- 1Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, United States
- 2Geriatric Research Education and Clinical Center, VA Maryland Health Care System, Baltimore, MD, United States
- 3Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD, United States
- 4Department of Physical Therapy and Rehabilitative Science, University of Iowa Carver College of Medicine, Iowa City, IA, United States
- 5Geriatric Research Education and Clinical Center, Central Arkansas Veterans Healthcare System, Little Rock, AR, United States
- 6Department of Pharmacy Practice, Southern Illinois University Edwardsville School of Pharmacy, Edwardsville, IL, United States
- 7Department of Family and Community Medicine, St. Louis University School of Medicine, St. Louis, MO, United States
- 8Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore, MD, United States
Introduction: Pain is highly prevalent in older adults and often contextualized by multiple clinical conditions (pain comorbidities). Pain comorbidities increase with age and this makes clinical decisions more complex. To address gaps in clinical training and geriatric pain management, we established the Pain in Aging—Educational Assessment of Need (PAEAN) project to appraise the impacts of medical and mental health conditions on clinical decision-making regarding older adults with pain. We here report development and pilot testing of the PAEAN survey instrument to assess clinician perspectives.
Methods: Mixed-methods approaches were used. Scoping review methodology was applied to appraise both research literature and selected Medicare-based data. A geographically and professionally diverse interprofessional advisory panel of experts in pain research, medical education, and geriatrics was formed to advise development of the list of pain comorbidities potentially impacting healthcare professional clinical decision-making. A survey instrument was developed, and pilot tested by diverse licensed healthcare practitioners from 2 institutions. Respondents were asked to rate agreement regarding clinical decision-making impact using a 5-point Likert scale. Items were scored for percent agreement.
Results: Scoping reviews indicated that pain conditions and comorbidities are prevalent in older adults but not universally recognized. We found no research literature directly guiding pain educators in designing pain education modules that mirror older adult clinical complexity. The interprofessional advisory panel identified 26 common clinical conditions for inclusion in the pilot PAEAN instrument. Conditions fell into three main categories: “major medical”, i.e., cardio-vascular-pulmonary; metabolic; and neuropsychiatric/age-related. The instrument was pilot tested by surveying clinically active healthcare providers, e.g., physicians, nurse practitioners, who all responded completely. Median survey completion time was less than 3 min.
Conclusion: This study, developing and pilot testing our “Pain in Aging—Educational Assessment of Need” (PAEAN) instrument, suggests that 1) many clinical conditions impact pain clinical decision-making, and 2) surveying healthcare practitioners about the impact of pain comorbidities on clinical decision-making for older adults is highly feasible. Given the challenges intrinsic to safe and effective clinical care of older adults with pain, and attendant risks, together with the paucity of existing relevant work, much more education and research are needed.
Introduction
Pain-associated conditions are prevalent in older adults who often experience high rates of medical and mental health conditions, i.e., pain comorbidities (1, 2). A range of professionals provide healthcare services to older adults; current models conceptualize this care in terms of interprofessional collaboration and view this care through the lens of interacting health conditions, i.e., multimorbidity, and systems of care, which taken together comprise multicomplexity (3–9). The multicomplexity intrinsic to healthcare for older adults increases the cognitive challenges which professional practitioners face in clinical decision-making (10–12). This is especially relevant with regards to pain management where failure to acknowledge and address the impacts of comorbidities and multicomplexity in the care of older adults may potentially diminish the effectiveness of educational efforts (13–18). At present, there is no evidence-based framework representing the real-world complexity of older adults living with pain and sufficient to support the construction of pain education modules for healthcare professionals (19).
Pain is so common in older adults that some have proposed that pain is a part of aging (20, 21). Others have argued that pain declines with age; however, the Global Burden of Disease studies indicate that pain rates rise steadily with age to decline only very late in life (2, 22, 23). The most prevalent pain-associated conditions affecting older adults relate to osteoarthritis, but other mechanisms, such as poor sleep quality, comorbid depression, and decreased recruitment of endogenous analgesia may contribute (8, 20, 24–26). Pain in older adults, separate from interactions with other conditions has intrinsic complexity (20, 27). This is compounded by the presence of comorbidities and the extent to which comorbidities increase the challenge of clinical decision-making in managing the pain of older adults is not well understood; the importance of understanding the context of pain has been highlighted by the IASP curricula (28–30). At the level of a single comorbid diagnosis, some diagnoses are known to be both highly prevalent and impactful in choosing therapies for older adults with pain (31, 32). Depression, for example, has a complex relation to pain, potentially increasing risks for and being increased by pain, as well as impacting compliance with pain therapies (25, 33–35). Heart disease, cerebrovascular disease, dementia, renal failure, and hepatic failure can all impact medication safety (36).
We and others have noted the need for intentionally designed educational curricula to address pain in older adults to prepare current and future healthcare practitioners (14, 37, 38). In order to create relevant and effective curricula, it is important to consider the real-world context in which practitioners treat chronic pain, i.e., a patient's medical and/or mental health comorbidities and the pharmacologic therapies used to treat them; in a formal curriculum development framework, this is a foundational preparation step termed “task assessment” (39). Needs assessment of the clinical contexts of pain management in older adults still requires additional refinement (40). Nonetheless, it is likely that comorbidities directly affect clinical decision-making in the treatment of chronic pain (1). In this study, we sought to formulate, and pilot test an instrument designed to assess the extent to which healthcare practitioners perceive common pain comorbidities as impacting decision-making pertaining to the treatment of pain in older adults.
Methods
This study followed an intentional mixed-methods process incorporating and integrating evidence from (1) an informationist-supported multi-step literature search, (2) review of Medicare-based population-level data about pain and comorbidities in older adults, and (3) advice from an interprofessional, subject matter expert panel (41–43).
Pain comorbidities literature search
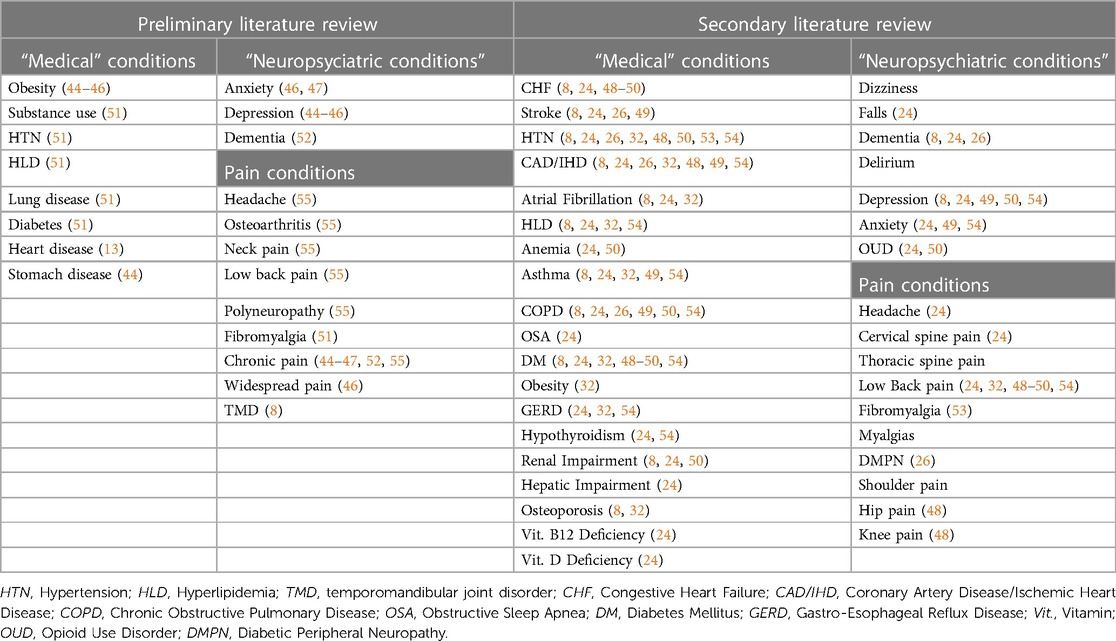
A multi-stage approach was required for the literature search of pain conditions and comorbidities. An initial literature search, directed by a health science librarian, sought to examine the prevalence of chronic pain comorbidities in older adults and used the search terms, “prevalence AND chronic pain AND comorbid or comorbidities.” Our target was to identify relevant literature encompassing pain-associated conditions with high prevalence in older adults, i.e., conditions for which prevalence was estimated to exceed 100 per 100,000. This search yielded 118 results, which were individual reviewed by BH and BS for relevance. A preliminary list of comorbidities was created after review of the articles with highest relevance, Table 1. Reference lists from these articles were reviewed to identify additional articles of interest. The references from these additional articles were reviewed to find further additional relevant articles. Comorbidities from the articles selected in this manner were evaluated. Another literature search sought to examine the prevalence of chronic pain and medical comorbidities in older adults. A health science librarian used the following pain terms (in alphabetical order), “Chronic pain, Chronic widespread pain, Diabetic peripheral neuropathy, Diabetic neuropathies, Fibromyalgia, Headache, headache disorders, Hip pain, Knee pain, Low back pain, Lower back pain, Neck pain, Patellofemoral pain syndrome, Shoulder pain” along with the following comorbidity terms: “Comorbidity terms: Comorbid, Co-morbid, Complexity, Co-diagnosis, Multimorbid, Multi-morbid.” A search utilizing pairs of chronic pain conditions and medical comorbidity terms yielded 104 unique literature results. Two study team members (BH and BS) reviewed results for relevance, and additional comorbidities were added to the preliminary list.
