
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pain Res. , 19 February 2024
Sec. Veterinary and Comparative Pain
Volume 5 - 2024 | https://doi.org/10.3389/fpain.2024.1347548
This article is part of the Research Topic Advancement in Equine Pain Management View all 12 articles
Effective management of postoperative pain is essential to ensure patient welfare, reduce morbidity and optimize recovery. Opioids are effective in managing moderate to severe pain in horses but concerns over their adverse effects on gastrointestinal (GI) motility and associated increased colic risk limit their widespread use. Studies investigating the impact of systemic opioids on both GI motility and colic incidence in horses have yielded inconclusive outcomes. Therefore, this retrospective study aims to assess the influence of systemic administration of butorphanol, morphine, and methadone on post-anaesthetic colic (PAC) incidence. Horses undergoing general anaesthesia for non-gastrointestinal procedures that were hospitalized for at least 72 h post-anaesthesia were included in this study. Anaesthetised horses were stratified by procedure type into horses undergoing diagnostic imaging without surgical intervention, emergency or elective surgery. In addition, patients were grouped by opioid treatment regime into horses receiving no opioids, intraanaesthetic, short- (<24 h) or long-term (>24 h) postoperative opioids. Administered opioids encompassed butorphanol, morphine and methadone. The number of horses showing signs of colic in the 72 h after anaesthesia was assessed for each group. A total of 782 horses were included, comprising 659 undergoing surgical procedures and 123 undergoing diagnostic imaging. The overall PAC incidence was 15.1%. Notably, horses undergoing diagnostic imaging without surgery had a significantly lower PAC rate of 6.5% compared to those undergoing surgery (16.7%, p = 0.0146). Emergency surgeries had a significantly lower PAC rate of 5.8% compared to elective procedures (18%, p = 0.0113). Of the 782 horses, 740 received intraoperative opioids and 204 postoperative opioids, 102 of which long-term (≥24 h). Neither intraoperative (p = 0.4243) nor short-term postoperative opioids (p = 0.5744) increased PAC rates. Notably, only the long-term (≥24 h) administration of morphine significantly increased PAC incidence to 34% (p = 0.0038). In contrast, long-term butorphanol (5.3% PAC, p = 0.8482) and methadone (18.4% PAC, p = 0.6161) did not affect PAC rates. In summary, extended morphine administration was the only opioid treatment associated with a significantly increased risk of PAC.
Postoperative pain is prevalent among the majority of patients undergoing surgical procedures. Effective pain management is crucial to mitigate suffering, reduce morbidity, and facilitate recovery and rehabilitation. Although the analgesic properties of opioids and opiates, such as butorphanol, buprenorphine, methadone, and morphine, are well-established, their use in managing perioperative and post-traumatic pain in equine patients is limited by concerns about potential adverse gastrointestinal side effects (1–9). Constipation is a widely recognized side effect of opioid treatment in all species, affecting up to 95% of human patients, attributed to diminished coordinated motility, prolonged transit time, enhanced fluid absorption from intestinal contents and decreased secretion of fluids and electrolytes into the intestinal lumen (10–20). While opioids provide analgesia by stimulating μ-, κ- and δ-opioid receptors in the brain, the dorsal horn of the spinal cord and peripheral tissues, the activation of opioid receptors within the gastrointestinal tract can decrease motility and induce alterations in the secretion, absorption and transport of electrolytes and fluids (9, 11–17, 21–24).
Post-anaesthetic colic (PAC) represents a common complication in equine patients, with reported incidence rates reaching up to 21.1% (5, 6, 25–28). Research into risk factors for the development of PAC and the influence of systemic opioids on gastrointestinal (GI) motility has produced equivocal results, ranging from decreased risk (25), no elevated risk (6, 26, 27, 29–32) to a fourfold rise in colic cases following perioperative opioid administration (5). The inconsistency in these outcomes could be attributed to differences in the GI side effects associated with various opioid agonists and variations in the dosage, frequency, administration method, and duration of opioid use across these studies.
Butorphanol, morphine, methadone and hydromorphone are commonly used in equine analgesia and their pharmacokinetics and pharmacodynamics have been thoroughly investigated (2, 24, 29, 30, 32, 33). Butorphanol, a synthetic strong κ-opioid receptor agonist and weak μ-opioid receptor antagonist (2, 34–38), has been observed to induce a transient reduction in gastrointestinal motility in anesthetized horses when used as CRI at a dosage of 13 μg/kg/h (34). In contrast, pre- or intraoperative butorphanol administration at a mean dosage of 0.007 mg/kg and 0.05 mg/kg respectively has been reported to reduce the risk of PAC (25).
Similarly, morphine, a μ-opioid agonist, has been shown to decrease gastrointestinal propulsive motility and to increase PAC rates after orthopaedic surgery four-fold when administered intravenously at a dosage of 0.08–0.3 mg/kg compared to no opioid or butorphanol (5, 9, 35, 39, 40). Conversely, two other studies found no association between peri-anaesthetic intravenous morphine administration at a dosage of 0.1–0.17 mg/kg and increased PAC risk (6, 41). Notably, the administration of epidural morphine after laparoscopy has been demonstrated to provide effective pain relief without compromising gastrointestinal motility (42).
