- Department of Health, Kinesiology, and Applied Physiology, Concordia University, Montreal, QC, Canada
Introduction: Chronic low back pain (CLBP) is the leading cause of years lived with disability worldwide. Transcutaneous electrotherapies have been widely used to treat CLBP but, with the partial exception of transcutaneous electrical nerve stimulation (TENS), their effect on pain, disability, quality-of-life, and psychosocial outcomes have not been systematically reviewed. The purpose of this systematic review and meta-analysis was to clarify the overall effect of transcutaneous electrotherapies on patient-reported outcome measures (PROMs) in CLBP patients.
Methods: Four databases and two study registries were searched for studies that utilized transcutaneous electrotherapies as a primary intervention for CLBP, compared against active or passive controls. Two reviewers independently extracted study data and assessed risk of bias. Studies were grouped by intervention vs. comparison, and by time of follow-up. Meta-analyses were conducted where appropriate.
Results: A total of 89 full-text were assessed for eligibility; 14 studies were included, with 6 in the meta-analyses (all TENS or mixed TENS). Pain: meta-analyses revealed no significant difference for TENS vs. active control, TENS vs. passive control, or mixed TENS vs. active control at post-intervention, nor for mixed TENS vs. active control at 1-month post-intervention. Interferential current (IFC) was more effective than active control (2 studies), while electromyostimulation (EMS) was generally superior to passive, but not active, controls (6 studies).
Disability: Meta-analyses revealed no significant difference for TENS vs. active control at post-intervention, mixed TENS vs. active control at post-intervention, or mixed TENS vs. active control at 1-month post-intervention. IFC was more effective than active control (2 studies), while the EMS results were mixed (6 studies). We were unable to perform meta-analyses for quality-of-life or psychosocial outcomes.
Conclusion: There is moderate evidence that TENS is similar to all controls for improving pain and disability. There is limited evidence that IFC is superior to active controls for improving pain and disability. There is limited evidence that EMS is superior to passive but not active controls for improving pain, and similar to all controls for improving disability.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=452851, Identifier (CRD42023452851).
1 Introduction
Chronic low back pain (CLBP) affects approximately 20% of the global population (1) and is the leading cause of years-lived with disability worldwide (2), in spite of large healthcare expenditures towards its treatment (3). CLBP is a multi-factorial condition (4–6) and psychological variables (e.g., pain catastrophizing, fear-avoidance beliefs) can mediate CLBP-related patient prognosis (5, 6). To date, however, guidelines for the development of systematic reviews for CLBP interventions have not recommended examining fear-avoidance beliefs, fear of movement, and pain catastrophizing as part of patient-centered outcomes (7, 8).
Transcutaneous electrotherapies are routine conservative interventions for CLBP, typically used for analgesic (e.g., transcutaneous electrical nerve stimulation, interferential current) or motor stimulation (e.g., neuromuscular electrical nerve stimulation, Russian current) purposes. Transcutaneous electrical nerve stimulation (TENS) aims to stimulate sensory nerve fibres, and is thought to promote analgesia by activating endogenous inhibitory pathways in the central nervous system and by reducing peripheral nociceptive output (9). Neuromuscular electrical nerve stimulation (NMES) is a form of electromyostimulation (EMS) that evokes visible muscle contractions (10–12) in order to improve muscle activation and strength (10). While NMES is traditionally delivered under static conditions (10, 11) or superimposed with voluntary isometric contractions (11), whole-body electromyostimulation (WB-EMS) is a recent iteration of NMES which delivers current through a form-fitted suit and allows for whole-body resistance training in conjunction with muscle stimulation (13) Handheld TENS and NMES units deliver current at frequencies that typically range from 2 to 300 Hz (10). In contrast, larger, medium-frequency, stand-alone units (14–16) can produce carrier frequencies ranging from 1 to 4 kHz, allowing for a variety of clinical applications (10, 14, 15, 17). For example, interferential current (IFC) is a medium-frequency treatment typically used for pain relief (10, 17, 18), while Russian (10, 12) and Aussie (14) current are medium-frequency iterations of EMS. Proponents of medium-frequency electrostimulation argue that it lowers skin impedance (19), allowing current to penetrate tissue more deeply than low-frequency alternatives, such as TENS and NMES, and providing a more comfortable experience for users (10, 12).
To date, although a wide spectrum of randomized controlled trials have investigated transcutaneous electrotherapies for CLBP, systematic reviews have overwhelmingly focused on the efficacy of TENS (20–26). Recommendations remain equivocal: some medical guidelines do not recommend TENS for CLBP (27, 28), while recent systematic reviews suggest it improves pain (25) and disability (26) under certain conditions. However, little information is known about the effect of other transcutaneous electrotherapies on PROMs for CLBP. A single meta-analysis that was not CLBP-specific examined the effect of IFC on pain in musculoskeletal conditions (17), and no systematic reviews have been able to evaluate the effect of EMS on PROMs in CLBP patients. The Philadelphia Panel (2001) was unable to find eligible studies investigating the effect of EMS in subjects with CLBP (29). Poitras and Brosseau (2008) attempted to assess the effect of EMS in CLBP, as part of a larger review article, but found no relevant studies (24). The lack of data regarding the efficacy of electrotherapies aside from TENS is clinically relevant: medium-frequency interventions may be more comfortable for patients than low-frequency interventions (10), and recent systematic reviews examining the effect of EMS on paraspinal muscle characteristics have reported that EMS improves paraspinal muscle strength (30, 31) and endurance (30, 31) in CLBP patients. Considered collectively, non-TENS electrotherapies might offer a compelling and more holistic alternative to TENS if they help improve patients' self-reported symptoms, but this remains to be determined.
Therefore, the aim of this systematic review was to assess the overall effect of transcutaneous electrotherapy (e.g., EMS, IFC and TENS) on PROMs for CLBP including pain, back-related disability, fear-avoidance behaviours, catastrophizing beliefs, and quality of life. Focus was paid to short (<3 months), medium (3–12 months), and long-term (>12 months) outcomes.
2 Methods
This systematic review protocol was registered with PROSPERO (ID number: CRD42023452851). In designing the protocol, we followed the recommendations suggested by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 explanation and elaboration guidebook (32). We also adhered to the guidelines proposed by Cochrane Neck and Back (33) for systematic reviews.
2.1 Selection criteria
2.1.1 Types of studies
We included randomized and quasi-randomized control trials assessing the effect of transcutaneous electrotherapy in CLBP patients in comparison with a passive or active control (defined below). In line with the Philadelphia Panel's consensus opinion (29), only studies with ≥5 participants per treatment group were included. To adequately assess the effect of interventions over time, we only included studies with ≥8 treatments per group. Only English or French language articles were included.
2.1.2 Participants
Participants aged 18–70 with a diagnosis of CLBP, defined as persistent pain between the lower ribs and gluteal fold, with or without leg pain, of at least 12 weeks duration were included in this review. Participants diagnosed with a specific spinal pathology, defined as one of the following: infection, tumour, previous lumbar surgery, osteoporosis, fracture, structural deformity (ex. scoliosis), inflammatory disorder (ex. ankylosis spondylosis), or cauda equina syndrome, were excluded. However, participants with lumbar disc herniation were included—provided they did not present with radicular symptoms—in line with evidence that disc degeneration and annulus tears (visible on T2-weighted MRI) are not necessarily painful (34). Studies that included participants with mixed lower and upper back pain were excluded. Additionally, studies that included a mix of acute (<4 weeks) and chronic LBP patients, as well as sub-acute (4–12 weeks) and chronic LBP patients were excluded, in line with suggestions that these conditions be considered separately (35).
2.1.3 Types of interventions
We included studies that use transcutaneous electrotherapy as the primary intervention for CLBP. In cases of studies using transcutaneous electrotherapy plus another intervention, transcutaneous electrotherapy had to account for at least 40% of the treatment program. This cut-off value was previously used by Macedo et al. (2009) in their systematic review of motor control exercises for persistent LBP (35). Studies that compared the effect of transcutaneous electrotherapy against a passive or active control were included. Passive controls were defined as the following: sham electrotherapy (defined as having the device modified so that no current passes to skin-surface electrodes), usual care, and/or no treatment. Actives controls were defined as the following: any non-transcutaneous electrotherapeutic intervention for CLBP, such as ultrasound, hot/cold packs, exercise, mobilization/manipulation, massage/soft tissue therapy, acupuncture, and non-transcutaneous electrotherapy. Studies comparing two transcutaneous electrotherapeutic treatments were be excluded, as were studies comparing the effect of transcutaneous electrotherapy plus another intervention against a third intervention (ex. TENS + hot pack vs. ultrasound), in line with recommendations by the Cochrane Back and Neck Group (33) Additionally, studies where the method of determining stimulation intensity is according to manufacturers' specifications, or where it is not described, were excluded.
2.1.4 Types of outcome measures
We included studies that assessed at least one of the following primary outcomes: pain (ex. VAS, NPRS), back-related disability (ex. Oswestry Disability Index, Roland-Morris disability questionnaire), fear-avoidance behaviours (ex. Fear Avoidance Belief Questionnaire), pain catastrophizing (ex. Pain Catastrophizing Scale), quality of life (ex. Short Health Form Survey), patient satisfaction (ex. Patient Satisfaction Survey), and depression (ex. PHQ-9).
2.2 Search strategy
The following bibliographic databases were searched for studies pertaining to CLBP and transcutaneous electrotherapy: PubMed, Scopus, Web of Science, and Embase. Additionally, the follow study registers were searched for protocols of the included studies: WHO International Clinical Trials Registry Platform1 and the US National Institute of Health.2 A search strategy was developed based on a literature review, and with help from a reference librarian at Concordia University affiliated with the department of Health, Kinesiology, and Applied Physiology. Mesh terms and key words related to: (1) Low back pain, (2) electrical stimulation therapy, (3) TENS, (4) NMES were used. The search strategy for PubMed and Embase is available in Appendix 1. The initial search was performed between February 1, 2022 and March 31, 2022, and the database was updated for the last time on September 15, 2022. No time limit was applied to publication dates. Search results were compiled in the reference management software Zotero (version 5.0.96.3).
2.3 Study selection
Two reviewers (DW, BR) initially screened the search results for potential studies based on the study title and, where reasonable, the abstract. After excluded articles during this first round, the full text of the remaining articles was read by the same reviewers, and a global yes/no decision was made for each potential study based on the inclusion criteria identified in Section 2.1. In the case of disagreement over the inclusion of an article, a third reviewer (MF) was consulted, and a consensus decision between the 3 reviewers was taken. Study screening was managed using SR Accelerator.3
2.4 Data extraction and risk of bias
Two reviewers (DW, BR) independently extracted data from each included study using a modified version of the extraction template developed by Cochrane Back and Neck (33). Participant characteristics, interventions, comparisons, outcomes, analysis approach, results, and study sponsorship were recorded.
