- 1Department of Emergency Medicine, Jiangxi Medical College, The Second Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, China
- 2Jiangxi Medical College, The Second Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, China
Pain is a common symptom of many clinical diseases; it adversely affects patients’ physical and mental health, reduces their quality of life, and heavily burdens patients and society. Pain treatment is one of the most difficult problems today. There is an urgent need to explore the potential factors involved in the pathogenesis of pain to improve its diagnosis and treatment rate. Piezo1/2, a newly identified mechanosensitive ion channel opens in response to mechanical stimuli and plays a critical role in regulating pain-related diseases. Inhibition or downregulation of Piezo1/2 alleviates disease-induced pain. Therefore, in this study, we comprehensively discussed the biology of this gene, focusing on its potential relevance in pain-related diseases, and explored the pharmacological effects of drugs using this gene for the treatment of pain.
1 Introduction
Pain is commonly defined as an unpleasant sensory and emotional experience linked to or similar to, the experience of actual or potential tissue damage (1). Pain is classified based on several factors. The time of onset is one such factor, with pain categorized as acute or chronic. Another factor is the underlying cause of the pain, which can be classified as inflammatory, neuropathic, or cancer-related. Pain can be categorized according to severity, ranging from mild to moderate to severe (2). In this study, we focused on pain resulting from various etiologies. Many diseases are known to cause pain; however, the areas of research that have garnered particular attention are inflammatory, neuropathic, and cancerous pain (3). The pain stemming from these causes is predominantly chronic in nature and may be accompanied by additional symptoms such as dizziness, nausea, vomiting, and anorexia (4). Undoubtedly, the experience of pain creates significant obstacles to daily activities (5), however, for a prolonged period, there was a lack of distinct diagnostic criteria for pain. Until the release of the International Classification of Diseases (ICD) in 2018, pain was not systematically classified and the diagnostic criteria for each type of pain were not summarized. This classification system provides a standardized framework for the diagnosis and treatment of various types of pain (6).
Piezo1/2 has been identified as a crucial element in cellular activity, and any mutations in the piezo1/2 gene can cause diseases in different parts of the human body. One illustrative example of this class of disorders is Hereditary Xerocytosis (HX), a condition precipitated by mutations in the Piezo1 gene, which is integral to the maintenance of red blood cell volume homeostasis (7). Furthermore, the dysfunction of Piezo1 in adult lymphatics culminates in parenchymal lymphatic valve degeneration (8). Additionally, diminished mRNA levels of Piezo1 have been implicated in the constriction of the outflow tract and aortic valve malformation (9) among other sequelae (10).
Piezo1/2 channels are crucial for pain perception and initiation. They play a key role in converting mechanical stimuli into neuronal signals that contribute to pain sensation. These channels are expressed in different types of sensory neurons, including those that respond to mechanical stimuli and can be activated by physical pressure or touch (11). Activation of these channels in sensory neurons leads to depolarization of the membrane potential and opening of voltage-gated ion channels, ultimately leading to the activation of action potential firing and the perception of pain (12).
The purpose of this review was to provide a comprehensive overview of the potential association between Piezo1/2 and pain, as well as their pharmacological properties in managing pain.
2 Structure and abnormal expression of Piezo1/2
Patapoutian et al. initially identified the Piezo family of proteins in Neuro2A cells and reported that it is present in numerous species. These proteins are expressed in various vertebrate tissues including the lungs, bladder, colon, bone, sensory neurons, and DRG neurons (13). Piezo1/2 is a well-conserved ion channel comprising 2,500 amino acids. Various cryo-electron microscopy studies revealed that Piezo1 has a homogeneous trimeric structure with three blades arranged in a propeller-like configuration. It also contains a central cap structure, three long beams connecting the cap and blade structures, and a TM region (14–18). Piezo2 is structurally similar to Piezo1 but contains additional contraction sites (13) that can function as transmembrane gateways regulated by the cap domain (18).
Several studies have demonstrated that mutations in Piezo1 can lead to various diseases. Dehydration in hereditary stem cell disorder and congenital lymphoid dysplasia are associated with such mutations (19). Moreover, mutations in Piezo2 can lead to the development of distal joint contracture type 5 (DA5), as well as other diseases such as Gordon syndrome (GS) and Marden-Walker syndrome (MWS) in humans. Knocking out Piezo1/2 leads to embryo damage and results in the death of mice shortly after birth (20). Piezo1/2 is also involved in the development of diseases under corresponding pathological conditions. For example, under high venous pressure conditions, Piezo1 ion channel proteins can disrupt pulmonary endothelial barrier function and promote arterial remodeling during hypertension. Additionally, it is associated with aortic stenosis and the development of atherosclerosis (21). In the gastrointestinal epithelium, Piezo1 channels function as pressure sensors that regulate cell crowding (21) and migration- (22, 23). Wang et al. investigated the role of Piezo1 in large omental metastasis of gastric cancer and its underlying mechanism. Piezo1 expression was notably higher in 110 metastatic gastric cancer tissue samples than in non-metastatic gastric cancer tissue samples. Silencing Piezo1 resulted in a significant reduction in gastric cancer metastasis, whereas overexpression of Piezo1 inhibited apoptosis and promoted cell proliferation in GC cells. Thus, Piezo1 plays a crucial role in the invasion and development of gastric cancer (24). Moreover, Piezo1 ion channels are linked to acute pancreatitis (AP)- (23). The pancreas has a low threshold for mechanical stimulation (25). According to Romac et al. (26), injection of the Piezo1 agonist Yoda1 reduced the secretion of pancreatic enzymes that cause pancreatitis, and blocking Piezo1 prevented pancreatitis caused by mechanical stimulation. Additionally, Piezo1 antagonists are effective in preventing pancreatitis.Piezo1 promotes early local invasion of pancreatic ductal adenocarcinoma cells through a combination of environmental pH, mechanical output of pancreatic stellate cells, and stromal mechanical stimulation (27–29).
3 Pain behavior evaluation methods
Pain exerts a profound influence on both the physiological and psychological aspects of an individual. Behavioral alterations in patients serve as indirect indicators of the intensity of experienced pain (30). To quantify the severity of pain, a plethora of clinical assessment scales have been devised, predominantly categorized into unidimensional and multidimensional scales. Unidimensional scales, while straightforward and user-friendly, are characterized by a stronger emphasis on a singular aspect of pain assessment, which may limit their comprehensiveness. In contrast, multidimensional scales adopt a holistic approach, integrating observations, physiological responses, and behavioral changes, along with other subjective and objective indicators. This comprehensive methodology has garnered significant attention from the scientific community in recent years, highlighting the complexity and multifaceted nature of pain assessment (31).
3.1 Unidimensional pain assessment tool
3.1.1 PAULA pain scale
The PAULA pain scale, introduced by Machata et al. in 2009, is a unidimensional tool designed for post-operative pain assessment (32). The scale comprises two sections: the upper section features a spectrum of five faces, transitioning from yellow to red, symbolizing increasing pain intensity. The lower section consists of a 20 cm long 100-grid ruler, linked to a movable scale. The scale ranges from 0 points, represented by a yellow face indicating “no pain,” to 100 points, depicted by a red face for “the most severe pain.” The PAULA scale is user-friendly, offering a seamless integration of the visual analog scale (VAS) and the facial rating scale (FRS). The initial study demonstrated that even first-time users of the PAULA scale exhibited less variability in their pain assessments compared to the VAS. In a retest involving only 23% of patients, the discrepancy in pain scores was also smaller than that observed with the VAS.
3.1.2 Memorized pain assessment card (MPAC)
MPAC was initially proposed by Fishman in 1987 as a tool for the rapid assessment of cancer pain (33). The MPAC incorporates elements of the Visual Analog Scale (VAS) format, featuring three 10-cm horizontal lines to represent the intensity of pain, the degree of pain relief, and the emotional impact of pain. Each line is anchored by endpoints labeled “least” and “most.” Additionally, the second part of the card utilizes a modified Tursky pain descriptor scale. Fishman examined the correlation of these four components with the Zung Anxiety Inventory, Hamilton Depression Scale, and MPO, confirming their consistency (34). The MPAC's reliability and validity have been established. While the MPAC integrates various dimensions of pain assessment, it is categorized as unidimensional due to its focus on pain intensity as the primary measure, akin to the PAULA Pain Scale. This integration enhances the comprehensiveness of pain assessment while maintaining a unidimensional approach.
3.1.3 Critical-pain observation tool (CPOT)
The CPOT was developed by Gelinas et al. in 2006, specifically for assessing pain in critically ill patients who may be unable to communicate their pain levels (35). The CPOT is characterized as a unidimensional scale focusing on behavioral indicators of pain. It comprises four behavioral items: facial expression, body movement, muscle tension, and compliance with ventilation (for intubated patients) or vocalization (for non-intubated patients). Each item is scored from 0 to 2 based on the patient's observed responses, resulting in a total score ranging from 0 to 8. A higher total score indicates a greater intensity of perceived pain. Although the CPOT assesses multiple behavioral aspects, it is considered unidimensional because it aggregates these observations into a single score representing the overall pain intensity.
3.2 Multidimensional pain assessment tool
There are three common pain assessment tools: the short-form McGill Pain Questionnaire (SF-MPQ) (36), the Brief Pain Inventory (BPI) (37), and the Global Pain Scale (GPS) (38). The SF-MPQ, developed by Melzack, includes a pain rating index, a visual analogy rating method, and existing pain intensity. It employs a pain rating scale with 15 descriptive pain words to reflect the nature of pain, and quantitatively describe the degree of pain. The BPI, however, is a quick multidimensional pain assessment scale with 15 entries for pain and pain levels (39). It has been translated into several versions, including a Chinese version, and is used to assess neuropathic pain in patients with urolithiasis and cancer. Another tool, the GPS, developed by Gentile et al., includes four dimensions: pain, emotion, clinical manifestations, and daily activities. Scores range from 0 to 10 for each item and can reach a maximum of 200, reflecting pain severity. It is primarily used to access chronic pain and can provide valuable assessment and treatment plans for healthcare professionals (40).
