
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 05 June 2018
Sec. Pediatric Cardiology
Volume 6 - 2018 | https://doi.org/10.3389/fped.2018.00167
Objectives: Masked hypertension (MH) is not uncommon in the youth and may increase risks of long-term cardiovascular impairment. However, little is known about the subclinical heart damage in this group of patients. Currently, 3-layer speckle tracking imaging based on two-dimensional echocardiography is feasible to detect the early signs of myocardial damage. We therefore aimed to investigate whether subtle changes of cardiac function occurred in the young MH patients by using advanced quantification with layer-specific speckle tracking.
Methods: A total of 40 adolescents with MH (age 18 ± 3 years, 73% males) and 40 age-, gender-, race-, and height-matched normotensive volunteers were enrolled in our study. MH was defined as one or more of the ambulatory blood pressure (BP) parameters (24-h, daytime and night-time average BPs) higher than ≥ 95th percentile for gender and height according to the local reference. Both comprehensive two-dimensional echocardiography with layer-specific strain analysis and 24-h ambulatory BP monitoring were performed. Longitudinal strain and circumferential strain in endocardial, mid-myocardial, and epicardial layers were determined accordingly with the dedicated software (EchoPAC software version 201, GE Healthcare, Horten, Norway).
Results: Compared with normotensive controls, youths with MH had higher ambulatory pulse rate and left ventricular mass index, and were more obese. Interestingly, similar ventricular volumes and ejection fraction were observed in the study groups, but further analysis with layer-specific strains revealed that endocardial and mid-myocardial longitudinal and circumferential mechanical function were decreased in the young MH subjects when compared to normotensive individuals (all p < 0.05). However, there were no difference regarding radial strain and apical rotation derived from traditional speckle tracking analysis.
Conclusion: Subclinical change of LV mechanic function assessed by layer-specific speckle tracking is present in youth with MH despite considered as normal with conventional ways.Thus, MH in youth should be monitored closely instead of labeling as an entirely benign entity.
Masked hypertension (MH) has recently become a topic of interest in youths; it refers to a condition in which office blood pressure (BP) is normal but being elevated during ambulatory BP monitoring (1). Actually, MH is not uncommon in the youth. The prevalence ranges from 7.6% in 592 unselected children to 9.4% in 85 youths referred for hypertension evaluation and to 11.9% in 1445 Chinese children from a large territory-wide school-based cohort in Hong Kong (2–4). A growing body of evidence suggests that MH in adults has been associated with more extensive target organ damage than in true normotensive subjects, and similar to that in people with sustained hypertension (5), such as higher left ventricular mass index and thickening of the carotid wall (2). Hence, it is reasonable to infer that MH in youths may increase risks of long-term cardiovascular impairment. Consequently, comprehensive assessment of myocardial function at an early stage may be of clinical importance in MH patients and might provide more information in the risk stratification of these patients.
Two-dimensional (2D) speckle-tracking echocardiography (STE), capable of obtaining quantitative measurements of whole-layer longitudinal, circumferential, and radial directional deformation without the angle dependency of one-dimensional techniques, has been well recognized to allow easily early detection of myocardial damage while left ventricular (LV) ejection fraction (LVEF) is still preserved in various pathologies (6–9). Recently, technological progression of 2D-STE software has enabled the assessment of layer-specific strain, thus allowing to comprehensively evaluate myocardial deformation layer by layer (endocardial, mid-myocardial and epicardial levels), which seems superior in detecting subtle deteriorations of cardiac contractility. Moreover, a report from the EACVI-ASE Strain Standardization Task Force released the feasibility, accuracy, and reproducibility of layer-specific global longitudinal strain measurement (10). Furthermore, the clinical usefulness of the layer-specific technology has already been successfully tested in some clinical settings, including coronary artery disease, myocardial infarction, arterial hypertension, and heart failure (11–16). Of note, recent analysis revealed the layer-specific impairment of LV mechanical function in adult MH patients (age > 50 years old) (8). However, the influence of MH on LV mechanics at its early stage has not been investigated yet.
Thus, this study for the first time provided the new insight to LV mechanical function in youths with MH, and assessed the occurrence of early echocardiographic signs of subclinical left ventricular dysfunction in this young group, using advanced quantification with layer-specific 2D speckle tracking.
