
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pain Res., 15 May 2024
Sec. Neuropathic Pain
Volume 5 - 2024 | https://doi.org/10.3389/fpain.2024.1352711
 Dilara Kersebaum1,2*†
Dilara Kersebaum1,2*† Manon Sendel1,†
Manon Sendel1,† Josephine Lassen1
Josephine Lassen1 Sophie-Charlotte Fabig1
Sophie-Charlotte Fabig1 Julia Forstenpointner1
Julia Forstenpointner1 Maren Reimer1
Maren Reimer1 Sima Canaan-Kühl3
Sima Canaan-Kühl3 Jens Gaedeke3,‡
Jens Gaedeke3,‡ Stefanie Rehm1
Stefanie Rehm1 Janne Gierthmühlen4
Janne Gierthmühlen4 Ralf Baron1
Ralf Baron1 Philipp Hüllemann1
Philipp Hüllemann1
Background: Fabry disease (FD) causes cold-evoked pain and impaired cold perception through small fiber damage, which also occurs in polyneuropathies (PNP) of other origins. The integrity of thinly myelinated fibers and the spinothalamic tract is assessable by cold-evoked potentials (CEPs). In this study, we aimed to assess the clinical value of CEP by investigating its associations with pain, autonomic measures, sensory loss, and neuropathic signs.
Methods: CEPs were examined at the hand and foot dorsum of patients with FD (n = 16) and PNP (n = 21) and healthy controls (n = 23). Sensory phenotyping was performed using quantitative sensory testing (QST). The painDETECT questionnaire (PDQ), FabryScan, and measures for the autonomic nervous system were applied. Group comparisons and correlation analyses were performed.
Results: CEPs of 87.5% of the FD and 85.7% of the PNP patients were eligible for statistical analysis. In all patients combined, CEP data correlated significantly with cold detection loss, PDQ items, pain, and autonomic measures. Abnormal CEP latency in FD patients was associated with an abnormal heart frequency variability item (r = −0.684; adjusted p = 0.04). In PNP patients, CEP latency correlated significantly with PDQ items, and CEP amplitude correlated with autonomic measures (r = 0.688, adjusted p = 0.008; r = 0.619, adjusted p = 0.024). Furthermore, mechanical pain thresholds differed significantly between FD (gain range) and PNP patients (loss range) (p = 0.01).
Conclusions: Abnormal CEPs were associated with current pain, neuropathic signs and symptoms, and an abnormal function of the autonomic nervous system. The latter has not been mirrored by QST parameters. Therefore, CEPs appear to deliver a wider spectrum of information on the sensory nervous system than QST alone.
Treating neuropathic pain (NP) conditions can be difficult, due to an oftentimes challenging diagnostic workup. Patients with NP present a wide range of sensory signs and symptoms. Various previous studies have employed quantitative sensory testing (QST) to perform sensory phenotyping and subgrouping of the patients to gain new insights into the underlying mechanisms of action and to drive phenotype-based treatment decisions (1–6).
There is an ongoing discussion about whether specific sensory markers are indicative of certain neuropathic syndromes (7). In the case of Fabry disease (FD), the impairment of cold sensation is one of the first evident sensory abnormalities and can serve as an early clinical sign of the disease. FD is an X-linked lysosomal storage disorder characterized by an early disease onset, affecting the nervous system apart from numerous other organ manifestations, such as kidneys, heart, cornea, and endothelial cells (8–11). The damage to organ and nerve tissue originates from an accumulation of toxic metabolites, i.e., globotriaosylceramide and globotriaosylsphingosine [lysoGb3; (12)] due to deficiency of the alpha-galactosidase enzyme, which leads to inflammation, fibrosis, and oxidative stress (13–16). Typically, FD patients report burning pain in the hands and feet (17) triggered by heat or cold stimuli and present abnormal cold detection thresholds in the upper limb (18) and cold sensory loss (19–21), indicative of a small fiber neuropathy (22). Early detection of the disease is of utmost importance since severe neuropathic and cardiovascular complications may be prevented by specific enzyme replacement therapy (9, 23). It has been reported that small fiber dysfunction is more common than large fiber dysfunction in the early stages of FD (24), making the detection of a small fiber dysfunction crucial in the early diagnosis of FD. Over the past decade, there has been growing interest in an electrophysiological method for examining cold-mediating, thinly myelinated Aδ-fibers and their central pathways: the recording of cold-evoked potentials (CEPs) (25–27). Previously, in healthy subjects, both our research group and colleagues from other research laboratories have demonstrated CEPs to be a reliable, non-invasive tool for measuring Aδ-fiber integrity with various thermodes and even stimulation parameters (25, 28–31).
While laser-evoked potentials (LEPs) have long been recognized for their assessment of nociceptive pathways (32) and given the progress with CEPs in healthy individuals (31, 33), the clinical relevance of cold-evoked potential (CEP) recordings—especially regarding diagnosing NP—is promising but subject to ongoing discussion, due to scarcity of patient data available (27, 34). In a previous study, we introduced cases of a patient with central pain and another with polyneuropathy (PNP), both demonstrating abnormal CEPs corresponding to their clinical findings and providing initial promising insights into their potential for clinical and diagnostic application (28). Recently, Perchet et al. reported on CEPs in patients with suspected NP compared to LEPs. They concluded that “for some patients suffering from symptoms limited only to cold, CEPs but not LEPs may allow the diagnosis of thin fiber pathology” (30).
In earlier projects, we conducted comparisons of CEPs between single patients with neuropathic conditions and healthy controls (28, 35) and found possible associations between abnormal CEPs and the occurrence of pain and sensory loss. However, we were unable to draw any definitive conclusions as these preliminary insights were based on single-patient cases. We assumed that abnormal CEPs reflect an abnormal small fiber function potentially due to a significant reduction of intraepidermal nerve fibers reflecting neuropathic damage. A similar reduction in intraepidermal Aδ-fibers was observed, for instance, in patients with chemotherapy-induced painful PNP, a group known to experience NP, especially thermal hyperalgesia (36–38). We proceeded with a CEP patient study in an attempt to validate these assumptions.
This study aims to determine whether CEPs are feasible in FD patients and, if applicable, whether they can detect abnormal function of cold-mediating Aδ-fibers using a CE-certified thermal stimulator for clinical/diagnostic use. Subsequently, we investigated patients with PNP as representatives of a more heterogeneous NP syndrome (7, 39). We focused on whether CEPs correlate with the sensory phenotype and whether abnormal CEP findings are associated with an altered or abnormal sensory phenotype (assessed by QST). We hypothesized that patients with abnormal CEPs would report higher pain levels, be more likely classified as neuropathic based on the painDETECT questionnaire, and, in the case of FD patients, achieve higher scores on the FabryScan.
Patients with FD and PNP and age-matched healthy subjects were recruited. According to the general inclusion/exclusion criteria (see Supplementary Material), subjects with current alcohol or drug abuse, who are pregnant or breastfeeding women, with insufficient language skills, or who are unable to give informed consent were not eligible for the study. Patients with FD, with and without pain, were included only with a mutation in the GLA gene and with a completed clinical evaluation. Both currently available medical laboratory testing services [ARCHIMEDlife (40) and CENTOGENE (41)] have been used in some of the patients, while others have been diagnosed by genetic panel testing. There was one patient with a stroke history (n = 1) who was not excluded from the analysis as organ manifestations of FD (including the endothelium) are broad. PNP patients with and without pain were recruited, and the inclusion criteria were based on the consensus criteria for a symmetric PNP (42). Healthy subjects were, inter alia, only eligible for participation if they met the following criteria: absence of neurological and pain disorders and the non-usage of analgesic medication within the past 14 days. Additionally, patients who were diagnosed with psychosis, depression, anxiety, panic attacks, eating disorders, chronic fatigue or exhaustion, addiction or dependence, or any other severe organ system failures were excluded as recommended by Gierthmühlen et al. (43). FD patients, PNP patients and healthy subjects underwent CEP and QST assessment. Apart from a few exceptions, QST and heart rate (HR) variability have been assessed in all subjects prior to this study (44). In patients, both feet were investigated (the most affected side was included for analysis). For healthy subjects, no side-dependent evoked potential (EP) differences were known (26, 45); therefore, the right body side was chosen for the test application. Both patient groups completed the PDQ. The Fabry group was additionally characterized with the FabryScan, whereas the PNP group underwent nerve conduction studies.
In accordance with the Declaration of Helsinki, patients and controls were first explained the aim and nature of the tests and provided their written informed consent. The study was approved by the Ethics Committee of the University Hospital of Kiel (study protocol number: A 101/15) and registered in the German Clinical Trials Register (DRKS00009013).
Age, sex, comorbidities, medication, and height of patients and healthy volunteers were assessed and are reported in the Results section.
The subjects were positioned on a comfortable stretcher. To prevent blinking or eye movement artifacts, they fixed their gaze on a marking on the ceiling and were asked not to blink or to move their eyes during stimulus application and 3 s until a ping tone chimed. The latter signaled the subjects to rate the perceived cold stimuli on a numerical rating scale (0 = not cold at all, 10 = most imaginable cold). The room temperature was maintained at a constant 22°C. The skin temperature of the testing sites was measured before the testing started and was >32°C at the test site (28, 35). There was no standard wait time before starting the recordings. Cold stimuli were applied to the dorsum of the hand and the foot using the PATHWAY Pain & Sensory Evaluation System (Medoc, Israel; CE-number 0473; software version 4.0.11.0). A baseline temperature of 30 °C, a destination temperature of 25°C, a destination rate of 20°C/s, and a return rate of 40°C/s were used. In total, 25 CEPs with an interstimulus interval of 8–12 s were applied on each test site. The thermode remained at a fixed position (28).
Gold cup electroencephalography (EEG) electrodes (Fz, Cz, Pz, C3, C5, C4, C6, T3, and T4) were attached according to the international 10–20 system and referenced to linked earlobes for the recording of potentials. A grounding electrode was attached to the torso. An electrooculogram (EOG) was used for detecting eye movement/blinking artifacts. An artifact correction step based on regression has been applied to account for these artifacts. The EEG was recorded with Brain Vision Recorder 1.2 using the BrainAmp MR plus EEG amplifier (Brain Products GmbH, Gilching, Germany) and analyzed with Brain Vision Analyzer 2.0 (Brain Products GmbH; Gilching, Germany, version 2.0.3.6367). The EEG was band-pass filtered with 0.3–35 Hz, and the sampling rate was 1,000 Hz (25, 35).
Both peak detection and artifact rejection were performed manually and framewise. For each frame, a baseline correction was performed using a pre-stimulus window from −500 to −100 ms to the N1 peak for the determination of the N1. The N2P2 amplitude was measured from the most negative (N2) to the most positive peak (P2). The latency of each component was measured from the stimulus onset (0 ms) to the most negative (N2) and most positive (P2) peaks of the averaged potentials, respectively. The amplitude and latency data were measured at the Cz channel. The other channels were used to support the correct identification of the averaged potential and for artifact detection.
All frames containing artifacts or analysis-hindering elements 0.5 s before the stimulus and 2 s afterward due to movement or blinking were excluded from the analysis. Blinking artifacts were identified and taken care of with an ocular correction filter. Disruptive factors such as the occurrence of alpha-EEG sequences or muscular artifacts were excluded during manual inspection. CEP recordings were included in the final analysis if the recording of the subjects contained at least 50% artifact-free EEG segments (i.e., 13/25 EEG frames). On average, 73.5 ± 20.4% of the segments from the foot and 73.6 ± 20.7% from the hand were used for the analysis.
Small fiber function was assessed with QST according to the protocol of the German Research Network on Neuropathic Pain (DFNS) (46–48). QST results were defined as abnormal if the Z value was outside the limits of a 95% confidence interval of healthy controls of the DFNS database (46).
Thermal stimuli were applied using a thermal testing device (TSA 2001-II, Medoc, Israel), which applied cold stimuli with a ramp of 1°C/s reaching a minimal temperature of 0°C. Cold detection threshold (CDT), the parameter for cold detection of the QST protocol, was assessed in both feet while the foot with the higher threshold and the ipsilateral hand dorsum were chosen for further testing (49–51).
