- 1Leiden University Center for Infectious Diseases (LUCID), Leiden University Medical Center, Leiden, Netherlands
- 2Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, Netherlands
- 3Mondial Diagnostics, Amsterdam, Netherlands
- 4Department of Public Health, Institute of Tropical Medicine, Antwerp, Belgium
- 5Institute of Tropical Medicine, University Hospital Tübingen, Tübingen, Germany
- 6German Center for Infection Research (DZIF), partner site Tübingen, Tübingen, Germany
- 7Centre de Recherches Médicales de Lambaréné, Lambaréné, Gabon
- 8Department of Infectious Disease Epidemiology, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany
- 9Centre d’Infectiologie Charles Mérieux, Antananarivo, Madagascar
- 10University of Antananarivo, Antananarivo, Madagascar
- 11University of Fianarantsoa, Fianarantsoa, Madagascar
- 12ISGlobal, Hospital Clínic, Universitat de Barcelona, Barcelona, Spain
Background: Schistosomiasis is caused by infection with parasitic Schistosoma worms and affects more than 250 million people globally. The detection of schistosome derived circulating cathodic and anodic antigens (CCA and CAA) has proven highly valuable for detecting active Schistosoma infections, causing both intestinal and urinary schistosomiasis.
Aim: The combined detection of CCA and CAA was explored to improve accuracy in detecting Schistosoma infections.
Methods: Parallel detection of CCA and CAA was performed on two banked sample sets with matching serum and urine samples from Schistosoma mansoni (Sm) and S. haematobium (Sh) infected individuals using the non-concentration based lateral flow (LF) test comprising the sensitive luminescent up-converting reporter particle (UCP) technology.
Results: Parallel detection of CCA and CAA increased the positivity rate for detecting both Sm and Sh infections compared to the detection of either antigen separately, demonstrating the added value of detecting both antigens in a single sample to confirm diagnosis, independent from the Schistosoma species. Significantly higher CCA concentrations in urine were observed in Sm infected individuals compared to Sh infected individuals, while serum CCA-concentrations were similar between species. CAA concentrations were higher in serum compared to those in urine, irrespective of species. When exploring the relationship of CCA and CAA in urine, the CCA/CAA ratio in Sm infected individuals was significantly higher than in Sh infected individuals, while no differences were observed in serum.
Discussion and conclusion: Parallel detection of CCA and CAA via the UCP-LF platform showed added diagnostic value through an increased positivity rate for the detection of Sm and Sh infections, compared to only detecting either of the antigens. The combined and quantitative detection of CCA and CAA is indicative for identifying the infecting species, but needs further exploration.
Highlights
● CCA and CAA were detected in parallel in urine and serum samples from S. mansoni and S. haematobium infected individuals.
● Urine CCA concentrations were significantly higher in S. mansoni infected individuals than in S. haematobium infected individuals, while urine CAA concentrations were similar.
● Serum CAA concentrations were generally higher than urine CAA concentrations.
● Detecting both CCA and CAA improved diagnostic value compared to detecting infection by either antigen separately.
1 Introduction
Schistosomiasis is a neglected tropical disease affecting more than 250 million people of which the majority reside in sub-Saharan Africa (WHO, 2023). The disease is caused by parasitic blood flukes of the Schistosoma genus. Using accurate diagnostic tests to correctly identify and treat those who are infected is crucial in order to successfully reduce schistosomiasis burden and to move toward elimination of schistosomiasis. Viable schistosomes release a range of antigens into the hosts’ blood circulation and detecting these antigens allows for accurate diagnosis of active infections. The two Schistosoma genus specific gut-associated antigens called ‘circulating cathodic antigen’ (CCA) and ‘circulating anodic antigen’ (CAA) are well acknowledged for diagnosing active infections in humans (Bergquist, 2013). CCA and CAA – both gut-associated glycoconjugates – are regurgitated by living Schistosoma worms into the hosts’ blood circulation (van Dam et al., 1996), and are cleared via the kidneys into the urine (van Lieshout et al., 2000) with little day-to-day variation (Polman et al., 1998). Both antigens are therefore detectable in the infected individual’s serum as well as in urine. CCA and CAA have been shown to be cleared within a few days to weeks after treatment with praziquantel (PZQ) (Kildemoes et al., 2017; Sousa et al., 2019; Hoekstra et al., 2020; Hoekstra et al., 2022a), making antigen detection a highly effective tool for treatment monitoring. The initial development of monoclonal antibody (mAb) based sandwich ELISAs resulted in sensitive and highly specific detection of CCA and CAA (Deelder et al., 1989; de Jonge et al., 1990; Deelder et al., 1994; Deelder et al., 1996; Polman et al., 2000).
Since early 2000, the anti-CCA ELISA has been transformed into a mAb based lateral flow (LF) assay, in which CCA is detected in a single drop of urine with high sensitivity and specificity. Subsequently, a point-of-care test was developed which detects CCA in urine (POC-CCA) and is commercially available via Rapid Medical Diagnostics (Pretoria, South Africa) since 2008. The POC-CCA test is a non-invasive, user-friendly, field-applicable and visually scored LF urine test. Even though CCA is excreted by all Schistosoma species, the POC-CCA test has been demonstrated to be particularly useful in detecting intestinal Schistosoma species (Ochodo et al., 2015; Coulibaly et al., 2011; Tchuem Tchuente et al., 2012; Colley et al., 2013; Coulibaly et al., 2013; Adriko et al., 2014; Danso-Appiah et al., 2016; Kabore et al., 2017; Bärenbold et al., 2018; Colley et al., 2020a). Test performance lacks sensitivity in detecting urogenital infections (Ochodo et al., 2015; Stothard et al., 2006; Ashton et al., 2011; Danso-Appiah et al., 2016; Sanneh et al., 2017), due to significantly lower urine CCA concentrations in case of a S. haematobium infection. However, in some S. haematobium endemic areas, the POC-CCA test was reported to be a valuable tool for field diagnosis of urogenital schistosomiasis (Ayele et al., 2008; Midzi et al., 2009; El-Ghareeb et al., 2016). Additionally, co-infection with S. haematobium does not seem to influence the accuracy of the POC-CCA for accurately detecting S. mansoni infections (Coulibaly et al., 2013). A few studies have pointed toward reduced specificity in specific populations, e.g. in pregnant women (Greter et al., 2016; Marti et al., 2020; Casacuberta-Partal et al., 2021), small children (Midzi et al., 2009) and individuals with urinary tract infections or hematuria (RMD, 2018), likely due to cross-reactivity with host components (Van Dam et al., 1994; Casacuberta-Partal et al., 2021). Other limitation of the test include the interpretation of the so-called ‘trace results’ (Bärenbold et al., 2018; Peralta and Cavalcanti, 2018; Clark et al., 2021; Graeff-Teixeira et al., 2021; Clark et al., 2022; Kabbas-Piñango et al., 2023) as well as recently observed batch-to-batch variations in test performance (Viana et al., 2019; Colley et al., 2020b; Colley et al., 2023; Kabbas-Piñango et al., 2023). However, the POC-CCA has been repeatedly reported as very useful for S. mansoni diagnosis in field settings in endemic countries and the test is currently being recommended by the WHO as a more user-friendly and more sensitive alternative tool to stool microscopy for mapping prevalence of intestinal schistosomiasis prevalence and for surveillance purposes in S. mansoni endemic areas (Bärenbold et al., 2018; WHO, 2022; WHO, 2023).
Detection of CAA was significantly improved by the introduction of a LF test platform combined with a unique and highly sensitive luminescent reporter label – up-converting reporter particles (UCP) – that improved sensitivity more than 10-fold compared to previous ELISA assays (Corstjens et al., 2008; Corstjens et al., 2014). An extraction step with trichloroacetic acid (TCA) was added, which leaves carbohydrate structures such as CAA (but also CCA) in the clear supernatant fluid while precipitating proteins, thereby increasing the specificity of the test. Furthermore, by including a concentration step (i.e. increasing the sample volume to be tested), the sensitivity of the UCP-LF CAA test was further improved and in experimental studies it has been shown that the test is able to accurately quantify CAA concentrations down to the level of a single worm infection (Corstjens et al., 2020). The UCP-LF CAA test has demonstrated high specificity and sensitivity for the detection of all Schistosoma species including Schistosoma hybrids and veterinary species (Knopp et al., 2015; van Dam et al., 2015a; van Dam et al., 2015b; Vonghachack et al., 2017; Clements et al., 2018; Sousa et al., 2019; Corstjens et al., 2020; Hoekstra et al., 2021). It is applicable to various sample types, including urine, serum, plasma and dried blood spots (Stete et al., 2018; Downs et al., 2015; de Dood et al., 2018; Corstjens et al., 2020). An advanced and robust version of the UCP-LF CAA test has become available, omitting the need for a cold chain and thus facilitating storage and worldwide shipment and its implementation in basic equipped central laboratories in endemic regions (van Dam et al., 2013; Corstjens et al., 2015; Corstjens et al., 2020).
