- 1Department of Dental Anesthesiology, Tokyo Dental College, Tokyo, Japan
- 2Department of Anesthesiology and Pain Relief Center, The University of Tokyo Hospital, Tokyo, Japan
- 3Department of Pain Medicine, Fukushima Medical University School of Medicine, Fukushima, Japan
- 4Department of Molecular Imaging and Theranostics, Institute for Quantum Medical Science, National Institutes for Quantum Science and Technology, Chiba, Japan
- 5Nursing Department, The University of Tokyo Hospital, Tokyo, Japan
- 6Institute of Engineering Innovation, School of Engineering, The University of Tokyo, Tokyo, Japan
- 7Department of Orthopaedic Surgery, Tailor Made Backpain Clinic, Tokyo, Japan
- 8Department of Psychiatry, Aizu Medical Center, Fukushima Medical University, Fukushima, Japan
- 9Division of Special Needs Dentistry and Orofacial Pain, Tokyo Dental College, Tokyo, Japan
Introduction: Burning mouth syndrome (BMS) is an idiopathic oral pain disorder characterized by burning sensations and dysesthesia, often complicated by psychosocial factors and psychiatric comorbidities, necessitating a multidisciplinary approach. BMS, classified as nociplastic pain (NcplP), frequently involves central sensitization. Attention-deficit/hyperactivity disorder (ADHD), a neurodevelopmental disorder, is commonly comorbid with NcplP, and ADHD-targeted treatment has shown efficacy in NcplP management. However, the role of ADHD diagnosis and treatment on BMS and associated brain function abnormalities remains unexplored. Therefore, we aimed to investigate the prevalence of ADHD comorbidity and its assessment using ADHD scales and the therapeutic efficacy of an ADHD-focused algorithm, including pre- and post-treatment cerebral blood flow single-photon emission computed tomography (SPECT) results, in patients with treatment-resistant BMS referred from the outpatient clinic of dental psychosomatic specialists at a tertiary care institution for multidisciplinary treatment.
Methods: We retrospectively analyzed data from 14 patients with treatment-resistant BMS who received multidisciplinary care, including psychiatric evaluation and SPECT imaging. Clinical assessments included the Conners' Adult ADHD Rating Scale (CAARS-S and CAARS-O), Pain Numerical Rating Scale, Hospital Anxiety and Depression Scale, and Pain Catastrophizing Scale. Algorithm-based pharmacotherapy using ADHD-effective medications (methylphenidate, atomoxetine, guanfacine, aripiprazole, venlafaxine, and duloxetine) was administered.
Results: ADHD was diagnosed in 13 patients (92.9%), with 57.2% exhibiting borderline or clinical-level symptoms. Clinically significant improvements were observed in all clinical scales among the 10 patients who completed algorithm-based treatment. Brain perfusion SPECT identified hypoperfusion in the frontal lobe and hyperperfusion in the perigenual anterior cingulate cortex, insular cortex, posterior cingulate gyrus, and precuneus in 90% of cases, with improvements noted following treatment.
Conclusions: ADHD is frequently comorbid in patients with treatment-resistant BMS, and ADHD-targeted pharmacotherapy may help alleviate pain, cognitive dysfunction, and brain perfusion abnormalities. These findings suggest that ADHD screening, diagnosis, and multidisciplinary management involving psychiatrists could play a crucial role in optimizing clinical outcomes in patients with BMS.
1 Introduction
Burning mouth syndrome (BMS) is defined as oral pain of unknown origin, characterized by a burning or abnormal sensation lasting at least 2 h daily for a minimum of 3 months (1). The global prevalence of BMS is estimated to range from 0.1%–3.9%, with postmenopausal women aged 50–70 years being the most affected demographic (2, 3). The burning pain and numbness in the oral cavity—including the tongue, lips, palate, and gums (4)—typically worsen throughout the day, peaking in the evening (5). Despite its clinical impact, BMS remains challenging to diagnose and treat, with a spontaneous remission rate as low as 3%–4% within 5–6 years of diagnosis (6). Its pathogenesis is not fully understood; however, its etiology in many patients has been suspected to involve local, systemic, and/or psychological contributions (4). Dysfunction of dopaminergic pathways in the central nervous system (CNS) has been implicated as one of the systemic factors involved (7). BMS is frequently associated with depression, and reduced cerebral blood flow (CBF) in the left parietal and temporal lobes has been observed in patients with both BMS and depression (8). Indeed, BMS is now recognized as closely linked to psychosocial factors and psychiatric comorbidities. Addressing the psychogenic component of the pain requires multidisciplinary research and treatment approaches incorporating the expertise of psychologists and psychiatrists (4, 9).
In 2017, pain traditionally categorized as psychogenic or related to somatoform disorders was redefined as nociplastic pain (NcplP) (10), which is now recognized as a third pain type alongside nociceptive and neuropathic pain. NcplP is thought to involve plastic changes in nociceptive central neural circuits, leading to central sensitization and amplification of external stimuli (11). A key clinical feature of NcplP is its frequent association with CNS symptoms, including hyperalgesia, fatigue, sensory hypersensitivity to sound or light, sleep disturbances, mood disorders, and cognitive dysfunctions, such as impaired attention and memory. Additionally, it is often influenced by various psychosocial factors (12).
Recent studies have reported that NcplP disorders—such as fibromyalgia (13–15), chronic low back pain (16–20), idiopathic orofacial pain (19, 21–23), temporomandibular joint disorders (24, 25), chronic chest pain (26), chronic abdominal pain (27–29), and irritable bowel syndrome (30)—frequently coexist with attention deficit-hyperactivity disorder (ADHD). ADHD has been suggested to contribute to the development of central sensitization and cognitive dysfunctions, including attention deficits and sensory overactivity, observed in NcplP disorders (31). Notably, when ADHD coexists with NcplP, symptoms of pain and related CNS dysfunctions, including cognitive impairments, have been shown to improve with ADHD medications (18, 19, 21–23, 28, 32–34). Furthermore, these medications have demonstrated the ability to modulate cerebral blood flow, improving reduced perfusion in the prefrontal cortex, increasing perfusion in the precuneus, and addressing blood flow imbalances in the anterior cingulate and insular cortices—regions collectively known as the pain matrix (18, 19, 28, 32). These findings suggest that ADHD medications suppress excessive activity in the default mode network (DMN) and enhance activity in the central executive network (35), making them a promising new treatment option for NcplP, which is often refractory to conventional therapies (36).
A previous study reported that 72.5% of patients with refractory NcplP had comorbid ADHD (33). BMS is now considered a representative NcplP disorder; however, no published reports exist on the prevalence of ADHD comorbidity in BMS or the potential efficacy of ADHD medications in improving BMS symptoms and related CNS dysfunctions. Additionally, no studies have investigated changes in CBF in patients with BMS before and after ADHD medication intervention.
In this study, we aimed to investigate the prevalence of ADHD comorbidity and its assessment using ADHD scales and the therapeutic efficacy of an ADHD-focused algorithm, including pre- and post-treatment CBF single-photon emission computed tomography (CBF-SPECT) results, in patients with treatment-resistant BMS referred from the outpatient clinic of dental psychosomatic specialists at a tertiary care institution for multidisciplinary treatment.
2 Materials and methods
2.1 Study design, setting, and patients
In this retrospective study, we enrolled 103 patients with BMS aged between 18 and 90 years who visited Tokyo Dental College Hospital between May 2020 to August 2022. Of these, 20 showed intractable symptoms despite treatment with clonazepam, capsaicin, pregabalin, amitriptyline, gargle liquid, and/or low-level laser therapy (LLLT) after ≥6 months. Fourteen of these 20 patients were referred to the Department of Anesthesiology and Pain Center, University of Tokyo Hospital. We retrospectively analyzed the records of these 14 patients who presented with tongue pain between July 2020 and August 2022.
