- 1Respiratory and Allergy Unit, 3rd Pediatric Department of National and Kapodistrian University of Athens, University General Hospital “Attikon,” Athens, Greece
- 22nd Pediatric Department of National and Kapodistrian University of Athens, “P & A Kiriakou” Children Hospital, Athens, Greece
- 3Faculty of Nursing, National and Kapodistrian University of Athens, Athens, Greece
Introduction: Poor adherence to inhaled medication is a commonly encountered problem among children with asthma. However, there is a relatively paucity of data regarding the adherence of children with severe asthma, as well as the merit of any interventions to improve this adherence.
Objectives: The aim of this systematic review was to identify the available literature on the rate of adherence and the influence of interventions in improving adherence to controller inhaled medication, in children with severe asthma.
Methods: A systematic literature search was performed in MEDLINE/PubMed, Cochrane Library, and Scopus databases. Studies were included in the present review if their target population were children and/or adolescents with severe asthma and presented data on medication adherence before and after a given intervention.
Results: A total of seven studies, conducted in USA, Canada, and UK, and published between 2012 and 2018, met the inclusion criteria. Adherence to controller medication was assessed via either objective or subjective measures (questionnaires), or a combination of them. Interventions included communication during pediatric visits and audio-taped medical visits, individualized care programs, electronic monitoring devices, interactive website and peak–flow prediction with feedback. Adherence rates for the baseline (before intervention) or for the control groups ranged from 28 to 67%. In general, there was a significant improvement of adherence after intervention with rates increasing to 49–81%.
Conclusion: Adherence rate in children with severe asthma is not satisfactory but it can be improved after proper interventions. Nevertheless, the heterogeneity among adherence assessment tools, and the variety of interventions, in combination with the lack of studies focusing on severe asthma, highlight the need for further research in this field.
Introduction
Asthma is a heterogeneous disease, usually characterized by chronic airway inflammation. It is defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness, and cough that vary over time and in intensity, together with variable expiratory airflow limitation (1). Asthma affects 1–18% of the population in different countries. It is one of the main causes of disability, health care services utilization, and quality of life impairment (2–5). It is estimated that about 14% of the children worldwide experience asthma symptoms. Asthma management aims to achieve good symptom control; maintain normal activities; minimize asthma attacks; reduce the side effects of treatment and as a result prevent the progression of obstructive lung damage during growth and then later in life (2).
Although the majority of asthma patients can be effectively controlled with the available medications, a substantial subset remains uncontrolled despite being offered the optimal therapy (6).
Severe asthma, according to European Respiratory Society (ERS)/American Thoracic Society (ATS) is asthma which requires Step 4 or 5 treatment [according to Global Initiative for Asthma (GINA) guidelines], e.g., high dose inhaled corticosteroids (ICS) and long-acting beta agonists (LABA) or leukotriene modifier/theophylline for the previous year or systemic ICS for ≥50% of the previous year to prevent it from becoming “uncontrolled” or which remains “uncontrolled” despite this therapy (6). Severe asthma includes patients with refractory or treatment-resistant asthma, or patients with incomplete response to treatment due to comorbidities (5). The primary approach of a child with problematic, severe respiratory symptoms, who is unresponsive to prescribed asthma therapy should be the confirmation of asthma diagnosis; secondly, one should explore if the child belongs to the category of “difficult-to-treat asthma” (2). The latter term is reserved for patients with ongoing factors that interfere with achieving good asthma control (allergen exposure, poor adherence), severe therapy-resistant asthma, asthma plus comorbidities (gastroesophageal reflux, obesity, obstructive apnea), or any combination of the above (7). Clinicians should also be aware of a prevalent cluster of chronic upper airway comorbidities, such as chronic rhinosinusitis and allergic rhinitis, which is recognized to patients with severe asthma but also seems that contribute to worsen asthma control and complicate asthma diagnosis and management (8). Distinguishing between severe asthma and uncontrolled asthma is crucial since the latter can be due to causes that can be more or less easily improved, such as the correction of a faulty inhaler technique and poor adherence (2, 9, 10).
Adherence is defined as the extent to which the patient's behavior matches the agreed recommendations from the prescriber. Patients can follow or not their doctors' recommendations, but failure to do so should not be a reason to blame the patient (11, 12). Adherence of asthmatics to long-term inhaled treatment has contributed substantial to asthma control and to morbidity reduction, yet, in general, it still remains suboptimal (13–16). The suboptimal adherence leads to poorer clinical outcomes and increased health care costs (17, 18).
Poor adherence (<60%) (19) to inhaled medication should be considered in all “difficult to control” patients. It has been reported that only 55% of children with moderate/severe persistent asthma use their controller medication daily (20).