Originally developed as a screening tool for the detection of a NP component in patients with chronic pain (52), the PDQ by now has also been shown to enable the identification of neuropathic subgroups and sensory profiles (6). This questionnaire assesses general pain intensity, the course and distribution of pain, and finally the following sensory signs: burning sensation, tingling/prickling, painful to light touch, sudden pain attacks, painful to cold or heat, numbness, and pressure pain. These are then rated on a six-point Likert scale (0 = never, 5 = very strongly). The sum of all components results in a score ranging from 0 to 38. A sum score of ≥19 indicates a >90% probability that a NP component is present while ≤12 point makes it unlikely.
We have used painDETECT in its version 2010.
The FabryScan is a screening tool specifically developed for the identification of FD patients (53). It consists of 10 items covering different typical symptoms for FD and differential diagnoses and three bedside exams. With a score of 16 or more, FD is considered likely. A score between 11 and 15 is interpreted to be an unclear result.
We have used the FabryScan in its original validated version.
We recorded the HRV by a three-lead electrocardiogram during a 5 min resting period and during controlled breathing for a period of 110 interbeat intervals. We also assessed the root mean square of successive square differences (RMSSD) in a resting and breathing (RMSSDb) condition. A computer-assisted equipment and software (ProSciCard III, MediSyst GmbH, Germany), developed according to the 1996 Task Force Guidelines, was used to process and analyze the HRV (1996). This analysis algorithm detected artifacts and extrasystoles as well as dismissed a series with an artifact percentage of >10% (54). The abovementioned software was also used to conduct a time-domain measurement (TDM) during orthostatic conditions, where out of 50 RR intervals, the respiratory cycle with the highest and lowest HRV is detected and the quotient is calculated (HR max/HR min).
For both the RMSSDb and orthostatic TDM, higher values were rated as favorable as patients with cardiac conduction abnormalities such as atrial fibrillation would have been recognized by the program and excluded from the analysis (neither PNP nor FD patients presented atrial fibrillation).
Statistical analysis was conducted using SPSS (SPSS 29.0; SPSS, Inc., Chicago, IL, USA). All parameters were displayed as mean (± standard deviation). CEPs were defined to be abnormal if their N2P2 amplitude was below the lower limit of a 95% confidence interval of age-matched controls' artifact-free average or if the N2 latency was above the upper limit of a 95% confidence interval of age-matched healthy controls.
For the comparison of group variables (height, N2 and P2 latencies, and N2P2 amplitudes of the hand and foot, the CDT of the hand and foot, and the PDQ and FabryScan score if applicable), the Mann–Whitney U (MWU) test was used. Subgroup analyses on painful vs. painless patients or FD patients with or without abnormal CEP parameters were also conducted using the MWU test. FD patients and healthy controls were age-matched during the recruitment phase and therefore did not differ significantly in age (first step of the study). As expected, the PNP cohort (average onset of disease in middle-aged and older patients) was significantly older than the Fabry cohort (average onset of disease in late childhood) and the controls (who were age-matched to FD). The PNP cohort was recruited during the second step of the study. Before performing group comparisons, we therefore performed an age matching procedure, excluding the oldest PNP patients and the youngest healthy controls, so that age would not differ significantly between PNP patients and controls or to FD patients.
To detect associations between CEP data, QST items, autonomic items, and questionnaire scores, a correlation analysis was performed using Spearman’s rho. Correlation analyses were performed in all patients and each patient group (PNP, FD) separately. The significance level (p < 0.05) was adjusted for multiple testing by multiplying it with the number of analyzed items.
A total of 16 patients with FD (age 44.25 ± 17.92 years, 11 females, 5 males), 21 patients with PNP (age 64.62 ± 11.19 years, 9 females, 12 males), and 23 controls (age 46.83 ± 19.53 years, 13 females, 10 males) were recruited. Five FD patients were on enzyme replacement therapy (agalsidase, n = 3; migalastat, n = 2). Although female patients were heterozygous (most common in FD), some received enzyme replacement therapy due to measurable organ damage. Some patients did not fulfill the criteria for treatment at the time of study conduction (e.g., low symptom burden in combination with a “non-classical mutation”). Some patients with eligible mutations received chaperone-therapy instead of enzyme replacement.
The PNP group offered the following etiologies: diabetic (n = 5), HMNS type II (n = 1), CIDP (n = 1), paraneoplastic (n = 2), alcohol-related (n = 2), and chemotherapy-induced (n = 2). Eight of the 21 patients remained of unclear origin. The MWU revealed no significant differences in height between patients with FD and controls/age-matched patients with PNP and controls.
Epidemiological data, EP values, and questionnaire scores are presented in Table 1. Since PNP patients had a significantly higher age as compared to FD patients and healthy controls, we performed a secondary age matching between the PNP group and healthy controls as shown in Table 2. Table 3 provides an age-matched comparison of patients with FD and PNP. Supplementary Tables A and B (see Supplementary Material) provide an overview of abnormal results (indicated through “X”). Figure 1 gives an overview of the studied groups and the performed statistical analyses with a brief summary of the significant results. Figure 2 shows the Z-score sensory profiles of both the patient groups and the controls. EEG data of the hand and foot for each group respectively is displayed in Figures 3, 4, and 5.
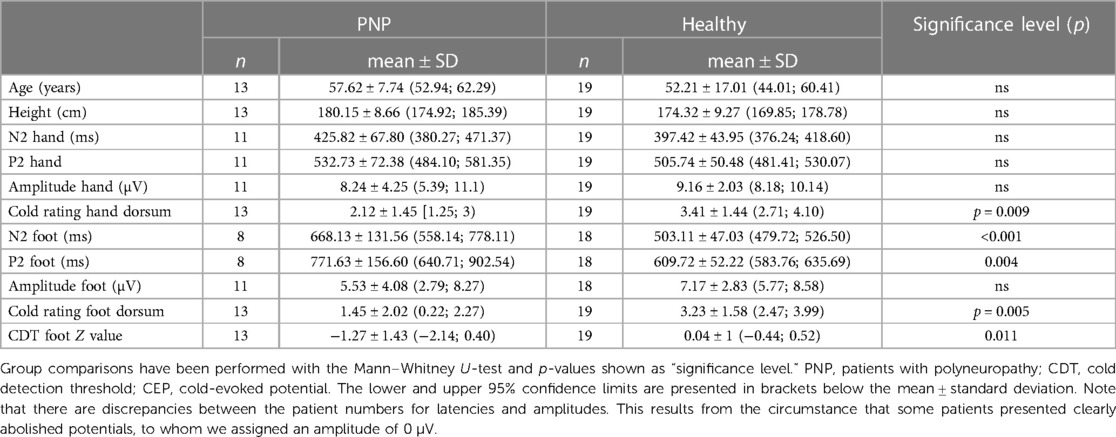
Table 2. Demographic data, CEP values, and questionnaire scores for PNP patients and healthy cohort after age matching.
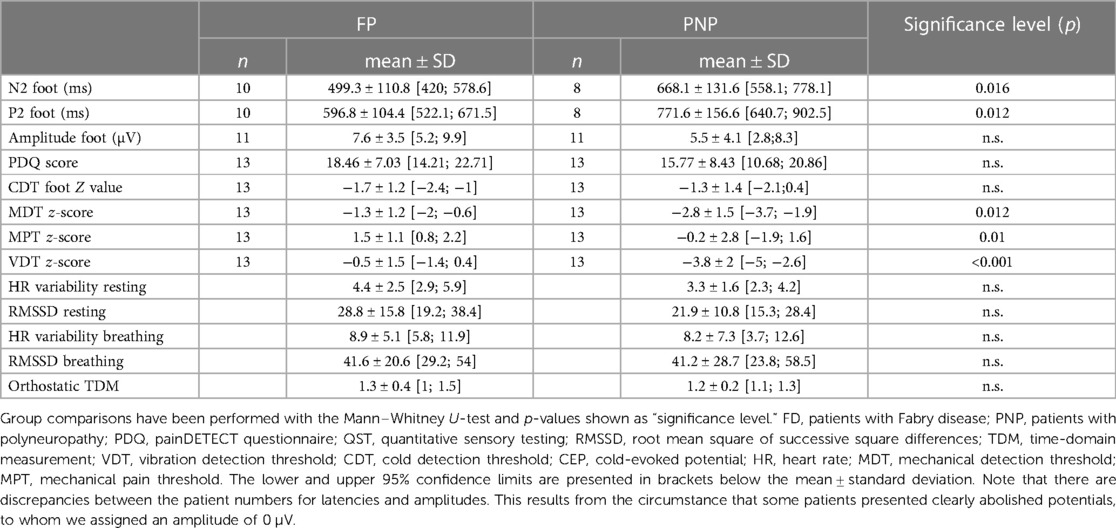
Table 3. CEP data, QST z-values, and questionnaire scores for each patient group after age matching.
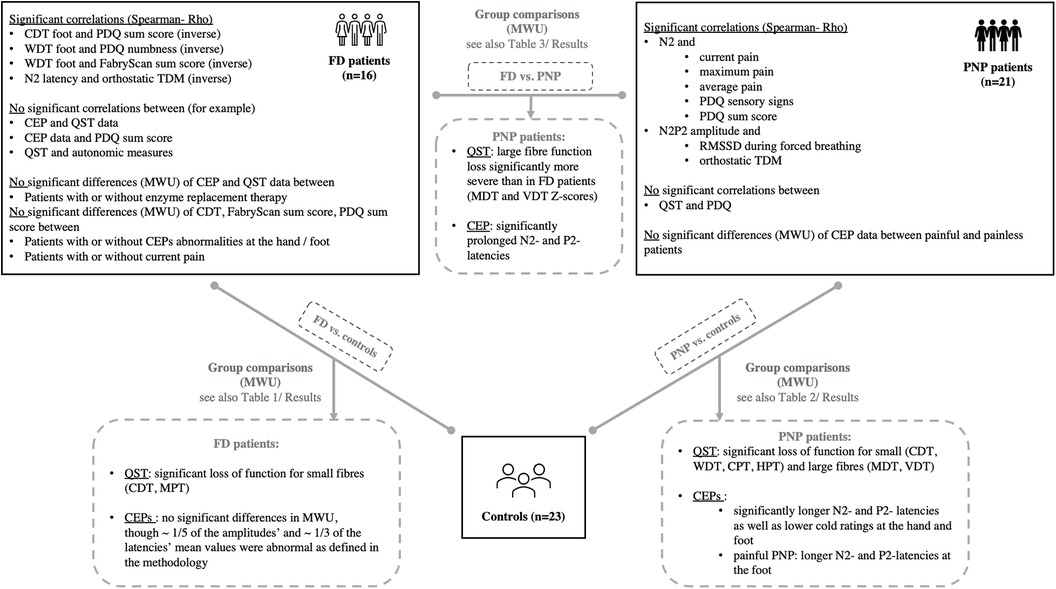
Figure 1. Overview of the study population (controls, PNP and FD patients) and the performed statistical analyses with a summary of significant results after correction for multiple testing. For comprehensive data, see the Results section of the manuscript. CDT, cold detection threshold; CEP, cold-evoked potential; CPT, cold pain threshold; HPT, heat pain threshold; FD, Fabry disease; MDT, mechanical detection threshold; MWU, Mann–Whitney U; PDQ, painDETECT questionnaire; PNP, polyneuropathy; TDM, time-domain measurement; QST, quantitative sensory testing; WDT, warm detection threshold; VDT, vibration detection threshold.