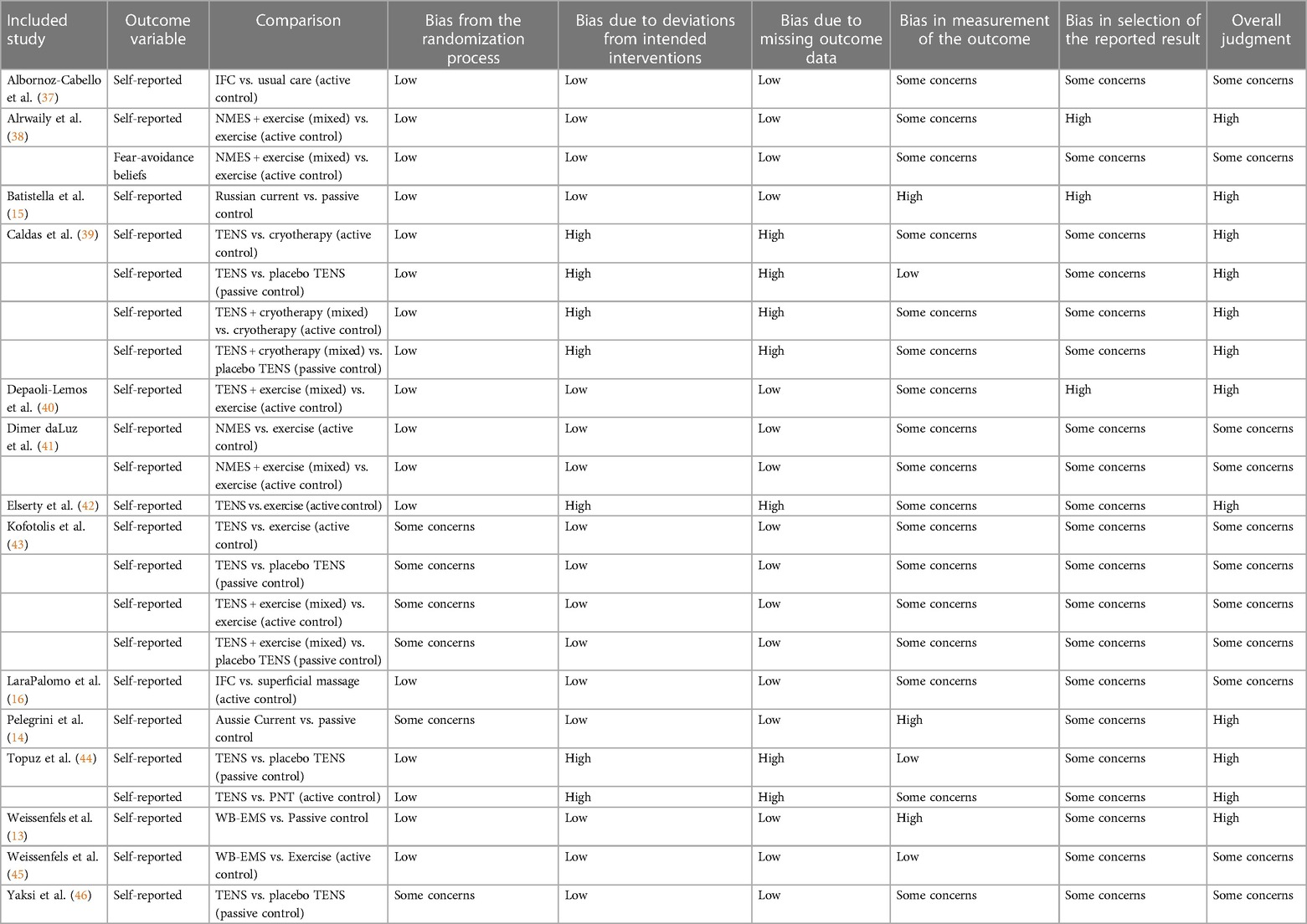
Risk of bias was assessed using the revised Cochrane risk-of-bias tool (RoB 2) (36) for randomized trials. This tool examines five domains: randomization bias, bias due to deviations from intended interventions, missing outcome data bias, measurement bias, and bias in selection of the reported result. Bias is assessed on a per-outcome basis, and the tool uses signaling questions and an algorithm to guide reviewers to judgment. Each outcome is rated as follows: low risk, some concerns, high risk. Both reviewers (DW, BR) strictly followed the instructions outlined in the full guidance document for the RoB 2 tool. In case of disagreement, a third reviewer (MF) was consulted.
2.5 Statistical analysis
Studies were grouped according to intervention vs. comparator (active or passive), at short, medium, and long term follow up (subgroup analysis). Since not all studies provided change scores, we based our between-group analyses on post-intervention or equivalent (e.g., 1-month post-intervention) scores. Outcome values were pooled where appropriate using StatstoDo4. Meta-analyses were conducted, using a random-effects model, when comparisons within a group were sufficiently homogenous with respect to PICO variables (population, intervention, comparator, outcome). A minimum of three comparisons were needed for a comparison to be eligible for meta-analysis; in such cases, the overall treatment effect of the intervention, with 95% confidence intervals, was calculated for each outcome. For continuous variables measured using different scales, the standardized mean difference (SMD) was calculated. Statistical heterogeneity was conducted using the Q-test, and reported as the I2 statistic. We interpreted the statistic as follows: <40% suggests a low risk of heterogeneity, 40%–75% a moderate heterogeneity, >75% a high risk of heterogeneity (33). We used the Review Manager statistical software (RevMan version 5.4.1) to conduct the meta-analyses.
3 Results
3.1 Search results
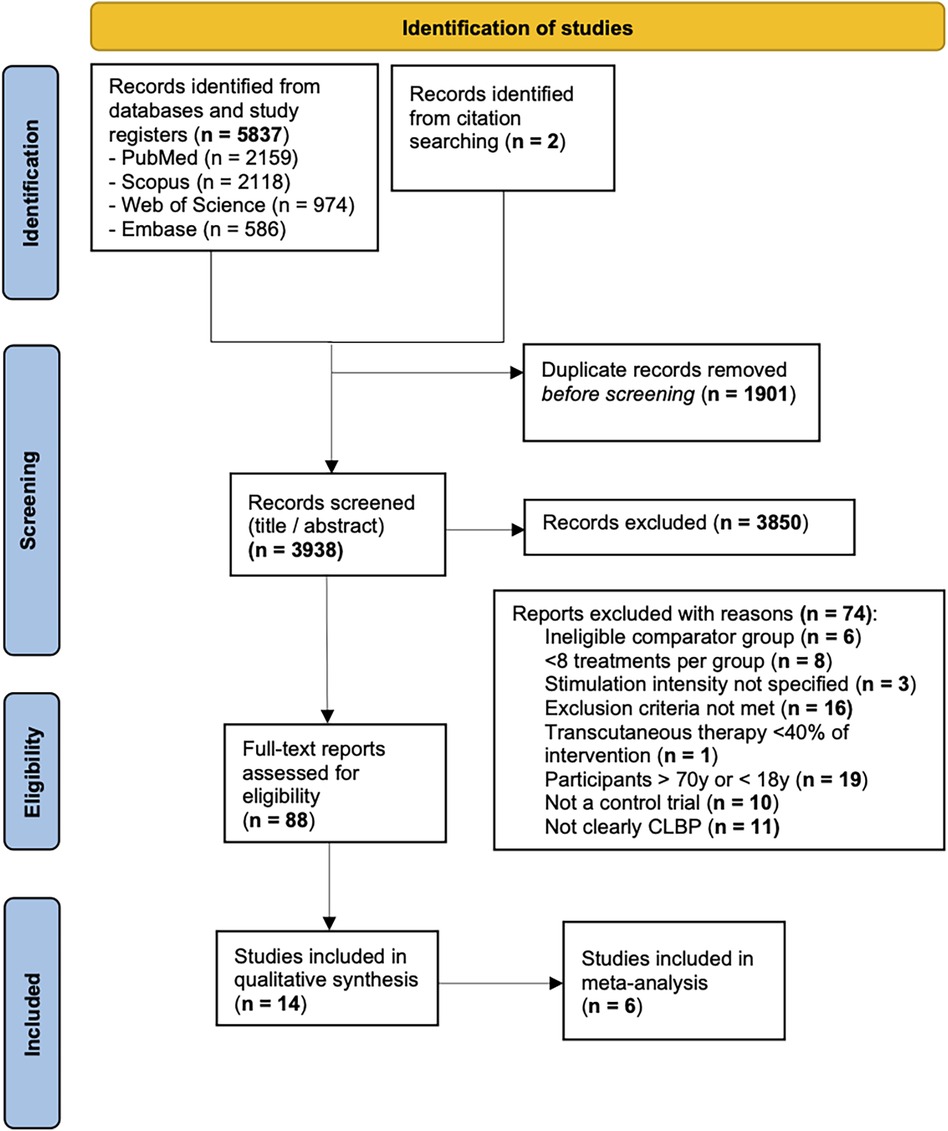
We performed an electronic search for eligible articles across the following four databases: PubMed, Embase, Scopus, and Web of Science. We also hand searched reference lists for articles that the electronic search might have missed. A total of 5,839 records were found through the search. After removing duplicates, 3,938 titles were screened and 88 were selected for a full-text review. Fourteen studies were included in the qualitative review and six were included in the meta-analysis. The full results of our search are presented in Figure 1.
3.2 Risk of bias
Risk of bias was evaluated on a per-outcome basis and ranged from “some concerns” to “high”. Specifically, bias in selected of the reported result was judged to be of at least “some concerns” for all outcomes, because no protocols for statistical analysis could be found for any of the included studies, and therefore a comparison between the published report and the protocol could not be performed, which automatically elevates the risk of bias for this domain. The risk-of-bias assessment is presented in Table 1.