Low adherence rates suggest an urgent need to increase adherence in order to reduce the burden of the disease. The improvement of adherence will result in better asthma control, and therefore, in a reduction of asthma severity (16, 21, 22). Shared decision making for medication/dose choice (23), inhaler reminders (24), home visits (25), prescribing ICS once daily versus twice (26), are all some of the interventions that are conducive to adherence improvement (2).
Although there are quite a few published reviews on the adherence of asthmatic children to controller medication and the effects of various interventions thereupon, there is still a lack of focus on severe asthma. Our aim in this systematic review was to identify the available literature on the rate of adherence and the influence of various interventions in improving adherence to controller inhaled medication, in children with severe asthma.
Methods
This systematic review was based on the Preferred Reporting Items for Systematic Reviews and Meta—Analyses (PRISMA) statement, which aims to be the most accurate elaboration of a systematic review (27).
Two independent reviewers searched for publications in three of the most commonly used databases in medicine: Pubmed/Medline, Cochrane Library and Scopus. Key word combinations of “children,” “severe asthma,” “difficult asthma,” “inhaled treatment,” and “adherence,” were used to retrieve articles with these key words in title and abstract.
The criteria included were as follows:
1) Articles which were published from January of 2012 to March of 2018;
2) Articles written in English;
3) Studies that targeted children and/or adolescents;
4) Studies which have focused on severe asthma (as their main aim or as a subcategory of the study population); and
5) Studies on the effect of an intervention on adherence rate (as their main aim or as a subcategory of the study population).
Data Extraction
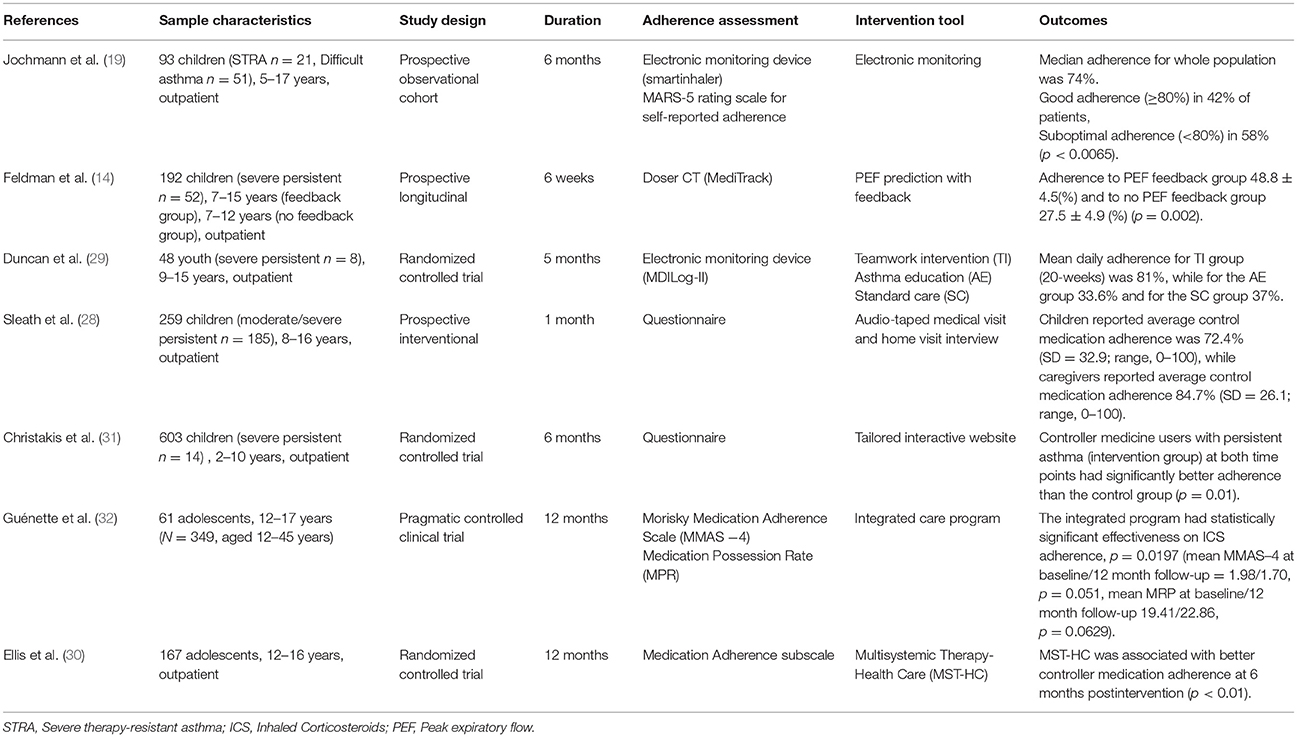
The data extraction was conducted by two reviewers. The characteristics collected for each study were references, sample characteristics, study design, duration, adherence assessment, intervention tools, and outcomes. Table 1 illustrates the main studies' characteristics.
