- 1Department of Biostatistics, Epidemiology, and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 2Center for Health Equity Research and Promotion, Corporal Crescenz VA Medical Center, Philadelphia, PA, United States
- 3Qualitative, Evaluation and Stakeholder Engagement Research Services, Center for Research on Health Care, University of Pittsburgh, Pittsburgh, PA, United States
- 4Department of Management, Policy, and Community Health, The University of Texas Health Science Center at Houston, Houston, TX, United States
- 5Department of Anesthesiology Perioperative and Pain Medicine, Brigham and Women’s Hospital, Boston, MA, United States
- 6Department of Pediatrics, University of Pittsburgh School of Medicine, Children’s Hospital of Pittsburgh, Pittsburgh, PA, United States
- 7School of Social Work, University of Pittsburgh, Pittsburgh, PA, United States
- 8Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, United States
- 9Division of General Internal Medicine, CHAllenges in Managing and Preventing Pain (CHAMPP) Clinical Research Center, University of Pittsburgh, Pittsburgh, PA, United States
Background: Most management of chronic pain, a serious illness affecting the physical and psychological wellbeing of millions, occurs in primary care settings. Primary care practitioners (PCPs) attempt to provide evidence-based practices to treat chronic pain. However, there continues to be a gap between the care people receive and the evidence. The objectives for this study were to (1) explore determinants of evidence-based chronic pain management and (2) develop a novel approach to using implementation science to address the evidence-practice gap.
Method: A convenience sample of twenty-one Pennsylvania PCPs participated in one-time semi-structured telephone interviews. Interviews were transcribed verbatim and both deductive and inductive approaches were used during analysis. We used the Consolidated Framework for Implementation Research (CFIR) and the Expert Recommendations for Implementing Change (ERIC) to inform our analysis and findings.
Results: We identified determinants of evidence-based chronic pain management across the CFIR domains of Intervention Characteristics, Characteristics of Individuals, and the Outer Setting and reported implementation strategies. Based on identified themes, we developed a three-step process to support the ongoing and pragmatic implementation of evidence-based chronic pain management in primary care settings.
Conclusions: Previous efforts exist to integrate implementation science into chronic pain management; yet a gap persists. Implementation approaches should prioritize the needs of people living with chronic pain and their families. Further, future approaches or strategies used should build on the current three-step model to include the fourth step of tailoring existing implementation strategies to the specific needs of chronic pain in the clinical context.
Introduction
Chronic pain is a common, serious illness often accompanied by psychological comorbidities including anxiety and depression (1, 2), decreased quality of life (1), reduced participation in the workforce (2), and strain on interpersonal relationships (2). It affects about 55 million people in the United States (3) and costs $635 billion annually in medical treatment and lost productivity (4). The consequences of chronic pain may be further complicated by environmental factors such as stress and lack of social support (5).
Evidence-based practices (EBPs) to treat chronic pain include pharmacologic therapies [e.g., antidepressants (6), amitriptyline, etc.], non-pharmacologic approaches such as physical therapy (7–9) and cognitive behavioral therapy (CBT) (10–12), and behavioral approaches such as self-management and cognitive behavioral therapy, yoga, and physical therapy (13). However, patients often do not receive evidence-based chronic pain management (14–16). A prime example is the treatment for chronic low back pain. A recent systematic review reported that chronic low back pain prevalence rises linearly from the third decade of life on with rates of 4.2% in individuals between 24 and 39 years old and 19.6% in those ages 20 and 59 (17), with an estimated 2.06 million cases per year (18). A 2018 series published by The Lancet examining current and best practices for treatment showed that low back pain results in 2.7 million emergency department visits each year, despite best practices for managing chronic pain in primary care and avoiding emergency care (19). Further, surgical procedures are often used as a strategy to manage chronic low back pain, which again contradicts guidance advising the reliance on noninvasive treatment strategies (19). One study found that only 12% of people with chronic low back pain have received psychological support and only 8.4% received CBT (19).