3.3 Characteristics of included studies
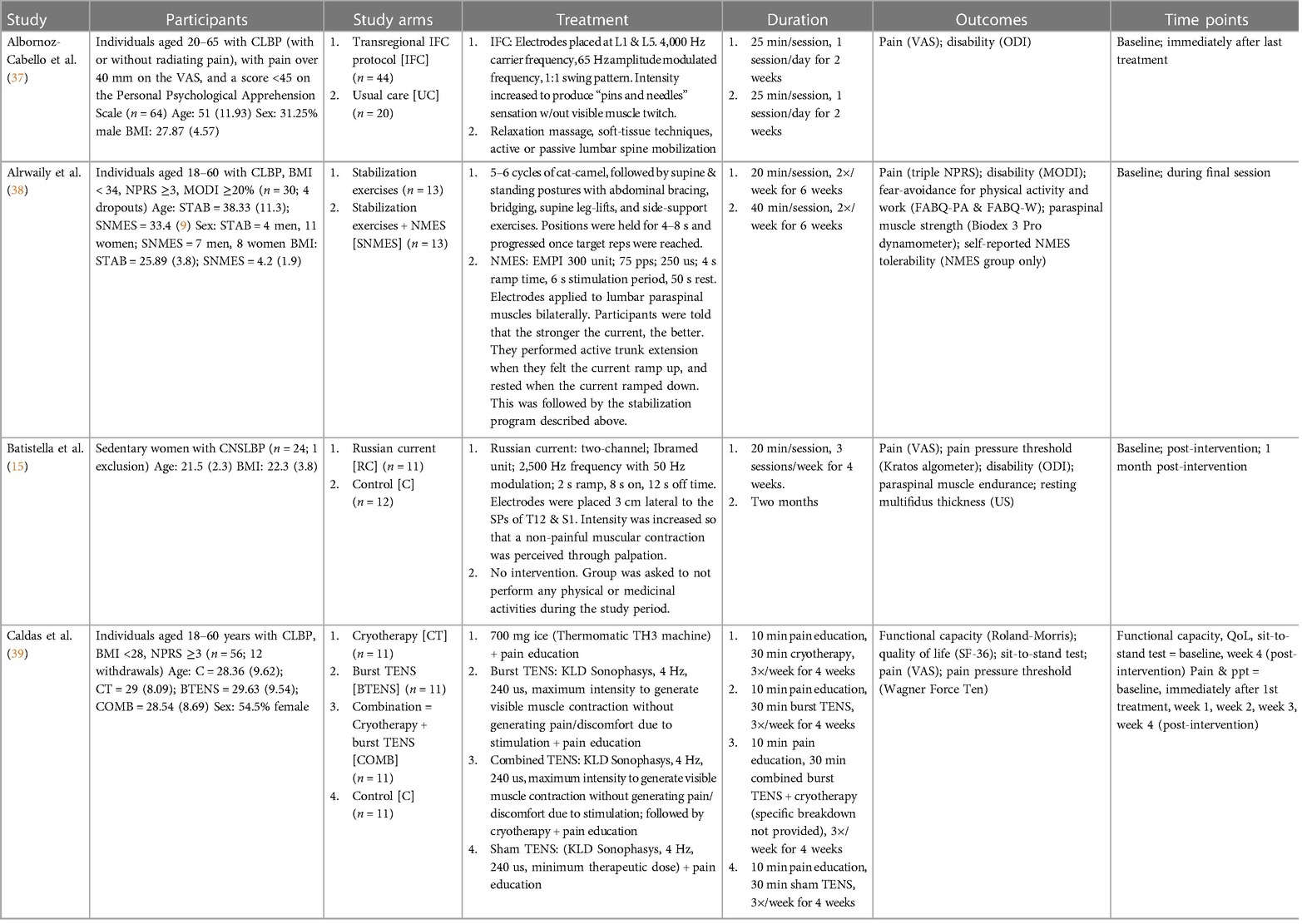
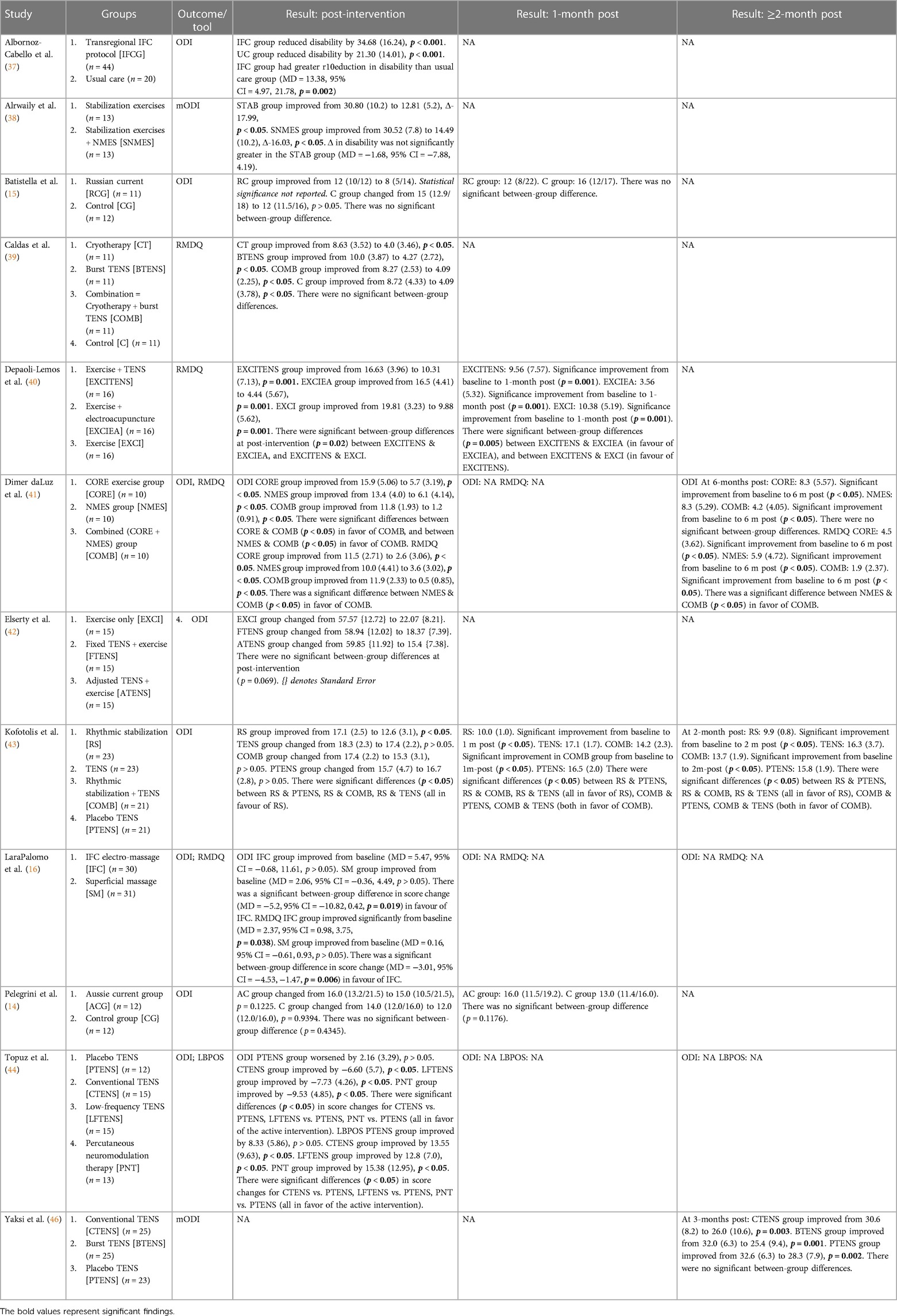
Of the fourteen included studies, twelve were stand-alone, and two formed a pair with a shared recruitment process and inclusion/exclusion criteria, but different interventions. Eight studies evaluated sensory electrotherapy: six used TENS (39, 40, 42–44, 46), and two used IFC (16, 37) The remaining six studies evaluated EMS: one used NMES (38), one used Russian current (15), one used Aussie current (14), one used mid-frequency (2,500 Hz) current with progressive low-frequency (LF) modulation (41), and two used WB-EMS (13, 45) Furthermore, eight studies were comprised of stand-alone transcutaneous electrotherapy interventions (13–16, 37, 44–46), three studies included only mixed interventions (38, 40, 42) (electrotherapy plus an additional intervention), and three studies evaluated both stand-alone and mixed interventions (39, 41, 43) Study characteristics are provided in Table 2, while the results are presented, per outcome, in Tables 3–7.
3.4 Outcome: pain
3.4.1 TENS vs. active controls at post-intervention
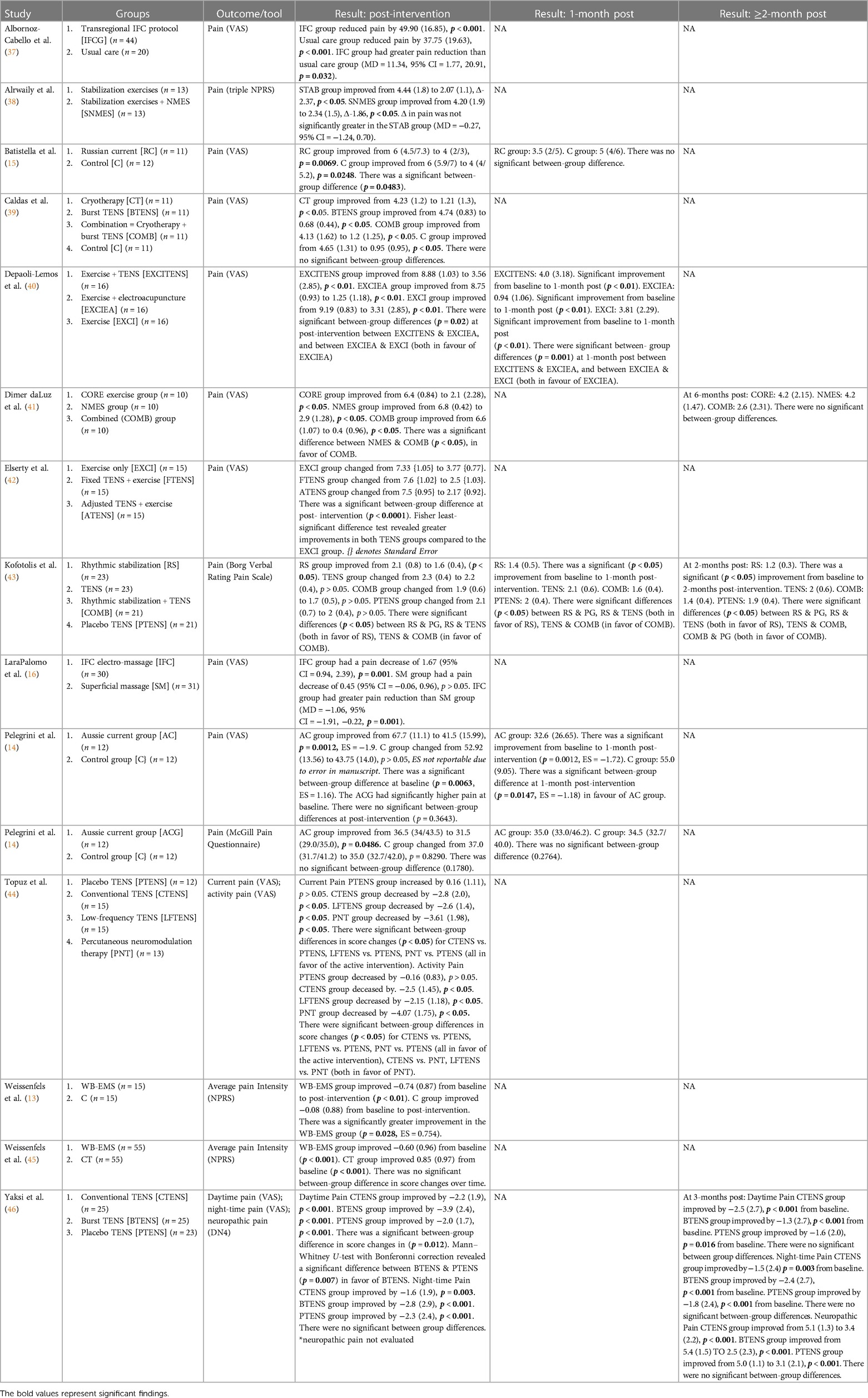
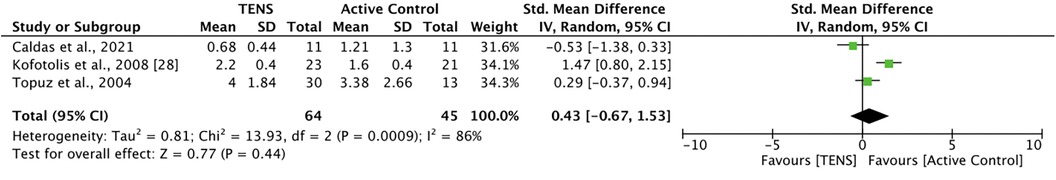
Three studies (39, 43, 44) compared TENS with an active control. Caldas et al. (39) found that 30 min of burst TENS plus 10 min of pain education was not significantly more effective than 30 min of cryotherapy plus 10 min of pain education at reducing pain. Kofotolis et al. (43) compared 40–45 min of TENS with 30–45 min of rhythmic stabilization exercises, finding that rhythmic stabilization was significantly more effective than TENS (p < 0.05). Topuz et al. (44) compared 20 min of conventional TENS and 20 min of low-frequency TENS with 20 min of percutaneous neuromodulation therapy (PNT). There were no significant between-group differences in current pain, but PNT was significantly more effective than both TENS interventions at improving activity pain (p < 0.05). Meta-analysis revealed that active control was not significantly better than TENS at improving pain at post-intervention [SMD = 0.43, 95% CI = −0.67, 1.53] (Figure 2). The comparisons made in this analysis had a high degree of heterogeneity (I2 = 86%). For meta-analysis, the means and standards deviations of both TENS groups in Topuz et al. (44) were pooled, and “current pain” was selected as the outcome measure.