4 Piezo1/2 and cancer pain
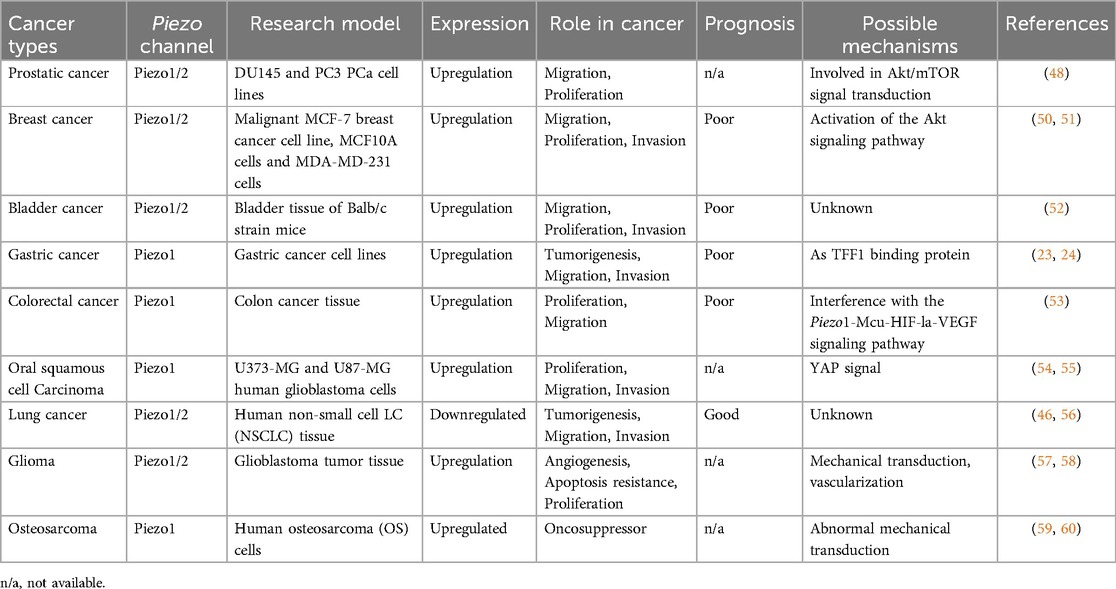
Pain associated with malignant tumors, including epithelial cancers and mesenchymal sarcomas, has become one of the most widespread health concerns worldwide (41). Tumors often occur as a result of clonal abnormalities in the level of gene regulation, which manifest themselves as developmental dedifferentiation, structural heterogeneity, persistent growth, and organismal incoordination (42–44). In recent years, evidence showing a high correlation between piezo genes and tumorigenesis has increased in a stepwise manner. Further, piezo ion channels are aberrantly expressed in a variety of cancers, including but not limited to respiratory cancers (45), the expression levels of piezoelectric proteins in different types of tumors and the related mechanisms are shown in Table 1, genitourinary cancers (46, 47), and gastrointestinal cancers (22), disrupting pre-existing normal electrical-mechanical signaling and participating in the development of pain in cancer (48).
4.1 Relationship between piezo channels and genitourinary tumors
Prostate cancer is a malignant tumor that predominantly affects men and is accompanied by painful mechanical compression in addition to invasion of the glandular tissue itself (49). The generation of pain is closely related to the pressure on the internal organs, infiltration of cancer cells, and expansion of cancerous tissue, which is highly diffuse. Immunohistochemical analysis revealed that the piezo1 gene is significantly higher in prostate cancer cells than in normal prostate and paracancerous tissues (47). Yu et al. compared TCGA and GENT1 databases and found that piezo1 expression levels in cancer tissues and normal tissues were not the same; in the TCGA database piezo1 was more expressed in normal tissues, whereas in the GENT1 database, the opposite was true (50). Elevated piezo1 activity is also observed in the cancerous tissues of bladder cancer patients, and excessively open mechanosensitive ion channels (MSC) contributes positively to the metastatic proliferation of cancer cells; however, this adversely affects the body's battle against cancer and ongoing survival (51, 52).
4.2 Relationship between piezo channels and digestive system tumors
Cancers of the digestive system, including those of the stomach (53), intestines (54), and upper gastrointestinal tract (55), are the most common malignancies. There are many causes of pain, including but not limited to compression pain and obstructive pain caused by rapid tumor growth, as well as pain caused by tumor infiltration of blood vessels, resulting in arterial occlusion, venous stasis, and local hypoxia (56, 57). In normal human gastric GES-1 cells, piezo1 can act as a trefoil factor family 1 (TFF1) binding protein that mediates cell proliferation and migration, whereas in gastric cancer cells, with knockdown of the piezo1 channel, the cell cycle is stalled in the G0/G1 phase and cell proliferation and migration are reduced (22, 23). Furthermore, if piezo1 extracted from gastric cancer cells is injected into mice, tumor growth can be inhibited (58). Colorectal and upper gastrointestinal tract cancers, such as oral squamous epithelial cancer, are two additional gastrointestinal cancers linked to piezo. The upregulation of piezo1 expression plays a role in both their development and progression, but the two act on slightly different signaling pathways, which achieve further tumor advancement by interfering with the Piezo1-Mcu-HIF- la-VEGF signaling pathway in colorectal cancer, and are associated with YAP signaling in oral epithelial cancer (59–61).
4.3 Relationship between piezo channels and lung cancer
In contrast to the aforementioned cancers, the development of lung cancer is associated with a significant downregulation of Piezo1 and Piezo2 gene expression. Specifically, in non-small cell lung cancer (NSCLC), both Piezo1 and Piezo2 mRNA translation levels are notably reduced compared to adjacent lung non-tumor cells. This downregulation indicates a high degree of deletion in gene expression within NSCLC. Furthermore, when Piezo1 and Piezo2 are experimentally knocked down, lung cancer tissue primary foci exhibit an increased growth trend and a significant enhancement of in vitro metastasis (45). Moreover, this metastatic trend and pain sensation were closely related to the degree of piezo gene deletion. In the early stage of lung cancer, that is, when piezo gene expression is initially lost, patients do not have pain sensation or feel mild pain, but as the cancer progresses, the pain sensation can progressively deepen and metastasize, causing diffuse pain in the chest wall, which can be accompanied by pain radiating to the shoulder and other places (45, 62). Despite the lack of understanding of the underlying mechanism, the use of appropriately increased piezo gene expression as a major direction for lung cancer treatment is promising.
4.4 Relationship between piezo channels and other cancers
Gliomas are neurologically related tumors that cause persistent and severe headaches and originate from glial cells in the brain, and piezo2 is involved in the regulation of the vascular architecture of their tissues (63, 64). This role is associated with calcium ions, and the inward flow of calcium ions enhances Wnt signaling activity, enabling serial signaling of Wnt and β-proteins and promoting vascular activity and permeability in glial cells (63). In a Drosophila model analysis, piezo1 could accelerate glioma development by enhancing the mechanical transduction of tissue (65). The pain caused by glioma is neuropathic cancer pain, which differs from the injurious pain caused by several of the aforementioned cancers in that, in addition to the onset of hypersensitivity symptoms (persistent tingling sensation as well as a burning sensation), it is accompanied by hypoallergenic symptoms (numbness and decreased electrical conduction ability) (66, 67). Osteosarcoma (OS) is the most common malignant bone tumor in adolescents. As an aggressive class of aberrations, their occurrence is closely associated with aberrant mechanotransduction, often accompanied by overexpression of piezo1 in OS cell lines, a feature also observed in the SW982 synovial sarcoma (68, 70). In addition to the pain caused by OS itself, bone pain is one of the most common types of pain in cancer patients, and its onset is often spontaneous and progressively worsens as the disease develops, progressing from an initial intermittent dull ache to unbearable pain (71–73). This pain often manifests as a breakthrough pain, that is, recurrent episodes of extreme pain (72).
Similarly, piezo1 showed differences in mRNA expression levels, with its expression being significantly higher in the PC3 and DU145 cell lines than in the normal cell line RWPE-1 (47). This implies that increased expression of the piezo1 gene contributes to prostate cancer progression, as evidenced by the invasive proliferation of cancer cells and escalated visceral pain. In the development of breast cancer, overexpression of piezo2 contributes to the low survival rate of triple-negative breast cancer (TNBC), suggesting that the opening of excessive piezo2 channels enhances the proliferative capacity and invasiveness of TNBC cells, which may be related to the activation of the Akt signaling pathway (46). The opening of gated piezo2 channels allows the release of calcium ions from the sarcoplasmic reticulum, accompanied by a Ca2+ inward flow, triggering the RhoA-mDia pathway, which in turn regulates the actin-constituted cytoskeleton (74, 75). Notably, piezo1 is also involved in breast cancer-related processes and plays different roles in changing breast cancer type and stage.