Primary care practitioners (PCPs; i.e., physicians, nurse practitioners, and physician assistants) (20) are often the first line of care for people living with chronic pain (20) and patients often do not receive chronic pain EBPs (14–16). In part, this is due to complex referral systems and insurance coverage (21) and a lack of pain specialists (4, 22). Therefore, understanding and integrating PCP perceptions of barriers to delivering these evidence-based therapies is a critically important next step toward improving implementation. Implementation science (IS) seeks to understand and close the gap between EBPs and clinical care (23) and may help to ensure that people living with chronic pain receive evidence-base management. The primary objectives for this study were to investigate PCP perceptions of barriers to evidence-based chronic pain management implementation, and PCP strategies (or facilitators) for addressing these barriers. The secondary objective was to develop a novel approach to using implementation science to address the evidence-practice gap in chronic pain management in primary care settings.
Method
We selected a qualitative approach to answer our research question. Qualitative data collection and analysis support both positivist and constructionist epistemological perspectives (24). We used qualitative data collection (i.e., interviews) and analyzed data using exploratory or content-driven analysis which answers the question, “what do x people think about y?” (24). We conducted one-time semi-structured telephone interviews with PCPs between May 2019 and August 2019. The COREQ (COnsolidated criteria for REporting Qualitative research) Checklist was used and can be found in the Supplementary File S2.
Participants and participant recruitment
Clinicians were eligible for participation if they (1) were a physician, physician assistant, or nurse practitioner; (2) practiced in an outpatient setting in Pennsylvania; and (3) treated adults. We targeted these groups as we wanted solicit insights from PCPs who are responsible for using evidence-based approaches to manage chronic pain with their patients. We limited interviews to clinicians in Pennsylvania to capture chronic pain management approaches across a diverse set of geographic areas, yet also within the same policy system (i.e., Medicaid reimbursement and prescription reporting via the PA Prescription Drug Monitoring Program). We purposively sampled for clinicians who self-reported working in either academic or non-academic settings (distinct from teaching responsibilities) to incorporate both perspectives. We used a convenience sampling approach primarily focused in Western and Central Pennsylvania agnostic to health system or affiliation with participants representing several health systems. PCPs were recruited via email and at meetings (i.e., staff meetings and in-person trainings). As such, we were unable to calculate a response rate due to the scale and broadcast nature of our recruitment strategy.
Development of the interview guide
We developed an interview guide (see Supplementary File S1) based on the chronic pain literature and factors influencing implementation from Diffusion of Innovations Theory (25) as further operationalized by the Consolidated Framework for Implementation Research (CFIR) (26). We used the CFIR interview guide template to draft an initial interview guide and tailored it based on barriers identified in the literature and the experiences of our investigator team. For example, we solicited information on what makes it difficult to treat chronic pain with follow-up prompts focused on individual (e.g., substance abuse, depression), group (e.g., organizational difficulties), and system factors (e.g., insurance coverage).
We then pilot tested the interview guide with two academic PCPs who provided extensive feedback, and modified the original guide based on that feedback. The resulting guide contained questions focused on general chronic pain management, agnostic to the type of chronic pain treatment (i.e., pharmacologic vs. non-pharmacologic). Participants were asked to think of the types of chronic pain they commonly see in their practice and use that as a reference point for other questions. Given that evidence-based approaches to chronic pain management rather than specific pain are more similar across types and locations, we chose to focus on chronic pain generally rather than a specific pain type (e.g., neuropathic) or location (e.g., low back). Participants were then specifically prompted on the role of co-morbidities, insurance, and organizational structures.
Data collection and transcription
The PI (LEA) conducted all interviews with iterative feedback from the research team (MEH, EM, SME, and JSM) with no field notes generated. The PI (LEA), who identifies as female, was a PhD candidate with a Master of Social Work degree, and five years of experience conducting qualitative data collection and analysis at the time of data collection. The PI introduced themselves to participants as a PhD candidate and social worker. The PI described the purpose of the study and their interest in learning more about how PCPs manage chronic pain, the challenges to that management, and ways they overcome those strategies. The PI emphasized the lived experience and expertise of each participant.
The interviews were conducted in private office with the door closed with only the PI (LEA) physically present and the participant on the phone. Participants were entered into a sweepstakes to win a $100 Amazon gift card as compensation. Interviews were audio recorded and transcribed verbatim and were continued until thematic saturation was reached and no new themes emerged. Transcripts were not returned to participants for review.
Data coding/codebook formation and data analysis
We used an inductive approach codebook development and coding to conduct an applied thematic analysis. First, the PI developed initial codes based on the first five interviews with additional codes added as new themes emerged. Two investigators (LEA and SSO) finalized the codebook by reviewing all transcripts in duplicate until a finalized codebook was agreed upon. The PI then coded all transcripts using NVivo 12 (QSR International). Once coding was complete, the PI developed an initial set of themes from the content of the interviews.