2.2 Inclusion and exclusion criteria
Eligible patients were aged ≥ 18 years, diagnosed with BMS, and exhibited no improvement after ≥ 6 months of treatment (n = 14). Exclusion criteria encompassed secondary BMS or symptom remission following treatment (n = 83), and the wish not to be referred to the Pain Centre (n = 6). Severe psychiatric conditions (depression, suicidal ideation, or psychosis) impairing reality judgment or inducing manic states were not included in this study.
2.3 Diagnostic assessments
2.3.1 BMS diagnosis and treatment
In this study, we defined an intraoral burning or dysesthetic sensation, recurring daily for >2 h for >3 months, without evident causative lesions on clinical examination and investigation, as described in the International Classification of Orofacial Pain—first edition (ICOP-1), as the diagnostic criterion for BMS. Currently, no definitive treatment for BMS is available; however, it is treated using methods consistent with those for neuropathic pain and NcplP. Reports have shown the effectiveness of clonazepam, capsaicin, pregabalin, and amitriptyline as pharmacological therapy for BMS (37).
2.3.2 ADHD diagnosis
Adult ADHD was assessed using the Conners’ Adult ADHD Rating Scale Self-Report (CAARS-S) and Observer-Report (CAARS-O) (38). These validated instruments comprise 66 items across eight subscales, leveraging T-scores based on a standardized, age-matched population. T-scores > 65 were considered indicative of clinically significant symptoms, while scores between 60 and 65 were categorized as borderline. ADHD traits such as “frequent careless mistakes”, “difficulty with organization”, or “impatience” were evaluated across average, borderline, and clinical levels.
A psychiatrist (S.K.) confirmed ADHD diagnoses based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria (39), and the Diagnostic Interview for ADHD in Adults 2.0 (DIVA 2.0), a semi-structured interview (40). The DIVA 2.0 exemplifies dysfunction in daily activities due to ADHD symptoms from childhood through adulthood across 18 diagnostic criteria within five domains: work/education, romantic/family relationships, social interactions, leisure/hobbies, and self-confidence/self-image. DSM-5 criteria require ≥ 5 of 9 inattention or hyperactivity/impulsivity symptoms for individuals aged ≥ 17 years. ADHD subtypes were classified as predominantly inattentive, predominantly hyperactive-impulsive, or combined. Other comorbid psychiatric disorders were also differentiated based on the DSM-5 diagnostic criteria.
2.3.3 Assessment of psychosocial factors
The Multidimensional Pain Inventory (MPI) (41, 42) was used to assess psychosocial factors, as it contributes to predicting treatment responsiveness in patients with chronic pain. The MPI calculates a Dysfunctional (DYS) score, which reflects the tendency of family members to exhibit overprotective behaviors that reinforce the patient's pain, and an Interpersonally Distressed (ID) score, which indicates the patient's perception of being blamed by their family. Based on the balance of these scores, patients are classified into one of the following three categories: DYS, ID, or Adaptive Coper (AC). If a response does not fit into any of these three categories, it is classified as Anomalous.
In the DYS category, a symbiotic relationship tends to develop between the patient and their family, making family conflicts less apparent. In contrast, patients in the ID category tend to feel blamed by their families and have a heightened awareness of family relationship conflicts. Patients classified as AC experience conflicts with their families but can maintain an appropriate interpersonal distance. Patients in the DYS category are considered to respond well to family-involved operant behavioral therapy, those in the ID category to assertiveness training, and those in the AC category to education on pain self-management for symptom improvement (43).
2.3.4 Pain assessment
Pain duration was measured as the time (in months) from BMS onset to the initial clinic visit. Pain intensity was evaluated using the Numerical Rating Scale (NRS) (44), with a minimum clinically important difference (MCID) of ≥2 points (45).
2.3.5 Mood state assessment
Mood disturbances, including anxiety and depression, were assessed using the Hospital Anxiety and Depression Scale (HADS) (46). Each subscale ranged from 0 to 21, with scores ≥ 11 indicating clinical levels (47). The MCID was set at 1.5 points (48).
2.3.6 Pain catastrophizing
Pain-related catastrophic thinking was assessed using the Pain Catastrophizing Scale (PCS) (49), which measures the degree to which individuals amplify or ruminate on pain experiences. Scores range from 0 to 52, with scores ≥ 30 indicating chronic pain within the 75th percentile. The MCID for PCS was set at 6.48 points (50).
2.3.7 BMS severity
BMS severity was evaluated using the Clinical Global Impression Severity (CGI-S) scale (51), assessing the condition's impact on daily activities and cognitive functions (anxiety, depression, insomnia, attention deficits, and sensory sensitivity). Scores range from 1 to 7, where 1 = normal, 2 = borderline illness, 3 = mildly ill, 4 = moderately ill, 5 = markedly ill, 6 = severely ill, and 7 = extremely ill.
2.4 Medication algorithm
The pharmacotherapy algorithm for ADHD (52, 53) is shown in Figure 1. For patients without contraindications to pharmacological treatment, the first-line medication administered was the ADHD stimulant methylphenidate (MP). If MP does not achieve sufficient improvement or causes intolerable side effects, patients either transition to combination therapy with MP and the selective norepinephrine reuptake inhibitor atomoxetine (ATX) or switch to ATX monotherapy. Should ATX fail to provide adequate improvement or result in intolerable side effects, patients either receive combination therapy with the α2 agonist guanfacine (GF) or switch to GF monotherapy. If GF administration does not yield satisfactory outcomes, patients either receive combination therapy with aripiprazole (APZ) or switch to APZ monotherapy. APZ, a partial agonist of dopamine D2 receptors and dopamine system stabilizer, can modulate dopamine activity, either by enhancing or suppressing it as needed. Suppose APZ fails to provide sufficient improvement or causes intolerable side effects. In that case, treatment progresses to combination therapy with the serotonin-norepinephrine reuptake inhibitors venlafaxine (VFX) or duloxetine (DXT) or switches to VFX/DXT monotherapy. In cases where ADHD diagnostic criteria were not met, the algorithm initiated treatment with APZ. Treatment effects on NRS, HADS, and PCS scores, as well as CBF measurements, were evaluated 2 months after medication adjustments, provided there was adequate improvement without adverse effects.
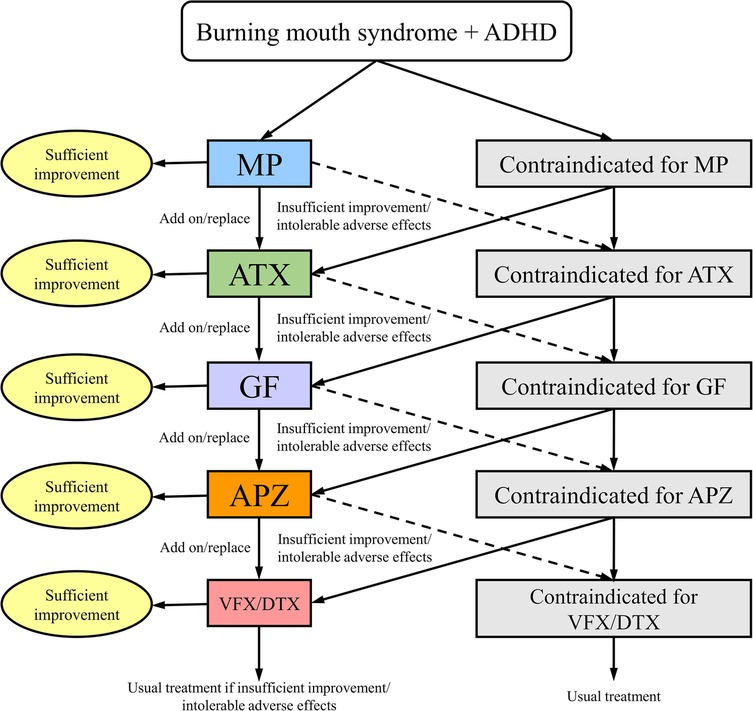
Figure 1. Algorithm for pharmacological management of patients with BMS and comorbid ADHD. ADHD, attention-deficit/hyperactivity disorder; APZ, aripiprazole; ATX, atomoxetine; DXT, duloxetine hydrochloride; GF, guanfacine; MP, methylphenidate; VFX, venlafaxine hydrochloride.
