- 1Anesthesia, Critical Care and Pain Management Research Center, Tehran University of Medical Sciences, Tehran, Iran
- 2School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
- 3Department of Emergency Medicine, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
- 4Department of Clinical Pharmacy, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Background: Renal colic is characterized by severe pain that is highly disabling. Gabapentin, an antiepileptic medication, is often recommended as a first-line therapy for neuropathic pain. However, its effectiveness in managing somatic pain, which is defined as the result of activity by pain receptors in the deep tissues, such as renal colic pain, is not as well-established.
Method: A phase 3 randomized clinical trial was conducted to evaluate the adjuvant analgesic effects of gabapentin on acute renal colic pain. Eligible patients participated in the study via random allocation to the control or gabapentin groups using the block randomization method. All patients received a shared regimen of ketorolac and rescue morphine as the conventional analgesic treatment for renal colic pain. Gabapentin was added as an adjuvant analgesic for the gabapentin group.
Result: A total of 63 individuals with an average age of 41.35 ± 13.08, were enrolled and completed the study. At the time of admission, there were no significant differences between the baseline characteristics of two groups, with exception of weight. The gabapentin group showed a significantly higher percentage of patients with pain severity of less than 5 after 60 and 90 min, as well as a significantly lower percentage of morphine rescue requirement and total morphine intake (mg) and mg/kg.
Conclusion: In cases of acute renal colic, gabapentin significantly decreases both the amount of morphine required and the degree of pain, indicating that it may be a useful adjutant to standard analgesic regimens. Treatment regimens that include gabapentin may help individuals manage their pain and become less reliant on opioids.
Clinical Trial Registration: https://irct.behdasht.gov.ir/trial/56066, identifier: IRCT20200322046833N2.
1 Introduction
Renal colic pain is an acute and catastrophic pain caused by kidney stones, affecting 5%–15% of people worldwide (1, 2). Treatment aims to lessen the patient's discomfort and preserve renal function by exerting the stone (1). The effective and safe management of renal colic pain remains a therapeutic challenge in the emergency department.
Since increased ureteral smooth muscle contractile activity is the primary cause of renal colic pain, smooth muscle relaxants are helpful for managing pain and facilitating the elimination of stones (1, 3, 4).
Currently, a range of medications can be used for the management of renal colic, such as calcium channel blockers, alpha receptor antagonists, and antimuscarinic medicines. However, opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) are the primary therapy options (1, 3, 4). NSAIDs are the initial choice for treating acute renal colic pain, which can decrease the frequency of hospital admissions but do not impact the passage of stones. However, they can cause renal dysfunction by decreasing renal blood flow and gastrointestinal discomforts and bleeding (5–7). On the other hand, opioids are useful but can cause drowsiness, respiratory depression, and dependency, hence alternatives are required (4).
Narcotics, which effectively manage pain via the central nervous system, are another medication useful for managing renal colic pain. However, due to their potential side effects, medical personnel should be in charge of administering them (8). A meta-analysis of randomized controlled trials showed that NSAIDs were equivalent to morphine alone in terms of reducing acute renal colic pain within the initial 30 min (5).
Gabapentin, an antiepileptic medication belonging to the class of drugs known as gabapentinoids, exhibits potential as an analgesic medication and is particularly recommended for managing neuropathic pain caused by diabetic neuropathy, restless legs syndrome, and other forms of central neuropathic pain (9–11). Recent research has demonstrated that gabapentin has analgesic properties for both acute pre- and postoperative somatic pain, reducing the need for opioids (12, 13). Many mechanisms, including calcium channel modulation, inhibition of excitatory neurotransmitter release, descending inhibition via enhancing serotonergic and noradrenergic systems, and reduction of temporal summation—the phenomenon where repeated stimuli cause progressively increased pain perception—are responsible for its effectiveness in managing somatic pain, especially in the context of postoperative and inflammatory pain (14–16).
Despite the administration of NSAIDs and morphine alone or in combination to patients with acute renal colic pain, referred to as ED, pain is frequently uncontrolled, necessitating frequent rescue doses of morphine.
The management of renal colic pain remains an issue in the emergency department. The addition of adjutant gabapentin to standard therapy may have beneficial impacts in lowering renal colic pain, but this effect has not been verified. This trial aims to evaluate the safety and efficacy of a 600 mg oral dose of gabapentin compared to a placebo in providing further pain relief for severe renal colic pain.
