- 1Faculty of Nursing, University of Regina, Regina, SK, Canada
- 2Department of Oncology, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
- 3Department of Psychology, University of Calgary, Calgary, AB, Canada
- 4Department of Psychology, University of Regina, Regina, SK, Canada
- 5Centre for Simulation and Visualization, University of Calgary, Calgary, AB, Canada
Background: Chronic cancer-related pain (CRP) can have a significant negative impact on quality of life. Mindfulness is hypothesized to mitigate chronic CRP by regulating both physical and emotional resistance to pain. In recent years, there has been interest in the use of virtual reality (VR) to deliver mindfulness meditation. VR provides an immersive and engaging environment, which may enhance one's focused attention to present-moment experiences, potentially making mindfulness less effortful and more efficacious for individuals with chronic pain. There has been little research in this area for people with a history of cancer.
Objective: The aim of this mixed methods study is to evaluate the feasibility of a VR-guided mindfulness (VRGM) intervention offered to adult cancer survivors with chronic CRP.
Methods: This mixed methods feasibility study will employ a single-arm, pretest-posttest design with semistructured interviews. In total, 15 cancer survivors will be enrolled in a 6-week home-based intervention that consists of 10–15 min of daily VRGM practice. The primary outcome is feasibility as assessed by accrual rates, retention in the study, intervention adherence, questionnaire completion, and side effect rates. Participants will be assessed on psychosocial outcome measures (i.e., pain, sleep, depressive and anxiety symptoms, fatigue, quality of life, and mindfulness) before and after the intervention, and 6 weeks post intervention (follow-up). Changes in pain will be described in relation to levels of immersion and presence in the virtual environment, trait mindfulness, and amount of VRGM practice. Qualitative information will provide subjective detail on participants’ experience with VRGM to complement quantitative data. This study has been approved by the Health Research Ethics Board of Alberta Cancer Committee (HREBA.CC-20-0411).
Conclusions: This novel intervention provides a potential alternative treatment to pharmacological pain management. Results from this study may inform future larger VGRM trials for chronic CRP to help reduce suffering in people with cancer. Study findings will be disseminated through open access publications, traditional conference presentations, professional cancer organizations, and social media platforms.
1 Introduction
1.1 Background
Improved survival owing to advances in cancer treatment has resulted in increased numbers of patients experiencing chronic pain (1), defined by the International Association for the Study of Pain as “persistent or recurrent pain lasting longer than 3 months” (2). The prevalence of chronic cancer-related pain (CRP) has been estimated at 30%–50% in patients undergoing cancer treatment and more than 70% in patients with advanced, metastatic, or terminal disease (3). CRP is often felt as moderate or severe. A 2016 systematic review on the prevalence of CRP (3) found that 38% of 32,261 patients across 52 studies reported at least moderate CRP, while a 2009 European survey study (4) indicated that of 5,084 adults with cancer, 56% reported moderate to severe CRP.
CRP can be due to the cancer itself or its treatment. CRP directly related to cancer can arise from tumor invasion that can affect the viscera, nerves, and bones (5). CRP related to treatment is generally due to peripheral neuropathy from chemotherapeutic agents (e.g., vincristine and taxane), radiotherapy-induced neural damage, and chronic postsurgical pain from mastectomy, amputation, or thoracotomy (5). Certain treatments for cancer, such as head and neck radiation or conditioning regimens administered prior to hematopoietic cell transplant, can also cause painful gastrointestinal mucositis (6).
CRP is associated with psychological distress and functional impairment. One study of 667 individuals with various cancer types found that pain, when present, interfered with participants’ daily activity and enjoyment of life to a moderate to severe extent (7). To this end, chronic pain research often emphasizes the importance of managing pain interference in addition to pain intensity in order to improve quality of life (8). Descriptive studies of women with breast cancer also link pain to poor emotional, physical, and social functioning, in addition to increased fatigue, anxiety, depression, and insomnia (9, 10). Studies also support the link between poor sleep and increased risk for chronic diseases and mortality (11, 12). Therefore, effective treatment of CRP can have broad positive effects on the health and well-being of patients with and survivors of cancer by improving other symptoms related to quality of life and morbidity.
Opioid analgesics are the standard of care for people with chronic CRP (13), but they are limited by negative side effects and neuroadaptations associated with long-term use, which reduces analgesic efficacy (14). Some psychological and physical therapies (e.g., cognitive behavioral therapy, hypnosis, and acupuncture) have shown the potential to reduce levels of CRP as a stand-alone treatment or an adjunct to pharmacological interventions (15, 16). In light of the high prevalence of CRP among patients with and survivors of cancer and the limitations of currently existing treatments, the establishment of safe and effective nonpharmacological interventions for chronic CRP management should be a research priority.
One style of training in mindfulness meditation is a psychological approach that involves directing focused attention to the internal and external experiences occurring in the present moment with an accepting attitude (17, 18). By bringing attention to the present moment, one can cultivate a nonjudgmental awareness to everyday situations that may be perceived as stressful (17). This is particularly important for people with chronic CRP, as pain persistence itself may cause stress (19). It has been hypothesized that mindfulness practice over time would enhance trait mindfulness (i.e., one’s predisposition to be mindful in daily life) and consequently potentially improve pain outcomes by regulating emotional reactions to pain and enhancing acceptance-related coping approaches (20, 21). The biological mechanism explaining this effect with the most supportive evidence is that endogenous opioid pathways mediate the analgesic effects of mindfulness on pain through orbitofrontal cortex activation and thalamic deactivation (22).
A pioneering 1985 single-group study of mindfulness-based stress reduction (MBSR) by Kabat-Zinn (23) showed a significant reduction in chronic pain among 90 patients with a variety of conditions, even up to 4 years post intervention (24). More recent randomized controlled trials (RCTs) have also supported the positive effects of mindfulness on chronic pain (25). For example, in a 2011 RCT of people with irritable bowel syndrome (IBS) (26), participants reported significant pain improvement following a mindfulness-based intervention, and benefits were maintained 3 months post intervention. Multivariate path analyses suggested that mindfulness practice mitigated pain through improving anxiety and emotional responses to IBS symptoms (26, 27). While very few mindfulness studies targeting pain have been conducted among cancer survivors, the evidence to date is promising with studies showing small to moderate effects (28). For example, in a 2016 RCT (29) comparing MBSR to an active control condition (psychoeducational support) for breast and colorectal cancer survivors, MBSR participants reported significant reduction in pain compared with control participants (Cohen d = 0.53).
