- 1Brody School of Medicine at East Carolina University, Greenville, NC, United States
- 2Department of Biostatistics, School of Public Health at East Carolina University, Greenville, NC, United States
- 3Department of Physiology, Brody School of Medicine at East Carolina University, Greenville, NC, United States
- 4Department of Emergency Medicine, Brody School of Medicine at East Carolina University, Greenville, NC, United States
Purpose: Despite their dangerous side effects, opioid drugs remain a standard of care for moderate to severe pain with few alternatives. Strategies to maintain the analgesic effects of opioids while minimizing the associated risks are needed. Pre-clinical studies have shown using a dopamine 3 receptor (D3R) agonist as an adjuvant to morphine provides superior analgesia against painful stimuli compared to morphine alone. Our objective was to test if adjunct treatment with a D3R agonist can lead to a reduction in opioid use while maintaining effective analgesia.
Patients and methods: This study was set up as a double-blinded, placebo-controlled randomized trial. Enrollment included acute renal colic patients presenting to the emergency department, from which patients were randomized to either the “control” or “study arm”. The control group received standard treatment of care (morphine, 0.1 mg/kg; i.v.) and an oral placebo pill. The experimental group received half-dosed morphine and oral pramipexole pill (0.25 mg). Pain measurements including a numerical pain scale and visual analog scale were collected from enrollees at baseline and every subsequent 15 min.
Results: A total of 19 patients completed the study, 10 in the experimental arm and 9 in the control arm. During the study period, effective analgesia (50% decrease from baseline) was achieved in 80% of patients in the experimental arm vs. 33.3% in the control arm.
Conclusion: Our pilot clinical trial demonstrated that D3R recruitment can serve as an effective adjuvant to low-dose morphine for control of renal colic pain and potentially other acute pain conditions.
Clinical Trial Registration: ClinicalTrials.gov, identifier, (NCT04160520).
Introduction
Opioid analgesics are among the most prescribed class of medications in the US. While opioids may be essential for controlling pain and other sensory disorders under acute conditions, the rates of misuse/abuse and accidental overdose involving prescription opioids has continuously increased since 1999, leading to the ongoing opioid epidemic (1). Clinicians have been challenged to find alternatives to opioid analgesics and current medical guidelines call for minimizing opioid doses in those cases where opioids are required for both acute and chronic pain (1–3). At the same time, the decision not to use opioids for certain conditions may lead to undertreatment of pain and reduced quality of life for those patients. Therefore, new regimens for pain that are highly effective but come with fewer risks are an urgent need for patients and physicians.
Pre-clinical studies have shown that using dopamine 2/3-like receptor agonists as an adjuvant to morphine provides superior analgesia against painful stimuli compared to morphine alone (4–6). This effect is maintained even when the dose of morphine is lowered to a dose that does not provide analgesia on its own (6). Pramipexole, a drug commonly used to treat Parkinson's disease (2, 7–9) and Restless Legs Syndrome (8, 10–12) is a dopamine 3 receptor (D3R) -preferring agonist that has also shown efficacy in fibromyalgia (13) and in enhancing morphine analgesia (6, 14–19). A role for the D3R but not the D2R in modulating pain-related spinal cord reflexes was previously identified in a functional D3R knockout mouse model, in which D2R remained unaltered over background controls (20), and in which application of D2R agonists was unable to rescue the behavioral readout of the D3KO animal (21). More recently, the D3R partial agonist (VK4–40) significantly decreased peak oxycodone self-administration in a nonhuman primate model of opioid use disorders (OUD) (22) and the highly selective and efficacious D3R partial agonist (S)-ABS01-113 demonstrated promising translational potential for the treatment of OUD (23). Together, these data suggest that the use of D3R-preferring agonist, such as pramipexole, as an adjuvant to morphine may allow for meaningful analgesia with minimal patient exposure to an opioid drug, reducing the risks associated with that exposure. To date, no data regarding the analgesic effect of this combination of FDA-approved drugs in humans has been described.
Using renal colic as a clinical acute condition that often presents with hallmark flank pain, we sought to compare the analgesic effect of a standard 0.1 mg/kg intravenous dose of morphine vs. a half-dose of intravenous morphine (0.05 mg/kg) in combination with oral pramipexole. This population was chosen as renal colic is often severe and frequently treated with morphine (24). The primary objective was to determine if combination therapy with a reduced level of the opioid plus pramipexole provided a similar amount of analgesia as the standard the opioid therapy alone (non-inferiority study).
