- 1N4 Solutions, LLC., Greenbelt, MD, United Stated
- 2National Institute of Neurological Disorders and Stroke, Office of Pain Policy and Planning, Bethesda, MD, United Stated
The National Institutes of Health and its independent advisors recognize the need to develop a strong pain research workforce and provide opportunities, particularly for clinicians, to pursue research careers. A survey was conducted to better understand the challenges facing the clinical pain research community. Respondents reported that time and funding to pursue research were the most critical factors either enabling or holding them back from a research career. Respondents who received some kind of formal research training or mentorship were more likely than those who did not to have federal research funding and to be at more advanced stages of their careers. The findings point to a need for all stakeholders in the pain research community to help formalize research training and provide funding or protected time to support the ambitions of aspiring researchers.
1. Introduction
In 2011, the Institute of Medicine issued a landmark report (1) on pain care in the United States, including a chapter devoted to research challenges. Among the points made by the report were that pain research needs more scientists from diverse disciplines and across basic, clinical, behavioral and social backgrounds. The authors recommended increasing the training of pain researchers, including through training grants from the National Institutes of Health (NIH), specifically advocating for pre- and postdoctoral fellows and junior investigators to promote pain research education.
In 2020, the Interagency Pain Research Coordinating Committee (IPRCC) discussed the promotion of a new generation of pain researchers at its November meeting (2).
Among IPRCC members' ideas for promoting a new generation of pain researchers was the suggestion to survey clinical and basic pain researchers to identify what factors could advance their interest in research rather than, for example, push them towards private practice.
The medical research community has been aware of challenges facing clinician-scientists for some time. In 2014, a working group convened by the NIH to make recommendations that could strengthen the physician-scientist workforce identified several challenges (3), including: the uncertainty of funding, the structure of training, debt, a poor work-life balance, a need for multiple mentors and pressure to increase institutional revenue through patient care.
The factors affecting participation and progression in clinical research range from personal (e.g., compensation, social capital, and confidence), orientation toward certain roles (e.g., preferences for administration, clinical care, and education) to interpersonal (e.g., mentorship and discriminatory behavior), to organizational (e.g., academic and clinical workplace culture) and policies (e.g., availability of mentors and active support for advancing careers) (4). There also can be challenges to finding a position that would help one gain clinical research experience without already having clinical research experience (5).
Some published literature has specifically examined the issue as it relates to pain research, but while pain management is a multidisciplinary field, the discourse on workforce development has primarily focused on anesthesiologists (6,7). One study (8) on the challenges facing the anesthesiology workforce notes that while protected time to develop research skills and conduct research is essential, the receipt of an NIH grant meant to support research training was rare. Other (9) obstacles that have been cited for pain researchers include financial challenges (financial disincentives to pursue research, as well as debt), the existence of adequate mentorship and the acquisition of research skills.
In response to the IPRCC's recommendation, the Office of Pain Policy and Planning (OPPP) within the National Institute of Neurological Disorders and Stroke (NINDS) developed a survey to examine the reasons why those with clinical degrees and an interest in pain might or might not embark on a career in clinical pain research.
We sought to expand on the currently published literature to include the broader multidisciplinary backgrounds that make up the clinical pain research workforce, which also includes nurses, dentists, psychologists, physical therapists and those from many other distinct medical fields.
2. Materials and methods
2.1. Sample
The survey population included pain clinicians or pain researchers across the career spectrum. Respondents could opt-in to taking the survey through non-specific invitations distributed across the pain research community through different pain management and research organizations: American Academy of Pain Medicine; American Psychological Association; American Society of Anesthesiologists; American Society of Regional Anesthesia and Pain Medicine; Foundation for Anesthesia Education and Research; Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials(IMMPACT)/Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks(ACTTION); Kaui'i Pain Conference; NIH Pain Consortium; Pain Research Forum; U.S. Association for the Study of Pain (USASP); and the Veterans Health Administration. There were no inclusion or exclusion criteria for participation in this survey.
2.2. Dissemination and data collection
The survey responses were collected in three waves. The first version of the survey was administered to attendees of the Kaua'i Pain Conference in March, 2021, using the Digitell platform (10). The second version of the survey was sent to members of the USASP. USASP leadership sent its members an invitation and link to the survey. The survey was accessible to anyone with access to the link. The final version of the survey was sent in a similar fashion to members of the aforementioned pain management and research organizations. Once again, anybody with access to the link was able to take the survey.