3.4.2 TENS vs. active controls at ≥1-month post-intervention
Of the three studies listed above, only Kofotolis et al. (43) continued to monitor participants post-intervention. At 1-month post-intervention, the rhythmic stabilization group has significantly less pain than the TENS group (MD = 0.70, 95% CI = 0.37, 1.03). At 2-months post-intervention, the rhythmic stabilization group continued to have significantly less pain than the TENS group (MD = 0.80, 95% CI = 0.52, 1.08).
3.4.3 TENS vs. passive controls at post-intervention
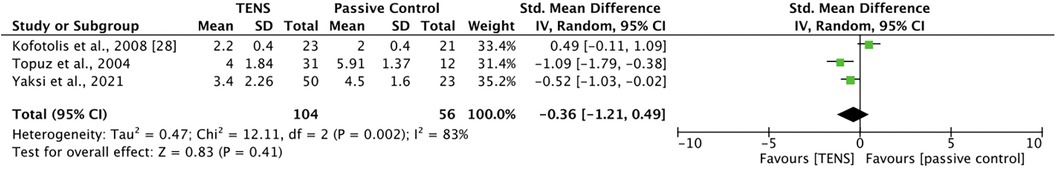
Three studies (43, 44, 46) compared TENS with a passive control. Kofotolis et al. (43) compared 40–45 min of TENS with 40–45 min of sham TENS. There were no significant between-group differences at post-intervention. Topuz et al. (44) compared 20 min of conventional TENS and 20 min of low-frequency TENS with 20 min of sham TENS. Both active TENS groups were significantly more effective than sham TENS at improving current pain (p < 0.05) and activity pain (p < 0.05), with no significant differences between the two active TENS groups. Yakzi et al. (46) compared the effect of 30 min of conventional TENS and burst TENS with 30 min of placebo TENS on daytime and night-time pain. At post-intervention, there was a significant difference in score change between the groups (p = 0.012). A Mann–Whitney U-test with Bonferroni correction revealed a significant difference between burst TENS and placebo TENS, with burst TENS significantly more effective (p = 0.007) at reducing pain. For night-time pain, there were no significant between-group differences. Additionally, Caldas et al. (39) compared TENS with sham TENS; however, the sham TENS group received the minimum therapeutic dose and was excluded from analysis. Meta-analysis revealed that TENS was not significantly better than passive control at improving pain at post-intervention [SMD = −0.36, 95% CI = −1.21, 0.49] (Figure 3). The comparisons made in this analysis had a high degree of heterogeneity (I2 = 83%). For meta-analysis, the means and standards deviations of both TENS groups in Topuz et al. (44) and Yaksi et al. (46) were pooled; additionally, “current pain” was selected as the outcome measure in Topuz et al. (44) and “daytime pain” was selected in Yaksi et al. (46).
3.4.4 TENS vs. passive controls at ≥1-month post-intervention
Two studies examined the effect of TENS at 1-month post-intervention and beyond. Kotofolist et al. (43) compared TENS with sham TENS at 1 and 2-months post-intervention. There were no significant differences between the groups at either time point. Yaksi et al. (46) compared conventional and burst TENS with sham TENS at 3-months post-intervention, and reported no significant between-group differences.
3.4.5 Mixed TENS vs. active controls at post-intervention
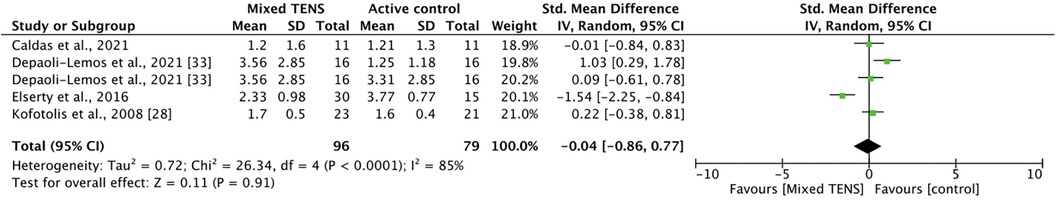
Five comparisons across four studies studied the effect of a mixed TENS intervention vs. active control. Caldas et al. (39) compared 30 min of burst TENS/cryotherapy + 10 min of pain education with 30 min of cryotherapy + 10 min of pain education alone. Depaoli-Lemos et al. (40) compared 30 min of exercise + 20 min of TENS with 30 min of exercise alone; additionally, they compared 30 min of exercise + 20 min of TENS with 30 min of exercise + 20 min of electro-acupuncture. Elserty et al. (42) compared fixed TENS + 40 min of exercise, adjusted TENS + 40 min of exercise, and 40 min of exercise alone (for meta-analysis, the results of the TENS groups were pooled). Finally, Kofotolis et al. (43) compared rhythmic stabilization exercises + TENS with TENS alone. Meta-analysis revealed that mixed TENS was not significantly better than active control at reducing pain [SMD = −0.04, 95% CI = −0.86, 0.77] (Figure 4). The comparisons made in this analysis had a high degree of heterogeneity (I2 = 85%).
3.4.6 Mixed TENS vs. active controls at 1-month post-intervention
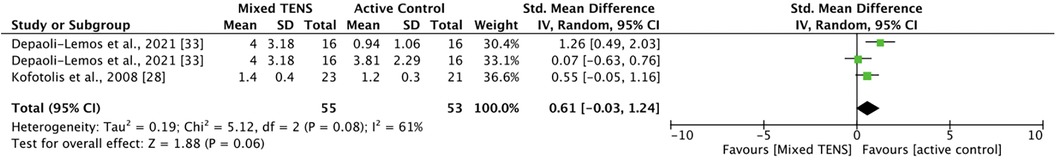
Two studies involving three comparisons examined mixed TENS vs. active control for pain at 1-month post-intervention: two from Depali-Lemos et al. (40) and one from Kofotolis et al. (43). Meta-analysis revealed that active control was not significantly better than mixed TENS at reducing pain 1-month post intervention [SMD = 0.61, 95% CI = −0.03, 1.24] (Figure 5). The comparisons made in this analysis had a moderate degree of heterogeneity (I2 = 61%).
3.4.7 Mixed TENS vs. active controls at >1-month post-intervention
Kofotolis et al. (43) compared TENS + rhythmic stabilization exercises with rhythmic stabilization exercises alone at 2-months post-intervention. There were no significant differences between the groups for pain at this timepoint.
3.4.8 Mixed TENS vs. passive controls at post-intervention
Kofotolis et al. (43) compared TENS + rhythmic stabilization exercises with sham TENS. There were no significant differences between the groups for pain at this timepoint.
3.4.9 Mixed TENS vs. passive controls at ≥1-month post-intervention
Kofotolis et al. (43) compared TENS + rhythmic stabilization exercises with sham TENS at 1- and 2-months post-intervention. At 1-month post-intervention, there were no significant between-group differences. However, at 2-months post-intervention, the combined group had significantly less pain than the sham TENS group (1.4 (0.4) vs. 1.9 (0.5), p < 0.05).
3.4.10 IFC vs. active controls at post-intervention
Two studies compared the effectiveness of IFC with an active control on pain. Albornoz-Cabello et al. (37) compared IFC with a usual care intervention; the IFC group had a significantly greater reduction in pain (MD = −11.34, 95% CI = −1.77, −20.91). Lara-Palomo et al. (16) compared IFC with superficial massage; the IFC group had a significantly greater reduction in pain (MD = 1.06, 95% CI = −1.91, −0.22).
3.4.11 EMS vs. active controls at post-intervention
Dimer daLuz et al. (41) compared NMES with core exercises for pain. At post-intervention, there were no significant differences between the groups.
3.4.12 EMS vs. active controls at ≥1-month post-intervention
Dimer daLuz et al. (41) re-examined pain outcomes at 6-months post-intervention and reported no significant differences between the groups.
3.4.13 EMS vs. passive controls at post-intervention
Batistella et al. (15) compared the effect of Russian Current with passive control (no intervention). At post-intervention, the Russian Current group had significantly less pain than the control group (median = 4, [2,4] vs. median = 4 [4,5.2], p = 0.0483). Pelegrini et al. (14) compared the effect of Aussie Current with passive control (no intervention). At post-intervention, there were no significant between-group differences noted on either the VAS or the McGill Pain Questionnaire. However, the Aussie Current group had significantly greater pain at baseline (p = 0.0063, ES = 1.16).