In this study, we aimed to combine the best of two worlds by evaluating the parallel detection of both CCA (being particularly sensitive for intestinal schistosomiasis) and CAA (highly sensitive and specific for all Schistosoma species), with the overall aim to explore the diagnostic potentials of a single UCP-LF based CCA/CAA duplex test. In order to shed further light into the presence of CCA and CAA in S. mansoni and S. haematobium mono-infected individuals, we applied the non-concentration, laboratory-based UCP-LF technique to qualitatively and quantitively assess both antigens in banked urine and serum samples. Furthermore, we investigated differences in species-specific excretion levels of CCA and CAA and if this could potentially provide information regarding the schistosome species.
2 Methods
This study utilized two sets of paired serum and urine samples available from two previous epidemiological studies, one performed in a heavily S. mansoni (Sm) infected community in Senegal (Stelma et al., 1993; Polman et al., 1995), and one performed in a high S. haematobium (Sh) endemic area in Cameroon (Kremsner et al., 1994). The original studies received ethical approval according to local standards and guidelines in place at the time of the study (Stelma et al., 1993; Kremsner et al., 1994; Polman et al., 1995). Extensive information was provided to the communities involved in the studies and oral informed consent was obtained from all participants (or from their parents in case of children) before sample collection. Urine and serum samples have been stored at -20°C and -80°C, respectively, since their arrival in the Netherlands, for detection of circulating antigen testing by ELISA as part of the original study and for future testing and validation of circulating antigen detection assays. Selection criteria were having a confirmed Sm or Sh infection (i.e. egg positive) as well as the availability of both a urine and serum sample. Egg positivity was based on microscopy, either by Kato-Katz (duplicate 25-mg slides from two stool samples collected within a time interval of 1-2 weeks) for the detection of Sm infections (Stelma et al., 1993; Polman et al., 1995) or by urine filtration (urine samples collected on 3 consecutive days) for the detection of Sh infections (Kremsner et al., 1994). A total of 105 and 97 matching urine and serum samples were available from Sm and Sh infected individuals, respectively.
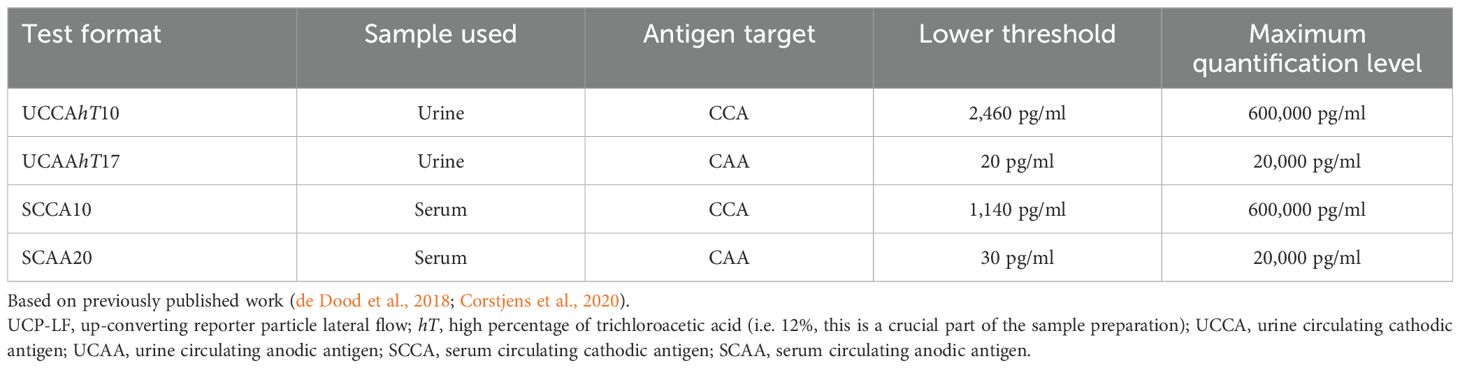
CCA and CAA concentration was determined in each serum and urine samples using in parallel the non-concentration, dry format of the UCP-LF CCA test and UCP-LF CAA test, respectively, as previously described (Corstjens et al., 2014; de Dood et al., 2018; Corstjens et al., 2020). Briefly, 50µl of urine was mixed with 10µl of 12% of trichloroacetic acid (TCA), incubated and centrifuged, while for serum 50µl of each serum sample was mixed with an equal volume of 4% TCA, incubated and centrifuged. Subsequently, for either the urine test or the serum test 20µl of the clear supernatant was added to microtiter plate wells containing UCP reporter particles labeled with anti-CCA or anti-CAA antibodies hydrated in 100µL LF assay buffer and incubated on a shaker at 37°C. After 1h, LF strips were added to the wells and incubated overnight, followed by scanning the strips using a multistrip benchtop reader (UPCON; Labrox Oy, Turku, Finland). Samples with known CCA/CAA concentrations were included as a reference standard to quantify individual antigen concentrations and to validate the lower limit of detection (cut-off), which was based on previously published results: 2,460 pg/ml for urine CCA and 1,140 pg/ml for serum CCA, 20 pg/ml for urine CAA, 30 pg/ml for serum CAA (de Dood et al., 2018; Corstjens et al., 2020), with maximum quantification levels of 600,000 pg/ml for CCA and 20,000 pg/ml for CAA, see also Table 1. The cut-off for CCA is based on biological background presence of CCA-like host antigens (i.e. Lewis-X trisaccharide units) in urine and serum (Van Dam et al., 1994; Polman et al., 2000), while the CAA cut-off is based on technical aspects of the assay (Corstjens et al., 2014; Corstjens et al., 2015; Corstjens et al., 2020). Samples with a concentration above the indicated cut-off were considered positive. The Chi-square test was used to compare proportions and to determine statistically significant differences between antigens (CCA and CAA), between sample types (urine and serum) as well as between sample sets (Sm and Sh) and the Mann-Whitney U-test was used to compare quantitative CCA and CAA levels between groups, using SPSS version 29 and GraphPad Prism Version 10.2.3. CCA/CAA ratios in urine and serum were determined in samples which showed detectable (above the cut-off) concentrations of both CCA and CAA (see also Supplementary Figure 1).
3 Results
Urine and serum samples from individuals with a microscopy-confirmed Sm or Sh infection were analyzed for the presence of CCA and CAA by the non-concentration, dry format of the laboratory-based UCP-LF test.
3.1 Percentage positive based on CCA and CAA
CCA was significantly more often detected in Sm infected individuals compared to Sh infected individuals, in urine (93% vs 40%, respectively) as well as in serum (93% vs 68%, respectively). In contrast, the proportion of CAA positives was comparable between Sm and Sh infected individuals, in urine (78% vs 70%, respectively) as well as in serum (83% vs 85%), see also Table 2 and Figure 1.