2 Material and methods
The investigation was conducted out as a double-blinded, randomized controlled clinical trial with two parallel treatment groups at Sina Hospital ED, which is affiliated with Tehran University of Medical Sciences in Tehran, Iran. This clinical trial was authorized by the Iranian Registry of Clinical Trials (registration code: IRCT20200322046833N2) and approved by the Research Ethics Committee of Tehran University of Medical Sciences (approval number: IR.TUMS.TIPS.REC.1399.154).
Prior to enrollment in the study, all eligible participants or their guardians provided written informed consent.
2.1 Study population
To be eligible to participate in the present study, all following precipitants, aged 18 to 85 years, who presented with renal colic pain on a visual analogue scale (VAS) (17) more than 6, which is confirmed by computed tomography (CT) scan or ultrasonography were screened. Patients having a VAS score (a numerical rating scale ranging from 0 (no pain) to 10 (worst pain imaginable)) of at least 6 (severe pain) were given parenteral ketorolac and morphine in accordance with our hospital's pain management strategy.
Exclusion criteria included the following: history of chronic opioid use, oral intake intolerance, active peptic ulcer, active COIVD-19 infection, history of renal failure (stage 4 and beyond in chronic kidney disease) or liver impairment (Child-Pugh grades B and C), pregnancy, lactation, recent trauma, use of any analgesia within 6 h of participation, taking Gabapentin or Pregabalin within the previous 7 days, chronic lung disease (e.g., pulmonary fibrosis, asthma, etc.), and concurrent participation in other studies were excluded.
2.2 Sample size calculation
Goodarzi et al.'s study (15) guided the sample size calculation, ensuring a power analysis to detect a clinically meaningful difference in pain reduction between the gabapentin and placebo groups. An effect size of 1 and standard deviation of 1.4 were considered, indicating a moderate difference in pain scores. At a significance level of 0.05 and a power of 80%, it was calculated that 66 patients would be enough to obtain the required statistical power. This computation, by reducing the possibility of Type II errors, ensures that the study has sufficient power to identify significant differences between the groups.
According to Goodarzi et al.'s study (18), assuming a confidence level of 0.05 and a margin of error of 80%, the minimum sample size for each group will be 33 patients.
2.3 Randomization
An online tool from https://www.sealedenvelope.com was used to execute out the block randomization process. A block size of four was selected in considering to guarantee a fair distribution of participants between the groups. The 66 individuals were distributed into 16 groups of 4 and one group of 2. In each block, a matched random sequence, such as AABB or ABAB, was generated. A non-affiliated researcher, who was not part of the trial, employed a random sequence to allocate patient to either the gabapentin or placebo group, so reducing selection bias and upholding the integrity of the study.
2.4 Blinding
The hard gelatin capsules containing gabapentin and the placebo were similar. The placebo capsules contained the same amount of powder.
The allocated groups were unknown to anyone (contain patients, physicians, research students, and statisticians) other than the principal investigator.
2.5 Intervention
The participants in the intervention and control groups were administered two capsule 300 mg (a total of 600 mg) of gabapentin (manufactured by Mehrdarou Company of Iran, under the trade name Neuropentin) or a placebo that was matched to the gabapentin, respectively, upon enrollment. Moreover, an intramuscular injection of 30 mg of ketorolac was administered to each patient at the same time with gabapentin (maximum 10 min apart).
Morphine sulphate was given intravenously as rescue therapy when the VAS was six or higher (0.05 mg/kg as the initial dose, then upgraded by 0.03 mg/kg) until the VAS was continuously decreased to less than 5.
2.6 Monitoring
The Visual Analog Scale (VAS), a commonly used pain intensity measure, was used to quantify pain severity. The VAS is a 10-cm horizontal line with “no pain” (0) and “worst imaginable pain” (10). We marked a point on the line for each post-intervention time period to indicate the severity of pain. The VAS score measured the centimeter distance from the left endpoint to the participant's mark, indicating pain severity. This approach measures a continuous range of pain intensity, enabling the precise measurement and comparison of gabapentin and placebo pain levels. The VAS assessment timepoints were at baseline, then every 5 min until their VAS score dropped to less than or equal to 7, then every 15 min until score 4 or lower.