Results
Studies Selection
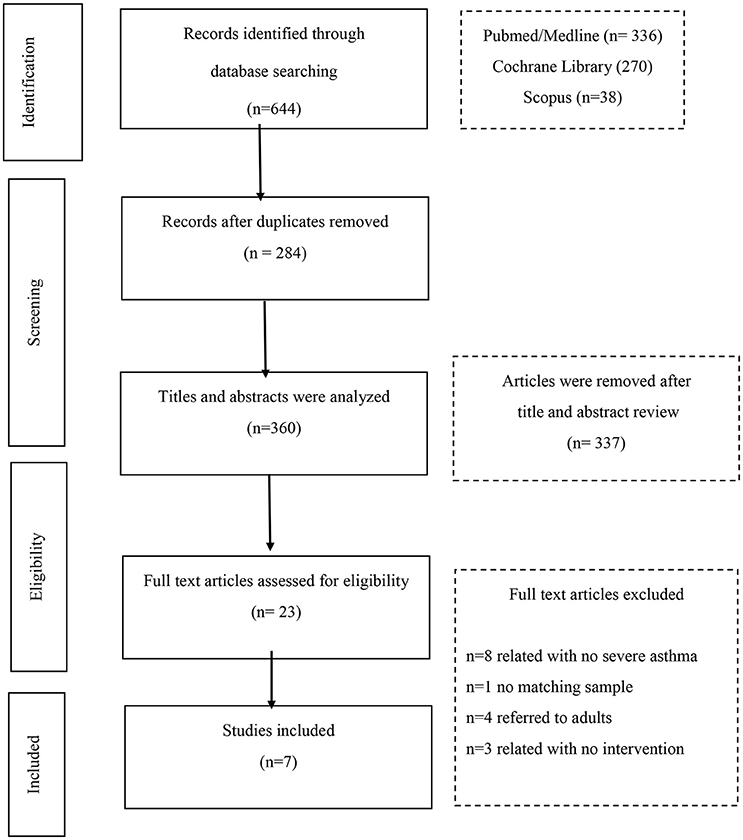
The search of the 3 databases retrieved 644 articles. Of these, 284 were duplicates and were excluded. The remaining 360 articles were screened for relevance. The full texts of 23 articles were assessed for eligibility; and finally 7 articles were chosen for the systematic review.
The reasons for the exclusions are listed on the flow chart (Figure 1) which also provides the publication retrieval process.
Studies Description
Seven articles were included in the systematic review. Five of them were conducted in USA (14, 28–31); 1 in Canada (32); and 1 in UK (19).
Three studies were randomized controlled clinical trials (29–31), 1 was a controlled pragmatic clinical trial (32), 1 was a prospective observational cohort (19), 1 was a prospective longitudinal study (14), and 1 was a prospective interventional study (28).
Patients' recruitment was held mainly during outpatient visits. Guénette et al. (32) recruited patients with mediation of community pharmacists. Patients were children and their age ranged from 2 to 16 years old.
The total sample for severe asthma from the 7 studies was n = 508 children/adolescents, with the lowest sample size being n = 8 (29) and the highest n = 185 (28).
Severe asthma was a subcategory of the selected sample in all seven studies (14, 19, 28–32).
Adherence Assessment
Adherence in ICS treatment was evaluated through objective (14, 29), or subjective measures (28, 30, 31) or as a combination of both (19, 32). Objective measures of adherence assessment included electronic monitoring devices (Smartinhaler, Doser CT, MDILog-II) used in 4 of the studies (14, 19, 29, 32) and Medication Possession Ratio (MPR) used by Guénette et al. (32).
All three pre-mentioned devices developed as a canister attachment that fits on top of the majority of inhalers. They can provide accurate information on medication usage, including timing and number of doses taken, and all these recorded data can be used to guide asthma management (19).
Medication Possession Ratio (MPR) is a validated objective measure based on pharmacy records expressing the percentage of days supply received divided by a period of time and has been found to be more accurate than self-report (33).
Questionnaires are subjective measures for assessing adherence that are convenient and relatively unobtrusive and rely on self-report (34). The questionnaires used in the studies reported in this review were:
(1) Brief Medication Questionnaire, a tool for screening patients' adherence as well as their barriers to adherence. The tool includes a 5-item Regimen Screen that asks patients how they took each medication in the past week, a 2-item Belief Screen that asks about drug effects and bothersome features, and a 2-item Recall Screen about potential difficulties remembering (35);
(2) Morisky medication adherence scale (MMAS-4) which is a generic self-reported, medication-taking behavior scale using four questions about past medication use patterns (36);
(3) Medication Adherence Rating Scale (MARS-5) is a self-reported measure which evaluates both attitudes about medications and actual medication-taking behavior and consists of 10 items (37); and
(4) Medication Adherence subscale which measures adherence to controller medication (38).
Intervention Tools
Among the 7 studies, 3 had as a main research objective the increase of adherence rate after an intervention (28, 29, 31) whereas in the other 4 studies (14, 19, 30, 32) the adherence improvement rate was a subcategory of the findings.