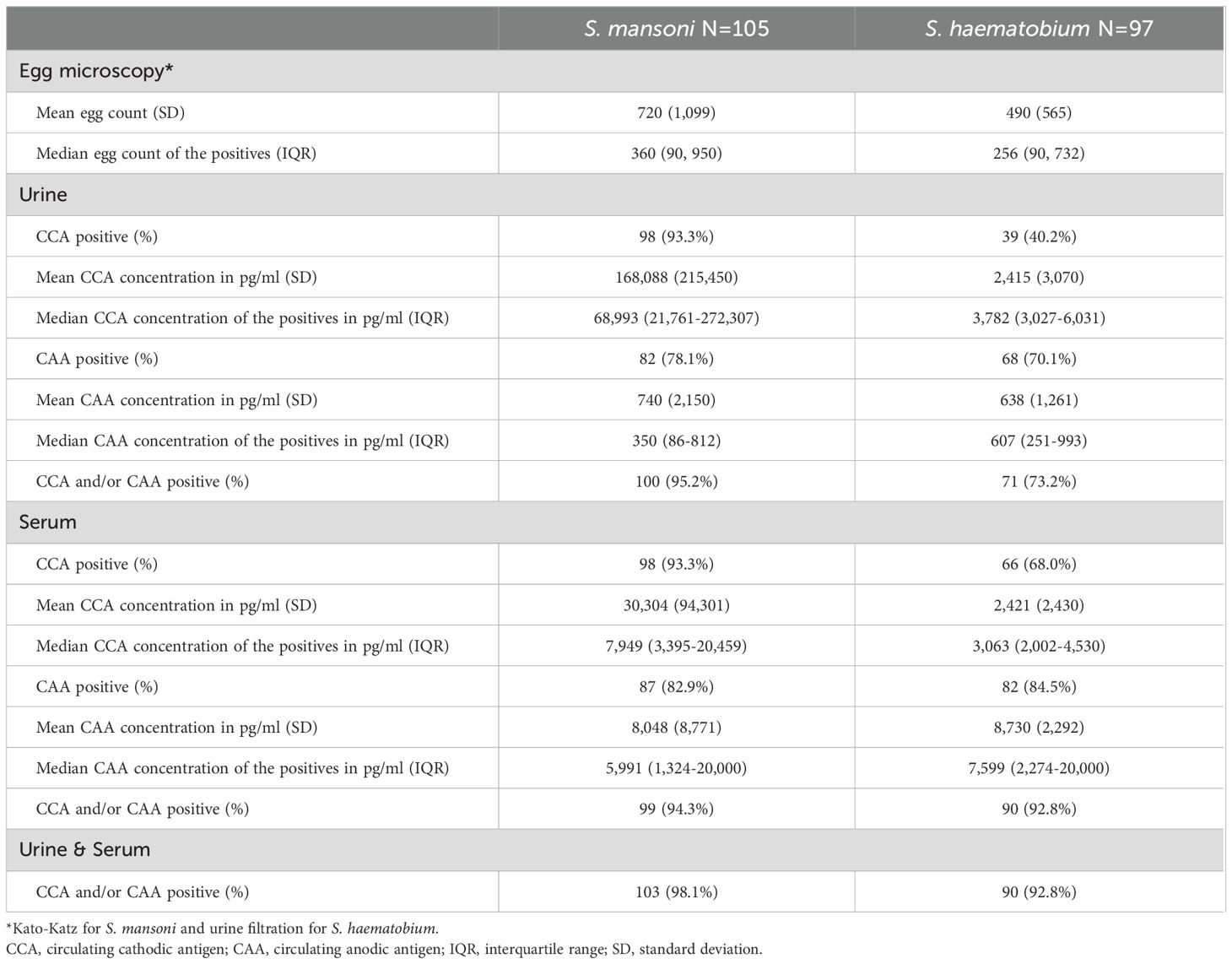
Table 2. CAA and CCA UCP-LF outcomes of urine and serum samples of S. mansoni infected (N=105) and S. haematobium infected (N=97) individuals.
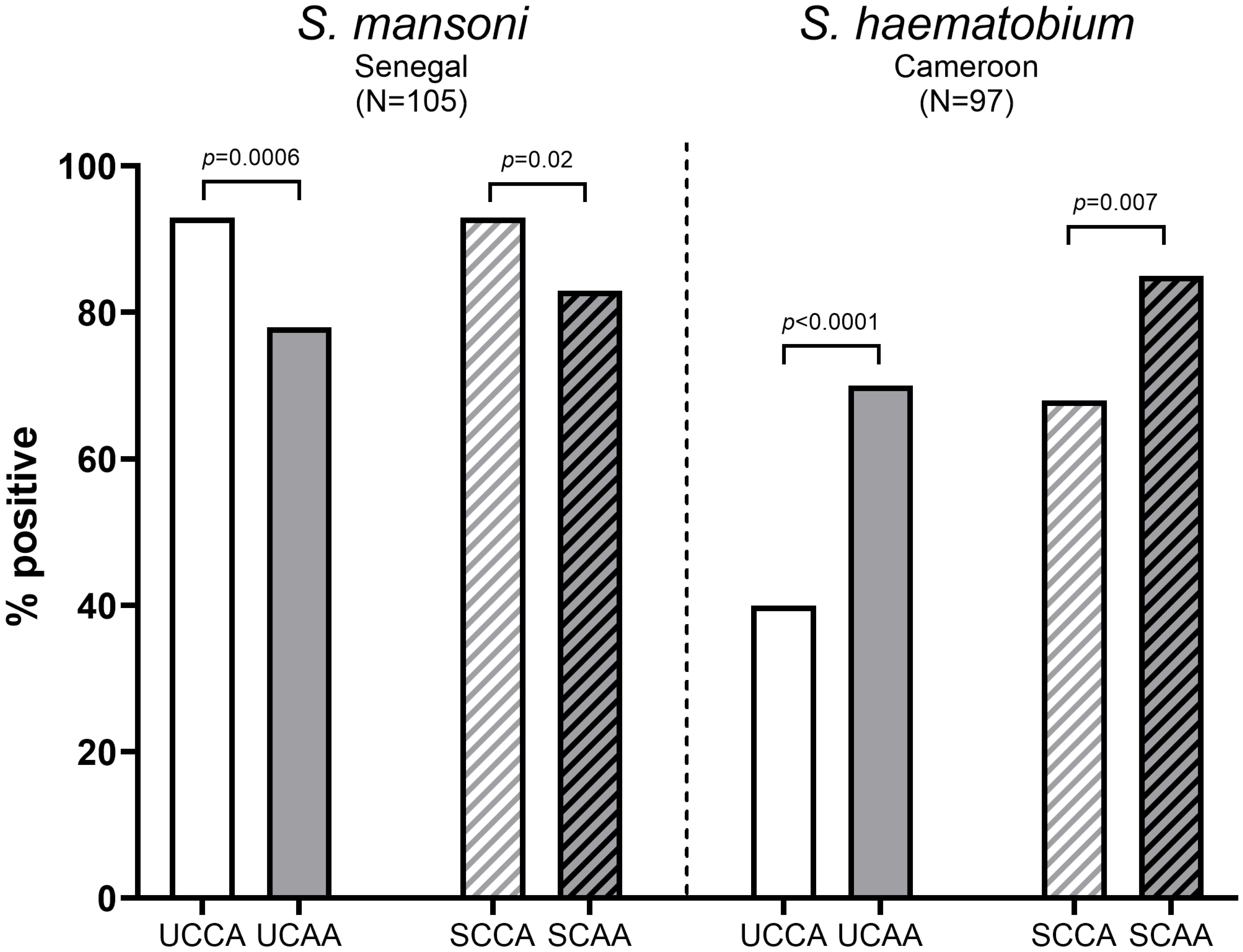
Figure 1. Proportion of CCA positive and CAA positive urine and serum samples from S. mansoni infected individuals (N=105) (left) and S. haematobium infected individuals (N=97) (right). Statistically significant differences between CCA and CAA are indicated in the figure by p-values. UCCA, urine circulating cathodic antigen; UCAA, urine circulating anodic antigen; SCCA, serum circulating cathodic antigen; SCAA, serum circulating anodic antigen.
3.1.1 S. mansoni infected individuals
As demonstrated in Figure 1, urine- and serum-based testing for Sm infections were significantly more accurate for CCA than for CAA (urine CCA 93% vs urine CAA 78%, p=0.0006 and serum CCA 93% vs serum CAA 83%, p=0.02). Overall, CCA and CAA were equally well detected in urine and serum (CCA: urine 93% vs serum 93%, p=1 and CAA: urine 76% vs serum 83%, p=0.23). Combining CCA and CAA outcomes increased positivity in urine (from 93% and 78% to 95%) as well as in serum (from 93% and 83% to 94%).
3.1.2 S. haematobium infected individuals
Contrary to Sm, for Sh infections, urine- and serum-based testing were significantly more accurate for CAA than for CCA (urine CAA 70% vs urine CCA 40%, p<0.0001 and serum CAA 85% vs CCA: 68%, p=0.007), see also Figure 1. CCA positives in urine were significantly lower than in serum (urine: 40% vs serum: 68%, p=0.0001), similarly to CAA (urine: 70% vs serum: 85%, p=0.02). Combining CCA and CAA outcomes increased positivity in urine (from 40% and 70% to 73%) as well as in serum (from 68% and 85% to 93%).
3.2 Intensity of infection based on CCA and CAA
Being a measure of intensity of infection, CCA and CAA concentrations were quantitatively analyzed and compared between Sm and Sh infected individuals, both for serum and urine samples (Figure 2). In Sm infected individuals, CCA concentrations were significantly higher compared to CAA concentrations, in particular in urine but also in serum. In Sh infected individuals, CCA concentrations in urine and serum were lower than in Sm infected individuals, while urine and serum CAA concentrations were similar between species. In all individuals, CAA concentrations in serum were significantly higher compared to urine.