Vital signs (including heart rate, blood pressure, respiration rate, and oxygen saturation) and probable side effects (including hypotension, bradypnea, nausea and vomiting, pruritus, a lowered state of consciousness, dizziness, and tiredness) were recorded during therapy at 15 min intervals of up to 120 min. If subjects experienced severe adverse reactions that necessitated awareness of the experimental medication, they were unblinded, which was not happened in this study.
2.7 Outcomes
The primary outcome of the study was the average pain severity measured using the VAS method at 40, 60, and 90 min.
The need for rescue morphine sulphate within the first 20 min of the study, the total amount of morphine sulphate used in milligrams (mg) and milligrams per kilograms (mg/kg) of the patient's weight, the average time taken to achieve a VAS score of less than 5, and a comparison of the two groups’ likely side effects were the secondary outcomes.
2.8 Statistical analysis
Potential confounders that could affect the study's results were taken into account in our analysis, including patient age, baseline pain levels, and hemodynamics statues.
Following the completion of data collection, statistical analysis was conducted using the STATA-17 statistical software program. The Kolmogorov-Smirnov test was used to examine the quantitative data for normal or non-normal distribution. If the data distribution followed a normal distribution, the findings were presented as the mean plus or minus the standard deviation (SD) and compared between the two groups using a t-test. If the data distribution deviated from normality, the findings were represented by the median [P25 ̗ P75], and their comparison between the two groups was conducted using the Mann-Whitney test. The qualitative data were presented as frequency and percentage, and Fisher's exact test was employed to compare them. A p-value below 0.05 was deemed statistically significant in this investigation.
3 Result
A total of 82 patients underwent screening from June 2021 to November 2021. Out of the total number, 66 patients met the requirements of the study and were selected at random to be divided into two groups: 33 patients received gabapentin and 33 patients received a placebo. This division occurred after the patients completed the informed consent form to participate in the trial. Three patients in the gabapentin group were excluded from this study due to their opioid's addiction (Figure 1).
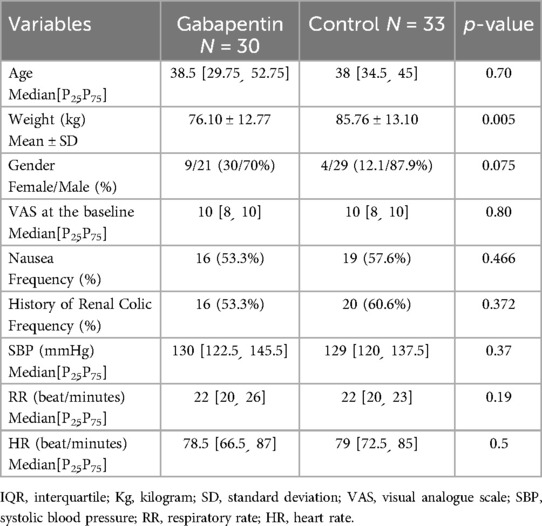
Except for weight, the two groups’ baseline characteristics were comparable. The average weight of patients in the placebo group is significantly higher (p-value = 0.005). It should be noted that there was no significant difference between the two groups’ baseline vital signs or history of prior renal colic pain.
It is noteworthy that both groups’ median VAS scores at the start of the intervention were between 8 and 10, and there was no statistically significant difference between them in this regard (p-value = 0.80) (Table 1).
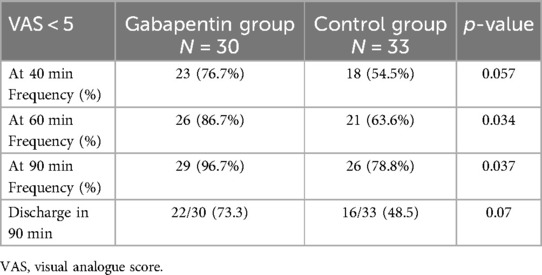
As described in Table 2, forty minutes after the intervention, patients in the gabapentin group had superior control over their pain intensity (VAS of less than five, 76.7% vs. 54.5% in the placebo group). However, the difference in VAS scores was not statistically significant (p-value = 0.057). After 60 and 90 min, respectively, the gabapentin group experienced a significant decrease in pain intensity (p = 0.03, 0.04 for VAS <5).
The discharge rate in 90 min of enrollment in the gabapentin and placebo groups was 73.3% and 48.5%, respectively, which is statistically marginally not significant (p-value = 0.07).
