- 1Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN, United States
- 2Department of Neuroscience, University of Minnesota, Minneapolis, MN, United States
- 3Department of Pharmaceutics, University of Minnesota, Minneapolis, MN, United States
- 4Department of Genetics Cell Biology and Development, University of Minnesota, Minneapolis, MN, United States
- 5Department of Pharmacology, University of Minnesota, Minneapolis, MN, United States
- 6Department of Dermatology, University of Minnesota, Minneapolis, MN, United States
Agmatine, a decarboxylated form of L-arginine, prevents opioid analgesic tolerance, dependence, and self-administration when given by both central and systemic routes of administration. Endogenous agmatine has been previously detected in the central nervous system. The presence of a biochemical pathway for agmatine synthesis offers the opportunity for site-specific overexpression of the presumptive synthetic enzyme for local therapeutic effects. In the present study, we evaluated the development of opioid analgesic tolerance in ICR-CD1 mice pre-treated with either vehicle control or intrathecally delivered adeno-associated viral vectors (AAV) carrying the gene for human arginine decarboxylase (hADC). Vehicle-treated or AAV-hADC-treated mice were each further divided into two groups which received repeated delivery over three days of either saline or systemically-delivered morphine intended to induce opioid analgesic tolerance. Morphine analgesic dose-response curves were constructed in all subjects on day four using the warm water tail flick assay as the dependent measure. We observed that pre-treatment with AAV-hADC prevented the development of analgesic tolerance to morphine. Peripheral and central nervous system tissues were collected and analyzed for presence of hADC mRNA. In a similar experiment, AAV-hADC pre-treatment prevented the development of analgesic tolerance to a high dose of the opioid neuropeptide endomorphin-2. Intrathecal delivery of anti-agmatine IgG (but not normal IgG) reversed the inhibition of endomorphin-2 analgesic tolerance in AAV-hADC-treated mice. To summarize, we report here the effects of AAV-mediated gene transfer of human ADC (hADC) in models of opioid-induced analgesic tolerance. This study suggests that gene therapy may contribute to reducing opioid analgesic tolerance.
1 Introduction
Opioid pharmacotherapy remains an important option for individuals with chronic pain arising from critical illnesses that are not alleviated by non-opioid or non-pharmacological pain management approaches (1, 2), particularly for malignant chronic pain. However, a panel of complex side effects accompanies long-term opioid pharmacotherapy, including, but not limited to, the development of opioid analgesic tolerance (1, 2). The development of analgesic tolerance for patients on chronic opioid therapy can result in significant dose escalation (3) resulting in increased adverse side effects (4), a need for more invasive delivery approaches, or the inability to adequately control pain [ceiling effect (5)]. Multiple strategies are used with varying success for these patients, such as opioid rotation, drug holiday, or inclusion of adjuvant analgesics such as ketamine (5). The application of ketamine as an analgesic adjuvant strategy (1, 2) to reduce the induction of opioid analgesic tolerance is based on the well-established observation that antagonism of NMDA receptors reduces the development of opioid analgesic tolerance (6). More than thirty years of pharmacological studies have shown that antagonism of the NMDA receptor (6–10) or inhibition of the nitric oxide synthase (NOS) enzyme (10–12) inhibits the induction of opioid-induced analgesic tolerance.
Agmatine was discovered in the central nervous system (CNS) in 1994 (13), closely followed by the cloning of its synthetic (14) and degradative enzyme systems (15) that are also expressed in CNS. Subsequently, neuropharmacological investigations have converged on an inhibitory role for agmatine in multiple models of behavioral neural plasticity (16). We and others have shown that agmatine inhibits the induction of opioid analgesic tolerance when given either systemically (17) or centrally (18–20). Evidence suggests that agmatine antagonizes the NMDA receptor (21, 22) and/or inhibits the NOS enzyme (21, 23–25) to exert its inhibition of opioid analgesic tolerence.
Since agmatine is a naturally occurring product with a presumptive synthetic enzyme that is expressed in the central nervous system (26), an opportunity arises to design an agmatine-based gene therapy with site-directed expression within the CNS. Toward this end, we developed an adeno-associated viral vector (AAV) that carries the presumptive synthetic gene for agmatine (27). We have demonstrated that AAV viral vectors delivered by intrathecal injection distribute through the central nervous system (28) and provide robust gene transfer to sensory neurons at all levels of the neuraxis (29). We have also shown that intrathecal delivery of an AAV vector containing the gene for human arginine decarboxylase (AAV-hADC) leads to persistent alleviation of neuropathic pain in rodents (27).
Since opioid analgesic tolerance and neuropathic pain and are both dependent on NMDA receptor activation (30) and agmatine reduces both neuropathic pain (31, 32) and opioid analgesic tolerance (18–20), we hypothesized that intrathecal delivery of AAV-hADC would reduce the induction of analgesic tolerance to opioids. To test this hypothesis, the development of chronic systemic morphine analgesic tolerance or acute intrathecal endomorphin-2 analgesic tolerance was compared between AAV-hADC-treated mice and vehicle-injected controls.
2 Materials and methods
2.1 Research subjects
All subjects were 20–25 g adult male ICR-CD1 mice (Envigo, Madison, WI). All experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee.
2.2 Chemicals
Morphine sulfate was a gift from the National Institute on Drug Abuse. Endomorphin-2 (ENDO-2, YPFF) was synthesized by the University of Minnesota's microchemical facility, Minneapolis, MN. Agmatine sulfate and NMDA were obtained from Sigma Chemical (St. Louis, MO) and dissolved in 0.9% NaCl.
2.3 AAV vectors
The development of the human ADC-containing vector was previously described (27). The vector titer was 8.24 × 1012 vector genomes/ml (vg/ml) for AAV5-hADC and 6.37 × 1012 vector genomes/ml (vg/ml) for AAV9-hADC which was diluted 1:3 in saline for a final injected titer of 2.1 × 1012 vector genomes/ml (vg/ml).
2.4 Gene expression by RT-PCR
2.4.1 PCR method used in AAV5-hADC experiments
Total RNA extraction and qualitative PCR has been previously described by Peterson and colleagues (27) and was based upon the method reported by Seybold and colleagues (33). The sequences of the primers used are as follows: AAV5 experiments: hADC forward primer: GCCTTGGACCTGTACTTCCC; hADC reverse primer: CTGGTCCGTGGATGGTTTCT.
2.4.2 PCR method used in AAV9-hADC experiments
Total RNA was extracted from tissue using RNAzol RT. Reverse-transcription (RT). Four hundred micrograms RNA was reverse-transcribed in RT master mixture (final concentrations: 2.5 mM MgCl2, 1× PCR Buffer II, 10 mM dithiothreitol, 1.25 μM random hexamers, 50 U RNase inhibitor, 250 µM dNTP and 75 U MMLV reverse transcriptase; Applied Biosystems/Life Technologies, Grand Island, NY) in a volume of 20 µl. The reactions were incubated at 25 °C for 10 min followed by 42 °C for 20 min followed by 65 °C for 3 min, cooled on ice and stored at −20 °C until used for PCR. Two microliters of RT product is added to PCR master mixture (final concentrations: 1× Lightcycler 480 SyBr green mastermix and 300 nM of each primer in a final volume of 20 µl and pipetted into a well of a 96-well plate (hADC forward primer: GTACCCGAGACCTGCTGAAG; hADC reverse primer: GGACCAACTCCATCTCTGCC). The plate is then inserted into a Lightcycler 480 II (Roche) and a PCR program was run. The program consisted of one cycle of 95°C for 8 min (polymerase activation) followed by 55 cycles of: 95 °C for 15 s, 60 °C for 15 s and 72 °C for 15 s. Following amplification, the built-in thermal melting (Tm) and second derivative maximum (to determine quantification cycle, Cq) programs were also run. The PCR products and data were analyzed using 1.5% agarose gel electrophoresis, Lightcycler 480 software v.1.5, Photoshop CS v.5.1 and GraphPad Prism v6f software.