Population-level pain comorbidities data
The 2017 Center for Medicare and Medicaid Services 5% standard analytical sample of carrier claims data were queried, as previously described, for the 20 most prevalent medical conditions in elderly adults (56). Our previously described data extraction approach was modified as follows, in brief, the extraction followed the sequence illustrated in the population flow chart, Figure 1. The total 2017 CMS beneficiaries numbered approximately 3 million, these were initially limited to those aged 65–100 who numbered approximately 2.5 million. The beneficiaries with claims present in either the Carrier or the Outpatient files were included for a total of approximately 1.5 million. This was further limited to the population of those 75–80 years old, participating in Medicare Part B but not in Part C, and alive for all 12 months of 2017, and the population of those with claims near the median, i.e., 40th–60th percentile for claims (56–60). Age was limited as we observe marked increases in variation in Medicare program usage and mortality at the younger and older extremes of old age respectively (57). The age cohort selected for study does span the median age for U.S. older adults (over 65 years old). Claims were limited as we have observed that beneficiaries with lower claims per year have lower diagnostic rates for common conditions, and those with many claims per year may have higher rates. The claims cohort spanning the median was selected as we seek here to define the properties of a “median” older adult population (57). The final study population for this unadjusted appraisal of rates of common pain conditions and common pain comorbidities was just under 50,000.
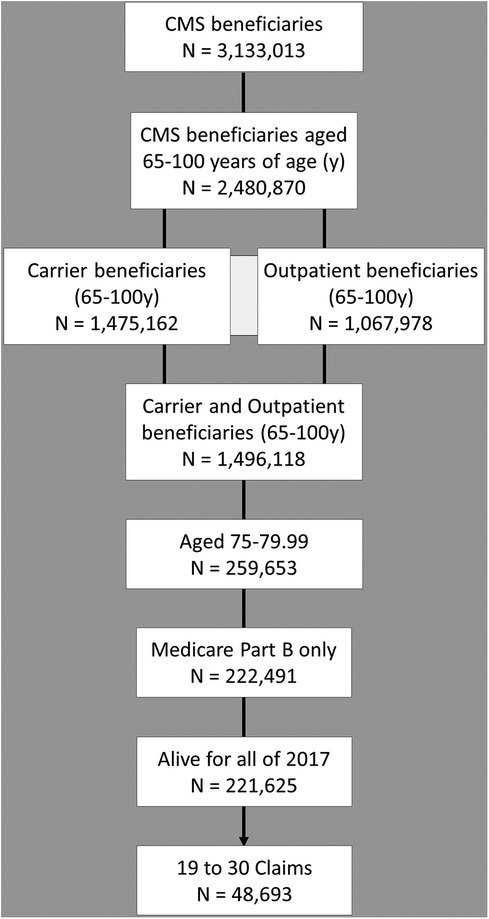
Figure 1. Population flow diagram for scoping data review. Medicare beneficiaries meeting study criteria were selected as illustrated.
Interprofessional advisory panel
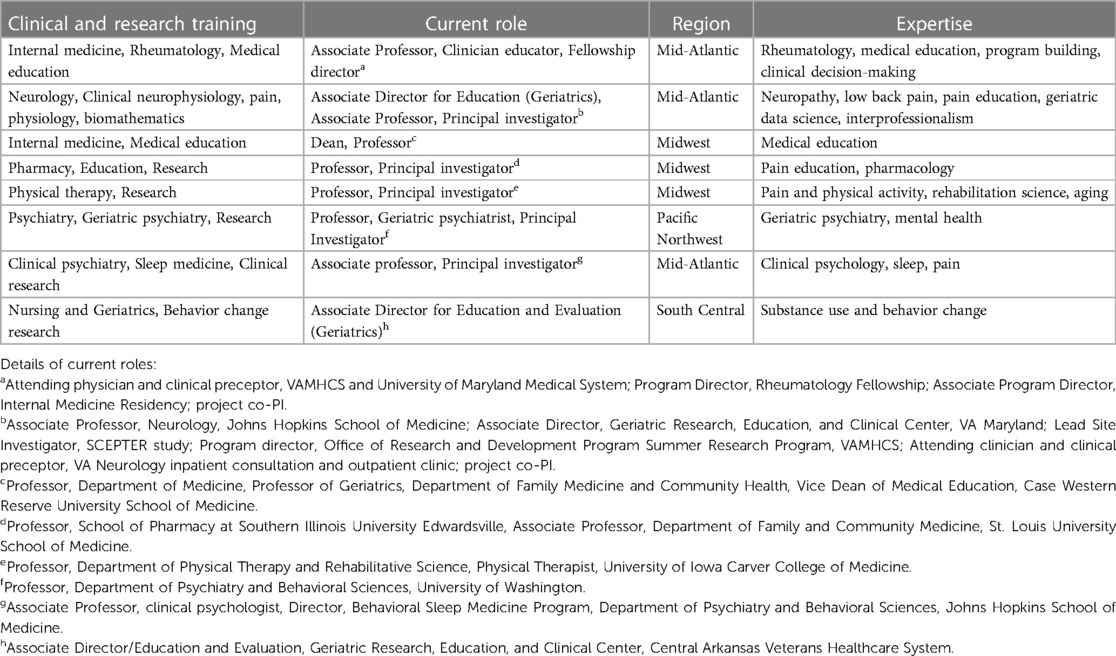
Through directed invitation, we assembled a geographically and professionally diverse subject matter expert interprofessional advisory panel (IAP) consisting of eight nationally recognized experts in pain care, health professions education, and gerontology. Criteria for invitation included: established expertise in a relevant area: academic appointment, presentation at national meetings, and peer-reviewed publications; interest in interprofessional collaboration, and responsiveness. Eight professionals were invited initially; all except one accepted the invitation who provided a reference to another, like professional who accepted our invitation. All professionals remained in contact throughout the study development period except for one physician who stepped back midway in the context of a job change. The IAP included 4 physicians (internist, neurologist, psychiatrist, and rheumatologist), one registered nurse, one pharmacist, one clinical psychologist, and one physical therapist, Table 2. The group met virtually to discuss the potential questions of interest, evaluate and comment on extracts of clinical data and review results of the literature search. The goals of the IAP were to develop a list of “high value” pain comorbidities based on prevalence and potential impact on care, and to advise on survey instrument construction.
Integration of literature review and data extracts with input from advisory panel
These results were combined with a list of common medical conditions found through literature searches above. Using an iterative review process, a final list of 26 medical conditions and 13 chronic pain conditions were included in the final survey. The instrument prompt was presented to the interprofessional advisory panel and revised for clarity and concision.
Survey instrument pilot testing
An interprofessional and multi-disciplinary group of 8 board-certified healthcare practitioners, including clinically active physicians and nurse practitioners providing general medical or geriatrics care, from 2 affiliated institutions (University of Maryland Medical Center and the VA Maryland Health Care System) were invited to pilot the survey. No members of the IAP were included in this group. Respondents were asked to rate their agreement with: “This is a common condition in older adults and potentially impacts my decision-making regarding treatment of pain” using a 5-point Likert scale. Individual conditions were scored in terms of the percentage of respondents who agreed or strongly agreed with the prompt statement. Respondents were asked to provide demographic information on their specialty, practice setting, professional title, institution, and number of years in practice.
We scored the survey results as the percent of respondents selecting “agree” or “strongly” agree. Data were processed using Excel (Microsoft) and SAS (Cary, NC). Results are reported as average percent agreement. This pilot study was not powered to detect differences between conditions but was intended to test the instrument for feasibility of use.
This study was approved by the University of Maryland Medical Center IRB and the VA Maryland Health Care System Research and Development Committee.