3.4.14 EMS vs. passive controls at 1-month post-intervention
At 1-month post-intervention, Batistella et al. (15) reported no significant differences in pain between the Russian Current and control groups, while Pelegrini et al. (14) found that the Aussie Current group had significantly less pain than the control group as reported on the VAS (32.60 (26.65) vs. 55.00 (9.05), p = 0.0147), but not the McGill Pain Questionnaire.
3.4.15 Mixed EMS vs. active controls at post-intervention
Three studies investigated the effect of mixed EMS vs. active control. Due to reporting differences, only two studies were eligible for meta-analysis, and therefore none was conducted. Alrwaily et al. (38) compared the effect of NMES + stabilization exercises with stabilization exercises alone and reported no significant between-group differences for pain at post-intervention. Dimer daLuz et al. (41) compared NMES + core exercises with core exercises alone. At post-intervention, there were no significant differences between the groups. Finally, Weissenfels et al. (45) compared WB-EMS with conventional back strengthening exercises. At post-intervention, there were no significant between-group differences in change in pain from baseline (p = 0.160).
3.4.16 Mixed EMS vs. active controls at ≥1-month post-intervention
Dimer daLuz et al. (41) re-examined pain outcomes at 6-months post-intervention and reported no significant differences between the groups.
3.4.17 Mixed EMS vs. passive controls at post-intervention
Weissenfels et al. (13) compared the effect of WB-EMS with passive control (no intervention). At post-intervention, the WB-EMS group had achieved a significantly greater reduction in pain from baseline compared to the control group (MD = 0.67, 95% CI = 0.18, 1.21, p = 0.028).
3.5 Outcome: disability
3.5.1 TENS vs. active controls at post-intervention
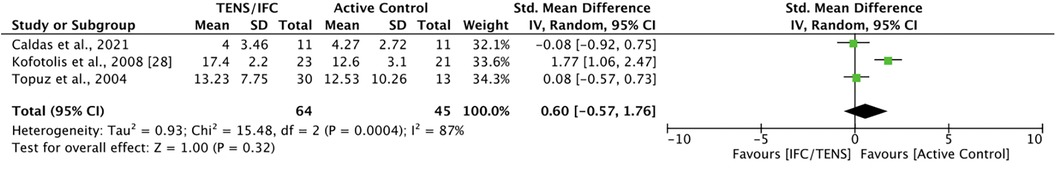
Three studies compared TENS with active controls for disability. Caldas et al. (39) did not find significant difference between TENS and cryotherapy at post-intervention. Kofotolis et al. (43) reported that rhythmic stabilization exercises were significantly more effective than TENS at improving disability (MD = 4.80, 95% CI = 3.20, 6.40). Topuz et al. (44) found no significant differences on both the ODI and LBPOS between PNT and either conventional or low-frequency TENS at post-intervention. Meta-analysis revealed no significant difference between TENS and active control [SMD = 0.60, 95% CI = −0.57, 1.76] (Figure 6). The comparisons in this analysis had a high degree of heterogeneity (I2 = 87%).
3.5.2 TENS vs. active controls at ≥1-month post-intervention
Only one study examined the effect of TENS at 1-month post-intervention and beyond. At 1-month post-intervention, Kofotolis et al. (43) reported that rhythmic stabilization exercises were significantly more effective than TENS at improving disability (MD = 7.10, 95% CI = 6.28, 7.92). At 2-months post-intervention, Kofotolis et al. (43) found that rhythmic stabilization exercises continued to be significantly more effective than TENS at improving disability (MD = 6.40, 95% CI = 4.85, 7.95).
3.5.3 TENS vs. passive controls at post-intervention
Two studies compared the effect of TENS with passive controls on disability. Kofotolis et al. (43) reported that TENS was not significantly more effective than sham TENS at improving disability. Topuz et al. (44) reported that both conventional TENS and low-frequency TENS were more effective than sham TENS at improving disability (using the ODI) at post-intervention (p < 0.05). After pooling the two TENS groups, the combined group continued to demonstrate greater effectiveness than sham TENS (MD = −5.14, 95% CI = −9.18, −1.10). However, when reporting with the LBPOS, while both conventional and low-frequency TENS were more effective than sham TENS at improving function at post-intervention (both p < 0.05), this effect was not significant when the groups were pooled (MD = 4.75, 95% CI = −2.95, 12.45).
3.5.4 TENS vs. passive controls at ≥1-month post-intervention
Two studies examined the effect of TENS at 1-month post-intervention and beyond. Kofotolis et al. (43) reported no difference in disability between TENS and sham TENS at 1-month and 2-months post-intervention. Yaksi et al. (46) reported no significant differences in disability between the conventional TENS, burst TENS, and placebo TENS groups at 3-months post-intervention.
3.5.5 Mixed TENS vs. active controls at post-intervention
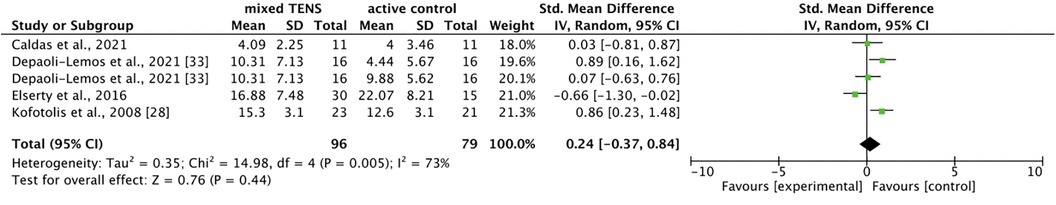
Five comparisons across four studies (39, 40, 42, 43) compared mixed TENS with active control for disability. Meta-analysis revealed no significant difference between mixed TENS and active control [SMD = 0.24, 95% CI = −0.37, 0.84] (Figure 7). The comparisons made in this analysis had a moderate degree of heterogeneity (I2 = 73%).
3.5.6 Mixed TENS vs. active controls at ≥1-month post-intervention
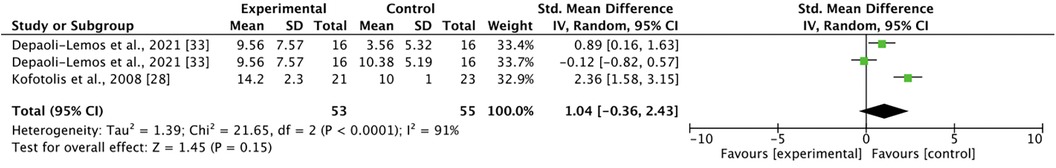
Two studies involving three comparisons examined mixed TENS vs. active control for pain at 1-month post-intervention: two from Depali-Lemos et al. (40) and one from Kofotolis et al. (43) Met-analysis revealed no significant differences between mixed TENS and active control [SMD = 1.04, 95% CI = −0.36, 2.43] (Figure 8). The comparisons in this analysis had a high degree of heterogeneity (I2 = 91%). At 2-months post-intervention, Kofotolis et al. (43) reported that rhythmic exercise continued to be significantly more effective than combined TENS + rhythmic stabilization exercises at reducing disability (9.9 (0.8) vs. 13.7 (1.9), p < 0.05).
3.5.7 Mixed TENS vs. passive controls at post-intervention
Kofotolis et al. (43) investigated the effect of TENS + rhythmic stabilization exercises vs. sham TENS on disability. At post-intervention, there were no between-group differences.
3.5.8 Mixed TENS vs. passive controls at ≥1-month post-intervention
At 1-month post-intervention, Kofotolis et al. (43) found that the mixed TENS group had significantly less disability than the sham TENS group (14.2 (2.3) vs. 16.5 (2.0), p < 0.05). At 2-months post-intervention, this finding continued to hold (13.7 (1.9) vs. 15.8 (1.9), p < 0.05).
3.5.9 IFC vs. active controls at post-intervention
Both Albornoz-Cabello et al. (37) and Lara-Palomo et al. (16) investigated the effect of their intervention on disability. Albornoz-Cabello et al. (37) reported that IFC was significantly more effective than usual care (MD = −13.38, 95% CI = −21.78, −4.97), while Lara-Palomo et al. (16) found that IFC was more effective than superficial massage as reported with both the ODI (MD = −5.20, 95% CI = −10.82, 0.42) and the RMDQ (MD = −3.01, 95% CI = −4.53, −1.47).
3.5.10 EMS vs. active controls at post-intervention
Dimer daLuz et al. (41) reported no significant differences between the NMES and core-exercise groups on both the ODI and RMDQ.
3.5.11 EMS vs. active controls at ≥1-month post-intervention
At 6-months post-intervention, Dimer daLuz et al. (41) continued to find no significant differences between the NMES and core-exercise groups.
3.5.12 EMS vs. passive controls at post-intervention
Neither Batistella et al. (15) nor Pelegrini et al. (14) reported significant differences between their active intervention (NMES and Aussie Current, respectively) and passive control for disability at post-intervention.
3.5.13 EMS vs. passive controls at 1-month post-intervention
Neither Batistella et al. (15) nor Pelegrini et al. (14) reported significant differences between their active intervention (NMES and Aussie Current, respectively) and passive control for disability at 1-month post-intervention.
3.5.14 Mixed EMS vs. active controls at post-intervention
Both Alrwaily et al. (38) and Dimer daLuz et al. (41) investigated the effect of their intervention on disability. Alrwaily et al. (38) reported no significant between-group differences. Dimer daLuz et al. (41) found that the mixed EMS group had significantly less disability than the core exercise group on the ODI [1.2 (0.91) vs. 5.17 (3.19), p < 0.05], but not on the RMDQ [0.5 (0.85) vs. 2.6 (3.06), p > 0.05].
3.5.15 Mixed EMS vs. active controls at ≥1-month post-intervention
Dimer daLuz et al. (41) re-examined disability outcomes at 6-months post-intervention and reported no significant differences between the groups on both the ODI and RMDQ.