2.5 Thermal nociception assay
Thermal nociception was assessed with the warm water (52.5°C) tail immersion assay (18, 20, 34). The time that lapses between the mouse tail entering the water to the mouse flicking its tail is the dependent measure. Initial tail-flick latencies were taken on each individual mice prior to treatment (for a sample of n = 42, mean = 3.32 s, and S.D. = 0.65 s). Each subject's baseline value was used as an internal control. The maximum possible effect (%MPE) was calculated accordingly: %MPE = (postdrug latency − predrug latency)/(cutoff − predrug latency) × 100%. A maximum score of 100% was given mice that do not flick their tails prior to a cutoff of 12 s. Twelve seconds is the maximum time for the tail to be allowed to remain in the water at that temperature in order to prevent thermal injury.
2.6 Rotarod assay
After two training sessions, mice were permitted to walk for a maximum of 300 s on an accelerating (4–40 rpm) rotarod (Ugo Basile, Varese, Italy). The dependent measure was the time spent remaining on the rotarod. We statistically compared the mean time that each subject remained on the rotarod across the four treatment groups using a one-way ANOVA with Tukey's post hoc test for comparisons between multiple groups.
2.7 Dose-response analysis
At least 3–4 doses were included in the development of each dose-response curve for each experimental group. The ED50 values and 95% confidence intervals (CIs) of drugs were calculated by parametric linear regression analysis of the log dose–response curves. These methods have been previously described (35). These calculations were performed using FlashCalc, a pharmacological statistics program written by Dr. Michael Ossipov, University of Arizona, Tucson, AZ.
2.8 Intrathecal injections
The AAV-hADC viral vectors or vehicle (0.9% normal saline) were given intrathecally (i.t.) by lumbar puncture in awake mice as previously described (36, 37). In order to conserve the AAV5-hADC vector we modified the delivery method (38). The needle (30-gauge, 0.5-inch) was connected to a length of PE10 tubing, which was itself connected to a second needle (a 30-gauge, 0.5-inch) that was fitted to a 50-µl Luer-hub Hamilton syringe. For both AAV-hADC experiments, 10 µl of the injectate which was comprised of 8.24 × 1012 viral vector genomes/ml (AAV5) or 2.1 × 1012 vector genomes/ml viral vector genomes (AAV9) were injected intrathecally. The injection was performed by gently grasping the hip bones of the rodent and inserting the needle (bevel side up) at about a 45° angle centered on the iliac crest. The penetration of the dura is accompanied by a reflexive flick of the tail. After intathecal delivery, the mice were returned to the animal housing area where they stayed until the the opioid analgesic tolerance experiment was initiated. Extraction of spinal cord, fourth ventricle choroid plexus, midbrain (PAG), and DRG for RT-PCR took place on the last day of the experiment after all analgesic testing had been completed.
2.9 Chronic morphine analgesic tolerance induction
Baseline tail flick latencies were taken on all mice prior to drug exposure. Mice acquired analgesic tolerance to morphine following repeated administration of subcutaneously delivered morphine over the course of three days. A single injection of morphine was delivered on day 1 (3 mg/kg) followed by three injections daily at 9 am, 5 pm, and 11 pm on Days 2 (3 mg/kg) and 3 (5 mg/kg). A total of seven subcutaneous injections were delivered over the course of the induction schedule. A cohort of control mice received an equivalent number of subcutaneous saline injections at the same time as the morphine treatment groups. All injections were given in a volume of 100 µl/10 grams. Cumulative dose-response curves of morphine were then constructed in both the morphine pre-treatment and saline pre-treatment groups in the following way: a single dose of morphine was injected, and the tail flick latency was assessed at 30 min after injection. A second dose of morphine was delivered and the process repeated. Doses were delivered in this manner until a complete dose-response curve was constructed in each treatment group.
2.10 Acute endomorphin-2 analgesic tolerance induction
Baseline tail flick latencies were assessed for all mice prior to drug exposure. Mice developed analgesic tolerance to endomorphin-2 (ENDO-2) following a single intrathecal injection of a large dose (10 nmol, 5 µl) of ENDO-2. A cohort of control mice were injected with an equivalent volume of intrathecal saline (5 µl) at the same time as the ENDO-2-treatment groups. Thirty minutes later, tail flick latencies were measured to verify that the responses were comparable to their baseline latencies. Cumulative dose-response curves of ENDO-2 were then constructed in both the ENDO-2 pre-treatment and Saline pre-treatment groups in the following manner. A single dose of ENDO-2 was injected, and the tail flick latency was taken at 2.5 min later (the peak analgesic time point). A second dose of ENDO-2 was delivered and the process repeated. Doses were delivered in this manner until a complete dose-response curve was constructed in each treatment group.
2.11 Intrathecal NMDA scratching and biting nocifensive behavioral assay
Intrathecal delivery of NMDA (0.3 nmol) results in biting and scratching of their hindlimbs that lasts for 1 min (39). These behaviors are counted and represent the dependent measure for this assay. Other agents intended to modify these behaviors may be co-administered with the NMDA or given as an intrathecal pre-treatment. In the present study, agmatine was given as a co-administration with NMDA. The anti-agmatine IgG was given as a five minute pre-treatment to the NMDA + agmatine combination.
2.12 Agmatine immunoneutralization
In these experiments, either normal guinea pig IgG (150 ng) or anti-agmatine IgG (150 ng) was injected intrathecally five minutes prior to the intrathecal injection of ENDO-2 (10 nmol), which was given to induce acute ENDO-2 tolerance. These IgGs were raised in-house and previously characterized and reported (20, 27, 31). Cumulative dose-response curves were performed 30 min after the ENDO-2 tolerance-inducing injection, as previously described in section 2.10.
3 Results
3.1 AAV5-hADC treatment prevents the acquisition of morphine analgesic tolerance
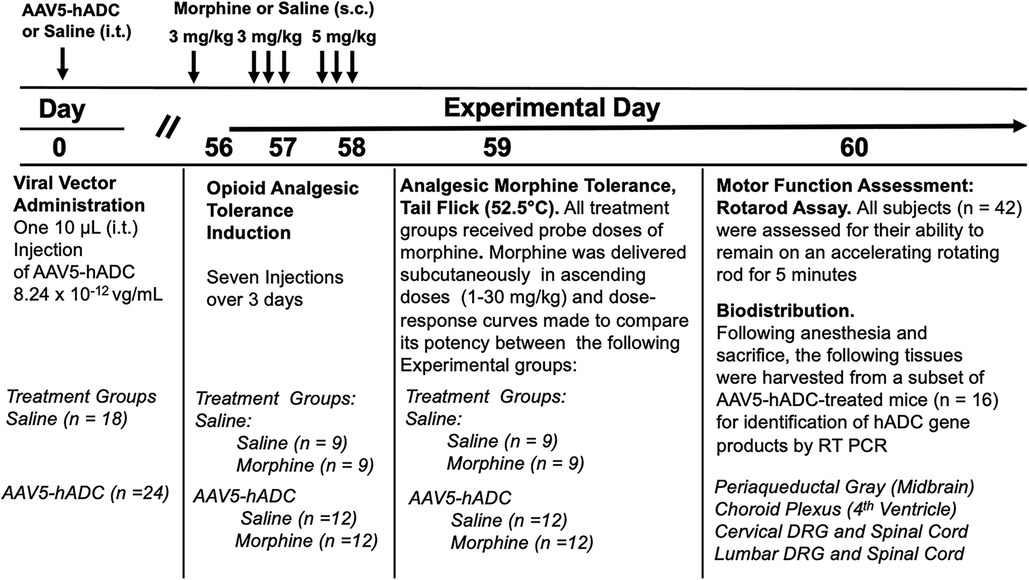
We proposed that expression of hADC in central nervous system reduces the acquisition of opioid analgesic tolerance. To test this hypothesis we compared the development of opioid analgesic tolerance in mice that had been injected before with either saline or AAV-hADC. The experimental plan is summarized in Figure 1.