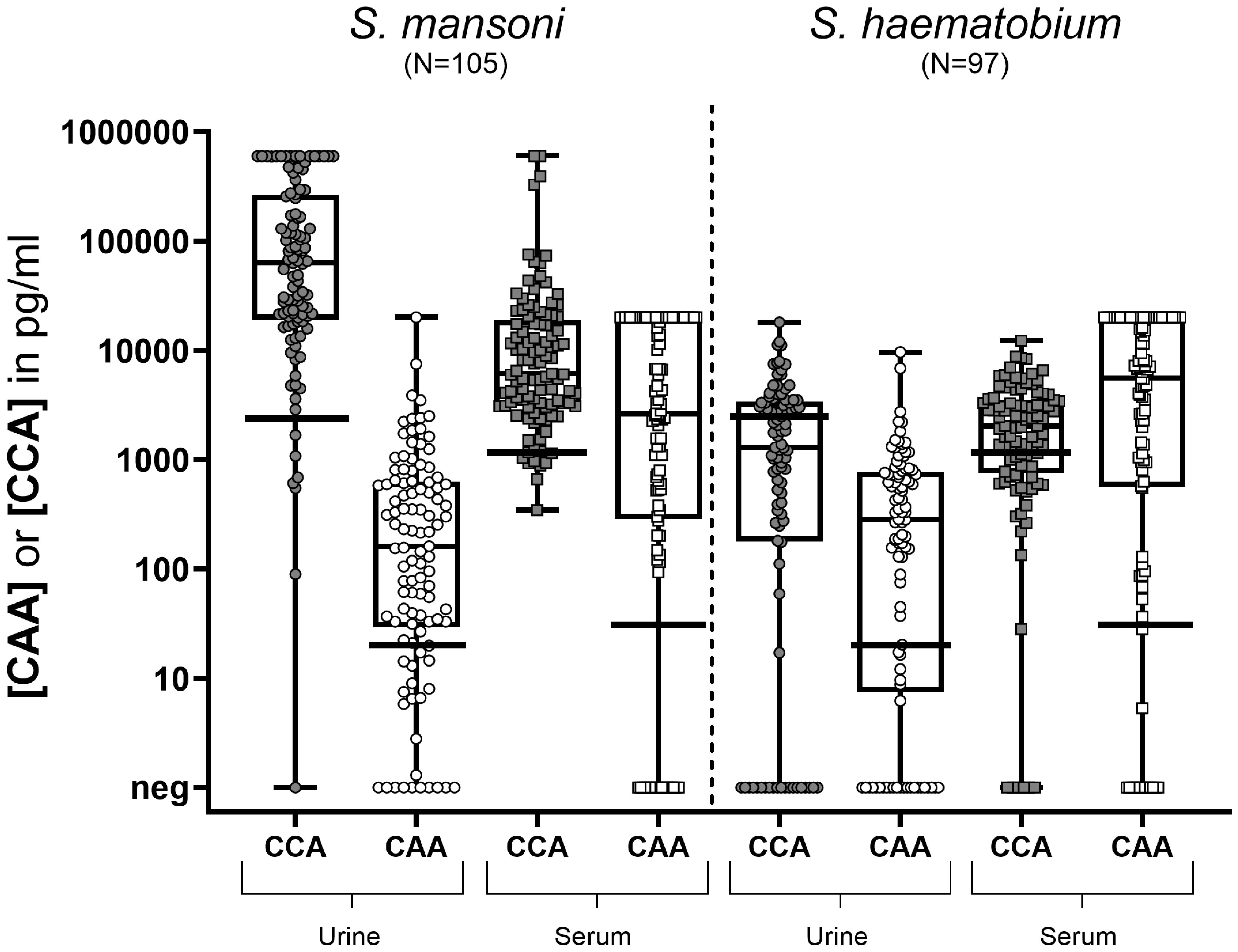
Figure 2. Box and whisker plot of individual CCA and CAA concentrations in urine (circles) and serum (squares) samples from S. mansoni infected individuals (N=105) (left) and S. haematobium infected individuals (N=97) (right). The box contains the 25th to 75th percentiles of the antigen concentrations, while the central lines denotes the median antigen concentration. The whiskers mark the minimum and maximum antigen concentration. The respective cut-offs (horizontal black lines) indicate the value above which a concentration is considered truly positive (i.e. 2,460 pg/ml for urine CCA; 20 pg/ml for urine CAA; 1,140 pg/ml for serum CCA and 30 pg/ml for serum CAA). UCCA, urine circulating cathodic antigen; UCAA, urine circulating anodic antigen; SCCA, serum circulating cathodic antigen; SCAA, serum circulating anodic antigen.
3.3 Identification of schistosome species based on CCA and CAA ratio
As significant differences in CCA and CAA concentrations between schistosome species were observed, the relation between CCA levels and CAA levels was analyzed (CAA/CCA ratios), in urine as well as in serum of Sm and Sh infected individuals (Figure 3). The highest CCA/CAA ratios were observed in urine of Sm infected individuals (median ratio of 327), being significantly higher than in urine of Sh infected individuals (median ratio 5.5, p<0.0001). Overall, CCA/CAA ratios in serum were lower compared to CCA/CAA ratios in urine. CCA/CAA ratios in serum of Sm infected individuals (median ratio 1.9) were also significantly higher than in serum of Sh infected individuals (median ratio 0.3, p<0.0001).
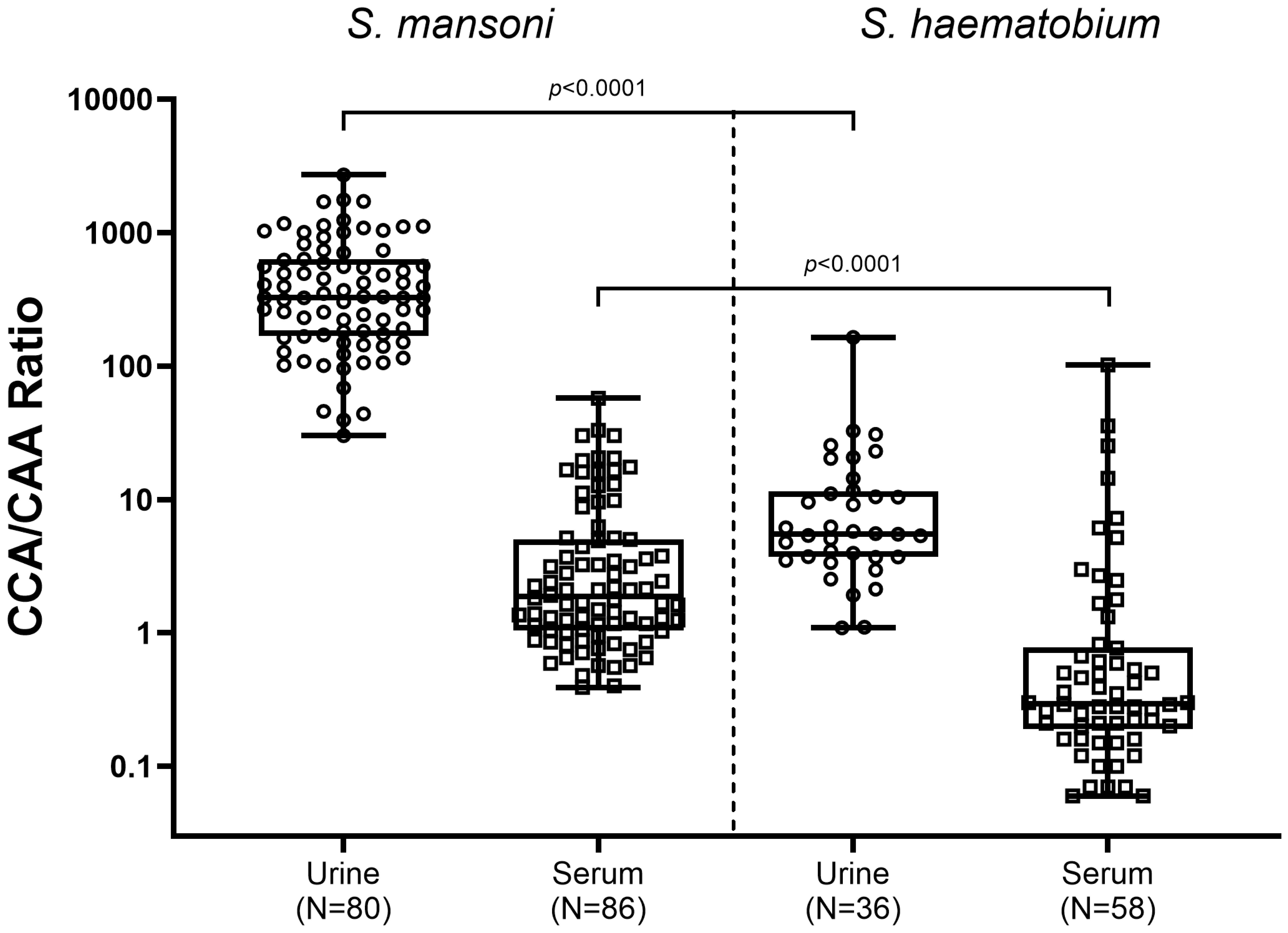
Figure 3. Box and whisker plot of individual CCA/CAA ratios in urine (circles) and serum (squares) samples from S. mansoni infected individuals (left) and S. haematobium infected individuals (right). The box contains the 25th to 75th percentiles of the CCA/CAA ratios, while the central lines denotes the median CCA/CAA ratio. The whiskers mark the minimum and maximum CCA/CAA ratio. CCA/CAA ratios were determined in urine and serum samples which showed detectable (above the cut-off) concentrations of both CCA and CAA (S. mansoni: N=80 urine and N=86 serum samples; S. haematobium: N=36 urine and N=58 serum samples. CCA, circulating cathodic antigen; CAA, urine circulating anodic antigen.