In the past few years, there has been a growing interest in using virtual reality (VR), a computer-generated 360° representation of a virtual environment displayed through a headset as a medium to support mindfulness practice (30). The multisensory information and stereo-visual image that VR delivers are meant to create a sense of space and depth, which can help users become fully present and immersed in the virtual atmosphere (31). Immersion is the degree to which the technological features of VR systems deliver an extensive, inclusive, surrounding, and vivid illusion of reality to the senses of a human participant, whereas presence is the perception of being present in a VR environment (i.e., a feeling of being there) (32). Although other platforms that provide guidance on mindfulness (e.g., web-based interventions or videos and smartphone apps) are available, environmental distractions (e.g., noisy surroundings) may pose limitations. VR has the potential to address those challenges by providing an engaging and immersive environment, potentially leading to sustained mindfulness and larger effects for reducing CRP (33).
Because humans have a limited amount of available conscious attention, VR competes for attention otherwise directed toward interpreting stimulation from the real environment, leaving lesser cognitive capacity available for processing external distractions (31, 33). It is also possible that VR-guided mindfulness (VRGM) can help participants self-initiate and continue mindfulness practice, as well as maintain conscious and cognitive efforts required for effective practice, all of which have previously been identified as barriers to mindfulness meditation practice, particularly among novice meditators (30, 33).
VRGM has the potential to overcome some of the limitations of traditional mindfulness-based interventions, such as MBSR, that involve an instructor within a group setting. External distractions from other group members may pose limitations required for effective mindfulness practice. Regularly attending sessions may be difficult for cancer patients and survivors with chronic pain due to pain related interference with daily activity, hospitalizations, or cancer treatments, in addition to personal commitments. The timing and frequency of sessions may not meet the meditative needs of participants. For example, some may prefer morning meditations to energize oneself, while others may prefer evening meditations to relax before sleep. A weekly session may not suffice the needs for people with cancer experiencing chronic pain, who may desire to practice mindfulness daily or multiple times per day for pain management. Those living in remote areas may have difficulties with accessibility, as local mindfulness-based interventions may not be available. Additional accessibility barriers may occur due to costs associated with attending sessions (i.e., session fees, transportation, parking, etc.), especially in countries without universal healthcare. VRGM has the potential to overcome these challenges by eliminating external group distractions and providing more flexibility in the timing, frequency, and accessibility of mindfulness practice through a self-delivered intervention.
In the last 10 years, innovations in technology have dramatically driven down costs for development and implementation of VR, which has given rise to its commercialization and affordability as a cost-effective tool for clinical practice (34–36). For example, in the 1990s VR systems used in clinical research could cost up to $90,000 USD, and today in 2024 the Meta Quest 2 standalone VR headset can be purchased for $250 USD (37). Major milestones for VR technology occurred in 2016 when companies in the technology industry began mass producing VR headsets and in 2019 when the industry shifted towards the development of standalone VR headsets (a wireless, easy to use, self-contained VR system not requiring an external computer to run the software) (34). Continued investment in VR technology by big tech companies, such as Meta, Apple, Microsoft, and Google, driven by competition for potentially new lucrative VR-related markets, have resulted in a considerable increase in the image quality and immersive experience year-over-year, while continuously improving affordability and availability (34). However, the current cost of a VR headset may still be financially constraining for some, and as such an ideal scenario to improve accessibility would have cancer support centres purchase a small number of VR headsets for people with cancer to use freely within the centre.
Despite its potential benefits for patients with chronic CRP, VRGM has received relatively limited attention in the VR literature. VR has primarily been used as a distraction technique for acute procedural pain relief, with some studies incorporating video games or virtual traveling into the VR experience as a way to enhance psychological distraction and reduce self-reported pain (31). Importantly, very few studies targeting chronic pain used VR as a medium to facilitate mindfulness meditation, which is less about distraction and more about awareness of current experience. A 2015 RCT of 13 participants (33) found VRGM to be superior to a mindfulness audio track alone in reducing chronic noncancer pain symptoms. Similarly, in a 2017 single-group study (38) evaluating the benefits of VRGM for 18 participants with chronic pain (16 patients with cancer and 2 patients with neurological conditions), all participants reported a reduction in pain intensity, ranging from a 20% to a 100% reduction.
Taken together, while VRGM could be a novel promising approach for chronic CRP management, additional research is needed to evaluate its efficacy and determine its acceptability and feasibility to guide the design and implementation of future trials. Research is also needed to understand the potential mechanisms of mindfulness-related improvement in pain intensity and pain interference and to test VRGM models that are self-deliverable and home-based to enhance effectiveness and sustainability. As a first step in a program of research, this mixed methods single-group feasibility study will evaluate a home-based 6-week VRGM intervention for cancer survivors with CRP lasting longer than 3 months. The following paragraphs outline the specific study objectives.
1.2 Primary objective
1.2.1 Objective 1: to evaluate the feasibility of a self-administered, at-home, 6-week VRGM program for chronic CRP
Feasibility outcomes include recruitment rates, intervention adherence, and study completion rates, as well as intervention safety as determined by the occurrence, type, and severity of negative side effects.
1.3 Secondary objectives
1.3.1 Objective 2: to explore the potential benefits of a 6-week VRGM program for patients with chronic CRP
To address this proof-of-concept objective, effect sizes and confidence intervals of changes in outcome measures (pain, sleep, depressive and anxiety symptoms, fatigue, quality of life, and analgesic use) will be described across different time points.
1.3.2 Objective 3: to explore potential factors contributing to the benefits of mindfulness practice for patients with chronic CRP
Specifically, pre- to post-VRGM changes in pain will be described in relation to levels of immersion and presence in the VR experience, trait mindfulness, and amount of VRGM practice.
1.3.3 Objective 4: to understand cancer survivors’ experiences with VRGM
Participants will take part in in-depth, semistructured interviews to evaluate the acceptability and perceived benefits of VRGM, and to identify obstacles and facilitators of adherence and the challenges faced in initiating and maintaining VRGM practice.
2 Methods
2.1 Design
2.1.1 Overview
This study will employ a prospective, single-arm, pretest-posttest, mixed methods design. Adults with chronic CRP will participate in a 6-week home-based VRGM intervention with a one-to-one interview upon intervention completion.
