
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pain Res. , 06 October 2023
Sec. Pediatric Pain
Volume 4 - 2023 | https://doi.org/10.3389/fpain.2023.1244609
This article is part of the Research Topic Insight in Pediatric Pain – 2023 View all 5 articles
 Amanda Cao1
Amanda Cao1 Raquel van Gool1
Raquel van Gool1 Emma Golden1
Emma Golden1 Benjamin Goodlett2
Benjamin Goodlett2 Carlos Camelo1
Carlos Camelo1 Simona Bujoreanu1
Simona Bujoreanu1 Walla Al-Hertani2
Walla Al-Hertani2 Jaymin Upadhyay1,3*
Jaymin Upadhyay1,3*
Pompe disease (PD) is a rare inherited metabolic disorder of deficient or absent acid alpha-glucosidase (GAA), resulting in defective lysosomal glycogen catabolism. Muscle weakness, respiratory deficiency and gastrointestinal symptoms are commonly monitored in PD. However, pain and associated psychological symptoms are less focused upon. A pediatric patient with late-onset Pompe disease (LOPD) comorbid with chronic pain is presented. Symptoms of pain in the feet were first reported between 6 and 7 years of age and were attributed to growing pains. Following progression of lower body pain, weakness, fatigue, and difficulties with ambulation, a thorough clinical assessment including genetic testing was performed, which led to a diagnosis of LOPD at 9 years of age. ERT with recombinant human alglucosidase alfa was subsequently started. The patient’s clinical status is compounded by depressed mood, anxiety, and attention deficit hyperactivity disorder, which may further exacerbate pain. A multidisciplinary pain treatment approach consisting of orthopedics, physical therapy, and psychosocial therapy aimed at enhancing pain coping skills is described for this LOPD patient. This case highlights the need for a greater understanding of pain generation and identification of optimized pain treatment approaches in children with LOPD that can be implemented alongside ERT.
Pompe disease results from an autosomal recessive mutation in the acid alpha glucosidase (GAA) gene (1). This mutation yields deficient or absent GAA, which results in glycogen accumulation, particularly in skeletal, cardiac, and smooth muscles, but also within the central nervous system (2–5). Patients with late-onset Pompe disease (LOPD) present with symptoms after the first year of life, typically demonstrating weakness in the limb-girdle, lower extremity, and trunk muscles (6). LOPD patients are often treated with enzyme-replacement therapy (ERT), exerting beneficial effects on the clinical course and survival (7–9). However, patients may still experience a range of physical and centrally based symptoms (10, 11). We present a patient with LOPD and a history of pain that initiated during early childhood.
This investigation was approved by the BCH Institutional Review Board (IRB). Informed consent was provided by the patient’s legal guardian and patient.
A pediatric patient diagnosed with LOPD presented with bilateral, lower body pain (Figure 1). Pain was first noted at approximately 6 years of age and localized to their feet. Pain was considered a result of normal growing pains by the patient’s primary care provider. During this period, the patient also demonstrated signs of disruptive behavior (diagnoses of attention deficit hyperactivity disorder/ADHD, Oppositional Defiant Disorder/ODD, and Disruptive Mood Dysregulation Disorder/DMDD) in school and was prescribed Dexmethylphenidate, Risperidone for behavior and mood regulation, and Clonidine for sleep. The patient’s pain progressively worsened over 2 years, later affecting the bilateral anterior pelvis, knees, and feet, resulting in inability to stand for extended periods of time on hard surfaces or take part in physical activity (i.e., currently, needs to rest after standing on their feet for 1 h). After extensive clinical workup and genetic testing, a diagnosis of LOPD was confirmed at 9 years of age. Radiographic examination and musculoskeletal magnetic resonance imaging (MRI) of the lumbar spine and lower limbs were unremarkable for any bony lesions, joint abnormalities, nerve impingements or soft tissue lesions. Neuroimaging was also unremarkable.
