- 1Centre for Osteoarthritis Pathogenesis Versus Arthritis, Kennedy Institute of Rheumatology, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences (NDORMS), University of Oxford, Oxford, United Kingdom
- 2Department of Rheumatology, Charing Cross Hospital, Imperial College Healthcare NHS Trust, London, United Kingdom
- 3Musculoskeletal Research Unit, University of Bristol, Bristol, United Kingdom
- 4Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, United Kingdom
- 5National Institute for Health Research Bristol Biomedical Research Centre (NIHR Bristol BRC), University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, United Kingdom
- 6Therapies Department, Imperial College Healthcare NHS Trust, London, United Kingdom
- 7Department of Immunology & Inflammation, Hammersmith Campus, Imperial College London, London, United Kingdom
Introduction: Hand osteoarthritis is more common in women, and its risk increases around the time of the menopause. We set out to describe the timing between menopause and the onset of symptomatic hand osteoarthritis (OA), and associations with the use of hormone replacement therapy (HRT) or its discontinuation, describing any identifiable subgroups of women.
Methods: Retrospective healthcare-records study of sequential women referred to a specialist hand OA clinic, 2007–2015. Confirmation of hand OA diagnosis was by clinican, by accepted criteria. Demographics and clinical variables were from healthcare-records, recorded by standardised proforma. Outcomes of interest were reported age of onset of hand symptoms, reported age at final menstrual period (FMP), time from FMP to reported onset of hand symptoms and time from cessation of HRT to reported onset of hand symptoms. Exposure categories for systemic HRT use were never users, current users, previous users. Analysis of Variance compared groups; linear regression analysed associations of exposure with outcome.
Results: 82/92(89%) of eligible women were post-menopausal, mean age at FMP 49.9 years (SD5.4). In these post-menopausal women, median time from FMP to hand symptom onset was 3 years. 48/82 (59%) developed hand symptoms within the defined peri-menopausal period (FMP ± 4 years), whilst some women developed their symptoms before or after (range −25, 30 years). In women who discontinued HRT prior to symptom onset, the median time from HRT cessation to onset of hand symptoms was 6 months. Past HRT users were older at hand symptom onset than women who had not taken HRT [coeff.4.7 years (0.92, 8.39); P = 0.015].
Conclusions: This study adds to evidence associating the menopause/sex hormone deficiency with hand OA symptom onset in a sizeable subgroup of women (but not all). HRT use/cessation appears to influence the timing of onset of hand OA symptoms. It is not possible to interpret from this type of study whether sex hormone deficiency is causative of disease or modulates its symptoms. It is also not possible to judge whether painful hand osteoarthritis in post-menopausal women is a subtype of disease. Further investigation is indicated of sex-specific subtypes and potential for personalised medicine for post-menopausal women with hand osteoarthritis, as a clearly definable high-risk subgroup.
Introduction
Osteoarthritis (OA) is in the top 20 leading causes of years lived with disability worldwide (1, 2). Hand OA is the commonest form, with symptoms affecting 47% of women and 25% of men over their lifetime (3). Of those over 45 years of age seeking treatment for hand OA, a large proportion are women [UK data, estimated 1.06 million (68%) (4)]. The disease can involve the interphalangeal joints (IPJs), the first carpometacarpal joint (CMCJ) or both, causing pain, deformity and functional impairment (3). Affected individuals have substantial impairment of quality of life which can be akin to rheumatoid arthritis (5). Those with symptomatic hand OA are more likely to experience cardiovascular disease (6). Evidence-based interventions for IPJ disease are limited and most commonly involve pain relief and exercise (7). There remains a poor understanding of disease pathogenesis of OA in general and hand OA in particular, with no pharmacological disease-modifying therapies for this disease, despite a number of recent clinical trials (8–10).
The apparent association between female sex, menopause and OA has long been noted (11). More recently, there has been growing circumstantial evidence for an association of OA with female sex: an increased risk in women in hand, hip and knee OA which becomes evident later in life is now well-documented (12–14). An upsurge in the relative risk of hand OA in women compared with men occurred around the age of 50 in a large Spanish study of electronic health records, the conserved typical age of menopause worldwide (14). In one Italian study in secondary specialist care, 90% of patients referred for “inflammatory”, erosive painful hand OA were women (15). In a large epidemiological study examining the association between hormone replacement therapy (HRT) and incident hand OA (using UK Community Practice Research Datalink data), current HRT use appeared protective when initiated around the time of the menopause, but this benefit diminished after discontinuing HRT (16).