Interventions design addressed solely the children (14, 19, 32), or there was also an element of parental involvement (28–31).
Briefly, Sleath et al. (28) audio-taped and coded communication during pediatric visits whether the provider included child or caregiver input into the asthma management treatment plan. Individualized care programs where health teams assess patients' and their caregivers' individual needs, share information and improve knowledge on asthma, were implemented in 3 of the studies (29, 30, 32). Christakis et al. (31) created a web-based tailored intervention aiming to increase children's positive beliefs about asthma management and Feldman et al. (14) used a piece of equipment where peak—flow prediction with feedback encouraged children to receive daily their inhaled treatment. Electronic monitoring devices were used in 1 study as an intervention (19) with the perception that adherence could improve following a period of monitoring.
The mean duration of interventions was 22 weeks, whereas the maximum (30, 32) and minimum duration (28) was 48 and 4 weeks, respectively.
Outcomes
Across the 7 studies, adherence rates for the baseline (before intervention) or for the control groups ranged from 28 to 67% (14, 29, 31); there was a remarkable improvement after intervention with adherence rates increasing to 49–81% (14, 28, 29, 31).
Feldman et al. (14) found a significant positive difference between intervention and the control group (p = 0.02) using PEF prediction with feedback. Christakis et al. (31) using an interactive website, also showed a significant correlation between intervention and adherence improvement. Concerning the implementation of individualized care programs, Guénette et al. (32) showed statistically significant effectiveness measuring the adherence with MMAS-4 Scale (p = 0.0151). Statistically significant correlation was also demonstrated in Ellis et al. (30) measurements (p < 0.01). Team Intervention in Duncan et al. (29) study had a positive impact in adherence rate in comparison with Standard Care (81 vs. 37% respectively). Suboptimal adherence rates after intervention were presented in Jochman et al. (19) (median adherence was 74%) similarly with Sleath et al. (28) (average control medication adherence as reported by children was 72.4%).
Discussion
This systematic review isolated seven articles from the recent literature, which focused on the improvement of adherence to inhaled medication after specific intervention, in children with severe asthma. Overall, the results of this systematic review highlight the importance of interventions in respect of adherence improvement, in children suffering from severe asthma.
Adherence can be assessed with objective or subjective measures (34). Objective measures used in some of the studies included in the current review were different kinds of electronic monitoring devices and the Medication Possession Ratio. There have also been included studies that used questionnaires for the subjective assessment of adherence. Some of the main objective adherence measuring tools for asthma medication adherence are the electronic monitoring devices, the canister weight, and the pharmacy refill data (39). Electronic monitoring has been labeled as the “gold standard” for assessing adherence due to the objective and detailed data it provides, but the cost and technology requirements (e.g., equipment, staff training) prohibit its widespread routine clinical use (40, 41). At the same time, some of the subjective measuring tools are interviews, questionnaires and diary/self-reporting (34). Subjective measures are inexpensive, convenient, and relatively unobtrusive and have the potential to provide information on related issues. Although, they mostly rely on self-report, their accuracy depends on psychometric properties and may mask variability of adherence across regimen components if assessed globally (40–42). Table 2 shows advantages and disavantages of methods for adherence assessment (34). As a great heterogeneity has appeared in asthma population regarding individualized capabilities, needs and preferences (43), researchers have concluded that more targeted and personalized methods of assessment are required (44).
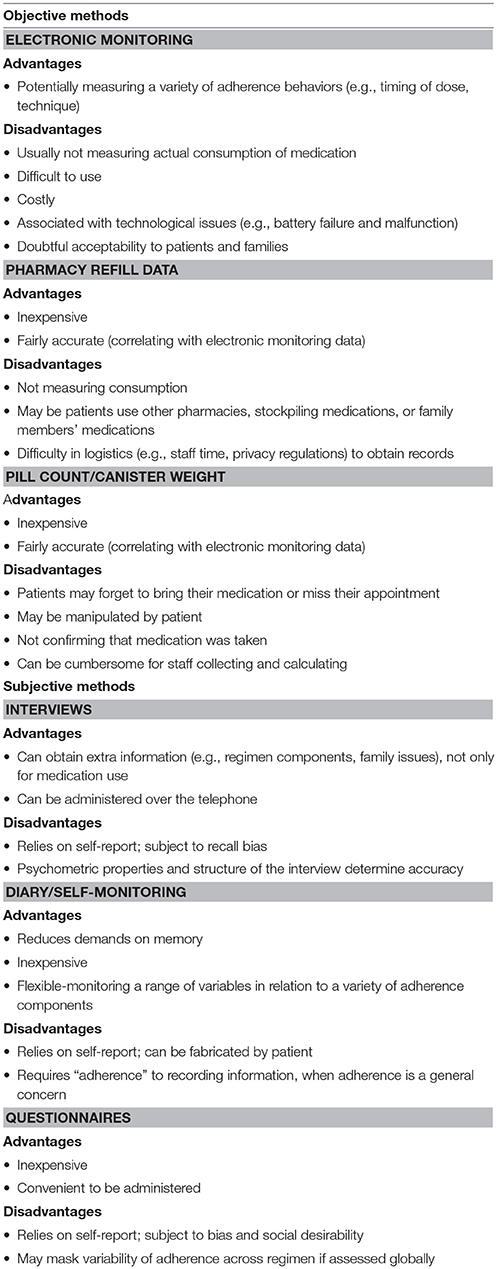
Table 2. Summary of objective and subjective methods for adherence assessment (34).