4 Discussion
This study is the first to thoroughly investigate the presence of two well-studied Schistosoma specific antigens – CCA and CAA – by utilizing the UCP-LF technique in matching urine and serum samples collected from Sm and Sh (microscopy-confirmed) infected individuals. The parallel detection of both CCA and CAA improved the positivity rate for detecting both Sm and Sh infections compared to detection of either antigen separately, confirming previous findings based on ELISA assays in Sm populations (Van Lieshout et al., 1992; Fillie et al., 1994), and thereby demonstrating the added value of detecting both antigens in a single sample for accurate diagnosis independent from the Schistosoma species. Furthermore, our results showed that quantitatively detecting both antigens can potentially provide information regarding the Schistosoma species, in particular identifying Sm infections in urine, provided that detectable concentrations (above the cut-off) are measured for both CCA and CAA.
Differences in CCA concentrations between the two Schistosoma species were observed: in Sm infected individuals CCA levels were significantly higher than in Sh infected individuals – with the greatest differences being observed in urine samples. Even though CCA is excreted by all Schistosoma species, the observation that the highest CCA concentrations (and highest CCA/CAA ratios) were observed in urines of Sm infected individuals confirm the usefulness of the currently available and WHO-recommended point-of-care (POC-CCA) test for diagnosing intestinal schistosomiasis (Bärenbold et al., 2018; WHO, 2022; WHO, 2023). In case of urogenital schistosomiasis, the median urine CCA concentration in this particular sample set was ca. 20 times lower, which explains that in particular for the low intensity infections, urine CCA concentrations most likely fall below the detection threshold of the POC-CCA test, resulting in lower diagnostic performance in these settings (Stothard et al., 2006; Ayele et al., 2008; Midzi et al., 2009; Stothard et al., 2009). This was confirmed in our study where, based on the more sensitive UCP-LF technique, detectable CCA concentrations were observed in urines from Sh infected individuals, although these concentrations were generally low and around the cut-off (Figure 2).
Overall, CAA concentrations were higher in serum compared to urine, irrespective of the Schistosoma species, which is similar to previous findings (Van Lieshout et al., 1995; van Dam et al., 1996; Polman et al., 1998; Polman, 2000; van Dam et al., 2015b; Sousa et al., 2019; Langenberg et al., 2020; Hoekstra et al., 2021; Casacuberta-Partal et al., 2022; Hoekstra et al., 2022b). The detection of CAA in serum is therefore highly suitable for diagnosing infection with all Schistosoma species. Currently, efforts are ongoing to develop a visually scored finger prick blood-based CAA rapid diagnostic test (CAA-RDT) in collaboration with FIND Dx (FIND, 2021). Combining the urine POC-CCA test with the upcoming finger prick blood CAA-RDT is expected to be a promising, field-friendly, diagnostic alternative for egg microscopy and would improve accuracy for diagnosing both Sm and Sh infections.
Based on species-specific excretion levels of CCA and CAA, the parallel detection of both antigens in serum and urine was hypothesized to be indicative for the infective species. In the current study, the urine CCA/CAA ratio in Sm infected individuals was significantly higher than the CCA/CAA ratio in Sh infected individuals, while in serum a similar trend but not a significant difference was observed. These findings indicate that if both CCA and CAA are detected in a urine sample and if the CCA/CAA ratio is high (>200 based on the results of our study), an infection with Sm is most likely, while no definitive conclusions can be drawn from serum. Although interesting as a rule of thumb, to further corroborate this conclusion, larger studies in different endemic settings and infection intensities are needed. Furthermore, antigen concentrations can vary from person to person and several factors should be taken into account when determining the CCA/CAA ratio, including the intensity of infection (i.e. worm burden) and individual antigen clearance mechanisms.
Limitations of this study include the pre-selection of the sample set based on the presence of Schistosoma eggs (in urine or stool, depending on the species). Some egg positive cases could not be confirmed with either CCA or CAA in urine and/or serum (~5% of cases). Discrepancies were observed over a range of different egg counts which should be further investigated by re-testing with higher sample volumes, which allows for the detection of a lower antigen concentration. As antigen diagnostics are in general more sensitive compared to microscopy, the opposite (egg negative and antigen positive) would be expected to occur more often, as also observed in previous studies (Knopp et al., 2015; Sousa et al., 2019; Hoekstra et al., 2020; Tamarozzi et al., 2021; Hoekstra et al., 2022a; Hoekstra et al., 2022b). Preferably, a larger sample set including egg positives as well as egg negatives from various schistosomiasis endemic settings should be explored, including areas where multiple species are prevalent and/or where hybrid infections occur. Because the available volume of urine and serum samples was limited, we were only able to apply the non-concentration format of the UCP-LF test. This format is less sensitive compared to the UCP-LF concentration format, resulting in lower accuracy when quantifying lower antigen concentrations. In case larger volumes are available, the most sensitive format of the UCP-LF CAA test should be performed, leading to a more accurate antigen measurement, in particular in the lower range around and below the currently used cut-off (Corstjens et al., 2020). On the other hand, in some cases the maximum level of detection (plateau value) was reached; this was mainly the case for CCA in urines from Sm infected individuals and CAA in serum from both Sm and Sh infected individuals. Ideally, these samples should be diluted and re-tested in order to obtain a more accurate quantitative measurement of the antigen concentration.
5 Conclusion
Parallel detection of CCA and CAA using the UCP-LF platform showed added value compared to detecting only one of the antigens. In particular in S. haematobium endemic areas, where a limited performance of the POC-CCA urine test is observed, additional detection of CAA – for example via the upcoming CAA-RDT – will result in a better estimation of the true prevalence of schistosomiasis. More studies are needed to confirm whether the quantitative detection of CCA and CAA can be used to specify the infecting species, thereby also including mixed and/or hybrid Schistosoma infections. Next steps include further optimization of the combined detection of both CCA and CAA in a single test format via the UCP-LF technique as well as to engage with industrial partners for potential commercialization of such a duplex test into a POC format for use in low-resource settings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The original studies received ethical approval according to local standards and guidelines in place at the time of the study. Extensive information was provided to the communities involved in the studies and oral informed consent was obtained from all participants (or from their parents in case of children) before sample collection.
Author contributions
PH: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing, Visualization. CD: Investigation, Writing – review & editing. TA: Investigation, Writing – review & editing. SH: Investigation, Writing – review & editing. AD: Writing – review & editing. KP: Resources, Writing – review & editing. PK: Resources, Writing – review & editing. LL: Writing – review & editing. AK: Funding acquisition, Writing – review & editing. AA: Writing – review & editing. DF: Writing – review & editing. TR: Writing – review & editing. MR: Writing – review & editing. RR: Writing – review & editing. RAR: Writing – review & editing. ES: Writing – review & editing. GD: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. PC: Conceptualization, Methodology, Supervision, Writing – review & editing.
freeBILy consortium
The freeBILy consortium consist of eight partners institutes including four from Africa and four from Europe, represented by the following members: G.J. van Dam, P.L.A.M. Corstjens, C.J. de Dood, P.T. Hoekstra, A.S. Amoah, M.I. Keshinro, S.T. Hilt, (LUMC); A. Kreidenweiss, (EKUT); N.G. Schwarz, D. Fusco, P. Klein, A. Jaeger, E. Lorenz, (BNITM); A.A. Adegnika, Y.J. Honkpehedji, J.C. Dejon-Agobe, R. Beh Mba, M. Mbong Ngwese, M. Nzamba Maloum, A. Nguema Moure, B.T. Meulah, J. Gerstenberg, R. Laclong Lontchi, (CERMEL); R.A. Rakotoarivelo, A. Ralaizandry, M. Radomanana, (UF); R. Rakotozandrindrainy, N. Rakotozandrindrainy, M.J. Solonirina, J. Randriamanjara, (UA); M. Rakoto Andrianarivelo, T. Rasamoelina, R. Razafindrakoto, (CICM); E. Sicuri, C. Aerts, and F. Roeder (ISGlobal).
