a detrusor window till bluish bladder mucosa is delineated. (B) Detrusor muscle separated with direct pinpoint electrocautery tip combined with blunt spreading of the muscle fibers. (C) Detrusor tunnel opened proximally to ureterovesical junction. (D) Passing suture underneath the ureter to advance the ureter into the detrusor trough (left to right).
Once the tunnel length of the detrusor is delineated, we begin dissecting the tunnel midway between the hitch stitch and ureterovesical junction until the bladder mucosal layer is defined (Figure 4A). Utilizing this window, we find that further progression proximally and distally proceeds more easily without the risk of inadvertent cystotomies (Figure 4C). We avoid medial and inferior dissection close to the VUJ, which poses damage to the nerve plexus and may increase the risk of detrusor injury and urinary retention. The ureters must be adequately mobilized in a cephalad direction in order to remove any proximal tension that may tease the ureter out of the tunnel.
Once the detrusorrhaphy begins, we prefer interrupted suturing with long term absorbable sutures (5–0 polydiaxone) in a “bottom up” approach, starting from uretero-vesical junction and then moving distally toward the hitch stitch (dome), as it allows for a tailored formation of the tunnel depending on the available ureter length and amount of tension. This technique can be confusing for the beginner and requires careful passing of suture underneath the ureter and back toward the initial bite side (Figure 4D) to allow placement of the ureter deep in the tunnel with close approximation of the detrusor edges. It is important to include ureteral adventitia in the initial and ending detrusor closure stitch. In cases of dismembered reimplants, solitary kidney, tapered or complex reimplants we prefer to leave a double J stent, but we do not stent typical RALUR cases. We also do not routinely place a drain. We leave a urinary catheter overnight and discharge the patient once they are able to void, leaving residuals that are <25% of the expected bladder capacity.
Table 4 summarizes the common pitfalls and technical modification adopted to address those concerns.
Challenges
In their recent review, Baek et al. analyzes the reasons for slower adoption of RALUR in comparison to the widespread and quickly adopted robotic prostatectomy among adult counterparts. The steeper learning curve and concerns about the efficacy compared to open reimplants were oft-cited reasons, and the authors suggested that the procedure be deferred to a later point in the robotic experience (30). However, from our learning curve experience, we deduce that careful adherence to outlined steps allows the RALUR to be safely and reproducibly performed. Standardization of salient steps with adequate training and judicious use of electrocautery will ensure the avoidance of common pitfalls.
Although there are debates regarding the efficacy of the RALUR in comparison to open ureteral reimplantation, we must be cautious while comparing the historical reports to the contemporary outcomes as the population characteristics (age group, voiding dysfunction, etc.) have been changing. Eventually, the trends suggest RALUR inexorably will be adopted more widely, and reports and ongoing multi-institutional consortiums will continue to provide further evidence regarding its safety and affirming its efficacy.
Conclusions
RALUR utilization has increased since its inception, but concerns over the procedure's technical difficulty, safety, risk of urinary retention and outcomes has limited its widespread use. Herein, we demonstrate that the learning curve of RALUR can be shortened with specific modifications predicated on experience, and that with technical adaptations, clinically significant improvements in surgical outcomes may be expected. In appropriately selected patients and with adequate preparation, we posit that the RALUR is a safe and effective technique that confers the well-described advantages of minimally invasive surgery to the treatment of VUR.
Data Availability
All datasets generated for this study are included in the manuscript and/or the supplementary files.