This was a community-based cohort study, which initially enrolled 1445 ethnic Chinese students for surveying 24-h ambulatory BP in Hong Kong during 2011/2012. The presented local reference was used for the definition of MH in Chinese adolescents as one or more of the ambulatory BP parameters (24-h, daytime and night-time average BPs) were higher than 95th percentile for age, gender and height, and the office BPs were normal (4, 17). Totally, 165 subjects (11.9%) were identified as MH at baseline. From June 2015 to July 2016, subjects diagnosed with MH at baseline were invited to recheck ambulatory BP in our center. However, fourteen subjects refused to consent and 7 subjects were lost contact. Among the remaining 144 cases, only 40 (aged from 13 to 22 years) were confirmed with persistent MH and performed echocardiography in our study. Accordingly, 40 normotensive controls, matched with cases for sex, age, race, and body height, were randomly selected from the original cohort. The office BP, 24-h, daytime and night-time average systolic blood pressure (SBP) and diastolic blood pressure (DBP) in controls were all below the sex-specific, age-specific and height-specific 95th percentile during the latest survey. Exclusion criteria were as follows: (1) concomitant pathologies known to impair myocardial contraction (e.g., diabetes, myocardial infarction, and hypertension); (2) uncontrolled arrhythmia; (3) other severe comorbidities that may affect cardiac function; (4) poor echocardiographic image quality or (5) patient unwillingness.
Anthropometric measurements (height, weight) and laboratory analyses (level of plasma fasting glucose, cholesterol and triglycerides) were obtained from all the subjects included in the study. Body mass index (BMI) and body surface area (BSA) were calculated for each subject. The study was approved by the Joint the Chinese University of Hong Kong and New Territories East Cluster Clinical Research Ethics Committee and the Ethics Committee of the Department of Health of the Hong Kong Government (CRE-2013.563). Informed consent was obtained from the participants and their parents before commencement of the monitoring.
Conventional BP measurements were obtained by measuring the average value of the 3 consecutive measurements in the sitting position, at least 1 min apart and after 5 min of rest, using the Datascope Accutorr Plus (Datascope Corporation, Mahwah, New Jersey, USA), which has been validated in our local Chinese children and adolescents (17, 18). Appropriate cuff size was used according to bladder width (at least 40% of the arm circumference) and length (6 × 12, 9 × 18, or 10 × 24 cm to cover 80–100% of the individual's arm circumference) (19). Two resting office BP measurements were taken before mounting and after dismounting the 24-h BP monitoring device on the following day. A third measurement was taken within 4 weeks for those who had both readings high. The reference values for defining high BP in the office are based on our previous local data (17).
All the participants underwent a 24-h BP monitoring. The non-invasive 24-h ambulatory BP monitoring was performed on the non-dominant arm during a weekday with typical activities, using a portable non-invasive oscillometric recorder (TM-2430, A&D Company Limited, Tokyo, Japan). The ABP readings will be obtained every 30 min over a 24-h period. Only ABP profiles with at least 40 recordings, including at least eight readings during sleep, will be accepted. Individual mean systolic, diastolic and mean arterial BP will be calculated for wake and sleep periods. All readings taken during 24 hours will be used to calculated mean 24-h, mean daytime and mean night-time SBP and DBP, in accordance with the method proposed by Soergel et al. (20). Local reference will be used for the definition of ambulatory hypertension as 24-h, daytime or night-time ambulatory BP higher than 95th percentile for gender and height (17). Subjects with office normotension and ambulatory hypertension will be characterized as MH.
Echocardiographic examination was performed using a Vivid E9 system (GE Healthcare, Horten, Norway), within 2–3 days after 24-h BP monitoring. Routine 2D cine loops of three consecutive cardiac cycles were obtained at end-expiratory apnea from parasternal long-axis and short-axis views and the standard three apical views (four-chamber, two-chamber, and long-axis) by the same operator. Grayscale recordings were optimized for LV evaluation at a frame rate between 50 and 70 fbp and saved for subsequent strain analyses. LV volume and LVEF were calculated with biplane Simpson method. Left ventricular mass index was determined according to the corrected method of the American Society of Echocardiography (ASE) and indexed to body height2.7 (2). The peak early (E) and late (A) transmitral flow velocities during diastole were obtained by pulsed-wave Doppler echocardiography, with the sample volume placed at the mitral leaflet tips, and then the ratio of early-to-late peak velocities (E/A) was calculated. Early diastolic septal mitral annular velocities (e′) were obtained from pulse wave velocity of spectral tissue Doppler imaging and the E/e′ ratio was then calculated (21).