Results
Literature review
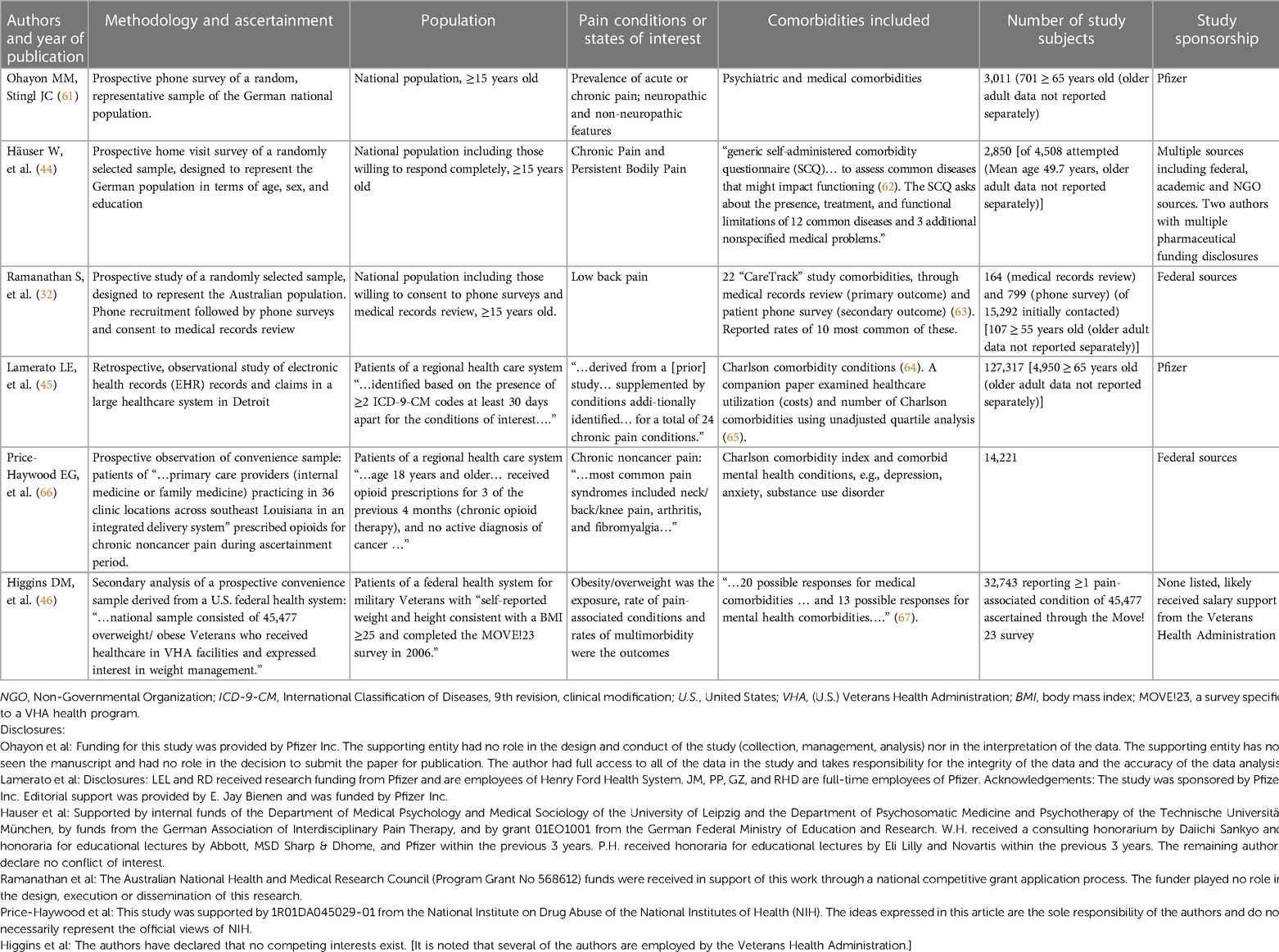
Extensive literature review did not identify articles directly addressing the impact of common comorbidities on pain treatment decision-making in older adults. A small number of articles addressed the occurrence of medical and mental health comorbidities in those with pain-associated diagnoses or pain states (reporting chronic or acute pain), Table 3. Study methodologies were largely cross sectional, with information gathering through population-based survey or health system database analysis or both.
Population-based survey studies
Ohayon and colleagues, using population-based phone survey methods, evaluated comorbidities in relation to acute vs. chronic and neuropathic vs. non-neuropathic pain (61). Survey respondents reporting obesity, diabetes, hypertension, and diseases of the cerebrovascular system, nervous system or blood had increased risk for neuropathic pain (61). Those who reported depression were 3-fold more likely to have non-neuropathic pain and 6-fold more likely to have neuropathic pain compared to those without depression. Häuser and colleagues, using population-based home visit methods, evaluated comorbidities in relation to cancer-related vs. non-cancer chronic pain and chronic disabling vs. chronic non-disabling pain (44). The investigators reported that depression was highly associated with chronic pain, as were stomach disease, rheumatic disease, obesity, and heart disease. Ramanathan and colleagues conducted a population-based ascertainment of participants consenting to survey and medical record review of persons reporting low back pain (32). The investigators observed that persons with low back pain had more medical comorbidities and those with more comorbidities described poorer health status. The presence of pain comorbidities increased the risk for provider non-compliance with 9 out of 10 quality indicators, including documentation of a medical history, performance of a physical or neurological examination, and assessment for infection or cancer (32).
Health records-based data analytics studies
Lamerato and colleagues extracted records for patients of a U.S.-based healthcare delivery system based on diagnosis with at least one of 24 chronic pain-associated conditions (45). Diabetes, chronic pulmonary disease, malignancy, and renal disease were the most prevalent comorbidities in those with chronic pain-associated diagnoses. In a companion paper, the authors present an unadjusted analysis suggesting that those with the highest healthcare costs have higher rates of comorbidities (65). Price-Haywood and colleagues extracted records for patients receiving primary care from a U.S.-based healthcare delivery system based on receipt of opioid prescriptions (66). A high Charlson comorbidity index was associated with a small decrease in the likelihood of providers prescribing opioids while substance use disorder diagnosis was associated with markedly increased likelihood of providers prescribing opioids (66). Higgins and colleagues extracted records for patients in a federal (nation-wide) U.S.-based healthcare delivery system based on participation in a national survey of U.S. veterans undergoing activity modification for weight management (46). The presence of multiple comorbid conditions increased the risks of low back pain and/or arthritis/joint pain with the likelihood of pain diagnoses increasing as the number of comorbid conditions increased, e.g., those veterans with 5 or more comorbid conditions had 7-fold likelihood of having both low back pain and arthritis/joint pain vs. having “no pain” when compared to those in the study with no comorbid conditions. The authors noted that pain comorbidities are likely to increase treatment complexity (46).
Clinical claims data scoping review
The data extraction for the purposes of this study included 48,693 Medicare beneficiaries 75–80 years old during 2017 meeting criteria for inclusion, 27,893 (57.3%) were recorded as female gender and 20,798 (42.7%) as male, Figure 1. The average age was 77.38 for females and 77.34 for males. The race and ethnicity distribution, utilizing the Research Triangle Index (%) was Undefined 0.18 and 0.12; White 83.46 and 85.83; Black 7.01 and 5.45; Other 0.81 and 1.15; Hispanic 3.05 and 2.87; Asian American/Pacific Islander 5.05 and 4.18; Native American 0.45 and 0.40 for females and males respectively (68). The rates of common pain diagnoses are shown in Figure 2A. Elbow, wrist, hand, and ankle/foot pain are included to illustrate the relative rates of pain at anatomical sites but these were not included in the group of common conditions which comprised headache, neck pain, thoracic spine pain, low back pain, shoulder, hip, and knee pain; type 2 diabetic polyneuropathy, and fibromyalgia and myalgias (muscle pains). The most common pain code used was M54.5, indicating low back pain. The rates of common medical and mental health diagnoses (comorbidities) selected for study are shown in Figure 2B for females and males. The rates represent the rates of diagnosis based the most common code utilized for each specified condition and are not expected to equate to more systematic appraisals of prevalence, but rather represent a scoping appraisal of ICD-10 code utilization to represent common conditions associated with aging, in the population studied. The most common cardio-vascular-pulmonary condition code used was I10, for Hypertension, which was utilized for over 75% of the studied beneficiaries; the most common metabolic condition code use was E11.9, Type 2 diabetes mellitus, unspecified in males, and E03.9, hypothyroidism, unspecified in females, although hyperlipidemia, unspecified (grouped with cardio-vascular-pulmonary conditions) exceeded both E11.9 and E03.9; and the most commonly used neuropsychiatric/aging-related code was R42, indicating dizziness. The least commonly noted condition incorporated here was hepatic impairment, included due to having a major impact on pain treatment choices, i.e., strict avoidance of acetaminophen and other selected analgesic agents. The extracted data showed some conditions having indications of increased rates in the older adults diagnosed with one or more common pain conditions, e.g., depression, however this was not the focus of this study and further analysis was not pursued.