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This publication was produced by freeBILy which is part of the EDCTP2 programme supported by the European Union (grant number RIA2016MC-1626-FREEBILY). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and opinions of authors expressed herein do not necessarily state or reflect those of EDCTP.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpara.2024.1460331/full#supplementary-material
Supplementary Figure 1 | Comparing CCA versus CAA concentrations in urine (circles) and serum (squares) samples from S. mansoni infected individuals [(A, B), N=105] and S. haematobium infected individuals [(C, D), N=97]. The red dotted lines indicate the cut-off above which a concentration is considered truly positive based on the test formats used in this study (i.e. 2,460 pg/ml for urine CCA; 20 pg/ml for urine CAA; 1,140 pg/ml for serum CCA and 30 pg/ml for serum CAA).
References
Adriko M., Standley C. J., Tinkitina B., Tukahebwa E. M., Fenwick A., Fleming F. M., et al. (2014). Evaluation of circulating cathodic antigen (CCA) urine-cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda. Acta tropica 136, 50–57. doi: 10.1016/j.actatropica.2014.04.001
Ashton R. A., Stewart B. T., Petty N., Lado M., Finn T., Brooker S., et al. (2011). Accuracy of circulating cathodic antigen tests for rapid mapping of Schistosoma mansoni and S. haematobium infections in Southern Sudan. Trop. Med. Int. health: TM IH 16, 1099–1103. doi: 10.1111/j.1365-3156.2011.02815.x
Ayele B., Erko B., Legesse M., Hailu A., Medhin G. (2008). Evaluation of circulating cathodic antigen (CCA) strip for diagnosis of urinary schistosomiasis in Hassoba school children, Afar, Ethiopia. Parasite 15, 69–75. doi: 10.1051/parasite/2008151069
Bärenbold O., Garba A., Colley D. G., Fleming F. M., Haggag A. A., Ramzy R. M. R., et al. (2018). Translating preventive chemotherapy prevalence thresholds for Schistosoma mansoni from the Kato-Katz technique into the point-of-care circulating cathodic antigen diagnostic test. PloS Negl. Trop. Dis. 12, e0006941. doi: 10.1371/journal.pntd.0006941
Bergquist R. (2013). Good things are worth waiting for. Am. J. Trop. Med. hygiene 88, 409–410. doi: 10.4269/ajtmh.12-0741
Casacuberta-Partal M., Beenakker M., de Dood C. J., Hoekstra P. T., Kroon L., Kornelis D., et al. (2021). Specificity of the point-of-care urine strip test for schistosoma circulating cathodic antigen (POC-CCA) tested in non-endemic pregnant women and young children. Am. J. Trop. Med. hygiene 104, 1412–1417. doi: 10.4269/ajtmh.20-1168
Casacuberta-Partal M., van Lieshout L., van Diepen A., Sijtsma J. C., Ozir-Fazalalikhan A., Koopman J. P. R., et al. (2022). Excretion patterns of Schistosoma mansoni antigens CCA and CAA by adult male and female worms, using a mouse model and ex vivo parasite cultures. Parasitology 149, 306–313. doi: 10.1017/S0031182021001839
Clark J., Moses A., Nankasi A., Faust C. L., Adriko M., Ajambo D., et al. (2022). Translating from egg- to antigen-based indicators for schistosoma mansoni elimination targets: A bayesian latent class analysis study. Front Trop Dis. 3, 825721. doi: 10.3389/fitd.2022.825721
Clark J., Moses A., Nankasi A., Faust C. L., Moses A., Ajambo D., et al. (2021). Reconciling egg- and antigen-based estimates of schistosoma mansoni clearance and reinfection: A modeling study. Clin. Infect. Dis. 74 (9), 1557–1563. doi: 10.1093/cid/ciab679
Clements M. N., Corstjens P., Binder S., Campbell C. H. Jr., de Dood C. J., Fenwick A., et al. (2018). Latent class analysis to evaluate performance of point-of-care CCA for low-intensity Schistosoma mansoni infections in Burundi. Parasit. Vectors 11, 111. doi: 10.1186/s13071-018-2700-4
Colley D. G., Binder S., Campbell C., King C. H., Tchuem Tchuenté L. A., N’Goran E. K., et al. (2013). A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am. J. Trop. Med. hygiene 88, 426–432. doi: 10.4269/ajtmh.12-0639
Colley D. G., Fleming F. M., Matendechero S. H., Knopp S., Rollinson D., Utzinger J., et al. (2020a). Contributions of the schistosomiasis consortium for operational research and evaluation (SCORE) to schistosomiasis control and elimination: key findings and messages for future goals, thresholds, and operational research. Am. J. Trop. Med. hygiene 103, 125–134. doi: 10.4269/ajtmh.19-0787
Colley D. G., King C. H., Kittur N., Ramzy R. M. R., Secor W. E., Fredericks-James M., et al. (2020b). Evaluation, validation, and recognition of the point-of-care circulating cathodic antigen, urine-based assay for mapping schistosoma mansoni infections. Am. J. Trop. Med. hygiene 103, 42–49. doi: 10.4269/ajtmh.19-0788
Colley D. G., Ramzy R. M. R., Maganga J., Kinung’hi S., Odiere M. R., Musuva R. M., et al. (2023). The POC-CCA assay for detection of Schistosoma mansoni infection needs standardization in production and proper quality control to be reliable. Acta tropica 238, 106795. doi: 10.1016/j.actatropica.2022.106795
Corstjens P., de Dood C. J., Knopp S., Clements M. N., Ortu G., Umulisa I., et al. (2020). Circulating anodic antigen (CAA): A highly sensitive diagnostic biomarker to detect active schistosoma infections-improvement and use during SCORE. Am. J. Trop. Med. hygiene 103, 50–57. doi: 10.4269/ajtmh.19-0819
Corstjens P. L., De Dood C. J., Kornelis D., Fat E. M., Wilson R. A., Kariuki T. M., et al. (2014). Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology 141, 1841–1855. doi: 10.1017/S0031182014000626
Corstjens P. L., Nyakundi R. K., de Dood C. J., Kariuki T. M., Ochola E. A., Karanja D. M., et al. (2015). Improved sensitivity of the urine CAA lateral-flow assay for diagnosing active Schistosoma infections by using larger sample volumes. Parasit. Vectors 8, 241. doi: 10.1186/s13071-015-0857-7
Corstjens P. L., van Lieshout L., Zuiderwijk M., Kornelis D., Tanke H. J., Deelder A. M., et al. (2008). Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. J. Clin. Microbiol. 46, 171–176. doi: 10.1128/JCM.00877-07
Coulibaly J. T., Knopp S., N’Guessan N. A., Silue K. D., Furst T., Lohourignon L. K., et al. (2011). Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Cote d’Ivoire. PloS Negl. Trop. Dis. 5, e1384. doi: 10.1371/journal.pntd.0001384
Coulibaly J. T., N’Gbesso Y. K., Knopp S., N’Guessan N. A., Silué K. D., van Dam G. J., et al. (2013). Accuracy of urine circulating cathodic antigen test for the diagnosis of Schistosoma mansoni in preschool-aged children before and after treatment. PloS Negl. Trop. Dis. 7, e2109. doi: 10.1371/journal.pntd.0002109
Danso-Appiah A., Minton J., Boamah D., Otchere J., Asmah R. H., Rodgers M., et al. (2016). Accuracy of point-of-care testing for circulatory cathodic antigen in the detection of schistosome infection: systematic review and meta-analysis. Bull. World Health Organ. 94, 522–33a. doi: 10.2471/BLT.15.158741
de Dood C. J., Hoekstra P. T., Mngara J., Kalluvya S. E., van Dam G. J., Downs J. A., et al. (2018). Refining diagnosis of schistosoma haematobium infections: antigen and antibody detection in urine. Front. Immunol. 9, 2635. doi: 10.3389/fimmu.2018.02635
Deelder A. M., De Jonge N., Boerman O. C., Fillie Y. E., Hilberath G. W., Rotmans J. P., et al. (1989). Sensitive determination of circulating anodic antigen in Schistosoma mansoni infected individuals by an enzyme-linked immunosorbent assay using monoclonal antibodies. Am. J. Trop. Med. hygiene 40, 268–272. doi: 10.4269/ajtmh.1989.40.268
Deelder A. M., Qian Z. L., Kremsner P. G., Acosta L., Rabello A. L., Enyong P., et al. (1994). Quantitative diagnosis of Schistosoma infections by measurement of circulating antigens in serum and urine. Trop. geographical Med. 46, 233–238.