However, there is no evidence for a direct association between the onset of hand OA and onset of menopause or direct evidence in humans for a causal relationship between the two. Whether the sex-specific epidemiology of this disease mean that either hand osteoarthritis in women or hand osteoarthritis in post-menopausal women might represent clinically relevant subgroups for study or personalised treatment has been inadequately explored.
Estrogen, or rather its deficiency, would appear to be important in the pathogenesis of OA and musculoskeletal pain. In animal models of surgically-induced knee OA, young female animals are protected from the disease compared with males, but lose this protection after ovariectomy (17). However several epidemiological studies examining the use of estrogen-containing HRT and its association with hand pain report conflicting findings (18–20). These studies are likely limited by substantial residual confounding (given that pain may be a reason to seek HRT prescription) and lack of validation cohorts (21). HRT use has been reported to have a significant protective effect on knee OA in Framingham cohort women (OR 0.31; 95% CI 0.11, 0.93) (22) and also in the Chingford cohort in the UK, where a weaker effect on hand joints was seen (23). Those in the unopposed estrogen arm of the Women's Health Initiative studies had reduced rates of total hip and knee replacement (24) and reduced musculoskeletal (MSK) pain of any cause (25). A systematic review found evidence for a protective effect of exogenous estrogen on hip OA (26). However there are very few randomized controlled trials (RCTs) testing the effects of estrogen-containing therapies in women included for MSK painful conditions (21). Only one study has been in symptomatic hand OA to our knowledge, which was a feasibility study so not designed to test efficacy (27).
We hypothesised that hand osteoarthritis in women around the time of menopause may represent an important phenotype of hand osteoarthritis. Within this phenotype, the onset of peri-menopause and menopause could influence the timing of onset of hand OA or its clinical presentation in the group as a whole, or in a subgroup of these women. Furthermore, use of HRT might also be associated with modified timing of hand symptom onset. Our aim was to explore these questions in a population of women with painful hand OA referred to a secondary care setting because of their hand symptoms, where relevant data were routinely collected. Our objectives were: (i) to describe the timing between the onset of menopause and the onset of hand OA symptoms; (ii) to explore if current or previous use of HRT was associated with the timing of onset of hand OA symptoms; (iii) to examine if cessation of HRT was associated with timing of onset of hand OA symptoms.
Materials and methods
Ethical approvals
Ethical approval was given for the analysis of usual care health records by the Sponsor Imperial College Healthcare NHS Trust in the UK for the purposes of this study (IRAS reference number 282499, REC 20/HRA/4630). The study conforms to STROBE guidelines (checklist in Supplementary Material) and included a pre-defined protocol for analysis which was reviewed as part of the ethical approval process.
Patients and setting
A retrospective study of individual healthcare records was carried out using the records of consecutive new patients attending a multidisciplinary specialist hand OA clinic at Charing Cross Hospital [Imperial College Healthcare National Health Service (NHS) Trust, London, UK].
Eligibility
Sequential records of those seen at the clinic as new patients for 8 years between 1 July 2007 and 30 June 2015 were reviewed and included if they were women aged 18 years and older and were diagnosed with hand OA by a rheumatologist. This diagnosis was in line with the American College of Rheumatology (ACR) definition of hand OA (28), considering clinical features including frequent hand joint pain with or without loss of function and consistent radiographic changes. Any case of hand OA was included (base of thumb OA only, interphalangeal joint OA only or having both sites affected). Those with other forms of arthritis (such as rheumatoid arthritis, gout or psoriatic arthritis), or other causes of hand pain as the main cause for pain (such as carpal tunnel syndrome or tenosynovitis) were excluded. Those using anti-estrogen treatments or chemotherapy were also excluded.
Data
These were extracted from a standardised proforma which had been completed at all initial consultations during this period which recorded medical and medication history, including specific questions on reported age of final menstrual period (FMP) and age at onset of hand OA symptoms. Patients were considered post-menopausal if their FMP was at least 1 year previous, in the absence of hormonal contraception (29, 30). In the case of surgically induced menopause, age at surgery was used as age at FMP. We defined the peri-menopausal period for our study as 4 years before FMP and up to 4 years after FMP (31, 32). Available medication information included the use and timing of systemic (estrogen-containing) HRT (specifically, when it was started and stopped, or whether there was ongoing use). Based on this information, HRT use was further categorised as “Current”, “Previous” or “Never” (16, 30). “Current” HRT use was a minimum of 6 months of use, taken within the last 12 months; “Previous” use was defined as minimum of 6 months of use, with cessation more than 12 months ago. “Never” use was defined as no use, or less than 6 months of use (pre-menopausal females were also included in this group). Missing data were recorded as such. Participants were not excluded due to missing data.