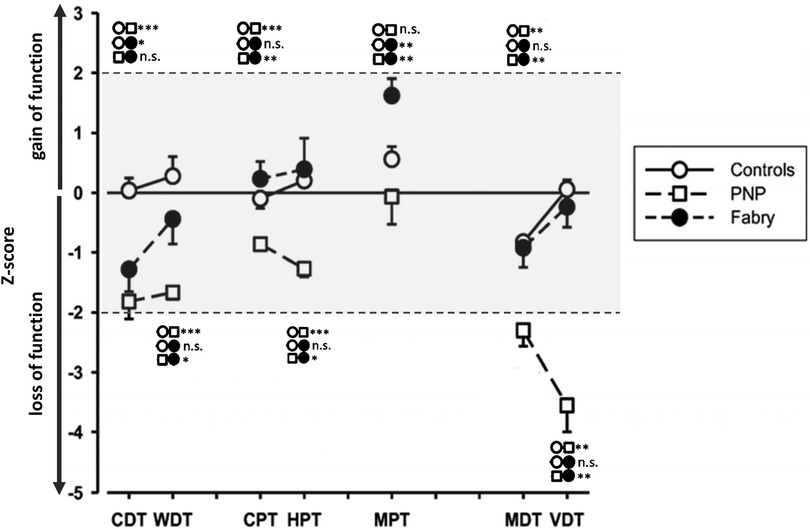
Figure 2. Quantitative sensory testing (QST) profiles of controls and PNP and FD patients. The asterisks indicate significant group differences; *** ≙ p < 0.001; ** ≙ p < 0.01; * ≙ p < 0.05; group symbols are indicated on the right side of the figure. FD patients exhibit a loss of Aδ-function, while the PNP group exhibits a wide range of dysfunction with functional loss of small and large fibers. The controls are within the normative range of Z-values. CDT, cold detection threshold; WDT, warm detection threshold; CPT, cold pain threshold; HPT, heat pain threshold; MPT, mechanical pain threshold; MDT, mechanical detection threshold; VDT, vibration detection threshold; FD, Fabry disease; PNP, polyneuropathy.
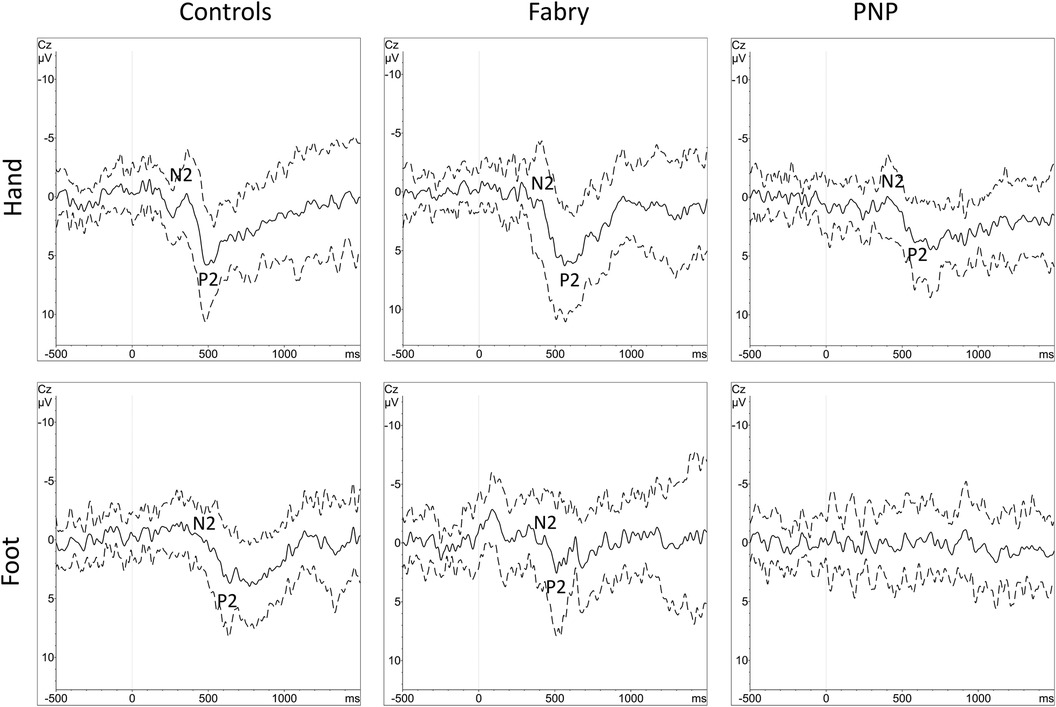
Figure 3. Grand averages of CEPs with standard deviations. Grand averages of CEPs derived from the hand and foot are displayed for each group (controls, FD and PNP patients). The black line equals the averaged EP of each cohort, the dashed line equals one standard deviation of the averaged EP data. N2 and P2 markers indicate the CEP potential. Within the PNP group, at the foot, there was no identifiable grand-average potential due to heterogenous EP latencies caused by heterogenous loss of function in different individuals. CEP, cold-evoked potential; EP, evoked potential; FD, Fabry disease; PNP, polyneuropathy.
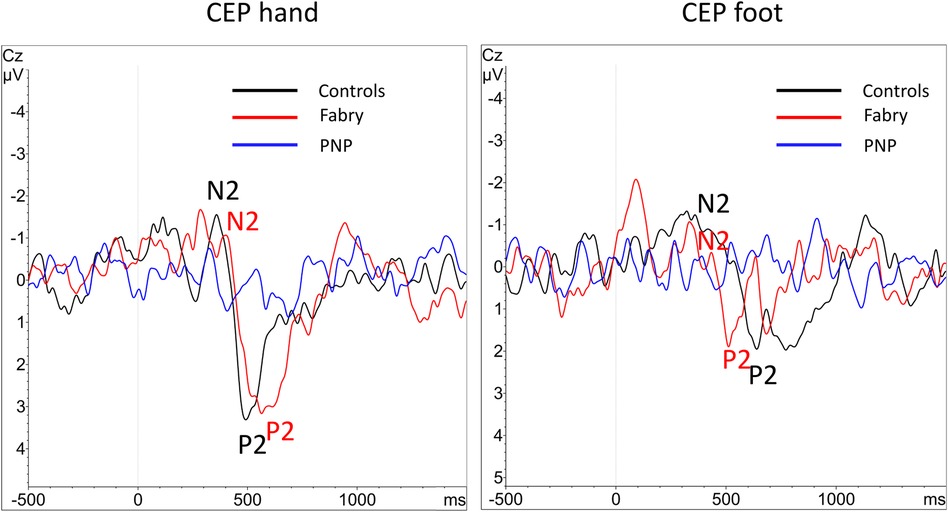
Figure 4. Overlay of grand-averaged CEPs. After performing the grand average of the CEP data there were no visible averaged CEPs within the PNP group at the hand and foot, due to the heterogeneity of normal and abnormal values. N2 and P2 markers indicate the CEP potential where applicable. CEP, cold-evoked potential; EP, evoked potential; PNP, polyneuropathy.
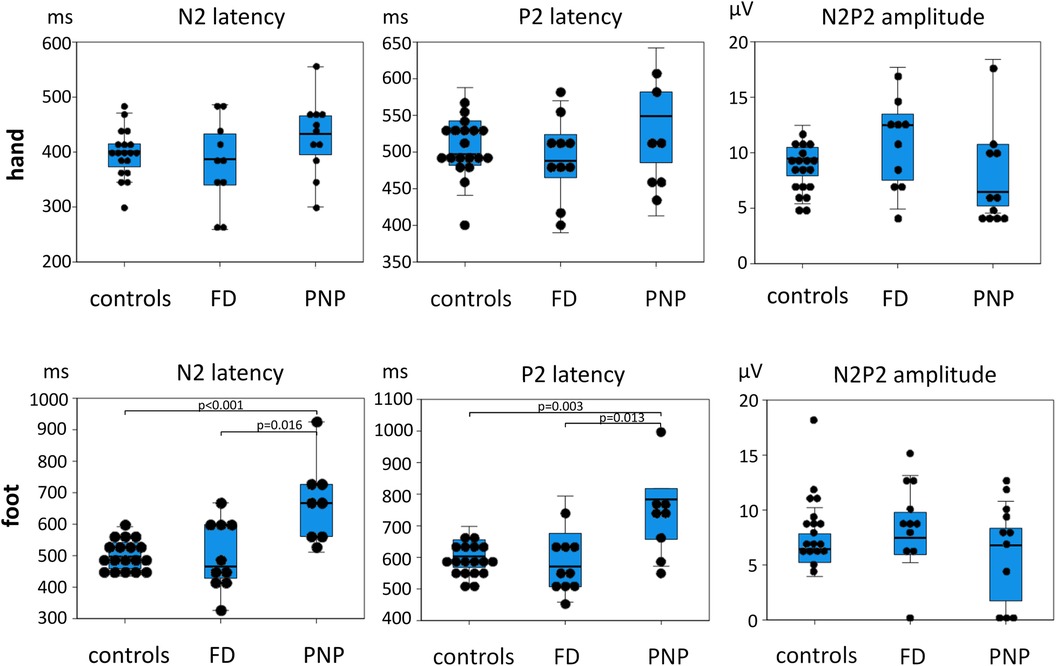
Figure 5. Dot blot and box blot of CEP data. N2 and P2 latencies as well as N2P2 amplitudes of controls and FD and PNP patients are shown here. The dots indicate individual patient data. The box blots indicate minimum and maximum values (T-bars) first quartile, median, and third quartile. There is a strong latency difference between PNP patients and controls, but not between FD patients and controls, supporting that PNP patients exhibit a more severe small fiber dysfunction than FD patients (see also Figure 2). FD, Fabry disease; PNP, polyneuropathy.
As proof of concept, we correlated N2 latency and height, which showed a significant positive correlation, i.e., the taller the subject, the longer the latency (r = 0.578; p = 0.003). The correlation analysis of both the autonomic measures (i.e., RMSSDb and orthostatic TDM) showed a significant positive correlation (r = 0.0626; p = 0.000035), confirming their coherent informative value.
Figure 6 shows the scatter plots for the significant QST correlations (after correction for multiple testing) and Figure 7 for the CEP correlations within all patients combined.
QST and pain [CDT, warm detection threshold (WDT), mechanical detection threshold (MDT), vibration detection threshold (VDT), mechanical pain threshold (MPT), current pain, max pain, average pain]
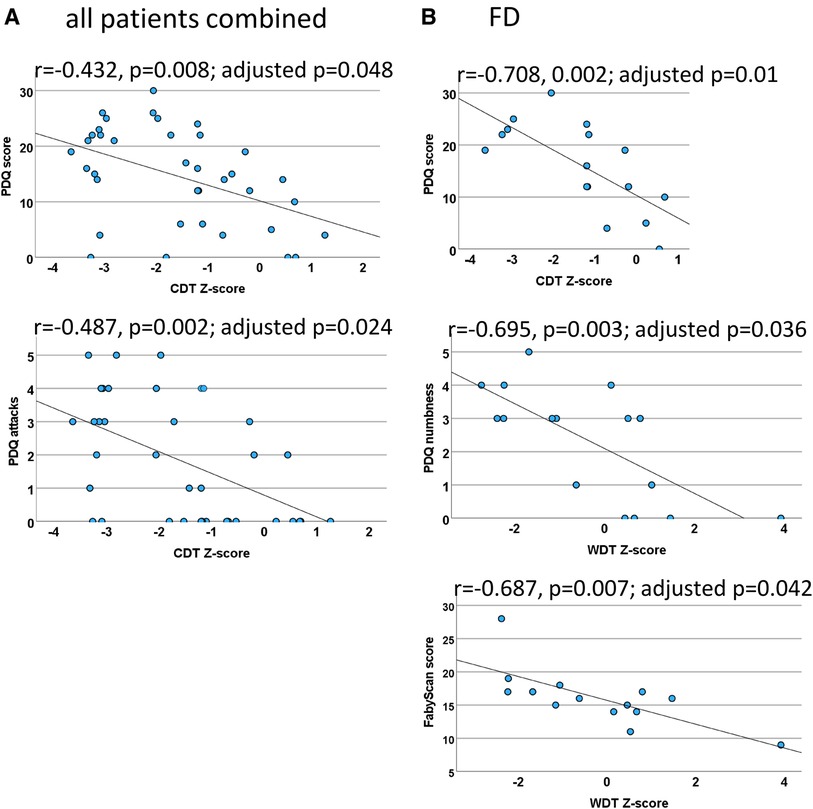
Figure 6. Quantitative sensory testing (QST) correlations. Scatter plots for the significant QST correlations (which withstood correction for multiple testing) within both patient groups combined (A) and FD patients (B). In the case of the PNP patients, the calculations did not withstand correction for multiple testing. (A) The correlation between CDT and the PDQ score indicates that a loss of cold fiber function is associated with a neuropathic pain (NP) component and the sensory symptom of pain attacks. (B) Cold fiber function loss is strongly correlated with NP in FD patients. The loss of C-fiber function was associated with higher ratings in the FabryScan and a stronger sensation of numbness. CDT, cold detection threshold; WDT, warm detection threshold; QST, quantitative sensory testing; FD, Fabry disease; PNP, polyneuropathy; PDQ, painDETECT questionnaire.