Laboratory tests showed elevated creatine kinase (CK; 1558 units/L, Normal Range: < 177 units/L), Hexose tetrasaccharide (Hex4; 14.5 mmol/mol creatinine, Normal Range: <4 mmol/mol creatinine), and Alpha Glucosidase (2.70 pmol/punch/h, Normal Range: 10.88 pmol/punch/hour). Through genetic testing, two heterozygous pathologic variants were identified (c.−32-13T > G (Intronic) and c.525del (p.Glu176Argfs*45) in GAA. ERT with recombinant human alglucosidase alfa (1400 mg IV every two weeks, 20 mg/kg) was initiated shortly after the patient's LOPD diagnosis after significant symptom presentation affecting muscle strength and function, pulmonary function (coughing, wheezing, shortness of breath), and other patient reported outcomes (12). At the time of evaluation, the patient had been receiving ERT for 22 months.
The patient’s mother sought additional clinical evaluation of the chronic pain. During a three-day examination period, the patient endorsed considerable pain, confirmed by the mother’s input, between the pelvis and feet, with pain severity experienced slightly more in the right compared to the left leg. The patient reported a pain level (0–10 scale) of 5 between hips and knees, a pain rating of 6 in the right ankle, and a pain rating of 9 in the right foot. Table 1 provides an overview of the presentation of pain [i.e., the Pain Frequency-Severity-Duration Scale (13)], as well as the psychological viewpoint of pain [i.e., the Pain Catastrophizing Scale and Fear of Pain Questionnaire (14, 15)]. The patient noted that pain is exacerbated when walking, standing, or sitting upright for prolonged periods (e.g., several minutes to an hour), but subsides with rest. Pain within both ankles and feet was described as aching, sharp, stabbing, and throbbing and at a level of 9. Pain localized to the bilateral anterior pelvis was described as sharp and ranged between 6 and 9. Presence of skin discoloration or tactile hyper- or hyposensitivity were absent in lumbar spine and lower limbs. However, mild bilateral trochanter and Achilles tendon tenderness was observed upon palpitation. Bilateral tightness of the Achilles tendon, bicep femoris, iliotibial band, and hip flexors was evident. At the time of the evaluation, pharmacological pain treatment included gabapentin (400 mg TID), baclofen (10 mg OD), and amitriptyline (25 mg OD).
Pain along with fatigue and muscle weakness, and the need to utilize assistive devices for ambulation has frequently limited or prohibited the patient from taking part in everyday physical activities at home or with peers at school—an aspect of the condition which has historically led to feelings of distress, embarrassment, irritability, social isolation, and low self-esteem. Additionally, the patient noted that physical symptoms were often elevated just prior to the bi-weekly ERT infusions.
In addition to the pain profile for this patient, upper and lower extremity motor functioning were assessed using the Patient-Reported Outcome Measurements Information System (PROMIS) questionnaires and the National Institutes of Health (NIH) Toolbox Motor Battery, respectively (Figure 2). The patient scored lower than the normative mean by approximately 1 standard deviation (SD) on upper (9-Hole Pegboard Dexterity Test and grip strength tests) and lower (2 min Walk Endurance Test and 4 m Walk Gait Test) extremity tasks. During upper extremity assessments, the patient showed little to no fatigue. However, for the lower extremity tasks, during the 2 min Walk Endurance Test and the Standing Balance Test, both pain and fatigue were evoked.