5 Piezo1/2 and inflammatory pain
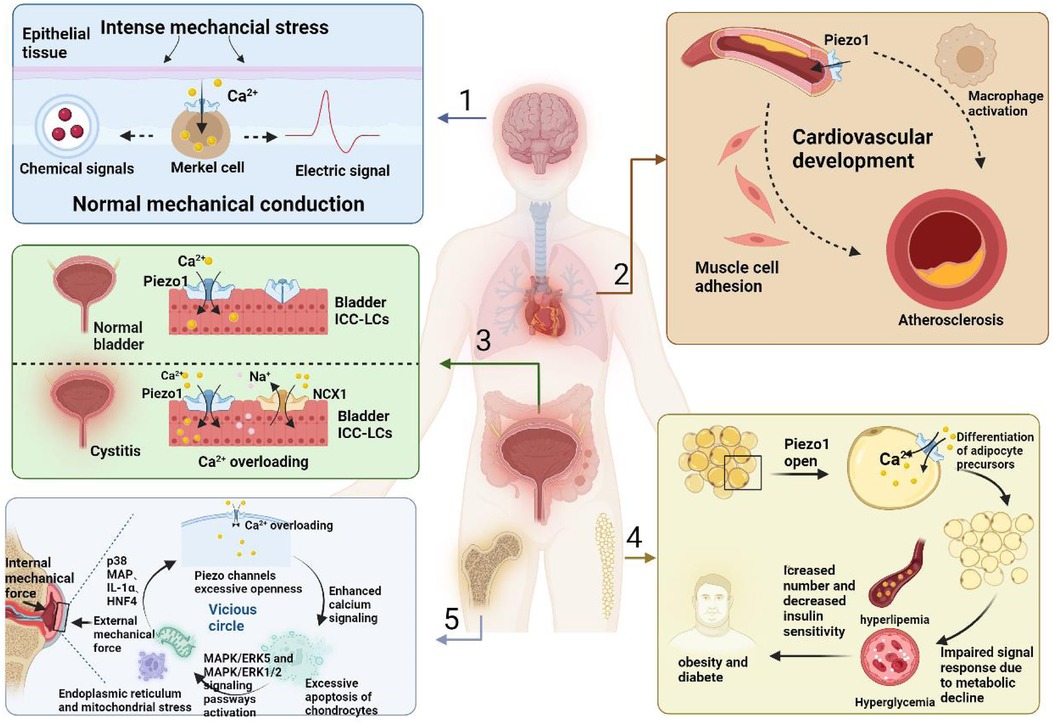
Stimulation by mechanical forces occurs throughout human life, and the source of this stimulation can be either passively applied or generated intracellularly (76, 77). When the stimulation increases beyond the maximum stimulation intensity that the tissue cells can withstand, the release of cytokines such as TNF-α, IL-1β, IL-6, and IL-8 can be observed, inducing inflammation (78–80). Cells can produce corresponding responses through the mechanical transduction of harmful stimuli (13). Inflammation can be divided into acute inflammation, which is dominated by metaplasia and exudation with visible infiltration of neutrophils (21, 81), and chronic inflammation, which is a class of proliferative reactions characterized by the infiltration of lymphocytes and plasma cells (82). Acute inflammation is essentially a defense response in living tissues, with the vascular system being stimulated by external and internal damage factors. This represents not only the predominant pathological phenomenon in humans and numerous animals, but also the key defensive mechanism that, significantly contributes to the defense against pathogenic incursion, infection, and mending of cell tissues (83). In contrast, chronic inflammation, which usually lasts six weeks or longer, brings about unpleasant subjective and emotional experiences associated with tissue damage or potential damage (84). During the transition from acute to chronic inflammation, cytokines mediate the migration of immune cells and their adhesion to cells at the site of injury, leading to the infiltration of inflammation-associated cells. Inflammatory cells, while removing necrotic cells, also cause damage to the surrounding normal cells, promoting the release of growth factors by the organism, stimulating the proliferation of new cells with structural morphology and physiological function different from normal cells, and transforming inflammation into chronic inflammation (85). The expression of piezoelectric protein 1/2 in various systemic inflammatory diseases is shown in Figure 1.
Piezo1/2 channels are a class of MSC capable of converting mechanical signals into electrochemical signals; piezo1 plays a role in pain that is still under investigation, while piezo2 has been shown to play a role in many types of pain (86, 87). For example, piezo1 is essential for controlling vascular blood pressure, mainly by mediating the release of ATP and participating in P2Y2/Gq/G11-mediated activation of downstream transduction signals, which leads to the phosphorylation and activation of protein kinase B(AKT) and endothelial NOS. In contrast, in mice with piezo1 KO, we observed a loss of vasodilatory capacity and an inability to form NO, manifesting as hypertension (88). Atherosclerosis (AS) is a multifactorial chronic inflammatory disease caused by damage to the vascular endothelial cells (ECs) and is essentially a class of chronic inflammatory diseases (89). During atherosclerosis development, piezo1 is involved in EC injury and mediates macrophage activation. At the same time, ECs can attract and adhere to muscle cells because of their stimulatory effect (21, 89). Inflammatory reactions in the bone and joints are a worldwide health problem, especially in weight-bearing joints, resulting in reduced mobility and significant medical costs (90). The researchers found that interleukin-1α (IL-1α) effectively upregulated piezo1 expression in chondrocytes and induced an increase in Ca2+ concentration in the intracellular fluid, accompanied by the opening of piezo1/2 ion channels during chondrocyte perception of inflammation and mechanical trauma (91). This results in a dilution of F-actin and greater susceptibility to mechanical trauma. In the development of osteoarthritis (OA), IL-1α does not act in isolation, but also in concert with several protein kinases and transcription factors, such as p38 MAP, HNF4, and ATF2/CREBP1 (91, 92).
Gentle mechanical stimulation, such as touch, does not cause pain but is accompanied by pain after tissue damage and inflammation occur (93). In contrast, when piezo2 KO mice and piezo2-deficient humans were similarly stimulated by touch, no signs of overactivity were observed (18). Recently, it has been shown that the knockdown of piezo2 channels can completely disable neurons that cause mechanical pain in mice (94) and that this removal not only results in reduced sensitivity to stroking stimuli but also an increase in the pain threshold (95, 96). This suggests that piezo2 is essential for mechanical and inflammatory pain because the body's response to small stimuli such as touch and vibration requires piezo2 involvement (94). One theory is that in many diseases of unknown origin, such as amyotrophic lateral sclerosis (ALS) and dry eye disease, piezo2 is involved in a signaling system that malfunctions. The changes in piezo2 channel, which is involved in transmitting proprioceptive signals, interrupt the transmission of consciousness proprioceptive signals to the hippocampus and disrupt the feedback process of motor neurons’ ultrafast proprioceptive signals (97). piezo2 has also been linked to acute pain. Studies on mice have shown that piezo2 is essential for the sensitivity of the mechanical stimuli that can cause acute pain, namely, that piezo2 depends on the nociception that is necessary for the mice to experience acute pain (98).
In addition to the typical inflammatory diseases described above, piezo1/2 channels can be expressed to some extent in various inflammatory conditions, such as fibrosis in the lung (a group of lesions characterized by an inflammatory response, fibroblast proliferation, lung tissue damage, and extracellular matrix deposition) (99), obesity and diabetes (hypertrophy and hyperplasia of adipocytes, which in turn cause inflammatory effects in adipose tissue with insulin resistance) (100) and chronic cystitis (101), which brings about painful reactions of varying degrees.
The numbered tissues are as follows: (1) CNS, (2) cardiovascular system, (3) genitourinary system, (4) adipose tissue, (5) bone tissue. When the organism receives external inflammatory stimuli, it first transmits them through the piezo1/2 channels and transforms them into electrical and chemical signals, which subsequently cause histological changes with different morphologies in different tissues. In the cardiovascular system, monocyte activation and myofibroblast adhesion are induced, accelerating the progression of atherosclerosis, in the genitourinary system there is an overload of calcium inward flow and dysfunction of ICC-LC receptors, in the adipose tissue there is a decrease in the hypertrophy and maturation of progenitors, and in the chondrocytes, there is inflammatory effusion, further damaging the newborn osteoblasts.
6 Piezo1/2 and neuropathic pain
The central nervous system (CNS), upon receiving mechanical signals from its environment, is capable of exhibiting diverse alterations, such as growth and diversification, movement and bonding, and structural modifications, encompassing nociceptive transmission (102–104). Neuropathic pain is a kind of pain caused by abnormal somatosensory system and disturbance of sensory signal transmission to the spinal cord and brain. Neuropathic pain is usually a chronic and secondary pain that can predict abnormalities in the peripheral nervous system or central nervous system (66). Approximately 500 million people worldwide suffer from it (105). For piezo1, Koser DE et al. showed that its presence is observed in retinal ganglion cells (RGCs) of African clawed toads (106, 107). RGCs are an important component of the CNS (108). RGC axons respond to mechanical signals transmitted in vivo, and when RGC embryos were treated with the piezo channel inhibitor GsMTx4, or when gene expression was downregulated by approximately 42% using morpholino knockdown, an irregular trend of axon spreading was observed, accompanied by a reduction in length and a decrease in the ability to transmit signals (107). Piezo2 is a class of genes homologous to piezo1 that, when aberrantly expressed, can render cells with low mechanical stimulation thresholds hypersensitive (109), and whose expression is more selective and enriched in somatosensory neurons (12).