Briefly, eight weeks after intrathecal delivery of saline or AAV5-hADC, the two groups were each divided into four experimental groups. Specifically, the saline-treated group and the AAV5-hADC-treated groups were each separated into two additional cohorts that received repeated doses of either morphine or saline over a 3 day period (Experimental Days 56–58, Figure 1). The following day (Experimental Day 59) all four subject groups were subcutaneously injected sequentially with increasing doses of probe morphine (1, 3, 10, 20 or 30 mg/kg.) mice. Probe dose-response curves for morphine were then constructed and the ED50 values and confidence intervals compared between the saline-injected and morphine-injected groups for each cohort (AAV5-hADC and vehicle pretreatment groups).
As expected, repeated injections of s.c. morphine produced an approximate 3-fold rightward shift in the probe morphine dose-response curve compared to the probe morphine dose-response curve in the group that received repeated s.c. injections of saline (control group) in the cohort of mice that had previously received an intrathecal injection of saline (control to the AAV-hADC) (Figure 2A, Table 1). The rightward shift in the ED50 values confirms the induction of systemic morphine tolerance in control conditions. In contrast, when mice were pre-treated with intrathecal AAV5-hADC, repeated injections with s.c. morphine did not produce a shift in the probe morphine dose-response curve compared to those repeatedly injected with s.c. saline (Figure 2B). The ED50 values for morphine were equivalent between the two repeated injection groups (Table 1). The observation of no shift in the morphine dose-response curves indicates that morphine tolerance did not develop in subjects treated with AAV5-hADC.
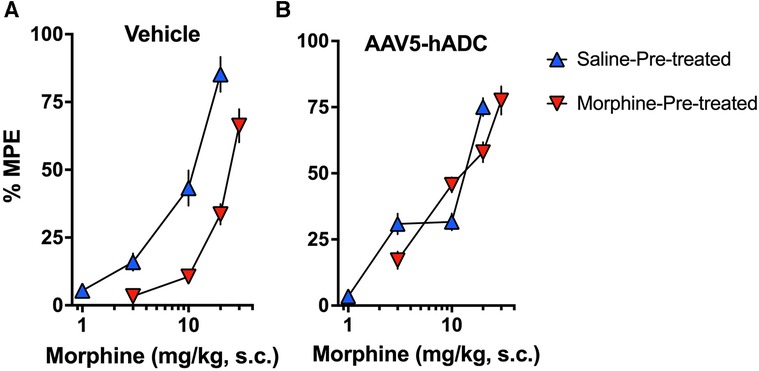
Figure 2. Effects of AAV5-hADC pre-treatment on acquisition of analgesic tolerance to morphine. Analgesic dose-response curves to acute subcutaneous (s.c.) probe morphine on Day 4 following repeated subcutaneous injection of either saline (blue triangles) or morphine (red inverted triangles). (A) Controls: Repeated s.c. injections of morphine (inverted red triangles) decreased acute probe morphine potency and efficacy compared with subjects repeatedly injected with saline (blue triangles), indicating induction of chronic morpine analgesic tolerance. (B) AAV5-hADC-treated subjects: Repeated s.c injections of morphine (inverted red triangles) demonstrated equivalent potency and efficacy compared with subjects repeatedly injected with saline (blue triangles), indicating that induction of chronic morpine analgesic tolerance did not occur in this experimental group.
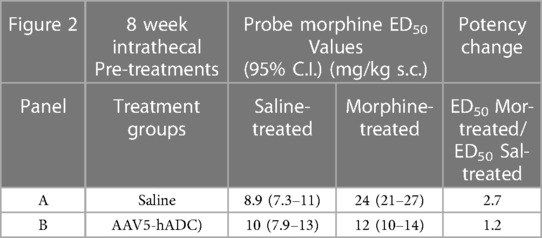
Table 1. ED50 values for the dose-response curves in Figure 2.
3.2 AAV5-hADC treatment does not impact rotarod performance
One day following completion of the morphine tolerance experiment, mice were assessed on the rotarod for any motor deficits. None was noted in any treatment group (Figure 3).
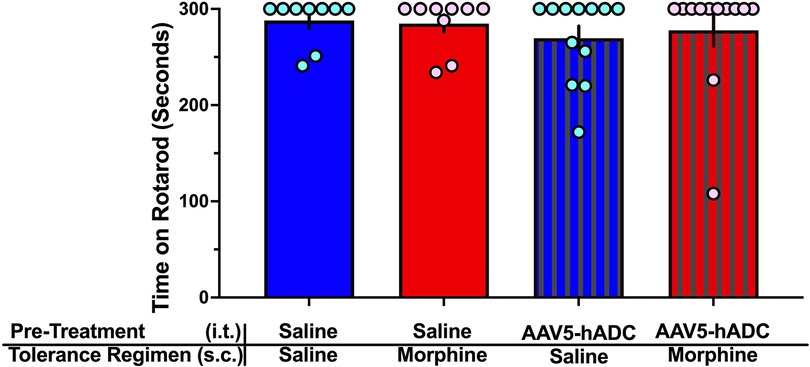
Figure 3. Rotarod assay of motor dysfunction/sedation. Comparison of rotarod performance in mice on Experimental Day 60 following treatment with (1) intrathecal saline and subcutaneous saline (blue bars, n = 9), (2) intrathecal saline and subcutaneous morphine (red bars, n = 9), (3) intrathecal AAV-hADC, and subcutaneous saline (blue striped bars, n = 12), and (4) intrathecal AAV-hADC and subcutaneous morphine (red striped bars, n = 12). There were no differences between groups as determined by a one-way ANOVA (F(3, 38) = 0.3967, p = 0.76) followed by Tukey's post hoc test for multiple comparisons between groups.
3.3 AAV5-hADC treatment results in hADC expression in the sensory system
Rodents express a form of ADC that is distinct from that of the human sequence. Since a human form of ADC was introduced, we are able to distinguish hADC from native mouse ADC (27). Therefore following conclusion of the rotarod experiments, subjects were sacrified and various tissues extracted and processed for presence of hADC mRNA by real-time PCR. hADC mRNA was detected in lumbar and cervical spinal cord and dorsal root ganglion tissue, choroid plexus (4th ventricle) and periaqueductal gray. The tissue distribution and the percentages of AAV5-hADC expressed in these tissues are summarized in Table 2.
3.4 AAV9-hADC treatment prevents the acquisition of morphine analgesic tolerance
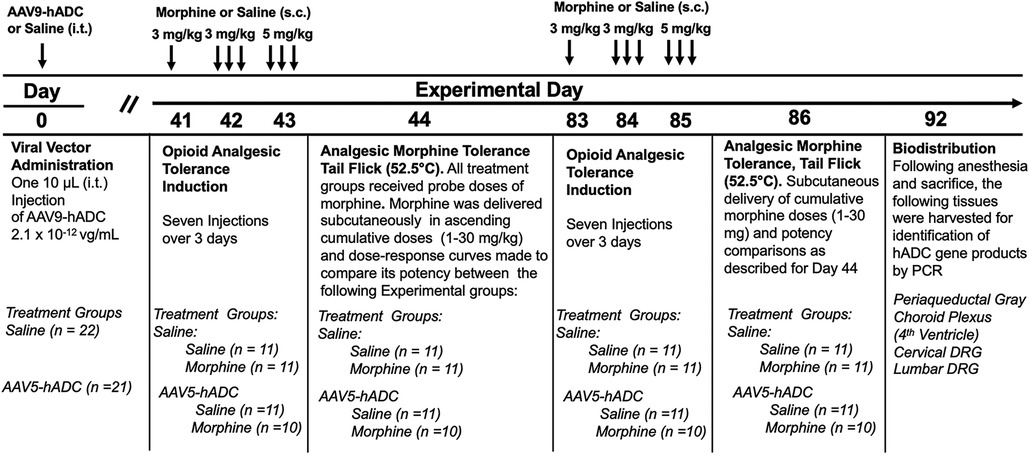
To determine whether the prevention of opioid tolerance in AAV-hADC-treated mice was generalizable to a second AAV serotype, we performed a similar experiment using an AAV9-hADC vector. In this case, we tested the subjects for opioid analgesic tolerance at approximately six weeks after AAV9-hADC treatment and then repeated the experiemnt at approximately 12 weeks post treatment. The general experimental design is as featured in Figure 4.