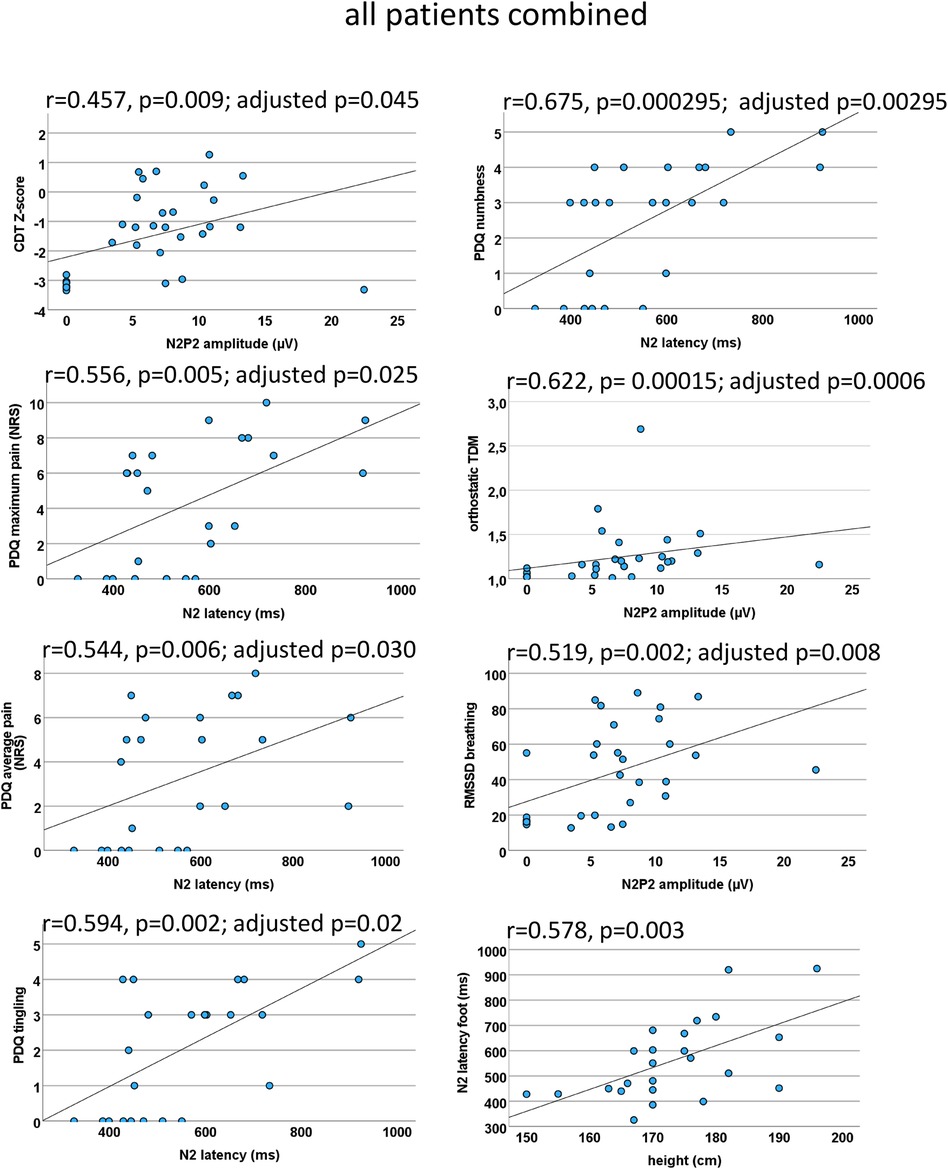
Figure 7. EP correlations within all patients combined. Scatter plots for the significant CEP correlations (which withstood correction for multiple testing) within both patient groups combined. A loss of function indicated by negative CDT Z-scores was associated with a CEP amplitude reduction. A prolonged N2 latency was indicative of higher average and maximum pain as well as stronger tingling sensation and numbness. Interestingly, a CEP amplitude decrease was associated with a functional loss of the autonomic nervous system. As a proof of concept, N2 correlated significantly with height (bottom right). CEP, cold-evoked potential; CDT, cold detection threshold; NRS, numeric rating scale; PDQ, painDETECT questionnaire; RMSSD, root mean square of successive square differences; TDM, time-domain measurement.
After correction for multiple testing, no significant correlations were found.
QST and PDQ (CDT, WDT, MDT, VDT, MPT, PDQ sum score)
Of the abovementioned QST parameters, a significant correlation was only found between CDT at the foot and the PDQ score (r = −0.432, p = 0.008; adjusted p = 0.048), indicating that a neuropathic component is associated with a loss of function of cold-mediating fibers.
QST and painDETECT sensory items (CDT, WDT, MDT, VDT, MPT, seven sensory questions)
After correction for multiple testing, a significant correlation has only been found between CDT and the item “do you have sudden pain attacks” (inverse, r = −0.487, p = 0.002; adjusted p = 0.024).
CEP and QST items (N2 latency, N2P2 amplitude, CDT, VDT, MPT)
We found a correlation between the CEP amplitude CDT (r = 0.457, p = 0.009; adjusted p = 0.045) and the MPT (r = 0.497, p = 0.01; adjusted p = 0.05), which did not reach valid significance after correction for multiple testing.
CEP and pain (N2 latency, N2P2 amplitude, current pain, max pain, average pain)
There was a moderate correlation between N2 latency and current pain (r = 0.508, p = 0.011; adjusted p = 0.055 not reaching significance), maximum pain (r = 0.556; p = 0.005; adjusted p = 0.025), and average pain (r = 0.544, p = 0.006; adjusted p = 0.030).
CEP and painDETECT (N2 latency, N2P2 amplitude, seven sensory questions, PDQ sum score)
When analyzing the seven PDQ questions separately, we found a significant correlation of the N2 latency with the painDETECT question “do you have a tingling sensation” (r = 0.594, p = 0.002; adjusted p = 0.02) and “do you suffer from a sensation of numbness”(r = 0.675, p = 0.000295; adjusted p = 0.00295). After correction for multiple testing, no other significant correlations were found.
CEP and autonomic measures (N2 latency, N2P2 amplitude, RMSSDb, orthostatic TDM)
The N2P2 amplitude correlated with RMSSD during breathing (r = 0.519, p = 0.002; adjusted p = 0.008) and with the orthostatic TDM (r = 0.622, p = 0.00015; adjusted p = 0.0006), indicating that patients with autonomic dysfunction also present small fiber loss of function.
There were no significant differences in CEP and QST data between patients with and without enzyme replacement therapy.
Abnormal z values were detected for CDT in 5/16 (31.3%), cold pain threshold (CPT) in 0/16, WDT in 5/15 (31.3%), heat pain threshold (HPT) in 4/16 (25%), VDT in 4/16 (25%), MDT in 5/16 (31.3%), and finally MPT in 6/16 (37.5%) patients. Compared to our healthy cohort, the MWU indicated a highly significant difference for the CDT (FD −1.29 ± 1.37 vs. controls 0.038 ± 0.99; p = 0.003) and MPT (FD 1.51 ± 1.06 vs. controls 0.49 ± 0.94; p = 0.006) at the foot.
Thirteen data sets (81.25%) of the hand and 14 data sets (87.5%) of the foot complied with our inclusion criteria for the statistical analysis. Out of these datasets, 38.5% of the N2 latencies, 23.1% of the P2 latencies, and 23.1% of the amplitudes of the hand dorsum were abnormal as defined in the experimental procedures. At the foot, 28.6% of the N2 and P2 latencies and 21.4% of the amplitudes were abnormal. In our analysis putting patient EP data into perspective with age-matched controls, the MWU revealed no significant differences. FD patients reported a mean cold rating of 2.6 (±2.27 SD) at the foot and 2.9 (±1.95 SD) at the hand dorsum. Their cold ratings were not significantly different from the controls. See also Table 2 for the descriptive statistics.
Figure 6 shows the scatter plots for the significant QST correlations (after correction for multiple testing) and Figure 8 for the CEP correlations within FD patients.
QST and pain (CDT, WDT, MDT, VDT, MPT, current pain, max pain, average pain)
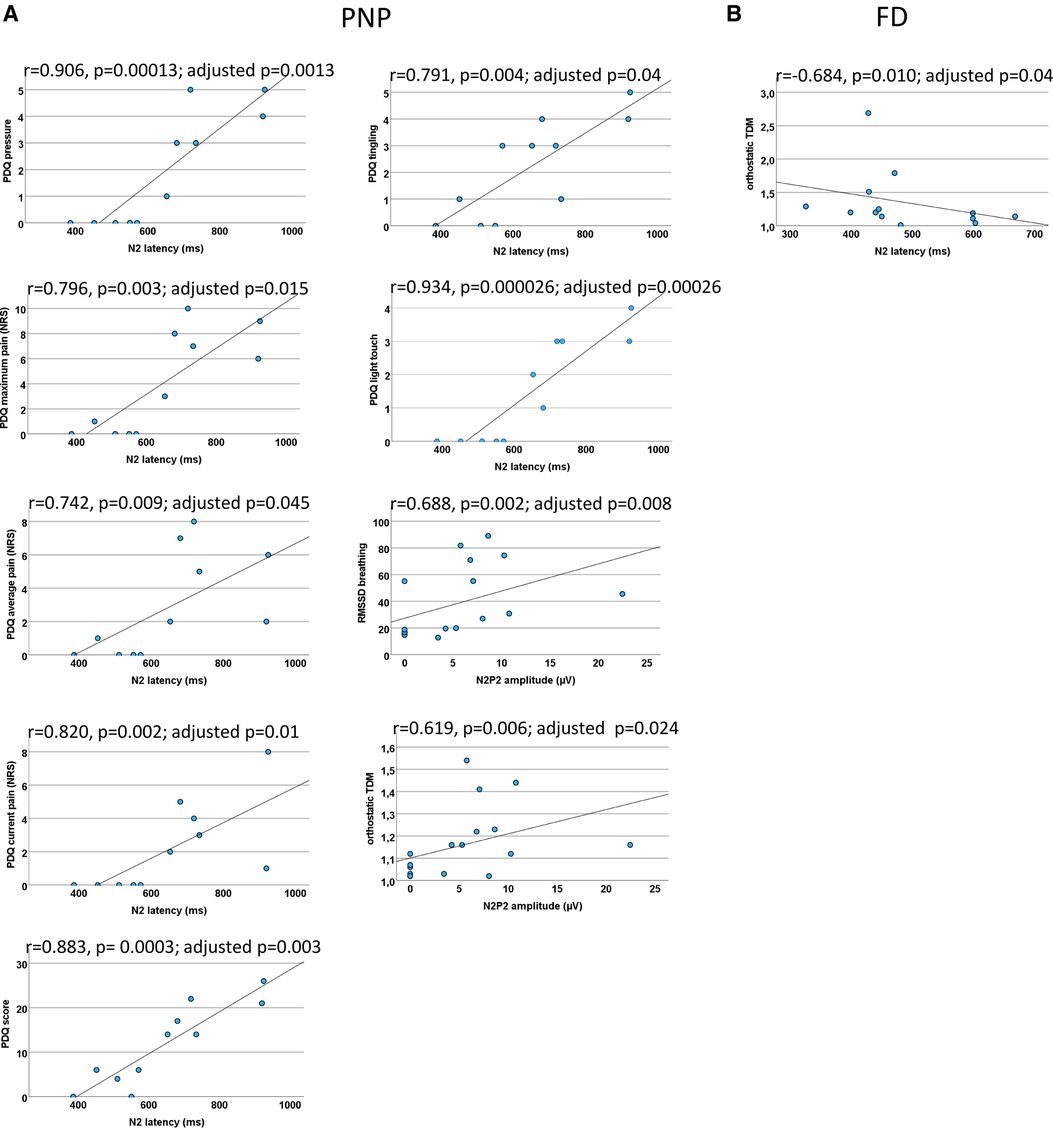
Figure 8. EP correlations for PNP and FD patients each. Scatter plots for the significant CEP correlations (which withstood correction for multiple testing) within PNP patients (A) and FD patients (B) each. (A) A prolonged N2 latency was associated with neuropathic pain (NP) and sensory symptoms (PDQ score and PDQ items). A CEP amplitude reduction was associated with a functional loss of the autonomic nervous system. (B) In FD patients, a prolonged N2 latency was associated with a functional loss of the autonomic nervous system. There were no other significant correlations. CEP, cold-evoked potential; FD, Fabry disease; NRS, numeric rating scale; PDQ, painDETECT questionnaire; PNP, polyneuropathy; RMSSD, root mean square of successive square differences; TDM, time-domain measurement.