6.1 Piezo channels and central neuropathic pain
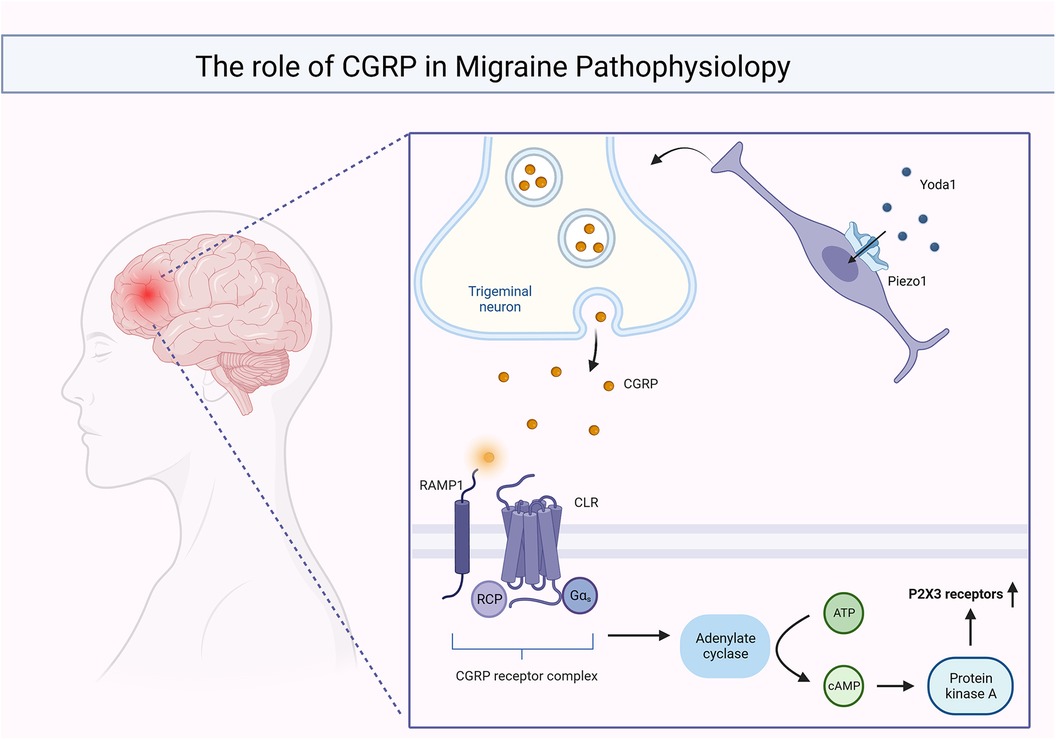
According to this classification, neuropathic pain can be further classified into central and peripheral nerve pain (110). The first type of pain is often caused by lesions in the spinal cord or brain. Migraine is the most common type of representative. Migraine is a common chronic neurovascular disease and one of the most serious disabling diseases in the world (111). As a group of neurological disorders characterized by persistent headaches, it originates from the vascular injury receptor system located within the trigeminal nerve and is closely associated with hypersensitivity reactions (112). Adriana Della Pietra and team found that in the sensory neurons of the trigeminal nerve (113), the development and closure of both piezo1 and piezo2 channels can be detected, which are involved in the control of facial muscles and, micro-expressions while being able to induce the production of pulsatile neuropathic pain, one of the qualities of migraine (114). The opening of the Piezo1 channel can be achieved using exogenous facilitators. Yoda1 is a class of small lipid-soluble substances that is specific for piezo1 and which cannot act on piezo2 (115). When Yoda1 binds to one of the three subunits of the piezo1 channel protein, it interacts with the carboxyl end of the peptide chain of the piezo2063 protein to open the channel (116, 117). Calcitonin Gene-Related Peptide (CGRP) is the main mediator of trigeminal nerve sensitization and its action depends on the activation of protein kinase A (PKA) and PKC (118). CGRP binds to its corresponding receptors and activates adenylate cyclase through G proteins, with the assistance of cAMP to activate PKA and PKC to achieve signal amplification and ultimately enhance the expression of P2X3 receptors, P2X3 receptors sensitizes sensory nerve fibers, increasing their responsiveness to pain signals and leading to allodynia (119–121). In summary, the onset and development of migraine are closely related to the activation of meningeal injury receptors (122), which are mechanosensitive and accompanied by the involvement of piezo channels (123). CGRP and piezoelectric channels are involved in the pathogenesis and different stages of migraine as shown in Figure 2.
6.2 Piezo channels and peripheral neuropathic pain
The other category is peripheral neuropathic pain, often caused by damage to peripheral conduction nerve fibers, especially Aα and Aβ fibers (124). After peripheral nerve injury, the anterior cingulate cortex (ACC) exhibits excessive excitability accompanied by the upregulation of piezo1 gene expression, which is involved in pain processing (125). Qiao-Yun Li et al. showed that, using a mouse sciatic nerve peripheral nerve injury model, it was able to effectively stimulate the intermediate neurons, increase the opening of piezo1 channels in the bilateral ACC, mediate the inward flow of extracellular Ca2+, release Ca2+ from the sarcoplasmic reticulum, and finally induce phagocytosis of microglia (125). Up to now, there are relatively few reports on the potential role of piezoelectric 1/2 in other neural injury models, such as chemotherapy induced peripheral neuropathy, spare nerve injury, sciatic nerve ligation, and even tumor bone metastasis. Recent studies have shown that mice lacking Piezo2 in their tail sensory neurons exhibit impaired nociceptive responses to mechanical stimuli, and suggest that targeting Piezo2 may be an effective strategy for treating mechanical allodynia (94). However, of note, excessive piezo1 expression often signals an influx of excess Ca2+ (126, 127), which may result in the emergence of oxidation or the weakening of the axonal skeleton during the process of repairing nerve damage- (128, 129), inducing neurodegeneration, which is often irreversible (130).
When Yoda1 acts on piezo1, it results in the release of large amounts of intra-synaptic CGRP, which binds to the CGRP receptor complex and activates the cAMP/PKA signaling pathway, ultimately leading to an increase in P2X3 receptor expression.
7 The related antagonists and pharmacological properties of Piezo1/2
Various types of pain are present in all aspects of life, not only causing substantial damage to the body but also being psychologically accompanied by great stress (131–133). The occurrence of cancerous pain predicts local tumor proliferation in the organism (42, 133). Inflammatory pain is accompanied by mechanical stimulation of various types of silver injury (83, 90) and neuropathic pain is closely related to the destruction of central or peripheral nerve fibers (134). Despite the strong self-healing capacity of the organism, it is somewhat helpless when confronted with these types of unfavorable pain, because they do not have a specific pathogen, such as a bacterial infection or viral invasion (135–137). Therefore it is necessary to conduct drug research on the implicated illnesses.
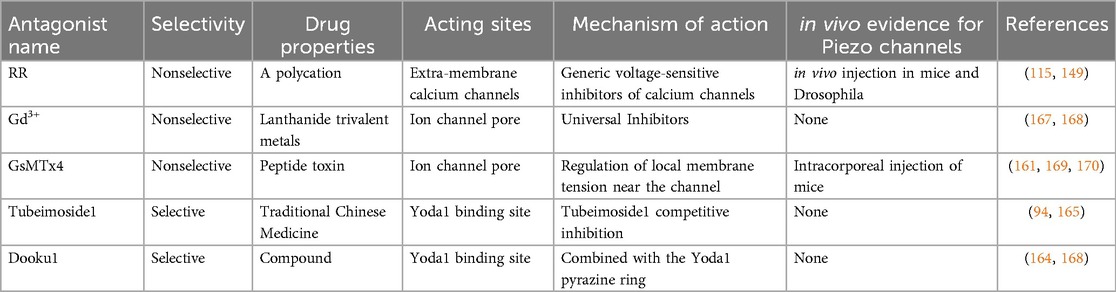
The involvement of piezo channels in mechanical signaling between various cells or tissues has been confirmed by animal experiments (138), such as the establishment of Drosophila (139, 111), birds (140), and mice (141, 142). As already mentioned, pain, whether physiological or pathological, is often accompanied by an abnormally large opening of piezo1/2 channels. Therefore, the use of efficient antagonists and blockers to inhibit the expression of piezo genes or the opening of channels will help treat related diseases (51, 90, 143). The details of this process are described below. A variety of piezoelectric channel antagonists and their related properties are shown in Table 2.
7.1 Antagonistic effects of non-specific drugs
piezo1/2 channels are a complex, novel, and unique class of MSCs, for which pharmacological studies are still in their infancy, and the antagonists identified so far lack specificity and cannot target piezo channels efficiently. Ruthenium red reagents (RR), gadolinium metal (Gd3+), and GsMTx4 are the most commonly used piezo1 and piezo2 blockers (144, 145). RR is a polycation (146). In previous studies, experimentalists did not explore the mechanism of action of RR clearly but only pointed out that the concentration of the reagents might be an influencing factor, and the opening of piezo channels showed a decreasing trend with increasing RR concentration (147). However, recent studies have shown that RR treatment of cells outside the cell membrane only blocks piezo1/2 channel-mediated inward currents but has no effect on outward currents, suggesting that our RR action may be based on an extra-membrane blocking mechanism (148). The lanthanide trivalent metal gadolinium is often found in various experiments on mesenchymal cell growth and development (51). which can inhibit not only TREK-1(A potassium channel that controls cell excitability and keeps the membrane potential below the depolarization threshold is involved in neuropathic pain) pathway and voltage-gated sodium channel, but also piezoelectric channel (149, 150).
Compared to RR and Gd3+, GsMTx4 is more widely used in MSC. This amphoteric polypeptide, abundant in cysteine (six), initially derived from the tarantula venom, is currently one of the blockers of MSC (151–153). GsMTx4 is an inhibitory cysteine linking (ICK) peptide, ICK peptides tend to have both hydrophobic and hydrophilic sides, and thought to act by inserting their hydrophobic side into the cell membrane and binding to the ion channel voltage sensor domain on their hydrophilic side (17, 25). This amphiphilic nature effectively facilitates their attachment to the lipid bilayer, enables the binding of membrane-gating elements, mediates the transduction of signaling molecules, and ultimately alters the kinetic effects on the cell membrane (154, 155). The ICK peptide GsMTx4 isn't the sole variant extracted from tarantula venom; it is merely a standard example that shows minor variations from other ICK peptide types (155, 156). Most ICK peptides act stereoisomerically, which is not the case for GsMTx4 (157, 158). We found that both L-GsMTx4 and D-GsMTx4 exerted almost equal inhibitory effects, and it is reasonable to assume that this is due to the high positive charge (+5) and hydrophobicity of GsMTx4 and the high proportion of lysine content (159, 160). While the previously described inhibitor RR exerts its effect by blocking action by binding to gating elements on biological membranes (148), GsMTx4 takes a very different approach in which its proximity to the piezo channel is not selectively inserted into the phospholipid bilayer (161), achieving an increase in tension on both sides of the membrane and a decrease in lateral pressure (147, 162), which ultimately induces relaxation of the outer membrane and decreases the efficiency of force transduction by mechanical stimulation (144).