Methadone, a synthetic μ-and δ-agonist with N-methyl-d-aspartate (NMDA) antagonist activity and the ability to inhibit serotonin and noradrenaline uptake, is not extensively approved for equine use and consequently, its utilization is less prevalent compared to morphine or butorphanol. Although it has also been shown to reduce borborygmi (2), the potential influence of perianaesthetic methadone administration on the occurrence of post-anaesthetic colic (PAC) remains unexplored.
Therefore, this study aims to assess and compare the impact of intra- and postoperative administration of the commonly used opioids—butorphanol, morphine and methadone—on the incidence of PAC in equine patients.
This retrospective chart review includes data from all horses over the age of one year that underwent general anaesthesia at the University of Veterinary Medicine Vienna's Equine Hospital in the 5-year period from October 2013 to December 2018 and remained hospitalized at the clinic for a minimum of 72 h post-anaesthesia. Patients undergoing abdominal surgery, presenting with gastrointestinal disorders, or requiring multiple surgeries such as repeated arthroscopic lavages within the initial 72-h window, were excluded from the study (Figure 1).
The cases were stratified into an elective and an emergency group. Patients in the emergency category were not fasted due to the immediate need for surgical intervention. In contrast, horses in the elective group were fasted for a minimum of 6 h before anaesthesia.
Furthermore, both elective and emergency surgeries were categorized based on the type of procedure, distinguishing between orthopaedic and soft tissue surgeries. Horses subjected to general anaesthesia for computer tomography or magnetic resonance imaging, without concurrent surgical intervention, constituted the control group.
In addition to the horses’ age, sex, breed and weight, details of intra- and post-anaesthetic opioid regimen, including drug type, dosage, route and duration of administration, duration of anaesthesia and intraoperative blood pressure and all physical exam parameters in the 72 h following the anaesthesia were extracted from the records. Opioids were categorized into intra- and post-anaesthetic administration, with further subdivision into short-term (<24 h) and long-term (≥24 h) treatment.
Patients were classified as experiencing post-anaesthetic colic if they displayed clinical symptoms such as depression, pawing, reduced appetite, or rolling, necessitating a comprehensive colic assessment in the 72 h following anaesthesia.
Data were summarized using standard descriptive statistics including mean, standard deviation, and range for continuous variables and for categorical variables frequency and proportion. Associations between categorical variables were examined by the Chi-Square test. The effect of age, sex, procedure type (anaesthesia only or surgery), emergency vs. elective surgery, anaesthesia duration, intra- and postoperative opioid administration and opioid type on PAC rate was assessed using multiple logistic regression with PAC as the outcome variable, anaesthesia without surgery as the reference level for procedure type, elective surgery as the reference level for surgery and gelding as the reference level for sex. All computations were carried out in Graphpad Prism (Version 10.0.2).
A total of 782 cases, 246 (31.5%) mares, 313 (40%) geldings and 223 (28.5%) stallions met the inclusion criteria. Horses' age ranged from 1 to 30 years (mean: 9.1 years, s.d.: 6 years).
Of the 782 cases, 659 (84.3%) horses were surgical patients, the remaining 123 (15.7%) were anesthetized for diagnostic imaging purposes without concurrent surgical intervention (Table 1). The 659 surgical patients were divided into 69 (10.5%) emergency and 590 (89.5%) elective cases. The 69 emergencies included 55 (79.7%) orthopaedic and 14 (20.3%) soft tissue emergencies. In contrast, the elective cases comprised 243 (41.2%) orthopaedic and 347 (58.8%) were soft tissue surgeries. Anaesthesia duration (overall mean: 107 min, s.d.: 65 min) was significantly different between horses undergoing anaesthesia for diagnostic imaging (mean: 42 min, s.d.: 37 min), elective (mean: 115 min, s.d.: 61 min) or emergency (mean: 145 min, s.d.: 67 min) surgery (p < 0.0001).