Author Contributions
ARS conceived the idea, laid the platform for the review with substantial additions to the manuscript, along with proofreading. RS prepared the initial manuscript and collected the raw data. KS prepared the literature review and part of data abstraction. CL and AKS reviewed the manuscript and made significant corrections.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Routh JC, Bogaert GA, Kaefer M, Manzoni G, Park JM, Retik AB, et al. Vesicoureteral reflux: current trends in diagnosis, screening, and treatment. Eur Urol. (2012) 61:773e82. doi: 10.1016/j.eururo.2012.01.002
2. Qureshi AH, Ajayi O, Schwaderer AL, Hains DS. Decreased Identification of vesicoureteral reflux: a cautionary tale. Front Pediatr. (2017) 5:175. doi: 10.3389/fped.2017.00175
3. Kurtz MP, Leow JJ, Varda BK, Logvinenko T, McQuaid JW, Yu RN, et al. The decline of the open ureteral reimplant in the United States: National data from 2003 to 2013. Urology. (2017) 100:193–7. doi: 10.1016/j.urology.2016.07.024
4. Paquin AJ. Ureterovesical anastomosis: the description and evaluation of a technique. J Urol. (1959) 82:573–83. doi: 10.1016/S0022-5347(17)65934-2
5. Cannon GM, Ost MC. Robot-assisted laparoscopic extravesical ureteral reimplantation for primary vesicoureteral reflux in children. J Urol. (2017) 197:1379–81. doi: 10.1016/j.juro.2017.03.112
6. Gargollo PC. Editorial comment regarding: Prospective multicenter study on robot-assisted laparoscopic extravesical ureteral reimplantation (RALUR-EV): outcomes and complications. J Ped Urol. (2018) 14:263–4. doi: 10.1016/j.jpurol.2018.02.018
7. Boysen W, Ellison JS, Kim C, Koh CJ, Noh P, Whittam B, et al. Multi-institutional review of outcomes and complications of robot-assisted laparoscopic extravesical ureteral reimplantation for treatment of primary vesicoureteral reflux in children. J Urol. (2017) 197:1555–61. doi: 10.1016/j.juro.2017.01.062
8. Boysen WR, Akhavan A, Ko J, Ellison JS, Lendvay TS, Huang J, et al. Prospective multicenter study on robot-assisted laparoscopic extravesical ureteral reimplantation (RALUR-EV): Outcomes and complications. J Pediatr Urol. (2018) 14:262.e1–6. doi: 10.1016/j.jpurol.2018.01.020
9. Peters CA. Robotically assisted surgery in pediatric urology. Urol Clin North Am. (2004) 31:743–52. doi: 10.1016/j.ucl.2004.06.007
10. Peters CA, Woo R. Intravesical robotically assisted bilateral ureteral reimplantation. J Endourol. (2005) 19:618–21. doi: 10.1089/end.2005.19.618
11. Marchini GS, Hong YK, Minnillo BJ, Diamond DA, Houck CS, Meier PM, et al. Robotic assisted laparoscopic ureteral reimplantation in children: case matched comparative study with open surgical approach. J Urol. (2011) 185:1870–5. doi: 10.1016/j.juro.2010.12.069
12. Chan KW, Lee KH, Tam YH, Sihoe JD. Early experience in robotic-assisted laparoscopic bilateral intravesical ureteral reimplantation for vesicoureteral reflux in children. J Robot Surg. (2012) 6:259–62. doi: 10.1007/s11701-011-0288-1
13. Casale P, Patel RP, Kolon TF. Nerve sparing robotic extravesical ureteral reimplantation. J Urol. (2008) 179:1987–9; discussion 1990. doi: 10.1016/j.juro.2008.01.062
14. Schomburg JL, Haberman K, Willihnganz-Lawson KH, Shukla AR. Robot-assisted laparoscopic ureteral reimplantation: a single surgeon comparison to open surgery. J Pediatr Urol. (2014) 10:875–9. doi: 10.1016/j.jpurol.2014.02.013
15. Srinivasan AK, Maass D, Shrivastava D, Long CJ, Shukla AR. Is robot-assisted laparoscopic bilateral extravesical ureteral reimplantation associated with greater morbidity than unilateral surgery? A comparative analysis. J Pediatr Urol. (2017) 13:494.e1–7. doi: 10.1016/j.jpurol.2017.01.021