Pain generation coincides with the activation of ion channel gates, indicating that there is a disproportion of cations, particularly calcium ions, with non-selective cationic MSC playing a key role in this occurrence.D-GsMTx4 is an effective protective agent in ischemia-reperfusion (causing myocardial pain with infarction) that occurs in the myocardium (163). In experiments exploring the relationship between piezo1 channels and the inflammatory response, it was found that the blockade of MSC channel opening with GsMTx4 was accompanied by a corresponding increase in certain cytokines that contributed to the reduction of inflammatory pain in the CNS, the more prominent ones being interleukins (such as IL-1β, IL-6, and IL-8) and tumor necrosis factor (164).
7.2 Potential specific blocking agents
Notably, these three inhibitors lack specificity; however, recent research indicates Tubeimoside1 and Dooku1 may specifically target piezo channels (164). Mechanical signaling is not the only factor that can activate piezo channels; the small molecules Yoda1 and Jedi2/1 can also selectively activate piezo1 channels (117). During the research conducted by Evans et al., the influence of Tubeimoside1 lies not in altering piezo1 channels’ function, but in its reliance on Yoda1, meaning the impact of Tubeimoside1 is based on Yoda1's existence, a dependency also observed in Dooku (165, 166). The effect of Tubeimoside1 decreased gradually with the increase of Yoda1 concentration and the inhibition was reversible, suggesting that Tubeimoside1 competes with Yoda1 to bind piezo1 channel, and when the same experiment was conducted on TRPV4, TRPM1, and TRPC5 belonging to MSC, no significant inhibition of Tubeimoside1 was found, which again proved that it might be a specific antagonist of the piezo channel (166, 167). Yoda1, a chemically synthesized small molecule, has a pyrazine ring within its structure that serves as the exact binding site for Dooku1. While Dooku1's sole activation of constitutive piezo1 channels does not lead to inhibition, it suppresses native Yoda1 and reduces the activation of associated channels (165).
8 Conclusion
Pain frequently occurs in numerous clinical scenarios, profoundly impacting a patient's quality of life. Consequently, researchers have focused extensively on employing efficient techniques to alleviate pain and distress in patients. Owing to the complex pathological mechanisms of pain, drugs are commonly used to relieve pain clinically; however, their therapeutic effects are poor. The role of Piezo1/2 in pain is widely accepted. In this review, we discuss the pathogenesis of pain and its relationship between Piezo1/2 and pain. Consequently, creating and employing targeted Piezo1/2 antagonists to inhibit its excessive expression may have clinical significance in pain management.
Author contributions
YX: Writing – original draft, Writing – review & editing. YW: Writing – original draft. SM: Data curation, Writing – review & editing. JH: Supervision, Writing – review & editing. LW: Supervision, Writing – review & editing. LX: Data curation, Formal Analysis, Writing – review & editing. LB: Investigation, Project administration, Writing – review & editing. XF: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by doctoral start-up fund of the Second Affiliated Hospital of Nanchang University (B3150), and the Natural Science Foundation of Jiangxi Province (20232BAB216042), the Science and Technology Project of Jiangxi Provincial Administration of Traditional Chinese Medicine (2023A0339, 2022A053, 2021A360).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ICD, international classification of diseases; HX, hereditary xerocytosis; DA5, distal joint contracture type 5; GS, gordon syndrome; MWS, Marden-Walker syndrome; AP, acute pancreatitis; FRS, faces rating scale; MAPC, memorized pain assessment card; CPOT, critical-pain observation tool; SF-MPQ, short-form McGill pain questionnaire; BPI, brief pain inventory; GPS, global pain scale; TNBC, triple-negative breast cancer; TFF1, trefoil factor family 1; NSCLC, non-small cell lung cancer; OS, osteosarcoma; MSC, mechanosensitive ion channels; AKT, protein kinase B; AS, atherosclerosis; ECs, endothelial cells; IL-1α, interleukin-1α; OA, osteoarthritis; CNS, central nervous system; RGCs, retinal ganglion cells; ACC, anterior cingulate cortex; RR, ruthenium red reagents; Gd3+, gadolinium metal; ICK, inhibitory cysteine junction; CGRP, calcitonin gene-related peptide; PKA, protein kinase A.
References
1. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161:1976–82. doi: 10.1097/j.pain.0000000000001939
2. Smart KM, Blake C, Staines A, Doody C. The discriminative validity of “nociceptive,” “peripheral neuropathic,” and “central sensitization” as mechanisms-based classifications of musculoskeletal pain. Clin J Pain. (2011) 27:655–63. doi: 10.1097/AJP.0b013e318215f16a
3. Sluka KA, George SZ. A new definition of pain: update and implications for physical therapist practice and rehabilitation science. Phys Ther. (2021) 101(4):pzab019. doi: 10.1093/ptj/pzab019
4. Lee GI, Neumeister MW. Pain: pathways and physiology. Clin Plast Surg. (2020) 47:173–80. doi: 10.1016/j.cps.2019.11.001
5. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. doi: 10.1016/S0140-6736(21)00393-7
6. Harrison JE, Weber S, Jakob R, Chute CG. ICD-11: an international classification of diseases for the twenty-first century. BMC Med Inform Decis Mak. (2021) 21:206. doi: 10.1186/s12911-021-01534-6
7. Del Orbe Barreto R, Arrizabalaga B, De la Hoz Rastrollo AB, García-Orad A, Gonzalez Vallejo I, Bento C, et al. Hereditary xerocytosis, a misleading anemia. Ann Hematol. (2016) 95:1545–6. doi: 10.1007/s00277-016-2716-9
8. Choi D, Park E, Jung E, Cha B, Lee S, Yu J, et al. Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight. (2019) 4(5):e125068. doi: 10.1172/jci.insight.125068
9. Faucherre A, Moha Ou Maati H, Nasr N, Pinard A, Theron A, Odelin G, et al. Piezo1 is required for outflow tract and aortic valve development. J Mol Cell Cardiol. (2020) 143:51–62. doi: 10.1016/j.yjmcc.2020.03.013
10. Song S, Zhang H, Wang X, Chen W, Cao W, Zhang Z, et al. The role of mechanosensitive Piezo1 channel in diseases. Prog Biophys Mol Biol. (2022) 172:39–49. doi: 10.1016/j.pbiomolbio.2022.04.006
11. Strzyz P. Cell death: bCL-2 proteins feed their own expression. Nat Rev Mol Cell Biol. (2017) 18:652–3. doi: 10.1038/nrm.2017.106
12. Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. (2014) 516:121–5. doi: 10.1038/nature13980
13. Fang XZ, Zhou T, Xu JQ, Wang YX, Sun MM, He YJ, et al. Structure, kinetic properties and biological function of mechanosensitive piezo channels. Cell Biosci. (2021) 11:13. doi: 10.1186/s13578-020-00522-z
14. Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. (2015) 527:64–9. doi: 10.1038/nature15247
15. Guo YR, MacKinnon R. Structure-based membrane dome mechanism for piezo mechanosensitivity. Elife. (2017) 6:e33660. doi: 10.7554/eLife.33660
16. Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. (2018) 554:481–6. doi: 10.1038/nature25453
17. Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. (2018) 554:487–92. doi: 10.1038/nature25743
18. Wang L, Zhou H, Zhang M, Liu W, Deng T, Zhao Q, et al. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature. (2019) 573:225–9. doi: 10.1038/s41586-019-1505-8
19. Wu J, Lewis AH, Grandl J. Touch, tension, and transduction—the function and regulation of piezo Ion channels. Trends Biochem Sci. (2017) 42:57–71. doi: 10.1016/j.tibs.2016.09.004
20. Volkers L, Mechioukhi Y, Coste B. Piezo channels: from structure to function. Pflugers Arch. (2015) 467:95–9. doi: 10.1007/s00424-014-1578-z
21. Baratchi S, Zaldivia MTK, Wallert M, Loseff-Silver J, Al-Aryahi S, Zamani J, et al. Transcatheter aortic valve implantation represents an anti-inflammatory therapy via reduction of shear stress-induced, piezo-1-mediated monocyte activation. Circulation. (2020) 142:1092–105. doi: 10.1161/CIRCULATIONAHA.120.045536
22. Yang XN, Lu YP, Liu JJ, Huang JK, Liu YP, Xiao CX, et al. Piezo1 is as a novel trefoil factor family 1 binding protein that promotes gastric cancer cell mobility in vitro. Dig Dis Sci. (2014) 59:1428–35. doi: 10.1007/s10620-014-3044-3
23. Zhang J, Zhou Y, Huang T, Wu F, Liu L, Kwan JSH, et al. PIEZO1 functions as a potential oncogene by promoting cell proliferation and migration in gastric carcinogenesis. Mol Carcinog. (2018) 57:1144–55. doi: 10.1002/mc.22831
24. Wang X, Cheng G, Miao Y, Qiu F, Bai L, Gao Z, et al. Piezo type mechanosensitive ion channel component 1 facilitates gastric cancer omentum metastasis. J Cell Mol Med. (2021) 25:2238–53. doi: 10.1111/jcmm.16217
25. Yao P, Zhao H, Cao J, Chen L. Piezo1: a novel mechanism of pressure-induced pancreatitis. Acta Biochim Biophys Sin (Shanghai). (2019) 51:344–5. doi: 10.1093/abbs/gmy173
26. Romac JM, Shahid RA, Swain SM, Vigna SR, Liddle RA. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat Commun. (2018) 9:1715. doi: 10.1038/s41467-018-04194-9
27. Fels B, Nielsen N, Schwab A. Role of TRPC1 channels in pressure-mediated activation of murine pancreatic stellate cells. Eur Biophys J. (2016) 45:657–70. doi: 10.1007/s00249-016-1176-4