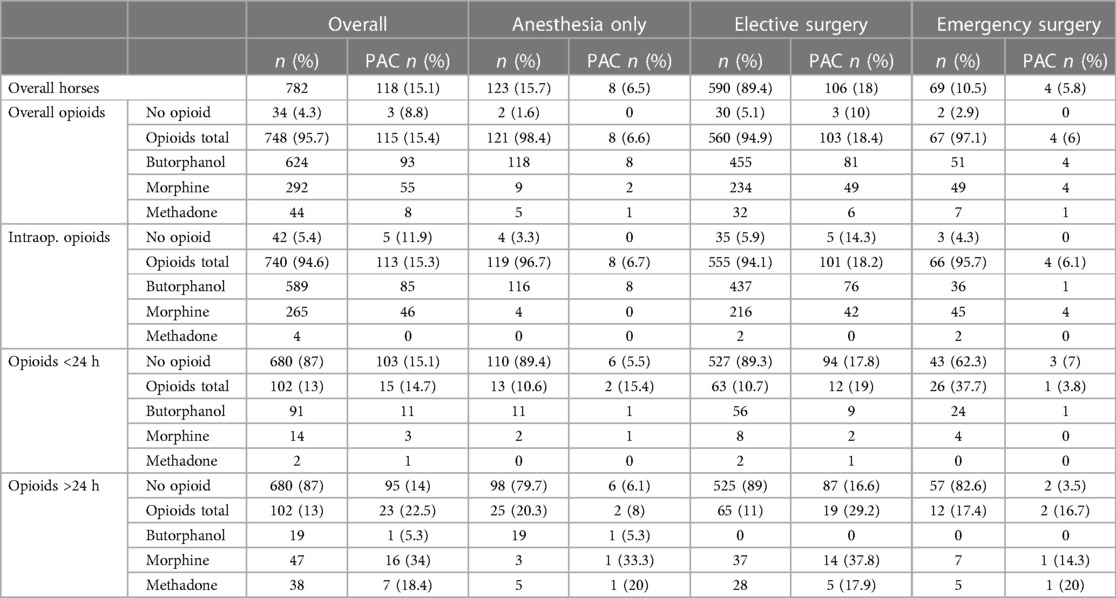
Table 1. Number of horses receiving opioids and developing PAC by overall, intraoperative and postoperative short-term (<24 h) and long-term (>24 h) opioid administration. As horses received multiple opioids as well as intra- and postoperative opioids, cumulative percentages can exceed 100%.
The overall incidence of post-anaesthetic colic (PAC) was 15.1% (118/782, Table 1). Age, sex and anaesthesia duration had no statistically significant effect on PAC rates (Table 2).
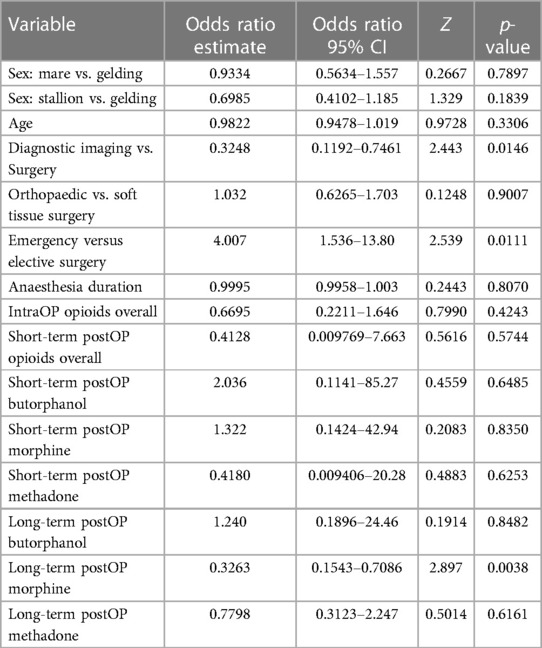
Table 2. Odds ratio estimates and 95% confidence intervals (CI, Z and p-values calculated using multiple logistic regression with PAC as the outcome variable.
However, horses anesthetized for diagnostic imaging purposes without concurrent surgical intervention had a significantly lower PAC rate of 6.5% (n = 8/123) compared to horses undergoing surgery with a PAC rate of 16.7% (n = 110/659, p = 0.0146, Table 2). Furthermore, horses anesthetized for emergency surgery had a significantly lower PAC rate of 5.8% (n = 4/69), than horses undergoing elective procedures with a PAC rate of 18% (n = 106/590) (p = 0.0113; Table 2). The type of surgery (soft tissue vs. orthopaedic) did not have a significant effect on PAC rate with 15.1% (45/298) orthopaedic and 18% (65/361) soft tissue surgery patients suffering from PAC (p = 0.9).
Overall, 34 horses (4.35%) received no opioids during their hospital stay. Of the 748 horses (95.65%) that were administered opioids, 196 (25.1%) received opioids intra- and postoperatively, 544 (69.6%) only intraoperatively and 8 (1%) only postoperatively, accumulating to 740 horses (94.6%9 with intraoperative and 204 (26.1%) with postoperative opioid treatment. Postoperative opioid therapy lasted for <24 h in 119 (58.3%) patients and long-term (≥24 h) in 85 (41.7%; Table 1).
Butorphanol dosage ranged from 0.01 mg/kg to 0.03 mg/kg intravenously as single (i.e., sedation) or repeated intravenous bolus injections. Morphine was administered at 0.1 mg/kg intramuscularly every 4–6 h. Methadone was utilized at 0.1 mg/kg intramuscularly every 4–6 h as a bolus (n = 43) or as part of a continuous rate infusion (CRI, n = 1) at a dosage of 0.017 mg/kg/h, combined with lidocaine 2% (1.2 mg/kg/h) and ketamine 10% (0.3 mg/kg/h) for post-anaesthetic pain management. No horse received methadone during anaesthesia (Table 1).