No significant correlations were found.
QST and PDQ (CDT, WDT, MDT, VDT, MPT, PDQ sum score)
Within the Fabry group, there was a significant inverse correlation between the CDT at the foot and the PDQ score (r = −0.708, 0.002; adjusted p = 0.01).
QST and painDETECT sensory items (CDT, WDT, MDT, VDT, MPT, seven sensory questions)
After correction for multiple testing, a significant correlation has only been found between WDT and the item “do you suffer from a sensation of numbness” (r = −0.695, p = 0.003; adjusted p = 0.036).
QST and FabryScan (FabryScan score, CDT, WDT, MDT, VDT, MPT)
Within the abovementioned QST parameters, only WDT showed a significant inverse correlation (r = −0.687, p = 0.007; adjusted p = 0.042).
QST and autonomic measures (CDT, WDT, MDT, VDT, MPT, RMSSDb, orthostatic TDM)
After correction for multiple testing, no significant correlations were found.
CEP and QST items (N2 latency, N2P2 amplitude, CDT, VDT, MPT)
No significant correlations were found.
CEP and pain (N2 latency, N2P2 amplitude, current pain, max pain, average pain)
N2 latency correlated with the average pain (r = 0.679, p = 0.011; adjusted p = 0.055), just not reaching significance after correction for multiple testing.
CEP and painDETECT (N2 latency, N2P2 amplitude, seven sensory questions, PDQ score)
No significant correlations were found.
CEP and autonomic measures (N2 latency, N2P2 amplitude, RMSSDb, orthostatic TDM)
N2 correlated inversely with the results of the orthostatic TDM (r = −0.684, p = 0.010; adjusted p = 0.04), indicating that FD patients with autonomic dysfunction were associated with abnormal small fiber function.
Of our 13 FD patients whose CEP data of the hand were included in the statistical analysis, 5 presented abnormal N2 latencies and 3 abnormal amplitudes. Of the 14 patients whose data of the foot were included, 4 presented abnormal N2 latencies and 3 abnormal amplitudes. Eleven FD patients presented current pain of numeric rating scale (NRS) ≥1, and three FD patients presented no current pain (NRS = 0). The subgroup analyses comparing the patient groups with or without CEP abnormalities at the hand or foot, or with or without current pain, showed no significant differences in terms of the total score of the FabryScan, PDQ score, or the pain items or the CDT.
Mirroring the PNP, abnormal z-values were detected for CDT in 11/21 (52.4%), WDT in 5/21 (23.81%), CPT in none, HPT in 3/21 (14.3%), VDT in 18/21 (85.7%), MDT in 14/21 (66.7%), and finally MPT in 6/21 (28.6%) patients. Between age-matched PNP patients and controls, the MWU showed a significant difference in CDT (p = 0.011), WDT (p = 0.002), CPT (p = 0.006), HPT (p = 0.016), and finally MDT and VDT (p < 0.001 each).
Sixteen out of 21 CEP data sets from the hand (76.2%, one exhibiting entirely abolished CEPs) and 18 out of 21 from the foot (85.7%, seven of them presenting entirely abolished CEPs) met our inclusion criteria for statistical analysis. After age matching, 11 data sets (52.4%) remained for analysis of the hand and foot, respectively. For the hand, 54.5% of the N2 and P2 latencies each and 63.6% of the N2P2 amplitudes were abnormal. For the foot, 87.5% of the N2 latencies, 75% of the P2 latencies, and 36.4% of the N2P2 amplitudes were abnormal. The MWU showed a significant difference between PNP patients and controls for the N2 (p < 0.001) and P2 (p = 0.004) latency. PNP patients reported a mean cold rating of 1.36 (±1.75 SD) at the foot and 2.07 (±1.47 SD) at the hand. In the age-matched comparisons, their cold ratings were significantly different (i.e., lower) from the controls both at the foot (p = 0.005) and the hand dorsum (p = 0.009). See also Table 2 for the descriptive statistics.
Figure 8 shows the scatter plots for the significant CEP correlations (after correction for multiple testing) within PNP patients.
QST and pain (CDT, WDT, MDT, VDT, MPT, current pain, max pain, average pain)
No significant correlations were found.
QST and PDQ (CDT, WDT, MDT, VDT, MPT, PDQ sum score)
No correlations were found between QST and the PDQ sum score.
QST and painDETECT sensory items (CDT, WDT, MDT, VDT, MPT, seven sensory questions)
After correction for multiple testing, no significant correlations were identified.
QST and autonomic measures (CDT, WDT, MDT, VDT, MPT, RMSSDb, orthostatic TDM)
After correction for multiple testing, no significant correlations were identified.
CEP and QST items (N2 latency, N2P2 amplitude, CDT, VDT, MPT)
A correlation between the N2P2 amplitude of the foot and CDT was observed but did not withstand correction for multiple testing (r = 0.558, p = 0.016; adjusted p = 0.08). No other significant correlations were found.
CEP and pain (N2 latency, N2P2 amplitude, current pain, max pain, average pain)
N2 significantly correlated with current pain (r = 0.820, p = 0.002; adjusted p = 0.01), maximum pain (r = 0.796, p = 0.003; adjusted p = 0.015), and average pain (r = 0.742, p = 0.009; adjusted p = 0.045).
CEP and painDETECT (N2 latency, N2P2 amplitude, seven sensory questions, PDQ score)
The N2 latency correlated significantly with the painDETECT questions “is light touch painful” (r = 0.934, p = 0.000026; adjusted p = 0.00026), “does slight pressure trigger pain” (r = 0.906, p = 0.00013; adjusted p = 0.0013), and “do you have a tingling sensation” (r = 0.791, p = 0.004, adjusted p = 0.04) and finally with the painDETECT sum score (r = 0.883, p = 0.0003; adjusted p = 0.003). All other results did not withstand correction for multiple testing.
CEP and autonomic measures (N2 latency, N2P2 amplitude, RMSSDb, orthostatic TDM)
The N2P2 amplitude correlated significantly with RMSSD during forced breathing (r = 0.688, p = 0.002; adjusted p = 0.008) and orthostatic TDM (r = 0.619, p = 0.006; adjusted p = 0.024).
The comparison of painful (n = 16) vs. painless (n = 5) PNP patients showed no significant differences in latencies or amplitudes. When compared to age-matched controls (n = 19), patients with painful PNP (n = 8) exhibited significantly longer N2 and P2 latencies (p < .001 each) at the foot.
As shown in Table 3 and Figure 5, PNP patients exhibited significantly longer CEP latencies (indicating a loss of small fiber function) and a more pronounced loss of large fiber function (MDT, VDT) compared to the FD group. Interestingly, MPT was in the gain range of the QST Z-scores in FD patients and the loss range in PNP patients.
The main goals of this study were to test the feasibility of CEPs in two patient groups and to identify clinical or sensory features associated with CEP abnormalities. By presenting data of patients with FD, a typical small fiber disorder, and patients with PNP, a typical mixed fiber condition, we hereby contribute new insights to the controversy of the clinical usefulness of CEPs. We found (1) a consistent informative quality in neurophysiological (i.e., CEPs) and psychophysical (i.e., QST) diagnostic measures and (2) an association of CEP data with self-reported sensory symptoms and a NP component, as reflected by the PDQ and finally (3) an association of CEP data (but not QST) with autonomic measures in both patient groups. The results are encouraging regarding the use of CEPs as an electrophysiological tool to detect NP in clinical practice, which will be discussed further below.
As Figure 8 shows, these associations varied among our patient groups. Although the functional loss of cold-mediating fibers is reported as a typical feature of FD (18, 20, 21), this loss of cold fiber function was more pronounced in our PNP group, suggesting an overall disease progression in this patient cohort with dysfunction of large and small fibers. The functional loss within the cold-mediating small fibers in PNP patients was reflected by significantly prolonged CEP latencies as well as in the QST profile. For instance, 52% of the PNP patients exhibited abnormal CDT and 86% abnormal VDT, compared to only 31% of FD patients with abnormal CDT and 25% with abnormal VDT. In summary, the QST results for the PNP group pointed to a somatosensory nervous system with more severe dysfunction, especially within the large fiber range—a finding consistent with the expected characteristic of PNP as noted in previous studies (4, 7).
Through the correlation analyses and group comparisons with CEP and QST data, patient-reported outcome measures (PROMs), and autonomous measures, several interesting observations were made as follows:
1) CEP amplitudes showed a significant correlation with the CDT when including all patients in the analysis, i.e., a reduced N2P2 amplitude was indicative of a loss of function as detected by the CDT within the QST protocol. This finding illustrates CEPs' capability to assess small fiber neuropathy through an impaired function of cool-sensitive Aδ fibers. These results are consistent with previous studies using noxious stimuli to examine NP in patients with FD [i.e., pain-related evoked potentials (PREPs) and LEPs (55, 56)]. In our cohort, when analyzing FD patients alone, there were no significant correlations between the CEP data and the QST items. The absence of correlation may be explained by a more intact sensory nervous system in FD patients compared to the PNP patients, or it could suggest that sensory function in FD patients varies more dynamically depending on pain exacerbations.
CEP latencies correlated significantly with current, maximum, and average pain, when including all patients in the analysis or when analyzing PNP patients alone. Even in the smaller FD group, there was a significant correlation with the average pain (notwithstanding correction for multiple testing). This finding is particularly noteworthy, as the functional loss of cold-mediating fibers appears to be indicative of the development of (chronic) pain (57–59). However, due to the small cohort, it is not possible to conclude mechanistic conclusions about ongoing spontaneous NP from our data and further studies with larger patient cohorts are necessary. Nonetheless, the following considerations can be made: CEPs allow for the examination of specialized, cool-sensitive Aδ- and C-fibers (60–62) and their central pathways (26, 28, 30, 31). While the conduction velocity offers insights into the state of the myelin sheaths, changes in the amplitudes can be due to axonal dysfunction or, as shown for nociceptive pathways (63), sensitization processes. Our findings illustrate how small fiber damage, reported by PROMs and assessed through psychophysiological methods, can indeed be mirrored by non-noxious, quantifiable electrophysiological parameters as shown here by CEP latencies and CEP amplitudes. A prolonged latency could result from distal loss of peripheral thermosensory input or perhaps the desynchronization of ascending information (64). In this case, the positive correlation of CEP latencies of the PNP group with the reported pain intensity suggests that the same mechanisms leading to an impairment of specialized, cool-sensitive Aδ-fibers will also affect nociceptors. Furthermore, our CEP data seem to support the mechanistic approach of central disinhibition pain that is thoroughly discussed in the review by Forstenpointner et al. (65) and comprises the disbalance between the lateral and the medial pain pathway, two phylogenetically different pain processing systems. An impaired Aδ fiber function may have an “unmasking” effect on the nociceptive C-fibers in our PNP group, notably where large fibers exhibited pronounced dysfunction.