2.2 Participants
2.2.1 Sample size
This protocol is designed as a mixed methods feasibility study. The recruitment goal is 15 participants, a sample which is comparable to other mindfulness studies primarily focusing on feasibility, reporting sample sizes of 13–19 participants (39, 40). This sample size can allow the evaluation of feasibility outcomes by providing crucial information about the ability to recruit this population to such an intervention, intervention safety, adherence, and study completion rates. Feasibility will also partly be determined by participants’ attitudes toward VRGM and level of satisfaction with the intervention during the semistructured interviews.
2.2.2 Eligibility criteria
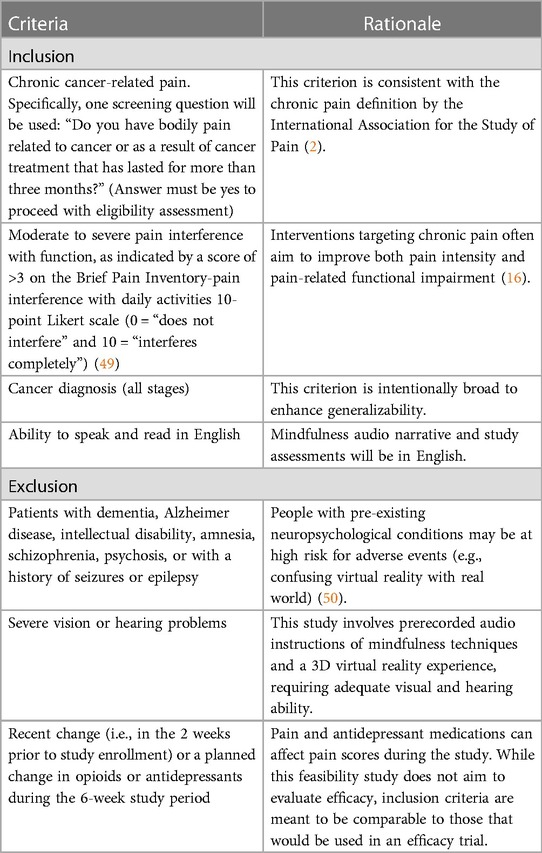
Patients with any type or stage of cancer, who have reported chronic CRP (i.e., for 3 months or longer), are eligible to participate. Patients could be on active treatment, posttreatment, or receiving palliative care (see Table 1 for rationale of all inclusion and exclusion criteria).
2.3 Procedures
2.3.1 Recruitment
The main recruitment strategy will be via oncology physician referral in the outpatient clinics of the Tom Baker Cancer Centre, a tertiary care teaching hospital affiliated with the University of Calgary (Calgary, Alberta, Canada). Social media platforms, such as Facebook, Instagram, and Twitter, and community cancer support organizations will also be used to enrich recruitment and reach underrepresented populations. The study coordinator will make a telephone call to referred patients or those who have expressed interest via advertisements to describe the purpose of the study, provide details about the intervention and the number and frequency of assessments to be completed, and screen for eligibility. Those who are interested in participating and eligible will sign an electronic consent form.
2.3.2 Baseline call
Consenting participants will be mailed or delivered a head-mounted VR display headset (Pico Neo 3 Pro) with the VRGM protocol preinstalled. Prior to starting the intervention, participants will complete a 20 min baseline in person or by videoconference call with the investigative team to ensure receipt of the VRGM kit and for an orientation session (e.g., how to wear and set up the headset, what are the buttons required, and what they do). After orientation, participants will complete a 15 min trial session with the study coordinator or a trained research assistant (RA) to assess how comfortable they are with the VR experience and if they develop simulator sickness, which is a possible side effect of VR (41). Simulator sickness is generally attributed to the discrepancy between simulated visual motion and the sense of movement stemming from the vestibular system, producing symptoms similar to motion sickness (41). After the trial session, participants will complete the Simulator Sickness Questionnaire (SSQ) (41) with the study coordinator or RA, a well-validated scale that assesses 16 symptoms on a 4-point scale (scores 0–3): nausea, general discomfort, stomach awareness, sweating, increased salivation, vertigo, burping, difficulty concentrating, difficulty focusing, eyestrain, fatigue, headache, blurred vision, dizziness with eyes open, dizziness with eyes closed, and fullness of head. A total SSQ score greater than 20 indicates that symptoms may be concerning (41), and thus participants may have high tendency to develop simulator sickness. Therefore, participants who score >20 on the SSQ will be deemed ineligible to proceed with study participation. They will be asked to stop using the VR headset and return it to investigators. Instead, they will be provided with resources for other mindfulness platforms.
2.3.3 Data collection
Consenting participants will be emailed a link before the baseline call to complete the pre- and poststudy surveys in the REDCap secure database. Daily diaries will be returned using a stamped, self-addressed envelope to the investigative team. With regard to qualitative data, semistructured interviews will be conducted in person or through videoconference call by a member of the research team using a pre-established set of questions (see Table 2) that cover the following areas: perceptions of VRGM, perceived benefits of VRGM, whether and how VRGM has impacted pain severity and interference with function, perception of pain and previous mindfulness meditation experience (42), and factors underlying satisfaction and dissatisfaction with study participation. Participants who drop out of the study will also be invited to participate in the semistructured interview. Interviews will be audio-recorded, and recordings will be transcribed verbatim.
2.4 Intervention
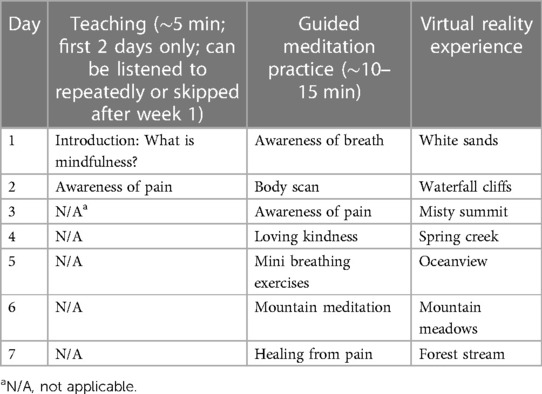
Consistent with previous similar studies (33, 43), the VRGM intervention will consist of a 10–15 min, once-daily session for 6 weeks. The VR headset will present a range of preinstalled 3D high-quality calming virtual environments (i.e., nature scenes and sounds), such as mountain meadows, white sands, spring creek, and other beautiful nature scenery (e.g., beaches, waterfalls, and tall trees). The headset includes a motion tracker that measures the position of the head and adjusts the visual image accordingly, which can make participants feel as if they can look around and move through the virtual environment. Built-in speakers on the VR headset will provide an audio narrative of guided mindfulness practice.