All data of 2D speckle tracking were analyzed off-line using the dedicated EchoPAC V201 software (GE Healthcare, Horten, Norway). The advanced software automatically tracked the contour of endocardium to cover the myocardial thickness of the entire LV wall at an end-diastolic frame, similarly to traditional 2D-STE method, but allowing investigation of three myocardial layers for longitudinal and circumferential strain: endocardial, mid-myocardial and epicardial level. Adequate tracking could be verified in real time and corrected by manual to ensure optimal tracking of endocardium and epicardium and inclusion of the entire LV thickness including endocardial, mid-myocardial and epicardial layers in all observed echocardiographic views (Figure 1). Layer-specific longitudinal strain (LS) was analyzed from the three standard apical views, whereas layer-specific circumferential strain (CS) and radial strain (RS) were evaluated in short axis view at the papillary muscles level. Global strain was calculated as the average of all three layer-specific strains (endocardial, mid-myocardial, and epicardial). The global RS was the mean value of 6 segments from papillary muscles level. The LV apical rotation was measured from parasternal apical level.
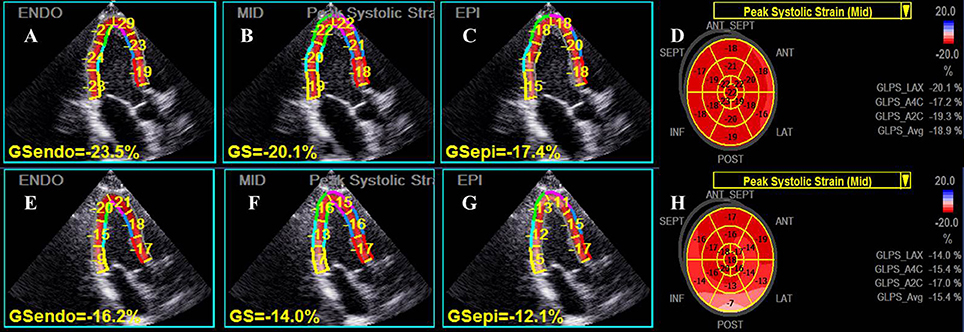
Figure 1. Representative layer-specific longitudinal strain (LS) analyses in apical three-chamber view. The upper row, from left to right panel (A–D), showed the endocardial, mid-myocardial and epicardial layer as well as global mid-myocardial LS results respectively in an individual from the control group, while the lower row (E–H) showed the corresponding LS values in a young patient with masked hypertension. Significantly reduced values of LS for all myocardial layers were presented in the patient.
Intra-observer and inter-observer variabilities of 2D specific-layer strain data were evaluated in 15 randomly subjects by two sonographers blindly. Intra- and inter-observer variabilities were evaluated by means of intraclass correlation coefficients (ICCs).
Collected data were computerized and analyzed using the IBM SPSS Statistics, version 20 (IBM) software. Continuous variables are expressed as mean ± SD and nominal variables as percentages. Data were compared between 2 groups using the independent sample t-tests for continuous variables, as well as the chi-square test and Fisher's exact test for categorical variables. The P < 0.05 was considered statistically significant.
None of subjects was found to be primary or secondary hypertensive. Patient characteristics are presented in Table 1. The study groups were of similar age, gender and height distribution. However, BSA and BMI were higher in young MH patients than in controls. Compared with normotensive subjects, participants with MH had higher office BPs as well as ambulatory pulse rates over 24 hours, daytime, and night-time. Also, young MH subjects had higher plasma glucose and triglycerides level than normal controls. There was no difference in heart rate and cholesterol levels between the both groups.
Routine echocardiographic features of the study population were shown in Table 2. Left ventricular mass indexed to body height2.7 was significantly higher in cases than in normotensive individuals. There was no significant difference in the LV wall thickness, ventricular volumes and LVEF between the observed groups. Interestingly, young subjects with MH had lower mitral E/A ratio and mitral annulus e′ when compared with normotensive individuals (all P < 0.05), but no difference in E/e′.
2D-STE derived LV global LS and CS were significantly lower in youth with MH than in controls. Moreover, comparing with true normotensives, LV systolic and early diastolic longitudinal strain rates in MH subjects were significantly decreased, while late diastolic strain rates tended to increase in MH, although without statistically significance (Table 3). However, radial and rotational mechanics was mostly similar between the observed groups.