2) CEP latencies showed a certain association with somatosensory signs and symptoms. The PDQ contains two questions reflecting dynamic mechanical allodynia [proposed as an important clinical marker for central sensitization (66)]: “is light touch painful (…)?” and “does slight pressure trigger pain (…)?”. We found a highly significant correlation between these items and N2 latency within the PNP cohort (see Figure 8). These findings may encourage further studies to explore a possible link between abnormal CEP latencies and the presence of central sensitization in PNP patients.
In this patient group, the CEP latency also correlated strongly with the item “do you have a tingling sensation” and the painDETECT sum score, suggesting an association of prolonged CEP latencies with a higher likelihood of the presence of an NP component [note: the higher the PDQ score, the higher the likelihood of NP (52)] and the occurrence of pain in this group. This is further supported by a subgroup analysis, showing that PNP patients with current pain presented significantly longer N2 latencies than those in age-matched controls, while the CEP data of painless PNP patients did not differ significantly from age-matched controls. These results, however, must be interpreted with caution due to the small number of painless PNP patients (n = 3). Nonetheless, our results are in line with previous reports on altered N2 latencies in NP conditions assessed through other evoked potentials (67, 68). In a previous study, we demonstrated how the N2 latency of LEPs in radiculopathy patients correlated with pain intensity and clinical severity in the affected dermatome (69). The FD group on the other side showed no correlation of CEP data with the items of the PDQ assessing sensory signs and symptoms, emphasizing the value of QST and how the examined methods are not interchangeable.
3) Abnormal CEPs indicated abnormal autonomic small fiber function, possibly leading to direct diagnostic consequences for further assessment and possible therapeutic implications. The analysis of the entire patient cohort and the PNP group alone revealed a significant correlation between the N2P2 amplitude of the foot and both the RMSSDb and orthostatic TDM. Similarly, the analysis of the FD group revealed an inverse correlation between the N2 latency and the orthostatic TDM. These findings consistently suggest that the electrophysiological integrity of intact cold-mediating fibers is linked to the functional integrity of the autonomic nervous system. In other words, a low CEP amplitude indicates a loss of function of the cold-mediating fibers, and lower RMSSD and TDM scores indicate abnormal parasympathetic/autonomic small fiber function, possibly with a direct diagnostic consequence of further assessment thereof and potential therapeutic implications. This correlation aligns with previous reports on the simultaneous involvement of somatic and autonomic small fibers in autonomic neuropathies (70). Although Thaisetthawatkul et al. (71) reported that somatic and autonomic small fibers require independent and complementary measures, their conclusion relied upon data acquired by QST. In this study, we have now been able to show that CEPs might provide information not only about cold-mediating fibers and their central pathways but, indirectly, also about the status of autonomic small fibers. In their consensus statement on the electrodiagnostic assessment of the autonomic nervous system, Cheshire et al. (72) stated that the “evaluation of disorders of the autonomic nervous system is both an art and a science, calling upon the physician's most astute clinical skills as well as knowledge of autonomic neurology and physiology.” Our findings propose that abnormal CEPs may signal abnormal autonomic function, warranting further autonomic diagnostic tests upon detecting abnormal CEP parameters.
4) Our correlation analyses with the QST data showed differing results for FD and PNP patients. In patients with FD, the significant inverse correlation between WDT and the FabryScan suggested an association between loss of function of warm-mediating small fibers and an increased likelihood of screening positive for FD. Similarly, we observed an association between a functional loss of cold-mediating small fibers and an increased probability of having a NP component (see also Figures 1, 6). Notably, these associations did not emerge among PNP patients. The self-reported pain intensities (current, maximum, and average pain) in neither the FD nor PNP patients were linked with QST parameters except for WDT and the item “do you suffer from a sensation of numbness” in FD patients. These findings seem to support a potential beneficiary capacity of CEPs, mirroring clinical indicators of chronic pain and providing insight into the cold-mediating pathways.
Interestingly, the MPT significantly varied between the FD and PNP groups, a metric associated with central sensitization (73, 74). The FD group's MPT fell within the Z-score's gain range, whereas the PNP group's MPT was in the loss range, indicating a pronounced sensory loss (and a progressed chronic state of the disease) in the PNP group, while FD patients showed stronger signs of central sensitization. A similar observation was observed previously in patients with painful radiculopathy, where signs of central sensitization were present in the early stages of the disease, which then were replaced by functional loss of the pain-mediating nerve fibers with disease progression (69).
Despite some patients not meeting EEG quality criteria for statistical analysis, we successfully conducted CEP recording and evaluation in over 80% of participants (Table 1). To comprehensively address the posed questions, further investigation in larger patient cohorts is essential. In line with previous reports on healthy individuals, our present study shows how the examination of CEPs has its technical limitations, i.e., CEPs were not recordable in all our patients due to various reasons (see above). Particularly, examining the distal lower extremities in elderly subjects remains a challenge (31). While improvements in CEP recording could be achieved with advanced thermal stimulators (featuring steep cooling ramps and low target temperatures (35, 75), for now, only the Medoc thermal stimulator possesses a CE certificate as a diagnostic tool on patients in clinical routine, facilitating their application in both research and clinical diagnostics.
As described above, early FD detection is crucial to prevent disease progression, emphasizing the need for diagnostic precision in identifying early-phase small fiber dysfunction. This is a notable challenge in clinical practice. Beyond QST and CEPs, various research groups focused on microneurography to gain a readout of specific sub-types of sensory nerve fibers (76–78). This method also allowed the description of a subset of cold-mediating C-fibers (79–81). Due to its time-consuming nature, its availability is only limited and unfit for routine usage in patients. Thus, microneurography remains a tool of few, specialized research centers. Currently, the gold standard for the electrophysiological assessment of small fibers is LEPs (3, 32). Both LEPs and contact-heat evoked potentials (CHEPs) (69, 82–85), allow an examination of the thermo-nociceptive nervous system by visualizing the pain-related brain potentials within the EEG (86). The quantifiable and reliable feature assigns these tools a potentially decisive stance for the classification of a given pain syndrome as “definitely neuropathic” (25, 87). Unfortunately, though, LEPs also failed to reach a broad clinical utilization partly due to potential skin damage and necessary safety precautions. This is where CEPs might step in: The advantages of CEP assessment as a non-invasive method to measure small fibers and their central pathways are comparable to those of LEPs, but with the upside of a pain-free examination. A study on the conduction velocity of the cold spinal pathway even suggested that CEPs may represent an alternative to LEPs (75).
Although we are not quite there yet, the appeal of electrophysiological spinothalamic tract examinations without painful stimuli is particularly attractive for sensitive or hyperalgesic skin areas in clinical settings, though further advancements are needed to integrate CEPs into routine electrophysiological diagnostics, necessitating enhanced CEP paradigms across diverse NP conditions and robust normative data collection. Notably, our results point to the capacity of CEPs to indirectly assess the status of autonomic small fibers. This observation is worth examining in further studies.
CEPs were successfully obtained from patients with NP and correlated with both QST results and PROMs. As expected, the application of CEPs and QST revealed that the somatosensory system of the PNP group was more severely affected by functional loss compared to the FD group. Moreover, abnormal CEPs, unlike QST, were associated with dysfunctional autonomic nervous system function in both FD and PNP patients. Thus, abnormal CEPs may indicate neuropathic and/or chronic pain conditions and could prompt further diagnostic actions (e.g., autonomic diagnostics), although additional studies are necessary for confirmation. It is worthwhile to further improve CEP paradigms to make CEP accessible to all patients. This may be achieved by modern cold stimulators with steep temperature ramps, so far lacking a CE certificate for a diagnostic and clinical purpose. In summary, CEPs hold significant potential as a diagnostic adjunct for NP.
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
The study was approved by the Ethics Committee of the University Hospital of Kiel (study protocol number: A 101/15) and registered in the German Clinical Trials Register (DRKS00009013). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
DK: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization. MS: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization. JL: Writing – original draft, Investigation, Formal Analysis, Data curation. S-CF: Writing – original draft, Investigation, Formal Analysis, Data curation. JF: Writing – original draft, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization. MR: Writing – original draft, Formal Analysis, Conceptualization. SC-K: Writing – original draft, Investigation, Data curation. JGa: Writing – original draft, Investigation, Data curation. SR: Writing – original draft, Supervision, Investigation, Data curation, Conceptualization. JGi: Writing – original draft, Supervision, Formal Analysis, Conceptualization. RB: Conceptualization, Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Funding acquisition. PH: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Methodology, Funding acquisition, Formal Analysis, Data curation, Conceptualization.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This research was supported by Medoc and Sanofi. The funding source was not involved in study design, data collection, data analysis, manuscript preparation, and publication decisions.