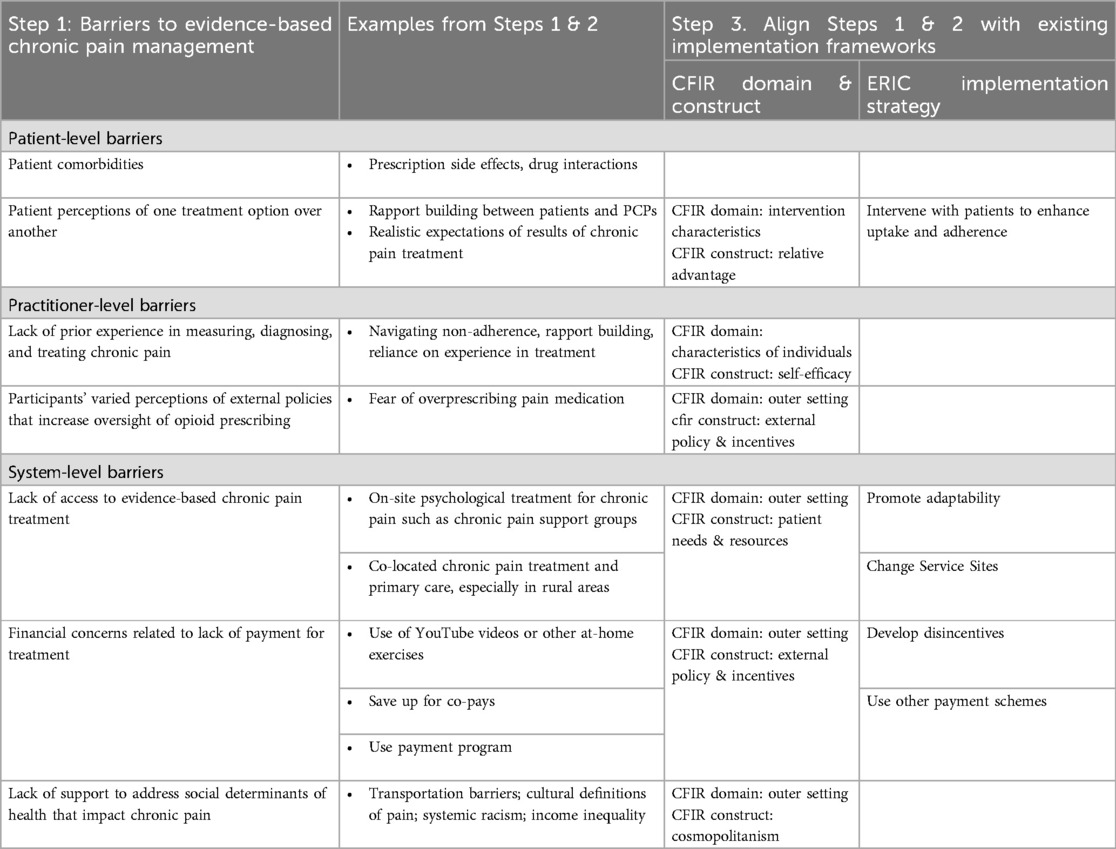
Following this initial inductive approach, we conducted a second deductive analysis. Implementation science examines how evidence-based practices are put into use (23) (p5). The Consolidated Framework for Implementation Research (CFIR) is a robust meta-theoretical framework often used in implementation science which helps to identify and understand barriers to utilizing evidence-based practices (26). The Expert Recommendations for Implementing Change (ERIC) taxonomy builds on the CFIR to describe strategies (or facilitators) for overcoming identified barriers (27). For example, themes related to beneficial perceptions of treatment were identified as fitting into the relative advantage portion of the CFIR framework. Themes related to any CFIR or ERIC elements are presented in Table 1. Using this deductive approach, we mapped determinants of evidence-based chronic pain management (e.g., what makes it difficult?) identified by PCPs and mapped these to CFIR domains and constructs and mapped strategies described by PCPs to utilize evidence-based approaches to understand how PCPs navigate their complex environment to ERIC implementation strategies with feedback from the co-authors, but not participants.