Further layer-specific 2D strain analysis revealed that endocardial and mid-myocardial longitudinal and circumferential mechanical functions during systole were significantly worsened in the young MH individuals when compared to normotensive controls (all p < 0.05) (Table 3 and Figure 2).
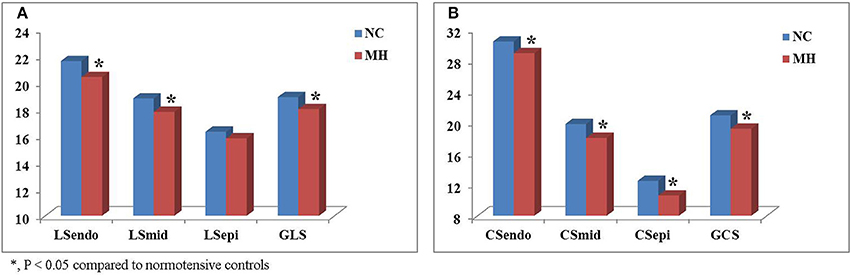
Figure 2. Comparison of layer-specific strains between young subjects with masked hypertension (MH) and normotensive controls (NC). Left ventricular global longitudinal (LS) (A) and circumferential strain (CS) (B) were significantly lower in youth with MH than in normotensive controls. Moreover, layer-specific strain analysis showed that endocardial and mid-myocardial LS (A) and CS (B) during systole were significantly worsened in the young MH individuals when compared to controls. LSendo, endocardial longitudinal strain; LSmid, mid-myocardial longitudinal strain; LSepi, epicardial longitudinal strain; GLS, global longitudinal strain; CSendo, endocardial circumferential strain; CSmid, mid-myocardial circumferential strain; CSepi, epicardial circumferential strain; GCS, global circumferential strain. *P < 0.05.
As it has been shown in Table 4, intra- and inter-observer agreement are high for all parameters of 2D-STE LV multilayer strains.
Recent studies have demonstrated that the prevalence of MH in youth is more common than previously reported and appears to be more closely associated with increased cardiovascular risk in later adulthood (2–5). Consequently detection of subtle LV contractile dysfunction at an early subclinical stage allows earlier intervention to avoid irreversible consequent LV deterioration. Of note, 2D-STE is a rapidly evolving tool for the assessment of myocardial deformation in children (22), without the angle dependency of one-dimensional technique. Moreover, the advanced strain analysis has been supported by the latest guidelines that recommended determination of LV strains in adult hypertension, due to its high predictive value that is even higher than for conventional LVEF (23). Recent clinical studies had reported that LV myocardial strain is a sensitive marker of early cardiac dysfunction, and is associated with outcomes in pediatric disease processes known to impair contractility, such as diabetes mellitus, chemotherapy cardiotoxicity, amyloidosis, Kawasaki disease, etc. (6, 24–26). Nevertheless, no data has provided any information on the MH in the youth.
In the present study, we have compared measurements of cardiac function between hypertensive and normotensive children. Similar to the previous studies, patients with MH had higher ambulatory pulse rate and plasma glucose level than normotensive subjects, and were more obese (3, 27). However, commonly used echocardiographic parameters of systolic function including LV volumes and LVEF were not significantly different between the study groups. Therefore, our study for the first time provided insight in LV structural, functional, and mechanical changes in young MH patients by using the advanced and more sensitive layer-specific strain analysis.
Importantly, our results revealed that the young MH subjects were associated with subclinical LV systolic dysfunction, which can be identified as a reduction in longitudinal and circumferential LV deformations and, particularly, in endocardial and mid-myocardial layers. Thus, our study confirms the application of these measurements for early detection of myocardial impairment consistent with recently published comparable results in adult MH patients (8), which demonstrated significantly reduced LS and CS of all three LV myocardial layers (endocardial, mid-myocardial and epicardial) in adult masked hypertensives.