Briefly, six weeks following intrathecal delivery of either saline or AAV9-hADC, the two groups were each divided further into four experimental groups as described in Figure 4. The saline-treated group and the AAV9-hADC-treated groups were each separated into two additional groups that received repeated doses of either morphine or saline over a 3 day period (Experimental Days 41–43). The following day (Experimental Day 44) all four subject groups were subcutaneously injected sequentially with increasing doses of probe morphine (1, 3, 10, 20 or 30 mg/kg.) mice. Probe morphine dose-response curves were then constructed and compared between the saline and morphine-injected groups for each cohort (AAV9-hADC and saline pretreatment groups).
In the intrathecal saline pre-treatment group, repeated injections of s.c. morphine produced a 1.7-fold rightward shift in the probe morphine dose-response curve (Figure 5A) relative to the probe morphine dose-response curve in the group that received repeated s.c. saline (control group). Repeated morphine injections increased the acute morphine ED50 value (Table 3). Similarly, when mice were pre-treated with intrathecal AAV9-hADC, repeated injections with morphine produced a 1.5-fold shift in the acute probe morphine dose-response curve when compared to those repeatedly injected with s.c. saline (Figure 5B). In this experiment, repeated morphine injections increased the acute morphine ED50 value in the AAV9-hADC-treated group to nearly the same magnitude as the control group (Table 3). We speculate that the six week pre-treatment was insufficient for AAV9-hADC delivered at a titer of 2.1 × 1012 vector genomes/ml to manifest the effect. Therefore, we re-tested the same subjects at 12 weeks post-injection in the same morphine analgesic tolerance induction schedule (Figures 5C,D).
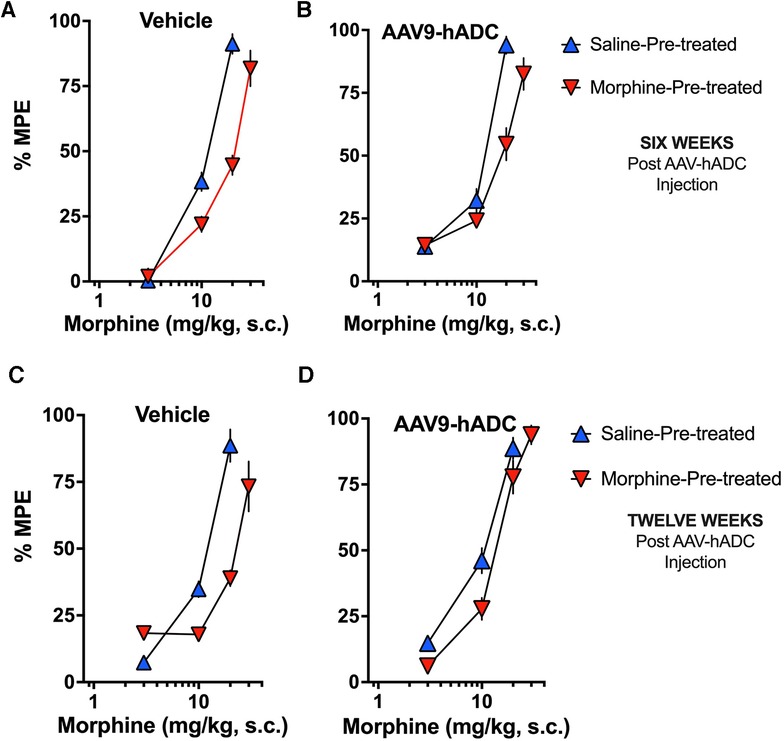
Figure 5. Effects of AAV9-hADC pre-treatment on development of morphine tolerance. Analgesic dose-response curves to acute subcutaneous (s.c.) probe morphine on Day 4 following repeated subcutaneous injection of either saline (blue triangles) or morphine (inverted red triangles). Six Weeks Post-Vehicle (Saline) or AAV9-hADC I.T. injection. (A) Controls: Repeated s.c. injections of morphine (inverted red triangles) decreased acute probe morphine potency and efficacy compared with subjects repeatedly injected with saline (blue triangles), indicating induction of chronic morphine analgesic tolerance. (B) AAV9-hADC-treated subjects: repeated s.c injections of morphine (inverted red triangles) decreased acute probe morphine potency compared with subjects repeatedly injected with saline (blue triangles), indicating induction of chronic morphine analgesic tolerance. (C, D) Twelve Weeks Post-Vehicle (Saline) or AAV9-hADC I.T. injection. Controls: Repeated s.c. injections of morphine (inverted red triangles) decreased acute probe morphine potency and efficacy compared with subjects repeatedly injected with saline (blue triangles), indicating induction of chronic morphine analgesic tolerance. (D) AAV9-hADC-treated subjects: repeated s.c injections of morphine (inverted red triangles) demonstrated equivalent potency and efficacy compared with subjects repeated injected with saline (blue triangles), indicating that induction of chronic morphine analgesic tolerance did not occur in this experimental group.
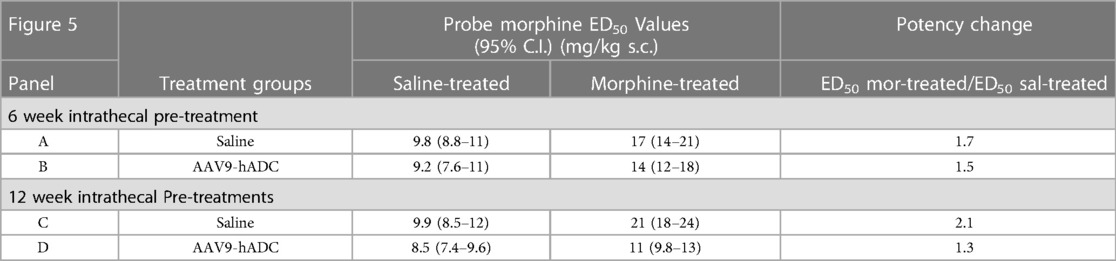
Table 3. ED50 values for the dose-response curves in Figure 5.
In the control mice, repeated injections of s.c. morphine produced a 2.1-fold rightward shift in the probe morphine dose-response curve relative to the probe morphine dose-response curve in the group that received repeated s.c. saline (Figure 5C). Repeated morphine injections doubled the acute morphine ED50 value (Table 3). This result demonstrates that morphine tolerance developed in the control group, as expected. In contrast, repeated injections with morphine produced a 1.3-fold shift in the probe morphine dose-response curve compared to those repeatedly injected with s.c. saline in the mice pre-treated with AAV9-hADC (Figure 5D, Table 3). The observation of no shift in the morphine dose-response curve indicates that morphine tolerance did not develop in subjects treated with AAV9-hADC, similar to what was observed in the AAV5-hADC treated subjects represented in Figure 2.
As in Figure 2, the subjects were sacrificed and various tissues extracted and processed for presence of hADC mRNA by real-time PCR. hADC mRNA was detected in cervical and lumbar dorsal root ganglion tissue and choroid plexus (4th ventricle). The tissue distribution and the percentages of AAV9-hADC expressed in these tissues are summarized in Table 4.