Material and methods
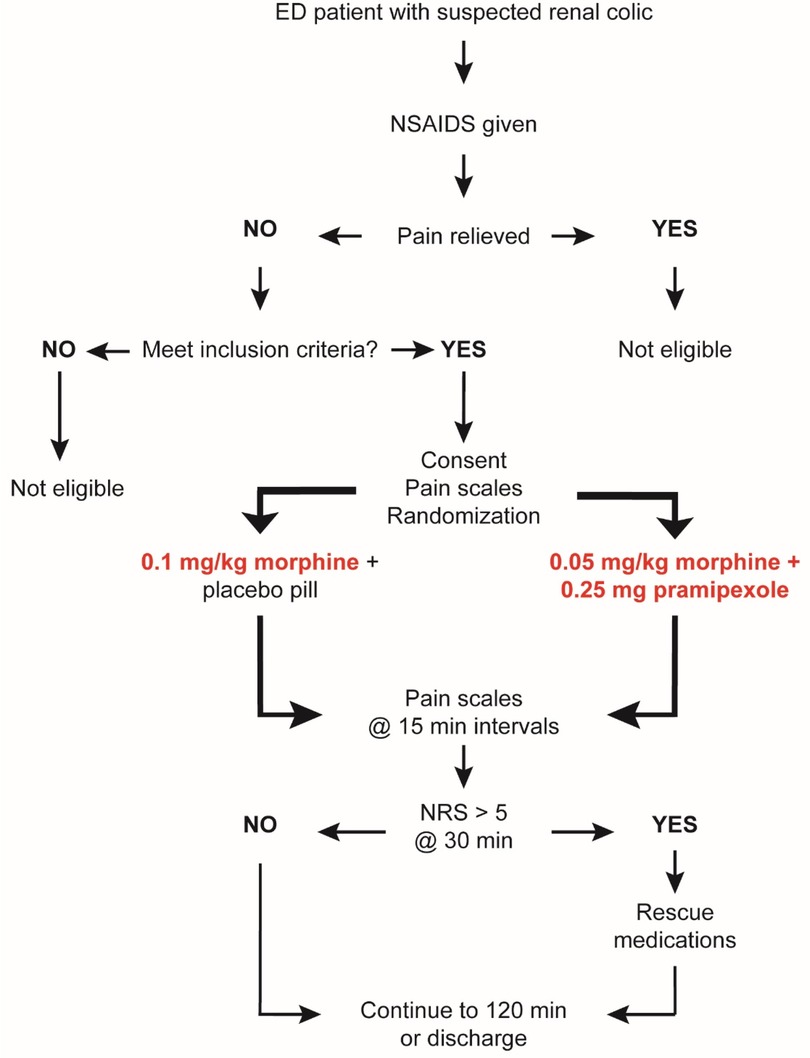
All study procedures were reviewed and approved by the University and Institutional Review Board of East Carolina University. This trial is registered on ClinicalTrials.gov (NCT04160520). A summary of the study protocol is shown in Figure 1.
Study design
This study was a double-blinded, placebo-controlled randomized controlled trial in an academic emergency department (ED) at a rural Level I trauma center.
Enrollment
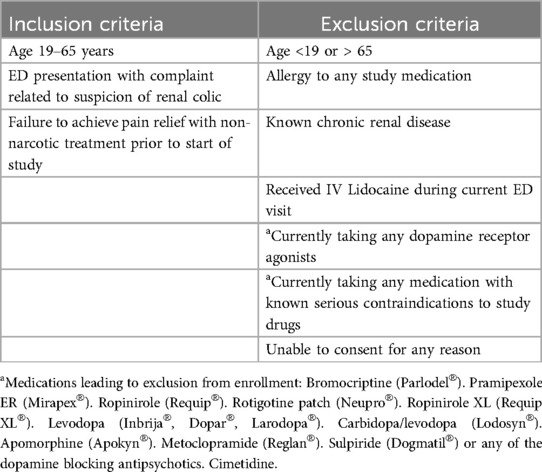
Patients between the ages of 19 and 65 years presenting to the ED with suspected renal colic were eligible for screening. A convenience sample of patients was screened based on the inclusion and exclusion criteria described in Table 1. Patients were eligible only if they failed to obtain adequate pain relief from non-narcotic analgesic given at least 30 min prior to enrollment. Patients determined by both the research and clinical team to be eligible to participate were approached for written consent and enrolled if consent was provided. Enrollment took place from October 2019 through December 2022, with an 18-month interruption from April 2020 to January 2022 due to COVID-19 restrictions.
Randomization
Enrolled participants were randomized into 2 arms at a 1:1 ratio using a SAS-based computer-generated randomization algorithm. The result of the randomization was blinded to the investigators, research team, and patient by having an integer (1–60) assigned to sealed envelopes that contained a card denoting if the randomization result was “control” or “study arm”. Envelopes with study assignment were secured in a locked ED pyxis that was only accessible to treating clinical team. The randomization result was only visible to the clinical team member responsible for ordering the appropriate medication and dosing. Study investigators were not in the room at the time of drug administration.
Study arms
Eligible patients were randomized to one of two study arms: (1) Control group: received a bolus of 0.1 mg/kg intravenous morphine and an oral placebo pill. (2) Experimental group: received a bolus of 0.05 mg/kg intravenous morphine and an active pramipexole pill (0.25 mg). This lower dose of morphine is not expected to be effective for acute pain in adults on its own based on the FDA dosing guidelines for morphine (0.1–0.2 mg/kg) (25) and studies that have demonstrated the mean effective dose across all sexes and ages for post-operative pain is 0.17 ± 0.1 mg/kg (26). The investigational drug or placebo was dispensed by the investigation site pharmacy.