The survey was hosted on the SurveyMonkey platform. While SurveyMonkey prevents the same user from completing the survey multiple times if they use the same browser, and a question was included to help screen out respondents who had taken it previously, there was no way to ensure that there were not multiple responses from the same individual.
All survey responses were anonymous. The NINDS Office of Science Policy and Planning (OSPP) determined this initiative's activities did not qualify as research requiring protections for human participants and did not require Institutional Review Board (IRB) review.
2.3. Survey
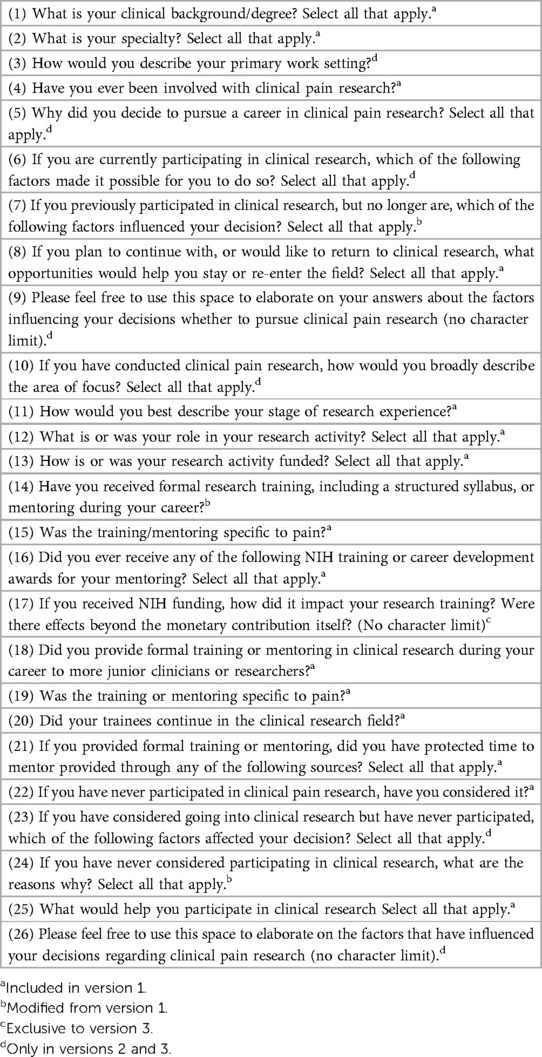
The final version of the survey had 29 total questions, with respondents answering a minimum of 10 questions and a maximum of 22 questions. Two earlier versions of the survey had fewer questions.
For a full list of questions asked in the final version of the survey, see Table 1. The table notes which questions were asked in the initial version of the survey and the differences between the second and final versions of the survey.
The questions were developed by the NIH OPPP with feedback from the NINDS Office of Science Policy and Planning, and a group of experienced clinical pain investigators from outside of NIH.
2.4. Analysis
Responses were aggregated using Microsoft Excel and R, with overall response data and crosstabulations produced using R. The analysis was based on responses stratified by the following variables: whether the respondent had ever been involved in clinical pain research; stage of research experience; receipt of formal research training or mentoring; and providing formal research training or mentoring. Significance tests were done using two-proportion Z-tests conducted in Microsoft Excel.
3. Results
A total of 433 responses were collected: 105 from attendees of Kaua'i Pain Conference, 120 from the US Association for the Study of Pain, and 208 from the broader clinical pain and pain research community. The final analysis included 430 responses, after some incomplete responses were excluded from the data set. Many questions permitted respondents to select all answers that could apply, and for some questions, the authors are reporting only the top responses, so in those cases the percentages reported in this paper might not add up to 100%.
3.1. Respondent profile
Among the respondents (Table 2), the most common degrees were MD (41%), PhD (20%), clinical PhD (17%), RN (6%) and PsyD (5%). The most common specialties were pain (48%), psychology (26%) and anesthesiology (18%). The most common work settings were universities (22%), teaching institutions (21%) and government hospitals (19%).