This pharmacotherapy algorithm was developed based on the following considerations. First, MP was selected as the first-line medication because it is recommended as a first-line treatment for ADHD in various national ADHD treatment guidelines (54). Furthermore, given that the participants in this study had been suffering from BMS for an average of 6.1 years without improvement, a rapid-acting medication was considered beneficial for enhancing patient motivation and adherence to treatment. Other ADHD medications that are not rapid-acting include ATX and GF. However, in Japan, a generic version of ATX is available, whereas GF does not have a generic version and is relatively expensive. To reduce the financial burden on patients, ATX was designated as the second-line treatment, whereas GF was administered as the third-line option. For the fourth-line treatment, APZ was selected based on its demonstrated efficacy against ADHD (53), chronic pain (55), BMS (56), and idiopathic oral facial pain (21). For the fifth-line treatment, VFX/DXT was selected based on the evidence that, although classified as antidepressants, both VFX and DXT have been shown to be effective in treating ADHD (53) and chronic pain (28, 57).
2.5 CBF-SPECT imaging
CBF was assessed using SPECT imaging with commercially available 99mTc-labeled ethyl cysteinate dimer (ECD; PDRadiopharma Inc., Chuo-ku, Japan). Patients were intravenously administered a 740-MBq (20 mCi) dose of ECD while resting supine with their eyes closed in a quiet environment. Approximately 5 min post-injection, a 30-min scan was conducted using a triple-head SPECT system (GCA-9300R; Cannon Medical Systems, Otawara, Japan) equipped with a high-resolution fan-beam collimator, achieving a spatial resolution of 7.2 mm full-width at half-maximum (FWHM).
Images were reconstructed using filtered back-projection with Butterworth and Ramp filters and collected in a 128 × 128 × 89 matrix with a voxel size of 1.72 × 1.72 × 3.44 mm. To minimize high-frequency noise, a 6-mm FWHM Gaussian filter was applied. The resulting images were rendered in axial, coronal, and sagittal views with rainbow color mapping. Two nuclear medicine experts (M.T., with approximately 20 years of experience, and T.M., with approximately 40 years of experience) independently conducted a qualitative visual analysis of the images to identify characteristic findings.
2.6 Statistical analysis
Changes in NRS, HADS, and PCS scores before and after treatment were evaluated using the Wilcoxon signed-rank test. Statistical significance was set at P < 0.05, with Bonferroni correction applied for multiple comparisons. Data analyses were performed using SPSS Statistics v25 (IBM Corp., Armonk, NY, USA).
2.7 Ethics approval and consent to participate
This study was approved by the University of Tokyo Hospital Research Ethics Committee (approval number 3,678). Verbal informed consent was obtained during the initial visit, followed by written informed consent for publication. Participants were informed of their right to decline participation or withdraw from the study at any time.
3 Results
3.1 Clinical characteristics
The demographic and clinical characteristics of the 14 participants (2 males, 12 females; mean age: 60.0 ± 16.3 years) are summarized in Supplementary Table S1. The average pain duration was 73.1 ± 71.5 months (approximately 6.1 years). The mean CGI-S score was 3.9 ± 1.1, indicating a range from mildly ill to markedly ill.
Based on the MPI classification of psychosocial factors, four patients (28.6%) were categorized as DYS, five (35.7%) as ID, four patients (28.6%) as AC, and one (7.1%) as Anomalous.
Previous pharmacological treatments for pain management included tricyclic antidepressants (64.3%), pregabalin (28.6%), clonazepam (14.3%), and other agents (7.1% each), such as anticonvulsants, tramadol hydrochloride, and acetaminophen. Furthermore, sleep medications (21.4%) and VFX were used as treatments for psychiatric conditions. Psychiatric treatment histories were noted in 5 participants (35.7%), including depression (21.4%), adjustment disorder (7.1%), and insomnia (7.1%). None of these treatments significantly alleviated BMS symptoms.
3.2 Assessment and diagnosis of ADHD
Thirteen participants (92.9%) met DSM-5 criteria for ADHD. ADHD subtypes were: predominantly inattentive (23.1%, 3 patients), predominantly hyperactive-impulsive (30.7%, 4 patients), and combined type (46.2%, 6 patients). Comorbid psychiatric conditions were present in five patients (35.7%), including autism spectrum disorder (28.6%, 4 patients) and depression (7.1%, 1 patient). ADHD symptoms assessed using the CAARS revealed that 28.6% of patients displayed clinical-level symptoms, 28.6% exhibited borderline symptoms, and 57.2% had ADHD symptoms at or above the borderline level. The highest CAARS subscale scores for each patient are detailed in Supplementary Table S1.
3.3 Medication regimens and outcomes
Four participants declined pharmacotherapy or discontinued early, opting for outpatient cognitive-behavioral therapy with S.K. The remaining 10 participants followed the study's pharmacotherapy algorithm. Post-treatment changes in pain-related and psychological scale scores are presented in Figure 2 and Table 1. No serious adverse events were reported. The observed improvements included: maximum pain NRS score decreased by 4.1 ± 1.0 points, minimum pain score by 2.7 ± 0.9 points, and mean pain score by 4.2 ± 1.0 points. Anxiety (HADS-A) scores improved by 3.6 ± 1.2 points, depression (HADS-D) scores by 1.6 ± 1.1 points (non-significant), and PCS scores by 10.3 ± 2.5 points. All mean changes exceeded the MCID.
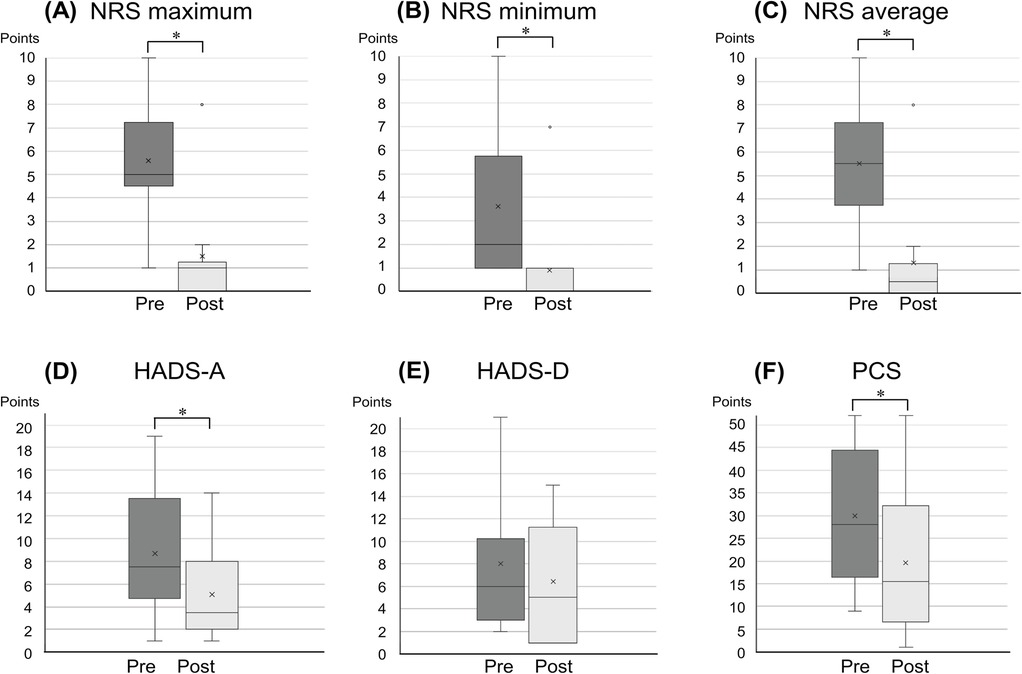
Figure 2. Changes in pain intensity and related symptoms pre- and post-treatment. Panels (A–F) illustrate the changes in pain NRS maximum, pain NRS minimum, pain NRS average, HADS-A, HADS-D, and PCS scores, respectively, pre- and post-treatment. *P < 0.05. HADS-A/D, hospital anxiety and depression scale anxiety and depression; NRS, numerical rating scale; PCS, Pain Catastrophizing Scale.