3.6 Outcome: quality of life
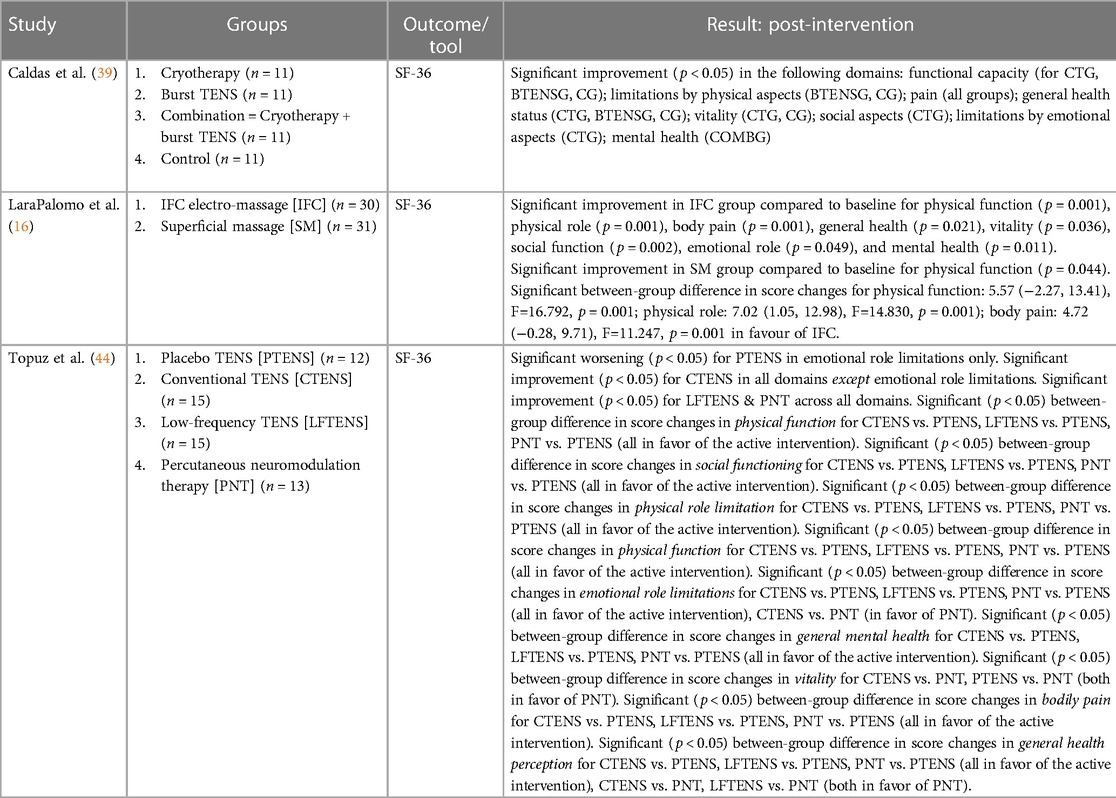
3.6.1 TENS vs. active controls at post-intervention
Two studies compared the effect of TENS with active controls on quality of life. Caldas et al. (39) reported no difference between TENS and cryotherapy on any of the SF-36 domains at post-intervention. Topuz et al. (44) found that PNT was significantly more effective than conventional TENS at improving the emotional role limitations domain of the SF-36 (p < 0.05), significantly more effective than conventional TENS at improving the vitality domain of the SF-36 (p < 0.05), and significantly more effective than both conventional and low-frequency TENS at improving the general health perception domain of the SF-36 (both p < 0.05) at post-intervention.
3.6.2 TENS vs. passive controls at post-intervention
One eligible study compared the effect of TENS with sham TENS on quality of life. Topuz et al. (44) reported that both conventional and low-frequency TENS were significantly more effective than sham TENS at improving the following quality-of-life domains: physical functioning, social functioning, physical role limitation, general mental, bodily pain, and general health perception (all p < 0.05). Low-frequency TENS was significantly more effective than sham TENS at improving the emotional role limitations domain (p < 0.05), but sham TENS was significantly more effective than conventional TENS (p < 0.05).
3.6.3 IFC vs. active controls at post-intervention
Lara-Palomo et al. (16) investigated the effect of IFC on quality-of-life. IFC was more effective than superficial massage in improving quality of life in the following domains: physical function (MD = 5.57, 95% CI = −2.27, 13.41), physical role (MD = 7.02, 95% CI = 1.05, 12.98), body pain (MD = 4.27, 95% CI = −0.28, 9.71).
3.7 Outcome: fear-avoidance beliefs
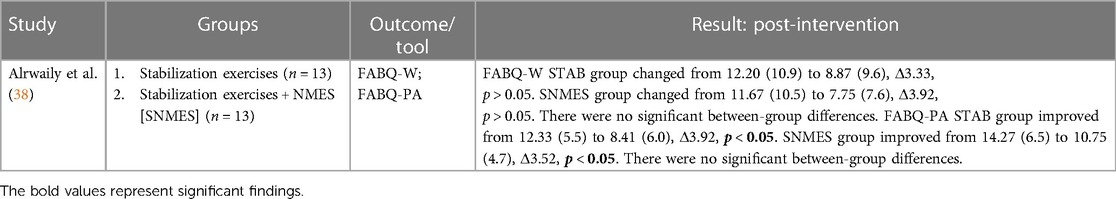
3.7.1 Mixed EMS vs. active controls at post-intervention
Alrwaily et al. (38) considered the effect of their intervention on work- and physical activity-related fear avoidance beliefs. At post-intervention, there were no significant between-group differences for either work- or physical activity-related fear avoidance beliefs.
3.8 Outcome: depression
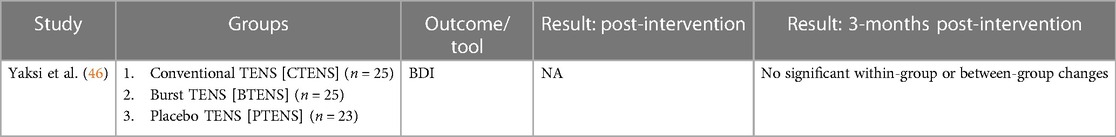
3.8.1 TENS vs. passive control at 3-months post-intervention
Yaksi et al. (46) reported no significant within- or between-group differences in depression at 3-months post-intervention.
4 Discussion
To our knowledge, this is the first meta-analysis to evaluate the effect of a variety of transcutaneous electrotherapies on several PROMs for CLBP. Previous systematic reviews reported on the effect of TENS for pain [Van Tulder et al. (20); Flowerdew et al. (21); Milne et al. (22); Khadlikar et al. (23); Poitras and Brosseau (24); Jauregui et al. (25); Wu et al. (26)) and TENS for disability/function (Milne et al. (22); Poitras and Brosseau (24); Khadilkar et al. (23); Wu et al. (26)] for CLBP patients. The most recent systematic reviews reported a modest but positive effect on pain and function under certain conditions. Jauregui et al. (2016) found significant weighted mean differences in pain intensity in patients treated with TENS for less than, but not more than, 5 weeks (25) Wu et al. (2018) found that TENS improves functional disability when follow-up is within 6-weeks of treatment, compared to controls (26) To date, no systematic reviews had examined the effect of TENS on pain catastrophizing or fear-avoidance beliefs in CLBP patients. Additionally, no systematic reviews had been able to report the effect of EMS on PROMs, while a single review reported on the effect of IFC for pain in a variety of musculoskeletal conditions including CLBP (17). An additional novelty of this meta-analysis is that stand-alone and mixed interventions were examined separately at multiple time-points.
The effect of transcutaneous electrotherapies on pain, compared to active and passive controls, appears to be intervention-dependant. The results of the meta-analyses for TENS on pain revealed no significant differences between TENS and either active or passive controls at post-intervention. This finding is similar to Wu et al. (26), who reported no significant differences between TENS and passive control for pain [SMD: −0.20, 95% CI: −0.58, 0.18, p = 0.293]. Wu et al. (26) additionally found that neural stimulation therapy (NST) was more effective than TENS at reducing pain [SMD: 0.68, 95% CI: 0.15, 1.57, p = 0.017]. Although our meta-analysis did not reveal a significant difference between TENS and active control, our definition of active control was very broad, which may explain the discrepancy with Wu et al. (2018). A single study (43) re-assessed pain at 1- and 2-months post-intervention and reported that the active control group had less pain than the TENS group at both time points. Similarly, meta-analyses for the effect of mixed TENS revealed no significant differences between active control at post-intervention and 1-month post-intervention. There were also no reported differences between mixed TENS and active control at 2-months post-intervention (43) or between mixed TENS and passive control and post intervention (43) and 1-month post intervention (43) However, one study reported that the mixed TENS group had significantly less pain than passive controls at 2-months post-intervention (43) On the other hand, the two studies that investigated IFC (16, 37) both reported that it was more effective than active control at reducing pain at post-intervention. Amongst EMS studies, stand-alone EMS was not superior to active control at post-intervention (41) and 6-months post-intervention (41), but it was more effective than passive control at post-intervention (15) and 1-month post-intervention (14). Similarly, mixed EMS was similar to active control at post-intervention (38, 41, 45) and 6-months post-intervention (41), but was more effective than passive control at post-intervention (13).
The results of this synthesis suggest that both IFC and EMS may be more suitable interventions for reducing pain at post-intervention than TENS, since they performed better than TENS vs. active controls (the IFC studies) and passive controls (the EMS studies). Nevertheless, this finding should be interpreted with caution, given that no meta-analysis for IFC or EMS was performed. Previously, a 2020 overview of Cochrane Reviews (47) examined eight Cochrane Reviews of TENS for chronic pain, involving 51 RCTs and spanning 2,895 participants, but was unable to conclude with confidence whether TENS was beneficial or safe for pain control. Similarly, a recent comparative review of electrical stimulation (ES) devices found that the efficacy of TENS for pain was low-to-insignificant (48). In our systematic review, an analysis of within-group changes for all active transcutaneous electrotherapy interventions suggests that TENS, IFC, and EMS are generally effective at reducing pain at post-intervention: 15 interventions resulted in a significant reduction in pain (6 TENS, 2 IFC, 7 EMS), while 2 did not (2 TENS). Additionally, a recent meta-analysis of TENS for chronic pain (the meta-TENS study) (49) reported that TENS was more effective than placebo for pain relief during or immediately post-application [SMD: −0.96, 95% CI: −1.14, 0.78; moderate-certainty evidence] and more effective than pharmacological and non-pharmacological treatment for pain relief during or immediately post-application [SMD: −0.72, 95% CI: −0.95, −0.50; low-certainty evidence]. Clinicians may want to prioritize the use of IFC or EMS as part of a longer-term treatment plan, especially since recent systematic reviews reported that EMS is effective at improving trunk muscle strength (30) and endurance (30, 31) in CLBP patients, but TENS may be suitable for short-term pain relief within a multi-modal treatment plan.
The efficacy of transcutaneous electrotherapies at reducing disability was mixed. For TENS interventions, one study found that active control was more effective at post-intervention and ≥1-month post-intervention (43), while another reported no significant differences at post-intervention (44). Some studies found that TENS was more effective than passive control (44), while others found no difference (43, 46). Mixed TENS was more effective than passive controls at ≥1-month post-intervention (43), but not more effective at earlier time points (43) or compared to active controls at any time point (42, 43) Additionally, there were no significant differences in effect on disability for any of the EMS interventions at any time point, with the exception of Dimer da Luz et al. (41), who reported that mixed EMS was significantly more effective than active control at post-intervention. On the other hand, both IFC studies (16, 37) reported that it was significantly more effective than active control at reducing disability at post-intervention. An analysis of within-group changes for active transcutaneous electrotherapy interventions suggests an overall benefit for disability at post-intervention: 8 interventions resulted in a significant reduction in disability (2 TENS, 2 IFC, 4 EMS), while 3 did not (2 TENS, 1 EMS). Previous systematic reviews reported no overall difference in disability between TENS and passive controls (22, 23, 26), although one found that TENS was superior to passive control when follow-up was less than 6-weeks [SMD: −1.24, 95% CI: −1.83, −0.65, p < 0.001] (26) Additionally, Wu et al. (26) reported no difference in disability between TENS and NST.