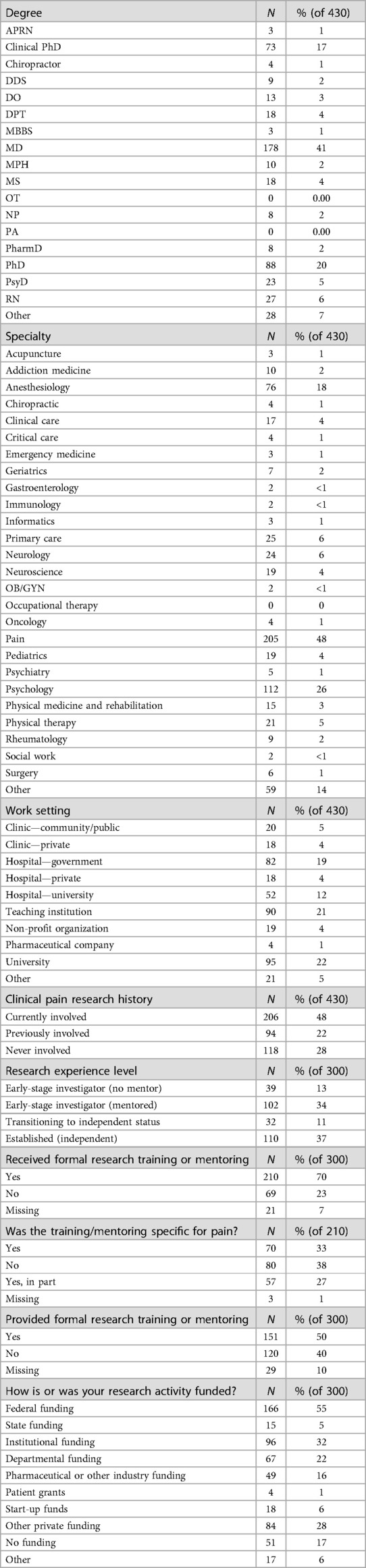
Table 2. Characteristics of survey respondents by degree, work setting, pain research experience and funding.
Most respondents reported some involvement with clinical pain research: 48% reported current involvement with clinical pain research, 22% were previously involved with clinical pain research, and 28% were never involved in clinical pain research.
Among those with research experience (n = 300), 37% were established, independent investigators; 11% were transitioning to independent status; 34% were early investigators with mentors, and 13% were early investigators with no mentor. Most respondents who identified as researchers (70%) received formal research training or mentoring, with a further 60% of those respondents reporting that the training or mentoring was related to pain.
Among the different work settings, a significantly larger proportion of respondents were engaged in research at teaching institutions compared to the overall sample: 31% to 21%, z = 2.75, p = .006) or universities (50% to 34%, z = 3.87, p < .001) were engaged in research when compared to the overall sample.
3.2. Funding for research
Federal dollars were the most common source of research funding, with 55% of those with research experience (n = 300) receiving federal funding. The next most common sources were institutional (32%) and other private funding (28%). Among those with research experience, 28% of respondents saying that they had received an NIH award meant for early-career researchers, mentored research projects or pre- and post-doctoral training.
3.3. Motivation for research
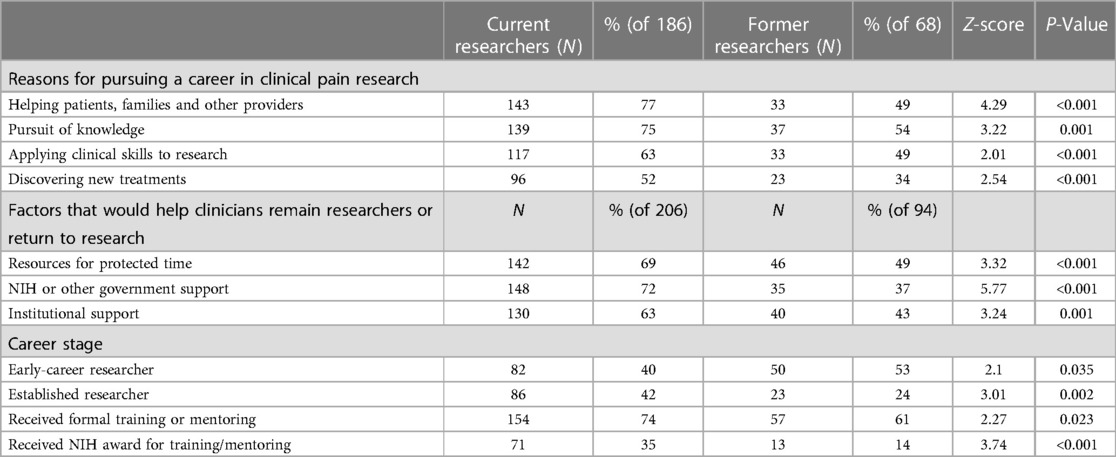
In response to the question, “Why did you decide to pursue a career in clinical pain research,” the top responses among all respondents who had ever been involved in research were: helping patients, families and other providers (69%); the pursuit of knowledge (69%); applying clinical skills to research (59%) and discovering new treatments (47%). However, there were differences between current researchers and former researchers in this regard (Table 3). Pursuit of knowledge was a reason for 75% of current researchers, compared to 54% of former researchers, z = 3.22, p = 0.001. While 77% of current researchers said that “helping patients, families and other providers” was a factor, just 49% of former researchers included this as a factor, z = 4.29, p < 0.001.