Analysis
All available data on all patients fulfilling criteria were analysed.
Predefined outcomes of interest were continuous variables of: (a) reported age of onset of hand symptoms; (b) reported age of FMP; (c) time from FMP to reported onset of hand symptoms; (d) time from cessation of HRT to reported onset of hand symptoms.
The main exposure of interest was use of systemic HRT (where groups were never users of HRT, current users and previous users).
One way Analysis of Variance (ANOVA) was used to compare the outcome, age of onset of hand symptoms (in years) between these 3 groups: those who had never used HRT, current users and previous users.
In addition, linear regression modelling was used to describe associations of HRT exposure groups with outcome, the age of onset of hand symptoms, in years. Effect size (regression coefficient of the difference in mean age at onset between groups, and its 95% confidence interval) therefore indicated the effect on additional years of age at onset of hand symptoms in each of two groups (previous users and current users) when compared with age of onset of hand symptoms in the reference group (never users of HRT). Sensitivity analysis included adjustment for menopausal status (pre, post) in the linear regression model.
Descriptive statistics were used to summarise patient characteristics (mean (SD) for normally distributed and median (IQR and/or range) for non-normally distributed continuous variables, and number (%) for categorical variables).
Pseudonymised data were recorded in a Microsoft Access database and statistical analysis was performed using STATA IC 13 (Stata, College Station, Texas, US).
This was an exploratory study including all available eligible patient data using the methods described. No power calculations were therefore carried out, no multiplicity testing adjustment made (33) and no replication cohort defined.
Results
Patient characteristics
Of 115 individuals fulfilling diagnostic criteria for hand OA during this defined time period, 92 (80%) were women and selected for further study based on fulfilling eligibility criteria (Table 1, Supplementary Figure S1). Eighty two of 92 (89%) were post-menopausal women, whilst 8 were pre-menopausal and for 2 status was unknown. 29 (31.5%) had used HRT in the past, but discontinued this, whilst 11 (12.0%) were current users and 49 (53.3%) had never used HRT (Table 1).
Hand OA and menopause
In these women with hand OA, hand symptom onset was at a mean of 54 years of age. The 82 post-menopausal women reported their FMP at a mean age of 49.9 years (SD 5.2) (Table 1, Figures 1A,B). In post-menopausal women, the median time from FMP to hand symptom onset was 3 years (range −25, 30 years, Figure 1C). 48/82 (59%) of these women developed their hand symptoms within the defined peri-menopausal period (FMP ± 4 years), whilst 34/82 (41%) did not.

Figure 1. The relationship between the reported onset of menopause and hand OA symptoms. Histograms and kernel density plots of the frequency in the study population of (A) Age of onset of the menopause (in years) (B) Age of onset of hand OA symptoms (in years) and (C) The time between FMP (onset of menopause) and onset of hand symptoms (in years).
Hand OA and HRT use
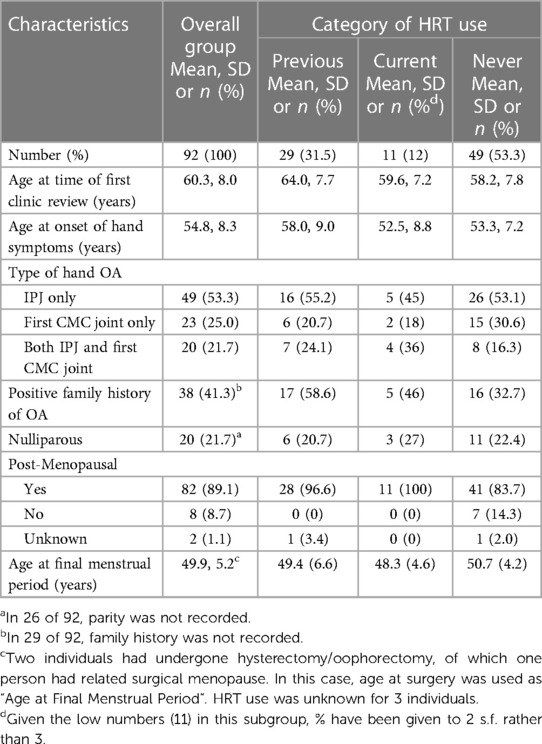
Of those 29 women with hand OA who had used HRT but discontinued it previously, 22 (76%) had developed their hand OA after discontinuing HRT therapy and 7 (24%) between 2 and 31 years prior to that time. In these women, the median time from cessation of HRT to self-reported age at onset of hand symptoms was 6 months (Figure 2A). The age of onset of hand symptoms was compared in those who had never used HRT, current users and previous users. There was a difference in age of onset of hand symptoms between these 3 groups, with those who were previous users of HRT appearing older at symptom onset than current users or never users [mean age (SD) 58.0 (9.0), 52.5 (8.8), 53.3 (7.2) respectively for each group; ANOVA between 3 groups, F2,86 = 3.57, P = 0.0323], Figure 2B. Of note, most (7/11) of the women in the current HRT use group had developed hand OA symptoms prior to HRT use (so were similar to the never use group). By linear regression, when compared with the group of women who had never taken HRT, those past users of HRT were older at onset of their reported hand symptoms [coeff. 4.7 years (0.92, 8.39); P = 0.015], whilst those using HRT currently did not differ in age [coeff. −0.85 years (−6.17, 4.47); P = 0.75]. Given that those who had not taken HRT could be younger and more likely to be pre-menopausal, in an additional hypothesis test, given the null hypothesis was rejected, post-menopausal status was added as a covariate in this regression model. The association between past use of HRT and older age at onset at hand symptoms remained [coeff. 7.7 years (1.3, 14.1); P = 0.019].