Neither opioids administered during anaesthesia (p = 0.4243) nor short-term opioid administration after anaesthesia (p = 0.5744) increased the incidence of PAC (Tables 1, 2). Notably, only the long-term administration of morphine resulted in a statistically significant increase of PAC (p = 0.0038) with 34% (16/47) horses that received morphine for ≥24 h developing PAC (Tables 1, 2, Figure 2). In contrast, long-term butorphanol [5.3% (1/19) PAC, p = 0.8482] and methadone [18.4% (7/38) PAC, p = 0.6161] had no significant effect on PAC rate, compared to horses receiving no long-term postoperative opioids [14% (95/680) PAC; Tables 1, 2, Figure 2].
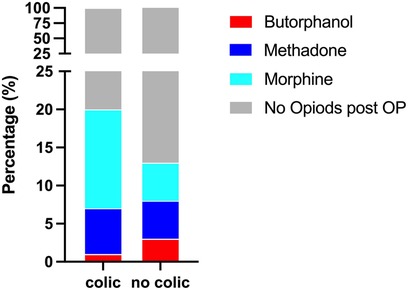
Figure 2. Percentage of horses receiving long-term (≥24 h) post-anaesthetic opioids with and without colic symptoms.
Morphine administration for longer than 24 h postoperatively emerged as the sole opioid significantly associated with increased PAC incidence in this study, in contrast to both butorphanol and methadone. This finding aligns with numerous human studies consistently ranking morphine highest in the opioid potency profile order for inducing constipation (30, 43–45). Notably, the dosage necessary for morphine's analgesic effect significantly surpasses that needed for its gastrointestinal side effects, approximately fifty-fold in humans and fourfold in experimental animals (46, 47). In horses, morphine administered intravenously at dosages as low as 0.05 mg/kg, half the dosage typically used clinically to provide analgesia, decreased propulsive gastrointestinal motility for up to 6 h and significantly reduced defecation frequency, faecal matter weight and moisture content (9, 39, 48–50).
Opioids affect gastrointestinal motility by activating the μ-, κ- and δ-opioid receptors of the myenteric and submucosal plexus neurons in the enteric nervous system, interstitial cells of Cajal and immune cells (13–15, 22, 51–58). Broadly, opioids induce delayed gastric emptying and constipation by disrupting neurotransmission between enteric neurons and their targets, namely, smooth muscles and epithelial cells (15). The inhibition of excitatory neurons in the myenteric plexus decreases propulsive peristalsis while the concomitant suppression of inhibitory neuromuscular transmission increases intestinal muscle tones and non-propulsive tonic contractions, which may cause abdominal cramps (13–15, 22, 51–58). Additionally, opioid-induced inhibition of submucosal secretomotor neurons diminishes epithelial secretion of Cl− and osmotic water movement, exacerbating constipation (13–15, 22, 51–58).
While all three classical opioid receptor types are found in the gastrointestinal tract and contribute to analgesic and adverse effects, mechanistic studies using µ-selective drugs and µ-receptor-knockout mice indicate that opioid-induced gastrointestinal tract dysfunction is primarily mediated by the µ-receptor (13–15, 22, 51–58). Strong μ-agonists, such as morphine and methadone are thus more likely to induce adverse gastrointestinal side effects. Correspondingly, butorphanol, a mixed agonist-antagonist opioid analgesic with an affinity ratio for the μ-, δ-, and κ-opioid receptor of 1:4:25 and a a 3-, 10-, and 30-fold lower half maximal inhibitory concentration (IC50) for these receptors than morphine (38, 59), had the lowest PAC rate in this study. The lower PAC incidence following methadone compared to morphine administration observed in this study, is consistent with reported constipation relief in 80% of human patients after switch from morphine to methadone (19, 31, 60, 61). The extraopioid analgesic effects caused by methadone's non-competitive antagonist activity at the N-methyl-D-aspartate receptor and its function as a serotonin re-uptake inhibitor, may contribute to the lower rate of gastrointestinal effects observed with methadone compared to morphine therapy (19, 31, 60, 61). Serotonin exerts a variety of effects on intrinsic enteric neurons, extrinsic afferents, enterocytes and smooth muscle cells and its agonists are used as promotility agents to promote gastric emptying and to alleviate constipation (62–65).
Interestingly, although anaesthesia duration had no influence on PAC rate, horses anesthetized for diagnostic imaging purposes had a significantly lower PAC rate (6.5%) than horses undergoing surgery (16.7% PAC) and horses receiving no opioids postoperatively had a higher PAC (14%) rate than horses receiving butorphanol (5.3%), underscoring the importance of postoperative pain management. Pain initiates a spinal reflex arc, increases sympathetic activity and cortisol and endogenous opioid release, thus inhibiting propulsive gastrointestinal motility (66–69). Effective pain management is thus crucial to ensure patients' welfare and minimize postoperative morbidity and complications.
Notably, emergency surgeries were associated with a significantly lower PAC rate than elective surgeries in our study, which is in contrast to previous studies in which out-of-hours surgeries and horses that were not fasted before anaesthesia carried an increased risk of PAC (5, 26). The exclusion of horses with gastrointestinal problems, the more intensive supportive care and monitoring provided to emergency cases may contribute to the lower PAC rate in emergency surgeries in our study. However, horses undergoing emergency surgery, in contrast to elective procedures, also were not fasted prior to anaesthesia. Therefore, the lower PAC rate may also be attributable to the ongoing provision of food rather than the horse being presented as an emergency. Although this interpretation is more plausible from a clinical perspective, the simultaneous occurrence of these two factors does not allow statistical analysis to test this hypothesis.