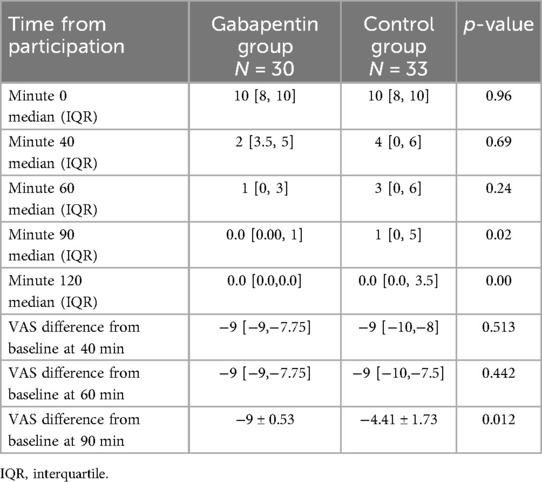
Table 3 shows the VAS measurements for pain at different time points in between groups. Although it was not statistically significant in minutes 40 and 60 (p-value = 0.69 and 0.24, respectively), pain alleviation began faster in the gabapentin group. The gabapentin group showed a significant decrease in pain at minute 90 when compared to the control group (p-value = 0.02), where all patients were pain-free at the 120th minute.
The VAS difference at 90 min was statistically lower in the gabapentin group compared to controls (−9 ± 0.53 vs. −4.41 ± 1.73, p-value = 0.012), despite the fact that the VAS difference from baseline at 40 and 60 min was not statistically different (p-value = 0.513 and 0.442, respectively), which is presented at Table 3.
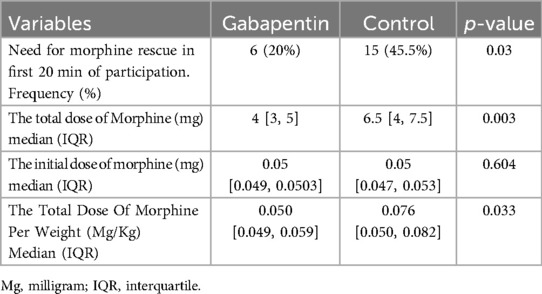
Table 4 demonstrates that the gabapentin group had considerably lower total morphine consumption and frequency of rescue morphine in the first 20 min of the study (p = 0.03).
Despite that the initial weight-based morphine dose in both groups was statistically comparable (p-value = 0.604), the placebo group's total morphine consumption (measured in milligrams) was considerably higher (p-value = 0.003). Although the higher total morphine intake may be related to the higher baseline weight of its patients, total morphine intake in terms of mg/kg in the placebo group was still higher than that in the gabapentin group (p-value = 0.033). Above finding was presented in Table 4.
None of the two groups’ patients experienced serious adverse effects during the trial that required stopping their medication or implementing therapeutic controls to manage the issue.
4 Discussion
This study was a double-blind randomized clinical trial that aimed to examine the impact of gabapentin, when used alongside the conventional analgesic regimen of ketorolac and morphine, on patients experiencing acute renal colic pain. According to the study's findings, patients with acute renal colic who receive gabapentin in addition to a conventional analgesic regimen report a significant reduction in pain intensity within the first hour of treatment. Additionally, patients use less morphine overall and require rescue morphine less frequently. Furthermore, no serious side effects or complications were reported in any of the individuals under study.
This study provides crucial insights into the management of acute renal colic pain by highlighting the potential advantages of gabapentin as an addition to standard analgesic regimens such as ketorolac and morphine. Severe pain is a hallmark of renal colic, which frequently necessitates significant analgesic intervention. Within the first hour of the study's enrollment, gabapentin dramatically lowered pain intensity and reduced the requirement for morphine; this suggests that gabapentin may play a key role in improving pain management measures for these individuals. These results have clinical value since they demonstrate that gabapentin may successfully reduce pain, which can lead to improved patient outcomes in emergency settings. For patients with conditions like renal colic, where the pain can be debilitating, pain management is an essential part of their therapy. Gabapentin may provide a safer alternative or complementary strategy to pain management by lowering reliance on opioids, which have been linked to side effects such as respiratory depression and possible dependency.