The University of Pittsburgh Human Research Protection Office approved this study (STUDY19010045). All participants provided verbal informed consent prior to participation.
Three-step approach to integrating implementation science
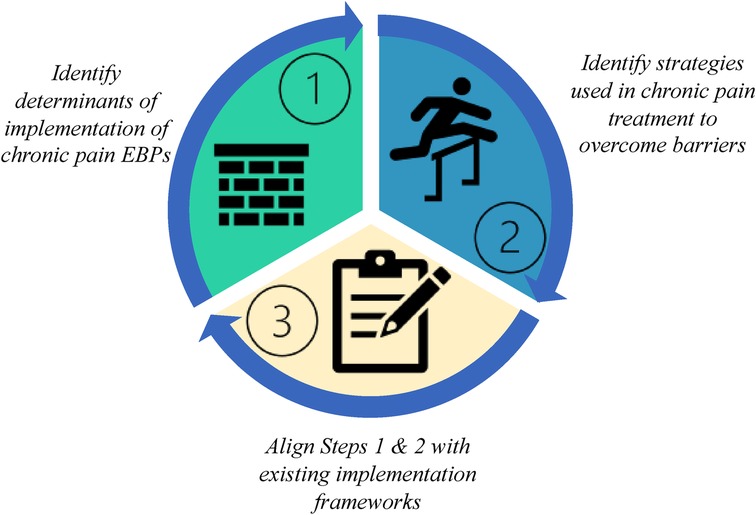
Based on our experience, we developed a three-step approach to identifying the evidence-practice gap through the process of analyzing the interview data and mapping it to existing implementation science theories and frameworks (Figure 1). The steps are as follows: Step 1: Identify determinants of implementation of chronic pain EBPs, Step 2: Identify strategies used by PCPs providing chronic pain treatment to overcome barriers, and Step 3: Align Steps 1 & 2 with existing implementation frameworks which leverages existing knowledge across the field of IS. In the current study, we tested the feasibility of using this approach for chronic pain management in primary care settings by conducting a qualitative study of PCPs.
Results
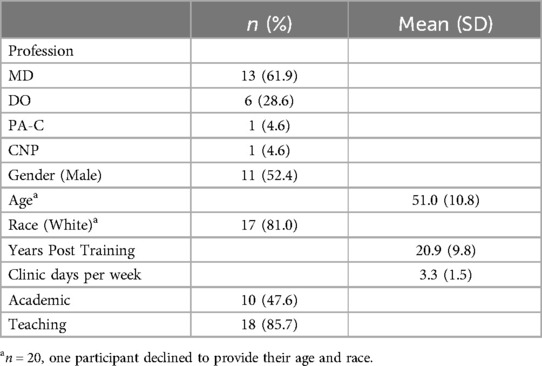
We interviewed 21 PCPs (see Table 2). The mean age was 51 (SD = 10.8). Half of participants (n = 11) identified as male, and most participants (n = 17) identified as white, with three participants identifying as Asian. The average number of years post-training was 21 (range 3–35); participants spent an average of 3.3 days (range 0.5–5) in the clinic per week. Participants were mostly physicians with 61% (n = 13) being Medical Doctors (MDs) and 28% (n = 6) as Doctors of Osteopathy (DOs). The remaining two participants were advanced practice providers (one Physician Assistant and one Nurse Practitioner). Half of the participants worked in an academic clinic (n = 10), and most (n = 18) were involved in some form of teaching (e.g., supervising medical residents). Interviews were an average of 21 min 14 s (range: 15:58–25:05). No participants refused participation nor dropped out for the duration of the interviews.