The relatively high overall PAC rate in this study (15.1%), which is at the high end of the reported range of 2.8–21.1% (5, 6, 25–28), may be due to the inclusion criteria necessitating hospitalisation for 72 h post anaesthesia, which may bias toward a patient population with more severe problems and the stringent definition of colic symptoms combined with the close monitoring in our clinic.
The study's limitations are inherent in its retrospective design, the non-randomized allocation of patients to the different opioid treatment groups, the absence of both standardized pain assessment and stratification based on pain severity, and the relatively low number of patients in the diverse treatment subgroups. Hence, the selection of opioid type, administration route, and duration was determined by the anticipated or subjectively perceived level of pain, potentially leading to both over- and undertreatment of pain. Moreover, given the absence of comprehensive data regarding equipotent dosages of these opioids in equines, the employed drug dosages likely lack equipotency, thereby hindering direct comparison of their analgesic properties. Studies assessing the equianalgesic potencies of different opioids in horses and associated side effects are required. Additionally, while all three classical opioid receptor types are found in the mammalian gastrointestinal tract, their distribution patterns exhibit inter-species variability (9, 11–17, 21–23, 43, 58). For the horse only the opioid receptor distribution pattern in the small intestine has been studied (70). Given the pivotal role of colonic dysfunction in opioid-induced gastrointestinal complications across species, further investigations to determine the opioid receptor distribution in the equine colon are needed.
Intraoperative opioids and postoperative pain management with butorphanol and morphine did not increase the incidence of PAC in our study. Long-term (>24 h) morphine was the only opioid increasing PAC rate. In addition, patients undergoing surgery had a significantly higher PAC incidence than horses anaesthetized for diagnostic imaging and horses receiving no postoperative opioids had a higher PAC rate than those receiving butorphanol, underscoring the role of pain and pain management in PAC.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
RH: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – review & editing. MJ: Data curation, Investigation, Writing – review & editing. AM: Data curation, Investigation, Writing – review & editing. FJ: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Clark L, Clutton RE, Blissitt KJ, Chase-Topping ME. Effects of peri-operative morphine administration during halothane anaesthesia in horses. Vet Anaesth Analg. (2005) 32:10–5. doi: 10.1111/j.1467-2995.2004.00174.x
2. Carregaro AB, Freitas GC, Ribeiro MH, Xavier NV, Dória RG. Physiological and analgesic effects of continuous-rate infusion of morphine, butorphanol, tramadol or methadone in horses with lipopolysaccharide (LPS)-induced carpal synovitis. BMC Vet Res. (2014) 10:966. doi: 10.1186/s12917-014-0299-z
3. Love EJ, Lane JG, Murison PJ. Morphine administration in horses anaesthetized for upper respiratory tract surgery. Vet Anaesth Analg. (2006) 33:179–88. doi: 10.1111/j.1467-2995.2005.00247.x
4. Love EJ, Taylor PM, Whay HR, Murrell J. Postcastration analgesia in ponies using buprenorphine hydrochloride. Vet Rec. (2013) 172:635–635. doi: 10.1136/vr.101440
5. Senior JM, Pinchbeck GL, Dugdale AHA, Clegg PD. Retrospective study of the risk factors and prevalence of colic in horses after orthopaedic surgery. Vet Rec. (2004) 155:321–5. doi: 10.1136/vr.155.11.321
6. Andersen MS, Clark L, Dyson SJ, Newton JR. Risk factors for colic in horses after general anaesthesia for MRI or nonabdominal surgery: absence of evidence of effect from perianaesthetic morphine. Equine Vet J. (2006) 38:368–74. doi: 10.2746/042516406777749263
7. Tessier C, Pitaud J-P, Thorin C, Touzot-Jourde G. Systemic morphine administration causes gastric distention and hyperphagia in healthy horses. Equine Vet J. (2019) 51:653–7. doi: 10.1111/evj.13090
8. Levionnois OL, Graubner C, Spadavecchia C. Colon constipation in horses after sustained-release buprenorphine administration. Vet Anaesth Analg. (2018) 45:876–80. doi: 10.1016/j.vaa.2018.08.004
9. Boscan P, Hoogmoed LMV, Farver TB, Snyder JR. Evaluation of the effects of the opioid agonist morphine on gastrointestinal tract function in horses. Am J Vet Res. (2006) 67:992–7. doi: 10.2460/ajvr.67.6.992
10. Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. (2008) 11:S105–20. doi: 10.36076/ppj.2008/11/S105
11. Chamie K, Golla V, Lenis AT, Lec PM, Rahman S, Viscusi ER. Peripherally acting μ-opioid receptor antagonists in the management of postoperative ileus: a clinical review. J Gastrointest Surg. (2021) 25:293–302. doi: 10.1007/s11605-020-04671-x
12. Corsetti M, Pannemans J, Whorwell P. Targeting mu opioid receptors to modulate gastrointestinal function: what have we learnt so far from the studies in functional bowel disorders? F1000Res. (2019) 8:F1000 Faculty Rev-257. doi: 10.12688/f1000research.15974.1
13. Galligan JJ, Sternini C. Insights into the role of opioid receptors in the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. (2017) 239:363–78. doi: 10.1007/164_2016_116
14. Mosiska P, Zieliska M, Fichna J. Expression and physiology of opioid receptors in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. (2016) 23:3–10. doi: 10.1097/med.0000000000000219
15. Sobczak M, Sałaga M, Storr MA, Fichna J. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J Gastroenterol. (2014) 49:24–45. doi: 10.1007/s00535-013-0753-x
16. Wade PR, Palmer JM, McKenney S, Kenigs V, Chevalier K, Moore BA, et al. Modulation of gastrointestinal function by MuDelta, a mixed µ opioid receptor agonist/µ opioid receptor antagonist. Br J Pharmacol. (2012) 167:1111–25. doi: 10.1111/j.1476-5381.2012.02068.x
17. Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept. (2009) 155:11–7. doi: 10.1016/j.regpep.2009.03.012
18. Yuan C-S. Clinical status of methylnaltrexone, a new agent to prevent and manage opioid-induced side effects. J Support Oncol. (2004) 2:111–7; discussion 119–22.15328815
19. Mercadante S, Casuccio A, Fulfaro F, Groff L, Boffi R, Villari P, et al. Switching from morphine to methadone to improve analgesia and tolerability in cancer patients: a prospective study. J Clin Oncol. (2001) 19:2898–904. doi: 10.1200/jco.2001.19.11.2898
20. Roger T, Bardon T, Ruckebusch Y. Colonic motor responses in the pony: relevance of colonic stimulation by opiate antagonists. Am J Vet Res. (1985) 46:31–5.3970439
21. Poole DP, Pelayo J-C, Scherrer G, Evans CJ, Kieffer BL, Bunnett NW. Localization and regulation of fluorescently labeled delta opioid receptor, expressed in enteric neurons of mice. Gastroenterology. (2011) 141:982–91.e18. doi: 10.1053/j.gastro.2011.05.042
22. Sternini C, Patierno S, Selmer I-S, Kirchgessner A. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil. (2004) 16:3–16. doi: 10.1111/j.1743-3150.2004.00553.x
23. Luca AD, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther. (1996) 69:103–15. doi: 10.1016/0163-7258(95)02053-5
24. Nannarone S, Giannettoni G, Laurenza C, Giontella A, Moretti G. Methadone or butorphanol as pre-anaesthetic agents combined with romifidine in horses undergoing elective surgery: qualitative assessment of sedation and induction. Animals (Basel). (2021) 11:2572. doi: 10.3390/ani11092572
25. Jago RC, Corletto F, Wright IM. Peri-anaesthetic complications in an equine referral hospital: risk factors for post anaesthetic colic. Equine Vet J. (2015) 47:635–40. doi: 10.1111/evj.12475
26. Senior JM, Pinchbeck GL, Allister R, Dugdale AHA, Clark L, Clutton RE, et al. Post anaesthetic colic in horses: a preventable complication? Equine Vet J. (2006) 38:479–84. doi: 10.2746/042516406778400673
27. Nelson BB, Lordan EE, Hassel DM. Risk factors for gastrointestinal dysfunction following elective anaesthesia. Equine Vet J. (2013) 45:8–14. doi: 10.1111/evj.12162
28. Patipa LA, Sherlock CE, Witte SH, Pirie GD, Berghaus RD, Peroni JF. Risk factors for colic in equids hospitalized for ocular disease. J Am Vet Méd Assoc. (2012) 240:1488–93. doi: 10.2460/javma.240.12.1488
29. Skrzypczak H, Reed R, Barletta M, Quandt J, Sakai D. A retrospective evaluation of the effect of perianesthetic hydromorphone administration on the incidence of postanesthetic signs of colic in horses. Vet Anaesth Analg. (2020) 47:757–62. doi: 10.1016/j.vaa.2020.06.003
30. Felden L, Walter C, Harder S, Treede R-D, Kayser H, Drover D, et al. Comparative clinical effects of hydromorphone and morphine: a meta-analysis. Br J Anaesth. (2011) 107:319–28. doi: 10.1093/bja/aer232
31. Mancini IL, Hanson J, Neumann CM, Bruera ED. Opioid type and other clinical predictors of laxative dose in advanced cancer patients: a retrospective study. J Palliat Med. (2000) 3:49–56. doi: 10.1089/jpm.2000.3.49