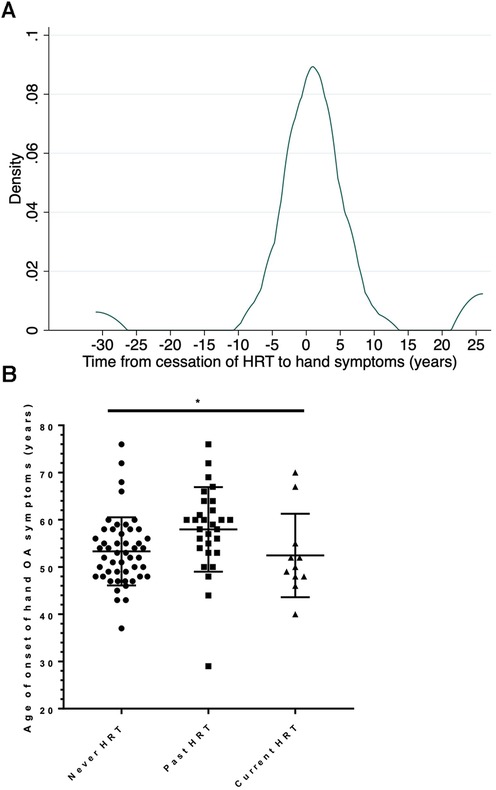
Figure 2. Effect of past use of HRT on the onset hand OA symptoms. (A) Kernel density plot of the frequency in the study population of the time between cessation of HRT and onset of symptoms in all women who were past users of HRT, n = 29. (B) The age of onset of hand symptoms is shown for 3 subgroups of postmenopausal women: those women who have never used HRT (Never HRT); those who have stopped HRT for at least 1 year (Past HRT) and those who are current users of HRT. The bar shows the mean and SD for each group. Comparison by 1 way ANOVA, F2,86 = 3.57, P = 0.0323.
Discussion
In this retrospective healthcare study of women referred to a secondary care clinic for symptomatic hand OA, we report that for a substantial subgroup (but not all), there is a short interval between their menopause onset and reported onset of hand OA symptoms. Furthermore, those who had taken previous HRT appeared to develop their hand symptoms at a higher age than those who had not. However, for the majority who had taken HRT, discontinued it and developed their symptoms after stopping, they developed their symptoms within one year of cessation of therapy. Jointly, these findings lend further support to an apparent association between menopause, female hormonal change and symptomatic hand OA. Though our discussion and conclusions are necessarily cautious in this limited study which require replication in other datasets, they support the further exploration of the potential for female subtypes of hand osteoarthritis which could have clinical potential for personalising the diagnosis and treatment of this condition.
It has long been reported that hand OA increases around the age of 50, which is the typical age of menopause (11–14). However, we are not aware of any studies previously examining the temporal relationship at an individual level between onset of hand OA symptoms specifically (rather than a diagnostic code) and onset of menopause. Many OA studies do not collect data on menstrual history or menopause. Some reports from historic cohorts which have collected this information tend to focus on hand radiographic change rather than hand symptoms (23). Explanations for our findings could include menopause influencing disease susceptibility/incidence, magnifying the symptoms or severity of prevalent disease, including other related factors that enhance pain (e.g., sleep disturbance, mood) (21). It is important to emphasise that there does not appear to be a temporal relationship with menopause for all women with the condition, but rather in a substantial subgroup (3 in 5 of those studied): it is not possible to say from this study if these two subgroups clinically vary from each other in any other regard or be otherwise differentiated, which would be an important question for onwards research.