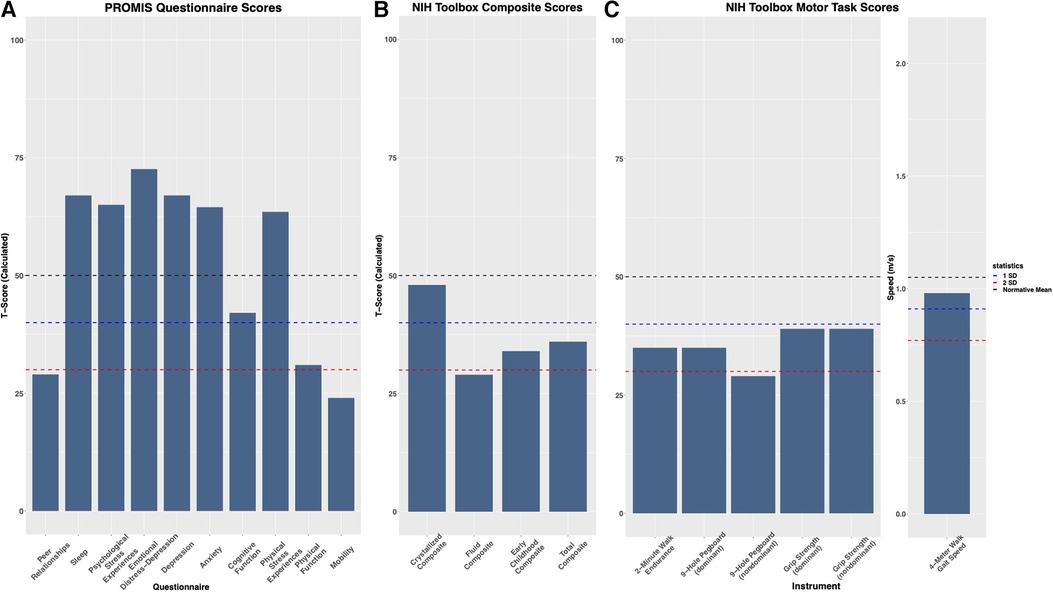
Figure 2. Evaluation of psychological and physical factors: (A) the patient shows higher level of depression, stress, anxiety, and sleep disturbances, and reduced quality of peer relationships, physical functioning, cognitive functioning, and mobility compared to the general population when assessed by patient-reported outcomes measurement information system (PROMIS) questionnaires. (B) On the NIH Toolbox Cognition Battery, the Total Composite Score and Early Childhood Composite scores factor in the Fluid Composite and Crystalized Composite Score to assess general cognitive function. For NIH Toolbox, T scores were corrected for gender, age, ethnicity, and education level. (C) Assessment of motor functioning utilizing the NIH Toolbox Motor Battery, evaluating dominant and nondominant function where accessible. On the 4 m Walk Gait Test, the patient scored (0.98 m/s) slightly below the age and gender-corrected mean (1.05 m/s).
The patient presented to the multidisciplinary clinic evaluation with a history of ADHD, ODD, and DMDD from six years of age. At the time of this evaluation, the mother reported that the patient had outgrown the ODD and DMDD diagnoses and was only treated with medication for ADHD and sleep. Based on the evaluation, the patient received a diagnosis of major depressive disorder (MDD), Generalized Anxiety Disorder (GAD), and Psychological and Behavioral Factors Affecting the Medical Condition, in addition to the child’s prior diagnosis of ADHD. Results on the PROMIS questionnaires further demonstrated elevated levels of depression, stress, anxiety, and sleep disturbances, along with reduced quality of peer relationships and cognitive functioning (Figure 2). The Total Composite and Early Childhood Composite score on the NIH Toolbox Cognition Battery was approximately 1 SD below the normative mean. The Fluid Composite T score, informing on problem-solving, reasoning, and memory encoding, was more than 2 SD below the normative mean. Treatment for the patient’s psychological symptoms includes dexmethylphenidate XR (40 mg OD, on school days only), sertraline (25 mg OD), clonidine (0.2 mg OD) for sleep, and psychotherapy (at the time of the multidisciplinary clinic evaluation, the bi-monthly psychotherapy was on hold due to logistical and health-care access barriers).
Psychological functioning was conceptualized through a biopsychosocial lens, to account for multiple contributing factors including physical/biological, psychological, and social, as well as developmental age, all likely contributing to the patient’s experience of and reaction to pain. The patient endures a severe and chronic illness that frequently imposes functional limitations leads to impairment across all relevant areas of life (school, peers, extracurricular activities, self-care, sleep, and family relationships and dynamics). In addition, their neurocognitive profile of ADHD and associated cognitive findings are likely to make them more susceptible than peers to feeling overwhelmed in the face of complex information and processing associated with the pain experience, pain management, and living with a chronic and potentially life limiting illness. Developmentally, this patient is at an age where the impact of medical issues can impact the sense of being a strong and healthy youth and further take a toll emotionally and socially by interfering with the achievement of developmentally appropriate milestones. Also, in relation to the young age, this patient presented with passive coping style (relying on distraction or asking for mother to do things for them), which becomes an additional vulnerability for emotional struggles in association with a lack of control over their own wellbeing.