28. Kuntze A, Goetsch O, Fels B, Najder K, Unger A, Wilhelmi M, et al. Protonation of Piezo1 impairs cell-matrix interactions of pancreatic stellate cells. Front Physiol. (2020) 11:89. doi: 10.3389/fphys.2020.00089
29. Xue R, Jia K, Wang J, Yang L, Wang Y, Gao L, et al. A rising star in pancreatic diseases: pancreatic stellate cells. Front Physiol. (2018) 9:754. doi: 10.3389/fphys.2018.00754
30. Song W, Eaton LH, Gordon DB, Hoyle C, Doorenbos AZ. Evaluation of evidence-based nursing pain management practice. Pain Manag Nurs. (2015) 16:456–63. doi: 10.1016/j.pmn.2014.09.001
31. Todd J, Aspell JE, Lee MC, Thiruchelvam N. How is pain associated with pelvic mesh implants measured? Refinement of the construct and a scoping review of current assessment tools. BMC Womens Health. (2022) 22:396. doi: 10.1186/s12905-022-01977-7
32. Machata AM, Kabon B, Willschke H, Fässler K, Gustorff B, Marhofer P, et al. A new instrument for pain assessment in the immediate postoperative period. Anaesthesia. (2009) 64:392–8. doi: 10.1111/j.1365-2044.2008.05798.x
33. Fishman B, Pasternak S, Wallenstein SL, Houde RW, Holland JC, Foley KM. The memorial pain assessment card. A valid instrument for the evaluation of cancer pain. Cancer. (1987) 60:1151–8. doi: 10.1002/1097-0142(19870901)60:5%3C1151::AID-CNCR2820600538%3E3.0.CO;2-G
34. Moore R, Miller ML, Weinstein P, Dworkin SF, Liou HH. Cultural perceptions of pain and pain coping among patients and dentists. Community Dent Oral Epidemiol. (1986) 14:327–33. doi: 10.1111/j.1600-0528.1986.tb01084.x
35. Gélinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. (2006) 15:420–7. doi: 10.4037/ajcc2006.15.4.420
36. Melzack R. The short-form McGill pain questionnaire. Pain. (1987) 30:191–7. doi: 10.1016/0304-3959(87)91074-8
37. Wang XS, Mendoza TR, Gao SZ, Cleeland CS. The Chinese version of the brief pain inventory (BPI-C): its development and use in a study of cancer pain. Pain. (1996) 67:407–16. doi: 10.1016/0304-3959(96)03147-8
38. Gentile DA, Woodhouse J, Lynch P, Maier J, McJunkin T. Reliability and validity of the global pain scale with chronic pain sufferers. Pain Physician. (2011) 14:61–70. doi: 10.36076/ppj.2011/14/61
39. Zelman DC, Gore M, Dukes E, Tai KS, Brandenburg N. Validation of a modified version of the brief pain inventory for painful diabetic peripheral neuropathy. J Pain Symptom Manage. (2005) 29:401–10. doi: 10.1016/j.jpainsymman.2004.06.018
40. Shen B, Chen H, Yang D, Yolanda O, Yuan C, Du A, et al. A structural equation model of health-related quality of life in Chinese patients with rheumatoid arthritis. Front Psychiatry. (2021) 12:716996. doi: 10.3389/fpsyt.2021.716996
41. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
43. Yin W, Wang J, Jiang L, James Kang Y. Cancer and stem cells. Exp Biol Med (Maywood). (2021) 246:1791–801. doi: 10.1177/15353702211005390
44. Vaghari-Tabari M, Ferns GA, Qujeq D, Andevari AN, Sabahi Z, Moein S. Signaling, metabolism, and cancer: an important relationship for therapeutic intervention. J Cell Physiol. (2021) 236:5512–32. doi: 10.1002/jcp.30276
45. Huang Z, Sun Z, Zhang X, Niu K, Wang Y, Zheng J, et al. Loss of stretch-activated channels, PIEZOs, accelerates non-small cell lung cancer progression and cell migration. Biosci Rep. (2019) 39(3):BSR20181679. doi: 10.1042/BSR20181679
46. Katsuta E, Takabe K, Vujcic M, Gottlieb PA, Dai T, Mercado-Perez A, et al. Mechano-sensing channel PIEZO2 enhances invasive phenotype in triple-negative breast cancer. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23179909
47. Han Y, Liu C, Zhang D, Men H, Huo L, Geng Q, et al. Mechanosensitive ion channel Piezo1 promotes prostate cancer development through the activation of the akt/mTOR pathway and acceleration of cell cycle. Int J Oncol. (2019) 55:629–44. doi: 10.3892/ijo.2019.4839
48. Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, Davey CF, et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. (2017) 543:118–21. doi: 10.1038/nature21407
49. Chang AJ, Autio KA, Roach M 3rd, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. (2014) 11:308–23. doi: 10.1038/nrclinonc.2014.68
50. Yu Y, Wu X, Liu S, Zhao H, Li B, Zhao H, et al. Piezo1 regulates migration and invasion of breast cancer cells via modulating cell mechanobiological properties. Acta Biochim Biophys Sin (Shanghai). (2021) 53:10–8. doi: 10.1093/abbs/gmaa112
51. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. (2010) 330:55–60. doi: 10.1126/science.1193270
52. Etem E, Ceylan GG, Özaydın S, Ceylan C, Özercan I, Kuloğlu T. The increased expression of Piezo1 and Piezo2 ion channels in human and mouse bladder carcinoma. Adv Clin Exp Med. (2018) 27:1025–31. doi: 10.17219/acem/71080
53. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. (2005) 55:74–108. doi: 10.3322/canjclin.55.2.74
54. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
55. McHugh BJ, Murdoch A, Haslett C, Sethi T. Loss of the integrin-activating transmembrane protein Fam38A (Piezo1) promotes a switch to a reduced integrin-dependent mode of cell migration. PLoS One. (2012) 7:e40346. doi: 10.1371/journal.pone.0040346
56. Zeppetella G, Ribeiro MD. Episodic pain in patients with advanced cancer. Am J Hosp Palliat Care. (2002) 19:267–76. doi: 10.1177/104990910201900412
57. Washington MK, Goldberg RM, Chang GJ, Limburg P, Lam AK, Salto-Tellez M, et al. Diagnosis of digestive system tumours. Int J Cancer. (2021) 148:1040–50. doi: 10.1002/ijc.33210
58. Yu JL, Liao HY. Piezo-type mechanosensitive ion channel component 1 (Piezo1) in human cancer. Biomed Pharmacother. (2021) 140:111692. doi: 10.1016/j.biopha.2021.111692
59. Sun Y, Li M, Liu G, Zhang X, Zhi L, Zhao J, et al. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. J Cancer Res Clin Oncol. (2020) 146:1139–52. doi: 10.1007/s00432-020-03179-w
60. Hasegawa K, Fujii S, Matsumoto S, Tajiri Y, Kikuchi A, Kiyoshima T. YAP signaling induces PIEZO1 to promote oral squamous cell carcinoma cell proliferation. J Pathol. (2021) 253:80–93. doi: 10.1002/path.5553
61. Rape AD, Kumar S. A composite hydrogel platform for the dissection of tumor cell migration at tissue interfaces. Biomaterials. (2014) 35:8846–53. doi: 10.1016/j.biomaterials.2014.07.003
62. Mercadante S, Vitrano V. Pain in patients with lung cancer: pathophysiology and treatment. Lung Cancer. (2010) 68:10–5. doi: 10.1016/j.lungcan.2009.11.004
63. Yang H, Liu C, Zhou RM, Yao J, Li XM, Shen Y, et al. Piezo2 protein: a novel regulator of tumor angiogenesis and hyperpermeability. Oncotarget. (2016) 7:44630–43. doi: 10.18632/oncotarget.10134
64. Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro-oncology. (2014) 16:896–913. doi: 10.1093/neuonc/nou087
65. Chen X, Wanggou S, Bodalia A, Zhu M, Dong W, Fan JJ, et al. A feedforward mechanism mediated by mechanosensitive Ion channel PIEZO1 and tissue mechanics promotes glioma aggression. Neuron. (2018) 100:799–815.e7. doi: 10.1016/j.neuron.2018.09.046
66. Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers. (2017) 3:17002. doi: 10.1038/nrdp.2017.2
67. Xu L, Zhang Y, Huang Y. Advances in the treatment of neuropathic pain. Adv Exp Med Biol. (2016) 904:117–29. doi: 10.1007/978-94-017-7537-3_9
68. Holenstein CN, Horvath A, Schär B, Schoenenberger AD, Bollhalder M, Goedecke N, et al. The relationship between metastatic potential and in vitro mechanical properties of osteosarcoma cells. Mol Biol Cell. (2019) 30:887–98. doi: 10.1091/mbc.E18-08-0545
69. Jiang L, Zhao YD, Chen WX. The function of the novel mechanical activated Ion channel Piezo1 in the human osteosarcoma cells. Med Sci Monit. (2017) 23:5070–82. doi: 10.12659/msm.906959
70. Suzuki T, Muraki Y, Hatano N, Suzuki H, Muraki K. PIEZO1 channel is a potential regulator of synovial sarcoma cell-viability. Int J Mol Sci. (2018) 19(5):1452. doi: 10.3390/ijms19051452
71. Zhu XC, Zhang JL, Ge CT, Yu YY, Wang P, Yuan TF, et al. Advances in cancer pain from bone metastasis. Drug Des Devel Ther. (2015) 9:4239–45. doi: 10.2147/DDDT.S87568
72. Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. (1997) 69:1–18. doi: 10.1016/S0304-3959(96)03267-8
73. Yoneda T, Hiasa M, Nagata Y, Okui T, White FA. Acidic microenvironment and bone pain in cancer-colonized bone. Bonekey Rep. (2015) 4:690. doi: 10.1038/bonekey.2015.58
74. Pardo-Pastor C, Rubio-Moscardo F, Vogel-González M, Serra SA, Afthinos A, Mrkonjic S, et al. Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc Natl Acad Sci U S A. (2018) 115:1925–30. doi: 10.1073/pnas.1718177115
75. Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. (2014) 156:1002–16. doi: 10.1016/j.cell.2014.01.040
76. Fischer LS, Rangarajan S, Sadhanasatish T, Grashoff C. Molecular force measurement with tension sensors. Annu Rev Biophys. (2021) 50:595–616. doi: 10.1146/annurev-biophys-101920-064756
77. Tschumperlin DJ. Why stress matters: an introduction. Methods Mol Biol. (2021) 2299:159–69. doi: 10.1007/978-1-0716-1382-5_12
78. Bourne S, Machado AG, Nagel SJ. Basic anatomy and physiology of pain pathways. Neurosurg Clin N Am. (2014) 25:629–38. doi: 10.1016/j.nec.2014.06.001
79. Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. (1992) 107:660–4. doi: 10.1111/j.1476-5381.1992.tb14503.x
80. Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. (1988) 334:698–700. doi: 10.1038/334698a0
81. Varela ML, Mogildea M, Moreno I, Lopes A. Acute inflammation and metabolism. Inflammation. (2018) 41:1115–27. doi: 10.1007/s10753-018-0739-1