This study suggests that there may be an interaction between HRT use and onset of hand symptoms and that both starting, taking and stopping are all relevant events. These observations may partly explain the conflicting literature around HRT use and hand OA, with some studies reporting that HRT use might be protective of disease, its associated pain or radiographic disease, or delay the occurrence of symptoms (23), whilst other studies report more hand OA in HRT users or past users (18, 19). These findings are not necessarily as contradictory as they first appear: sudden cessation of HRT could be a risk factor for development of symptomatic hand OA, or its use might simply delay the onset of OA which would have occurred at some point anyway. In a recent feasibility RCT of a form of HRT, more women in the active arm developed worse symptoms than those on placebo on tapering the study medication over a 4 week period, supporting this notion of symptomatic flare on estrogen withdrawal (27). A recent review highlighted that improved advice to the subgroup of women with osteoarthritis stopping HRT could have potential practical therapeutic relevance (21). Future studies might investigate the relative severity and duration of hand symptoms in previous, current and never users of HRT.
Our data highlights the potential for treatment bias which may influence some epidemiological studies: that users of HRT may have sought HRT after onset of MSK symptoms including hand symptoms, as we saw in those with current use. This may be a reason for the apparent increased prevalence of OA in those taking HRT in some population studies (18, 19). Our findings support earlier reports from the Chingford study (a UK cohort of women) which suggested a trend towards association of HRT use and protection from radiographic distal IPJ OA (OR 0.48; 95% CI 0.17, 1.42) (symptomatic data was not reported for this cohort) (23). It also provides additional evidence to complement that from RCTs, which analyse general MSK symptoms or large joint (hip or knee) OA outcomes, but where neither symptomatic nor radiographic hand OA data were specifically collected (24, 25).
There are a number of potential mechanisms by which menopause itself and HRT use could influence the manifestation of hand OA symptoms or disease. These include changes in estrogen levels and its relative deficiency. Testosterone levels also tend to fall in menopause, and an association between low serum testosterone and presence of hand OA has been previously reported (19). Estrogen is known to fluctuate intermittently to low levels for as long as 10 years prior to menopause (29), which may be an important consideration in those developing hand symptoms several years in advance of FMP. Estrogens (principally estradiol) are produced and sensed by connective tissues including articular cartilage and bone (34). Low level estrogen replacement or its receptor modulators have been described to promote cartilage growth and new bone formation (35, 36). Estrogen is known to be anti-inflammatory and mildly immunosuppressive (20, 21). Estrogen, progesterone and testosterone also directly regulate the experience of pain (37). All of these mechanisms could be relevant to the development of symptomatic hand OA in this potential subgroup of the condition (20, 21).
Finally it is worth reflecting on the very high overall proportion of women (80%) in the sample during this period in a secondary care clinic with hand OA diagnosis. High female preponderance has been previously reported in the context of erosive OA in a secondary care setting (15). This study, which considered all forms of hand OA, raises the question of whether sex-specific phenotypes of the disease exist, which has not been particularly considered in “clinical phenotypes” to date (38).
Limitations
It is important that in a study of this nature we do not over interpret our findings. First, this was a cross-sectional study of usually collected healthcare data: this means that no causal link can be inferred and that the apparent association between past use of HRT and increased age of hand OA symptom onset could have occurred by chance, or be influenced by unidentified or uncontrolled confounding. Second, the study relies on a retrospective recalled history of age of onset of hand symptoms and of FMP. This could be open to a lack of precision, recall bias (patient) or reporting bias (physician) if either attributed a link between the two. It is our belief that at the time of data collection (i.e., 2015 and before) there had been little publicised about any putative link, and so patients and clinicians would not have anticipated this association; also these data were systematically acquired for all patients using a standard proforma which seeks to address reporting bias.
Third, our sample size was relatively small particularly when examining pre-defined subgroups. It was not sufficient to further stratify, for example into those with IPJ or CMC joint disease only. The potential for differences (or equivalence) in these two important clinical subgroups of hand OA would be of high interest. However, combining these subgroups is frequently done in studies and trials, plus many of our patients had involvement at both sites. Similarly, it was not possible to stratify for non surgical and surgical menopause, given the very low numbers (one) in this latter group. Only 11 current users of HRT mean results relating to the association with timing of onset of hand OA symptoms in this small subgroup should be interpreted with caution.