Among the 10 patients, the medications used included APZ (6 patients; 60.0%), MP (5 patients; 50.0%), ATX (3 patients; 30.0%), VFX (3 patients; 30.0%), GF (1 patient; 10.0%), and DXT (1 patient; 10.0%). Monotherapy was employed in 60.0% of cases, while 40.0% received combination therapy. Mean monotherapy doses were: MP, 18 mg/day; ATX, 20 mg/day; APZ, 5.8 ± 4.6 mg/day; VFX, 225 mg/day; and DXT, 20 mg/day.
3.4 CBF-SPECT results
Abnormal CBF-SPECT findings included hyperperfusion in the bilateral perigenual anterior cingulate cortex (pgACC), insular cortex, precuneus, and posterior cingulate cortex (PCC), with relative hypoperfusion in other frontal regions. The representative case's brain perfusion images are shown in Figure 3. These abnormalities were observed in all patients except Patients 3 and 6. Patient 6 exhibited preserved frontal regions, minimizing abnormal findings, while Patient 3 showed predominant frontal lobe hypoperfusion without pgACC or insular perfusion. Details for each patient are listed in Supplementary Table S1.
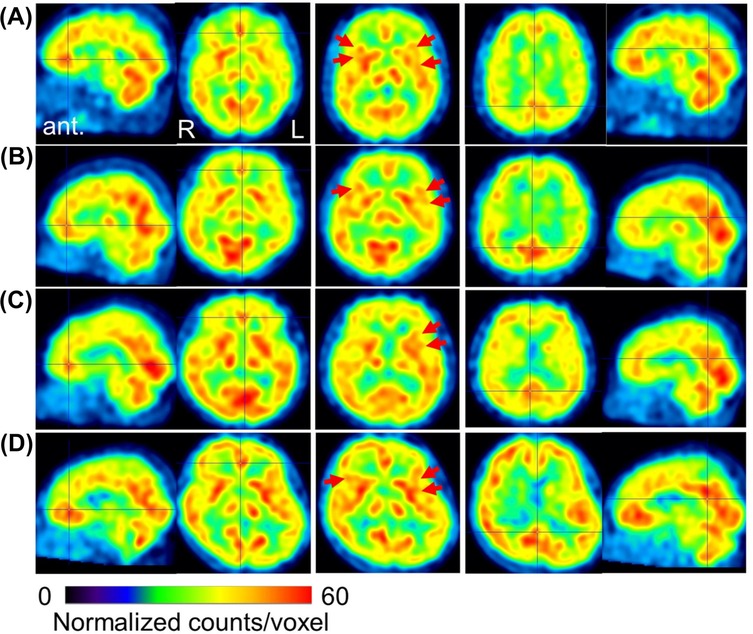
Figure 3. Representative cerebral blood flow SPECT images before treatment. Voxel values are normalized to average counts-per-voxel, with a cerebellar reference count of 50. The color bar represents count values ranging from 0 to 60. Panels (A–C) show images from patients #4, #5, and #12, respectively (all with a CGI-S score of 5), exhibiting focal hyperperfusion in the perigenual anterior cingulate cortex (crossbar) and the insular cortex (red arrows), alongside hypoperfusion in other frontal regions. Panel (D) displays images from patient #6 (CGI-S score of 2), who exhibited preserved CBF in the frontal and parietal lobes. The sagittal and axial views on the left indicate focal hyperperfusion, while the central axial views highlight increased perfusion in the insular cortex. The sagittal and axial views on the right show perfusion in the precuneus and posterior cingulate cortex. SPECT, single-photon emission computed tomography; CGI-S, clinical global impression severity; ant, anterior; R right; L, left.
Pre- and post-treatment CBF-SPECT evaluations were available for six patients (Patients 1, 4, 6, 11, 13, and 14) with a mean interval of 18 ± 7 months (range: 7–25 months). While no significant differences were observed between pre- and post-treatment images, abnormalities appeared less severe following ADHD medication therapy.
4 Discussion
This study highlights three key findings in the field of psychiatry. First, a high comorbidity rate of ADHD (92.9%, 13/14 patients) was observed among individuals with refractory BMS. Second, a pharmacotherapy algorithm effectively alleviated pain, anxiety, depression, and pain-related catastrophic thinking in these patients. Third, pre-treatment SPECT imaging in 10 patients revealed reduced frontal lobe CBF in 90.0% of cases, with localized hyperperfusion commonly identified in the pgACC, insula, precuneus, and PCC. Among the six patients who underwent post-treatment SPECT, 83% demonstrated improved CBF abnormalities, achieving a distribution closer to that of healthy individuals.
To our knowledge, this study is the first to report a high diagnostic rate of ADHD in patients with refractory BMS. The findings suggest that ADHD may frequently coexist in patients with BMS. The etiopathogenesis of primary BMS remains unclear and is probably of multifactorial origin (4). In this study, we targeted patients with treatment-resistant BMS who did not show improvement with pharmacotherapy, mouthwashes for pain relief, or LLLT, a homeopathic approach. Anxiety and depressive states are prominent complaints in adult ADHD, with over 80% of cases estimated to be overlooked, even in psychiatric clinical practice (58). Since BMS treatment is often managed by dentists or pain clinicians unfamiliar with ADHD management, it can be inferred that comorbid ADHD is frequently unrecognized. This study's results offer a new perspective on BMS in clinical and research settings. The tendency of patients with BMS to seek invasive procedures or narcotics for immediate pain relief or to engage in doctor shopping may reflect the impulsivity associated with ADHD and the challenges in awaiting gradual results (21).
The reported comorbidity rates of chronic pain and ADHD include approximately 83.3% in treatment-resistant idiopathic orofacial pain (21), 72.5% in treatment-resistant chronic pain (33), 80.2% among psychiatric outpatients with chronic pain (59), and 80% in fibromyalgia (60). The ADHD comorbidity rate in this study aligns with these figures but is comparatively higher. However, the prevalence of ADHD in fibromyalgia has varied, with Reyero et al. reporting 32.3% (61) and Yilmaz and Tamam reporting 29.5% (62). These variations highlight the influence of the study population and methodology.