82. Leuti A, Fazio D, Fava M, Piccoli A, Oddi S, Maccarrone M. Bioactive lipids, inflammation and chronic diseases. Adv Drug Delivery Rev. (2020) 159:133–69. doi: 10.1016/j.addr.2020.06.028
83. Muley MM, Krustev E, McDougall JJ. Preclinical assessment of inflammatory pain. CNS Neurosci Ther. (2016) 22:88–101. doi: 10.1111/cns.12486
84. Patrick N, Emanski E, Knaub MA. Acute and chronic low back pain. Med Clin North Am. (2014) 98:777–89. xii. doi: 10.1016/j.mcna.2014.03.005
85. Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. (1997) 92:5s–11.9395346
86. Satoh K, Hata M, Takahara S, Tsuzaki H, Yokota H, Akatsu H, et al. A novel membrane protein, encoded by the gene covering KIAA0233, is transcriptionally induced in senile plaque-associated astrocytes. Brain Res. (2006) 1108:19–27. doi: 10.1016/j.brainres.2006.06.050
87. Syeda R. Physiology and pathophysiology of mechanically activated PIEZO channels. Annu Rev Neurosci. (2021) 44:383–402. doi: 10.1146/annurev-neuro-093020-120939
88. Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest. (2016) 126:4527–36. doi: 10.1172/JCI87343
89. Liu H, Hu J, Zheng Q, Feng X, Zhan F, Wang X, et al. Piezo1 channels as force sensors in mechanical force-related chronic inflammation. Front Immunol. (2022) 13:816149. doi: 10.3389/fimmu.2022.816149
90. Lee W, Nims RJ, Savadipour A, Zhang Q, Leddy HA, Liu F, et al. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc Natl Acad Sci U S A. (2021) 118(13):e2001611118. doi: 10.1073/pnas.2001611118
91. Wei S, Siegal GP. Mechanisms modulating inflammatory osteolysis: a review with insights into therapeutic targets. Pathol Res Pract. (2008) 204:695–706. doi: 10.1016/j.prp.2008.07.002
92. Chuderland D, Seger R. Protein-protein interactions in the regulation of the extracellular signal-regulated kinase. Mol Biotechnol. (2005) 29:57–74. doi: 10.1385/MB:29:1:57
93. Szczot M, Liljencrantz J, Ghitani N, Barik A, Lam R, Thompson JH, et al. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci Transl Med. (2018) 10(462):eaat9892. doi: 10.1126/scitranslmed.aat9892
94. Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kühnemund J, et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci Transl Med. (2018) 10(462):eaat9897. doi: 10.1126/scitranslmed.aat9897
95. Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. (2006) 26:1833–43. doi: 10.1523/JNEUROSCI.4584-05.2006
96. Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, et al. Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron. (2017) 93:179–93. doi: 10.1016/j.neuron.2016.11.027
97. Sonkodi B. Progressive irreversible proprioceptive Piezo2 channelopathy-induced lost forced peripheral oscillatory synchronization to the hippocampal oscillator may explain the onset of amyotrophic lateral sclerosis pathomechanism. Cells. (2024) 13(6):492. doi: 10.3390/cells13060492
98. Bouchatta O, Brodzki M, Manouze H, Carballo GB, Kindström E, de-Faria FM, et al. PIEZO2-dependent rapid pain system in humans and mice. bioRxiv [Preprint]. (2023) 2023.12.01.569650. doi: 10.1101/2023.12.01.569650
99. Smith ML. The histologic diagnosis of usual interstitial pneumonia of idiopathic pulmonary fibrosis. Where we are and where we need to go. Mod Pathol. (2022) 35:8–14. doi: 10.1038/s41379-021-00889-5
100. Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. (2019) 20:242–58. doi: 10.1038/s41580-018-0093-z
101. Geng J, Zhao Q, Zhang T, Xiao B. In touch with the mechanosensitive piezo channels: structure, Ion permeation, and mechanotransduction. Curr Top Membr. (2017) 79:159–95. doi: 10.1016/bs.ctm.2016.11.006
102. Tyler WJ. The mechanobiology of brain function. Nat Rev Neurosci. (2012) 13:867–78. doi: 10.1038/nrn3383
103. Suter DM, Miller KE. The emerging role of forces in axonal elongation. Prog Neurobiol. (2011) 94:91–101. doi: 10.1016/j.pneurobio.2011.04.002
104. Pfister BJ, Iwata A, Meaney DF, Smith DH. Extreme stretch growth of integrated axons. J Neurosci. (2004) 24:7978–83. doi: 10.1523/JNEUROSCI.1974-04.2004
105. Szok D, Tajti J, Nyári A, Vécsei L. Therapeutic approaches for peripheral and central neuropathic pain. Behav Neurol. (2019) 2019:8685954. doi: 10.1155/2019/8685954
106. Franze K, Janmey PA, Guck J. Mechanics in neuronal development and repair. Annu Rev Biomed Eng. (2013) 15:227–51. doi: 10.1146/annurev-bioeng-071811-150045
107. Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, et al. Mechanosensing is critical for axon growth in the developing brain. Nat Neurosci. (2016) 19:1592–8. doi: 10.1038/nn.4394
108. Tatomir A, Cuevas J, Badea TC, Muresanu DF, Rus V, Rus H. Role of RGC-32 in multiple sclerosis and neuroinflammation—few answers and many questions. Front Immunol. (2022) 13:979414. doi: 10.3389/fimmu.2022.979414
109. Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, et al. The role of PIEZO2 in human mechanosensation. N Engl J Med. (2016) 375:1355–64. doi: 10.1056/NEJMoa1602812
110. Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. Neuropathic pain: central vs. Peripheral mechanisms. Curr Pain Headache Rep. (2017) 21:28. doi: 10.1007/s11916-017-0629-5
111. Li F, Lo TY, Miles L, Wang Q, Noristani HN, Li D, et al. The Atr-Chek1 pathway inhibits axon regeneration in response to piezo-dependent mechanosensation. Nat Commun. (2021) 12(1):3845. doi: 10.1038/s41467-021-24131-7
112. Benatto MT, Florencio LL, Carvalho GF, Dach F, Bigal ME, Chaves TC, et al. Cutaneous allodynia is more frequent in chronic migraine, and its presence and severity seems to be more associated with the duration of the disease. Arq Neuropsiquiatr. (2017) 75:153–9. doi: 10.1590/0004-282x20170015
113. Della Pietra A, Mikhailov N, Giniatullin R. The emerging role of mechanosensitive piezo channels in migraine pain. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21030696
114. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
115. Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, et al. Chemical activation of the mechanotransduction channel Piezo1. eLife. (2015) 4:e07369. doi: 10.7554/eLife.07369
116. Lacroix JJ, Botello-Smith WM, Luo Y. Probing the gating mechanism of the mechanosensitive channel Piezo1 with the small molecule Yoda1. Nat Commun. (2018) 9:2029. doi: 10.1038/s41467-018-04405-3
117. Botello-Smith WM, Jiang W, Zhang H, Ozkan AD, Lin YC, Pham CN, et al. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat Commun. (2019) 10:4503. doi: 10.1038/s41467-019-12501-1
118. Fabbretti E, D'Arco M, Fabbro A, Simonetti M, Nistri A, Giniatullin R. Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci. (2006) 26:6163–71. doi: 10.1523/JNEUROSCI.0647-06.2006
119. D'Arco M, Giniatullin R, Simonetti M, Fabbro A, Nair A, Nistri A, et al. Neutralization of nerve growth factor induces plasticity of ATP-sensitive P2X3 receptors of nociceptive trigeminal ganglion neurons. J Neurosci. (2007) 27:8190–201. doi: 10.1523/JNEUROSCI.0713-07.2007
120. Giniatullin R, Nistri A, Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol. (2008) 37:83–90. doi: 10.1007/s12035-008-8020-5
121. Dussor G. New discoveries in migraine mechanisms and therapeutic targets. Curr Opin Physiol. (2019) 11:116–24. doi: 10.1016/j.cophys.2019.10.013
122. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. (2015) 35:6619–29. doi: 10.1523/JNEUROSCI.0373-15.2015
123. LaPaglia DM, Sapio MR, Burbelo PD, Thierry-Mieg J, Thierry-Mieg D, Raithel SJ, et al. RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia. (2018) 38:912–32. doi: 10.1177/0333102417720216
124. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. (2009) 32:1–32. doi: 10.1146/annurev.neuro.051508.135531
125. Li QY, Duan YW, Zhou YH, Chen SX, Li YY, Zang Y. NLRP3-mediated Piezo1 upregulation in ACC inhibitory parvalbumin-expressing interneurons is involved in pain processing after peripheral nerve injury. Int J Mol Sci. (2022) 23(21):13035. doi: 10.3390/ijms232113035
126. Cinar E, Zhou S, DeCourcey J, Wang Y, Waugh RE, Wan J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc Natl Acad Sci U S A. (2015) 112:11783–8. doi: 10.1073/pnas.1507309112
127. Shao FB, Fang JF, Wang SS, Qiu MT, Xi DN, Jin XM, et al. Anxiolytic effect of GABAergic neurons in the anterior cingulate cortex in a rat model of chronic inflammatory pain. Mol Brain. (2021) 14:139. doi: 10.1186/s13041-021-00849-9
128. Azuma M, Shearer TR. The role of calcium-activated protease calpain in experimental retinal pathology. Surv Ophthalmol. (2008) 53:150–63. doi: 10.1016/j.survophthal.2007.12.006
129. Wojda U, Salinska E, Kuznicki J. Calcium ions in neuronal degeneration. IUBMB Life. (2008) 60:575–90. doi: 10.1002/iub.91
130. Frati A, Cerretani D, Fiaschi AI, Frati P, Gatto V, La Russa R, et al. Diffuse axonal injury and oxidative stress: a comprehensive review. Int J Mol Sci. (2017) 18(12):2600. doi: 10.3390/ijms18122600
131. Loeser JD, Melzack R. Pain: an overview. Lancet. (1999) 353:1607–9. doi: 10.1016/S0140-6736(99)01311-2
132. Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, et al. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol. (2011) 67:942–68. doi: 10.1002/jclp.20816
133. Glare PA, Davies PS, Finlay E, Gulati A, Lemanne D, Moryl N, et al. Pain in cancer survivors. J Clin Oncol. (2014) 32:1739–47. doi: 10.1200/JCO.2013.52.4629
134. Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. (2021) 101:259–301. doi: 10.1152/physrev.00045.2019