The content of the audio is based on the research team's previous work with the evidence-based mindfulness-based cancer recovery program (17) and will include instructions to perform weekly mindfulness exercises (one exercise per day) for 6 weeks (see Table 3). Each meditation exercise has its own unique preinstalled virtual environment, selected on the basis of which was best suited for each type of meditation. All virtual environments will also be customized to synchronize with the meditation audio (e.g., mixing movement with sound).
2.5 VR hardware specifications
The Pico Neo 3 Pro (PN3P) is a 6 degrees of freedom standalone VR headset with a high resolution 4k liquid crystal display (1,832 × 1,920 pixels per eye) and 90 Hz refresh rate (44). The PN3P was released in May 2021 and retailed for $699 USD. It accommodates for a wide range of different eye features, as the user is able to adjust the lens spacing at three settings (58 mm, 63.5 mm, and 69 mm) to line up with the user's interpupillary distance to ensure image clarity and allow for a more immersive experience (45). The PN3P is also suitable for a wide range of head shapes and facial features, as it uses an adjustable rear strap and polyurethane leather foam face cushion to properly position the VR device on the user's face (45). The all-hygienic materials of the P3NP make the VR headset easy to clean and ideal to share between multiple users (45). It is ergonomically designed for users to comfortably wear for long periods of time by counterbalancing the weight of the front display with the rear battery (44). The total weight of the PN3P is 673 g (46), which is in the middle range for other 4k standalone VR headsets with similar specifications and release date, such as the Oculus Quest 2 at 503 g (47) and HTC Vive Focus 3 at 785 g (48). Non-standalone (tethered) VR headsets require a cable connection to a personal computer (PC) to run the VR software, which increases the weight of the VR headset, restricts user mobility, and can be cumbersome for cable management. Tethered VR headsets also increase the complexity in setup for users and have additional costly and bulky hardware requirements (PC, graphics card, etc.), thus making a standalone system the preferred choice for a home-based VRGM intervention.
2.6 Feasibility evaluation
A log containing information on the number of patients approached for participation, the number referred to the research team, the number successfully contacted by the research team, and those who consented will be maintained. The number of patients who declined or did not meet the eligibility criteria and the underlying reasons will also be recorded. Another log will be maintained for the number of participants who dropped out during the study. Home practice will be tracked through engagement data from the VR device, which include session length, total time spent using the headset, and number of daily VRGM sessions. Data collected from the VR device will be verified through a daily home practice log. The log will provide space to input the date, number of VRGM sessions completed each day, total number of minutes per session, and comments about their experience and side effects. Participants will also be contacted once weekly to ask whether they have experienced any side effects. Data obtained from study logs will be used to calculate recruitment and study completion rates. Data collected from the VR system, home practice logs, and weekly emails will be used to evaluate intervention adherence and safety.
2.7 Outcome measures
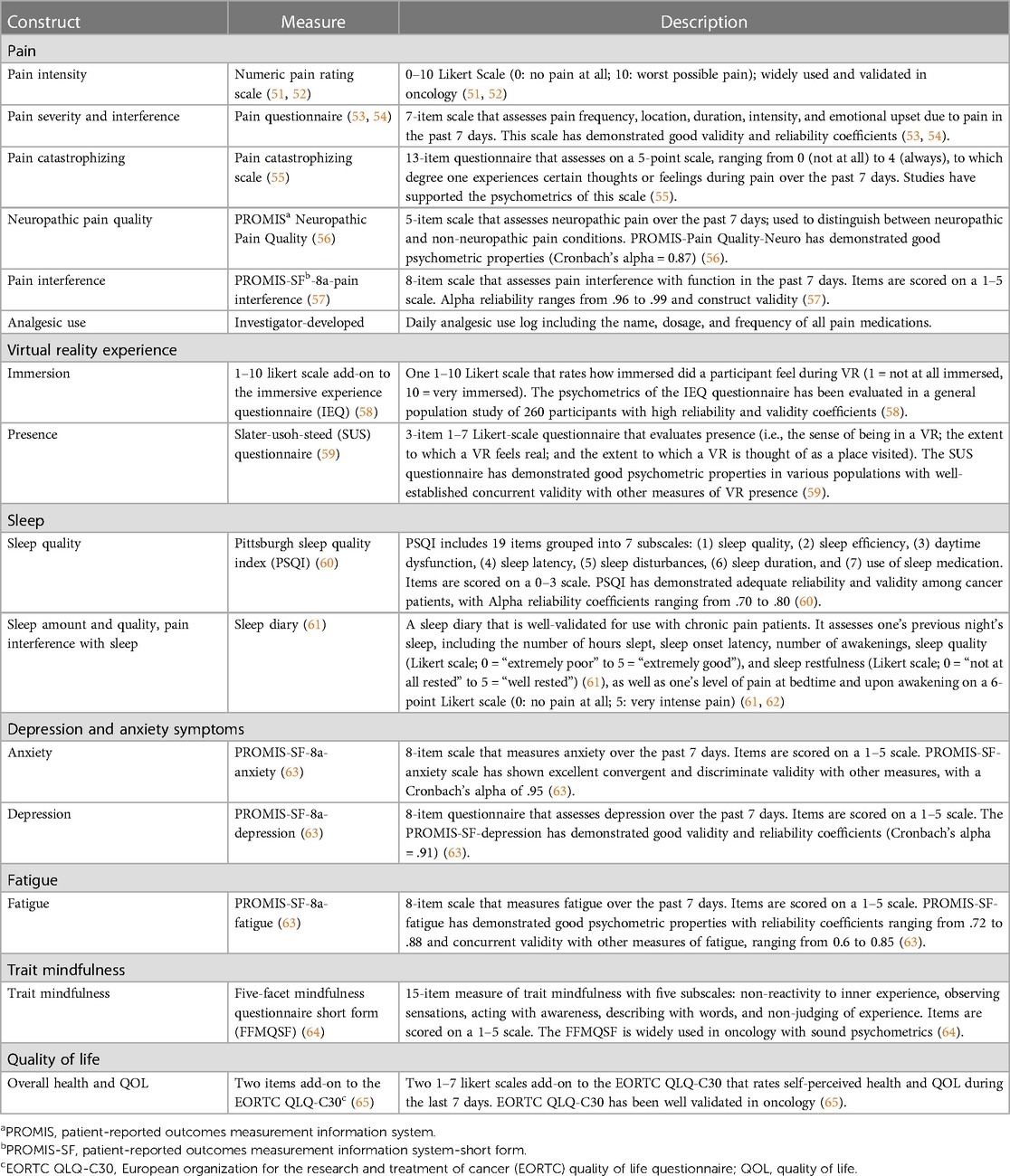
All the study measures are described in Table 4 (51–65). Standardized measures of pain, sleep, depressive and anxiety symptoms, fatigue, and quality of life (objective 2), as well as trait mindfulness and levels of immersion and presence in the VR experience (objective 3), will be used. Sociodemographic and medical information collected from participants at baseline will include age, sex, gender, race and ethnicity, cancer type and stage, time since diagnosis, time since last treatment, presence and severity of comorbidities, educational level, marital status, occupational status, and type and frequency of nonpharmacological interventions used for pain in the last 3 months. The prescribed analgesic regimen (standing and as needed) will also be obtained. This will include the number of analgesics prescribed, types of analgesics, frequency of administration, route of administration, and prescribed dose. The entire battery of questionnaires has been user-tested and requires approximately 30 min to complete.