It is still controversial regarding the number of layers in myocardial wall. Nevertheless, there is the agreement that the myocyte arrangement in different layers is different (8, 13). Endocardial and epicardial fibers are mainly longitudinally oriented, whereas the mid-myocardial fibers are circumferentially directed (8, 20). Moreover, previous study revealed a decreasing gradient layer-specific strain from the endocardium to the epicardium in both controls and adult patients with hypertension (13–28). Using animal model, investigators found that the endocardium was most vulnerable to the effect of LV filling pressure, and its function was easily impaired in patients with hypertension (29). Furthermore, considering that LV hypertrophy occurs from endocardium to epicardium, with myocytes hypertrophy, fibroblast proliferation and interstitial changes (29–31), it is essential to assess the influence of hypertrophy on each myocardial layer. Interestingly, our findings showed that, LS and CS of endocardial and mid-myocardial layers have been affected in young MH subjects, which indicated that deterioration of LV mechanical function was spreading from endocardium to epicardium even in subjects who do not have sustained BP elevation.
On the other hand, we observed that LV apical rotation was preserved in the MH group. This is similar with the previous hypertension study in pediatrics, which demonstrated no statistical difference of LV rotation in children and adolescents with systemic arterial hypertension compared with those with normotension (32). A potential explanation could be that in the young cohort with MH or hypertension, the disease state had not been presented for long enough to induce an impact on the myocardium to impair LV apical rotation.
As for LV diastolic function, the conventional echocardiographic parameters like E/A ratio and mitral annulus e′, were decreased in the MH group, when compared to the normal controls. Also, the E/e′ ratio, a surrogate for filling pressure, tended to be higher in masked hypertensions despite no statistically difference between the observed groups. Of note, we for the first time applied the advanced speckle-tacking analysis to evaluate LV diastolic function in subjects with MH. In line with the previous studies in adult MH patients, our findings showed that LV longitudinal strain rates during systole and early diastole were significantly lower in this young MH group than in confirmed normotensive subjects, whereas the late diastolic strain rate tended to increase in MH, although without statistically significance.
Previous studies have demonstrated MH is closely associated with obesity and target-organ damage in adolescents (33–35). Moreover, obesity and higher plasma glucose level in youth has been found to be linked to decreased myocardial deformation even in the absence of comorbidities in early stages (24, 36–38). Therefore, it is reasonable to indicate that the observation of reduction of strain in asymptomatic young MH patients may contribute to the comprehensive effects of higher ambulatory pulse rate and glucose level as well as body weight. The studies regarding the influence of LV mechanics on cardiovascular outcome in youths with MH have not been done so far. However, considering our results that revealed the decrements of both longitudinal and circumferential layer-specific strain and abnormal diastolic LV filling in asymptomatic young MH subjects, and the findings of recent longitudinal study regarding the predictive value of myocardial strain (39), it could be hypothesized that LV mechanical changes might contribute to higher morbidity and mortality of MH patients later in life. Hence, MH in youths should be regarded as a condition that requires periodic follow-up.
The main limitation in the present study is lack of a “gold standard” like cardiac magnetic resonance imaging (MRI) to validate the layer-specific strain determined by 2D-STE. However, Altiok E et al. reported that the layer-specific strain using cardiac MRI demonstrated good correlations with the layer-specific strain using 2D echocardiography (40). Of note, a report from the EACVI-ASE Strain Standardization Task Force has also released the feasibility, accuracy, and reproducibility of layer-specific global longitudinal strain measurement (10). Second, 2D-STE assessment of LV mechanics could be influenced by the quality of ultrasound images. Manual drawing of the cardiac border also needs special care. Also, 2D-STE analysis was performed on a single analysis platform, so our results may not be able to be extrapolated to different analysis software. Third, the reference values for defining high BP in the office are based on our previous local data, instead of established BP normative data for children (including CDC and WHO data). However, it was contributed to the age of studied subjects were ranged from 13 to 22 years, and using different references of the ambulatory blood pressure might reduce or increase the number of hypertensive patients. Moreover, the BP normative data from CDC or WHO did not include the data from the Chinese, while the ethnic difference might cause misleading in the interpretation. Finally, the investigation only covered a relatively small number of young subjects with MH in a single-center study and a larger study is required to further confirm our results. Nevertheless, major strengths of our study are the nature of school-based population, instead of hospital-based cohorts.
With layer-specific 2D-STE, subclinical impairment of LV mechanical function in different myocardial layers is present in youth with MH, despite considered as normal with conventional ways. Thus, periodic follow-up and early detection of LV functional deterioration in young MH patients may help to prevent the natural progression of the disease.