One factor contributing to the high comorbidity rate of ADHD in this study was the systematic and comprehensive evaluation of ADHD symptoms using the CAARS-S/O, which incorporated input from patients and their family members. Gathering information from multiple sources is crucial for accurate ADHD diagnosis, and combining self-reported data and observer-reported data is recommended (38). Additionally, using the CAARS-O when collecting information from family members allows for a thorough review of all 18 diagnostic criteria for ADHD as outlined in the DSM-5. Therefore, the use of the CAARS-O in combination with structured interviews for adult ADHD diagnosis is recommended. Furthermore, tailoring questions based on the responses to the CAARS-O helps focus inquiries during patient interviews, making it easier to extract relevant information, which is highly useful for diagnosis (63).
The ADHD comorbidity rate of 92.9% in this study may appear remarkably high; however, it likely reflects the systematic use of the CAARS-S/O, which allowed for precise identification of ADHD-related episodes. We recommend collaboration with psychiatrists and the use of the CAARS-S and CAARS-O for thorough ADHD screening in patients with chronic pain, including BMS.
Among the 14 patients with BMS screened using CAARS, four (28.6%) exhibited ADHD symptoms at a clinical level, and another four (28.6%) demonstrated borderline symptoms. ADHD traits at borderline levels, even below diagnostic thresholds, have been shown to affect pain (59). Thus, including borderline traits in ADHD evaluations of patients with chronic pain is essential. Our findings support the high diagnostic rate of ADHD in this population.
The ADHD subtype distribution in this study was 23.1% predominantly inattentive, 30.8% predominantly hyperactive-impulsive, and 46.2% combined. While no standard ADHD subtype distribution exists for adults, ratios in children have been reported as 3.6:1.3:2.2 for inattentive, hyperactive-impulsive, and combined types, respectively (64). Among individuals with ADHD and fibromyalgia, one-third are inattentive, and two-thirds are combined type (13). In this study, patients with BMS had a higher proportion of the hyperactive-impulsive type, potentially explaining their “impatient”, “active”, and “irritable” phenotypes, which may predispose them to seek invasive procedures or engage in doctor shopping.
Based on our clinical experience and the list of ADHD behavioral characteristics outlined in DIVA 2.0 (40), we identified behavioral traits commonly observed in patients with ADHD during consultations for chronic pain conditions, such as BMS. Figure 4 shows a sample of ADHD behavioral characteristics that clinicians should be mindful of and assess during consultations. These characteristics underline the importance of ADHD screening in this population.
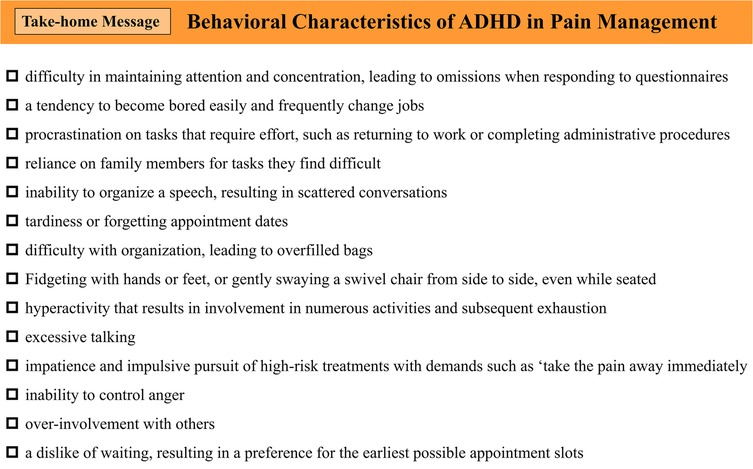
Figure 4. Behavioral characteristics of ADHD in pain management. ADHD, attention-deficit/hyperactivity disorder.
However, in dental and pain clinics that frequently treat patients with BMS, resources are often limited. In addition, the CAARS used in this study requires payment and consists of 66 questions, making it a challenging tool for routine screening. Therefore, when ADHD behavioral characteristics are observed in patients with BMS and comorbid ADHD is suspected, it is preferable to first use the Adult ADHD Self-Report Scale (ASRS) (65) for screening. ASRS is a free tool consisting of only six questions, making it a more feasible option for initial screening.
If a patient tests positive on ASRS, a consultation with a psychiatrist should be considered. However, ASRS has a sensitivity and specificity of 68.7% and 99.5% (65), respectively, indicating relatively low sensitivity and a possibility of false negatives. Therefore, even if ASRS results are negative, if a patient exhibits prominent ADHD behavioral traits, it may still be appropriate to consider the possibility of ADHD comorbidity and consult a psychiatrist while presenting the findings of this study.
The pharmacotherapy algorithm used in this study led to significant improvements in pain, anxiety, depression, and catastrophic thinking in treatment-resistant patients with BMS presenting with comorbid ADHD.
ADHD pathophysiology involves dopaminergic and noradrenergic dysfunctions (66). Medications in this study's algorithm enhanced dopamine and norepinephrine neurotransmission, likely improving both ADHD and pain symptoms. Dopamine and norepinephrine are key neurotransmitters in pain modulation (67), and their activation likely contributed to symptom improvement.
Traditionally, TCAs have been employed in the management of idiopathic orofacial pain, including BMS (68). Their mechanism of action involves norepinephrine reuptake inhibition, which enhances both norepinephrine and dopamine neurotransmission in the prefrontal cortex (69), potentially benefiting individuals with undiagnosed ADHD (21). Furthermore, ADHD medications such as MP and ATX have been shown to alleviate hyperalgesia and reduce pain thresholds through modulation of central sensitization (70–72). The high utilization of APZ and MP in this study underscores the role of enhanced dopaminergic neurotransmission in the nucleus accumbens (52), aligning with the dopamine dysfunction hypothesis of BMS pathophysiology (7).
Pre-treatment CBF-SPECT imaging revealed consistent patterns of regional hyperperfusion in the pgACC, insular cortex, precuneus, and PCC, alongside relative hypoperfusion in other frontal regions. Notably, reduced blood flow in the frontal lobe was observed in nine of 10 patients (90.0%). These findings are noteworthy as the pgACC and insular cortex are integral components of the pain matrix, while the precuneus and PCC are part of the DMN, which modulates internal cognitive processes related to external stimuli. Electrophysiological studies have shown that neuronal activity in the pgACC and PCC begins prior to voluntary reactions to noxious stimuli and persists beyond stimulus cessation, suggesting their involvement in perceiving pain stimuli as emotional experiences (73). Specifically, the pgACC has been implicated in responses to pathological skin stimuli (74) and ruminative thought processes (75), both of which can exacerbate the emotional experience of pain. We have previously demonstrated decreased precuneus CBF with chronic pain improvement following ADHD pharmacotherapy (35). ADHD medications in this study reduced activity in the DMN, likely attenuating pgACC hyperactivity and contributing to symptomatic improvement in BMS. Furthermore, reduced CBF in the frontal lobe has been identified as a potential biomarker distinguishing patients with ADHD from healthy individuals (76). Therefore, the SPECT findings in this study suggest that individuals with ADHD comorbid with BMS may exhibit physiological vulnerability to pain in the brain.
In this study, ADHD medications were associated with the normalization of CBF distribution, as shown in Figure 3 and Supplementary Table S1. Patients 4, 5, and 12 exhibited pronounced hyperperfusion in the pgACC and insula, while Patient 6, who lacked such hyperperfusion, maintained higher social and occupational functioning despite experiencing BMS.