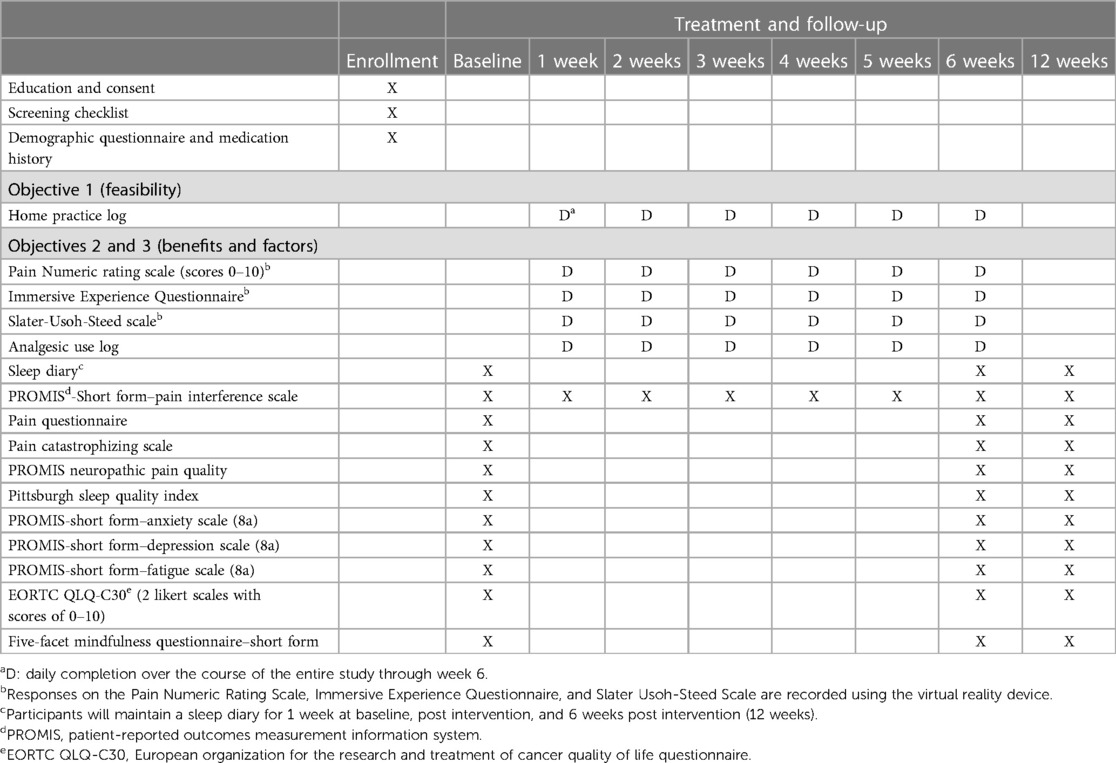
2.8 Timing of assessments
Timing of assessments is fully described in Table 5 (schedule of measures).
2.8.1 Objective 2
Psychosocial outcome measures (i.e., pain, sleep, fatigue, depressive and anxiety symptoms, and quality of life) will be assessed before and after the intervention and 6 weeks post intervention (follow-up). As noted above, pain-related functional impairment is often a priority in chronic pain mitigation efforts and is thus an important outcome for this feasibility trial. Therefore, pain interference with function will be assessed weekly during the 6-week intervention period and then again at follow-up. The VR device will record the intensity of participants’ pain on a Numeric Rating Scale (scores of 0–10) (51) before and immediately after each VRGM session. Participants will maintain analgesic use in the home practice logs to record their use of pain medications and dosages daily, until completion of the 6-week VRGM intervention. Additionally, they will maintain a sleep diary for 1 week before and after the intervention, as well as at 6 weeks post intervention (follow-up), including assessments of sleep duration and quality and pain interference with sleep. Since sleep diaries evaluate sleep on a daily basis, many of the limitations of retrospective self-reported sleep measures are minimized, such as recall bias and recency bias (tendency to recall last few nights) (66).
2.8.2 Objective 3
The VR device will record participants’ level of immersion and presence in each VRGM session. Trait mindfulness will be evaluated before and after the intervention and 6 weeks post intervention (follow-up).
2.9 Data analysis
This is a small feasibility study; therefore, analyses will be descriptive.
2.9.1 Quantitative analysis
Descriptive statistics will be used to calculate accrual rates (and reasons for not consenting to the trial), retention in the trial, intervention adherence, questionnaire completion, and adverse event rates (objective 1). Means and SDs will be calculated for continuous variables and frequency distributions for categorical variables. The profile of each outcome (means and SDs) will be displayed to visualize the pattern of changes over time (objective 2). Effect sizes and confidence intervals of changes in scores for outcome measures (i.e., pain, sleep, fatigue, depressive and anxiety symptoms, and quality of life) will be described across different time points (objective 2). Exploratory analyses will be used to describe the strength of associations of pre- to post-VRGM pain changes with the levels of immersion and presence in the VR experience, total amount of VRGM practice, and change in pre- to post-VRGM trait mindfulness (objective 3).