4.1 Limitations
Due to the small number of studies that investigated quality-of-life, fear-avoidance belief, and depression outcomes, no meta-analyses were performed for any of these outcome measures. We were also unable to find eligible studies that investigated pain catastrophizing. Therefore, we could not draw meaningful conclusions about the effectiveness of transcutaneous electrotherapies on these outcome measures. Another limitation of this systematic review is a lack of consistency with the included studies' definition of CLBP and inclusion criteria. Ten studies defined CLBP as low back pain of at least 3 months duration (14–16, 37–42, 44), two defined it as low back pain experienced on ≥ 50% of days in the previous 3 months (13, 45), one defined it as mechanical back pain of at least 3 months duration from degenerative disc disease or disc herniation without radicular compression (46), and one defined it as “chronic”—without specifying a baseline for symptom duration—whilst noting that participants were recruited from a pool of individuals who had LBP for at least 6 months (43). Studies also differed with respect to baseline levels of pain or disability. Five studies required participants to have at least a moderate amount of pain or disability at baseline (16, 37–39, 41) and the remaining nine did not (13–15, 40, 42–46). Requiring study participants to have at least a moderate degree of pain or disability allows for minimum important changes (MIC) to be detected. This value has been reported for several LBP questionnaires that our included studies used: the Visual Analogue Scale (MIC = 15), Numerical Pain Rating Scale (MIC = 2), the Oswestry Disability Index (MIC = 20%), and the Roland Morris Disability Questionnaire (MIC = 5) (50). Of the ten studies that used the ODI, participants in at least one study arm of four studies (14, 15, 43, 44) had baseline ODI scores of <20%, which may have limited improvements in disability.
A significant limitation to the generalizability of our findings was the high level of heterogeneity (> 80%) that was observed in five out the seven meta-analyses we conducted. This heterogeneity is likely due to between-study differences in active controls and sample characteristics. For example, in the comparison between TENS and passive controls for immediate pain relief, the one study (43) that did not report a significant between-group difference in pain had a sample comprised entirely of women, while the other two (44, 46) were comprised of sexes. While it is possible that the outcomes observed reflect sex-based differences in response to TENS, meta-analysis with a random-effects model—which we used—tends to give each sample a similar weight when calculating the mean difference (51). The broad nature of our study question—how effective are common transcutaneous electrotherapies for CLBP compared with controls?—meant that we were likely to include studies with parameters that differed in significant ways, leading to limits to the interoperability of our results, especially when they were not significant.
One feature of this systematic review was our stringent exclusion criteria. We only included studies with at least 5 participants per study arm, at least 8 treatments per group, with participants no older than 70 years without radicular CLBP or spinal abnormalities, and where the method of electrotherapy application (including stimulation intensity) was precisely described. As a result, we excluded several studies that were in included in previous systematic reviews (23, 24, 26) to increase the specificity of our findings. Although our TENS results were not significantly from those previously reported with respect to pain intensity and disability, our methodology provides insight into the effect of longer-term treatment with transcutaneous electrotherapies, which has not been the focus of previous systematic reviews.
Lastly, all our included studies had at least a moderate risk of bias, in large part because of the lack of availability of study protocols, which elevated the risk of reporting bias. Future RCTs can minimize the risk of bias by publishing the study protocol alongside the final report.
4.2 Conclusion
In sum, there is moderate evidence that TENS is similar to both active and passive controls for improving pain and disability in CLBP patients. There is limited evidence that IFC is superior to active controls for improving pain and disability. There is limited evidence that EMS is superior to passive but not active controls for improving pain, and similar to all controls for improving disability. Finally, there is inconclusive evidence regarding the effect of transcutaneous electrotherapies on quality-of-life and fear avoidance beliefs due both to the limited number of studies investigating these outcomes and, for quality-of-life, the high number of domains listed on the SF-36. Future transcutaneous electrotherapy investigations may want to prioritize EMS and medium-frequency interventions, since these are understudied compared to TENS, appear to be more promising for PROMs, and can also lead to functional improvements in strength and endurance (30, 31). Additionally, research into promising but less-studied forms of transcutaneous electrotherapy is justified; for example, into H-wave Device Stimulation, which was recently reported to improve pain, sleep and work performance in CLBP patients (52). Finally, the impact of all transcutaneous electrotherapy interventions on psychological mediators such as kinesiophobia and pain catastrophizing warrants further study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
DW: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Data curation. BR: Data curation, Investigation, Writing – review & editing. MF: Conceptualization, Formal Analysis, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
MF is supported by Fonds de Recherche du Québec—Santé (FRQS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
2. ^https://clinicaltrials.gov
3. ^https://sr-accelerator.com
4. ^https://www.statstodo.com/CombineMeansSDs.php)
References
1. Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. (2012) 64(6):2028–37. doi: 10.1002/art.34347
2. Wong JJ, Côté P, Tricco AC, Rosella LC. Examining the effects of low back pain and mental health symptoms on healthcare utilisation and costs: a protocol for a population-based cohort study. BMJ Open. (2019) 9(9):e031749. doi: 10.1136/bmjopen-2019-031749
3. Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al. Expenditures and health status among adults with back and neck problems. JAMA. (2008) 299(6):656–64. doi: 10.1001/jama.299.6.656
4. Carriere JS, Martel MO, Meints SM, Cornelius MC, Edwards RR. What do you expect? Catastrophizing mediates associations between expectancies and pain-facilitatory processes. Eur J Pain (United Kingdom). (2019) 23(4):800–11. doi: 10.1002/ejp.1348
5. Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, Weiser S. Catastrophizing—a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. (2014) 14(11):2639–57. doi: 10.1016/j.spinee.2014.03.003
6. Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. What low back pain is and why we need to pay attention. Lancet. (2018) 391:2356–67. doi: 10.1016/S0140-6736(18)30480-X
7. Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. (2009) 146(3):238–44. doi: 10.1016/j.pain.2009.08.019
8. Deyo RA, Battie M, Beurskens AJHM, Bombardier C, Croft P, Koes B, et al. Outcome measures for low back pain research: a proposal for standardized use. Spine (Phila Pa 1976). (1998) 23(18):2003–13. doi: 10.1097/00007632-199809150-00018
9. Vance CGT, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. (2014) 4:197–209. doi: 10.2217/pmt.14.13
10. Knight KL, Draper DO. Therapeutic Modalities: The art and Science: Second Edition. Baltimore, Philadelphia: Lippincott Williams & Williams (2012). p. 1–472.
11. Vanderthommen M, Duchateau J. Electrical stimulation as a modality to improve performance of the neuromuscular system. Exerc Sport Sci Rev. (2007) 35:180–5. doi: 10.1097/jes.0b013e318156e785
12. Lake DA. Neuromuscular electrical stimulation an overview and its application in the treatment of sports injuries. Sports Med. (1992) 13:320–36. doi: 10.2165/00007256-199213050-00003
13. Weissenfels A, Teschler M, Willert S, Hettchen M, Fröhlich M, Kleinöder H, et al. Effects of whole-body electromyostimulation on chronic nonspecific low back pain in adults: a randomized controlled study. J Pain Res. (2018) 11:1949–57. doi: 10.2147/JPR.S164904
14. Aparecida Pelegrini AC, Gasoto E, Bussolaro JM, Segatti G, De Albuquerque CE, Flor Bertolini GR. The analgesic action of aussie current in women with non-specific chronic lumbar pain. Int J Ther Rehabil. (2019) 26(7):1–10. doi: 10.12968/ijtr.2018.0063
15. Batistella CE, Bidin F, Giacomelli I, Nunez MA, Gasoto E, de Albuquerque CE, et al. Effects of the Russian current in the treatment of low back pain in women: a randomized clinical trial. J Bodyw Mov Ther. (2020) 24(2):118–22. doi: 10.1016/j.jbmt.2019.10.009
16. Lara-Palomo IC, Aguilar-Ferrándiz ME, Matarán-Peñarrocha GA, Saavedra-Hernández M, Granero-Molina J, Fernández-Sola C, et al. Short-term effects of interferential current electro-massage in adults with chronic non-specific low back pain: a randomized controlled trial. Clin Rehabil. (2013) 27(5):439–49. doi: 10.1177/0269215512460780
17. Fuentes JP, Olivo SA, Magee DJ, Gross DP. Effectiveness of interferential current therapy in the management of musculoskeletal pain: a systematic review and meta-analysis. J Phys Ther Rehabil. (2010) 90:1219–38. doi: 10.2522/ptj.20090335
18. Rampazo ÉP, Liebano RE. Analgesic effects of interferential current therapy: a narrative review. Medicina (Lithuania). (2022) 58:1–14. doi: 10.3390/medicina58010141
19. Low JL, Reed A. Electrotherapy Explained: Principles and Practice. Oxford, UK: Butterworth-Heinemann (2000). Available online at: https://books.google.ca/books?id=e6wAMQAACAAJ (Accessed November 24, 2023).
20. van Tulder M, Koes BW, Assendelft WJJ, Bouter LM. The Effectiveness of Conservative Treatment of Acute and Chronic low Back Pain. EMGO-instituut: Amsterdam (1999).