It was not our intention to analyse further the exact nature or duration of HRT given the limitations of sample size. This could be considered in further larger studies, although it should be noted that there could be a risk of confounding unless this is controlled in some way. Available data suggest that the majority were on oral preparations of either unopposed estrogen or estrogen-progestin combinations, depending on uterine status. Some individuals had used the oral contraceptive pill (OCP) in the past. However, a systematic history of this was not routinely captured in the same way as HRT, so this could not be reported. It may be that those previously using the OCP were more likely to seek HRT than those who had not for example, and that there could be some residual confounding here with other lifetime exogenous hormone use. However, the effects of OCP use on risk of hand osteoarthritis are also not well understood.
Fourth, this study was carried out in a secondary care setting, so likely to be open to referral bias, and is therefore not necessarily generalizable to all people with hand OA symptoms. Patients in this clinic (within rheumatology outpatients) typically had a high symptom burden and disease load, often with high inflammatory symptoms in many IP joints with radiological change justifying referral. The advantage of examining such a highly symptomatic population is the associated substantial socioeconomic cost and unmet treatment need. Some people may experience more transient, or self-limiting symptoms which never reach medical review. The only possible referral routes were from primary care (family doctors) or from other MSK practitioners (hand surgeons or other rheumatologists). No self referrals or direct NHS referrals from gynaecologists or menopause clinics were possible.
Lastly, data on height was not collected at that time and therefore body mass index (BMI) was not routinely available. Increased BMI is known to affect the development of OA, including of the hand. Adipose tissue can also synthesise estrogen. In this relatively small study, sex and age have been considered but we have not attempted to adjust in a multivariable model for the influence of additional factors which could have been different between groups. In the case of BMI, individuals who were obese or have other associated comorbidities would be less likely to be prescribed oral HRT (29). Larger studies are needed to test these findings and assess their robustness and generalisability. In a larger prospective sample, one could pursue association analysis of the paired variables “age at onset of menopause” and “age at onset of hand OA symptoms” with appropriate adjustment of confounders, to understand the approximation of the distributions further.
In summary, this study reports an apparent association between the onset of menopause and timing of onset of hand OA symptoms in hand OA, which may be influenced by past use of HRT. These findings should be tested in independent larger cohorts and clinical datasets, and also examined longitudinally. Ideally these longitudinal studies would be prospective and commence prior to individuals' natural, drug or surgery induced menopause, to more fully disentangle the effects of ageing and menopause. Ultimately, only RCTs in this population may adequately control all confounding to answer important remaining questions in this area definitively.
Data availability statement
The datasets presented in this article are not readily available because the ethical permissions granted by Imperial Healthcare NHS Trust for this work did not include the sharing of datasets. Queries relating to the datasets should be directed toZi53YXR0MUBuaHMubmV0.
Ethics statement
The studies involving humans were approved by Imperial College Healthcare NHS Trust, UK (IRAS reference number 282499, REC 20/HRA/4630). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this was a retrospective study using existing healthcare record data.
Author contributions
MG: Data curation, Formal Analysis, Investigation, Writing – review & editing, Visualization. GB: Data curation, Investigation, Writing – review & editing, Methodology, Project administration. AJ: Methodology, Writing – review & editing, Formal Analysis. DK: Writing – review & editing, Conceptualization, Investigation, Project administration, Resources. TV: Conceptualization, Investigation, Resources, Writing – review & editing, Data curation, Funding acquisition, Methodology, Supervision. FW: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing, Formal Analysis, Project administration, Software, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Centre for Osteoarthritis Pathogenesis Versus Arthritis (grant numbers 20205 and 21621). FW is supported by a UKRI Future Leaders Fellowship (MR/S016538/1 and MR/S016538/2) and has also received support from Kennedy Trust for Rheumatology Research and the NIHR Oxford Biomedical Research Centre (BRC). MG is supported by a Versus Arthritis Clinical Research Fellowship (grant number 21604). FW is a member of the Centre for Sport, Exercise and Osteoarthritis Research Versus Arthritis, University of Oxford (grant number 21595). AJ was supported by the NIHR Bristol Biomedical Research Centre, University Hospitals Bristol and Weston NHS Foundation Trust and University of Bristol. The funding sources had no involvement in the study design, collection, analysis or interpretation of data, or the decision to submit the article for publication. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgments
We thank Imperial College Healthcare NHS Trust for permitting this work. We acknowledge Katharine Carlisle for their clinical expertise.
Conflict of interest
FW had received consulting fees from Pfizer in 2020 for an unrelated programme in hip and knee osteoarthritis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2024.1331187/full#supplementary-material
References
1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Long H, Liu Q, Yin H, Wang K, Diao N, Zhang Y, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. Arthritis Rheumatol. (2022) 74(7):1172–83. doi: 10.1002/art.42089
3. Marshall M, Watt FE, Vincent TL, Dziedzic K. Hand osteoarthritis: clinical phenotypes, molecular mechanisms and disease management. Nat Rev Rheumatol. (2018) 14(11):641–56. doi: 10.1038/s41584-018-0095-4
4. Arthritis Research UK. Osteoarthritis in General Practice: Data and Perspectives. Chesterfield, United Kingdom: University of Keele (2013).