32. Reed R, Trenholme N, Skrzypczak H, Chang K, Ishikawa Y, Barletta M, et al. Comparison of hydromorphone and butorphanol for management of pain in equine patients undergoing elective arthroscopy: a randomized clinical trial. Vet Anaesth Analg. (2022) 49:490–8. doi: 10.1016/j.vaa.2022.05.006
33. Emanuel D, Kästner SBR, Delarocque J, Grob AJ, Bienert-Zeit A. Influence of butorphanol, buprenorphine and levomethadone on sedation quality and postoperative analgesia in horses undergoing cheek tooth extraction. Vet Sci. (2022) 9:174. doi: 10.3390/vetsci9040174
34. Dias BP, de Araújo MA, Deschk M, Trein TA, Pinheiro NC, Perri SHV, et al. Effects of a continuous rate infusion of butorphanol in isoflurane-anesthetized horses on cardiorespiratory parameters, recovery quality, gastrointestinal motility and serum cortisol concentrations. Acta Cir Bras. (2014) 29:801–6. doi: 10.1590/s0102-86502014001900006
35. Sojka JE, Adams SB, Lamar CH, Eller LL. Effect of butorphanol, pentazocine, meperidine, or metoclopramide on intestinal motility in female ponies. Am J Vet Res. (1988) 49:527–9.3377314
36. Natalini CC, Robinson EP. Evaluation of the analgesic effects of epidurally administered morphine, alfentanil, butorphanol, tramadol, and U50488H in horses. Am J Vet Res. (2000) 61:1579–86. doi: 10.2460/ajvr.2000.61.1579
37. Sellon DC, Roberts MC, Blikslager AT, Ulibarri C, Papich MG. Effects of continuous rate intravenous infusion of butorphanol on physiologic and outcome variables in horses after celiotomy. J Vet Intern Med. (2004) 18:555. doi: 10.1892/0891-6640(2004)18%3C555:eocrii%3E2.0.co;2
38. Commiskey S, Fan L-W, Ho IK, Rockhold RW. Butorphanol: effects of a prototypical agonist-antagonist analgesic on κ-opioid receptors. J Pharmacol Sci. (2005) 98:109–16. doi: 10.1254/jphs.crj05001x
39. Bacon EK, Donnelly CG, Bellone RR, Finno CJ, Velie BD. Melanocortin-1 receptor influence in equine opioid sensitivity. Equine Vet Educ. (2023) 35:152–62. doi: 10.1111/eve.13661
40. Figueiredo JP, Muir WW, Sams R. Cardiorespiratory, gastrointestinal, and analgesic effects of morphine sulfate in conscious healthy horses. Am J Vet Res. (2012) 73:799–808. doi: 10.2460/ajvr.73.6.799
41. Mircica E, Clutton RE, Kyles KW, Blissitt KJ. Problems associated with perioperative morphine in horses: a retrospective case analysis. Vet Anaesth Analg. (2003) 30:147–55. doi: 10.1046/j.1467-2995.2003.00092.x
42. Martin-Flores M, Campoy L, Kinsley MA, Mohammed HO, Gleed RD, Cheetham J. Analgesic and gastrointestinal effects of epidural morphine in horses after laparoscopic cryptorchidectomy under general anesthesia. Vet Anaesth Analg. (2014) 41:430–7. doi: 10.1111/vaa.12133
43. Imam MZ, Kuo A, Ghassabian S, Smith MT. Progress in understanding mechanisms of opioid-induced gastrointestinal adverse effects and respiratory depression. Neuropharmacology. (2018) 131:238–55. doi: 10.1016/j.neuropharm.2017.12.032
44. Wolff RF, Aune D, Truyers C, Hernandez AV, Misso K, Riemsma R, et al. Systematic review of efficacy and safety of buprenorphine versus fentanyl or morphine in patients with chronic moderate to severe pain. Curr Méd Res Opin. (2012) 28:833–45. doi: 10.1185/03007995.2012.678938
45. Kuo A, Wyse BD, Meutermans W, Smith MT. In vivo profiling of seven common opioids for antinociception, constipation and respiratory depression: no two opioids have the same profile. Br J Pharmacol. (2013) 172:532–48. doi: 10.1111/bph.12696
46. Mori T, Shibasaki Y, Matsumoto K, Shibasaki M, Hasegawa M, Wang E, et al. Mechanisms that underlie μ-opioid receptor agonist–induced constipation: differential involvement of μ-opioid receptor sites and responsible regions. J Pharmacol Exp Ther. (2013) 347:91–9. doi: 10.1124/jpet.113.204313
47. Matsumoto K, Umemoto H, Mori T, Akatsu R, Saito S, Tashima K, et al. Differences in the morphine-induced inhibition of small and large intestinal transit: involvement of central and peripheral μ-opioid receptors in mice. Eur J Pharmacol. (2016) 771:220–8. doi: 10.1016/j.ejphar.2015.12.033
48. Combie JD, Nugent TE, Tobin T. Pharmacokinetics and protein binding of morphine in horses. Am J Vet Res. (1983) 44:870–4.6869996
49. Hamamoto-Hardman BD, Steffey EP, Weiner D, McKemie DS, Kass P, Knych HK. Pharmacokinetics and selected pharmacodynamics of morphine and its active metabolites in horses after intravenous administration of four doses. J Vet Pharmacol Ther. (2019) 42:401–10. doi: 10.1111/jvp.12759