21. Flowerdew MW, Gadsby JG. A review of the treatment of chronic low back pain with acupuncture-like transcutaneous electrical nerve stimulation and transcutaneous electrical nerve stimulation. Complement Ther Med. (1997) 5(4):193–201. doi: 10.1016/S0965-2299(97)80029-5
22. Milne S, Welch VA, Brosseau L, Saginur M, Shea B, Tugwell P, et al. Transcutaneous electrical nerve stimulation (TENS) for chronic low-back pain. Cochrane Database Syst Rev. (2000) 4:1–25. doi: 10.1002/14651858.CD003008
23. Khadilkar A, Milne S, Brosseau L, Wells G, Tugwell P, Robinson V, et al. Transcutaneous electrical nerve stimulation for the treatment of chronic low back pain: a systematic review. Spine. (2005) 30(23):2657–66. doi: 10.1097/01.brs.0000188189.21202.0f
24. Poitras S, Brosseau L. Evidence-informed management of chronic low back pain with transcutaneous electrical nerve stimulation, interferential current, electrical muscle stimulation, ultrasound, and thermotherapy. Spine J. (2008) 8:226–33. doi: 10.1016/j.spinee.2007.10.022
25. Jauregui JJ, Cherian JJ, Gwam CU, Chughtai M, Mistry JB, Elmallah RK, et al. A meta-analysis of transcutaneous electrical nerve stimulation for chronic low back pain. Surg Technol Int. (2016) 28:296–302. PMID: 27042787.27042787
26. Wu LC, Weng PW, Chen CH, Huang YY, Tsuang YH, Chiang CJ. Literature review and meta-analysis of transcutaneous electrical nerve stimulation in treating chronic back pain. Reg Anesth Pain Med. (2018) 43(4):425–33. doi: 10.1097/AAP.0000000000000740
27. Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, et al. Chapter 4: european guidelines for the management of chronic nonspecific low back pain. Eur Spine J. (2006) 15:s192–300. doi: 10.1007/s00586-006-1072-1
28. Kreiner DS, Matz P, Bono CM, Cho CH, Easa JE, Ghiselli G, et al. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of low back pain. Spine J. (2020) 20:998–1024. doi: 10.1016/j.spinee.2020.04.006
29. Albright J, Allman R, Bonfiglio RP, Conill A, Dobkin B, Guccione AA, et al. Philadelphia Panel evidence-based clinical practice guidelines on selected rehabilitation interventions for low back pain. Phys Ther. (2001) 81(10):1641–74. doi: 10.1093/ptj/81.10.1641
30. Linzmeyer A, Coracini CA, Bertolini GRF, Carvalho AR. Effect of neuromuscular electrical stimulation on muscle function in chronic low back pain patients: systematic review. Brazilian Journal of Pain. (2022) 5(2):161–7. doi: 10.5935/2595-0118.20220025-en
31. Wolfe D, Rosenstein B, Fortin M. The effect of transcutaneous electrotherapy on lumbar range of motion and paraspinal muscle characteristics in chronic low back pain patients: a systematic review and meta-analysis. J Clin Med. (2023) 12(14):1–16. doi: 10.3390/jcm12144680
32. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 Explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Br Med J. (2021) 372:n160. doi: 10.1136/bmj.n160
33. Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, et al. 2015 Updated method guideline for systematic reviews in the cochrane back and neck group. Spine (Phila Pa 1976). (2015) 40(21):1660–73. doi: 10.1097/BRS.0000000000001061
34. Simon J, McAuliffe M, Shamim F, Vuong N, Tahaei A. Discogenic low back pain. Phys Med Rehabil Clin N Am. (2014) 25(2):305–17. doi: 10.1016/j.pmr.2014.01.006
35. Macedo LG, Maher CG, Latimer J, Mcauley JH. Motor control exercise for persistent, nonspecific low back pain: a systematic review. J Phys Ther Rehabil. (2009) 89:9–25. doi: 10.2522/ptj.20080103
36. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). BMJ (2019) 366(4898):1–8. doi: 10.1136/bmj.l4898
37. Albornoz-Cabello M, Maya-Martín J, Domínguez-Maldonado G, Espejo-Antúnez L, Heredia-Rizo AM. Effect of interferential current therapy on pain perception and disability level in subjects with chronic low back pain: a randomized controlled trial. Clin Rehabil. (2017) 31(2):242–9. doi: 10.1177/0269215516639653
38. Alrwaily M, Schneider M, Sowa G, Timko M, Whitney SL, Delitto A. Stabilization exercises combined with neuromuscular electrical stimulation for patients with chronic low back pain: a randomized controlled trial. Braz J Phys Ther. (2019) 23(6):506–15. doi: 10.1016/j.bjpt.2018.10.003
39. Caldas VVDA, MacIel DG, Cerqueira MS, Barboza JAM, Neto JBV, Dantas G, et al. Effect of pain education, cryotherapy, and transcutaneous electrical nerve stimulation on the pain, functional capacity, and quality of life in patients with nonspecific chronic low back pain: a single-blind randomized controlled trial. Am J Phys Med Rehabil. (2021) 100(3):243–9. doi: 10.1097/PHM.0000000000001552
40. Depaoli Lemos VJ, Selau RC, Blos C, Baptista Dohnert M, Boff Daitx R, de Almeida Brito V. Electroacupuncture and transcutaneous electrical nerve stimulation in chronic nonspecific low back pain: a blind randomized clinical trial. Muscles Ligaments Tendons J. (2021) 11(4):719–27. doi: 10.32098/mltj.04.2021.15
41. Dimer da Luz R, da Silva Santos M, Steffen Evaldt A, da Silva Matos L, Boff Daitx R, Döhnert MB. Neuromuscular electrical stimulation associated with core stability exercises in nonspecific postural low back pain: a randomized clinical trial. Muscles Ligaments Tendons J. (2019) 9(3):446–56. doi: 10.32098/mltj.03.2019.20
42. Elserty N, Kattabei O, Elhafez H. Effect of fixed versus adjusted transcutaneous electrical nerve stimulation amplitude on chronic mechanical low back pain. J Altern Complement Med. (2016) 22(7):557–62. doi: 10.1089/acm.2015.0063
43. Kofotolis ND, Vlachopoulos SP, Kellis E. Sequentially allocated clinical trial of rhythmic stabilization exercises and TENS in women with chronic low back pain. Clin Rehabil. (2008) 22(2):99–111. doi: 10.1177/0269215507080122
44. Topuz O, Özfidan E, Ozgen M, Ardic F. Efficacy of transcutaneous electrical nerve stimulation and percutaneous neuromodulation therapy in chronic low back pain. J Back Musculoskelet Rehabil. (2004) 17:127–33. doi: 10.3233/BMR-2004-173-407
45. Weissenfels A, Wirtz N, Dörmann U, Kleinöder H, Donath L, Kohl M, et al. Comparison of whole-body electromyostimulation versus recognized back-strengthening exercise training on chronic nonspecific low back pain: a randomized controlled study. Biomed Res Int. (2019) 2019:1–9. doi: 10.1155/2019/5745409
46. Yakşi E, Ketenci A, Baslo MB, Orhan EK. Does transcutaneous electrical nerve stimulation affect pain, neuropathic pain, and sympathetic skin responses in the treatment of chronic low back pain? A randomized, placebo-controlled study. Korean J Pain. (2021) 34(2):217–28. doi: 10.3344/kjp.2021.34.2.217
47. Travers MJ, O’Connell NE, Tugwell P, Eccleston C, Gibson W. Transcutaneous electrical nerve stimulation (TENS) for chronic pain: the opportunity to begin again. Cochrane Database Syst Rev. (2020) 4:1–3. doi: 10.1002/14651858.ED000139
48. Allen CB, Williamson TK, Norwood SM, Gupta A. Do electrical stimulation devices reduce pain and improve function?—a comparative review. Pain Ther. (2023) 12:1339–54. doi: 10.1007/s40122-023-00554-6
49. Johnson MI, Paley CA, Jones G, Mulvey MR, Wittkopf PG. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: a systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open. (2022) 12(2):e051073. doi: 10.1136/bmjopen-2021-051073
50. Ostelo RWJG, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). (2008) 33(1):90–4. doi: 10.1097/BRS.0b013e31815e3a10
51. Imrey PB. Limitations of meta-analyses of studies with high heterogeneity. JAMA Netw Open. (2020) 3(1):e1919325. doi: 10.1001/jamanetworkopen.2019.19325
52. Norwood SM, Han D, Gupta A. H-wave® device stimulation for chronic low back pain: a patient-reported outcome measures (PROMs) study. Pain Ther. (2024) 13(1):113–26. doi: 10.1007/s40122-023-00570-6
Appendix 1 Sample Search Strategy
(PubMed)
#1 Search: “Low Back Pain” [Mesh] OR “Back Pain” [Mesh:NoExp] OR “Hernia” [Mesh:NoExp] OR “Intervertebral Disc Displacement” [Mesh] OR “Intervertebral Disc Degeneration”[Mesh] OR “lumbosacral region” [Mesh] OR “Low back pain” OR “lumbago” OR “disc herniation” OR “intervertebral disc herniation” OR “disc degeneration” OR “degenerative disc” OR “lower back pain” OR “back ache” OR “backache” OR “backaches” OR “sacral pain” OR “lumbar pain” OR “lumbosacral pain” OR “LBP”
#2 Search: “Transcutaneous Electric Nerve Stimulation” [Mesh] OR “Electric Stimulation Therapy” [Mesh:NoExp] OR “Electric Stimulation” [Mesh:NoExp] OR “electric stimulation” OR “electrical stimulation” OR “electrostimulation” OR “TENS” OR “nerve stimulation” OR “electrotherapy” OR “interferential current” OR “electroanalgesia” OR “IFC” OR “neuromuscular electrical stimulation therapy” OR “NMES” OR “electromyostimulation” OR “EMS” OR “ES” OR “functional electrical stimulation” OR “FES” OR “Whole body electromyostimulation” OR “whole-body electromyostimulation” OR “WB-EMS”
#3 Search: (Animal[Mesh] NOT Human[Mesh])
#4 Search: #1 AND #2
#5: #4 NOT #3
(Embase)
#1 Search: (low back pain or low back pain).mp
#2 Search: (transcutaneous electric* nerve stimulation or transcutaneous electrical nerve stimulation).mp
#3 Search: (neuromuscular electric* stimulation or neuromuscular electric* nerve stimulation or neuromuscular electrical stimulation).mp
#4 Search: electrotherapy/or high frequency electrotherapy/or low frequency electrotherapy/or electrotherapy.mp
#5 Search: (interferential current or IFC or functional electric* stim* or FES or whole-body electromyostim* OR WB-EMS).mp
#6 Search: 2 or 3 or 4 or 5
#7 Search: 1 and 6.
Keywords: TENS, EMS, NMES, CLBP, chronic low back pain, IFC, pain
Citation: Wolfe D, Rosenstein B and Fortin M (2024) The effect of EMS, IFC, and TENS on patient-reported outcome measures for chronic low back pain: a systematic review and meta-analysis. Front. Pain Res. 5:1346694. doi: 10.3389/fpain.2024.1346694
Received: 29 November 2023; Accepted: 31 May 2024;
Published: 24 June 2024.
Edited by:
Mark Henry Pitcher, National Center for Complementary and Integrative Health (NIH), United StatesReviewed by:
Ashim Gupta, Future Biologics, United StatesMarco Ulises Martínez-Martinez, Mexican Social Security Institute, Mexico
© 2024 Wolfe, Rosenstein and Fortin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maryse Fortin, bWFyeXNlLmZvcnRpbkBjb25jb3JkaWEuY2E=
 Daniel Wolfe
Daniel Wolfe Brent Rosenstein
Brent Rosenstein Maryse Fortin
Maryse Fortin