Assessments
To assess analgesic effects of the 2 treatments, the following measurements were recorded: 1–10 numerical pain scale (NRS) anchored with “no pain” and “worst possible pain,” and a visual analog scale (VAS) anchored with “no pain” and “pain as bad as it could possibly be.” NRS was measured at baseline prior to administration of study drugs. VAS was not measured at baseline as the IRB would not allow for 2 pain scales to be administered prior to treatment due to the potential for delay in care. NRS and VAS were both measured every 15 min after drug treatment until patient was discharged or for 2 h (whichever came first). At 30 min after drug treatment, any patient with a NRS >5 was eligible for rescue medications (outside of study medications) at the discretion of the treating clinical team. Research team members were not involved in the decision to provide rescue medication. All patients continued in the study until the endpoint regardless of the use of rescue medications. Patients were monitored continuously for the development of any adverse event related or unrelated to study medications.
Primary outcome measures
(1) The proportion of patients achieving effective analgesia defined as a 50% or greater improvement in pain score on the 100 mm VAS, or the NRS within 120 min of treatment in experimental vs. control groups (27). This endpoint is designed to surpass the 30% reduction in pain suggested to be the minimally important change to imply effective analgesia (28). (2) The need for rescue medications at 30 min post-treatment.
An overview of the study design is shown in Figure 1.
Sample size calculation
Power analysis for non-inferiority trial of binary outcome (50% improvement in pain score, Yes vs. No) was performed using the following conditions:
Percent success of treatment in control group = 100%; Percent success of treatment in experimental group = 80%; Non-inferiority limit = 10; Alpha = 0.05; Power = 90%.
If there is a true difference in favor of the experimental group of 20%, then 32 patients are required to be 90% sure that the upper limit of a one-sided 95% confidence interval will exclude a difference in favor of the standard group of more than 10%.
To account for overestimation of effect in experimental group, the planned target number to enroll was set at 30/group (n = 60 total enrollment).
Statistical analysis
Per protocol, the primary outcome was proportion of patients achieving at least a 50% improvement in pain score on the 100 mm VAS, and the NRS within 120 min of treatment in study vs. control groups. Initially designed as a non-inferiority trial, this approach was abandoned due to recruitment issues. A 2 × 2 contingency table (Fisher's Exact test) analyzed treatment group by effective analgesia response (Yes, No). Time to first effective analgesia response was computed as elapsed from start of treatment and analyzed using a logrank Mantel-Cox test and plotted using the Kaplan-Meier method. Due to missing data points over time, change in NRS and VAS pain scores over time was analyzed using a mixed linear model to detect the effect of treatment group on pain scores over time with slope estimates reported for each group as a measure of change over time. All statistical analysis was performed using SPSS v.27 (IBM®) and graphs created in GraphPad Prism (v.10.2.3, Dotmatics).
Results
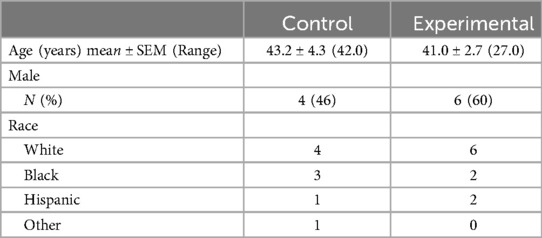
Twenty-one patients were enrolled in the study. 2 enrollees were withdrawn due to protocol deviations, leaving 19 patients who were randomized, 10 to the experimental arm and 9 to the control arm. Groups were similar with respect to age, gender, and race (Table 2). All patients received oral ibuprofen (800 mg) for pain control prior to enrollment and subsequent administration of study drugs.
Analgesia outcomes
Pain ratings over time for each group are shown in Figure 2. When measured using the NRS, change in pain over time in the experimental group showed a significant slope estimate (−0.461; p < 0.001) while the control group did not (−0.080; p = 0.32). The same was seen when pain was measured on the VAS, with the experimental group showing a slope estimate of −2.84 (p < 0.001) and a slope estimate of −1.10 in the control group (p = 0.169). Not all patients provided a pain scale at all time points. This mainly resulted from patients being out of the emergency department for imaging or other treatment during a data collection time. Two patients in the experimental group and 2 patients in the control group were discharged prior to study completion. Therefore, the 90, 105 and 120-minute assessments included 8 patients in the experimental group and 7 in the control group.