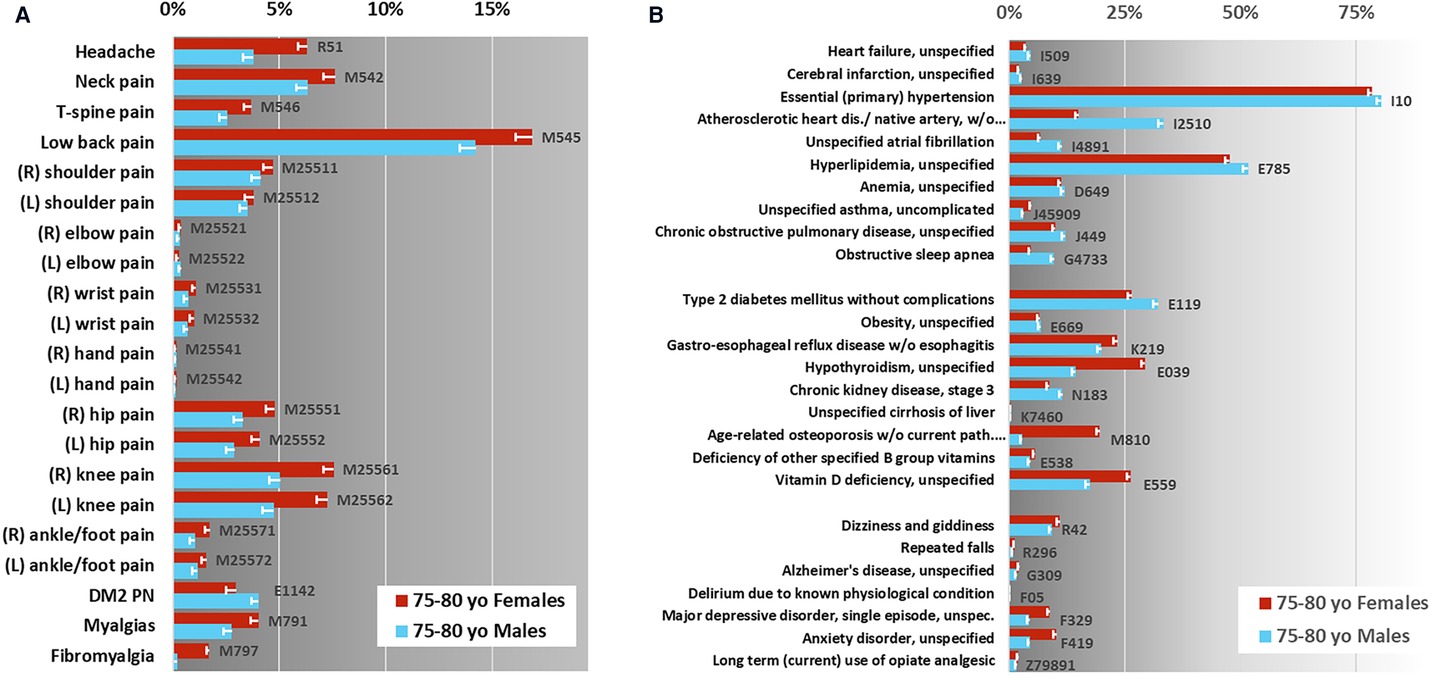
Figure 2. Claims data scoping review—identification of potential key elements. Diagnostic rate estimates for common condition ICD-10 codes in CMS Older adults 75–80 years old (yo). (A) Pain condition rate estimates, shown here are the most commonly utilized ICD-10 codes associated with these diagnoses. Pain-associated conditions marked with “*” were included in the assessment instrument. (B) Non-pain conditions selected for this study, shown here are the rate estimates for the most commonly used ICD-10 codes associated with each. Error bars indicate corrected 95% confidence interval, n = 100, net p = 0.05. The gray-scale background in each figure is to alert the viewer to the different x-axis scales.
Interprofessional advisory panel
The interprofessional advisory panel met 6 times over two years to review and discuss the data obtained and to strategize for and advise the construction of the Pain in Aging, Educational Assessment of Need (PAEAN) survey instrument, Table 2. The inclusion of diverse professional and geographic perspectives increased the number of conditions viewed as comorbid with and potentially significant for pain clinical decision-making in older adults.
Survey instrument construction
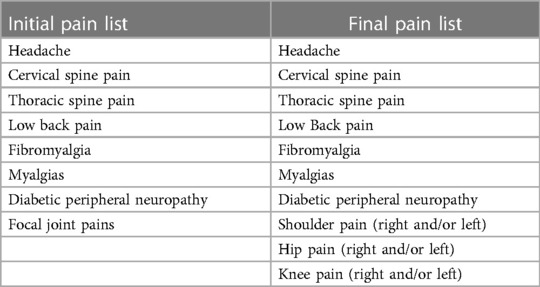
The interprofessional advisory panel (IAP) reviewed and revised the list of conditions integrating literature review and clinical claims data scoping analysis, Table 3. Using a focus group process, respondents iteratively responded with potential comorbidity additions, omissions, and nomenclature until the list finalized. The final decision was to include 26 common clinical [19 medical and 7 neuropsychiatric (mental health)] conditions and 13 common pain-associated conditions in the pilot instrument, Tables 4, 5.
The draft survey instrument was presented to the IAP for final input and advice. The final version of the instrument consisted of a section for rating pain comorbidities, a section for rating prevalence of common pain conditions, and a section on respondent demographics, Figure 3. Questions about respondent demographics (not reported here) were placed at the end of the instrument in order to improve responder engagement. Respondents reported professional title, institution, years in practice, and primary specialty to validate inclusion.
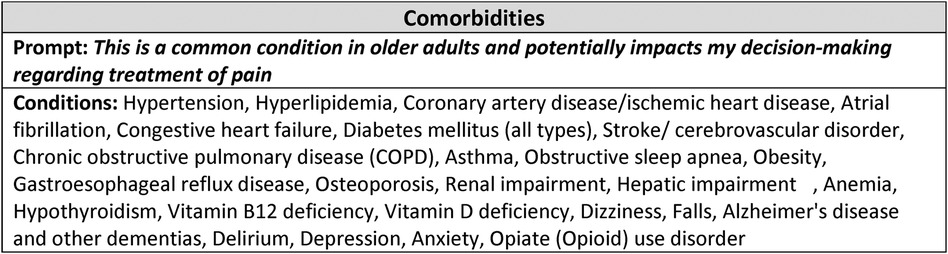
Figure 3. Pilot PAEAN instrument. Shown is the stem (Prompt) and list of conditions included in the final instrument. The instrument is constructed by placing the stem at the top of the page with the list of conditions along the left margin, each with a Likert scale to the right (one scale associated with each condition). The instruction for the instrument was: “Please rate your agreement with the following statement regarding each of the conditions below:” A 5-point Likert scale (strongly disagree to strongly agree) was used.
Pilot testing
Eight clinically active healthcare practitioners were invited to participate in the pilot survey, all responded to the survey (100% response rate). The median time to complete the survey was 2 min and 45 s, with a range of 1 min and 28 s to 8 min and 34 s. All conditions received a rating from each participant (no missing data). Participants were more likely to select strongly agree than strongly disagree; three conditions had 4 of 8 participants selecting strongly agree, these were “Falls”, “Delirium”, and “Opioid Use Disorder” as impacting pain clinical-decision making. For visualization of the pilot survey results, conditions were grouped together according to main clinical categories as: (1) “Major medical”, i.e., cardio-vascular-pulmonary; (2) “Metabolic”, i.e., involving metabolism, vitamin deficiency syndromes, and endocrine-mediated conditions; and (3) “Neuropsychiatric/age-related”, e.g., falls, dementia. All neuropsychiatric/age-related conditions including dementia and opioid use; selected cardio-vascular-pulmonary conditions, e.g., hypertension and stroke; and selected metabolic conditions, e.g., renal impairment and diabetes mellitus, were rated as impactful (“Agree” or “Strongly Agree”) by most of the practitioners completing the survey, Figure 4.
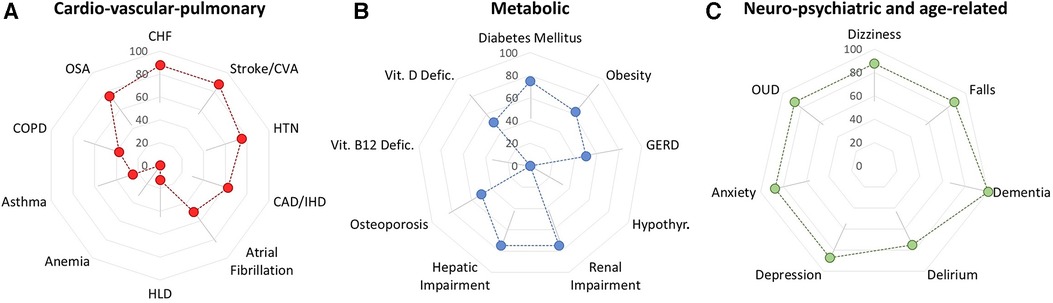
Figure 4. Preliminary assessment of respondent agreement that specified conditions impact pain-related clinical decision-making. (A) Cardio-vascular-pulmonary conditions may be viewed a variably impactful. (B) Metabolic conditions may be viewed as relatively less impactful although pilot data suggest that diabetes, renal impairment and hepatic impairment may have a strong impact on decision-making. (C) Pilot data suggest that neuro-psychiatric conditions have a major impact on pain-related clinical decision-making. Error bars represent the 95% confidence interval, corrected for multiple comparisons, n = 26, net p = 0.05. For clarity, error bars are shown in one direction only but pertain bidirectionally, with adjustments for floor (near zero) and ceiling (near 100%) effects.
Discussion
In this study, we demonstrate that there are many clinical conditions that potentially impact pain clinical decision-making by health care providers caring for older adults, and that this area requires additional study. The outcomes of this study are the pilot instrument as well as a demonstration of the comorbidity data for the study population, the literature review, and an appraisal of the instrument feasibility. The pilot instrument may be used by others, however, in current work we are using a revised stem version, replacing the “and” with “that”. The comorbidity data may be used by others to design pain education cases which incorporate the common and relevant comorbidities of pain in older adults aged 75–80 years. The literature review demonstrated that few articles address the importance of older adult pain comorbidities in clinical decision-making, this was the primary impetus for our study. Finally, we included a demonstration of the type of data that could be obtained with this instrument. We note that this data is pilot data so that the error bars are wide and we do not draw summative conclusions from these values. The time to complete the survey was less than three minutes including demographics items and questions about overall pain condition prevalence. Taken together, we conclude that future studies using this PAEAN instrument are highly feasible and the knowledge gained will improve educational pain case development and ultimately strengthen pain clinical decision-making by those treating older adults. We postulate that medical and mental health comorbidities increase the cognitive burden of pain clinical decision-making, increasing the risk of harms and narrowing the scope of acceptable and feasible therapeutic options (69). The net impact of this cognitive burden remains unknown, but formal needs assessment is essential to the creation of more realistic and clinically useful pain education scenarios (39).