M-CY, H-KS, and FF conceived and designed the experiments. X-XL, H-KS, and YZ organized the database. X-XL and YS performed the echocardiographic images analysis and statistical analysis. X-XL wrote the first draft of the manuscript. X-XL, YS, QG, and JS wrote sections of the manuscript. All authors have read and revised the manuscript, which was coordinated by FF and YZ, who also produced the final draft for submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would also like to thank the Health and Medical Research Fund (Ref No.: 12131351), Food and Health Bureau, Hong Kong SAR Government, Peoples' Republic of China for supporting this research project. Also, the authors are indebted to all the school principals, teachers, parents, and students who participated in this study.
MH, masked hypertension; BP, blood pressure; 2D, two-dimensional.2D-STE, two-dimensional speckle-tracking echocardiography; LV, left ventricle; LVEF, left ventricular ejection fraction; 2DE, two-dimensional echocardiography; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; BSA, body surface area; ASE, the American Society of Echocardiography; E, peak early transmitral flow velocities during diastole; A, peaklatetransmitral flow velocities during diastole; e′, early diastolic septal mitral annular velocities; LS, longitudinal strain; CS, circumferential strain; RS, radial strain; ICCs, intraclass correlation coefficients; MRI, magnetic resonance imaging.
1. Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension (2002) 40:795–6. doi: 10.1161/01.HYP.0000038733.08436.98
2. Stabouli S, Kotsis V, Toumanidis S, Papamichael C, Constantopoulos A, Zakopoulos N. White-coat and masked hypertension in children: association with target-organ damage. Pediatr Nephrol. (2005) 20:1151–5. doi: 10.1007/s00467-005-1979-5
3. Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, Redon J, et al. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension (2005) 45:493–8. doi: 10.1161/01.HYP.0000160320.39303.ab
4. So HK, Yip GW, Choi KC, Li AM, Leung LC, Wong SN, et al. Association between waist circumference and childhood-masked hypertension: a community-based study. J Paediatr Child Health (2016) 52:385–90. doi: 10.1111/jpc.13121
5. Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. (2008) 21:969–75. doi: 10.1038/ajh.2008.221
6. Buss SJ, Emami M, Mereles D, Korosoglou G, Kristen AV, Voss A, et al. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol. (2012) 60:1067–76. doi: 10.1016/j.jacc.2012.04.043
7. Luo XX, Fang F, Yu CM. Advantageous effect of biventricular pacing on cardiac function and coronary flow: a case report. Int J Cardiol. (2015) 190:236–8. doi: 10.1016/j.ijcard.2015.04.151
8. Tadic M, Cuspidi C, Vukomanovic V, Celic V, Tasic I, Stevanovic A, et al. Does masked hypertension impact left ventricular deformation? J Am Soc Hypertens. (2016) 10:694–701. doi: 10.1016/j.jash.2016.06.032
9. Luo XX, Fang F, Lee AP, Sun JP, Li S, Zhang ZH, et al. What can three-dimensional speckle-tracking echocardiography contribute to evaluate global left ventricular systolic performance in patients with heart failure? Int J Cardiol. (2014) 172:132–7. doi: 10.1016/j.ijcard.2013.12.314
10. Ünlü S, Mirea O, Duchenne J, Pagourelias ED, Bézy S, Thomas JD, et al. Comparison of feasibility, accuracy, and reproducibility of layer-specific global longitudinal strain measurements among five different vendors: a report from the EACVI-ASE strain standardization task force. J Am Soc Echocardiogr. (2018) 31:374–80.e1. doi: 10.1016/j.echo.2017.11.008
11. Sarvari SI, Haugaa KH, Zahid W, Bendz B, Aakhus S, Aaberge L, et al. Layer-specific quantification of myocardial deformation by strain echocardiography may reveal significant CAD in patients with non-ST-segment elevation acute coronary syndrome. JACC Cardiovasc Imaging (2013) 6:535–44. doi: 10.1016/j.jcmg.2013.01.009
12. Tarascio M, Leo LA, Klersy C, Murzilli R, Moccetti T, Faletra FF. Speckle-tracking layer-specific analysis of myocardial deformation and evaluation of scar transmurality in Chronic Ischemic Heart disease. J Am Soc Echocardiogr. (2017) 30:667–75. doi: 10.1016/j.echo.2017.03.015.