5. Slatkowsky-Christensen B, Mowinckel P, Loge JH, Kvien TK. Health-related quality of life in women with symptomatic hand osteoarthritis: a comparison with rheumatoid arthritis patients, healthy controls, and normative data. Arthritis Rheum. (2007) 57(8):1404–9. doi: 10.1002/art.23079
6. Haugen IK, Ramachandran VS, Misra D, Neogi T, Niu J, Yang T, et al. Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: data from the Framingham heart study. Ann Rheum Dis. (2015) 74(1):74–81. doi: 10.1136/annrheumdis-2013-203789
7. Kloppenburg M, Kroon FP, Blanco FJ, Doherty M, Dziedzic KS, Greibrokk E, et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. (2019) 78(1):16–24. doi: 10.1136/annrheumdis-2018-213826
8. Kingsbury SR, Tharmanathan P, Keding A, Ronaldson SJ, Grainger A, Wakefield RJ, et al. Hydroxychloroquine effectiveness in reducing symptoms of hand osteoarthritis: a randomized trial. Ann Intern Med. (2018) 168(6):385–95. doi: 10.7326/M17-1430
9. Kloppenburg M, Ramonda R, Bobacz K, Kwok WY, Elewaut D, Huizinga TWJ, et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. (2018) 77(12):1757–64. doi: 10.1136/annrheumdis-2018-213202
10. Kloppenburg M, Peterfy C, Haugen IK, Kroon F, Chen S, Wang L, et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1alpha and anti-interleukin-1beta dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann Rheum Dis. (2019) 78(3):413–20. doi: 10.1136/annrheumdis-2018-213336
11. Cecil RL, Archer BH. Arthritis of the menopause: a study of fifty cases. J Am Med Assoc. (1925) 84(2):75–9. doi: 10.1001/jama.1925.02660280001001
12. Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. (1995) 38(8):1134–41. doi: 10.1002/art.1780380817
13. Zhang Y, Niu J, Kelly-Hayes M, Chaisson CE, Aliabadi P, Felson DT. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: the Framingham study. Am J Epidemiol. (2002) 156(11):1021–7. doi: 10.1093/aje/kwf141
14. Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. (2014) 73(9):1659–64. doi: 10.1136/annrheumdis-2013-203355
15. Punzi L, Ramonda R, Sfriso P. Erosive osteoarthritis. Best Pract Res Clin Rheumatol. (2004) 18(5):739–58. doi: 10.1016/j.berh.2004.05.010
16. Burkard T, Rauch M, Spoendlin J, Prieto-Alhambra D, Jick SS, Meier CR. Risk of hand osteoarthritis in new users of hormone replacement therapy: a nested case-control analysis. Maturitas. (2020) 132:17–23. doi: 10.1016/j.maturitas.2019.11.006
17. Ma HL, Blanchet TJ, Peluso D, Hopkins B, Morris EA, Glasson SS. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. (2007) 15(6):695–700. doi: 10.1016/j.joca.2006.11.005
18. Maheu E, Dreiser RL, Guillou GB, Dewailly J. Hand osteoarthritis patients characteristics according to the existence of a hormone replacement therapy. Osteoarthritis Cartilage. (2000) 8(Suppl A):S33–7. doi: 10.1053/joca.2000.0334
19. Sowers MF, Hochberg M, Crabbe JP, Muhich A, Crutchfield M, Updike S. Association of bone mineral density and sex hormone levels with osteoarthritis of the hand and knee in premenopausal women. Am J Epidemiol. (1996) 143(1):38–47. doi: 10.1093/oxfordjournals.aje.a008655
20. Watt FE. Hand osteoarthritis, menopause and menopausal hormone therapy. Maturitas. (2016) 83:13–8. doi: 10.1016/j.maturitas.2015.09.007
21. Gulati M, Dursun E, Vincent V, Watt FE. The influence of sex hormones on musculoskeletal pain and osteoarthritis. Lancet Rheumatol. (2023) 5(4):E225–38. doi: 10.1016/S2665-9913(23)00060-7
22. Zhang Y, McAlindon TE, Hannan MT, Chaisson CE, Klein R, Wilson PW, et al. Estrogen replacement therapy and worsening of radiographic knee osteoarthritis: the framingham study. Arthritis Rheum. (1998) 41(10):1867–73. doi: 10.1002/1529-0131(199810)41:10%3C1867::AID-ART20%3E3.0.CO;2-W
23. Spector TD, Nandra D, Hart DJ, Doyle DV. Is hormone replacement therapy protective for hand and knee osteoarthritis in women?: The Chingford study. Ann Rheum Dis. (1997) 56(7):432–4. doi: 10.1136/ard.56.7.432