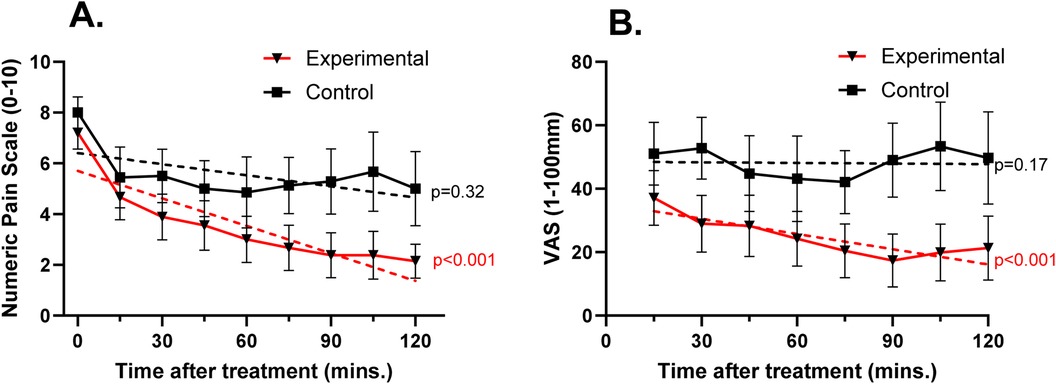
Figure 2. Mean (± SEM) pain ratings over time using the 0–10 numeric pain scale (A) and the 0–100 mm visual analog scale (B) dotted lines represent predicted slope based on a mixed linear model of change in score within each group over time. On the NRS, groups had similar pain ratings prior to treatment and showed a similar decrease at 15 min after treatment. Beyond 15 min, NRS and VAS pain ratings for patients in the experimental group continued to decrease while those in the control group remained constant. p-values indicate significance of predicted slope.
The primary outcome measure of reaching effective analgesia by 120 min was achieved in 80% (n = 8) of patients in the experimental arm vs. 33.3% (n = 3) in the control arm (p = 0.07). No patients required rescue medications at the 30-minute time point. One patient in the control group received rescue medication (2 mg intravenous morphine) at 60 min after study drug administration and remained in the study to the 120-minute time point.
Figure 3 shows the percent who had not achieved effective analgesic response at each time point. Descriptive values of the predicted mean and median time to first effective analgesic response, had there been no time cut off, is shown in Table 3. The curves were not significantly different (p = 0.180). However, while at the 15-minute measurement the two arms are similar, overall more subjects in the experimental group reached first effective analgesic response than in the control group.
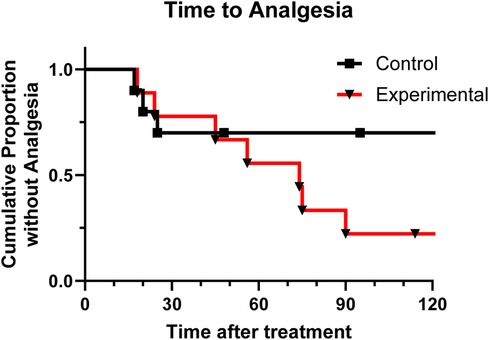
Figure 3. Kaplan-Meier analysis shows proportion of patients in each group that have not achieved effective analgesia over time.
Adverse events
No adverse events occurred in either group.
Discussion
In the first clinical trial of this combination of 2 FDA-approved and commonly used drugs, we demonstrated that the D3R agonist pramipexole may be an effective adjuvant to morphine for acute pain control, demonstrated here in renal colic patients. These results support the growing literature on the ability of D3Rs to enhance opioid-based analgesia (4–6, 29, 30). This combination of morphine and pramipexole represents a potential novel therapeutic intervention for pain conditions as it reduces patient exposure to opioids and lessens the risks associated with standard opioid dosing.
The importance of these results lies in the potential to reduce the doses of opioids needed to control pain. The CDC’s 2022 Clinical Practice Guideline for Prescribing Opioids for Pain emphasizes the need for physicians to reduce the risks associated with opioid pain therapy while maintaining flexible, patient-centered care. Specifically, the risk of overdose is known to be opioid dose dependent (31), and studies have described the primary factor associated with long-term use in opioid-naïve patients as being higher initial prescribed doses (32, 33). The CDC Guideline specifically states that when opioids are initiated in opioid-naïve patients with pain, “clinicians should prescribe the lowest effective dosage” and that the benefit of pain relief should outweigh the risk in all patients prescribed opioids (3). While there is evidence to show that non-opioid therapies can be as effective as opioids for many types of acute pain (7, 34–36), opioids will remain important for treating severe acute pain related to traumatic injury, postoperative pain, burns or other conditions for which alternative pain treatments (i.e., NSAIDS) are contraindicated or ineffective. In these cases, the addition of pramipexole as an adjuvant treatment may provide a means for clinicians to harness the analgesia that opioids provide while still meeting the goal of minimizing the doses needed to provide effective pain relief.