Improved preparation of healthcare providers is a high priority educational goal as the number of older adults is expected to increase (4, 70, 71). In addition to reporting on the conceptualization, development, and pilot testing of a pain clinical decision-making survey instrument, the data presented here are designed to increase awareness of and provide scoping-level data regarding those conditions most likely to increase the complexity of managing persistent pain in older adults (56, 70). Curriculum developers can use information gleaned in this study, together with other research findings, to take pragmatic steps towards improvements in evidence-based pain education initiatives (28, 39, 72–74). As a long-term goal, this study envisions better understanding of and preparation for providers facing real-world challenges in managing pain in and with older adults.
Although it might be assumed that pain clinical decision-making for those treating older adults focuses primarily on pharmacological management, it is important to note that non-pharmacological therapies may result in substantive reductions in pain intensity and interference, although the data specifically, focusing on older adults is limited (23, 75–77). The benefits of nonpharmacological therapies, e.g., exercise, mindfulness-based stress reduction, yoga, and tai chi, may extend to other health benefits, such as improved mobility and balance, reduction of blood pressure, preservation of muscle mass, especially impactful for older adults (78–82). Because of the high prevalence of medical and mental health comorbidities in older adults with pain, a comprehensive approach to pain management, proactively incorporating nonpharmacological as well as pharmacologically based therapies, where appropriate, is often needed and comprehensive approaches should be widely incorporated into pain curricula (69, 83–86).
This study lays the groundwork for considering multi-morbidity in the treatment of chronic pain through an educational curricular development lens. We envision creating a clearer appraisal of the complexities of clinical practice by surveying healthcare professionals who regularly treat older adults many of whom experience persistent pain. These results will help to inform the development of clinical cases, accurately representing patients by accounting for real-world comorbidity and ultimately improving clinical skillfulness at entry to practice and beyond. Educational curricula which ignore the effect of comorbidities and multicomplexity cannot be expected to adequately prepare practitioners for real-world clinical challenges (4, 16, 28, 74, 87).
Comparison to existing literature
The existing literature on the effect of medical comorbidities and chronic pain conditions on treatment decisions for chronic pain conditions in older adults is sparse (32, 46). The literature suggests that practitioners have a limited understanding of the scale of this problem which is profound. There was no consensus regarding a standard set of comorbidities of relevance. Two studies cited the Charlson comorbidities list which was specifically developed for clinical prognostication in older adults, utilizing this list for the purpose of assessing comorbidities of pain in adults across a broad age-range may not be sufficiently expansive. We show here that there is a small number of studies addressing the co-existence of medical comorbidities and chronic pain conditions and very few examine this phenomenon comprehensively, and we did not identify any other studies that investigate how comorbidities affect pain clinical decision-making. Some studies have asked about comorbidities in other populations, not specifically focusing on older adults—a population where the multiplicity of comorbidities expands the challenge and risk of medication-based management (88, 89). This study offers an important addition in systematically developing a survey instrument designed to characterize the impact of pain comorbidities in older adults on treatment decisions.
Integrating literature, data, and expert opinion
We utilized a 3-pronged approach to survey instrument development and combined evidence-based methods with the subject-matter expertise of our interprofessional working group, aiming at a robust instrument with clinical and real-world contextual relevance. First, peer-reviewed literature provided the initial framework of comorbidities that was further refined by the professional experience of our advisory group. With their input, the terms falls, dizziness, and delirium, were added due to relevance in the context of our study aims (90–93). Vitamin D deficiency, vitamin B12 deficiency, and hepatic impairment have significant clinical relevance in the treatment of chronic pain-associated conditions, e.g., enthesiopathies, neuropathies, yet were not prominently included in the literature (94–98). When addressing conditions, such as pain, that impact a large percentage of older adults and have profound impacts on many domains of function, it is important to include a diverse range of healthcare professionals in projects which require appraisal and integration of complex data (4, 16). Finally, the utilization of real-world claims data codes provided statistical evidence and confirmation of the prevalence of comorbidities in the United States and further validated inclusion in our instrument (56, 60). A deliberate, interprofessional process led to the development of this research instrument (99).
Limitations
This is a pilot study describing the use of an intentional interprofessional process to develop a survey to assess pain clinical decision-making in older adults with single highly prevalent comorbidities. Some limitations are noted. The Medicare data which was reviewed by the interprofessional advisory panel was drawn from a demographically representative population of older adults, nonetheless, it is acknowledged that claims data may underestimate or overestimate the prevalence of certain conditions (100–102). Some “conditions” are defined by nonspecific terms, e.g., headache and hypertension, whereas others were more specific such as obstructive sleep apnea and opioid use disorder, so that the broader classes pertaining to these diagnoses, i.e., sleep disturbances and substance use disorders, may not be well captured by the survey (56, 103). This reflects the real world complexity of clinical practice wherein both detailed specification as well as the capacity to abstract to the more general are important skills (104). This data was useful in familiarizing non-medical providers with an estimate of condition prevalence from contemporary data and is intended in this article to provide the reader with actionable data to enhance pain education module development. We did select a “typical” population from the Medicare data focusing on the older adult aged 75–80 who was alive for all of the study year, was enrolled in Part B but not in Medicare Advantage (Part C), and who had between 19 and 30 claims. The latter restriction was included because we and others have noted that diagnostic rates vary widely with claim rates; the number of claims selected for this study included the median 20% of claims, e.g., claim rates ranging from the 40%ile to the 60%ile as our goal was to evaluate the “median” diagnostic rates for the population. It acknowledged that older adults vary tremendously in terms of health and morbidity so that no single number can capture the full flavor, we seek to present a single number that is representative of the typical morbidity burden in the age group studied. It was challenging to develop an effective literature search strategy. Much of the pain literature focuses on “complex pain”, e.g., temporomandibular joint disorder, but does not address “medical complexity” and pain (105). Several meetings with the healthcare informationist were necessary to develop an effective strategy which ultimately included searching for pairs, i.e., a pain condition paired with a medical condition, for several of the high prevalence conditions. Although an effort was made to include several professions in the study group, the study team was led by two specialty physicians whereas the workforce for primary care is increasingly comprised of a broader range of healthcare providers including nurse practitioners, physical therapists, and physician assistants (106–108). This pilot study included a limited number of study subjects and a larger scale test of this instrument is underway, this report serves to explain the construction of the instrument and report feasibility (99). Finally, this study examines the impact of single comorbidities, however it is common for older adults, especially those of advanced age, to experience multiple serious health conditions simultaneously, i.e., multimorbidity, and to face health system challenges in coping with the medical instructions and treatments, i.e., multicomplexity (3, 46). We posit that clinical decision-making burdens likely increase as comorbidities multiply, thus it is important to examine the impact of multimorbidity and multicomplexity, it is our intention that this study provides an important foundation for that future work.
Conclusions
Comorbidities such as dementia, depression, anxiety, opioid use disorder, dizziness, falls, delirium, congestive heart failure, stroke, hypertension, diabetes, renal and hepatic impairment are likely to have a strong influence on clinical decision-making for healthcare providers working to address pain in older adults. Relatively understudied, the prevalence and impact of comorbidities present in older patients with pain should be proactively incorporated when creating educational curricula; in addition, the impact on clinical guidelines merits substantive consideration. Our survey instrument may be useful to those engaged in pain education research and content development, and improved understanding of pain-related clinical reasoning. We have provided the scoping Medicare data here so that educators can use this information to immediately begin to build more realistic cases incorporating the most common and impactful pain comorbidities. We conclude that further study is essential, and we propose the use of surveys, data analytics, focus groups, and literature reviews as well as systematic development and study of educational materials dedicated to improved clinical pain care, especially focusing on the question of how varying comorbid complexity impacts the decision-making processes of clinicians caring for older adults with pain.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The dataset is restricted by an existing data use agreement. Requests to access these datasets should be directed toYmV0aC5ob2dhbnNAdmEuZ292.
Ethics statement
The studies involving humans were approved by University of Maryland Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because of Exempt status.
Author contributions
BS: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. BH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LF-L: Conceptualization, Investigation, Methodology, Visualization, Writing – review & editing. LMB: Conceptualization, Investigation, Visualization, Writing – review & editing. CH: Conceptualization, Investigation, Methodology, Visualization, Writing – review & editing. LFB: Conceptualization, Investigation, Methodology, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the Baltimore VA Medical Center GRECC (Geriatric research, Education, and Clinical Center, VA 5I21RX003169), and the Johns Hopkins Blaustein Pain Research fund.