135. Armstrong SA, Herr MJ. Physiology, nociception. StatPearls. Treasure Island, FL: StatPearls Publishing LLC (2023).
136. Modarresi Chahardehi A, Masoumi SA, Bigdeloo M, Arsad H, Lim V. The effect of exercise on patients with rheumatoid arthritis on the modulation of inflammation. Clin Exp Rheumatol. (2022) 40:1420–31.34874837
137. Wawrzyniak-Gramacka E, Hertmanowska N, Tylutka A, Morawin B, Wacka E, Gutowicz M, et al. The association of anti-inflammatory diet ingredients and lifestyle exercise with inflammaging. Nutrients. (2021) 13(11):3696. doi: 10.3390/nu13113696
138. Kefauver JM, Ward AB, Patapoutian A. Discoveries in structure and physiology of mechanically activated ion channels. Nature. (2020) 587:567–76. doi: 10.1038/s41586-020-2933-1
139. Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila piezo in mechanical nociception. Nature. (2012) 483:209–12. doi: 10.1038/nature10801
140. Schneider ER, Mastrotto M, Laursen WJ, Schulz VP, Goodman JB, Funk OH, et al. Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. Proc Natl Acad Sci U S A. (2014) 111:14941–6. doi: 10.1073/pnas.1413656111
141. Nie X, Chung MK. Piezo channels for skeletal development and homeostasis: insights from mouse genetic models. Differentiation. (2022) 126:10–5. doi: 10.1016/j.diff.2022.06.001
142. Zhang M, Wang Y, Geng J, Zhou S, Xiao B. Mechanically activated piezo channels mediate touch and suppress acute mechanical pain response in mice. Cell Rep. (2019) 26:1419–1431.e4. doi: 10.1016/j.celrep.2019.01.056
143. Velasco-Estevez M, Gadalla KKE, Linan-Barba N, Cobb S, Dev KK, Sheridan GK. Inhibition of Piezo1 attenuates demyelination in the central nervous system. Glia. (2020) 68:356–75. doi: 10.1002/glia.23722
144. Suchyna TM. Piezo channels and GsMTx4: two milestones in our understanding of excitatory mechanosensitive channels and their role in pathology. Prog Biophys Mol Biol. (2017) 130:244–53. doi: 10.1016/j.pbiomolbio.2017.07.011
145. Li X, Hu J, Zhao X, Li J, Chen Y. Piezo channels in the urinary system. Exp Mol Med. (2022) 54:697–710. doi: 10.1038/s12276-022-00777-1
146. Tang H, Zeng R, He E, Zhang I, Ding C, Zhang A. Piezo-type mechanosensitive Ion channel component 1 (Piezo1): a promising therapeutic target and its modulators. J Med Chem. (2022) 65:6441–53. doi: 10.1021/acs.jmedchem.2c00085
147. Hamill OP, McBride DW Jr. The pharmacology of mechanogated membrane ion channels. Pharmacol Rev. (1996) 48:231–52. doi: 10.1038/s12276-022-00777-1
148. Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. (2012) 483:176–81. doi: 10.1038/nature10812
149. Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Lysophospholipids open the two-pore domain mechano-gated K(+) channels TREK-1 and TRAAK. J Biol Chem. (2000) 275:10128–33. doi: 10.1074/jbc.275.14.10128
150. Biagi BA, Enyeart JJ. Gadolinium blocks low- and high-threshold calcium currents in pituitary cells. Am J Physiol. (1990) 259:C515–20. doi: 10.1152/ajpcell.1990.259.3.C515
151. Beech DJ, Kalli AC. Force sensing by piezo channels in cardiovascular health and disease. Arterioscler Thromb Vasc Biol. (2019) 39:2228–39. doi: 10.1161/ATVBAHA.119.313348
152. Schrenk-Siemens K, Wende H, Prato V, Song K, Rostock C, Loewer A, et al. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nat Neurosci. (2015) 18:10–6. doi: 10.1038/nn.3894
153. Faucherre A, Nargeot J, Mangoni ME, Jopling C. Piezo2b regulates vertebrate light touch response. J Neurosci. (2013) 33:17089–94. doi: 10.1523/JNEUROSCI.0522-13.2013
154. Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol (Lond). (2016) 594:641–55. doi: 10.1113/JP271714
155. Ostrow KL, Mammoser A, Suchyna T, Sachs F, Oswald R, Kubo S, et al. cDNA sequence and in vitro folding of GsMTx4, a specific peptide inhibitor of mechanosensitive channels. Toxicon. (2003) 42:263–74. doi: 10.1016/S0041-0101(03)00141-7
156. Caputo GA, London E. Cumulative effects of amino acid substitutions and hydrophobic mismatch upon the transmembrane stability and conformation of hydrophobic alpha-helices. Biochemistry. (2003) 42:3275–85. doi: 10.1021/bi026697d
157. Bosmans F, Swartz KJ. Targeting voltage sensors in sodium channels with spider toxins. Trends Pharmacol Sci. (2010) 31:175–82. doi: 10.1016/j.tips.2009.12.007
158. Lee SY, MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. (2004) 430:232–5. doi: 10.1038/nature02632
159. Posokhov YO, Gottlieb PA, Morales MJ, Sachs F, Ladokhin AS. Is lipid bilayer binding a common property of inhibitor cysteine knot ion-channel blockers? Biophys J. (2007) 93:L20–2. doi: 10.1529/biophysj.107.112375
160. Kimura T. Stability and safety of inhibitor cystine knot peptide, GTx1-15, from the tarantula spider grammostola rosea. Toxins (Basel). (2021) 13(9):621. doi: 10.3390/toxins13090621
161. Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS, et al. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophys J. (2017) 112:31–45. doi: 10.1016/j.bpj.2016.11.013
162. Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. (2011) 50:6295–300. doi: 10.1021/bi200770q
163. Wang J, Ma Y, Sachs F, Li J, Suchyna TM. GsMTx4-D is a cardioprotectant against myocardial infarction during ischemia and reperfusion. J Mol Cell Cardiol. (2016) 98:83–94. doi: 10.1016/j.yjmcc.2016.07.005
164. Shinge SAU, Zhang D, Din AU, Yu F, Nie Y. Emerging Piezo1 signaling in inflammation and atherosclerosis; a potential therapeutic target. Int J Biol Sci. (2022) 18:923–41. doi: 10.7150/ijbs.63819
165. Evans EL, Cuthbertson K, Endesh N, Rode B, Blythe NM, Hyman AJ, et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br J Pharmacol. (2018) 175:1744–59. doi: 10.1111/bph.14188
166. Liu S, Pan X, Cheng W, Deng B, He Y, Zhang L, et al. Tubeimoside I antagonizes Yoda1-evoked Piezo1 channel activation. Front Pharmacol. (2020) 11:768. doi: 10.3389/fphar.2020.00768
167. Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ, et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun. (2017) 8:350. doi: 10.1038/s41467-017-00429-3
168. Ridone P, Vassalli M, Martinac B. Piezo1 mechanosensitive channels: what are they and why are they important. Biophys Rev. (2019) 11(5):795–805. doi: 10.1007/s12551-019-00584-5
169. Ma T, Wang YY, Lu Y, Feng L, Yang YT, Li GH, et al. Inhibition of Piezo1/Ca2+/calpain signaling in the rat basal forebrain reverses sleep deprivation-induced fear memory impairments. Behav Brain Res. (2022) 417:113594. doi: 10.1016/j.bbr.2021.113594
Keywords: Piezo1, Piezo2, therapeutic target, pain, Ca2+
Citation: Xu Y, Wang Y, Mei S, Hu J, Wu L, Xu L, Bao L and Fang X (2024) The mechanism and potential therapeutic target of piezo channels in pain. Front. Pain Res. 5:1452389. doi: 10.3389/fpain.2024.1452389
Received: 20 June 2024; Accepted: 2 September 2024;
Published: 27 September 2024.
Edited by:
Jenny Wilkerson, Texas Tech University Health Sciences Center, United StatesReviewed by:
Chilman Bae, Southern Illinois University Carbondale, United StatesYuma TeAdoro Ortiz, Texas Tech University Health Sciences Center, United States
Copyright: © 2024 Xu, Wang, Mei, Hu, Wu, Xu, Bao and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luyang Xu, OTUxNzI4NzEzQHFxLmNvbQ==; Lijie Bao, NTI3MjEzMjcwQHFxLmNvbQ==; Xiaowei Fang, MzMxMzM1Njc4QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yi Xu1,2,†
Yi Xu1,2,† Jialing Hu
Jialing Hu