There is considerable overlap between dopaminergic and mu-opioid receptors (MORs) expression and pathways in brain and spinal cord (37, 38), and we have shown in preclinical studies that the use of dopamine D3Rs in combination with an opioid to treat pain improved analgesia without the typical side effects of opioids. Specifically, we demonstrated that the D3R agonist pramipexole enhanced morphine's analgesic effects in animal models of acute and chronic pain conditions (6). Importantly, we could produce effective analgesia in this neuropathic pain model with doses of morphine and pramipexole that on their own were ineffective. This is mirrored in the current study in which we were able to achieve analgesia in patients with renal colic using half of a commonly used dose of morphine.
The current literature fails to fully explain how D3R modulation impacts opioid-induced analgesia, but we demonstrated earlier that functional D3Rs are necessary to achieve morphine analgesia (39, 40). D3Rs and mu-opioid receptors (MORs) can form functional heterodimers in the ventral horn of the spinal cord (41), and they are co-localized in the dorsal horn of the spinal cord (17), a site critical for modulating nociceptive input. D2 receptors are also co-expressed with MORs in the same neurons in the striatum (38) where extensive interactions between the dopamine and opioid systems have been demonstrated.
While the current study used an acute pain condition in which to test this drug combination, there is pre-clinical data to suggest that dopamine modulation may also contribute to the effective management of chronic conditions such as neuropathic pain (9, 42–44) and migraine pain (44). In rodent studies, this drug combination did not lead to the development of tolerance with chronic administration (17). Additionally, on a test of reward potential (conditioned place preference), animals receiving the drug combination do not develop a preference for the drug-paired chamber while those receiving morphine alone did (6).
Our study did not include a pramipexole alone group, but neither pre-clinical or clinical data support the idea that pramipexole is a strong analgesic on its own. In rodent models, pramipexole has only shown to provide pain control at doses well above the human equivalent dose of 0.25 mg used in our study (45, 46). The ability of pramipexole to provide pain relief in humans has also been studied and, when administered chronically, was shown to provide some degree of pain relief in patients with fibromyalgia (13). However, the dose of pramipexole in that study was increased weekly to a dose 18 times higher than that used in our study, with effects on pain measured weekly. Additionally, more than 50% of the patients in this study were also taking a narcotic during treatment with pramipexole so these data may further demonstrate a synergistic effect of D3-agonists and opioids (13). The CDC recommends nonopioid therapies for subacute and chronic pain, noting that opioids should only be used if the expected benefits for pain relief outweigh the risks to the patient. With the addition of pramipexole as an adjuvant, it may be possible to maintain the benefit of potent analgesic effect of opioids over time at doses that do not risk the development of tolerance, dependence, and addiction. Clinical trials will be required to determine if this is the case.
The results of this clinical study support what has been described in animal models regarding the enhancement of morphine analgesia with the addition of pramipexole. However, several questions remain that need to be addressed prior to full translation of this research to the clinic. Just one dose combination of drugs was tested. This work must be replicated on a larger scale, optimizing the ratio of drugs used so that the doses of both morphine and pramipexole can be minimized. In addition, while pramipexole is highly effective in treating RLS in the short term, long-term exposure is often associated with the gradual emergence of augmentation (8, 11, 47–50). However, this typically occurs only with doses much higher than those used in this study, and after treatment durations that span years. Additionally, the combination of drugs needs to be tested across a wide range of painful conditions to determine if the effect is universal. Most importantly, it is critical to fully assess the addictive potential and abuse potential of this drug combination with acute and long-term use to ensure that effective doses do not put patients at the same risk as existing opioid regimens do.
The study is primarily limited by low power created by the small sample size. For the primary outcome of 50% pain reduction at 120 min, our study reached a power of 55% to detect a significant difference between treatment groups. Based on these preliminary results, post-hoc power analysis indicates that a minimum of 16 patients per group would be needed to reach 80% power with p < 0.05. This goes beyond our goal of showing non-inferiority of the experimental treatment would suggest that the experimental treatment is potentially superior to morphine alone for renal colic.
We also report limited data with regards to the effects of both treatments. There is a need to also collect data on short-term side effects of the treatments, including nausea, sedation, euphoria and effects on standard vital signs. It is also critical to study other types of acute and chronic pain conditions before generalizing the potential of this treatment beyond renal colic. This study does not address the issue of tolerance and dependence that might occur with long-term use of the drug combination. Additionally, other D3 agonists may have better selectivity and perform better as an adjunct to morphine. While pre-clinical trials have addressed these issues, there is no data in humans on the effects of chronic use of this combination.
Conclusion
This small-scale pilot clinical trial demonstrated that activation of the D3R system through administration of pramipexole can enhance opioid-mediated analgesia in patients with renal colic, an acute pain condition, while at the same time reducing the overall amount of the opioid used. This novel therapeutic approach has the potential to minimize the quantity of prescribed opioids and thus reduce the risks of side adverse side effects of these drugs, including overdose, tolerance and addiction.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University Medical Center and Institutional Review Board of East Carolina University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CG: Formal Analysis, Investigation, Writing – original draft, Data curation. RP: Data curation, Investigation, Writing – review & editing. JD: Data curation, Investigation, Writing – review & editing. KO: Writing – review & editing, Formal Analysis, Methodology, Writing – original draft. SC: Writing – review & editing, Conceptualization, Funding acquisition, Visualization. KB: Conceptualization, Visualization, Writing – review & editing, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding provided by the North Carolina Biotechnology Center, Translational Research (Grant no: AWD-20-1435-002).