16. Sillén U. Bladder dysfunction and vesicoureteral reflux. Adv Urol. (2008) 8:815472. doi: 10.1155/2008/815472
17. Smith RP, Oliver JL, Peters CA. Pediatric robotic extravesical ureteral reimplantation: comparison with open surgery. J Urol. (2011) 185:1876–81. doi: 10.1016/j.juro.2010.12.072
18. Akhavan A, Avery D, Lendvay TS. Robot-assisted extravesical ureteral reimplantation: outcomes and conclusions from 78 ureters. J Pediatr Urol. (2014) 10:864–8. doi: 10.1016/j.jpurol.2014.01.028
19. Chalmers D, Herbst K, Kim C. Robotic-assisted laparoscopic extravesical ureteral reimplantation: an initial experience. J Pediatr Urol. (2012) 8:268–71. doi: 10.1016/j.jpurol.2011.04.006
20. Gundeti MS, Boysen WR, Shah A. Robot-assisted laparoscopic extravesical ureteral reimplantation: technique modifications contribute to optimized outcomes. Eur Urol. (2016) 70:818–23. doi: 10.1016/j.eururo.2016.02.065
21. Silay MS, Baek M, Koh CJ. Robot-assisted laparoscopic extravesical ureteral reimplantation in children: top-down suturing technique without stent placement. J Endourol. (2015) 29:864–6. doi: 10.1089/end.2014.0815
22. Kasturi S, Sehgal SS, Christman MS, Lambert SM, Casale P. Prospective long-term analysis of nerve-sparing extravesical robotic-assisted laparoscopic ureteral reimplantation. Urology. (2012) 79:680–3. doi: 10.1016/j.urology.2011.10.052
23. Dangle PP, Shah A, Gundeti MS. Robot-assisted laparoscopic ureteric reimplantation: extravesical technique. BJU Int. (2014) 114:630–2. doi: 10.1111/bju.12813
24. Grimsby GM, Dwyer ME, Jacobs MA, Ost MC, Schneck FX, Cannon GM, et al. Multi-institutional review of outcomes of robot-assisted laparoscopic extravesical ureteral reimplantation. J Urol. (2015) 193(5 Suppl.):1791–5. doi: 10.1016/j.juro.2014.07.128
25. Hayashi Y, Mizuno K, Kurokawa S, Nakane A, Kamisawa H, Nishio H, et al. Extravesical robot-assisted laparoscopic ureteral reimplantation for vesicoureteral reflux: initial experience in Japan with the ureteral advancement technique. Int J Urol. (2014) 21:1016–21. doi: 10.1111/iju.12483
26. Arlen AM, Broderick KM, Travers C, Smith EA, Elmore JM, Kirsch AJ. Outcomes of complex robot-assisted extravesical ureteral reimplantation in the pediatric population. J Pediatr Urol. (2016) 12:169.e1–6. doi: 10.1016/j.jpurol.2015.11.007
27. Herz D, Fuchs M, Todd A, McLeod D, Smith J. Robot-assisted laparoscopic extravesical ureteral reimplant: A critical look at surgical outcomes. J Pediatr Urol. (2016) 12:402.e1–402.e9. doi: 10.1016/j.jpurol.2016.05.042
28. Pruthi R, Smith A, Wallen E. Evaluating the learning curve for robot-assisted laparoscopic radical cystectomy. J Endourol. (2008) 22:2469–74. doi: 10.1089/end.2008.0320
29. Kaul S, Shah N, Menon M. Learning curve using robotic surgery. Curr Urol Reports. (2016) 7:125–29. doi: 10.1007/s11934-006-0071-4
Keywords: robotic, ureteral reimplantation, RALUR, learning curve, extravesical approach
Citation: Sahadev R, Spencer K, Srinivasan AK, Long CJ and Shukla AR (2019) The Robot-Assisted Extravesical Anti-reflux Surgery: How We Overcame the Learning Curve. Front. Pediatr. 7:93. doi: 10.3389/fped.2019.00093
Received: 25 December 2018; Accepted: 04 March 2019;
Published: 29 March 2019.
Edited by:
Miguel Alfedo Castellan, University of Miami, United StatesReviewed by:
Andrew J. Kirsch, Children's Healthcare of Atlanta, United StatesYuval Bar-Yosef, Dana-Dwek Children's Hospital, Israel
Copyright © 2019 Sahadev, Spencer, Srinivasan, Long and Shukla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aseem Ravindra Shukla, c2h1a2xhYUBlbWFpbC5jaG9wLmVkdQ==
 Ravindra Sahadev
Ravindra Sahadev Katelyn Spencer
Katelyn Spencer Arun K. Srinivasan
Arun K. Srinivasan Aseem Ravindra Shukla
Aseem Ravindra Shukla