There are a variety of different mechanisms that contribute to the efficacy of gabapentin in the management of somatic pain in postoperative and inflammatory conditions. These approaches are accountable for the effectiveness. These mechanisms include the modulation of calcium channels, the inhibition of excitatory neurotransmitter release, the descending inhibition that occurs through the enhancement of serotonergic and noradrenergic systems, and the reduction of temporal summation, which is the phenomenon in which repeated stimuli cause progressively increased pain perception (14–16).
In only one relevant study at the time of this trial, gabapentin was shown to considerably lower pain levels and the need for opioids in patients with renal colic when assessed in a comparable setting by Goodarzi et al. (18). In contrast to the current trial, where over 85% of patients in the gabapentin group had mild pain (VAS < 5) after an hour, the study found that the majority of patients continued to feel moderate pain (VAS > 5). Given that the current trial utilized ketorolac, a strong NSAID, which may have led to more effective pain management, whereas Goodarzi et al. used pethidine without NSAIDs, the gap may be explained by changes in the baseline analgesic regimes.
Neuropathic pain and reflex sympathetic dystrophy are the only conditions in which gabapentin is primarily used to treat pain (19). Due to their ability to lower perioperative hyperalgesia, gabapentinoids have been included in postoperative pain therapy over the last decade (20).
Numerous research investigations have demonstrated that gabapentin exhibits a noteworthy impact on pain severity and the amount of opioids used post-operatively, in addition to working in concert with other painkillers (21–23). These effects could be resulting from a variety of ways, although the specific mechanisms remain unknown.
Unlike opioids, gabapentin acts by binding to the alpha-2-delta subunits of voltage-gated calcium channels, lowering spinal cord irritation and the release of neurotransmitters such substance P, noradrenaline, and glutamate in the pain pathways (23).
According to a 2015 systematic review, the most effective approach for minimizing postoperative pain after various types of surgeries is to administer gabapentin before to the procedure (24), but its effectiveness as adjutant medicine for lowering postoperative pain was mainly demonstrated to be unreliable by a thorough meta-analysis and review of all trials on this topic and the evidence is not sufficient yet (25–28).
By interfering with calcium channels, GABA analogs have the potential to effectively treat both peripheral and central pain as well as renal colic pain. They can also ease pain and facilitate the passage of kidney stones by widening the ureteral smooth muscle vessels (29).
These mechanisms suggest that gabapentin, which was also identified in our investigation, deserves to be able to effectively reduce acute renal colic pain. Thus, patients with renal colic pain may expect a faster reduction in pain intensity and a decrease in morphine intake when gabapentin is added to the conventional analgesic regimen.
A limitation of this study was the limited sample size of the study, which may have resulted in imprecise findings. While blinding all trial participants helped to mitigate this issue, it appears that a multicenter investigation with a bigger sample size is required. Although the VAS is a common grading system for determining pain severity, using it in conjunction with functional (30) and behavioral (31) indications can increase accuracy.
Overall, this study adds to the broadening body of data supporting the use of gabapentin as an adjuvant in conventional analgesic regimens, especially in acute pain conditions where opioid-sparing methods are becoming increasingly important. According to the research, gabapentin may improve pain management and lessen the demand for opioids, providing a potentially safer and more efficient method of treating acute renal colic pain.