DK reports a grant, non-financial support, and a personal fee for a podcast episode from Grünenthal GmbH outside this study. MS is a consultant for Takeda Pharmaceutical and Merz Pharma; she reports personal fees from Alnylam Pharmaceuticals, Sanofi Genzyme, Grünenthal GmbH, Amicus Therapeutics, and Akcea, outside the submitted work. JL has received personal fees from Pfizer OFG Germany GmbH. S-CF reports grants from Grünenthal GmbH, during the conduct of this study as well as personal fees from Grünenthal GmbH outside the submitted work (speaker fees). Furthermore, she received financial support from Pfizer OFG Germany GmbH (personal fees). JF reports a grant (FO 1311/1-1) from the German Research Foundation (DFG); personal fees and non-financial support from Grünenthal GmbH and Sanofi Genzyme GmbH, personal fees from Bayer, non-financial support from Novartis, outside the submitted work. SC-K has received honoraria from Amicus Therapeutics, Chiesi Farmaceutici, Sanofi, and Takeda Pharmaceuticals. JGa reports grants from Amicus Therapeutics, Takeda Pharmaceuticals, and Sanofi Genzyme. JGi reports speaker fees from TAD Pharma, Insignia, Lilly GmbH, and Neurotech GmbH; consultant fees from Omega Pharma and Certkom; and travel support from Novartis, Lilly GmbH and Teva, outside the submitted work. RB reports grants/research support [EU Projects: “Europain“ (115007). DOLORisk (633491), IMI Paincare (777500), German Federal Ministry of Education and Research (BMBF): Verbundprojekt: Frühdetektion von Schmerzchronifizierung (NoChro) (13GW0338C), German Research Network on Neuropathic Pain (01EM0903), Pfizer Pharma GmbH, Genzyme GmbH, Grünenthal GmbH, Mundipharma Research GmbH und Co. KG., Novartis Pharma GmbH, Alnylam Pharmaceuticals Inc., Zambon GmbH, Sanofi-Aventis Deutschland GmbH]; speaker fees (Pfizer Pharma GmbH, Genzyme GmbH, Grünenthal GmbH, Mundipharma, Sanofi Pasteur, Medtronic Inc. Neuromodulation, Eisai Co. Ltd., Lilly GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Astellas Pharma GmbH, Desitin Arzneimittel GmbH, Teva GmbH, Bayer-Schering, MSD GmbH, Seqirus Australia Pty. Ltd., Novartis Pharma GmbH, TAD Pharma GmbH, Grünenthal SA Portugal, Sanofi-Aventis Deutschland GmbH, Agentur Brigitte Süss, Grünenthal Pharma AG Schweiz, Grünenthal B.V. Niederlande, Evapharma, Takeda Pharmaceuticals International AG Schweiz, Ology Medical Education Netherlands, Ever Pharma GmbH, Amicus Therapeutics GmbH); and consultant fees (Pfizer Pharma GmbH, Genzyme GmbH, Grünenthal GmbH, Mundipharma Research GmbH und Co. KG, Allergan, Sanofi Pasteur, Medtronic, Eisai, Lilly GmbH, Boehringer Ingelheim Pharma GmbH&Co. KG, Astellas Pharma GmbH, Novartis Pharma GmbH, Bristol Myers Squibb, Biogenidec, AstraZeneca GmbH, Merck, Abbvie, Daiichi Sankyo, Glenmark Pharmaceuticals S.A., Seqirus Australia Pty. Ltd., Teva Pharmaceuticals Europe Niederlande, Teva GmbH, Genentech, Mundipharma International Ltd. UK, Astellas Pharma Ltd. UK, Galapagos NV, Kyowa Kirin GmbH, Vertex Pharmaceuticals Inc., Biotest AG, Celgene GmbH, Desitin Arzneimittel GmbH, Regeneron Pharmaceuticals Inc. USA, Theranexus DSV CEA Frankreich, Abbott Products Operations AG Schweiz, Bayer AG, Grünenthal Pharma AG Schweiz, Mundipharma Research Ltd. UK, Akcea Therapeutics Germany GmbH, Asahi Kasei Pharma Corporation, AbbVie Deutschland GmbH & Co. KG, Air Liquide Sante International Frankreich, Alnylam Germany GmbH, Lateral Pharma Pty Ltd., Hexal AG, Angelini, Janssen, SIMR Biotech Pty Ltd. Australien, Confo Therapeutics N. V. Belgium, Merz Pharmaceuticals GmbH, Neumentum Inc., F. Hoffmann-La Roche Ltd. Switzerland, AlgoTherapeutix SAS France). PH reports grants from BMBF, Medoc, and Zambon outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2024.1352711/full#supplementary-material
1. Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. (2017) 158:261–72. doi: 10.1097/J.PAIN.0000000000000753
2. Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. (2014) 155:2263–73. doi: 10.1016/J.PAIN.2014.08.014
3. Garcia-Larrea L, Hagiwara K. Electrophysiology in diagnosis and management of neuropathic pain. Rev Neurol (Paris). (2019) 175:26–37. doi: 10.1016/j.neurol.2018.09.015
4. Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1,236 patients with different neuropathic pain syndromes. Pain. (2010) 150:439–50. doi: 10.1016/J.PAIN.2010.05.002
5. Verdugo RJ, Matamala JM, Inui K, Kakigi R, Valls-Solé J, Hansson P, et al. Review of techniques useful for the assessment of sensory small fiber neuropathies: report from an IFCN expert group. Clin Neurophysiol. (2022) 136:13–38. doi: 10.1016/J.CLINPH.2022.01.002
6. Vollert J, Kramer M, Barroso A, Freynhagen R, Haanpää M, Hansson P, et al. Symptom profiles in the painDETECT questionnaire in patients with peripheral neuropathic pain stratified according to sensory loss in quantitative sensory testing. Pain. (2016) 157:1810–8. doi: 10.1097/J.PAIN.0000000000000588
7. Vollert J, Maier C, Attal N, Bennett DLH, Bouhassira D, Enax-Krumova EK, et al. Stratifying patients with peripheral neuropathic pain based on sensory profiles: algorithm and sample size recommendations. Pain. (2017) 158:1446. doi: 10.1097/J.PAIN.0000000000000935
8. Eikrem Ø, Skrunes R, Tøndel C, Leh S, Houge G, Svarstad E, et al. Pathomechanisms of renal Fabry disease. Cell Tissue Res. (2017) 369:53–62. doi: 10.1007/S00441-017-2609-9/FIGURES/2
9. Kok K, Zwiers KC, Boot RG, Overkleeft HS, Aerts JMFG, Artola M. Fabry disease: molecular basis, pathophysiology, diagnostics and potential therapeutic directions. Biomolecules. (2021) 11:1–20. doi: 10.3390/biom11020271
10. Mehta A, Orteu C. Chapter 136. Fabry disease|Fitzpatrick’s dermatology in general medicine, 8e|AccessMedicine|McGraw Hill Medical. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick’s Dermatology in General Medicine. 8th ed. McGraw Hill (2012). Available online at: https://accessmedicine.mhmedical.com/content.aspx?sectionid=41138857&bookid=392 (accessed February 13, 2022).
11. Ortiz A, Germain DP, Desnick RJ, Politei J, Mauer M, Burlina A, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. (2018) 123:416–27. doi: 10.1016/j.ymgme.2018.02.014
12. Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. (2008) 105:2812–7. doi: 10.1073/PNAS.0712309105
13. Rozenfeld P, Feriozzi S. Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol Genet Metab. (2017) 122:19–27. doi: 10.1016/J.YMGME.2017.09.004
14. Shen JS, Meng XL, Moore DF, Quirk JM, Shayman JA, Schiffmann R, et al. Globotriaosylceramide induces oxidative stress and up-regulates cell adhesion molecule expression in Fabry disease endothelial cells. Mol Genet Metab. (2008) 95:163–8. doi: 10.1016/J.YMGME.2008.06.016
15. Shu L, Vivekanandan-Giri A, Pennathur S, Smid BE, Aerts JMFG, Hollak CEM, et al. Establishing 3-nitrotyrosine as a biomarker for the vasculopathy of Fabry disease. Kidney Int. (2014) 86:58–66. doi: 10.1038/KI.2013.520
16. Weidemann F, Sanchez-Niño MD, Politei J, Oliveira JP, Wanner C, Warnock DG, et al. Fibrosis: a key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis. (2013) 8:116. doi: 10.1186/1750-1172-8-116
17. Choi L, Vernon J, Kopach O, Minett MS, Mills K, Clayton PT, et al. The Fabry disease-associated lipid lyso-Gb3 enhances voltage-gated calcium currents in sensory neurons and causes pain. Neurosci Lett. (2015) 594:163–8. doi: 10.1016/J.NEULET.2015.01.084
18. Biegstraaten M, Hollak CEM, Bakkers M, Faber CG, Aerts JMFG, van Schaik IN. Small fiber neuropathy in Fabry disease. Mol Genet Metab. (2012) 106:135–41. doi: 10.1016/J.YMGME.2012.03.010
19. Burand AJ, Stucky CL. Fabry disease pain: patient and preclinical parallels. Pain. (2021) 162:1305–21. doi: 10.1097/j.pain.0000000000002152
20. Maag R, Binder A, Baron R. Assessment of pain and somatosensory function in Fabry disease: early diagnosis. Clin Ther. (2008) 30:2006–7. doi: 10.1016/S0149-2918(08)80042-2
21. Maag R, Binder A, Maier C, Scherens A, Toelle T, Treede RD, et al. Detection of a characteristic painful neuropathy in Fabry disease: a pilot study. Pain Med. (2008) 9:1217–23. doi: 10.1111/j.1526-4637.2008.00470.x
22. Lauria G, Merkies ISJ, Faber CG. Small fibre neuropathy. Curr Opin Neurol. (2012) 25:542–9. doi: 10.1097/WCO.0B013E32835804C5
23. Hilz MJ, Brys M, Marthol H, Stemper B, Dütsch M. Enzyme replacement therapy improves function of C-, adelta-, and abeta-nerve fibers in Fabry neuropathy. Neurology. (2004) 62:1066–72. doi: 10.1212/01.WNL.0000118207.84514.40
24. Dütsch M, Marthol H, Stemper B, Brys M, Haendl T, Hilz MJ. Small fiber dysfunction predominates in Fabry neuropathy. J Clin Neurophysiol. (2002) 19:575–86. doi: 10.1097/00004691-200212000-00011
25. Hüllemann P, Nerdal A, Binder A, Helfert S, Reimer M, Baron R. Cold-evoked potentials—ready for clinical use? Eur J Pain. (2016) 20:1730–40. doi: 10.1002/ejp.896
26. De Keyser R, van den Broeke EN, Courtin A, Dufour A, Mouraux A. Event-related brain potentials elicited by high-speed cooling of the skin: a robust and non-painful method to assess the spinothalamic system in humans. Clin Neurophysiol. (2018) 129:1011–9. doi: 10.1016/j.clinph.2018.02.123
27. Leone C, Dufour A, Di Stefano G, Fasolino A, Di Lionardo A, La Cesa S, et al. Cooling the skin for assessing small-fibre function. Pain. (2019) 160:1967–75. doi: 10.1097/J.PAIN.0000000000001584
28. Hüllemann P, Nerdal A, Sendel M, Dodurgali D, Forstenpointner J, Binder A, et al. Cold-evoked potentials versus contact heat-evoked potentials—methodological considerations and clinical application. Eur J Pain. (2019) 23:1209–20. doi: 10.1002/ejp.1389
29. Lithfous S, Trocmet L, Pebayle T, Després O, Dufour A. Investigating cold Aδ fibers in the 0–40°C temperature range: a quantitative sensory testing and evoked potentials study. Clin Neurophysiol. (2022) 134:81–7. doi: 10.1016/j.clinph.2021.11.076
30. Perchet C, Hagiwara K, Salameh C, Garcia-Larrea L. Cold-evoked potentials in clinical practice: a head-to-head contrast with laser-evoked responses. Eur J Pain. (2023) 27(8):1006–22. doi: 10.1002/EJP.2142
31. Scheuren PS, Nauer N, Rosner J, Curt A, Hubli M. Cold evoked potentials elicited by rapid cooling of the skin in young and elderly healthy individuals. Sci Rep. (2022) 12(1):4137. doi: 10.1038/S41598-022-07967-X
32. Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. (2010) 17:1010–8. doi: 10.1111/J.1468-1331.2010.02969.X
33. Rosner J, Rinert J, Ernst M, Curt A, Hubli M. Cold evoked potentials: acquisition from cervical dermatomes. Neurophysiol Clin. (2019) 49:49–57. doi: 10.1016/j.neucli.2018.11.003
34. Jamal GA, Hansen S, Weir AI, Ballantyne JP. Cerebral cortical potentials to pure non-painful temperature stimulation: an objective technique for the assessment of small fibre pathway in man. J Neurol Neurosurg Psychiatry. (1989) 52:99–105. doi: 10.1136/JNNP.52.1.99
35. Fabig SC, Kersebaum D, Lassen J, Sendel M, Jendral S, Muntean A, et al. A modality-specific somatosensory evoked potential test protocol for clinical evaluation: a feasibility study. Clin Neurophysiol. (2021) 132:3104–15. doi: 10.1016/J.CLINPH.2021.08.017
36. Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C. Terminal arbor degeneration–a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci. (2011) 33:1667–76. doi: 10.1111/J.1460-9568.2011.07652.X