Acknowledgments
The work presented here was supported by generous funding from the North Carolina Biotechnology Center (Translational Research Grant AWD-20-1435-002). The authors would like to acknowledge Ms. Allison Mainhart for her tireless work as the program manager and clinical coordinator for this project as well as Dr. Cassandra Bradby, Dr. Claudia Daly and Dr. Jeffrey Stayer for their input, support and efforts toward enrollment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Coyle DT, Pratt CY, Ocran-Appiah J, Secora A, Kornegay C, Staffa J. Opioid analgesic dose and the risk of misuse, overdose, and death: a narrative review. Pharmacoepidemiol Drug Saf. (2018) 27:464–72. doi: 10.1002/pds.4366
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. (2016) 65:1–49. doi: 10.15585/mmwr.rr6501e1
3. Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain - United States, 2022. MMWR Recomm Rep. (2022) 71:1–95. doi: 10.15585/mmwr.rr7103a1
4. Aira Z, Barrenetxea T, Buesa I, Gomez-Esteban JC, Azkue JJ. Synaptic upregulation and superadditive interaction of dopamine D2- and mu-opioid receptors after peripheral nerve injury. Pain. (2014) 155:2526–33. doi: 10.1016/j.pain.2014.09.012
5. Bonifazi A, Battiti FO, Sanchez J, Zaidi SA, Bow E, Makarova M, et al. Novel dual-target mu-opioid receptor and dopamine D(3) receptor ligands as potential nonaddictive pharmacotherapeutics for pain management. J Med Chem. (2021) 64:7778–808. doi: 10.1021/acs.jmedchem.1c00611
6. Rodgers HM, Yow J, Evans E, Clemens S, Brewer KL. Dopamine D1 and D3 receptor modulators restore morphine analgesia and prevent opioid preference in a model of neuropathic pain. Neuroscience. (2019) 406:376–88. doi: 10.1016/j.neuroscience.2019.03.034
7. Chou R, Wagner J, Ahmed AY, Blazina I, Brodt E, Buckley DI, et al. Treatments for Acute Pain: A Systematic Review. Comparative Effectiveness Review no. 240. Rockville, MD: Agency for Healthcare Research and Quality (2020).
8. Manconi M, Ferri R, Zucconi M, Oldani A, Fantini ML, Castronovo V, et al. First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Med. (2007) 8:491–7. doi: 10.1016/j.sleep.2006.10.008
9. Mitsi V, Zachariou V. Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience. (2016) 338:81–92. doi: 10.1016/j.neuroscience.2016.05.017
10. Jama L, Hirvonen K, Partinen M, Alakuijala A, Hublin C, Tamminen I, et al. A dose-ranging study of pramipexole for the symptomatic treatment of restless legs syndrome: polysomnographic evaluation of periodic leg movements and sleep disturbance. Sleep Med. (2009) 10:630–6. doi: 10.1016/j.sleep.2008.05.014
11. Manconi M, Garcia-Borreguero D, Schormair B, Videnovic A, Berger K, Ferri R, et al. Restless legs syndrome. Nat Rev Dis Primers. (2021) 7:80. doi: 10.1038/s41572-021-00311-z
12. Winkelman JW, Sethi KD, Kushida CA, Becker PM, Koester J, Cappola JJ, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology. (2006) 67:1034–9. doi: 10.1212/01.wnl.0000231513.23919.a1
13. Holman AJ, Myers RR. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. (2005) 52:2495–505. doi: 10.1002/art.21191
14. Chwieduk CM, Curran MP. Pramipexole extended release: in Parkinson’s disease. CNS Drugs. (2010) 24:327–36. doi: 10.2165/11204570-000000000-00000
15. Eisenreich W, Sommer B, Hartter S, Jost WH. Pramipexole extended release: a novel treatment option in Parkinson’s disease. Parkinsons Dis. (2010) 2010:612619. doi: 10.4061/2010/612619
16. Relja M, Klepac N. A dopamine agonist, pramipexole, and cognitive functions in Parkinson’s disease. J Neurol Sci. (2006) 248:251–4. doi: 10.1016/j.jns.2006.05.027
17. Rodgers HM, Lim SA, Yow J, Dinkins ML, Patton R, Clemens S, et al. Dopamine D1 or D3 receptor modulators prevent morphine tolerance and reduce opioid withdrawal symptoms. Pharmacol Biochem Behav. (2020) 194:172935. doi: 10.1016/j.pbb.2020.172935
18. Shannon KM, Bennett JP Jr., Friedman JH. Efficacy of pramipexole, a novel dopamine agonist, as monotherapy in mild to moderate Parkinson’s disease. The pramipexole study group. Neurology. (1997) 49:724–8. doi: 10.1212/WNL.49.3.724