3.5 Agmatine immunoneutralization prevents AAV5-hADC-mediated inhibition of opioid analgesic tolerance
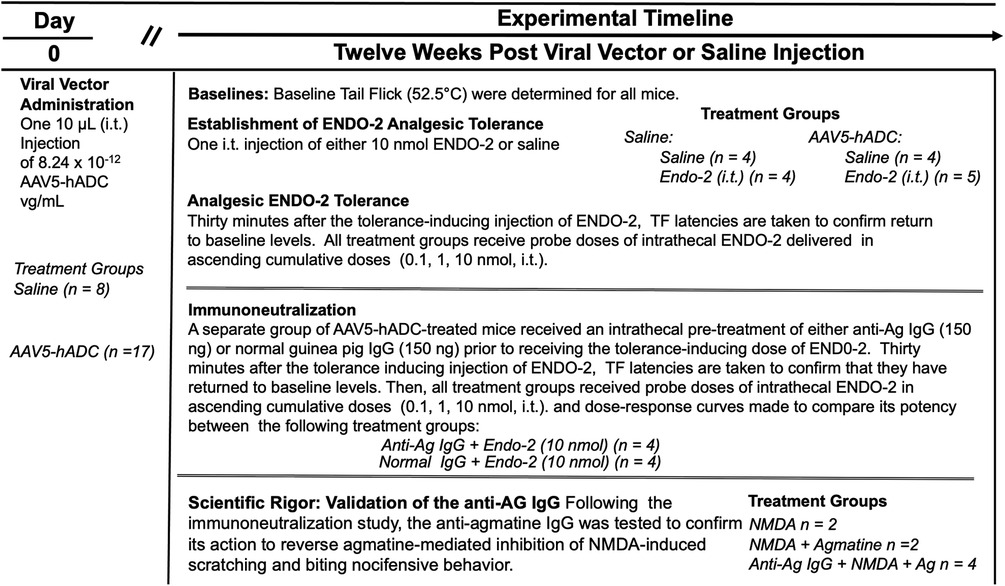
We used an immunoneutralization strategy to determine whether the prevention of opioid tolerance in AAV5-hADC-treated mice was due to an effect of the intended product, agmatine. The impact of intrathecal delivery of an immunoneutralizing antibody (anti-Ag IgG) was assessed in a model of opioid-induced analgesic tolerance as previously described (20). For this experiment, the opioid neuropeptide, endomorphin-2 (ENDO-2) (YPFF) (40), was used as the analgesic tolerance-inducing agent. It was previously demonstrated (20, 41) that mice given higher intrathecal doses of ENDO-2 (10–30 nmol, i.t.) develop acutely induced analgesic tolerance. It was also shown previously that intrathecal pre-treatment of an antibody raised to selectively target agmatine (20, 31) (anti-Ag IgG) sensitizes mice to the development of ENDO-2 analgesic tolerance induced by low doses (20). We used the ENDO-2 model of acute opioid tolerance to test for the effect of agmatine immunoneutralization in the hADC-treated mice. The overall experimental design is summarized in Figure 6.
Approximately twelve weeks following intrathecal delivery of either saline or AAV5-hADC, the two groups were each divided into four experimental groups. The saline-treated group and the AAV5-hADC-treated groups were each divided into two additional groups that received a single intrathecal injection of either ENDO-2 (10 nmol) or saline. ENDO-2 has a very short duration of action (41). Thirty minutes after the initial high dose injection, mice were tested cumulatively with increasing doses of ENDO-2. The tail flick test was performed 2.5 min after the test injection. All four subject groups were intrathecally injected sequentially with increasing doses of probe ENDO-2 (1, 3, 10, or 20 nmol). Probe ENDO-2 dose-response curves were then constructed and the ED50 values and confidence intervals compared between the saline-injected and ENDO-2-injected groups for each cohort (AAV5-hADC and vehicle pretreatment groups).
In control mice that had received a single i.t. injection of saline (as controls to AAV5-hADC), probe doses of ENDO-2 produced dose-dependent analgesia in the tail flick test in mice that received a single 30 min i.t. pre-treatment with saline. However, probe ENDO-2 doses failed to produce analgesia in control mice that had previously received a single high dose injections of i.t. ENDO-2 (Figure 7A). These data confirm the induction of acute ENDO-2 tolerance in control conditions. In contrast, when mice were pre-treated with intrathecal AAV5-hADC, a single high dose injection with i.t. ENDO-2 did not produce a reduction in either efficacy or potency in the probe ENDO-2 dose-response curve compared to those that received a single i.t. dose of saline (Figure 7B). The ED50 values for ENDO-2 were equivalent between the two injection groups (Table 5). The observation of no shift in the ENDO-2 dose-response curves or no reduction in efficacy indicates that ENDO-2 tolerance did not develop in subjects treated with AAV5-hADC.
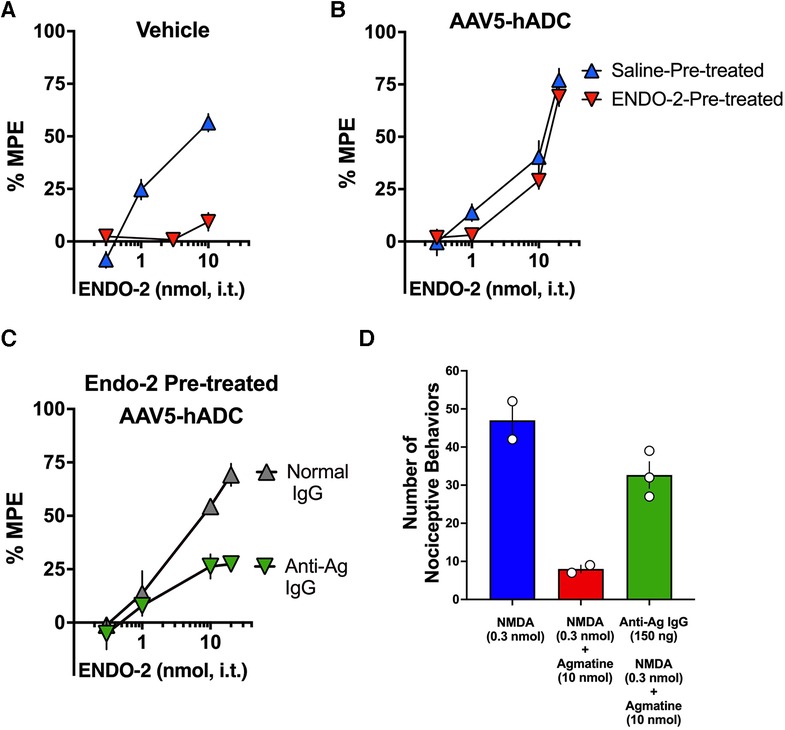
Figure 7. Effects of AAV5-hADC pre-treatment on the development of ENDO-2 tolerance. Analgesic dose-response curves to acute intrathecal (i.t.) probe ENDO-2 30 min following a single acute injection of either saline (blue triangles) or ENDO-2 (red inverted triangles). (A) Vehicle-Treated Controls: a single acute injection of ENDO-2 (inverted red triangles) decreased acute probe ENDO-2 potency compared with subjects injected with saline (blue triangles), indicating induction of acute ENDO-2 analgesic tolerance. (B) AAV5-hADC-treated subjects that received a single i.t. injection of ENDO-2 (inverted red triangles) demonstrated equivalent potency compared with AAV5-hADC-treated subjects subjects injected with saline (blue triangles), indicating that induction of acute ENDO-2 analgesic tolerance did not occur when subjects where pre-treated with AAV5-hADC. (C) AAV5-hADC-treated subjects injected with tolerance-inducing dose of ENDO-2. Subjects that received a pre-treatment with normal IgG (grey triangles) demonstrated equivalent potency and efficacy compared with AAV5-hADC-treated that received a tolerance-inducing dose of ENDO-2 (Panel B, red inverted triangles). Subjects that received a pre-treatment with anti-agmatine IgG (inverted green triangles) demonstrated greatly reduced probe ENDO-2 efficacy. (D) NMDA (0.3 nmol i.t) induces nociceptive behaviors (blue bar), which is reduced with co-injection with agmatine (10 nmol, red bar). Pre-treatment with anti-agmatine IgG attenuates the effect of agmatine (green bar).