Acknowledgments
The authors thank Patricia A. Thomas, Stephen Thielke, Leslie Katzel, Raya Kheirbek, John D. Sorkin, Marc Hochberg, and Brock Beamer for useful discussions of this work. The authors acknowledge the contributions of informationist Andrea Shipper in developing the innovative search strategy used for this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor JWW declared a past collaboration with one of the authors BH.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Geetha D, Jaisoorya TS, Sunil Kumar G, Manoj L, Gokul GR, Aakash B, et al.. Disentangling comorbidity in chronic pain: a study in primary health care settings from India. PLoS One. (2020) 15(11):e0242865. doi: 10.1371/journal.pone.0242865
2. GBD 2019 Ageing Collaborators. Global, regional, and national burden of diseases and injuries for adults 70 years and older: systematic analysis for the global burden of disease 2019 study. Br Med J. (2022) 376:e068208. doi: 10.1136/bmj-2021-068208
3. Ellis G, Whitehead MA, Robinson D, O’Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. Br Med J. (2011) 343:d6553. doi: 10.1136/bmj.d6553
4. Fishman SM, Young HM, Lucas Arwood E, Chou R, Herr K, Murinson BB, et al. Core competencies for pain management: results of an interprofessional consensus summit. Pain Med Malden Mass. (2013) 14(7):971–81. doi: 10.1111/pme.12107
5. Cacchione PZ. Innovative care models across settings: providing nursing care to older adults. Geriatr Nurs. (2020) 41(1):16–20. doi: 10.1016/j.gerinurse.2020.01.011
6. Hellman T, Jensen I, Bergström G, Brämberg EB. Essential features influencing collaboration in team-based non-specific back pain rehabilitation: findings from a mixed methods study. J Interprof Care. (2016) 30(3):309–15. doi: 10.3109/13561820.2016.1143457
7. Salsbury SA, Goertz CM, Vining RD, Hondras MA, Andresen AA, Long CR, et al. Interdisciplinary practice models for older adults with back pain: a qualitative evaluation. Gerontologist. (2018) 58(2):376–87. doi: 10.1093/geront/gnw188
8. Salive ME. Multimorbidity in older adults. Epidemiol Rev. (2013) 35:75–83. doi: 10.1093/epirev/mxs009
9. Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. (2009) 7(4):357–63. doi: 10.1370/afm.983
10. Luijks HD, Loeffen MJW, Lagro-Janssen AL, van Weel C, Lucassen PL, Schermer TR. GPs’ considerations in multimorbidity management: a qualitative study. Br J Gen Pract J R Coll Gen Pract. (2012) 62(600):e503–510. doi: 10.3399/bjgp12X652373
11. Zulman DM, Slightam CA, Brandt K, Lewis ET, Asch SM, Shaw JG. “They are interrelated, one feeds off the other”: a taxonomy of perceived disease interactions derived from patients with multiple chronic conditions. Patient Educ Couns. (2020) 103(5):1027–32. doi: 10.1016/j.pec.2019.11.020
12. Zanini C, Maino P, Möller JC, Gobbi C, Raimondi M, Rubinelli S. Enhancing clinical decisions about care through a pre-consultation sheet that captures patients’ views on their health conditions and treatments: a qualitative study in the field of chronic pain. Patient Educ Couns. (2016) 99(5):747–53. doi: 10.1016/j.pec.2015.11.029
13. Murinson BB, Agarwal AK, Haythornthwaite JA. Cognitive expertise, emotional development, and reflective capacity: clinical skills for improved pain care. J Pain. (2008) 9(11):975–83. doi: 10.1016/j.jpain.2008.07.010
14. Hogans BB, Watt-Watson J, Wilkinson P, Carr ECJ, Gordon DB. Perspective: update on pain education. Pain. (2018) 159(9):1681–2. doi: 10.1097/j.pain.0000000000001297
15. Gereau RW, Sluka KA, Maixner W, Savage SR, Price TJ, Murinson BB, et al. A pain research agenda for the 21st century. J Pain. (2014) 15(12):1203–14. doi: 10.1016/j.jpain.2014.09.004
16. Gordon DB, Watt-Watson J, Hogans BB. Interprofessional pain education-with, from, and about competent, collaborative practice teams to transform pain care. Pain Rep. (2018) 3(3):e663. doi: 10.1097/PR9.0000000000000663
17. Jacobs ZG, Elnicki DM, Perera S, Weiner DK. An E-learning module on chronic low back pain in older adults: effect on medical resident attitudes, confidence, knowledge, and clinical skills. Pain Med Malden Mass. (2018) 19(6):1112–20. doi: 10.1093/pm/pnx333
18. Watt-Watson J, McGillion M, Lax L, Oskarsson J, Hunter J, MacLennan C, et al. Evaluating an innovative eLearning pain education interprofessional resource: a Pre-post study. Pain Med Malden Mass. (2019) 20(1):37–49. doi: 10.1093/pm/pny105
19. Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. Boutron I, ed. PLoS One. (2012) 7(8):e41601. doi: 10.1371/journal.pone.0041601
20. Dagnino APA, Campos MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. (2022) 16:736688. doi: 10.3389/fnhum.2022.736688
21. Ogboli-Nwasor E, Hogans B. Pain Education in Low-Resource Countries - International Association for the Study of Pain (IASP) Pain Education in Low-Resource Countries|IASP. International Association for the Study of Pain (IASP). Available online at: https://www.iasp-pain.org/resources/fact-sheets/pain-education-in-low-resource-countries/ (Accessed January 4, 2024).
22. Thielke S, Sale J, Reid MC. Aging: are these 4 pain myths complicating care? J Fam Pract. (2012) 61(11):666–70.23256096
23. Tinnirello A, Mazzoleni S, Santi C. Chronic pain in the elderly: mechanisms and distinctive features. Biomolecules. (2021) 11(8):1256. doi: 10.3390/biom11081256
24. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Lond Engl. (2016) 388(10053):1545–602. doi: 10.1016/S0140-6736(16)31678-6
25. Marcuzzi A, Skarpsno ES, Nilsen TIL, Mork PJ. The interplay between multisite pain and insomnia on the risk of anxiety and depression: the HUNT study. BMC Psychiatry. (2022) 22(1):124. doi: 10.1186/s12888-022-03762-0
26. Sinclair AJ, Conroy SP, Bayer AJ. Impact of diabetes on physical function in older people. Diabetes Care. (2008) 31(2):233–5. doi: 10.2337/dc07-1784
27. Zis P, Daskalaki A, Bountouni I, Sykioti P, Varrassi G, Paladini A. Depression and chronic pain in the elderly: links and management challenges. Clin Interv Aging. (2017) 12:709–20. doi: 10.2147/CIA.S113576
28. Hogans BB, Gallagher RM. A global year for pain education: progress, trends, and the way forward. Pain Med Malden Mass. (2018) 19(8):1507–11. doi: 10.1093/pm/pny102
29. Watt-Watson J, Interprofessional Pain Curriculum Task Force. IASP Interprofessional Pain Curriculum Outline. Available online at: https://www.iasp-pain.org/education/curricula/iasp-interprofessional-pain-curriculum-outline/ (Accessed January 4, 2024).
30. Watt-Watson J, Hogans BB. Current Status of Pain Education and Implementation Challenges - International Association for the Study of Pain (IASP) Current Status of Pain Education and Implementation Challenges | IASP. Available online at: https://www.iasp-pain.org/resources/fact-sheets/current-status-of-pain-education-and-implementation-challenges/ (Accessed January 4, 2024).
31. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. (2018) 9(1):143–50. doi: 10.14336/AD.2017.0306
32. Ramanathan S, Hibbert P, Wiles L, Maher CG, Runciman W. What is the association between the presence of comorbidities and the appropriateness of care for low back pain? A population-based medical record review study. BMC Musculoskelet Disord. (2018) 19(1):391. doi: 10.1186/s12891-018-2316-z
33. Jin J, Zhang T, Xiong X, Chen H, Jiang Y, He S. A prospective study of chronic postsurgical pain in elderly patients: incidence, characteristics and risk factors. BMC Geriatr. (2023) 23(1):289. doi: 10.1186/s12877-023-04006-w
34. Helgadóttir B, Hallgren M, Kullberg CLE, Forsell Y. Sticking with it? Factors associated with exercise adherence in people with mild to moderate depression. Psychol Sport Exerc. (2018) 35:104–10. doi: 10.1016/j.psychsport.2017.11.011
35. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. (2000) 160(14):2101. doi: 10.1001/archinte.160.14.2101
36. Fanelli A, Ghisi D, Aprile PL, Lapi F. Cardiovascular and cerebrovascular risk with nonsteroidal anti-inflammatory drugs and cyclooxygenase 2 inhibitors: latest evidence and clinical implications. Ther Adv Drug Saf. (2017) 8(6):173–82. doi: 10.1177/2042098617690485
37. Schofield P. Pain education and current curricula for older adults. Pain Med Malden Mass. (2012) 13(Suppl 2):S51–56. doi: 10.1111/j.1526-4637.2011.01283.x
38. Hogans BB. Demanding competence. Pain Med Malden Mass. (2017) 18(10):1831–3. doi: 10.1093/pm/pnw359
39. Chen BY, Hughes MT, Kern DE, Thomas PA. Curriculum development for medical education: a six-step approach. 3rd volume. Johns Hopkins University Press. (2016). p. 16. doi: 10.1353/book.44600
40. Watt-Watson J, Murinson BB. Current challenges in pain education. Pain Manag. (2013) 3(5):351–7. doi: 10.2217/pmt.13.39
41. Palsbo SE, Hurtado MP, Levine RE, Barrett KA, Mastal MF. Enabling a survey of primary care to measure the health care experiences of adults with disabilities. Disabil Rehabil. (2011) 33(1):73–85. doi: 10.3109/09638288.2010.485671
42. Fridberg H, Wallin L, Wallengren C, Kottorp A, Forsman H, Tistad M. Development and evaluation of the measurement properties of a generic questionnaire measuring patient perceptions of person-centred care. BMC Health Serv Res. (2020) 20(1):960. doi: 10.1186/s12913-020-05770-w
43. Aber A, Phillips P, Lumley E, Radley S, Thomas SM, Nawaz S, et al. Mixed methods study to develop the content validity and the conceptual framework of the electronic patient-reported outcome measure for vascular conditions. BMJ Open. (2020) 10(8):e034154. doi: 10.1136/bmjopen-2019-034154
44. Häuser W, Schmutzer G, Hilbert A, Brähler E, Henningsen P. Prevalence of chronic disabling noncancer pain and associated demographic and medical variables: a cross-sectional survey in the general German population. Clin J Pain. (2015) 31(10):886–92. doi: 10.1097/AJP.0000000000000173
45. Lamerato LE, Dryer RD, Wolff GG, Hegeman-Dingle R, Mardekian J, Park PW, et al. Prevalence of chronic pain in a large integrated healthcare delivery system in the U.S.A. Pain Pract Off J World Inst Pain. (2016) 16(7):890–8. doi: 10.1111/papr.12334
46. Higgins DM, Buta E, Dorflinger L, Masheb RM, Ruser CB, Goulet JL, et al. Prevalence and correlates of painful conditions and multimorbidity in national sample of overweight/obese veterans. J Rehabil Res Dev. (2016) 53(1):71–82. doi: 10.1682/JRRD.2014.10.0251
47. Burri A, Ogata S, Vehof J, Williams F. Chronic widespread pain: clinical comorbidities and psychological correlates. Pain. (2015) 156(8):1458–64. doi: 10.1097/j.pain.0000000000000182
48. Calders P, Van Ginckel A. Presence of comorbidities and prognosis of clinical symptoms in knee and/or hip osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. (2018) 47(6):805–13. doi: 10.1016/j.semarthrit.2017.10.016
49. Puts MTE, Deeg DJH, Hoeymans N, Nusselder WJ, Schellevis FG. Changes in the prevalence of chronic disease and the association with disability in the older Dutch population between 1987 and 2001. Age Ageing. (2008) 37(2):187–93. doi: 10.1093/ageing/afm185
50. Lentz TA, Marlow NM, Beneciuk JM, Fillingim RB, George SZ. Comorbidity subgroups among medicare beneficiaries seeking health care for musculoskeletal pain. J Gerontol A Biol Sci Med Sci. (2019) 74(8):1310–5. doi: 10.1093/gerona/gly202
51. Rustøen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain: differences in health and quality of life among younger, middle-aged, and older adults. Clin J Pain. (2005) 21(6):513–23. doi: 10.1097/01.ajp.0000146217.31780.ef
52. Corsi N, Roberto A, Cortesi L, Nobili A, Mannucci PM, Corli O, et al. Prevalence, characteristics and treatment of chronic pain in elderly patients hospitalized in internal medicine wards. Eur J Intern Med. (2018) 55:35–9. doi: 10.1016/j.ejim.2018.05.031
53. Ghavidel-Parsa B, Bidari A, Amir Maafi A, Ghalebaghi B. The iceberg nature of fibromyalgia burden: the clinical and economic aspects. Korean J Pain. (2015) 28(3):169–76. doi: 10.3344/kjp.2015.28.3.169
54. Ritzwoller DP, Crounse L, Shetterly S, Rublee D. The association of comorbidities, utilization and costs for patients identified with low back pain. BMC Musculoskelet Disord. (2006) 7:72. doi: 10.1186/1471-2474-7-72
55. Romanelli RJ, Shah SN, Ikeda L, Lynch B, Craig TL, Cappelleri JC, et al. Patient characteristics and healthcare utilization of a chronic pain population within an integrated healthcare system. Am J Manag Care. (2017) 23(2):e50–6.28245659
56. Hogans BB, Siaton BC, Taylor MN, Katzel LI, Sorkin JD. Low back pain and substance use: diagnostic and administrative coding for opioid use and dependence increased in U.S. Older adults with low back pain. Pain Med. (2021) 22(4):836–47. doi: 10.1093/pm/pnaa428
57. Hogans B, Siaton B, Sorkin J. Diagnostic rate estimation from medicare records: dependence on claim numbers and latent clinical features. J Biomed Inform. (2023) 145:104463. doi: 10.1016/j.jbi.2023.104463
58. Institute of Medicine (US) Committee to Design a Strategy for Quality Review and Assurance in Medicare. In: Lohr KN, editor. Medicare: A Strategy for Quality Assurance. Volume 1. Washington, DC: National Academies Press (1990). PMID: 25144047.
59. Hoy D, March L, Brooks P, Woolf A, Blyth F, Vos T, et al. Measuring the global burden of low back pain. Best Pract Res Clin Rheumatol. (2010) 24(2):155–65. doi: 10.1016/j.berh.2009.11.002
60. Davis PA. Medicare Financing. Congr Res Serv. (2014). Available online at: https://crsreports.congress.gov/product/details?prodcode=R41436 (Accessed February 21, 2022).
61. Ohayon MM, Stingl JC. Prevalence and comorbidity of chronic pain in the German general population. J Psychiatr Res. (2012) 46(4):444–50. doi: 10.1016/j.jpsychires.2012.01.001
62. Streibelt M, Schmidt C, Brünger M, Spyra K. Comorbidity from the patient perspective - does it work? Validity of a questionnaire on self-estimation of comorbidity (SCQ-D). Orthopade. (2012) 41(4):303–10. doi: 10.1007/s00132-012-1901-3
63. Runciman WB, Hunt TD, Hannaford NA, Hibbert PD, Westbrook JI, Coiera EW, et al. Caretrack: assessing the appropriateness of health care delivery in Australia. Med J Aust. (2012) 197(2):100–5. doi: 10.5694/mja12.10510
64. Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. (2008) 61(12):1234–40. doi: 10.1016/j.jclinepi.2008.01.006
65. Park PW, Dryer RD, Hegeman-Dingle R, Mardekian J, Zlateva G, Wolff GG, et al. Cost burden of chronic pain patients in a large integrated delivery system in the United States. Pain Pract. (2016) 16(8):1001–11. doi: 10.1111/papr.12357
66. Price-Haywood EG, Burton J, Burstain T, Harden-Barrios J, Lefante J, Shi L, et al. Clinical effectiveness of decision support for prescribing opioids for chronic noncancer pain: a prospective cohort study. Value Health J Int Soc Pharmacoeconomics Outcomes Res. (2020) 23(2):157–63. doi: 10.1016/j.jval.2019.09.2748
67. Kinsinger LS, Jones KR, Kahwati L, Harvey R, Burdick M, Zele V, et al. Design and dissemination of the MOVE! weight-management program for veterans. Prev Chronic Dis. (2009) 6(3):A98.19527600
68. Eicheldinger C, Bonito A. More accurate racial and ethnic codes for medicare administrative data. Health Care Financ Rev. (2008) 29(3):27–42.18567241
69. Hogans BB. Chapter 16. Planning therapy: coordinated, comprehensive care. In: Pain Medicine at a Glance. Oxford: Wiley Blackwell Press; (2022). p. 32–3.
70. Kaasalainen S, Zacharias R, Hill C, Wickson-Griffiths A, Hadjistavropoulos T, Herr K. Advancing the pain management in older adults agenda forward through the development of key research and education priorities: a Canadian perspective. Can J Pain Rev Can Douleur. (2017) 1(1):171–82. doi: 10.1080/24740527.2017.1383139
71. Morrissey MB, Herr K, Levine C. Public health imperative of the 21st century: innovations in palliative care systems, services, and supports to improve health and well-being of older Americans. Gerontologist. (2015) 55(2):245–51. doi: 10.1093/geront/gnu178
72. Murinson BB, Gordin V, Flynn S, Driver LC, Gallagher RM, Grabois M, et al. Recommendations for a new curriculum in pain medicine for medical students: toward a career distinguished by competence and compassion. Pain Med Malden Mass. (2013) 14(3):345–50. doi: 10.1111/pme.12051
73. Patel KV, Guralnik JM, Phelan EA, Gell NM, Wallace RB, Sullivan MD, et al. Symptom burden among community-dwelling older adults in the United States. J Am Geriatr Soc. (2019) 67(2):223–31. doi: 10.1111/jgs.15673
74. Turner GH, Weiner DK. Essential components of a medical student curriculum on chronic pain management in older adults: results of a modified delphi process. Pain Med Malden Mass. (2002) 3(3):240–52. doi: 10.1046/j.1526-4637.2002.02030.x
75. Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, et al. Noninvasive Nonpharmacological Treatment for Chronic Pain: a Systematic Review. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK519953/ (Accessed November 29, 2022).
76. Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, et al. Noninvasive nonpharmacological treatment for chronic pain: a systematic review update. Rockville, MD: Agency for Healthcare Research and Quality (US); 2020. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK556229/ (Accessed August 30, 2021).