24. Cirillo DJ, Wallace RB, Wu L, Yood RA. Effect of hormone therapy on risk of hip and knee joint replacement in the women’s health initiative. Arthritis Rheum. (2006) 54(10):3194–204. doi: 10.1002/art.22138
25. Chlebowski RT, Cirillo DJ, Eaton CB, Stefanick ML, Pettinger M, Carbone LD, et al. Estrogen alone and joint symptoms in the women’s health initiative randomized trial. Menopause. (2013) 20(6):600–8. doi: 10.1097/GME.0b013e31828392c4
26. de Klerk BM, Schiphof D, Groeneveld FP, Koes BW, van Osch GJ, van Meurs JB, et al. Limited evidence for a protective effect of unopposed oestrogen therapy for osteoarthritis of the hip: a systematic review. Rheumatology (Oxford). (2009) 48(2):104–12. doi: 10.1093/rheumatology/ken390
27. Williams JAE, Chester-Jones M, Minns Lowe C, Goff MV, Francis A, Brewer G, et al. Hormone replacement therapy (conjugated oestrogens plus bazedoxifene) for post-menopausal women with symptomatic hand osteoarthritis: primary report from the HOPE-e randomised, placebo-controlled, feasibility study. Lancet Rheumatol. (2022) 4(10):e725–37. doi: 10.1016/S2665-9913(22)00218-1
28. Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American college of rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. (1990) 33(11):1601–10. doi: 10.1002/art.1780331101
29. Davis SR, Lambrinoudaki I, Lumsden M, Mishra GD, Pal L, Rees M, et al. Menopause. Nat Rev Dis Primers. (2015) 1:15004. doi: 10.1038/nrdp.2015.4
30. National Institute for Health and Care Excellence (NICE U). Menopause: Diagnosis and Management [NG23]. (2015). (Last updated: December 5, 2019).
31. Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the stages of reproductive aging workshop+10: addressing the unfinished agenda of staging reproductive aging. Menopause. (2012) 19(4):387–95. doi: 10.1097/gme.0b013e31824d8f40
32. Randolph JF Jr., Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. (2011) 96(3):746–54. doi: 10.1210/jc.2010-1746
33. Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet. (2005) 365(9470):1591–5. doi: 10.1016/S0140-6736(05)66461-6
34. Richette P, Corvol M, Bardin T. Estrogens, cartilage, and osteoarthritis. Joint Bone Spine. (2003) 70(4):257–62. doi: 10.1016/S1297-319X(03)00067-8
35. Sniekers YH, Weinans H, Bierma-Zeinstra SM, van Leeuwen JP, van Osch GJ. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment—a systematic approach. Osteoarthritis Cartilage. (2008) 16(5):533–41. doi: 10.1016/j.joca.2008.01.002
36. Wluka AE, Davis SR, Bailey M, Stuckey SL, Cicuttini FM. Users of oestrogen replacement therapy have more knee cartilage than non-users. Ann Rheum Dis. (2001) 60(4):332–6. doi: 10.1136/ard.60.4.332
37. Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Brain imaging reveals that engagement of descending inhibitory pain pathways in healthy women in a low endogenous estradiol state varies with testosterone. Pain. (2013) 154(4):515–24. doi: 10.1016/j.pain.2012.11.016
Keywords: phenotype, menopause, hormone replacement therapy (HRT), estrogen, female
Citation: Gulati M, Brewer G, Judge A, Kennedy D, Vincent TL and Watt FE (2024) Could sex-specific subtypes of hand osteoarthritis exist? A retrospective study in women presenting to secondary care. Front. Pain Res. 5:1331187. doi: 10.3389/fpain.2024.1331187
Received: 31 October 2023; Accepted: 25 January 2024;
Published: 12 February 2024.
Edited by:
Lingxiao Chen, Shandong University, ChinaReviewed by:
Bin Xu, Seoul National University, Republic of KoreaGe Li, Tianjin University of Traditional Chinese Medicine, China
© 2024 Gulati, Brewer, Judge, Kennedy, Vincent and Watt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fiona E. Watt Zi53YXR0QGltcGVyaWFsLmFjLnVr
 Malvika Gulati
Malvika Gulati Gretchen Brewer1
Gretchen Brewer1 Fiona E. Watt
Fiona E. Watt