Table 5. ED50 values for the dose-response curves in Figure 7.
We used an immunoneutralization strategy to determine whether the prevention of ENDO-2 acute tolerance by the AAV5-hADC pre-treatment was due to an effect of agmatine. We tested the impact of intrathecal delivery of an anti-agmatine IgG on the prevention of opioid tolerance observed in AAV-hADC-treated mice. We have previously shown that intrathecal delivery of anti-agmatine IgG dose-dependently reverses agmatine-mediated inhibition of scratching and biting nociceptive behaviors induced by intrathecal delivery of NMDA (20). To perform the immunoneutralization experiment, we compared the analgesic efficacy of probe ENDO-2 in AAV5-hADC-injected mice that had all received a high dose of ENDO-2 but that had also received an intrathecal pre-treatment of either anti-agmatine IgG or control normal IgGs.
One group of AAV5-hADC-treated mice received an intrathecal injection of normal guinea pig IgG (150 ng) prior to the high dose of ENDO-2 that is used to induce acute tolerance. Similar to the result of Figure 7B, the probe analgesic potency of ENDO-2 in these mice was not reduced. These data suggest that the AAV5-hADC pretreatment protected against the acquisition of ENDO-2 analgesic tolerance. In contrast, the probe analgesic potency of ENDO-2 in AAV-hADC-treated mice that received a pre-treatment of anti-agmatine IgG was greatly reduced (Figure 7C). As a point of scientific rigor, we tested the anti-Ag IgG used in Figure 7C for reversal of agmatine-mediated inhibition of NMDA-evoked biting and scratching behaviors. NMDA (0.3 nmol) elicited biting and scratching nociceptive behaviors which were attenuated with co-administration with agmatine (10 nmol). In mice pre-treated with anti-agmatine IgG (150 ng), the inhibitory effect of agmatine was greatly diminished (Figure 7D). This outcome verified the integrity of the anti-agmatine IgG aliquot that was used for the immunoneutralization experiment. We interpret these findings to mean that the anti-agmatine IgG immunoneutralized the protective effect of the AAV5-hADC pretreatment in the development of ENDO-2 analgesic tolerance, presumably through rendering elevating agmatine unavailable to exert its effects.
4 Discussion
We have recently demonstrated that intrathecal delivery of an AAV vector containing the gene for human arginine decarboxylase (hADC) persistently reduces neuropathic pain in mice (27). The present study reveals that intrathecal delivery of the same AAV vector containing the hADC gene also prevents the acquisition of analgesic tolerance to opioids in mice. It is noteworthy that the effect was observed with two distinct opioid agonists (morphine and endomorphin-2) and with two distinct AAV serotypes.
Both neuropathic pain and opioid analgesic tolerance share a well-established dependence on the NMDA receptor/NOS cascade (30). The presumptive product of hADC, decarboxylated L-arginine or agmatine, is a known antagonist of the NMDA receptor (22, 42) and the NOS enzyme (23). We have previously shown that agmatine reduces neuropathic pain (27, 31, 32) and prevents the development of opioid analgesic tolerance (18–20). We have also demonstrated that agmatine reduces spinal long-term potentiation evoked by high frequency stimulation (27). The present observation of reduction of opioid tolerance by gene transfer of hADC is consistent with the prior reported pharmacology of agmatine.
To determine whether endogenous agmatine accounted for the prevention of opioid tolerance observed in subjects treated with the AAV-hADC, we used an immunoneutralization approach. We observed that pre-treatment with an anti-agmatine IgG (but not normal IgG) reversed the anti-opioid analgesic tolerance effect observed in AAV-hADC-treated mice. Immunoneutralization strategies using scavenging antisera have been applied extensively to characterize the actions of endogenous analgesic substances (43–45) and recently endogenous pro-nociceptive substances (46). A recent study also used an immunoneutralization approach to evaluate pharmacological actions of a presumptive endogenous NMDA receptor antagonist, serine histogranin, arising from intraspinal delivery of an AAV2/AAV8 vector carrying its synthetic gene (47). We previously demonstrated that the structure-specific anti-agmatine immunoglobulin G (anti-Ag IgG) dose-dependently reversed the pharmacological effects of exogenously applied intrathecal agmatine in the NMDA test of nocifensive scratching and biting behavior (20). Therefore, we theorized that intrathecal delivery of the anti-Ag IgG antibody would prevent the pharmacological activity of agmatine presumed to be generated by the expression of hADC. In our previous report (27) we demonstrated that hADC gene transfer reduced tactile hypersensitivity arising from nerve injury. We demonstrated that intrathecal anti-Ag IgG (but not normal IgG) transiently reversed the alleviation of mechanical allodynia observed in AAV5-hADC-treated mice. We also observed that anti-Ag IgG potentiated the magnitude of spinal long-term potentiation induced in rat by low frequency stimulation (27), presumably due to reducing the concentration of spinal agmatine available to moderate excitation. Consistent with this observation, in this present study, intrathecally delivered anti-agmatine IgG (but not normal IgG) increased the magnitude of ENDO-2 analgesic tolerance induced by high dose spinal ENDO-2. These observations support the proposal that elevated endogenous agmatine contributes to the anti-neuroplasticity effects arising from gene transfer associated with the AAV5-hADC treatment.
It is notable that agmatine robustly inhibits the development of opioid-induced tolerance (17–19), but without motor toxicity (31, 48), which is commonly associated with NMDA receptor antagonists, such as MK801. Consistent with those prior findings, AAV5-hADC-treated mice did not demonstrate altered performance in rotarod compared to control subjects at the time point assessed.
In our previous report (27) we detected hADC mRNA expression in the sensory system (e.g., spinal cord and dorsal root ganglia) and supraspinal regions (choroid plexus) following intrathecal delivery of AAV-hADC vectors. In the present study we similarly detected hADC mRNA in dorsal root ganglia, spinal cord and choroid plexus, but also in periaqueductal gray (with the hADC-AAV5 vector), a region important to opioid action. Prior studies of the biodistribution of green fluorescent protein immunoreactivity in spinal cord and DRG (29, 49, 50) suggest that the AAV5 and AAV9 serotypes used in these experiments differentially target sensory neurons. Additionally, following intrathecal delivery of either AAV5 (49) or AAV9 (50), we have observed expression of GFP in various regions of the brain, including the choroid plexus, presumably from rostral diffusion of the viral particles (49). Therefore, the mRNA expression data presented here are consistent with the prior biodistribution studies using the GFP marker following intrathecal delivery of AAV5-GFP and AAV9-GFP. We speculate that hADC expressed in sensory neurons terminating in the dorsal horn of the spinal cord elevated agmatine production that exerted an effect similar to exogenously injected agmatine in models of morphine tolerance (18). Additionally, we further speculate that the expression of hADC in epithelial cells of the choroid plexus contributed to the elevation of agmatine via release of agmatine into the cerebrospinal fluid (CSF). This could cause a broad distribution of the molecule throughout the CNS that could act in a manner comparable to that observed in prior pharmacological studies demonstrating efficacy of i.c.v.-injected agmatine in blocking morphine analgesic tolerance (19).