13. Kim SA, Park SM, Kim MN, Shim WJ. Assessment of left ventricular function by layer-specific strain and its relationship to structural remodelling in patients with hypertension. Can J Cardiol. (2016) 32:211–6. doi: 10.1016/j.cjca.2015.04.025
14. Alcidi GM, Esposito R, Evola V, Santoro C, Lembo M, Sorrentino R, et al. Normal reference values of multilayer longitudinal strain according to age decades in a healthy population: a single-centre experience. Eur Heart J Cardiovasc Imaging (2017). doi: 10.1093/ehjci/jex306. [Epub ahead of print].
15. Zhang L, Wu WC, Ma H, Wang H. Usefulness of layer-specific strain for identifying complex CAD and predicting the severity of coronary lesions in patients with non-ST-segment elevation acute coronary syndrome: compared with syntax score. Int J Cardiol. (2016) 223:1045–52. doi: 10.1016/j.ijcard.2016.08.277
16. Nishi T, Funabashi N, Ozawa K, Takahara M, Fujimoto Y, Kamata T, et al. Resting multilayer 2D speckle-tracking transthoracic echocardiography for the detection of clinically stable myocardial ischemic segments confirmed by invasive fractional flow reserve. Part 1: vessel-by-vessel analysis. Int J Cardiol. (2016) 218:324–32. doi: 10.1016/j.ijcard.2016.05.016
17. Yip GW, Li AM, So HK, Choi KC, Leung LC, Fong NC, et al. Oscillometric 24-h ambulatory blood pressure reference values in Hong Kong Chinese children and adolescents. J Hypertens. (2014) 32:606–19. doi: 10.1097/HJH.0000000000000062
18. Wong SN, Tz Sung RY, Leung LC. Validation of three oscillometric blood pressure devices against auscultatory mercury sphygmomanometer in children. Blood Press Monit. (2006) 11:281–91. doi: 10.1097/01.mbp.0000209082.09623.b4
19. Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. (2016) 34:1887–920. doi: 10.1097/HJH.0000000000001039
20. Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, et al. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. (1997) 130:178–84.
21. Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Doppler quantification task force of the nomenclature and standards committee of the american society of echocardiography. recommendations for quantification of doppler echocardiography: a report from the doppler quantification task force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. (2002) 15:167–84.
22. Friedberg MK, Mertens L. Tissue velocities, strain, and strain rate for echocardiographic assessment of ventricular function in congenital heart disease. Eur J Echocardiogr. (2009) 10:585–93. doi: 10.1093/ejechocard/jep045
23. Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). Eur Heart J Cardiovasc Imaging (2015) 16:577–605. doi: 10.1093/ehjci/jev076
24. Yoldaş T, Örün UA, Sagsak E, Aycan Z, Kaya Ö, Özgür S, et al. Subclinical left ventricular systolic and diastolic dysfunction in type 1 diabetic children and adolescents with good metabolic control. Echocardiography (2018). 35:227–33. doi: 10.1111/echo.13764
25. Armstrong GT, Joshi VM, Ness KK, Marwick TH, Zhang N, Srivastava D, et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. jude lifetime cohort study. J Am Coll Cardiol.(2015) 65:2511–22. doi: 10.1016/j.jacc.2015.04.013
26. Azak E, Cetin II, Gursu HA, Kibar AE, Surucu M, Orgun A, et al. Recovery of myocardial mechanics in Kawasaki disease demonstrated by speckle tracking and tissue Doppler methods. Echocardiography (2018) 35:380–7. doi: 10.1111/echo.13773
27. Björklund K, Lind L, Zethelius B,rén B, Lithell H. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation (2003) 107:1297–302. doi: 10.1161/01.CIR.0000054622.45012.12
28. Sun JP, Xu T, Yang Y, Yang XS, Shang Q, Li Y, et al. Layer-specific quantification of myocardial deformation may disclose the subclinical systolic dysfunction and the mechanism of preserved ejection fraction in patients with hypertension. Int J Cardiol. (2016) 219:172–6. doi: 10.1016/j.ijcard.2016.06.035