2.9.2 Qualitative analysis (objective 4)
Qualitative data will be analyzed using the principles of Thematic Analysis (67), which is a rigorous inductive approach to identify and describe implicit and explicit patterns within qualitative data. The steps of Thematic Analysis (67) will be applied, including (1) reading the transcripts actively and repeatedly to become familiar with the depth and breadth of the content, (2) identifying patterns and developing initial codes (subthemes), (3) searching for and identifying overarching themes, (4) comparing and contrasting emergent themes within and across transcripts, and (5) developing and reviewing the thematic scheme. A number of strategies will be used to improve methodological rigor. To minimize researcher bias, a self-reflexivity journal will be maintained during data collection and analysis. Two members of the research team will independently code all of the transcripts and develop interim themes. Discrepancies will be discussed and resolved by consensus.
2.10 Ethics approval
This research has been approved by the Health Research Ethics Board of Alberta Cancer Committee (HREBA.CC-20-0411). Potential participants will be informed of study details, including confidentiality and the voluntary nature of participation, risks and benefits, and study requirements prior to signing the consent form. Participants will be made aware of their right to withdraw at any time, and that this will not affect their future care. As previously noted, VR technology is known to be safe, but it may be associated with simulator sickness. Measures are in place to minimize this risk as outlined in the protocol. The potential benefits will likely outweigh the side effects that may result from VR technology. Although the efficacy of mindfulness practices for reducing chronic CRP is an outstanding issue when delivered through an immersive virtual environment, in general, the mental and physical health benefits of mindfulness are well documented.
3 Discussion
3.1 Strengths and limitations of this study
A strength of this study is employing a mixed methods design, which can enhance the rigor of the study by including findings from different methodological perspectives. This study has and will continue to involve patient research partners throughout the project to guide research questions, design, and implementation, and add context to the research findings. This study will provide the foundation needed to design and conduct a larger study to examine the efficacy of VRGM in improving chronic CRP. A limitation of this study is its single-group design, which is necessary to evaluate the acceptability of VRGM by as many cancer survivors as possible. This feasibility study is not statistically powered to evaluate the intervention efficacy for clinically relevant outcomes.
3.2 Dissemination
The results will be widely disseminated to stakeholders through presentations at national and international conferences, publications in open access scientific journals, social media platforms (e.g., Twitter, LinkedIn, ResearchGate, and Academia.edu), and webinars and discussion groups through patient advocacy networks and professional oncology organizations.
4 Conclusions
Survivors of cancer experience increased levels of psychosocial symptoms and pain interference. This novel intervention provides a potential alternative treatment to opioid analgesics. Results from this study may inform future larger VGRM trials for chronic CRP to help reduce suffering in people with cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MB: Writing – review & editing, Writing – original draft. ZG: Writing – review & editing. MP: Writing – review & editing. AM: Writing – review & editing. SC: Writing – review & editing. LC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research received funding from the Enbridge Research Chair in Psychosocial Oncology, Alberta Innovates Graduate Student Scholarship, and Cumming School of Medicine Graduate Scholarship. VR software development and eight Pico Neo 3 Pro headsets were provided on loan in-kind by Novobeing.
Acknowledgments
The study concept was initiated by patient partners Craig Green and Paulina Szewczyk who brought the idea of VR pain management to the research team. These patient partners were also engaged in the development of this protocol through attending regular meetings with the researchers, providing input, and contributing to decision-making about the proposed study design including priority of the research question, methods of recruitment, and choice of outcome measures. We would like to thank them for the time and effort they contributed to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kurtin S, Fuoto A. Pain management in the cancer survivor. Semin Oncol Nurs. (2019) 35(3):284–90. doi: 10.1016/j.soncn.2019.04.010
2. International Society for the study of pain. Pain Definition (2020). Available online at: https://www.iasp-pain.org/ (accessed September 2, 2020).
3. van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. (2016) 51(6):1070–1090.9. doi: 10.1016/j.jpainsymman.2015.12.340
4. Breivik H, Cherny N, Collett B, de Conno F, Filbet M, Foubert AJ, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. (2009) 20(8):1420–33. doi: 10.1093/annonc/mdp001
5. Lam DK. Emerging factors in the progression of cancer-related pain. Pain Manag. (2016) 6(5):487–96. doi: 10.2217/pmt-2015-0003
6. Harris DJ. Cancer treatment-induced mucositis pain: strategies for assessment and management. Ther Clin Risk Manag. (2006) 2(3):251–8. doi: 10.2147/tcrm.2006.2.3.251
7. Daut RL, Cleeland CS. The prevalence and severity of pain in cancer. Cancer. (1982) 50(9):1913–8. doi: 10.1002/1097-0142(19821101)50:9%3C1913::aid-cncr2820500944%3E3.0.co;2-r
8. Sheinfeld Gorin S, Krebs P, Badr H, Janke EA, Jim HS, Spring B, et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol. (2012) 30(5):539–47. doi: 10.1200/JCO.2011.37.0437
9. Costa WA, Monteiro MN, Queiroz JF, Gonçalves AK. Pain and quality of life in breast cancer patients. Clinics (Sao Paulo). (2017) 72(12):758–63. doi: 10.6061/clinics/2017(12)07
10. Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat. (2018) 167(1):157–69. doi: 10.1007/s10549-017-4485-0
11. Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 national sleep foundation sleep in America survey. J Psychosom Res. (2004) 56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010
12. Bertisch SM, Pollock BD, Mittleman MA, Buysse DJ, Bazzano LA, Gottlieb DJ, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: sleep heart health study. Sleep. (2018) 41(6):zsy047. doi: 10.1093/sleep/zsy047