50. Knych HK, Steffey EP, McKemie DS. Preliminary pharmacokinetics of morphine and its major metabolites following intravenous administration of four doses to horses. J Vet Pharmacol Ther. (2014) 37:374–81. doi: 10.1111/jvp.12098
51. Camilleri M, Lembo A, Katzka DA. Opioids in gastroenterology: treating adverse effects and creating therapeutic benefits. Clin Gastroenterol Hepatol. (2017) 15:1338–49. doi: 10.1016/j.cgh.2017.05.014
52. Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. (2004) 16:17–28. doi: 10.1111/j.1743-3150.2004.00554.x
53. Lay J, Carbone SE, DiCello JJ, Bunnett NW, Canals M, Poole DP. Distribution and trafficking of the μ-opioid receptor in enteric neurons of the Guinea pig. Am J Physiol-Gastrointest Liver Physiol. (2016) 311:G252–66. doi: 10.1152/ajpgi.00184.2016
54. Schepper HUD, Cremonini F, Park M-I, Camilleri M. Opioids and the gut: pharmacology and current clinical experience. Neurogastroenterol Motil. (2004) 16:383–94. doi: 10.1111/j.1365-2982.2004.00513.x
55. Beckett EAH, Staikopoulos V, Hutchinson MR. Differential effect of morphine on gastrointestinal transit, colonic contractions and nerve-evoked relaxations in toll-like receptor deficient mice. Sci Rep. (2018) 8:5923. doi: 10.1038/s41598-018-23717-4
56. Shook JE, Pelton JT, Lemcke PK, Porreca F, Hruby VJ, Burks TF. Mu opioid antagonist properties of a cyclic somatostatin octapeptide in vivo: identification of mu receptor-related functions. J Pharmacol Exp Ther. (1987) 242:1–7.2886635
57. Roy S, Liu H-C, Loh HH. μ-Opioid receptor-knockout mice: the role of μ-opioid receptor in gastrointestinal transit. Mol Brain Res. (1998) 56:281–3. doi: 10.1016/s0169-328x(98)00051-5
58. Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett. (2004) 361:192–5. doi: 10.1016/j.neulet.2003.12.004
59. Horan PJ, Ho IK. Comparative pharmacological and biochemical studies between butorphanol and morphine. Pharmacol Biochem Behav. (1989) 34:847–54. doi: 10.1016/0091-3057(89)90284-0
60. Daeninck PJ, Bruera E. Reduction in constipation and laxative requirements following opioid rotation to methadone. J Pain Symptom Manag. (1999) 18:303–9. doi: 10.1016/s0885-3924(99)00086-x
61. Leppert W. The impact of opioid analgesics on the gastrointestinal tract function and the current management possibilities. Contemp Oncol. (2012) 16:125–31. doi: 10.5114/wo.2012.28792
62. Foong A-L, Grindrod KA, Patel T, Kellar J. Demystifying serotonin syndrome (or serotonin toxicity). Can Fam Physician Med Fam Can. (2018) 64:720–7.
63. Tonini M, Pace F. Drugs acting on serotonin receptors for the treatment of functional GI disorders. Dig Dis. (2006) 24:59–69. doi: 10.1159/000090309
64. Gershon MD. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. (2004) 20:3–14. doi: 10.1111/j.1365-2036.2004.02180.x
65. Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. (2007) 50:376–88. doi: 10.1007/s10350-006-0763-3
66. Kuo CP, Jao SW, Chen KM, Wong CS, Yeh CC, Sheen MJ, et al. Comparison of the effects of thoracic epidural analgesia and i.v. Infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J Anaesth. (2006) 97:640–6. doi: 10.1093/bja/ael217
67. Hasuo H, Kusunoki H, Kanbara K, Abe T, Yunoki N, Haruma K, et al. Tolerable pain reduces gastric fundal accommodation and gastric motility in healthy subjects: a crossover ultrasonographic study. Biopsychosoc Med. (2017) 11:4. doi: 10.1186/s13030-017-0089-5
68. Luckey A, Livingston E, Taché Y. Mechanisms and treatment of postoperative ileus. Arch Surg. (2003) 138:206–14. doi: 10.1001/archsurg.138.2.206
69. Carroll J, Alavi K. Pathogenesis and management of postoperative ileus. Clin Colon Rectal Surg. (2009) 22:047–50. doi: 10.1055/s-0029-1202886
Keywords: opioids, morphine, butorphanol, methadone, horse, equine, colic
Citation: Haralambus R, Juri M, Mokry A and Jenner F (2024) The impact of opioid administration on the incidence of postanaesthetic colic in horses. Front. Pain Res. 5:1347548. doi: 10.3389/fpain.2024.1347548
Received: 1 December 2023; Accepted: 8 February 2024;
Published: 19 February 2024.
Edited by:
Rachel Anne Reed, University of Georgia, United StatesReviewed by:
Stephanie Kleine, The University of Tennessee, United States© 2024 Haralambus, Juri, Mokry and Jenner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rhea Haralambus UmhlYS5IYXJhbGFtYnVzQHZldG1lZHVuaS5hYy5hdA== Florien Jenner Rmxvcmllbi5KZW5uZXJAdmV0bWVkdW5pLmFjLmF0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.