Results from Steps 1-3 are as follows: Step 1. Identify determinants of clinician's implementation of chronic pain EBPs. Participants reported barriers to implementing chronic pain EBPs in primary care settings across patient, provider, and system levels. A major issue cited was access to non-pharmacologic treatment such as cognitive behavioral therapy and physical therapy. One participant described these barriers in this way:
“I would say insurance coverage is always an issue, especially when people are looking for things like aqua therapy and acupuncture and things that may not be covered well by insurance. Also, physical therapy, although it's “covered,” a lot of times, the out-of-pocket expense, though, is still too high for patients to afford. So, some things are covered but not to the point of being able to be affordable.” Participant # 9; Physician, MD
Step 2. Identify strategies clinicians used in providing chronic pain treatment to overcome barriers. PCPs reported strategies they use to overcome these barriers such as using existing resources, rapport with patients, and understanding of the health system. For the barrier of access, some participants described developing in-house psychological treatment for people living with chronic pain. A participant described this approach used by their clinic to address access to psychological treatment for chronic pain management:
“So often times we'll talk about– we actually have a local group that meets with a counselor or a psychologist too. He just talks about chronic pain and how do you live with it and what do you do personally to manage the pain. Because we know that oftentimes we can't completely eliminate or most of the time we can't eliminate pain we could just make it bearable. So, starting with offering that group.” Participant #11; Physician, MD
Step 3. Align Steps 1 & 2 with existing implementation frameworks. The PI (LEA) reviewed the barriers and strategies identified from our inductive coding of interview transcripts and mapped them onto existing CFIR domains and constructs and ERIC implementation strategies. These selections were then reviewed by co-investigators (LEA, MEH, SME, and JSM) for acceptability from a qualitative (MEH) and chronic pain (JSM) perspective. We did this by reviewing the definitions of CFIR domains and constructs and implementation strategies with the identified themes from the data and matched definitions. For example, the barrier to access aligned with the CFIR Outer Setting domain and construct of Patient Needs and Resources because the PCP recognized the access need of patients living with chronic pain. The strategy to overcome aligned with the ERIC implementation strategy of Change Service Site because they moved treatment to a setting easily accessible by patients (i.e., in-house treatment). In the interviews, participants did not suggest any strategies to address the patient-level barrier of co-morbidities nor practitioner-level barriers. Table 1 has a list of themes that arose through our data collection and analysis and their alignment with existing implementation frameworks.
Discussion
Our results, while not generalizable, align with previous research within chronic pain management in primary care settings: the evidence-practice gap persists, particularly for implementation of non-pharmacologic approaches, despite ongoing efforts to integrate IS principles into chronic pain management (12, 28–34) and providers using creative approaches to ensure their patients receive the care they need. The importance of the therapeutic relationship between the patient and PCPs is well-known. The ongoing patient-practitioner relationship often helps to frame and interpret difficult conversations, including those about chronic pain (35). Conversations about chronic pain management can be difficult as PCPs may struggle to believe patients' reported pain (36) and patients may struggle to feel trusted in their own experience (35).
However challenging, participants in our study expressed a degree of hope and at times desire to push through the hard conversations to address a patient's pain or possible substance dependency. Chronic pain management still exists within the shadow of the opioid epidemic. The consequences of opioid use (and misuse) continue to be pervasive in national dialogue, continuing education, and funding—especially given the uptick in opioid-related deaths during the COVID-19 pandemic (37, 38). Again, given these challenges, across the board, practitioners in our study expressed the desire to navigate the complex challenges of treating chronic pain with their patients despite these contextual barriers. Participants demonstrated an immense desire to work with patients to navigate complex systems and manage their chronic pain. Unexpectedly, PCPs in this study were open to identifying and using non-opiate treatments to help manage their patient's chronic pain, even as these treatment options were typically harder to access. Participants showed surprising resourcefulness in overcoming known barriers to get patients the help they need, such as hosting chronic pain support groups in the primary care clinic. These strategies create an innovative foundation for future testing and implementation in more diverse clinical settings.
We developed a three-step approach as one way to interpret limited samples of provider experiences specifically within chronic pain to begin to address implementation gaps within a given clinical context. Our approach attempts to leverage the field of implementation science by providing a way for researchers and practitioners alike to address this gap by taking a strengths-based approach to the experience of PCPs and how they continue to find innovative ways to navigate complex chronic pain management. We then used the field of implementation science to complement and build on the front-line knowledge of barriers to treatment and the navigation of those barriers. Alignment of current barriers and strategies with the larger field of implementation science may provide avenues for additional strategy discovery or approaches used in other settings which can promote the ongoing evolution of navigating the complex barriers to treating chronic pain.