Severe asthma in children is known to cause great morbidity, and raise asthma costs. The exact prevalence is unknown although it is estimated that 2–5% of asthmatic children have severe disease (45, 46). There is an interrelated relationship between severe asthma and adherence and clinicians can overestimate the severity of asthma if they do not assess adherence. Furthermore, poor adherence can lead to severe asthma if it is not corrected (47). According to our findings, adherence rate at the baseline for children with severe asthma ranged between 28 and 67%, which is in agreement with Celano et al. (48) who studied children with persistent asthma. The adherence of children with any kind of asthma is approximately 30–70% (49).
In our systematic review, we retrieved studies with interventions aimed at improving adherence. Some of the intervention tools that had been used were: home interviews and audio-taped medical visits (28); individualized care programs (29, 30, 32); electronic monitoring device use (19); interactive website (31) and peak–flow prediction with feedback. In another systematic review and meta-analysis various behavioral interventions, e.g., providing families with specific strategies to manage the regimen; educational interventions providing basic information to families about the patient's illness and the importance of adherence; organizational interventions such as introducing calendars for self-monitoring and facilitating discussion with caregivers about their child's illness or supporting caregiver-health care provider interactions were meta-analyzed and discussed (50). Furthermore, two other studies investigated the efficacy and safety of text messages for dose reminding (51, 52).
Asthma control is associated with adherence level. Jochmann et al. (19) found that children with poor adherence maintained poor control, while Ellis et al. (30) showed that children with asthma knowledge and controller device use skills had better medication adherence. Similarly, in other studies, adolescents report that it is more likely to adhere to treatment when they feel hopeful (53), view management tasks as important and feel competent (54), or when they intend to follow treatment recommendations (55, 56). Increased levels of self-efficacy are associated with better adherence, as well (57, 58). Adherence to ICS was an independent strong predictor of long term asthma control, with highest levels of asthma control found when the adherence raised above 80% of prescribed doses (16).
Our review showed that adherence rate in children with severe asthma can be increased after a proper intervention. In the studies included in our review, there was a significant increase of adherence rate, from 28–67% to 49–81%. There is a relatively lack of studies focusing on severe asthma, but the above findings are in agreement with the results of studies dealing with all kinds of asthma in children (24, 59). Although, the majority of studies have shown a positive effect, there are some instances where intervention with electronic asthma medication reminders did not improve the adherence rate (32, 51). Also, a scheduled follow up visit in combination with a comprehensive asthma management care program implemented in preschool children with asthma in the Netherlands did not correct adherence rates (60). A systematic review and meta-analysis found the studies that applied adherence education as intervention achieved a benefit of 20% points over control, while electronic trackers or reminders led to better adherence rates of 10% points. Researchers concluded that interventions' results depend on the group target, method and duration of intervention (61).
The main limitations of this review is the lack of studies focusing on severe asthma in children as most of the studies investigate adherence in children in the community and adult severe asthmatics rather than severely asthmatic children. Additionally, there is heterogeneity in the definitions of severe asthma among studies, a problem that has already been noticed by other researchers (62). Studies used different adherence assessment tools, therefore results were presented in various ways.
A weak point of all 7 studies is that it is not clear whether the adherence improvement was clinically meaningful. Literature suggests that in order to maintain asthma control, adherence rates have to be in excess of 80% (63).
Conclusion
Adherence interventions have a positive impact on adherence rate in children with severe asthma. The remarkable heterogeneity between adherence assessment tools, and interventions, combined with the lack of studies focused on severe asthma, highlight the research gap in this field. There is a great need for further research focused exclusively on severe asthma and adherence treatment, in children.