3.4. Factors influencing clinical pain research careers
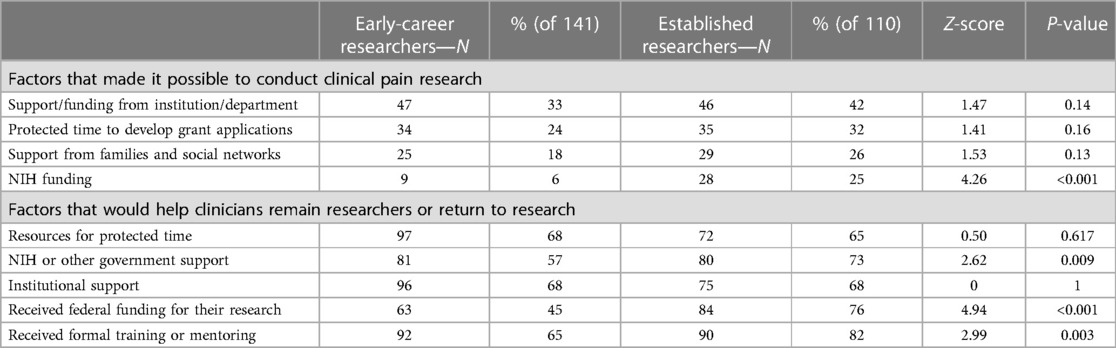
Asked about factors that made it possible to conduct clinical pain research, the most common responses among current researchers (n = 206) were support/funding from institution/department (53%); protected time to develop grant applications (40%); and support from families and social networks (35%). Just 21% reported that funding from NIH was a factor.
Those who no longer participate in clinical pain research (n = 94) most commonly reported a lack of time to prepare grant applications (34%) as a factor influencing their decision to quit research. Other factors included limited funding opportunities (29%), complexity associated with clinical trials (19%) and undesirable work/life balance (17%).
The most common factors reported that would help clinicians remain researchers or return to research (n = 300) were resources for protected time (63%), NIH or other government support (61%) and institutional support (57%). For any of these factors, however, those who were former researchers (n = 94) were less likely than current researchers (n = 206) to say that any of these factors would be helpful (Table 3): 69% of current researchers said that resources to support protected time would be helpful, vs. 49% of former researchers, z = 3.32, p < 0.001; 72% of current researchers said NIH or other government support vs. 37 percent of former researchers, z = 5.77, p < .001; and 63% of current researchers said institutional support vs. 43% of former researchers, z = 3.24, p = .001.
For those who were never researchers, but have considered it (n = 68), 51% said that a deciding factor was a lack of protected time, followed by a lack of time to prepare grant applications (40%), lack of opportunities (32%) and lack of adequate training (31%).
3.5. Early-career challenges
Among former researchers (n = 94), 53% said they were early in their careers (Table 3), while 40% of current researchers (n = 206) were early in their career, z = 2.1, p = 0.035 In contrast, 24% of former researchers are established compared to 42% of current researchers that are established, z = 3.01, p = 0.002. Established researchers (n = 110) were also be more likely than early-career researchers (n = 141) to receive federal funding (Table 4): 76% of established researchers reported receiving federal funding, compared to 45% of early-career researchers, z = 4.94, p < 0.001.
The importance of the aforementioned factors that help enable research varied based on career stage (Table 4). Established researchers were more likely than early career researchers to say that funding from their institution (42% vs. 33%, z = 1.47, p = 0.14), protected time to develop applications (32% to 24%, z = 1.41, p = 0.16), or funding from NIH (25% to 6%, z = 4.26, p < .001) were factors that made it possible for them to pursue clinical research.