Clinicians, scholars, and quality improvement evaluators can leverage existing knowledge by aligning existing barriers and strategies with the larger literature. Future practice and research should focus on building on the existing model to include Step 4 tailor existing implementation strategies to the specific needs of chronic pain in the clinical context, perhaps by formalizing changes in service location (per our earlier example) and expanding it to include virtual support groups and teletherapy (see Figure 2). Based on our findings, approaches should be patient-centered and solicit guidance from people living with chronic pain and their families and support systems. Strategies to overcome barriers and the interventions themselves should be adaptable and tailored to the given clinical context, type of chronic pain, and patient preferences. Important considerations include the degree to which the policy setting may impact PCPs ability to navigate barriers to evidence-based chronic pain management. Some contextual factors may include the degree to which mental health services reimbursed at the same or similar rates to physical health services (mental health parity) or the impact of the expansion of Medicaid influence the proportion of people with insurance coverage.
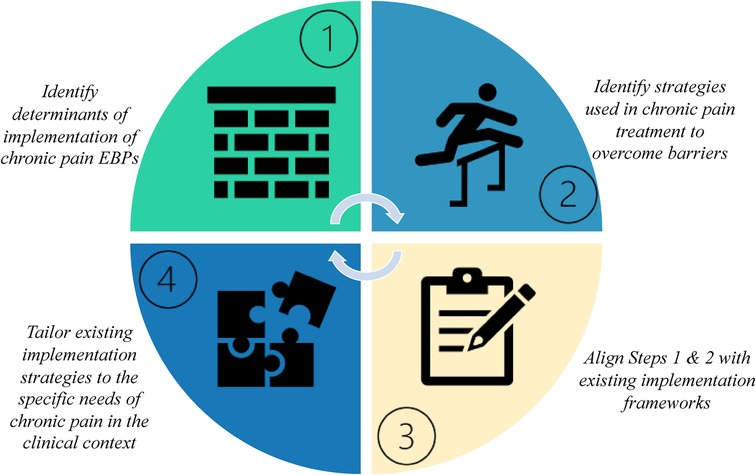
Figure 2. Putting implementation science into action to improve care for people living with chronic pain.
Limitations
The current study has several limitations. First, these findings represent a limited convenience sample and the reported experiences of participants in Pennsylvania—which are not generalizable. Most participants self-identified as white. Additionally, we did not solicit feedback on the identified implementation strategies from participants and instead relied on the qualitative and clinical experience of the investigative team. Future research should solicit the experiences of non-white PCPs to assess whether their approaches to managing patients with chronic pain differ from those of their white counterparts. These data were collected before the COVID-19 pandemic and therefore we may be missing some now critical updates to the way in which chronic pain management occurs. The potential for social desirability bias is also a concern. The PI developed rapport with each participant throughout the recruitment, screening, and interview process by highlighting the lived experience and expertise of the participants as PCPs.
Conclusion
The gap between evidence-based chronic pain management and care for chronic pain persists even with ongoing efforts to integrate IS. We developed a three-step process to show barriers that PCPs continue to face and strategies to overcome with the goal to integrate local information and IS knowledge to improve patient outcomes.
Data availability statement
De-identified data supporting the conclusions of this article will be made available upon reasonable request to the authors.
Ethics statement
The studies involving humans were approved by University of Pittsburgh Human Research Protection Office (STUDY19010045). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the research was deemed to be low risk. Participants provided verbal consent prior to participation.
Author contributions
LA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MH: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing. SO: Formal Analysis, Validation, Writing – review & editing. VH: Conceptualization, Methodology, Resources, Writing – review & editing. EM: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing. SE: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JM: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by NIH TL1TR001858.
Acknowledgments
The authors would like to acknowledge the millions of people who live with chronic pain and the diligence and dedication of primary care practitioners who continually navigate complex systems to do their best for their patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2024.1376462/full#supplementary-material
Supplementary File S1 | Interview Guide.
Supplementary File S2 | COREQ Checklist.
References
1. Fine PG. Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med. (2011) 12(7):996–1004. doi: 10.1111/j.1526-4637.2011.01187.x
2. Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. (2007) 105(1):205. doi: 10.1213/01.ane.0000268145.52345.55
3. Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morb Mortal Wkly Rep. (2018) 67(36):1001–6. doi: 10.15585/mmwr.mm6736a2
4. Institute of Medicine of the National Academies. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. USA: Inst Med Natl Acad (2012). Available online at: http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=115064741&site=ehost-live (Accessed February 14, 2019).