77. Chou R, Deyo R, Friedly J, Skelly A, Weimer M, Fu R, et al. Systemic pharmacologic therapies for low back pain: a systematic review for an American college of physicians clinical practice guideline. Ann Intern Med. (2017) 166(7):480. doi: 10.7326/M16-2458
78. Addison O, Serra MC, Katzel L, Giffuni J, Lee CC, Castle S, et al. Mobility improvements are found in older veterans after 6 months of gerofit regardless of body mass Index classification. J Aging Phys Act. (2019) 27(6):848–54. doi: 10.1123/japa.2018-0317
79. Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, et al. Exercise for preventing falls in older people living in the community. Cochrane bone, joint and muscle trauma group, ed. Cochrane Database Syst Rev. (2019) 1(1):CD012424. doi: 10.1002/14651858.CD012424.pub2
80. Merchant RA, Chen MZ, Wong BLL, Ng SE, Shirooka H, Lim JY, et al. Relationship between fear of falling, fear-related activity restriction, frailty, and sarcopenia. J Am Geriatr Soc. (2020) 68(11):2602–8. doi: 10.1111/jgs.16719
81. Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study ⋆. Pain. (2008) 134(3):310–9. doi: 10.1016/j.pain.2007.04.038
82. Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, et al. International exercise recommendations in older adults (ICFSR): expert consensus guidelines. J Nutr Health Aging. (2021) 25(7):824–53. doi: 10.1007/s12603-021-1665-8
83. Bruckenthal P, Marino MA, Snelling L. Complementary and integrative therapies for persistent pain management in older adults: a review. J Gerontol Nurs. (2016) 42(12):40–8. doi: 10.3928/00989134-20161110-08
84. Peretti J, Sibley A, Dobert R, Brennan M. Interprofessional team (IPT) offers supportive care to older adults (OA) in persons living with dementia (PLWD) in the community. J Am Geriatr Soc. 2020;68(SUPPL 1):S23–4. doi: 10.1111/jgs.16431
85. Karmali RN, Skinner AC, Trogdon JG, Weinberger M, George SZ, Hassmiller Lich K. The association between the supply of nonpharmacologic providers, use of nonpharmacologic pain treatments, and high-risk opioid prescription patterns among medicare beneficiaries with persistent musculoskeletal pain. Med Care. (2020) 58(5):433–44. doi: 10.1097/MLR.0000000000001299
86. Husebo BS, Kerns RD, Han L, Skanderson M, Gnjidic D, Pain AH. Complex chronic conditions and potential inappropriate medication in people with dementia. Lessons learnt for pain treatment plans utilizing data from the veteran health administration. Brain Sci. (2021) 11(1):86. doi: 10.3390/brainsci11010086
87. Fishman SM, Carr DB, Hogans B, Cheatle M, Gallagher RM, Katzman J, et al. Scope and nature of pain- and analgesia-related content of the United States medical licensing examination (USMLE). Pain Med Malden Mass. (2018) 19(3):449–59. doi: 10.1093/pm/pnx336
88. Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J Pain. (2019) 20(2):146–60. doi: 10.1016/j.jpain.2018.07.006
89. Shade MY, Herr K, Kupzyk K. Self-Reported pain interference and analgesic characteristics in rural older adults. Pain Manag Nurs Off J Am Soc Pain Manag Nurses. (2019) 20(3):232–8. doi: 10.1016/j.pmn.2019.03.001
90. Fernando E, Fraser M, Hendriksen J, Kim CH, Muir-Hunter SW. Risk factors associated with falls in older adults with dementia: a systematic review. Physiother Can. (2017) 69(2):161–70. doi: 10.3138/ptc.2016-14
91. Peretti J, Sibley A, Dobert R, Ang S, Brennan M. Potential inappropriate medication(s) (PIM) in frail older adults in the community. J Am Geriatr Soc. (2019) 67:S128. doi: 10.1111/jgs.15898
92. Jeffery MM, Hooten WM, Jena AB, Ross JS, Shah ND, Karaca-Mandic P. Rates of physician coprescribing of opioids and benzodiazepines after the release of the centers for disease control and prevention guidelines in 2016. JAMA Netw Open. (2019) 2(8):e198325. doi: 10.1001/jamanetworkopen.2019.8325
93. Kugler X. Recognizing delirium: the value of having geriatric training as an advanced practice clinician and using a framework of age-friendly care. J Gerontol Nurs. (2020) 46(11):49–50. doi: 10.3928/00989134-20201012-06
94. Shipton EE, Shipton EA. Vitamin D deficiency and pain: clinical evidence of low levels of vitamin D and supplementation in chronic pain states. Pain Ther. (2015) 4(1):67–87. doi: 10.1007/s40122-015-0036-8
95. Regland B, Forsmark S, Halaouate L, Matousek M, Peilot B, Zachrisson O, et al. Response to vitamin B12 and folic acid in myalgic encephalomyelitis and fibromyalgia. PLoS One. (2015) 10(4):e0124648. doi: 10.1371/journal.pone.0124648
96. Hemmer B, Glocker FX, Schumacher M, Deuschl G, Lücking CH. Subacute combined degeneration: clinical, electrophysiological, and magnetic resonance imaging findings. J Neurol Neurosurg Psychiatry. (1998) 65(6):822–7. doi: 10.1136/jnnp.65.6.822
97. Chandok N, Watt KDS. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. (2010) 85(5):451–8. doi: 10.4065/mcp.2009.0534
98. Holman A, Parikh N, Clauw DJ, Williams DA, Tapper EB. Contemporary management of pain in cirrhosis: toward precision therapy for pain. Hepatol Baltim Md. (2023) 77(1):290–304. doi: 10.1002/hep.32598
99. Loewenthal KM, Lewis CA. An Introduction to Psychological Tests and Scales. London: Psychology Press (2018).
100. Erler A, Beyer M, Muth C, Gerlach FM, Brennecke R. Garbage in - garbage out? Validity of coded diagnoses from GP claims records. Gesundheitswesen Bundesverb Arzte Offentlichen Gesundheitsdienstes Ger. (2009) 71(12):823–31. doi: 10.1055/s-0029-1214399
101. Patra BG, Sharma MM, Vekaria V, Adekkanattu P, Patterson OV, Glicksberg B, et al. Extracting social determinants of health from electronic health records using natural language processing: a systematic review. J Am Med Inform Assoc. (2021) 28(12):2716–27. doi: 10.1093/jamia/ocab170
102. Agarwal AR, Prichett L, Jain A, Srikumaran U. Assessment of use of ICD-9 and ICD-10 codes for social determinants of health in the US, 2011-2021. JAMA Netw Open. (2023) 6(5):e2312538. doi: 10.1001/jamanetworkopen.2023.12538
103. Koleck TA, Tatonetti NP, Bakken S, Mitha S, Henderson MM, George M, et al. Identifying symptom information in clinical notes using natural language processing. Nurs Res. (2021) 70(3):173–83. doi: 10.1097/NNR.0000000000000488
104. Bordage G. Elaborated knowledge: a key to successful diagnostic thinking. Acad Med J Assoc Am Med Coll. (1994) 69(11):883–5. doi: 10.1097/00001888-199411000-00004
105. Sanders AE, Greenspan JD, Fillingim RB, Rathnayaka N, Ohrbach R, Slade GD. Associations of sleep disturbance, atopy, and other health measures with chronic overlapping pain conditions. J Oral Facial Pain Headache. (2020) 34:s73–84. doi: 10.11607/ofph.2577
106. Tonelli M, Wiebe N, Manns BJ, Klarenbach SW, James MT, Ravani P, et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. (2018) 1(7):e184852. doi: 10.1001/jamanetworkopen.2018.4852
107. Morgan PA, Smith VA, Berkowitz TSZ, Edelman D, Van Houtven CH, Woolson SL, et al. Impact of physicians, nurse practitioners, and physician assistants on utilization and costs for Complex patients. Health Aff (Millwood). (2019) 38(6):1028–36. doi: 10.1377/hlthaff.2019.00014
Keywords: interprofessional, interdisciplinary, pain education, clinical decision-making, geriatric, multimorbidity, survey instrument, chronic pain
Citation: Siaton BC, Hogans BB, Frey-Law LA, Brown LM, Herndon CM and Buenaver LF (2024) Pain, comorbidities, and clinical decision-making: conceptualization, development, and pilot testing of the Pain in Aging, Educational Assessment of Need instrument. Front. Pain Res. 5:1254792. doi: 10.3389/fpain.2024.1254792
Received: 7 July 2023; Accepted: 26 January 2024;
Published: 22 February 2024.
Edited by:
Judy Watt-Watson, University of Toronto, CanadaReviewed by:
Sean D. Rundell, University of Washington, United StatesMark I. Johnson, Leeds Beckett University, United Kingdom
© 2024 Siaton, Hogans, Frey-Law, Brown, Herndon and Buenaver. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beth B. Hogans YmJAamhtaS5lZHU=
†These authors have contributed equally to this work and share first authorship
 Bernadette C. Siaton1,2,†
Bernadette C. Siaton1,2,† Beth B. Hogans
Beth B. Hogans Laura A. Frey-Law
Laura A. Frey-Law Christopher M. Herndon
Christopher M. Herndon Luis F. Buenaver
Luis F. Buenaver