Author Contributions
BB and DK contributed equally to this review. They did the literature research and the main writing of the article. VM, KP, and KD did all the academic support and the corrections during the whole process. KD did the finally fixing in English also.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brussell G, et al. After asthma: redefining airways diseases. Lancet (2017) 391:350–400. doi: 10.1016/S0140-6736(17)30879-6
2. ISAAC steering Committee. Worldwide variations in the prevalence of asthma symptoms: the Intrenational Study of Asthma and Allergies in Childhood (ISAAC). Eur Respir J. (1998) 12:315–35. doi: 10.1183/09031936.98.12020315
3. Bender BG, Rand C. Medication non-adherence and asthma treatment cost. Curr Opin Allergy Clin Immunol. (2004) 4:191–5. doi: 10.1097/01.all.0000129449.73727.cf
4. Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. (2015) 45:396–407. doi: 10.1183/09031936.00075614
5. GINA Report, Global Strategy for Asthma Management and Prevention. From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) (2018). Available online at: http://www.ginaasthma.org (Accessed April 4, 2018).
6. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. (2014) 43:343–73. doi: 10.1183/09031936.00202013
7. Bush A, Fleming L, Saglani S. Severe asthma in children. Respirology (2017) 22:886–97. doi: 10.1111/resp.13085
8. Licari A, Brambilla I, De Filippo M, Poddighe D, Castagnoli R, Marseglia GL. The role of upper airway pathology as a co-morbidity in severe asthma. Expert Rev Respir Med. (2017) 11:855–65. doi: 10.1080/17476348.2017.1381564
9. Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. (2011) 105:930–8. doi: 10.1016/j.rmed.2011.01.005
10. Boulet LP, Vervloet D, Magar Y, Foster JM. Adherence: the goal to control asthma. Clin Chest Med. (2012) 33:405–17. doi: 10.1016/j.ccm.2012.06.002
11. Haynes RB. Determinants of compliance: the disease and the mechanics of treatment. In: Haynes RB, Taylor DW, Sackett DL, editors. Compliance in Health Care. Baltimore, MD: Johns Hopkins University Press (1979). p. 49–62.
12. Horne R. Compliance, adherence and concordance: Implications for asthma treatment. Chest (2006) 130:65–72. doi: 10.1378/chest.130.1_suppl.65S
13. Klok T, Kaptein AA, Duiverman EJ, Brand PL. High inhaled corticosteroids adherence in childhood asthma: the role of medication beliefs. Eur Respir J. (2012) 40:1149–55. doi: 10.1183/09031936.00191511
14. Feldman JM, Kutner H, Matte L, Lupkin M, Steinberg D, Sidora-Arcoleo K, et al. Prediction of peak flow values followed by feedback improves perception of lung function and adherence to inhaled corticosteroids in children with asthma. Thorax (2012) 67:1040–5. doi: 10.1136/thoraxjnl-2012-201789
15. Vasbinder E, Dahhan N, Wolf B, Zoer J, Blankman E, Bosman D, et al. The association of ethnicity with electronically measured adherence to inhaled corticosteroids in children. Eur J Clin Pharmacol. (2013) 69:683–90. doi: 10.1007/s00228-012-1380-9
16. Klok T, Kaptein AA, Duiverman EJ, Brand PL. Long-term adherence to inhaled corticosteroids in children with ashma: observational study. Respir Med. (2015) 109:1114–9. doi: 10.1016/j.rmed.2015.07.016
17. Kew KM, Normansell R, Stovold E. Intervention to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev. (2016) 6:CD012226. doi: 10.1002/14651858.CD012226.
18. World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization (2003). Available online at: http://www.who.int/chp/knowledge/publications/adherence_report/en/ (Assessed April 8, 2018).
19. Jochmann A, Artusio L, Jamalzadeh A, Nagakumar P, Delgado-Eckert E, Saglani S, et al. Electronic monitoring of adherence to inhaled corticosteroids: an essential tool in identifying severe asthma in children. Eur Respir J. (2017) 50:1700910. doi: 10.1183/13993003.00910-2017
20. Diette GB, Skinner EA, Markson LE, Algatt-Bergstrom P, Nguyen TT, Clark RD, et al. Consistency of care with national guidelines for children with asthma in managed care. J Pediatr. (2001) 138:59–64. doi: 10.1067/mpd.2001.109600
21. Jentzsch NS, Camargos P, Sarinho E, Bousquet J. Adherence rate to beclomethasone dipropionate and the level of asthma control. Respir Med. (2012) 106:338–43. doi: 10.1016/j.rmed.2011.12.001
22. Klok T, Kaptein AA, Duiverman EJ, Brand PL. It's adherence, stupid (that determines asthma control in preschool children) Eur Respir J. (2014) 43:783–91. doi: 10.1183/09031936.00054613
23. Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence: systematic review and meta-analysis. BMJ (2003) 326:1308–9. doi: 10.1164/rccm.200906-0907OC
24. Chan AH, Stewart AW, Harrison J, Camargo CA Jr, Black PN, Mitchell EA. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respir Med. (2015) 3:210–9. doi: 10.1016/S2213-2600(15)00008-9
25. Otsuki M, Eakin MN, Rand CS, Butz AM, Hsu VD, Zuckerman IH, et al. Adherence feedback to improve asthma outcomes among inner-city children: a randomized trial. Pediatrics (2009) 124:1513–21. doi: 10.1542/peds.2008-2961
26. Price D, Robertson A, Bullen K, Rand C, Horne R, Staudinger H. Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: a randomized open-label study. BMC Pulm Med. (2010) 10:1. doi: 10.1186/1471-2466-10-1
27. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
28. Sleath B, Carpenter DM, Slota C, Williams D, Tudor G, Yeatts K, et al. Communication during pediatric asthma visits and self-reported asthma medication adherence. Pediatrics (2012) 130:627–33. doi: 10.1542/peds.2012-0913
29. Duncan CL, Hogan MB, Tien KJ, Graves MM, Chorney JM, Zettler MD, et al. Efficacy of a parent-youth teamwork intervention to promote adherence in pediatric asthma. J Pediatr Psychol. (2012) 38:617–28. doi: 10.1093/jpepsy/jss123
30. Ellis DA, King P, Naar-King S. Mediators of treatment effects in a randomized clinical trial of multisystemic therapy-health care in adolescents with poorly controlled asthma: disease knowledge and device use skills. J Pediatr Psychol. (2016) 41:522–30. doi: 10.1093/jpepsy/jsv114
31. Christakis DA, Garrison MM, Lozano P, Meischke H, Zhou C, Zimmerman FJ. Improving parental adherence with asthma treatment guidelines: a randomized controlled trial of an interactive website. Acad Pediatr. (2012) 12:302–11. doi: 10.1016/j.acap.2012.03.006
32. Guénette L, Breton MC, Grégoire JP, Jobin MS, Bolduc Y, Boulet LP, et al. Effectiveness of an asthma integrated care program on asthma control and adherence to inhaled corticosteroids. J Asthma (2015) 52:638–45. doi: 10.3109/02770903.2014.999084
33. Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros (2006) 5:117–85. doi: 10.1016/j.jcf.2006.03.002
34. Duncan CL, Mentrikoski JM, Wu YP, Fredericks EM. Practice-based approach to assessing and treating nonadherence in pediatric regimens. Clin Pract Pediatr Psychol. (2014) 2:322–36. doi: 10.1037/cpp0000066
35. Svarstad BL, Chewning BA, Sleath BL, Claesson C. The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns. (1999) 37:113–24.
36. Morisky DE. Nonadherence to medical recommendations for hypertensive patients: problems and potential solutions. J Compliance Health Care (1986) 1:5–32.
37. Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of ‘illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health (2002) 17:17–32. doi: 10.1080/08870440290001502
38. Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) (2008) 10(5):348-54.
39. Jentzsch NS, Camargos PA. Methods of assessing adherence to inhaled corticosteroid therapy in children and adolescents: adherence rates and their implications for clinical practice. J Bras Pneumol. (2008) 34:614-21. doi: 10.1590/S1806-37132008000800012
40. Hommel KA, Greenley RN, Maddux MH, Gray WN, Mackner LM. Self-management in pediatric inflammatory bowel disease: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. (2013) 57:250–7. doi: 10.1097/MPG.0b013e3182999b21
41. Quittner AL, Modi AC, Lemanek KL, Ievers-Landis CE, Rapoff MA. Evidence-based assessment of adherence to medical treatments in pediatric psychology. J Pediatr Psychol. (2008) 33:916–36. doi: 10.1093/jpepsy/jsm064
42. Garfield S, Clifford S, Eliasson L, Barber N, Willson A. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. (2011) 11:149. doi: 10.1186/1471-2288-11-149
43. Thomas M. Why aren't we doing better in asthma: time for personalized medicine? NPJ Prim Care Respir Med. (2015) 25:15004. doi: 10.1038/npjpcrm.2015.4
44. van Boven JF, Trappenburg JC, van der Molen T, Chavannes NH. Towards tailored and targeted adherence assessment to optimise asthma management. NPJ Prim Care Respir Med. (2015) 25:15046. doi: 10.1038/npjpcrm.2015.46
45. Lang A, Carlsen KH, Haaland G, Devulapalli CS, Munthe-Kaas M, Mowinckel P, et al. Severe asthma in childhood: assessed in 10 year olds in a birth cohort study. Allergy (2008) 63:1054–60. doi: 10.1111/j.1398-9995.2008.01672.x
46. Nordlund B, Melen E, Schultz ES, Gronlund H, Hedlin G, Kull I. Prevalence of severe childhood asthma according to the WHO. Respir Med. (2014) 108:1234–37. doi: 10.1016/j.rmed.2014.05.015