Deelder A. M., van Dam G. J., Kornelis D., Fillié Y. E., van Zeyl R. J. (1996). Schistosoma: analysis of monoclonal antibodies reactive with the circulating antigens CAA and CCA. Parasitology 112, 21–35. doi: 10.1017/S0031182000065045
de Jonge N., Kremsner P. G., Krijger F. W., Schommer G., Fillie Y. E., Kornelis D., et al. (1990). Detection of the schistosome circulating cathodic antigen by enzyme immunoassay using biotinylated monoclonal antibodies. Trans. R. Soc. Trop. Med. Hygiene 84, 815–818. doi: 10.1016/0035-9203(90)90094-U
Downs J. A., Corstjens P. L., Mngara J., Lutonja P., Isingo R., Urassa M., et al. (2015). Correlation of serum and dried blood spot results for quantitation of Schistosoma circulating anodic antigen: a proof of principle. Acta tropica 150, 59–63. doi: 10.1016/j.actatropica.2015.06.026
El-Ghareeb A. S., Abd El Motaleb G. S., Waked N. M., Osman Hany Kamel N., Aly N. S. (2016). Circulating cathodic antigen cassette test versus haematuria strip test in diagnosis of urinary schistosomiasis. J. Parasitic Diseases 40, 1193–1198. doi: 10.1007/s12639-015-0648-2
Fillie Y. E., Van Lieshout L., Kornelis D., Deelder A. M. (1994). Evaluation of an ELISA for combined measurement of CAA and CCA in schistosomiasis mansoni. Acta tropica 57, 279–287. doi: 10.1016/0001-706X(94)90073-6
FIND (2021). Rapid tests for schistosomiasis control & elimination. Available online at: https://www.finddx.org/marginalized-populations/schisto-rdts/. (Accessed August 2022).
Graeff-Teixeira C., Favero V., Pascoal V. F., de Souza R. P., Rigo F. V., Agnese L. H. D., et al. (2021). Low specificity of point-of-care circulating cathodic antigen (POCCCA) diagnostic test in a non-endemic area for schistosomiasis mansoni in Brazil. Acta tropica 217, 105863. doi: 10.1016/j.actatropica.2021.105863
Greter H., Krauth S. J., Ngandolo B. N., Alfaroukh I. O., Zinsstag J., Utzinger J. (2016). Validation of a point-of-care circulating cathodic antigen urine cassette test for Schistosoma mansoni diagnosis in the Sahel, and potential cross-reaction in pregnancy. Am. J. Trop. Med. hygiene 94, 361–364. doi: 10.4269/ajtmh.15-0577
Hoekstra P. T., Casacuberta-Partal M., van Lieshout L., Corstjens P., Tsonaka R., Assaré R. K., et al. (2020). Efficacy of single versus four repeated doses of praziquantel against Schistosoma mansoni infection in school-aged children from Côte d’Ivoire based on Kato-Katz and POC-CCA: An open-label, randomised controlled trial (RePST). PloS Negl. Trop. Dis. 14, e0008189. doi: 10.1371/journal.pntd.0008189
Hoekstra P. T., Casacuberta-Partal M., van Lieshout L., Corstjens P., Tsonaka R., Assaré R. K., et al. (2022a). Limited efficacy of repeated praziquantel treatment in Schistosoma mansoni infections as revealed by highly accurate diagnostics, PCR and UCP-LF CAA (RePST trial). PloS Negl. Trop. Dis. 16, e0011008. doi: 10.1371/journal.pntd.0011008
Hoekstra P. T., Chernet A., de Dood C. J., Brienen E. A. T., Corstjens P., Labhardt N. D., et al. (2022b). Sensitive diagnosis and post-treatment follow-up of schistosoma mansoni infections in asymptomatic Eritrean refugees by circulating anodic antigen detection and polymerase chain reaction. Am. J. Trop. Med. hygiene 106, 1240–1246. doi: 10.4269/ajtmh.21-0803
Hoekstra P. T., van Esbroeck M., de Dood C. J., Corstjens P. L., Cnops L., van Zeijl-van der Ham C. J., et al. (2021). Early diagnosis and follow-up of acute schistosomiasis in a cluster of infected Belgian travellers by detection of antibodies and circulating anodic antigen (CAA): A diagnostic evaluation study. Travel Med. Infect. disease 41, 102053. doi: 10.1016/j.tmaid.2021.102053
Kabbas-Piñango E., Arinaitwe M., van Dam G. J., Moses A., Namukuta A., Nankasi A. B., et al. (2023). Reproducibility matters: intra- and inter-sample variation of the point-of-care circulating cathodic antigen test in two Schistosoma mansoni endemic areas in Uganda. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 378, 20220275. doi: 10.1098/rstb.2022.0275
Kabore A., Ibikounle M., Tougoue J. J., Mupoyi S., Ndombe M., Shannon S., et al. (2017). Initiating NTD programs targeting schistosomiasis and soil-transmitted helminthiasis in two provinces of the Democratic Republic of the Congo: Establishment of baseline prevalence for mass drug administration. Acta tropica 166, 177–185. doi: 10.1016/j.actatropica.2016.11.023
Kildemoes A. O., Vennervald B. J., Tukahebwa E. M., Kabatereine N. B., Magnussen P., de Dood C. J., et al. (2017). Rapid clearance of Schistosoma mansoni circulating cathodic antigen after treatment shown by urine strip tests in a Ugandan fishing community - relevance for monitoring treatment efficacy and re-infection. PloS Negl. Trop. Dis. 11, e0006054. doi: 10.1371/journal.pntd.0006054
Knopp S., Corstjens P. L., Koukounari A., Cercamondi C. I., Ame S. M., Ali S. M., et al. (2015). Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of schistosoma haematobium in low endemic settings. PloS Negl. Trop. Dis. 9, e0003752. doi: 10.1371/journal.pntd.0003752
Kremsner P. G., Enyong P., Krijger F. W., De Jonge N., Zotter G. M., Thalhammer F., et al. (1994). Circulating anodic and cathodic antigen in serum and urine from Schistosoma haematobium-infected Cameroonian children receiving praziquantel: a longitudinal study. Clin. Infect. Dis. 18, 408–413. doi: 10.1093/clinids/18.3.408
Langenberg M. C. C., Hoogerwerf M. A., Koopman J. P. R., Janse J. J., Kos-van Oosterhoud J., Feijt C., et al. (2020). A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat. Med. 26, 326–332. doi: 10.1038/s41591-020-0759-x
Marti H., Halbeisen S., Bausch K., Nickel B., Neumayr A. (2020). Specificity of the POC-CCA urine test for diagnosing S. mansoni schistosomiasis. Travel Med. Infect. disease 33, 101473. doi: 10.1016/j.tmaid.2019.101473
Midzi N., Butterworth A. E., Mduluza T., Munyati S., Deelder A. M., van Dam G. J. (2009). Use of circulating cathodic antigen strips for the diagnosis of urinary schistosomiasis. Trans. R. Soc. Trop. Med. Hygiene 103, 45–51. doi: 10.1016/j.trstmh.2008.08.018
Ochodo E. A., Gopalakrishna G., Spek B., Reitsma J. B., van Lieshout L., Polman K., et al. (2015). Circulating antigen tests and urine reagent strips for diagnosis of active schistosomiasis in endemic areas. Cochrane Database systematic Rev. 2015 (3), CD009579. doi: 10.1002/14651858.CD009579.pub2
Peralta J. M., Cavalcanti M. G. (2018). Is POC-CCA a truly reliable test for schistosomiasis diagnosis in low endemic areas? The trace results controversy. PloS Negl. Trop. Dis. 12, e0006813. doi: 10.1371/journal.pntd.0006813
Polman K. (2000). Epidemiological application of ciculating antigen detection in schistosomiasis (Leiden, the Netherlands: Leiden University).
Polman K., Diakhate M. M., Engels D., Nahimana S., Van Dam G. J., Falcao Ferreira S. T., et al. (2000). Specificity of circulating antigen detection for schistosomiasis mansoni in Senegal and Burundi. Trop. Med. Int. health: TM IH 5, 534–537. doi: 10.1046/j.1365-3156.2000.00600.x
Polman K., Engels D., Fathers L., Deelder A. M., Gryseels B. (1998). Day-to-day fluctuation of schistosome circulating antigen levels in serum and urine of humans infected with Schistosoma mansoni in Burundi. Am. J. Trop. Med. hygiene 59, 150–154. doi: 10.4269/ajtmh.1998.59.150
Polman K., Stelma F. F., Gryseels B., Van Dam G. J., Talla I., Niang M., et al. (1995). Epidemiologic application of circulating antigen detection in a recent Schistosoma mansoni focus in northern Senegal. Am. J. Trop. Med. hygiene 53, 152–157. doi: 10.4269/ajtmh.1995.53.152
RMD Technical Brochure - Rapid test for qualitative detection of: Bilharzia (Schistosomiasis). Available online at: http://www.rapid-diagnostics.com/updates_15_09_2019/RMD_Pamphlet_13_12_2018_Colourweb.pdf (2018). (Accessed December 2023).