29. Nakamura N, Hirata K, Imanishi T, Kuroi A, Arita Y, Ikejima H, et al. Electrocardiographic strain and endomyocardial radial strain in hypertensive patients. Int J Cardiol. (2011) 150:319–24. doi: 10.1016/j.ijcard.2010.04.049
30. Tadic M, Cuspidi C, Ivanovic B, Ilic I, Celic V, Kocijancic V. Influence of white-coat hypertension on left ventricular deformation 2- and 3-dimensional speckle tracking study. Hypertension (2016) 67:592–6. doi: 10.1161/HYPERTENSIONAHA.115.06822
31. Tadic M, Cuspidi C, Vukomanovic V, Celic V, Pavlovic T, Kocijancic V. The influence of masked hypertension on the right ventricle: is everything really masked? J Am Soc Hypertens. (2016) 10:318–24. doi: 10.1016/j.jash.2016.02.001
32. Navarini S, Bellsham-Revell H, Chubb H, Gu H, Sinha MD, Simpson JM. Myocardial deformation measured by 3-dimensional speckle tracking in children and adolescents with systemic arterial hypertension. Hypertension (2017) 70:1142–7. doi: 10.1161/HYPERTENSIONAHA.117.09574
33. Lurbe E, Invitti C, Torro I, Maronati A, Aguilar F, Sartorio A, et al. The impact of the degree of obesity on the discrepancies between office and ambulatory blood pressure values in youth. J Hypertens. (2006) 24:1557–64. doi: 10.1097/01.hjh.0000239291.32883.e3
34. Stabouli S, Kotsis V, Papamichael C, Constantopoulos A, Zakopoulos N. Adolescent obesity is associated with high ambulatory blood pressure and increased carotid intimal-medial thickness. J Pediatr. (2005) 147:651–56. doi: 10.1016/j.jpeds.2005.06.008
35. Li AQ, Zhao ZY, Zhang LL, Lu FH, Yan ZH, Li YY, et al. Overweight influence on circadian variations of ambulatory blood pressure in Chinese adolescents. Clin Exp Hypertens. (2005) 27:195–201. doi: 10.1081/CEH-48777
36. Kulkarni A, Gulesserian T, Lorenzo JMMD, Haroonian Y, Ngyuyen M, Lo Y, et al. Left ventricular remodelling and vascular adaptive changes in adolescents with obesity. Pediatr Obes. (2018). doi: 10.1111/ijpo.12278. [Epub ahead of print].
37. Mangner N, Scheuermann K, Winzer E, Wagner I, Hoellriegel R, Sandri M, et al. Childhood obesity: impact on cardiac geometry and function. JACC Cardiovasc Imaging (2014) 7:1198–205. doi: 10.1016/j.jcmg.2014.08.006
38. Hensel KO, Grimmer F, Jenke AC, Wirth S, Heusch A. The influence of real-time blood glucose levels on left ventricular myocardial strain and strain rate in pediatric patients with type 1 diabetes mellitus-a speckle tracking echocardiography study. BMC Cardiovasc Disord. (2015) 15:175. doi: 10.1186/s12872-015-0171-5
39. Lee WH, Liu YW, Yang LT, Tsai WC. Prognostic value of longitudinal strain of subepicardial myocardium in patients with hypertension. J Hypertens. (2016) 34:1195–200. doi: 10.1097/HJH.0000000000000903
40. Altiok E, Neizel M, Tiemann S, Krass V, Kuhr K, Becker M, et al. Quantitative analysis of endocardial and epicardial left ventricular myocardial deformation-comparison of strain-encoded cardiac magnetic resonance imaging with two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. (2012) 25:1179–88. doi: 10.1016/j.echo.2012.07.019
Keywords: masked hypertension, youth, two-dimensional echocardiography, layer-specific speckle tracking analysis, left ventricular function
Citation: Luo X-X, Zhu Y, Sun Y, Ge Q, Su J, So H-K, Yam M-C and Fang F (2018) Does Masked Hypertension Cause Early Left Ventricular Impairment in Youth? Front. Pediatr. 6:167. doi: 10.3389/fped.2018.00167
Received: 05 February 2018; Accepted: 21 May 2018;
Published: 05 June 2018.
Edited by:
Bo Xi, Shandong University, ChinaReviewed by:
Ioannis Germanakis, University of Crete, GreeceCopyright © 2018 Luo, Zhu, Sun, Ge, Su, So, Yam and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hung-Kwan So, aHVuZ2t3YW5zb0BnbWFpbC5jb20=
Man-Ching Yam, eWFtbWNAY3Voay5lZHUuaGs=
Fang Fang, ZmFuZ2ZhbmdfZmZAaG90bWFpbC5jb20=
†Co-first author.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.