It has been established that co-administration of NMDA receptor antagonists with opioids prevents the development of opioid tolerance (6). This strategy has been used clinically to “reset” a patient's opioid analgesic efficacy and potency when opioid analgesic tolerance has arisen (51, 52). However, initial efforts to develop a combined preparation of opioid + NMDA receptor antagonist have not yet advanced to practice (54). As stated previously, what distinguishes agmatine from other NMDA receptor antagonists/NOS inhibitors is that it is endogenous and, therefore, may present a highly localized and effective gene therapy to counter maladaptive neuroplasticity. The present study features an application of gene transfer to sensory neurons, the midbrain, and choroid plexus epithelial cells using direct lumbar puncture. As gene therapeutics are further developed, optimized, and translated into clinical applications, intrathecal gene therapy as an adjuvant to chronic opioid pharmacotherapy may become more broadly recognized as a potentially effective pain management strategy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by University of Minnesota Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CC: Conceptualization, Formal Analysis, Project administration, Writing – original draft, Writing – review & editing, Data curation, Investigation, Methodology. CP: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. KK: Formal Analysis, Investigation, Writing – review & editing. KP: Formal Analysis, Investigation, Writing – review & editing. LB: Writing – review & editing, Conceptualization. RM: Conceptualization, Writing – review & editing, Formal Analysis. LV: Conceptualization, Formal Analysis, Writing – review & editing. GW: Formal Analysis, Writing – review & editing, Supervision. CF: Formal Analysis, Supervision, Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Resources, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the National Institute on Drug Abuse (DA025164 to CF and DA035931 to CF). NIDA training grant T32DA007097 supported CC.
Acknowledgments
We are grateful to Ms. H. Oanh Nguyen for exquisite research laboratory management that facilitated these experiments as well as the laboratory animal care teams at University of Minnesota's Research Animal Resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Martyn JAJ, Mao J, Bittner EA. Opioid tolerance in critical illness. N Engl J Med. (2019) 380:365–78. doi: 10.1056/NEJMra1800222
2. Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. (2019) 393:1558–68. doi: 10.1016/S0140-6736(19)30430-1
3. Chen L, Sein M, Vo T, Amhmed S, Zhang Y, Hilaire KS, et al. Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag. (2014) 10:383–93. doi: 10.5055/jom.2014.0235
4. Chu LF, D'Arcy N, Brady C, Zamora AK, Young CA, Kim JE, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain. (2012) 153:1583–92. doi: 10.1016/j.pain.2012.02.028
5. Lorenz M, Hussein S, Verner L. Continuous intraventricular clonidine infusion in controlled morphine withdrawal–case report. Pain. (2002) 98:335–8. doi: 10.1016/S0304-3959(02)00099-4
6. Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. (1991) 251:85–7. doi: 10.1126/science.1824728
7. Bilsky EJ, Inturrisi CE, Sadee W, Hruby VJ, Porreca F. Competitive and non-competitive NMDA antagonists block the development of antinociceptive tolerance to morphine, but not to selective mu or delta opioid agonists in mice. Pain. (1996) 68:229–37. doi: 10.1016/S0304-3959(96)03185-5
8. Elliott K, Minami N, Kolesnikov YA, Pasternak GW, Inturrisi CE. The NMDA receptor antagonists, LY274614 and MK-801, and nitric oxide synthase inhibitor, NG-nitro-L-arginine, attenuate analgesic tolerance to the mu-opioid morphine but not kappa opioids. Pain. (1994) 56:69–75. doi: 10.1016/0304-3959(94)90151-1
9. Popik P, Kolasiewicz W. Mesolimbic NMDA receptors are implicated in the expression of conditioned morphine reward. Naunyn Schmiedebergs Arch Pharmacol. (1999) 359:288–94. doi: 10.1007/PL00005354
10. Babey AM, Kolesnikov Y, Cheng J, Inturrisi CE, Trifilletti RR, Pasternak GW. Nitric oxide and opioid tolerance. Neuropharmacology. (1994) 33:1463–70. doi: 10.1016/0028-3908(94)90050-7
11. Kolesnikov YA, Pick CG, Pasternak GW. N-G-Nitro-L-arginine prevents morphine tolerance. Eur J Pharmacol. (1992) 221:399–400. doi: 10.1016/0014-2999(92)90732-J
12. Gledhill LJ, Babey AM. Synthesis of the mechanisms of opioid tolerance: do we still say NO? Cell Mol Neurobiol. (2021) 41:927–48. doi: 10.1007/s10571-021-01065-8
13. Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain [see comments]. Science. (1994) 263:966–9. doi: 10.1126/science.7906055
14. Morrissey J, McCracken R, Ishidoya S, Klahr S. Partial cloning and characterization of an arginine decarboxylase in the kidney. Kidney Int. (1995) 47:1458–61. doi: 10.1038/ki.1995.204
15. Iyer RK, Kim HK, Tsoa RW, Grody WW, Cederbaum SD. Cloning and characterization of human agmatinase. Mol Genet Metab. (2002) 75:209–18. doi: 10.1006/mgme.2001.3277
16. Piletz JE, Aricioglu F, Cheng JT, Fairbanks CA, Gilad VH, Haenisch B, et al. Agmatine: clinical applications after 100 years in translation. Drug Discov Today. (2013) 18:880–93. doi: 10.1016/j.drudis.2013.05.017
17. Kolesnikov Y, Jain S, Pasternak GW. Modulation of opioid analgesia by agmatine. Eur J Pharmacol. (1996) 296:17–22. doi: 10.1016/0014-2999(95)00669-9
18. Fairbanks CA, Wilcox GL. Acute tolerance to spinally administered morphine compares mechanistically with chronically induced morphine tolerance. J. Pharmacol. Exp. Ther. (1997) 282:1408–17.9316854
19. Kitto KF, Fairbanks CA. Supraspinally administered agmatine prevents the development of supraspinal morphine analgesic tolerance. Eur J Pharmacol. (2006) 536:133–7. doi: 10.1016/j.ejphar.2006.01.053
20. Wade CL, Eskridge LL, Nguyen HO, Kitto KF, Stone LS, Wilcox G, et al. Immunoneutralization of agmatine sensitizes mice to micro-opioid receptor tolerance. J Pharmacol Exp Ther. (2009) 331:539–46. doi: 10.1124/jpet.109.155424
21. Roberts JC, Grocholski BM, Kitto KF, Fairbanks CA. Pharmacodynamic and pharmacokinetic studies of agmatine after spinal administration in the mouse. J Pharmacol Exp Ther. (2005) 314:1226–33. doi: 10.1124/jpet.105.086173
22. Yang XC, Reis DL. Agmatine selectively blocks the N-methyl-D-aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J. Pharmacol. Exp. Ther. (1999) 288(2):544–9.9918557
23. Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J. (1996) 316:247–9. doi: 10.1042/bj3160247
24. Demady DR, Jianmongkol S, Vuletich JL, Bender AT, Osawa Y. Agmatine enhances the NADPH oxidase activity of neuronal NO synthase and leads to oxidative inactivation of the enzyme. Mol Pharmacol. (001) 59:24–9. doi: 10.1124/mol.59.1.24
25. Auguet M, Viossat I, Marin JG, Chabrier PE. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn J Pharmacol. (1995) 69:285–7. doi: 10.1254/jjp.69.285
26. Regunathan S, Reis DJ. Characterization of arginine decarboxylase in rat brain and liver: distinction from ornithine decarboxylase. J Neurochem. (2000) 74:2201–8. doi: 10.1046/j.1471-4159.2000.0742201.x
27. Peterson CD, Waataja JJ, Kitto KF, Erb SJ, Verma H, Schuster DJ, et al. Long-term reversal of chronic pain behavior in rodents through elevation of spinal agmatine. Mol Ther. (2023) 31:1123–35. doi: 10.1016/j.ymthe.2023.01.022
28. Pflepsen KR, Peterson CD, Kitto KF, Riedl MS, McIvor RS, Wilcox GL, et al. Biodistribution of adeno-associated virus serotype 5 viral vectors following intrathecal injection. Mol Pharm. (2021) 18:3741–9. doi: 10.1021/acs.molpharmaceut.1c00252
29. Vulchanova L, Schuster DJ, Belur LR, Riedl MS, Podetz-Pedersen KM, Kitto KF, et al. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol Pain. (2010) 6:31. doi: 10.1186/1744-8069-6-31
30. Mayer DJ, Mao J, Price DD. The association of neuropathic pain, morphine tolerance and dependence, and the translocation of protein kinase C. NIDA Res Monogr. (1995) 147:269–98.8742791
31. Fairbanks CA, Schreiber KL, Brewer KL, Yu CG, Stone LS, Kitto KF, et al. Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proc Natl Acad Sci USA. (2000) 97:10584–9. doi: 10.1073/pnas.97.19.10584
32. Peterson CD, Kitto KF, Verma H, Pflepsen K, Delpire E, Wilcox GL, et al. Agmatine requires GluN2B-containing NMDA receptors to inhibit the development of neuropathic pain. Mol Pain. (2021) 17:17448069211029171. doi: 10.1177/17448069211029171
33. Seybold VS, Coicou LG, Groth RD, Mermelstein PG. Substance P initiates NFAT-dependent gene expression in spinal neurons. J Neurochem. (2006) 97:397–407. doi: 10.1111/j.1471-4159.2006.03744.x
34. Janssen PA, Niemegeers CJ, Dony JG. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittelforschung. (1963) 13:502–7.13957426
35. Tallarida RJ, Murray RB. Manual of pharmacological calculations with computer programs. New York: Springer Verlag (1987). p. 26–31.
36. Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. (1980) 67:313–6. doi: 10.1016/0014-2999(80)90515-4
37. Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. (2003) 55:1007–41. doi: 10.1016/S0169-409X(03)00101-7
38. Pflepsen KR, Peterson CD, Kitto KF, Vulchanova L, Wilcox GL, Fairbanks CA. Detailed method for intrathecal delivery of gene therapeutics by direct lumbar puncture in mice. Methods Mol Biol. (2019) 1937:305–12. doi: 10.1007/978-1-4939-9065-8_20
39. Aanonsen LM, Wilcox GL. Phencyclidine selectively blocks a spinal action of N-methyl-D-aspartate in mice. Neurosci Lett. (1986) 67:191–7. doi: 10.1016/0304-3940(86)90396-4
40. Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. (1997) 386:499–502. doi: 10.1038/386499a0
41. Stone LS, Fairbanks CA, Laughlin TM, Nguyen HO, Bushy TM, Wessendorf MW, et al. Spinal analgesic actions of the new endogenous opioid peptides endomorphin-1 and -2. Neuroreport. (1997) 8:3131–5. doi: 10.1097/00001756-199709290-00025
42. Reynolds IJ. Arcaine uncovers dual interactions of polyamines with the N-methyl-D-aspartate receptor. J Pharmacol Exp Ther. (1990) 255:1001–7.2148185
43. Ohsawa M, Mizoguchi H, Narita M, Nagase H, Kampine JP, Tseng LF. Differential antinociception induced by spinally administered endomorphin-1 and endomorphin-2 in the mouse. J Pharmacol Exp Ther. (2001) 298:592–7.11454920
44. Tseng LF, Narita M, Suganuma C, Mizoguchi H, Ohsawa M, Nagase H, et al. Differential antinociceptive effects of endomorphin-1 and endomorphin-2 in the mouse. J Pharmacol Exp Ther. (2000) 292:576–83.10640294
45. Vanderah TW, Lai J, Yamamura HI, Porreca F. Antisense oligodeoxynucleotide to the cckb receptor produces naltrindole- and [leu(5)]enkephalin antiserum-sensitive enhancement of morphine antinociception. Neuroreport. (1994) 5:2601–5. doi: 10.1097/00001756-199412000-00049
46. Fairbanks CA, Peterson CD, Speltz RH, Riedl MS, Kitto KF, Dykstra JA, et al. The VGF-derived peptide TLQP-21 contributes to inflammatory and nerve injury-induced hypersensitivity. Pain. (2014) 155:1229–37. doi: 10.1016/j.pain.2014.03.012
47. Jergova S, Gordon CE, Gajavelli S, Sagen J. Experimental gene therapy with serine-histogranin and endomorphin 1 for the treatment of chronic neuropathic pain. Front Mol Neurosci. (2017) 10:406. doi: 10.3389/fnmol.2017.00406
48. Nguyen HO, Goracke-Postle CJ, Kaminski LL, Overland AC, Morgan AD, Fairbanks CA. Neuropharmacokinetic and dynamic studies of agmatine (decarboxylated arginine). Ann N Y Acad Sci. (2003) 1009:82–105. doi: 10.1196/annals.1304.009
49. Schuster DJ, Belur LR, Riedl MS, Schnell SA, Podetz-Pedersen KM, Kitto KF, et al. Supraspinal gene transfer by intrathecal adeno-associated virus serotype 5. Front Neuroanat. (2014) 8:66. doi: 10.3389/fnana.2014.00066
50. Schuster DJ, Belur LR, Riedl MS, Schnell SA, Podetz-Pedersen KM, Kitto KF, et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat. (2014) 8:42. doi: 10.3389/fnana.2014.00042
51. Quinlan J. The use of a subanesthetic infusion of intravenous ketamine to allow withdrawal of medically prescribed opioids in people with chronic pain, opioid tolerance and hyperalgesia: outcome at 6 months. Pain Med. (2012) 13:1524–5. doi: 10.1111/j.1526-4637.2012.01486.x
52. Prommer EE. Ketamine for pain: an update of uses in palliative care. J Palliat Med. (2012) 15:474–83. doi: 10.1089/jpm.2011.0244
53. Galer BS, Lee D, Ma T, Nagle B, Schlagheck TG. Morphidex (morphine sulfate/dextromethorphan hydrobromide combination) in the treatment of chronic pain: three multicenter, randomized, double-blind, controlled clinical trials fail to demonstrate enhanced opioid analgesia or reduction in tolerance. Pain. (2005) 115:284–95. doi: 10.1016/j.pain.2005.03.004
Keywords: AAV5, agmatine, human arginine decarboxylase, intrathecal, opioid tolerance, AAV9, NMDA receptor antagonism
Citation: Churchill CC, Peterson CD, Kitto KF, Pflepsen KR, Belur LR, McIvor R.S, Vulchanova L, Wilcox GL and Fairbanks CA (2024) Adeno-associated virus-mediated gene transfer of arginine decarboxylase to the central nervous system prevents opioid analgesic tolerance. Front. Pain Res. 4:1269017. doi: 10.3389/fpain.2023.1269017
Received: 28 July 2023; Accepted: 19 December 2023;
Published: 9 February 2024.
Edited by:
Stanislava Jergova, University of Miami, United StatesReviewed by:
Christopher Michael Peters, Wake Forest University, United StatesOhannes K. Melemedjian, University of Maryland, United States
© 2024 Churchill, Peterson, Kitto, Pflepsen, Belur, McIvor, Vulchanova, Wilcox and Fairbanks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolyn A. Fairbanks Y2FyZmFpckB1bW4uZWR1
 Caroline C. Churchill
Caroline C. Churchill Cristina D. Peterson
Cristina D. Peterson Kelley F. Kitto
Kelley F. Kitto Kelsey R. Pflepsen
Kelsey R. Pflepsen Lalitha R. Belur
Lalitha R. Belur R. Scott McIvor
R. Scott McIvor Lucy Vulchanova
Lucy Vulchanova George L. Wilcox
George L. Wilcox Carolyn A. Fairbanks
Carolyn A. Fairbanks