Sanneh B., Joof E., Sanyang A. M., Renneker K., Camara Y., Sey A. P., et al. (2017). Field evaluation of a schistosome circulating cathodic antigen rapid test kit at point-of-care for mapping of schistosomiasis endemic districts in The Gambia. PloS One 12, e0182003. doi: 10.1371/journal.pone.0182003
Sousa M. S., van Dam G. J., Pinheiro M. C. C., de Dood C. J., Peralta J. M., Peralta R. H. S., et al. (2019). Performance of an ultra-sensitive assay targeting the circulating anodic antigen (CAA) for detection of Schistosoma mansoni infection in a low endemic area in Brazil. Front. Immunol. 10. doi: 10.3389/fimmu.2019.00682
Stelma F. F., Talla I., Polman K., Niang M., Sturrock R. F., Deelder A. M., et al. (1993). Epidemiology of schistosoma mansoni infection in a recently exposed community in northern Senegal. Am. J. Trop. Med. hygiene 49, 701–706. doi: 10.4269/ajtmh.1993.49.701
Stete K., Glass T. R., van Dam G. J., Ntamatungiro A., Letang E., de Dood C. J., et al. (2018). Effect of schistosomiasis on the outcome of patients infected with HIV-1 starting antiretroviral therapy in rural Tanzania. PloS Negl. Trop. Dis. 12 (10), e0006844. doi: 10.1371/journal.pntd.0006844
Stothard J. R., Kabatereine N. B., Tukahebwa E. M., Kazibwe F., Rollinson D., Mathieson W., et al. (2006). Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis. Acta tropica 97, 219–228. doi: 10.1016/j.actatropica.2005.11.004
Stothard J. R., Sousa-Figueiredo J. C., Standley C., Van Dam G. J., Knopp S., Utzinger J., et al. (2009). An evaluation of urine-CCA strip test and fingerprick blood SEA-ELISA for detection of urinary schistosomiasis in schoolchildren in Zanzibar. Acta tropica 111, 64–70. doi: 10.1016/j.actatropica.2009.02.009
Tamarozzi F., Ursini T., Hoekstra P. T., Silva R., Costa C., Gobbi F., et al. (2021). Evaluation of microscopy, serology, circulating anodic antigen (CAA), and eosinophil counts for the follow-up of migrants with chronic schistosomiasis: a prospective cohort study. Parasit. Vectors 14, 149. doi: 10.1186/s13071-021-04655-z
Tchuem Tchuente L. A., Kuete Fouodo C. J., Kamwa Ngassam R. I., Sumo L., Dongmo Noumedem C., Kenfack C. M., et al. (2012). Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in Cameroon. PloS Negl. Trop. Dis. 6, e1758. doi: 10.1371/journal.pntd.0001758
Van Dam G. J., Bergwerff A. A., Thomas-Oates J. E., Rotmans J. P., Kamerling J. P., Vliegenthart J. F., et al. (1994). The immunologically reactive O-linked polysaccharide chains derived from circulating cathodic antigen isolated from the human blood fluke Schistosoma mansoni have Lewis x as repeating unit. Eur. J. Biochem. 225, 467–482. doi: 10.1111/j.1432-1033.1994.00467.x
van Dam G. J., Bogitsh B. J., van Zeyl R. J., Rotmans J. P., Deelder A. M. (1996). Schistosoma mansoni: in vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. J. parasitol. 82, 557–564. doi: 10.2307/3283780
van Dam G. J., de Dood C. J., Lewis M., Deelder A. M., van Lieshout L., Tanke H. J., et al. (2013). A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp. parasitol. 135, 274–282. doi: 10.1016/j.exppara.2013.06.017
van Dam G. J., Odermatt P., Acosta L., Bergquist R., de Dood C. J., Kornelis D., et al. (2015a). Evaluation of banked urine samples for the detection of circulating anodic and cathodic antigens in Schistosoma mekongi and S. japonicum infections: a proof-of-concept study. Acta tropica 141, 198–203. doi: 10.1016/j.actatropica.2014.09.003
van Dam G. J., Xu J., Bergquist R., de Dood C. J., Utzinger J., Qin Z. Q., et al. (2015b). An ultra-sensitive assay targeting the circulating anodic antigen for the diagnosis of Schistosoma japonicum in a low-endemic area, People’s Republic of China. Acta tropica 141, 190–197. doi: 10.1016/j.actatropica.2014.08.004
Van Lieshout L., De Jonge N., el Masry N. A., Mansour M. M., Krijger F. W., Deelder A. M. (1992). Improved diagnostic performance of the circulating antigen assay in human schistosomiasis by parallel testing for circulating anodic and cathodic antigens in serum and urine. Am. J. Trop. Med. hygiene 47, 463–469. doi: 10.4269/ajtmh.1992.47.463
Van Lieshout L., Panday U. G., De Jonge N., Krijger F. W., Oostburg B. F., Polderman A. M., et al. (1995). Immunodiagnosis of schistosomiasis mansoni in a low endemic area in Surinam by determination of the circulating antigens CAA and CCA. Acta tropica 59, 19–29. doi: 10.1016/0001-706X(94)00084-E
van Lieshout L., Polderman A. M., Deelder A. M. (2000). Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta tropica 77, 69–80. doi: 10.1016/S0001-706X(00)00115-7
Viana A. G., Gazzinelli-Guimarães P. H., Castro V. N., Santos Y., Ruas A. C. L., Bezerra F. S. M., et al. (2019). Discrepancy between batches and impact on the sensitivity of point-of-care circulating cathodic antigen tests for Schistosoma mansoni infection. Acta tropica 197, 105049. doi: 10.1016/j.actatropica.2019.105049
Vonghachack Y., Sayasone S., Khieu V., Bergquist R., van Dam G. J., Hoekstra P. T., et al. (2017). Comparison of novel and standard diagnostic tools for the detection of Schistosoma mekongi infection in Lao People’s Democratic Republic and Cambodia. Infect. Dis. poverty 6, 127. doi: 10.1186/s40249-017-0335-x
WHO (2022). WHO guideline on control and elimination of human schistosomiasis (Geneva: World Health Organization).
WHO (2023). Schistosomiasis. Available online at: https://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis. (Accessed January 2024).
Keywords: schistosomiasis, diagnostics, circulating cathodic antigen (CCA), circulating anodic antigen (CAA), duplex test
Citation: Hoekstra PT, de Dood CJ, Abdoel T, Hilt S, van Diepen A, Polman K, Kremsner P, van Lieshout L, Kreidenweiss A, Adegnika AA, Fusco D, Rasomoelina T, Rakoto Andrianarivelo M, Rakotozandrindrainy R, Rakotoarivelo RA, Sicuri E, van Dam GJ and Corstjens PLAM (2024) Detecting two Schistosoma circulating antigens – CCA and CAA – in urine and serum to improve diagnosis of human schistosomiasis. Front. Parasitol. 3:1460331. doi: 10.3389/fpara.2024.1460331
Received: 05 July 2024; Accepted: 16 September 2024;
Published: 04 October 2024.
Edited by:
Alex Loukas, James Cook University, AustraliaReviewed by:
Wannaporn Ittiprasert, United States Army Medical Research Institute of Infectious Diseases (USAMRIID), United StatesElfadil Abass, Imam Abdulrahman Bin Faisal University, Saudi Arabia
Copyright © 2024 Hoekstra, de Dood, Abdoel, Hilt, van Diepen, Polman, Kremsner, van Lieshout, Kreidenweiss, Adegnika, Fusco, Rasomoelina, Rakoto Andrianarivelo, Rakotozandrindrainy, Rakotoarivelo, Sicuri, van Dam and Corstjens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pytsje T. Hoekstra, cHRob2Vrc3RyYUBsdW1jLm5s
 Pytsje T. Hoekstra
Pytsje T. Hoekstra Claudia J. de Dood2
Claudia J. de Dood2 Angela van Diepen
Angela van Diepen Peter Kremsner
Peter Kremsner Lisette van Lieshout
Lisette van Lieshout Andrea Kreidenweiss
Andrea Kreidenweiss Ayola Akim Adegnika
Ayola Akim Adegnika Daniela Fusco
Daniela Fusco Mala Rakoto Andrianarivelo
Mala Rakoto Andrianarivelo Govert J. van Dam
Govert J. van Dam Paul L. A. M. Corstjens
Paul L. A. M. Corstjens