5. Bushnell MC, Case LK, Ceko M, Cotton VA, Gracely JL, Low La, et al. Effect of environment on the long-term consequences of chronic pain. Pain. (2015) 156 Suppl 1(0 1):S42–9. doi: 10.1097/01.j.pain.0000460347.77341.bd
6. Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med. (2002) 162(1):19–24. doi: 10.1001/archinte.162.1.19
7. Hayden JA, Van Tulder MW, Malmivaara AV, Koes BW. Meta-analysis: exercise therapy for nonspecific low back pain. Ann Intern Med. (2005) 142(9):765. doi: 10.7326/0003-4819-142-9-200505030-00013
8. Kinney M, Seider J, Beaty AF, Coughlin K, Dyal M, Clewley D. The impact of therapeutic alliance in physical therapy for chronic musculoskeletal pain: a systematic review of the literature. Physiother Theory Pract. (2018) 0(0):1–13. doi: 10.1080/09593985.2018.1516015
9. Rubinstein SM, van Middelkoop M, Assendelft WJ, de Boer MR, van Tulder MW. Spinal manipulative therapy for chronic low-back pain. Cochrane Database Syst Rev. (2011) (2):CD008112. doi: 10.1002/14651858.CD008112.pub2
10. Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. (2007) 26(1):1–9. doi: 10.1037/0278-6133.26.1.1
11. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. (1999) 80(1):1–13. doi: 10.1016/S0304-3959(98)00255-3
12. Niesen CR, Olson DM, Nowdesha KD, Tynsky DA, Loftus CG, Meiers SJ. Enhancing self-management for adults with functional abdominal pain: a registered nurse-led cognitive-behavioral therapy approach. Gastroenterol Nurs. (2018) 41(4):321–32. doi: 10.1097/SGA.0000000000000322
13. Mills S, Torrance N, Smith BH. Identification and management of chronic pain in primary care: a review. Curr Psychiatry Rep. (2016) 18:1–9. doi: 10.1007/s11920-015-0659-9
14. Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med. (2013) 173(17):1573. doi: 10.1001/jamainternmed.2013.8992
15. Agnoli A, Jerant A, Becker W, Franks P. Opioid prescriptions and short-term mortality: a U.S. National study. J Gen Intern Med. (2020) 35(3):656–61. doi: 10.1007/s11606-019-05501-w
16. McCalmont JC, Jones KD, Bennett RM, Friend R. Does familiarity with CDC guidelines, continuing education, and provider characteristics influence adherence to chronic pain management practices and opioid prescribing? J Opioid Manag. (2018) 14(2):103–16. doi: 10.5055/jom.2018.0437
17. Meucci RD, Fassa AG, Faria NMX. Prevalence of chronic low back pain: systematic review. Rev Saúde Pública. (2015) 49:1. doi: 10.1590/S0034-8910.2015049005874
18. Waterman BR, Belmont PJ, Schoenfeld AJ. Low back pain in the United States: incidence and risk factors for presentation in the emergency setting. Spine J. (2012) 12(1):63–70. doi: 10.1016/j.spinee.2011.09.002
19. Foster NE, Anema JR, Cherkin D, Chou R, Cohen SP, Gross DP, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. (2018) 391(10137):2368–83. doi: 10.1016/S0140-6736(18)30489-6
20. Breuer B, Cruciani R, Portenoy RK. Pain management by primary care physicians, pain physicians, chiropractors, and acupuncturists: a national survey. South Med J. (2010) 103(8):738–47. doi: 10.1097/SMJ.0b013e3181e74ede
21. Pizzo PA, Clark NM. Alleviating suffering 101—pain relief in the United States. N Engl J Med. (2012) 366(3):197–9. doi: 10.1056/NEJMp1109084
22. Schottenfeld JR, Waldman SA, Gluck AR, Tobin DG. Pain and addiction in specialty and primary care: the bookends of a crisis. J Law Med Ethics. (2018) 46(2):220–37. doi: 10.1177/1073110518782923
23. National Institutes of Health. PAR-19-276: Dissemination and Implementation Research in Health (R03 Clinical Trial Not Allowed). 2019. Available online at: https://grants.nih.gov/grants/guide/pa-files/PAR-19-276.html (Accessed August 21, 2019).