47. Pike KC, Levy ML, Moreiras J, Fleming L. Managing problematic severe asthma: beyond the guidelines. Arch Dis Child. (2018) 103:392–397. doi: 10.1136/archdischild-2016-311368
48. Celano MP, Linzer JF, Demi A, Bakeman R, Smith CO, Croft S, et al. Treatment adherence among low-income, African American children with persistent asthma. J Asthma (2010) 47:317–322. doi: 10.3109/02770900903580850
49. Jentzsch NS, Camargos PA, Colosimo EA, Bousquet J. Monitoring adherence to beclomethasone in asthmatic children and adolescents through four different methods. Allergy (2009) 64:1458–62. doi: 10.1111/j.1398-9995.2009.02037.x
50. Wu YP, Pai AL. Health care provider-delivered adherence promotion interventions: a meta-analysis. Pediatrics (2014) 133:1698–707. doi: 10.1542/peds.2013-3639
51. Mosnaim G, Li H, Martin M, Richardson D, Belice PJ, Avery E, et al. The impact of peer support and mp3 messaging on adherence to inhaled corticosteroids in minority adolescents with asthma: a randomized, controlled trial. J Allergy Clin Immunol Pract. (2013) 1:485–493. doi: 10.1016/j.jaip.2013.06.010
52. Kenyon CC, Gruschow SM, Quarshie WO, Griffis H, Leach MC, Zorc JJ, et al. Controller adherence following hospital discharge in high risk children: A pilot randomized trial of text message reminders. J Asthma (2018) 13:1–9. doi: 10.1080/02770903.2018.1424195
53. Burgess SW, Sly PD, Morawska A, Devadason SG. Assessing adherence and factors associated with adherence in young children with asthma. Respirology (2008) 13:559–63. doi: 10.1111/j.1440-1843.2008.01292.x
54. Armstrong ML, Duncan CL, Stokes JO, Pereira D. Association of caregiver health beliefs and parenting stress with medication adherence in preschoolers with asthma. J Asthma (2014) 51:366–72. doi: 10.3109/02770903.2013.876431
55. Capo-Ramos DE, Duran C, Simon AE, Akinbami LJ, Schoendorf KC. Preventive asthma medication discontinuation among children enrolled in fee-for-service Medicaid. J Asthma (2014) 51:618–26. doi: 10.3109/02770903.2014.895010
56. Mulvaney SA, Ho YX, Cala CM, Chen Q, Nian H, Patterson BL, et al. Assessing adolescent asthma symptoms and adherence using mobile phones. J Med Internet Res. (2013) 15:141. doi: 10.2196/jmir.2413
57. Edgecombe K, Latter S, Peters S, Roberts G. Health experiences of adolescents with uncontrolled severe asthma. Arch Dis Child. (2010) 95:985–91. doi: 10.1136/adc.2009.171579
58. Blaakman SW, Cohen A, Fagnano M, Halterman JS. Asthma medication adherence among urban teens: a qualitative analysis of barriers, facilitators and experiences with school-based care. J Asthma (2014) 51:522–9. doi: 10.3109/02770903.2014.885041
59. Vasbinder EC, Goossens LM, Rutten-van Mölken MP, de Winter BC, van Dijk L, Vulto AG, et al. e-Monitoring of asthma therapy to improve compliance in children (e-MATIC): a randomised controlled trial. Eur Respir J. (2016) 48:758–67. doi: 10.1183/13993003.01698-2015
60. Keemink YS, Klok T, Brand PL. Long-term adherence to daily controller medication in children with asthma: the role of outpatient clinic visits. Pediatr Pulmonol. (2015) 50:1060–4. doi: 10.1002/ppul.23138
61. Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev. (2017) 4:CD012226. doi: 10.1002/14651858.CD012226.pub2
62. Hedlin G, Bush A, Lødrup Carlsen K, Wennergren G, De Benedictis FM, Melén E, et al. Problematic severe asthma in children, not one problem but many: a GA2LEN initiative. Eur Respir J. (2010) 36:196–201. doi: 10.1183/09031936.00104809
Keywords: children, severe asthma, difficult asthma, inhaled treatment, adherence
Citation: Boutopoulou B, Koumpagioti D, Matziou V, Priftis KN and Douros K (2018) Interventions on Adherence to Treatment in Children With Severe Asthma: A Systematic Review. Front. Pediatr. 6:232. doi: 10.3389/fped.2018.00232
Received: 22 May 2018; Accepted: 31 July 2018;
Published: 21 August 2018.
Edited by:
Renato Cutrera, Bambino Gesù Ospedale Pediatrico (IRCCS), ItalyReviewed by:
Gian Luigi Marseglia, Policlinico San Matteo Fondazione (IRCCS), ItalyPaolo Bottau, Azienda Unità Sanitaria Locale (AUSL) Imola, Italy
Copyright © 2018 Boutopoulou, Koumpagioti, Matziou, Priftis and Douros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Boutopoulou, Ym1wb3V0b3BvdWxvdUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Barbara Boutopoulou
Barbara Boutopoulou Despoina Koumpagioti2†
Despoina Koumpagioti2† Kostas N. Priftis
Kostas N. Priftis Konstantinos Douros
Konstantinos Douros