19. Weiner WJ, Factor SA, Jankovic J, Hauser RA, Tetrud JW, Waters CH, et al. The long-term safety and efficacy of pramipexole in advanced Parkinson’s disease. Parkinsonism Relat Disord. (2001) 7:115–20. doi: 10.1016/S1353-8020(00)00031-6
20. Zhu H, Clemens S, Sawchuk M, Hochman S. Unaltered D1, D2, D4, and D5 dopamine receptor mRNA expression and distribution in the spinal cord of the D3 receptor knockout mouse. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. (2008) 194:957–62. doi: 10.1007/s00359-008-0368-5
21. Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci. (2004) 24:11337–45. doi: 10.1523/JNEUROSCI.3698-04.2004
22. Woodlief K, Allen MI, Cornelissen JC, Banks ML, Newman AH, Nader MA. Effects of selective dopamine D3 receptor partial agonist/antagonists on oxycodone self-administration and antinociception in monkeys. Neuropsychopharmacology. (2023) 48:1716–23. doi: 10.1038/s41386-023-01590-8
23. Galaj E, Bi GH, Klein B, Hempel B, Shaik AB, Gogarnoiu ES, et al. A highly D(3)R-selective and efficacious partial agonist (S)-ABS01-113 compared to its D(3)R-selective antagonist enantiomer (R)-ABS01-113 as potential treatments for opioid use disorder. Neuropsychopharmacology. (2022) 47:2309–18. doi: 10.1038/s41386-022-01379-1
24. Pathan SA, Mitra B, Cameron PA. A systematic review and meta-analysis comparing the efficacy of nonsteroidal anti-inflammatory drugs, opioids, and paracetamol in the treatment of acute renal colic. Eur Urol. (2018) 73:583–95. doi: 10.1016/j.eururo.2017.11.001
25. Hospira. Highlights of Prescribing Information. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202515s000lbl.pdf (Accessed February 24, 2024).
26. Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. (2005) 103:156–60. doi: 10.1097/00000542-200507000-00023
27. Chang AK, Bijur PE, Baccelieri A, Gallagher EJ. Efficacy and safety profile of a single dose of hydromorphone compared with morphine in older adults with acute, severe pain: a prospective, randomized, double-blind clinical trial. Am J Geriatr Pharmacother. (2009) 7:1–10. doi: 10.1016/j.amjopharm.2009.02.002
28. Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine. (2008) 33:90–4. doi: 10.1097/BRS.0b013e31815e3a10
29. Bonifazi A, Saab E, Sanchez J, Nazarova AL, Zaidi SA, Jahan K, et al. Pharmacological and physicochemical properties optimization for dual-target dopamine D(3) (D(3)R) and mu-opioid (MOR) receptor ligands as potentially safer analgesics. J Med Chem. (2023) 66:10304–41. doi: 10.1021/acs.jmedchem.3c00417
30. Sanchez JE, Noor S, Sun MS, Zimmerly J, Pasmay A, Sanchez JJ, et al. The FDA-approved compound, pramipexole and the clinical-stage investigational drug, dexpramipexole, reverse chronic allodynia from sciatic nerve damage in mice, and alter IL-1beta and IL-10 expression from immune cell culture. Neurosci Lett. (2023) 814:137419. doi: 10.1016/j.neulet.2023.137419
31. Chou R, Deyo R, Devine B, Hansen R, Sullivan S, Jarvik JG, et al. The Effectiveness and Risks of Long- Term Opioid Treatment of Chronic Pain. Evidence Report/Technology Assessment no. 218. AHRQ Publication no 14-E005-EF. Rockville MD: Agency for Healthcare Research and Quality (2014).
32. Deyo RA, Hallvik SE, Hildebran C, Marino M, Dexter E, Irvine JM, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: a statewide retrospective cohort study. J Gen Intern Med. (2017) 32:21–7. doi: 10.1007/s11606-016-3810-3
33. Deyo RA, Hallvik SE, Hildebran C, Marino M, Springer R, Irvine JM, et al. Association of prescription drug monitoring program use with opioid prescribing and health outcomes: a comparison of program users and nonusers. J Pain. (2018) 19:166–77. doi: 10.1016/j.jpain.2017.10.001
34. Chou R, Hartung D, Turner J, Blazina I, Chan B, Levander X, et al. Opioid Treatments for Chronic Pain. Comparative Effectiveness Review, No. 229. Rockville MD: Agency for Healthcare Research and Quality (2020).
35. VanderPluym JH, Singh RBH, Urtecho M, Morrow AS, Nayfeh T, Roldan VDT, et al. Acute Treatments for Episodic Migraine. Comparative Effectiveness Review no. 239. Rockville, MD: Agency for Healthcare Research and Quality (2020).
36. Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, et al. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Update. Comparative Effectiveness Review no. 227. Rockville, MD: Agency for Healthcare Research and Quality (2020).
37. Aira Z, Barrenetxea T, Buesa I, Del Cano G G, Azkue JJ. Dopamine D1-like receptors regulate constitutive, mu-opioid receptor-mediated repression of use-dependent synaptic plasticity in dorsal horn neurons: more harm than good? J Neurosci. (2016) 36:5661–73. doi: 10.1523/JNEUROSCI.2469-15.2016
38. de Gortari P, Mengod G. Dopamine D1, D2 and mu-opioid receptors are co-expressed with adenylyl cyclase 5 and phosphodiesterase 7B mRNAs in striatal rat cells. Brain Res. (2010) 1310:37–45. doi: 10.1016/j.brainres.2009.11.009
39. Brewer KL, Baran CA, Whitfield BR, Jensen AM, Clemens S. Dopamine D3 receptor dysfunction prevents anti-nociceptive effects of morphine in the spinal cord. Front Neural Circuits. (2014) 8:62. doi: 10.3389/fncir.2014.00062
40. Keeler BE, Baran CA, Brewer KL, Clemens S. Increased excitability of spinal pain reflexes and altered frequency-dependent modulation in the dopamine D3-receptor knockout mouse. Exp Neurol. (2012) 238:273–83. doi: 10.1016/j.expneurol.2012.09.002
41. Clemens S, Belin-Rauscent A, Simmers J, Combes D. Opposing modulatory effects of D1- and D2-like receptor activation on a spinal central pattern generator. J Neurophysiol. (2012) 107:2250–9. doi: 10.1152/jn.00366.2011
42. Huang S, Borgland SL, Zamponi GW. Dopaminergic modulation of pain signals in the medial prefrontal cortex: challenges and perspectives. Neurosci Lett. (2019) 702:71–6. doi: 10.1016/j.neulet.2018.11.043
43. Martikainen IK, Hagelberg N, Jaaskelainen SK, Hietala J, Pertovaara A. Dopaminergic and serotonergic mechanisms in the modulation of pain: in vivo studies in human brain. Eur J Pharmacol. (2018) 834:337–45. doi: 10.1016/j.ejphar.2018.07.038
44. Zhang W, Lei M, Wen Q, Zhang D, Qin G, Zhou J, et al. Dopamine receptor D2 regulates GLUA1-containing AMPA receptor trafficking and central sensitization through the PI3K signaling pathway in a male rat model of chronic migraine. J Headache Pain. (2022) 23:98. doi: 10.1186/s10194-022-01469-x
45. Edwards S, Callicoatte CN, Barattini AE, Cucinello-Ragland JA, Melain A, Edwards KN, et al. Pramipexole treatment attenuates mechanical hypersensitivity in male rats experiencing chronic inflammatory pain. Neuropharmacology. (2022) 208:108976. doi: 10.1016/j.neuropharm.2022.108976
46. Mihaylova A, Kostadinov I, Doncheva N, Delev D, Zlatanova H. Pramipexole and tolcapone alleviate thermal and mechanical nociception in naive rats. Folia Med (Plovdiv). (2021) 63:377–84. doi: 10.3897/folmed.63.e55136
47. Garcia-Borreguero D, Allen RP, Benes H, Earley C, Happe S, Hogl B, et al. Augmentation as a treatment complication of restless legs syndrome: concept and management. Mov Disord. (2007) 22(Suppl 18):S476–484. doi: 10.1002/mds.21610
48. Montplaisir J, Nicolas A, Denesle R, Gomez-Mancilla B. Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology. (1999) 52:938–43. doi: 10.1212/WNL.52.5.938
49. Silber MH, Girish M, Izurieta R. Pramipexole in the management of restless legs syndrome: an extended study. Sleep. (2003) 26:819–21. doi: 10.1093/sleep/26.7.819
Keywords: morphine, dopamine, D3 agonist, adjuvant, rats, opioid
Citation: Girardi C, Duronio J, Patton R, O’Brien K, Clemens S and Brewer KL (2024) A novel opioid/pramipexole combination treatment for the management of acute pain: a pilot study. Front. Pain Res. 5:1422298. doi: 10.3389/fpain.2024.1422298
Received: 23 April 2024; Accepted: 12 September 2024;
Published: 24 September 2024.
Edited by:
George Latimer Wilcox, University of Minnesota Twin Cities, United StatesReviewed by:
Ratan Banik, University of Minnesota Twin Cities, United StatesDaniel Manvich, Rowan University School of Osteopathic Medicine, United States
Copyright: © 2024 Girardi, Duronio, Patton, O'Brien, Clemens and Brewer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kori L. Brewer, YnJld2Vya0BlY3UuZWR1
 Cara Girardi
Cara Girardi Joseph Duronio1
Joseph Duronio1 Kevin O’Brien
Kevin O’Brien Stefan Clemens
Stefan Clemens Kori L. Brewer
Kori L. Brewer