24. Guest G, MacQueen KM, Namey EE. Applied Thematic Analysis. Thousand Oaks, CA: SAGE Publications, Inc. (2012). doi: 10.4135/9781483384436
26. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. (2009) 4(1):50. doi: 10.1186/1748-5908-4-50
27. Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implement Sci. (2015) 10(1):21. doi: 10.1186/s13012-015-0209-1
28. Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med. (2019) 20(7):1311–20. doi: 10.1093/pm/pny229
29. Becker WC, Dorflinger L, Edmond SN, Islam L, Heapy AA, Fraenkel L. Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Fam Pract. (2017) 18(1):41. doi: 10.1186/s12875-017-0608-2
30. Jacobson N, Johnson R, Deyo B, Alagoz E, Quanbeck A. Systems consultation for opioid prescribing in primary care: a qualitative study of adaptation. BMJ Qual Saf. (2019) 28(5):397–404. doi: 10.1136/bmjqs-2018-008160
31. Midboe AM, Wu J, Erhardt T, et al. Academic detailing to improve opioid safety: implementation lessons from a qualitative evaluation. Pain Med. (2018) 19:S46–53. doi: 10.1093/pm/pny085
32. Shea CM, Gertner AK, Green SL. Barriers and perceived usefulness of an ECHO intervention for office-based buprenorphine treatment for opioid use disorder in North Carolina: a qualitative study. Subst Abuse. (2021) 42(1):54–64. doi: 10.1080/08897077.2019.1694617
33. Varley AL, Lappan S, Jackson J, Goodin BR, Cherrington AL, Copes H, et al. Understanding barriers and facilitators to the uptake of best practices for the treatment of co-occurring chronic pain and opioid use disorder. J Dual Diagn. (2020) 16(2):239–49. doi: 10.1080/15504263.2019.1675920
34. Becker WC, Mattocks KM, Frank JW, Bair MJ, Jankowski RL, Kerns RD, et al. Mixed methods formative evaluation of a collaborative care program to decrease risky opioid prescribing and increase non-pharmacologic approaches to pain management. Addict Behav. (2018) 86:138–45. doi: 10.1016/j.addbeh.2018.03.009
35. Matthias M, Krebs E, Bergman A, Coffing J, Bair M. Communicating about opioids for chronic pain: a qualitative study of patient attributions and the influence of the patient–physician relationship. Eur J Pain. (2014) 18(6):835–43. doi: 10.1002/j.1532-2149.2013.00426.x
36. Matthias MS, Parpart AL, Nyland KA, Huffman MA, Stubbs DL, Sargent C, et al. The patient–provider relationship in chronic pain care: providers’ perspectives. Pain Med. (2010) 11(11):1688–97. doi: 10.1111/j.1526-4637.2010.00980.x
37. Kelley MA, Lucas J, Stewart E, Goldman D, Doctor JN. Opioid-related deaths before and after COVID-19 stay-at-home orders in Los Angeles county. Drug Alcohol Depend. (2021) 228:109028. doi: 10.1016/j.drugalcdep.2021.109028
Keywords: primary care, chronic pain, implementation science, qualitative research, methods
Citation: Ashcraft LE, Hamm ME, Omowale SS, Hruschak V, Miller E, Eack SM and Merlin JS (2024) The perpetual evidence-practice gap: addressing ongoing barriers to chronic pain management in primary care in three steps. Front. Pain Res. 5:1376462. doi: 10.3389/fpain.2024.1376462
Received: 25 January 2024; Accepted: 23 September 2024;
Published: 8 October 2024.
Edited by:
Flavia Di Pietro, Curtin University, AustraliaReviewed by:
Teresa Damush, United States Department of Veterans Affairs, United StatesPeng Zhao, Liberty Mutual Insurance, United States
Copyright: © 2024 Ashcraft, Hamm, Omowale, Hruschak, Miller, Eack and Merlin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Ellen Ashcraft, bGF1cmFlbGxlbi5hc2hjcmFmdEBwZW5ubWVkaWNpbmUudXBlbm4uZWR1
†ORCID:
Laura Ellen Ashcraft
orcid.org/0000-0001-9957-0617
Serwaa S. Omowale
orcid.org/0000-0002-1331-5111
Valerie Hruschak
orcid.org/0000-0003-0791-6970
Shaun M. Eack
orcid.org/0000-0001-6368-7004
Jessica S. Merlin
orcid.org/0000-0003-3207-4875
 Laura Ellen Ashcraft
Laura Ellen Ashcraft Megan E. Hamm3
Megan E. Hamm3 Elizabeth Miller
Elizabeth Miller