37. Burakgazi AZ, Messersmith W, Vaidya D, Hauer P, Hoke A, Polydefkis M. Longitudinal assessment of oxaliplatin-induced neuropathy. Neurology. (2011) 77:980–6. doi: 10.1212/WNL.0B013E31822CFC59
38. Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol. (2006) 201:507–14. doi: 10.1016/J.EXPNEUROL.2006.05.007
39. Vollert J, Attal N, Baron R, Freynhagen R, Haanpää M, Hansson P, et al. Quantitative sensory testing using DFNS protocol in Europe: an evaluation of heterogeneity across multiple centers in patients with peripheral neuropathic pain and healthy subjects. Pain. (2016) 157:750–8. doi: 10.1097/J.PAIN.0000000000000433
40. ARCHIMEDlife—Medical Laboratory Services. (n.d.). Available online at: https://www.archimedlife.com/ (accessed February 11, 2024)
41. CENTOGENE—The Rare Disease Company: centogene.com. (n.d.). Available online at: https://www.centogene.com/ (accessed February 11, 2024)
42. England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetrical polyneuropathy: definition for clinical research. Muscle Nerve. (2005) 31:113–23. doi: 10.1002/MUS.20233
43. Gierthmühlen J, Enax-Krumova EK, Attal N, Bouhassira D, Cruccu G, Finnerup NB, et al. Who is healthy? Aspects to consider when including healthy volunteers in QST–based studies-a consensus statement by the EUROPAIN and NEUROPAIN consortia. Pain. (2015) 156:2203–11. doi: 10.1097/J.PAIN.0000000000000227
44. Forstenpointner J, Sendel M, Moeller P, Reimer M, Canaan-Kühl S, Gaedeke J, et al. Bridging the gap between vessels and nerves in Fabry disease. Front Neurosci. (2020) 14:448. doi: 10.3389/FNINS.2020.00448
45. Lenoir C, Huang G, Vandermeeren Y, Hatem SM, Mouraux A. Human primary somatosensory cortex is differentially involved in vibrotaction and nociception. J Neurophysiol. (2017) 118:317–30. doi: 10.1152/JN.00615.2016/ASSET/IMAGES/LARGE/Z9K0071741890005.JPEG
46. Magerl W, Krumova EK, Baron R, Tölle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. (2010) 151:598–605. doi: 10.1016/J.PAIN.2010.07.026
47. Pfau DB, Geber C, Birklein F, Treede RD. Quantitative sensory testing of neuropathic pain patients: potential mechanistic and therapeutic implications. Curr Pain Headache Rep. (2012) 16:199–206. doi: 10.1007/S11916-012-0261-3
48. Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. (2006) 10:77. doi: 10.1016/J.EJPAIN.2005.02.003
49. Baumgärtner U, Magerl W, Klein T, Hopf HC, Treede RD. Neurogenic hyperalgesia versus painful hypoalgesia: two distinct mechanisms of neuropathic pain. Pain. (2002) 96:141–51. doi: 10.1016/S0304-3959(01)00438-9
50. Chan AW, MacFarlane IA, Bowsher D, Campbell JA. Weighted needle pinprick sensory thresholds: a simple test of sensory function in diabetic peripheral neuropathy. J Neurol Neurosurg Psychiatry. (1992) 55:56–9. doi: 10.1136/JNNP.55.1.56
51. Fruhstorfer H, Gross W, Selbmann O. von Frey hairs: new materials for a new design. Eur J Pain. (2001) 5:341–2. doi: 10.1053/EUJP.2001.0250
52. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. (2006) 22:1911–20. doi: 10.1185/030079906X132488
53. Arning K, Naleschinski D, Maag R, Biegstraaten M, Kropp P, Lorenzen J, et al. Fabryscan: a screening tool for early detection of Fabry disease. J Neurol. (2012) 259:2393–400. doi: 10.1007/S00415-012-6619-Y
54. Ziegler D, Laux G, Dannehl K, Spüler M, Mühlen H, Mayer P, et al. Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med. (1992) 9:166–75. doi: 10.1111/J.1464-5491.1992.TB01754.X
55. Üçeyler N, Kahn AK, Kramer D, Zeller D, Casanova-Molla J, Wanner C, et al. Impaired small fiber conduction in patients with Fabry disease: a neurophysiological case–control study. BMC Neurol. (2013) 13:47. doi: 10.1186/1471-2377-13-47
56. Valeriani M, Mariotti P, Le Pera D, Restuccia D, De Armas L, Maiese T, et al. Functional assessment of A delta and C fibers in patients with Fabry’s disease. Muscle Nerve. (2004) 30:708–13. doi: 10.1002/MUS.20174
57. Cho EB, Seok JM, Park K-J, Min J-H, Suh BC, Kim BJ. Skin coldness and painful cold: the common symptom in patients with clinically suspected small fiber neuropathy in Korea (P2.429). Neurology. (2018) 90(15 Suppl). doi: 10.1212/WNL.90.15_supplement.P2.429
58. MacDonald DI, Wood JN, Emery EC. Molecular mechanisms of cold pain. Neurobiol Pain. (2020) 7:100044. doi: 10.1016/J.YNPAI.2020.100044
59. Vale TA, Symmonds M, Polydefkis M, Byrnes K, Rice ASC, Themistocleous AC, et al. Chronic non-freezing cold injury results in neuropathic pain due to a sensory neuropathy. Brain. (2017) 140(10):2557–69. doi: 10.1093/brain/awx215
60. Baumgärtner U, Greffrath W, Treede RD. Contact heat and cold, mechanical, electrical and chemical stimuli to elicit small fiber-evoked potentials: merits and limitations for basic science and clinical use. Neurophysiol Clin. (2012) 42:267–80. doi: 10.1016/j.neucli.2012.06.002
61. Kenshalo DR, Duclaux R. Response characteristics of cutaneous cold receptors in the monkey. J Neurophysiol. (1977) 40(2):319–32. doi: 10.1152/JN.1977.40.2.319
62. Schepers RJ, Ringkamp M. Thermoreceptors and thermosensitive afferents. Neurosci Biobehav Rev. (2010) 34:177–84. doi: 10.1016/J.NEUBIOREV.2009.10.003
63. Liang M, Lee MC, O’Neill J, Dickenson AH, Iannetti GD. Brain potentials evoked by intraepidermal electrical stimuli reflect the central sensitization of nociceptive pathways. J Neurophysiol. (2016) 116:286. doi: 10.1152/JN.00013.2016
64. Creac’H C, Bertholon A, Convers P, Garcia-Larrea L, Peyron R. Effects of aging on laser evoked potentials. Muscle Nerve. (2015) 51:736–42. doi: 10.1002/MUS.24458
65. Forstenpointner J, Berry D, Baron R, Borsook D. The cornucopia of central disinhibition pain—an evaluation of past and novel concepts. Neurobiol Dis. (2020) 145:105041. doi: 10.1016/j.nbd.2020.105041
66. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. (2010) 9:807–19. doi: 10.1016/S1474-4422(10)70143-5
67. Mueller D, Obermann M, Koeppen S, Kavuk I, Yoon MS, Sack F, et al. Electrically evoked nociceptive potentials for early detection of diabetic small-fiber neuropathy. Eur J Neurol. (2010) 17:834–41. doi: 10.1111/J.1468-1331.2009.02938.X
68. Pupe C, Davidovich E, Vianna F, Amaral C, Pires K, Nogueira CB, et al. Contact heat evoked potentials in painful autoimmune neuropathy (PAIN) (P6.253). Neurology. (2016) 86(16 Suppl). doi: 10.1212/WNL.86.16_supplement.P6.253
69. Hüllemann P, von der Brelie C, Manthey G, Düsterhöft J, Helmers AK, Synowitz M, et al. Laser-evoked potentials in painful radiculopathy. Clin Neurophysiol. (2017) 128:2292–9. doi: 10.1016/J.CLINPH.2017.09.100
70. Singer W, Spies JM, McArthur J, Low J, Griffin JW, Nickander KK, et al. Prospective evaluation of somatic and autonomic small fibers in selected autonomic neuropathies. Neurology. (2004) 62:612–8. doi: 10.1212/01.WNL.0000110313.39239.82
71. Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Autonomic evaluation is independent of somatic evaluation for small fiber neuropathy. J Neurol Sci. (2014) 344:51–4. doi: 10.1016/J.JNS.2014.06.017
72. Cheshire WP, Freeman R, Gibbons CH, Cortelli P, Wenning GK, Hilz MJ, et al. Electrodiagnostic assessment of the autonomic nervous system: a consensus statement endorsed by the American Autonomic Society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin Neurophysiol. (2021) 132:666–82. doi: 10.1016/J.CLINPH.2020.11.024
73. Rehm S, Sachau J, Hellriegel J, Forstenpointner J, Børsting Jacobsen H, Harten P, et al. Pain matters for central sensitization: sensory and psychological parameters in patients with fibromyalgia syndrome. Pain Rep. (2021) 6(1):e901. doi: 10.1097/PR9.0000000000000901
74. Schuttert I, Timmerman H, Petersen KK, McPhee ME, Arendt-Nielsen L, Reneman MF, et al. The definition, assessment, and prevalence of (human assumed) central sensitisation in patients with chronic low back pain: a systematic review. J Clin Med. (2021) 10:5931. doi: 10.3390/JCM10245931/S1
75. Leone C, Di Lionardo A, Diotallevi G, Mollica C, Di Pietro G, Di Stefano G, et al. Conduction velocity of the cold spinal pathway in healthy humans. Eur J Pain. (2020) 24:1923–31. doi: 10.1002/EJP.1640
76. Campero M, Bostock H. Unmyelinated afferents in human skin and their responsiveness to low temperature. Neurosci Lett. (2010) 470:188–92. doi: 10.1016/J.NEULET.2009.06.089
77. Jørum E, Schmelz M. Chapter 29 microneurography in the assessment of neuropathic pain. Handb Clin Neurol. (2006) 81:427–38. doi: 10.1016/S0072-9752(06)80033-3
78. Schmelz M, Forster C, Schmidt R, Ringkamp M, Handwerker HO, Torebjörk HE. Delayed responses to electrical stimuli reflect C-fiber responsiveness in human microneurography. Exp Brain Res. (1995) 104:331–6. doi: 10.1007/BF00242018
79. Campero M, Serra J, Ochoa JL. C-polymodal nociceptors activated by noxious low temperature in human skin. J Physiol. (1996) 497:565. doi: 10.1113/JPHYSIOL.1996.SP021789
80. Schmelz M, Schmidt R. Microneurographic single-unit recordings to assess receptive properties of afferent human C-fibers. Neurosci Lett. (2010) 470:158–61. doi: 10.1016/J.NEULET.2009.05.064
81. Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol. (1999) 515:799. doi: 10.1111/J.1469-7793.1999.799AB.X
82. Garcia-Larrea L, Convers P, Magnin M, André-Obadia N, Peyron R, Laurent B, et al. Laser-evoked potential abnormalities in central pain patients: the influence of spontaneous and provoked pain. Brain. (2002) 125:2766–81. doi: 10.1093/BRAIN/AWF275
83. Jutzeler CR, Ulrich A, Huber B, Rosner J, Kramer JLK, Curt A. Improved diagnosis of cervical spondylotic myelopathy with contact heat evoked potentials. J Neurotrauma. (2017) 34:2045–53. doi: 10.1089/NEU.2016.4891
84. Di Stefano G, La Cesa S, Leone C, Pepe A, Galosi E, Fiorelli M, et al. Diagnostic accuracy of laser-evoked potentials in diabetic neuropathy. Pain. (2017) 158:1100–7. doi: 10.1097/J.PAIN.0000000000000889
85. de Tommaso M, Libro G, Guido M, Losito L, Lamberti P, Livrea P. Habituation of single CO2 laser-evoked responses during interictal phase of migraine. J Head Pain. (2005) 6:195–8. doi: 10.1007/S10194-005-0183-0/METRICS
86. Bromm B, Lorenz J. Neurophysiological evaluation of pain. Electroencephalogr Clin Neurophysiol. (1998) 107:227–53. doi: 10.1016/S0013-4694(98)00075-3
Keywords: neuropathic pain, Fabry disease, polyneuropathy, cold-evoked potentials, diagnostic workup, neurophysiology
Citation: Kersebaum D, Sendel M, Lassen J, Fabig S-C, Forstenpointner J, Reimer M, Canaan-Kühl S, Gaedeke J, Rehm S, Gierthmühlen J, Baron R and Hüllemann P (2024) Cold-evoked potentials in Fabry disease and polyneuropathy. Front. Pain Res. 5:1352711. doi: 10.3389/fpain.2024.1352711
Received: 8 December 2023; Accepted: 2 April 2024;
Published: 15 May 2024.
Edited by:
Paulina S. Scheuren, University of British Columbia, CanadaReviewed by:
Barbara Namer, University Hospital RWTH Aachen, Germany© 2024 Kersebaum, Sendel, Lassen, Fabig, Forstenpointner, Reimer, Canaan-Kühl, Gaedeke, Rehm, Gierthmühlen, Baron and Hüllemann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dilara Kersebaum, ZGlsYXJhLmtlcnNlYmF1bUB1a3NoLmRl
†These authors share first authorship
‡Present Addresses: Jens Gaedeke, Klinik für Nephrologie und Dialyse, Evangelisches Krankenhaus Königin Elisabeth Herzberge gGmbH, Berlin, Germany
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.