The patient’s pain in its current form is considered multifactorial, and thus a multidisciplinary pain treatment approach has been recommended. A rehabilitative pain treatment which combines physical and psychological therapies and focuses on improving function (as opposed to eliminating pain) was considered optimal for this patient (16). The biopsychosocial or functional-restoration approach entails a re-training of the nervous system to subside afferent drive or lower pain, and equally important, provide the patient with behavioral tools and strategies to enhance the mind-body interface, learn coping skills, regulate emotional and behavioral regulation, and improve communication with care providers. Learning strategies such as pacing activities are important parts of this process and can be incorporated as part of physical therapy treatment. A Cognitive Behavioral Therapy (CBT) strategy inclusive of deep breathing, guided imagery, and stress reduction was also recommended (17, 18). In parallel, considering biomechanical factors (i.e., tightness in the lower extremities), orthotic support for the feet and physical therapy involving aquatic therapy, home-based exercise, and a stretching routine with gradual pacing were indicated. No changes in the patient’s pharmacological treatment regimen were recommended.
Herein, a pediatric patient with LOPD and severe persistent pain as well as cognitive and physical difficulties is described. Upon receiving a LOPD diagnosis and being placed on ERT, improvements in overall clinical status and physical functioning were noted, yet the patient’s pain as well as fatigue continue despite utilization of pharmacological (analgesics). Specifically, symptoms of pain as well as physical disability have greatly reduced quality of life, negatively impacting the child’s experience and performance at school, and introducing challenges in terms of taking part in social activities or developing social interactions. Further complicating the patient’s overall clinical status is a diagnosis of depression and anxiety, which may stem from living with a severe chronic illness, psychosocial limitations, presence of persistent pain, or environmental factors. While any conclusions on any causal interactions among pain and depression or anxiety are difficult to make, we propose that the current patient and likely others that present with a similar phenotype are prone to a cyclical and persistent allosteric load that can involve maladaptive neurobiological and physiological processes (19–22). Moreover, although the current patient’s pain-related symptoms and physical exam findings are not specific to LOPD, this case highlights the importance of early work up when presentation of pain or pain interference occurs alongside other signs and symptoms such as progressive muscle weakness.
Notably, the patient received an ADHD diagnosis at a young age and near the time that he first reported pain, which was initially solely attributed to growing pains. We speculate that a child’s early reports of pain likely warranted careful focus, specifically in relation to difficulties with attention or psychological presentation in general. Chronic pain has an interruptive effect on executive functioning, which, in school-age children, is frequently manifested in academic settings (23, 24). As such, pediatric pain may, in some instances, be the hidden cause behind behavioral and academic difficulties, specifically those attributed to problems with attention. Correct diagnosis and early intervention are important, more so in rare diseases and particularly when symptoms present during childhood development. Furthermore, Barr and colleagues (25, 26) hypothesized that early exposure to pain may redirect neurodevelopment in a way that renders an individual vulnerable to pain. This may likely contribute to one’s perception of pain in ways similar to that of the patient described here; mainly, high levels of pain catastrophizing and an increased fear of pain. Given the rarity of LOPD, accurate diagnoses of this illness may be difficult in the primary care setting and treating related symptoms. Additionally, providing incorrect diagnoses or inaccurate causes of symptoms likely further delay specialist evaluation. Significant diagnostic delays during childhood may be detrimental for disease progression but also increase the chances of developing physical and psychosocial symptoms that could potentially be mitigated with early and optimize treatment intervention.