13. Wright EM, El-Jawahri A, Temel JS, Carr A, Safren SA, Park ER, et al. Patient patterns and perspectives on using opioid regimens for chronic cancer pain. J Pain Symptom Manage. (2019) 57(6):1062–70. doi: 10.1016/j.jpainsymman.2019.02.023
14. Ballantyne JC. “Safe and effective when used as directed”: the case of chronic use of opioid analgesics. J Med Toxicol. (2012) 8(4):417–23. doi: 10.1007/s13181-012-0257-8
15. Kravits K. Hypnosis: adjunct therapy for cancer pain management. J Adv Pract Oncol. (2013) 4(2):83–8. doi: 10.6004/jadpro.2013.4.2.2
16. Eaton LH, Hulett JM. Mind-body interventions in the management of chronic cancer pain. Semin Oncol Nurs. (2019) 35(3):241–52. doi: 10.1016/j.soncn.2019.04.005
17. Carlson LE. Mindfulness-based interventions for coping with cancer. Ann N Y Acad Sci. (2016) 1373(1):5–12. doi: 10.1111/nyas.13029
18. Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. (2008) 12(4):163–9. doi: 10.1016/j.tics.2008.01.005
19. Zaza C, Baine N. Cancer pain and psychosocial factors: a critical review of the literature. J Pain Symptom Manage. (2002) 24(5):526–42. doi: 10.1016/s0885-3924(02)00497-9
20. Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC. Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett. (2012) 520(2):165–73. doi: 10.1016/j.neulet.2012.03.082
21. Adler-Neal AL, Zeidan F. Mindfulness meditation for fibromyalgia: mechanistic and clinical considerations. Curr Rheumatol Rep. (2017) 19(9):59. doi: 10.1007/s11926-017-0686-0
22. Sharon H, Maron-Katz A, Ben Simon E, Flusser Y, Hendler T, Tarrasch R, et al. Mindfulness meditation modulates pain through endogenous opioids. Am J Med. (2016) 129(7):755–8. doi: 10.1016/j.amjmed.2016.03.002
23. Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. (1985) 8:163–90. doi: 10.1007/BF00845519
24. Kabat-Zinn J, Lipworth L, Burney R, Sellers W. Four-year follow-up of a meditation based program for the self-regulation of chronic pain: treatment outcomes and compliance. Clin J Pain. (1986) 2:159–73. doi: 10.1097/00002508-198602030-00004
25. Reiner K, Tibi L, Lipsitz JD. Do mindfulness-based interventions reduce pain intensity? A critical review of the literature. Pain Med. (2013) 14(2):230–42. doi: 10.1111/pme.12006
26. Gaylord SA, Palsson OS, Garland EL, Faurot KR, Coble RS, Mann JD, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. (2011) 106(9):1678–88. doi: 10.1038/ajg.2011.184
27. Garland EL, Gaylord SA, Palsson O, Faurot K, Douglas Mann J, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med. (2012) 35(6):591–602. doi: 10.1007/s10865-011-9391-z
28. Ngamkham S, Holden JE, Smith EL. A systematic review: mindfulness intervention for cancer-related pain. Asia Pac J Oncol Nurs. (2019) 6(2):161–9. doi: 10.4103/apjon.apjon_67_18
29. Johns SA, Brown LF, Beck-Coon K, Talib TL, Monahan PO, Giesler RB, et al. Randomized controlled pilot trial of mindfulness-based stress reduction compared to psychoeducational support for persistently fatigued breast and colorectal cancer survivors. Support Care Cancer. (2016) 24(10):4085–96. doi: 10.1007/s00520-016-3220-4
30. Seabrook E, Kelly R, Foley F, Theiler S, Thomas N, Wadley G, et al. Understanding how virtual reality can support mindfulness practice: mixed methods study. J Med Internet Res. (2020) 22(3):e16106. doi: 10.2196/16106
31. Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clin Psychol Rev. (2010) 30(8):1011–8. doi: 10.1016/j.cpr.2010.07.001
32. Rose T, Nam CS, Chen KB. Immersion of virtual reality for rehabilitation—review. Appl Ergon. (2018) 69:153–61. doi: 10.1016/j.apergo.2018.01.009
33. Gromala D, Tong X, Choo C, Karamnejad M, Shaw CD. The virtual meditative walk: virtual reality therapy for chronic pain management. In: Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems. New York: Association for Computing Machinery (2015). p. 521–4. doi: 10.1145/2702123.2702344
34. Hitching R, Hoffman HG, Garcia-Palacios A, Adamson MM, Madrigal E, Alhalabi W, et al. The emerging role of virtual reality as an adjunct to procedural sedation and anesthesia: a narrative review. J Clin Med. (2023) 12(3):843. doi: 10.3390/jcm12030843
35. Trost Z, France C, Anam M, Shum C. Virtual reality approaches to pain: toward a state of the science. Pain. (2021) 162:325–31. doi: 10.1097/j.pain.0000000000002060
36. Hoffman HG, Chambers GT, Meyer WJ 3rd, Arceneaux LL, Russell WJ, Seibel EJ, et al. Virtual reality as an adjunctive non-pharmacologic analgesic for acute burn pain during medical procedures. Ann Behav Med. (2011) 41(2):183–91. doi: 10.1007/s12160-010-9248-7
37. Hoffman HG, Boe DA, Rombokas E, Khadra C, LeMay S, Meyer WJ, et al. Virtual reality hand therapy: a new tool for nonopioid analgesia for acute procedural pain, hand rehabilitation, and VR embodiment therapy for phantom limb pain. J Hand Ther. (2020) 33(2):254–62. doi: 10.1016/j.jht.2020.04.001
38. Popert S, Riat H. Hodges EP-35 can virtual reality (VR) guided meditation reduce pain? A feasibility and acceptability study. BMJ Support Palliat Care. (2017) 7:A22. doi: 10.1136/bmjspcare-2017-00133.5
39. Eyles C, Leydon GM, Hoffman CJ, Copson ER, Prescott P, Chorozoglou M, et al. Mindfulness for the self-management of fatigue, anxiety, and depression in women with metastatic breast cancer: a mixed methods feasibility study. Integr Cancer Ther. (2015) 14(1):42–56. doi: 10.1177/1534735414546567
40. Lucas AR, Focht BC, Cohn DE, Buckworth J, Klatt MD. A mindfulness-based lifestyle intervention for obese, inactive endometrial cancer survivors: a feasibility study. Integr Cancer Ther. (2017) 16(3):263–75. doi: 10.1177/1534735416668257
41. Balk SA, Bertola MA, Inman VW. Simulator sickness questionnaire: twenty years later. In: Proceedings of the Seventh International Driving Symposium on Human Factors in Driver Assessment, Training, and Vehicle Design; 2013 Jun 17–20; McLean, VI, United States. New York: Public Policy Center, University of Iowa (2013). p. 257–63.
42. Poletti S, Abdoun O, Zorn J, Lutz A. Pain regulation during mindfulness meditation: phenomenological fingerprints in novices and experts practitioners. Eur J Pain. (2021) 25(7):1583–602. doi: 10.1002/ejp.1774
43. Pekyavas NO, Ergun N. Comparison of virtual reality exergaming and home exercise programs in patients with subacromial impingement syndrome and scapular dyskinesis: short term effect. Acta Orthop Traumatol Turc. (2017) 51(3):238–42. doi: 10.1016/j.aott.2017.03.008
44. Pico Immersive Pte. Pico Neo 3 Pro Overview (2023). Available online at: https://www.picoxr.com/global/products/neo3-pro-eye (accessed February 19, 2024).