This study has some limitations. First, the highly specific patient cohort drawn from a tertiary care center and the small sample size (n = 14) limit the generalizability of the findings. The participants in this study were unique in that they had proved difficult to treat even by dental specialists at a tertiary care university hospital; therefore, extrapolating these results to patients with BMS in more general settings, such as in typical dental clinics, should be performed with caution. The small sample size of only 14 participants means that the observed ADHD comorbidity rate of 92.9% may be a specific result limited to this study population. Furthermore, in this study, ADHD diagnoses were made by a single psychiatrist, posing a risk of overdiagnosis and diagnostic bias. Ideally, to ensure the validity of ADHD diagnoses, assessments by an additional psychiatrist should have been conducted. However, owing to limited human resources, this was not feasible. To compensate for this limitation, we utilized the CAARS-O, a paid assessment tool that provides an objective evaluation of ADHD symptoms as observed by significant others in daily life, rather than relying solely on free self-report ADHD scales (65). This approach allowed us to assess ADHD in a setting different from the clinical consultation environment. Second, the absence of a control group precludes definitive attribution of the observed improvements to the pharmacotherapy algorithm vs. spontaneous remission. Further studies involving larger, more diverse cohorts and randomized controlled designs while controlling for comorbid conditions other than ADHD are necessary to validate these findings. Third, among the participants in this study, 28.6% were classified as DYS and 35.7% as ID. Ideally, the implementation of family-involved operant behavioral therapy and assertiveness training as part of a multidisciplinary treatment approach could have further improved their symptoms (43). However, our pain center does not have a dedicated psychologist, making it impossible to implement these interventions. In the future, dentists will need to adopt a multidisciplinary approach, not only utilizing the ADHD treatment methods provided by psychiatrists, as demonstrated in this study, but also collaborating with clinical psychologists while considering patients' psychosocial factors. Furthermore, there have been reports on the effectiveness of homeopathic approaches, such as acupuncture and repetitive transcranial magnetic stimulation (37), which are alternative treatments for BMS that were not examined in this study. Therefore, these treatment options should also be explored in the future.
In conclusion, this study highlights the frequent comorbidity of ADHD in refractory BMS and its association with aberrant CBF patterns. It further demonstrates the potential of ADHD pharmacotherapy to ameliorate pain, cognitive dysfunction, and abnormal CBF in these patients. Given the high prevalence of undiagnosed ADHD in adults, routine screening for ADHD symptoms in patients with BMS using the CAARS-S/O is imperative. Collaborative, multidisciplinary approaches, incorporating other psychiatric expertise and targeted ADHD treatment, are essential to optimize outcomes in this complex population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of the University of Tokyo Hospital (approval number 3,678). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KT: Writing – original draft, Writing – review & editing. SK: Writing – original draft, Writing – review & editing. MT: Writing – original draft, Writing – review & editing. TM: Writing – original draft, Writing – review & editing. NS: Writing – review & editing, Writing – original draft. TM: Writing – review & editing, Writing – original draft. KM: Writing – review & editing, Writing – original draft. S-IN: Writing – review & editing, Writing – original draft. KU: Writing – review & editing, Writing – original draft. TH: Writing – original draft, Writing – review & editing. TI: Writing – original draft, Writing – review & editing. K-iF: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science [grant numbers: 20K07755 and 24K13083].
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing. We would also like to express our gratitude to the outpatient nurse, Mayumi Imamura, for her support in caring for difficult patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2025.1536584/full#supplementary-material
Abbreviations
ADHD, attention-deficit/hyperactivity disorder; APZ, aripiprazole; ATX, atomoxetine; CAARS-S/O, Connors' adult ADHD rating scale self-report/observer rated; CGI-S, clinical global impression severity; DMN, default mode network; DSM-5, diagnostic and statistical manual of mental disorders, fifth edition; DXT, duloxetine hydrochloride; ECD, ethyl cysteinate dimer; GF, guanfacine; HADS-A/D, hospital anxiety and depression scale-anxiety/depression; MCID, minimum clinically important difference; MP, methylphenidate; NcplP, nociplastic pain; NRS, numerical rating scale; PCC, posterior cingulate cortex; PCS, pain catastrophizing scale; pgACC, perigenual anterior cingulate cortex; SPECT, single-photon emission computed tomography; VFX, venlafaxine.
References
1 International classification of orofacial pain, 1st edition (ICOP). Cephalalgia. (2020) 40(2):129–221. doi: 10.1177/0333102419893823
2. Bergdahl M, Bergdahl J. Burning mouth syndrome: prevalence and associated factors. J Oral Pathol Med. (1999) 28:350–4. doi: 10.1111/j.1600-0714.1999.tb02052.x
3. Kohorst JJ, Bruce AJ, Torgerson RR, Schenck LA, Davis MDP. The prevalence of burning mouth syndrome: a population-based study. Br J Dermatol. (2015) 172:1654–6. doi: 10.1111/bjd.13613
4. Scala A, Checchi L, Montevecchi M, Marini I, Giamberardino MA. Update on burning mouth syndrome: overview and patient management. Crit Rev Oral Biol Med. (2003) 14:275–91. doi: 10.1177/154411130301400405
5. Forssell H, Teerijoki-Oksa T, Kotiranta U, Kantola R, Bäck M, Vuorjoki-Ranta TR, et al. Pain and pain behavior in burning mouth syndrome: a pain diary study. J Orofac Pain. (2012) 26:117–25.22558611
6. Sardella A, Lodi G, Demarosi F, Bez C, Cassano S, Carrassi A. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. (2006) 12:152–5. doi: 10.1111/j.1601-0825.2005.01174.x
7. Taiminen T, Kuusalo L, Lehtinen L, Forssell H, Hagelberg N, Tenovuo O, et al. Psychiatric (axis I) and personality (axis II) disorders in patients with burning mouth syndrome or atypical facial pain. Scand J Pain. (2011) 2:155–60. doi: 10.1016/j.sjpain.2011.06.004
8. Liu BL, Yao H, Zheng XJ, Du GH, Shen XM, Zhou YM, et al. Low regional cerebral blood flow in burning mouth syndrome patients with depression. Oral Dis. (2015) 21:602–7. doi: 10.1111/odi.12322
9. Drage LA, Rogers RS 3rd. Burning mouth syndrome. Dermatol Clin. (2003) 21:135–45. doi: 10.1016/s0733-8635(02)00063-3
10. Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice ASC, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain. (2016) 157:1382–6. doi: 10.1097/j.pain.0000000000000507
11. Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, et al. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain. (2021) 162:2629–34. doi: 10.1097/j.pain.0000000000002324
12. Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. (2021) 397:2098–110. doi: 10.1016/S0140-6736(21)00392-5