As with many rare diseases, however, the focus of therapeutic interventions is rightfully placed on cardinal features of the disease. However, comorbid conditions such as chronic pain or neuropsychiatric manifestations may in some cases receive less attention despite their interference with physical or psychosocial health. Although longitudinal progress or treatment outcomes are unavailable at this time for this patient, we hope to underline in this investigative case the complexities regarding the spectrum of pain symptoms patients with Pompe disease may experience as well as the urgency for more focused attention to mental and physical health issues that may worsen the patients' experience with pain. Finally, while an immature neurological system in children with LOPD may on one hand be vulnerable to pathobiological processes either because of direct insult or a downstream effect, the neurodevelopmental status may in some cases, represent a therapeutic window for preventing or reversing maladaptive process that underpin conditions such as chronic pain.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This investigation was approved by the BCH Institutional Review Board (IRB). Informed consent was obtained from the patient’s legal guardian and the patient for the publication of this case report (including all text, images, and tables).
JU, AC, and BG contributed to conceptualization of the study. JU and WA acquired funding. JU and AC designed methodology. JU acquired resources and JU and WA supervised the research investigation. JU, AC, e.g., BG, CC, SB, and WA contributed to the investigation process. AC, RV, e.g., BG, CC, and SB curated the data, and AC and RV conducted formal analysis. AC and RV wrote the original draft. JU, e.g., BG, CC, SB, and WA reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
JU and WA receive funding support from SanofiGenzyme. WA is a site Principal Investigator for the Early Access Program with Arimoclomol in US Patients with NPC (NCT04316637), which is sponsored by KemPharm Denmark A/S WA is on the advisory board for Beam Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Reuser AJJ, Hirschhorn R, Kroos MA. Pompe disease: glycogen storage disease type II, acid α-glucosidase (acid maltase) deficiency. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA, editors. The online metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill Education (2019).
2. Bhengu L, Davidson A, du Toit PJ, Els C, Gerntholtz T, Govendrageloo K, et al. Diagnosis and management of pompe disease. S Afr Med J. (2014) 104(4):273–4. doi: 10.7196/samj.7386
3. Kohler L, Puertollano R, Raben N. Pompe disease: from basic science to therapy. Neurotherapeutics. (2018) 15(4):928–42. doi: 10.1007/s13311-018-0655-y
4. DeRuisseau LR, Fuller DD, Qiu K, DeRuisseau KC, Donnelly WH Jr., Mah C, et al. Neural deficits contribute to respiratory insufficiency in pompe disease. Proc Natl Acad Sci U S A. 2009;106(23):9419–24. doi: 10.1073/pnas.0902534106
5. Mancall EL, Aponte GE, Berry RG. Pompe’s disease (diffuse glycogenosis) with neuronal storage. J Neuropathol Exp Neurol. (1965) 24:85–96. doi: 10.1097/00005072-196501000-00008
6. Tarnopolsky M, Katzberg H, Petrof BJ, Sirrs S, Sarnat HB, Myers K, et al. Pompe disease: diagnosis and management. Evidence-based guidelines from a Canadian expert panel. Canadian Journal of Neurological Sciences / Journal Canadien des Sciences Neurologiques. (2016) 43(4):472–85. doi: 10.1017/cjn.2016.37
7. Güngör D, Kruijshaar ME, Plug I, Rizopoulos D, Kanters TA, Wens SC, et al. Quality of life and participation in daily life of adults with pompe disease receiving enzyme replacement therapy: 10 years of international follow-up. J Inherit Metab Dis. (2016) 39(2):253–60. doi: 10.1007/s10545-015-9889-6
8. Güngör D, Kruijshaar ME, Plug I, D’Agostino RB, Hagemans MLC, van Doorn PA, et al. Impact of enzyme replacement therapy on survival in adults with pompe disease: results from a prospective international observational study. Orphanet J Rare Dis. (2013) 8(1):49. doi: 10.1186/1750-1172-8-49
9. Winkler M, von Landenberg C, Kuchenbecker K, Reimann J, Kornblum C. Long-term effects of enzyme replacement therapy in an elderly cohort of late-onset pompe disease. Neuromuscul Disord. (2022) 32(3):195–205. doi: 10.1016/j.nmd.2022.01.001
10. Tucker-Bartley A, Lemme J, Gomez-Morad A, Shah N, Veliu M, Birklein F, et al. Pain phenotypes in rare musculoskeletal and neuromuscular diseases. Neurosci Biobehav Rev. (2021) 124:267–90. doi: 10.1016/j.neubiorev.2021.02.009
11. Korlimarla A, Lim JA, Kishnani PS, Sun B. An emerging phenotype of central nervous system involvement in pompe disease: from bench to bedside and beyond. Ann Transl Med. (2019) 7(13):289. doi: 10.21037/atm.2019.04.49
12. Schoser B., Laforêt P. 208th ENMC international workshop: formation of a European network to develop a European data sharing model and treatment guidelines for pompe disease naarden, The Netherlands, 26–28 September 2014. (2015) 25(8):674–8.25998612
13. SSkco K,aG9iYXJ0QHV3bS5lZHU=WHD, RFmro M, JWsco S, RHkco K. The pain frequency-severity-duration scale as a measure of pain: preliminary validation in a pediatric chronic pain sample. Pain Res Treat. (2014) 2014.
14. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7(4):524–32. doi: 10.1037/1040-3590.7.4.524
15. Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The fear of pain questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. J Pain. (2011) 12(6):677–86. doi: 10.1016/j.jpain.2010.12.008
16. Randall ET, Smith KR, Conroy C, Smith AM, Sethna N, Logan DE. Back to living: long-term functional Status of pediatric patients who completed intensive interdisciplinary pain treatment. Clin J Pain. (2018) 34(10):890–9. doi: 10.1097/AJP.0000000000000616
17. Chow ET, Otis JD, Simons LE. The longitudinal impact of parent distress and behavior on functional outcomes among youth with chronic pain. J Pain. (2016) 17(6):729–38. doi: 10.1016/j.jpain.2016.02.014
18. Coakley R, Wihak T. Evidence-based psychological interventions for the management of pediatric chronic pain: new directions in research and clinical practice. Children (Basel. (2017) 4(2).28165415
19. McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metab Clin Exp. (2010) 59(Suppl 1):S9–15. doi: 10.1016/j.metabol.2010.07.012
20. Vachon-Presseau E. Effects of stress on the corticolimbic system: implications for chronic pain. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 87(Pt B):216–23. doi: 10.1016/j.pnpbp.2017.10.014
21. Rabey M, Moloney N. “I don't know why i've got this pain!” allostasis as a possible explanatory model. Phys Ther. (2022) 102(5):pzac017. doi: 10.1093/ptj/pzac017
22. Nelson S, Bento S, Enlow MB. Biomarkers of allostatic load as correlates of impairment in youth with chronic pain: an initial investigation. Children (Basel). (2021) 8(8).34438600
23. Moore DJ, Keogh E, Eccleston C. The interruptive effect of pain on attention. Quarterly Journal of Experimental Psychology. (2012) 65(3):565–86. doi: 10.1080/17470218.2011.626865
24. Beckmann EA, Jastrowski Mano KE. Advancing the measurement of executive functioning in pediatric chronic pain. Children. (2021) 8(8):630. doi: 10.3390/children8080630
25. Barr GA, Hunter DA. Interactions between glia, the immune system and pain processes during early development. Dev Psychobiol. (2014) 56(8):1698–710. doi: 10.1002/dev.21229
Keywords: pompe disease, lysosomal storage disease, pain, analgesia, depression, ADHD
Citation: Cao A, van Gool R, Golden E, Goodlett B, Camelo C, Bujoreanu S, Al-Hertani W and Upadhyay J (2023) Case report: Chronic pain in a pediatric patient with late-onset pompe disease. Front. Pain Res. 4:1244609. doi: 10.3389/fpain.2023.1244609
Received: 27 June 2023; Accepted: 21 September 2023;
Published: 6 October 2023.
Edited by:
Edoardo Malfatti, Hôpitaux Universitaires Henri Mondor, FranceReviewed by:
Erin E. Young, University of Kansas School of Medicine, United States© 2023 Cao, van Gool, Golden, Goodlett, Camelo, Bujoreanu, Al-Hertani and Upadhyay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaymin Upadhyay amF5bWluLnVwYWRoeWF5QGNoaWxkcmVucy5oYXJ2YXJkLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.