45. Qingdao Pico Technology Co. Pico Neo 3 User Guide (2022). Available online at: https://www.picoxr.com/cn/neo3/pdf/PicoNeo3UserGuide.pdf (accessed February 19, 2024)
46. PICO Immersive Pte. Pico Neo 3 Pro Product Specs (2023). Available online at: https://www.picoxr.com/global/products/neo3-pro-eye/specs (accessed February 19, 2024).
47. Hicks ML. Here’s why the Skinnier Quest 3 Weighs more than the Quest 2. Yahoo Finance (2024). Available online at: https://finance.yahoo.com/news/heres-why-skinnier-quest-3-210553727.html?guccounter=1#:∼:text=The%20Oculus%20Quest%202%20weighs,to%20the%20small%20weight%20gain (accessed February 20, 2024)
48. Carbotte K. HTC Vive Focus 3 Review: Standalone VR Done Right. Tom’s Hardware, Future US (2021). Available online at: https://www.tomshardware.com/reviews/vive-focus-3-vr-headset (accessed on February 20, 2024)
49. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. (1994) 23(2):129–38. 8080219.8080219
50. Gorini A, Riva G. Virtual reality in anxiety disorders: the past and the future. Expert Rev Neurother. (2008) 8(2):215–33. doi: 10.1586/14737175.8.2.215
51. Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. (1997) 20(2):88–93. doi: 10.1097/00002820-199704000-00002
52. Haefeli M, Elfering A. Pain assessment. Eur Spine J. (2006) 15(Suppl 1):S17–24. doi: 10.1007/s00586-005-1044-x
54. von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the numerical rating scale (NRS-11) for children’s self-reports of pain intensity. Pain. (2009) 143(3):223–7. doi: 10.1016/j.pain.2009.03.002
55. Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The pain catastrophizing scale: further psychometric evaluation with adult samples. J Behav Med. (2000) 23(4):351–65. doi: 10.1023/a:1005548801037
56. Askew RL, Cook KF, Keefe FJ, Nowinski CJ, Cella D, Revicki DA, et al. A PROMIS measure of neuropathic pain quality. Value Health. (2016) 19(5):623–30. doi: 10.1016/j.jval.2016.02.009
57. Teresi JA, Ocepek-Welikson K, Cook KF, Kleinman M, Ramirez M, Reid MC, et al. Measurement equivalence of the patient reported outcomes measurement information system® (PROMIS®) pain interference short form items: application to ethnically diverse cancer and palliative care populations. Psychol Test Assess Model. (2016) 58(2):309–52. 28983449.28983449
58. Jennett C, Cox AL, Cairns P, Dhoparee S, Epps A, Tijs T, et al. Measuring and defining the experience of immersion in games. Int J Hum Comput Stud. (2008) 66(9):641–61. doi: 10.1016/j.ijhcs.2008.04.004
59. Usoh M, Catena E, Arman S, Slater M. Using presence questionnaires in reality. Presence. (2000) 9(5):497–503. doi: 10.1162/105474600566989
60. Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh sleep quality Index in cancer patients. J Pain Symptom Manage. (2004) 27(2):140–8. doi: 10.1016/j.jpainsymman.2003.12.002
61. Haythornthwaite JA, Hegel MT, Kerns RD. Development of a sleep diary for chronic pain patients. J. Pain Symptom Manage. (1991) 6:65–72. doi: 10.1016/0885-3924(91)90520-E
62. Wilson KG, Watson ST, Currie SR. Daily diary and ambulatory activity monitoring of sleep in patients with insomnia associated with chronic musculoskeletal pain. Pain. (1998) 75(1):75–84. doi: 10.1016/S0304-3959(97)00207-8
63. Quach CW, Langer MM, Chen RC, Thissen D, Usinger DS, Emerson MA, et al. Reliability and validity of PROMIS measures administered by telephone interview in a longitudinal localized prostate cancer study. Qual Life Res. (2016) 25(11):2811–23. doi: 10.1007/s11136-016-1325-3
64. Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. (2006) 13(1):27–45. doi: 10.1177/1073191105283504
65. Osoba D, Aaronson N, Zee B, Sprangers M, te Velde A. Modification of the EORTC QLQ-C30 (version 2.0) based on content validity and reliability testing in large samples of patients with cancer. The study group on quality of life of the EORTC and the symptom control and quality of life committees of the NCI of Canada clinical trials group. Qual Life Res. (1997) 6(2):103–8. doi: 10.1023/a:1026429831234
66. Short MA, Arora T, Gradisar M, Taheri S, Carskadon MA. How many sleep diary entries are needed to reliably estimate adolescent sleep? Sleep. (2017) 40(3):zsx006. doi: 10.1093/sleep/zsx006
Keywords: cancer pain, chronic pain, integrative oncology, mind-body intervention, mindfulness meditation, virtual reality
Citation: Baydoun M, Gajtani Z, Patton M, McLennan A, Cartwright S and Carlson LE (2024) Virtual reality–guided mindfulness for chronic pain in cancer survivors: protocol for the virtual mind study—a single-group feasibility trial. Front. Pain Res. 5:1291374. doi: 10.3389/fpain.2024.1291374
Received: 9 September 2023; Accepted: 19 March 2024;
Published: 4 April 2024.
Edited by:
Carole Helissey, Hôpital d'Instruction des Armées Bégin, FranceReviewed by:
Oussama Abdoun, INSERM U1028 Centre de Recherche en Neurosciences de Lyon, FranceBenedict Kolber, The University of Texas at Dallas, United States
© 2024 Baydoun, Gajtani, Patton, McLennan, Cartwright and Carlson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda E. Carlson bC5jYXJsc29uQHVjYWxnYXJ5LmNh
†These authors have contributed equally to this work and share first authorship
Abbreviations CRP, cancer-related pain; IBS, irritable bowel syndrome; MBSR, mindfulness-based stress reduction; PN3P, Pico Neo 3 Pro; RA, research assistant; RCT, randomized controlled trial; SSQ, simulator sickness questionnaire; VR, virtual reality; VRGM, virtual reality–guided mindfulness.
 Mohamad Baydoun
Mohamad Baydoun Zen Gajtani
Zen Gajtani Michaela Patton3
Michaela Patton3