5 Conclusion
This study shows that adding gabapentin to a ketorolac-based regimen significantly reduces acute renal colic pain severity and opioid analgesic use. Gabapentin may help manage acute renal colic pain, reducing narcotic consumption and improving emergency patient outcomes. Further study is needed to validate these findings and expand this approach's usefulness.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of Tehran University of Medical Sciences (approval number: IR.TUMS.TIPS.REC.1399.154). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PK: Methodology, Supervision, Writing – original draft, Writing – review & editing, Conceptualization, Validation. PV: Data curation, Writing – review & editing. PP: Investigation, Writing – review & editing. AS: Investigation, Writing – review & editing. YB: Investigation, Writing – review & editing. HH: Investigation, Supervision, Writing – review & editing. MM: Supervision, Writing – review & editing. KB: Investigation, Writing – review & editing. Ek-R: Writing – review & editing. FN: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express their sincere gratitude to the participants who volunteered for this clinical trial, demonstrating a commitment to advancing medical knowledge and patient care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shokeir AA. Renal colic: pathophysiology, diagnosis and treatment. Eur Urol. (2001) 39(3):241–9. doi: 10.1159/000052446
2. Raja AS, Pourjabbar S, Ip IK, Baugh CW, Sodickson AD, O'Leary M, et al. Impact of a health information technology-enabled appropriate use criterion on utilization of emergency department CT for renal colic. AJR Am J Roentgenol. (2019) 212(1):142–5. doi: 10.2214/AJR.18.19966
3. Ziaee SAM, Simforoosh N, Zand S. Renal colic: pathophysiology, diagnosis and management. J Med Council I.R.I. (2007) 24(4):412–22. Available online at: https://sid.ir/paper/41527/en
4. Brown J. Diagnostic and treatment patterns for renal colic in US emergency departments. Int Urol Nephrol. (2006) 38(1):87–92. doi: 10.1007/s11255-005-3622-6
5. Pathan SA, Mitra B, Cameron PA. A systematic review and meta-analysis comparing the efficacy of nonsteroidal anti-inflammatory drugs, opioids, and paracetamol in the treatment of acute renal colic. Eur Urol. (2018) 73(4):583–95. doi: 10.1016/j.eururo.2017.11.001
6. Gu HY, Luo J, Wu JY, Yao QS, Niu YM, Zhang C. Increasing nonsteroidal anti-inflammatory drugs and reducing opioids or paracetamol in the management of acute renal colic: based on three-stage study design of network meta-analysis of randomized controlled trials. Front Pharmacol. (2019) 10:96. doi: 10.3389/fphar.2019.00096
7. Davenport K, Waine E. The role of non-steroidal anti-inflammatory drugs in renal colic. Pharmaceuticals (Basel). (2010) 3(5):1304–10. doi: 10.3390/ph3051304
8. Abdolrazaghnejad A, Banaie M, Tavakoli N, Safdari M, Rajabpour-Sanati A. Pain management in the emergency department: a review article on options and methods. Front Emerg Med. (2018) 2(4):e45. doi: 10.22114/AJEM.v0i0.93
9. Rheims S, Ryvlin P. Pharmacotherapy for tonic-clonic seizures. Expert Opin Pharmacother. (2014) 15(10):1417–26. doi: 10.1517/14656566.2014.915029
10. Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, et al. EFNS Guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. (2010) 17(9):1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x
11. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. (2015) 14(2):162–73. doi: 10.1016/S1474-4422(14)70251-0
12. Peng PW, Wijeysundera DN, Li CC. Use of gabapentin for perioperative pain control—a meta-analysis. Pain Res Manag. (2007) 12(2):85–92. doi: 10.1155/2007/840572
13. Dierking G, Duedahl TH, Rasmussen ML, Fomsgaard JS, Møiniche S, Rømsing J, et al. Effects of gabapentin on postoperative morphine consumption and pain after abdominal hysterectomy: a randomized, double-blind trial. Acta Anaesthesiol Scand. (2004) 48(3):322–7. doi: 10.1111/j.0001-5172.2004.0329.x
14. Chincholkar M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth. (2018) 120(6):1315e1334. doi: 10.1016/j.bja.2018.02.066
15. Arendt-Nielsen L, Frøkjaer JB, Staahl C, Graven-Nielsen T, Huggins JP, Smart TS, et al. Effects of gabapentin on experimental somatic pain and temporal summation. Reg Anesth Pain Med. (2007) 32(5):382–8. doi: 10.1016/j.rapm.2007.05.002
16. Russo M, Graham B, Santarelli DM. Gabapentin-Friend or foe? Pain Pract. (2023) 23(1):63–9. doi: 10.1111/papr.13165
17. Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. European Palliative care research collaborative (EPCRC). studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. (2011) 41(6):1073–93. doi: 10.1016/j.jpainsymman.2010.08.016
18. Goodarzi D, Cyrus A, Baghinia MR, Sameni D. Gabapentin as an adjuvant treatment in renal colic: a randomized double-blind clinical trial. Afr J Pharm Pharmacol. (2013) 7(24):1677–9. doi: 10.5897/AJPP12.242
19. Parikh HG, Dash SK, Upasani CB. Study of the effect of oral gabapentin used as preemptive analgesia to attenuate post-operative pain in patients undergoing abdominal surgery under general anesthesia. Saudi J Anaesth. (2010) 4(3):137–41. doi: 10.4103/1658-354X.71409
20. Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. (2016) 17(2):131–57. doi: 10.1016/j.jpain.2015.12.008 Erratum in: J Pain. 2016 Apr;17(4):508-10. Dosage error in article text. PMID: 26827847.26827847
21. Menda F, Köner O, Sayın M, Ergenoğlu M, Küçükaksu S, Aykaç B. Effects of single-dose gabapentin on postoperative pain and morphine consumption after cardiac surgery. J Cardiothorac Vasc Anesth. (2010) 24(5):808–13. doi: 10.1053/j.jvca.2009.10.023
22. Montazeri K, Kashefi P, Honarmand A. Pre-emptive gabapentin significantly reduces postoperative pain and morphine demand following lower extremity orthopaedic surgery. Singapore Med J. (2007) 48(8):748–51.17657384
23. Pandey CK, Sahay S, Gupta D, Ambesh SP, Singh RB, Raza M, et al. Preemptive gabapentin decreases postoperative pain after lumbar discoidectomy. Can J Anaesth. (2004) 51(10):986–9. doi: 10.1007/BF03018484
24. Penprase B, Brunetto E, Dahmani E, Forthoffer JJ, Kapoor S. The efficacy of preemptive analgesia for postoperative pain control: a systematic review of the literature. AORN J. (2015) 101(1):94–105.e8. doi: 10.1016/j.aorn.2014.01.030
25. Fabritius ML, Geisler A, Petersen PL, Wetterslev J, Mathiesen O, Dahl JB. Gabapentin in procedure-specific postoperative pain management—preplanned subgroup analyses from a systematic review with meta-analyses and trial sequential analyses. BMC Anesthesiol. (2017) 17(1):85. doi: 10.1186/s12871-017-0373-8
26. Joshi GP, Kehlet H. Meta-analyses of gabapentinoids for pain management after knee arthroplasty: a caveat emptor? A narrative review. Acta Anaesthesiol Scand. (2021) 65(7):865–9. doi: 10.1111/aas.13820
27. Verret M, Lauzier F, Zarychanski R, Perron C, Savard X, Pinard A-M, et al. Turgeon, the Canadian perioperative anesthesia clinical trials (PACT) group; perioperative use of gabapentinoids for the management of postoperative acute pain: a systematic review and meta-analysis. Anesthesiology. (2020) 133:265–79. doi: 10.1097/ALN.0000000000003428
28. Aubrun F, Nouette-Gaulain K, Fletcher D, Belbachir A, Beloeil H, Carles M, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. (2019) 38(4):405–11. doi: 10.1016/j.accpm.2019.02.011
29. Angello JT. The treatment of renal colic with gaba analogs. Patent: EP1094804. (2005). Available online at: http://www.freepatentsonline.com/EP1094804B1.html
30. Jensen MP, Smith DG, Ehde DM, Robinsin LR. Pain site and the effects of amputation pain: further clarification of the meaning of mild, moderate, and severe pain. Pain. (2001) 91(3):317–22. doi: 10.1016/S0304-3959(00)00459-0
Keywords: renal colic pain, gabapentin, ketorolac, morphine, somatic pain
Citation: Kianpour P, Valavioun P, Payandemehr P, Safaei A, Borhani Y, Honarmand H, Mojtahedzadeh M, Basiri K, karimpour-Razkenari E and Najmeddin F (2024) Enhancing analgesia in acute renal colic pain: a randomized controlled trial of gabapentin adjunct to ketorolac-based regimen. Front. Pain Res. 5:1427711. doi: 10.3389/fpain.2024.1427711
Received: 1 July 2024; Accepted: 24 September 2024;
Published: 14 October 2024.
Edited by:
Ajmer Singh Grewal, Guru Gobind Singh College of Pharmacy, IndiaReviewed by:
Geeta Deswal, Guru Gobind Singh College of Pharmacy, IndiaBhawna Chopra, Guru Gobind Singh College of Pharmacy, India
Copyright: © 2024 Kianpour, Valavioun, Payandemehr, Safaei, Borhani, Honarmand, Mojtahedzadeh, Basiri, karimpour-Razkenari and Najmeddin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farhad Najmeddin, ZmFyaGFkLm5ham1AZ21haWwuY29t
 Parisa Kianpour1
Parisa Kianpour1 Arash Safaei
Arash Safaei Elahe karimpour-Razkenari
Elahe karimpour-Razkenari Farhad Najmeddin
Farhad Najmeddin