13. Pallanti S, Porta F, Salerno L. Adult attention deficit hyperactivity disorder in patients with fibromyalgia syndrome: assessment and disabilities. J Psychiatr Res. (2021) 136:537–42. doi: 10.1016/j.jpsychires.2020.10.027
14. van Rensburg R, Meyer HP, Hitchcock SA, Schuler CE. Screening for adult ADHD in patients with fibromyalgia syndrome. Pain Med. (2018) 19:1825–31. doi: 10.1093/pm/pnx275
15. Kasahara S, Matsudaira K, Sato N, Niwa SI. Pain and attention-deficit/hyperactivity disorder: the case of Margaret Mitchell. Psychosom Med. (2021) 83:492–3. doi: 10.1097/PSY.0000000000000947
16. Kasahara S, Niwa SI, Matsudaira K, Sato N, Oka H, Fujii T, et al. High attention-deficit/hyperactivity disorder scale scores among patients with persistent chronic nonspecific low back pain. Pain Physician. (2021) 24:E299–307.33988951
17. Kasahara S, Matsudaira K, Sato N, Niwa SI. Attention-deficit/hyperactivity disorder and centralized pain: a review of the case of john F. Kennedy. Clin Case Rep. (2022) 10:e6422. doi: 10.1002/ccr3.6422
18. Kasahara S, Kanda S, Takahashi M, Fujioka M, Morita T, Matsudaira K, et al. Case report: guanfacine and methylphenidate improved chronic lower back pain in autosomal dominant polycystic kidney disease with comorbid attention deficit hyperactivity disorder and autism spectrum disorder. Front Pediatr. (2023) 11:1283823. doi: 10.3389/fped.2023.1283823
19. Kasahara S, Kato Y, Takahashi M, Matsudaira K, Sato N, Niwa SI, et al. Case report: remission of chronic low back pain and oral dysesthesia comorbid with attention deficit/hyperactivity disorder by treatment with atomoxetine and pramipexole. Front Pain Res. (2023) 4:1159134. doi: 10.3389/fpain.2023.1159134
20. Ibrahim ME, Hefny MA. Central sensitization and adult attention deficit hyperactivity disorder in medical students with chronic back pain: a cross-sectional study. Egypt Rheumatol Rehabil. (2022) 49:1–7. doi: 10.1186/s43166-022-00124-2
21. Kasahara S, Takahashi K, Matsudaira K, Sato N, Fukuda KI, Toyofuku A, et al. Diagnosis and treatment of intractable idiopathic orofacial pain with attention-deficit/hyperactivity disorder. Sci Rep. (2023) 13:1678. doi: 10.1038/s41598-023-28931-3
22. Kasahara S, Kato Y, Takahashi K, Matsudaira K, Sato N, Fukuda K-I, et al. Improvement in persistent idiopathic facial pain with comorbid ADHD using the combination of a dopamine system stabilizer and psychostimulant: a case report. Clin Case Rep. (2023) 11:e7552. doi: 10.1002/ccr3.7552
23. Kasahara S, Takao C, Matsudaira K, Sato N, Tu TTH, Niwa S-I, et al. Case report: treatment of persistent atypical odontalgia with attention deficit hyperactivity disorder and autism spectrum disorder with risperidone and atomoxetine. Front Pain Res (Lausanne). (2022) 3:926946. doi: 10.3389/fpain.2022.926946
24. Wu X, Li Z, Cui Y, Yan Z, Lu T, Cui S. Neurodevelopmental disorders as a risk factor for temporomandibular disorder: evidence from Mendelian randomization studies. Front Genet. (2024) 15:1365596. doi: 10.3389/fgene.2024.1365596
25. Kasahara S, Morita T, Niwa S-I. Letter to the editor on: “relationship between attention deficit hyperactivity disorder and temporomandibular disorders in adults: a questionnaire-based report”. Eur Rev Med Pharmacol Sci. (2024) 28:4227–8. doi: 10.26355/eurrev_202409_36711
26. Zain E, Sugimoto A, Egawa J, Someya T. Case report: methylphenidate improved chronic pain in an adult patient with attention deficit hyperactivity disorder. Front Psychiatry. (2023) 14:1091399. doi: 10.3389/fpsyt.2023.1091399
27. Asztély K, Kopp S, Gillberg C, Waern M, Bergman S. Chronic pain and health-related quality of life in women with autism and/or ADHD: a prospective longitudinal study. J Pain Res. (2019) 12:2925–32. doi: 10.2147/JPR.S212422
28. Kasahara S, Takahashi M, Takahashi K, Morita T, Matsudaira K, Sato N, et al. Case report: methylphenidate and venlafaxine improved abdominal nociplastic pain in an adult patient with attention deficit hyperactivity disorder, autism spectrum disorder, and comorbid major depression. Front Pain Res. (2024) 5:1394131. doi: 10.3389/fpain.2024.1394131
29. Lipsker CW, Bölte S, Hirvikoski T, Lekander M, Holmström L, Wicksell RK. Prevalence of autism traits and attention-deficit hyperactivity disorder symptoms in a clinical sample of children and adolescents with chronic pain. J Pain Res. (2018) 11:2827–36. doi: 10.2147/JPR.S177534 30519085
30. Instanes JT, Klungsøyr K, Halmøy A, Fasmer OB, Haavik J. Adult ADHD and comorbid somatic disease: a systematic literature review. J Atten Disord. (2018) 22:203–28. doi: 10.1177/1087054716669589 27664125
31. Lipsker CW, Hirvikoski T, Balter LJT, Bölte S, Lekander M, Holmström L, et al. Autistic traits and attention-deficit hyperactivity disorder symptoms associated with greater pain interference and depression, and reduced health-related quality of life in children with chronic pain. Front Neurosci. (2021) 15:716887. doi: 10.3389/fnins.2021.716887
32. Kasahara S, Takahashi M, Morita T, Matsudaira K, Sato N, Momose T, et al. Case report: atomoxetine improves chronic pain with comorbid post-traumatic stress disorder and attention deficit hyperactivity disorder. Front Psychiatry. (2023) 14:1221694. doi: 10.3389/fpsyt.2023.1221694
33. Kasahara S, Niwa SI, Matsudaira K, Sato N, Oka H, Yamada Y. Attention-deficit/hyperactivity disorder and chronic pain. Psychosom Med. (2020) 82:346–7. doi: 10.1097/PSY.0000000000000789
34. Kasahara S, Okamura Y, Matsudaira K, Oka H, Suzuki Y, Murakami Y, et al. Diagnosis and treatment of attention-deficit hyperactivity disorder in patients with chronic pain. Open J Psychiatr. (2017) 7:261–75. doi: 10.4236/ojpsych.2017.74023
35. Takahashi M, Kasahara S, Soma T, Morita T, Sato N, Matsudaira K, et al. Precuneal hyperperfusion in patients with attention-deficit/hyperactivity disorder-comorbid nociplastic pain. Front Pharmacol. (2024) 15:1480546. doi: 10.3389/fphar.2024.1480546
36. Kasahara S, Takahashi M, Suto T, Morita T, Obata H, Niwa SI. Innovative therapeutic strategies using ADHD medications tailored to the behavioral characteristics of patients with chronic pain. Front Pharmacol. (2025) 16:1500313. doi: 10.3389/fphar.2025.1500313
37. Tan HL, Renton T. Burning mouth syndrome: an update. Cephalalgia Rep. (2020) 3:2515816320970143. doi: 10.1177/2515816320970143
38. Conners CK, Erhardt D, Sparrow EP. Conners’ Adult ADHD Rating Scales (CAARS): Technical Manual. NY: Multi-Health Systems North Tonawanda (1999).
39. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Publishing (2013).
40. Kooij JJS, Francken MH. DIVA 2.0. Diagnostic Interview Voor ADHD in Adults bij volwassenen [DIVA 2 0 Diagnostic Interview ADHD in Adults] DIVA Foundation (http://www.ivacenter.eu/DIVA.aspx). (2010). Available at: http://www.casan.ro/cjastm/media/pageFiles/2016-05-09%20interviul%20diva.pdf (Accessed March 18, 2025).
41. Kerns RD, Turk DC, Rudy TE. The west haven-yale multidimensional pain inventory (WHYMPI). Pain. (1985) 23:345–56. doi: 10.1016/0304-3959(85)90004-1
42. Kasahara S, Takahashi N, Matsudaira K, Oka H, Takatsuki K, Yabuki S. Psychometric properties of the multidimensional pain inventory: japanese language version (MPI-J). Pain Physician. (2022) 25:E105–12.35051157
43. Flor H, Turk DC. Chronic Pain: An Integrated Biobehavioral Approach. Philadelphia, PA: Lippincott Williams & Wilkins (2015).
44. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press (2011). p. 19–44.
45. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. (2004) 8:283–91. doi: 10.1016/j.ejpain.2003.09.004
46. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
47. Kugaya A, Akechi T, Okuyama T, Okamura H, Uchitomi Y. Screening for psychological distress in Japanese cancer patients. Jpn J Clin Oncol. (1998) 28:333–8. doi: 10.1093/jjco/28.5.333
48. Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. (2008) 6(46). doi: 10.1186/1477-7525-6-46
49. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7:524–32. doi: 10.1037/1040-3590.7.4.524
50. Suzuki H, Aono S, Inoue S, Imajo Y, Nishida N, Funaba M, et al. Clinically significant changes in pain along the pain intensity numerical rating scale in patients with chronic low back pain. PLoS One. (2020) 15:e0229228. doi: 10.1371/journal.pone.0229228
51. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry. (2007) 4:28–37.20526405
52. Stahl SM. Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge: Cambridge University Press (2021).
53. Buoli M, Serati M, Cahn W. Alternative pharmacological strategies for adult ADHD treatment: a systematic review. Expert Rev Neurother. (2016) 16:131–44. doi: 10.1586/14737175.2016.1135735
54. Boesen K, Paludan-Müller AS, Gøtzsche PC, Jørgensen KJ. Extended-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. (2022) 2:CD012857. doi: 10.1002/14651858.CD012857.pub2
55. Kasahara S, Kunii Y, Mashiko H, Otani K, Konno S-I, Niwa S-I. Four cases of chronic pain that improved dramatically following low-dose aripiprazole administration. Prim Care Companion CNS Disord. (2011) 13:PCC.10l01078. doi: 10.4088/PCC.10l01078
56. Umezaki Y, Takenoshita M, Toyofuku A. Low-dose aripiprazole for refractory burning mouth syndrome. Neuropsychiatr Dis Treat. (2016) 12:1229–31. doi: 10.2147/NDT.S94426
57. Rej S, Dew MA, Karp JF. Treating concurrent chronic low back pain and depression with low-dose venlafaxine: an initial identification of “easy-to-use” clinical predictors of early response. Pain Med. (2014) 15:1154–62. doi: 10.1111/pme.12456
58. Ginsberg Y, Quintero J, Anand E, Casillas M, Upadhyaya HP. Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: a review of the literature. Prim Care Companion CNS Disord. (2014) 16:PCC.13r01600. doi: 10.4088/PCC.13r01600
59. Udal ABH, Stray LL, Stray T, Bertelsen TB, Pripp AH, Egeland J. ADHD-pain: characteristics of chronic pain and association with muscular dysregulation in adults with ADHD. Scand J Pain. (2024) 24(1):20240015. doi: 10.1515/sjpain-2024-0015
60. Young JL, Redmond JC. ADHD Fibromyalgia: related conditions? In: Ostalecki S, editor. Fibromyalgia: A Complete Guide from Medical Experts and Patients. Sudbury, MA: Jones & Bartlett (2007). p. 165–77.
61. Derksen MT, Vreeling MJW, Tchetverikov I. High frequency adult attention deficit hyperactivity disorder among fibromyalgia patients in The Netherlands: should systematic collaboration between rheumatologists psychiatrists sought? Clin Exp Rheumatol. (2015) 33:S141.25152225
62. Yılmaz E, Tamam L. Attention-deficit hyperactivity disorder and impulsivity in female patients with fibromyalgia. Neuropsychiatr Dis Treat. (2018) 14:1883–9. doi: 10.2147/NDT.S159312
63. Conners. Conners’ Adult ADHD Diagnostic Interview DSM IV. ADHD Diagnostic Interview DSM IV (1999).
64. Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. (2012) 9:490–9. doi: 10.1007/s13311-012-0135-8
65. Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. The world health organization adult ADHD self-report scale (ASRS): a short screening scale for use in the general population. Psychol Med. (2005) 35:245–56. doi: 10.1017/s0033291704002892
66. Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. (2011) 69:e145–57. doi: 10.1016/j.biopsych.2011.02.036
67. Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. (2008) 8:781–97. doi: 10.1586/14737175.8.5.781
68. Feinmann C, Harris M. Psychogenic facial pain. Part 2: management and prognosis. Br Dent J. (1984) 156:205–8. doi: 10.1038/sj.bdj.4805304
69. Banaschewski T, Roessner V, Dittmann RW, Santosh PJ, Rothenberger A. Non-stimulant medications in the treatment of ADHD. Eur Child Adolesc Psychiatry. (2004) 13:I102–16. doi: 10.1007/s00787-004-1010-x
70. Treister R, Eisenberg E, Demeter N, Pud D. Alterations in pain response are partially reversed by methylphenidate (Ritalin) in adults with attention deficit hyperactivity disorder (ADHD). Pain Pract. (2015) 15:4–11. doi: 10.1111/papr.12129
71. Sifeddine W, Ba-M’hamed S, Landry M, Bennis M. Effect of atomoxetine on ADHD-pain hypersensitization comorbidity in 6-OHDA lesioned mice. Pharmacol Rep. (2023) 75:342–57. doi: 10.1007/s43440-023-00459-3
72. Suto T, Kato D, Koibuchi I, Arai Y, Ohta J, Hiroki T, et al. Rat model of attention-deficit hyperactivity disorder exhibits delayed recovery from acute incisional pain due to impaired descending noradrenergic inhibition. Sci Rep. (2023) 13:5526. doi: 10.1038/s41598-023-32512-9
73. Bastuji H, Frot M, Perchet C, Magnin M, Garcia-Larrea L. Pain networks from the inside: spatiotemporal analysis of brain responses leading from nociception to conscious perception. Hum Brain Mapp. (2016) 37:4301–15. doi: 10.1002/hbm.23310
74. Lorenz J, Casey KL. Imaging of acute versus pathological pain in humans. Eur J Pain. (2005) 9:163–5. doi: 10.1016/j.ejpain.2004.07.009
75. Alexander L, Jelen LA, Mehta MA, Young AH. The anterior cingulate cortex as a key locus of ketamine’s antidepressant action. Neurosci Biobehav Rev. (2021) 127:531–54. doi: 10.1016/j.neubiorev.2021.05.003
Keywords: burning mouth syndrome, nociplastic pain, attention deficit hyperactivity disorder, methylphenidate, multidisciplinary approach, single-photon emission computed tomography, frontal hypoperfusion, precuneal hyperperfusion
Citation: Takahashi K, Kasahara S, Takahashi M, Morita T, Sato N, Momose T, Matsudaira K, Niwa S-I, Uchida K, Handa T, Ichinohe T and Fukuda K-i (2025) Recognition and treatment of attention deficit-hyperactivity disorder in patients with treatment-resistant burning mouth syndrome: a retrospective case study. Front. Pain Res. 6:1536584. doi: 10.3389/fpain.2025.1536584
Received: 29 November 2024; Accepted: 25 March 2025;
Published: 23 April 2025.
Edited by:
Yojiro Umezaki, Fukuoka Dental College, JapanReviewed by:
Soraya M. C. Grossmann, Pontifical Catholic University of Minas Gerais, BrazilTakayuki Suga, Institute of Science Tokyo, Japan
Motoko Watanabe, Tokyo Medical and Dental University, Japan
Copyright: © 2025 Takahashi, Kasahara, Takahashi, Morita, Sato, Momose, Matsudaira, Niwa, Uchida, Handa, Ichinohe and Fukuda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoshi Kasahara, bmFtYWhhZ2VuYXRvckBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Kaori Takahashi1,†
Kaori Takahashi1,† Satoshi Kasahara
Satoshi Kasahara Miwako Takahashi
Miwako Takahashi Taito Morita
Taito Morita Ko Matsudaira
Ko Matsudaira Shin-Ichi Niwa
Shin-Ichi Niwa