Some factors that would help established researchers (n = 110) continue their research careers were also less likely to be factors that would help early-stage researchers (n = 94) continue their research. Notably, while 73% of established researchers said NIH or other government support would continue their research careers, only 57% of early-career researchers said NIH funding would help them continue their careers, z = 2.62, p = .009.
3.6. Importance of training and mentoring
Among all who have ever engaged in research (n = 300), 70% have had formal training or mentoring. Among those who had received formal training or mentoring (n = 210), 33% said it was specific to pain; 27% said it was related to pain “in part” and 38% said it was not specific to pain (Table 1). But current researchers (n = 206) were more likely than former researchers (n = 94) to have received research training or mentoring (74% vs. 61%, z = 2.28, p = .023, Table 3). Among established researchers (n = 110), 82% received research training or mentoring, and 65% of early researchers (n = 141) received research training or mentoring, z = 2.99, p = .003, Table 4.
Those who received formal research training or mentoring were also more likely to receive federal funding. While the percentage of all researcher respondents (n = 300) who received federal funding was 55%, 67% of those who received formal research training and mentoring (n = 210) received federal funding, compared to 39% of those who did not receive formal research training or mentoring (n = 69), z = 3.96, p < .001).
Those who were still in research (Table 3) were more likely to have received an NIH training or career development award: 34% of current researchers (n = 206) received an award, compared to 14% of former researchers (n = 94), z = 3.59, p < .001.
3.7. Characteristics of mentors
Among respondents who have ever been involved in research (n = 300), 50 percent have provided formal training or mentoring. The percentage is greater among current researchers (n = 206, 61%), but less among former researchers (n = 94, 44%, z = 2.55, p = .011). Among those who are established in their careers (n = 109, current and former researchers), 91% provided formal training or mentoring. Mentors (n = 151) were more likely than the overall sample (n = 430) to be working at teaching institutions (32% vs. 21%, z = 2.49, p = .013).
Among those who provided mentoring (n = 151), 85% said it was specific to pain. Asked if their trainees continued in the clinical research field, 24% said “most” (75% or more) continued; 30% said “some” (25% to 75%) continued; and 30% said “a few” (less than 25%) continued; and 7% said “none” continued.
While mentoring is fairly common, especially among more established researchers, few reported having funded protected time to support their mentoring activities. Among those who reported providing training or mentorship (n = 151), the most common sources of funding for protected time to mentor was from their departments (25%) or institutions (23%), followed by a federally-funded award (16%). But the most common answer was “no funding received” (49%).
4. Discussion
This was the first survey asking multidisciplinary research clinicians who specialize in pain to identify the factors that have helped them pursue clinical pain research as a career, or, conversely, held them back from pursuing research alongside their responsibilities in patient care.
The findings in this survey are similar to the findings of Hall (11), who wrote that time and lack of formal research training were the most likely barriers to neurologists hoping to pursue clinical research. In that study, it was found that the amount of time spent on research training may not be adequate for those who wish to conduct more complex or larger studies, which would affect the type of clinical research being done.
Based on the responses in this study, it could be inferred that those who attempted to pursue research careers but have returned to focus exclusively on patient care tend to be earlier in their careers (53% of former researchers were early in their careers, vs. 40% of current researchers, while 42% of current researchers are established vs. 24% of former researchers) and are less likely than current researchers to have received any formal research training or mentoring (among current researchers, 74% received training or mentoring, vs. 61% of former researchers).
Other workforce researchers also have found that quality mentorship and exposure to research during medical school or earlier have a positive effect on factors like efficacy—belief in one's own ability to pursue scientific research—that can inspire physicians to enter clinical research (12–15). In addition, a lack of specific training programs and mentorship (16) can be an obstacle.
This survey's findings also are compatible with the assertion by Adams and Memtsoudis (7) that the mentorship model, while valuable for many, may have drawbacks for some compared to more regular, formalized research training. Our findings showed that those who were still in research were more likely to received formal training and mentoring (74% of current researchers vs. 61% of former researchers); are more likely to have received federal research funding (67% of those who received training and mentoring vs. 39% among those who didn't); and that there may be a disparity between the training and mentoring that was available to those who are further along in their careers compared to those who are at the beginning of their careers today (82% of established researchers received training or mentoring vs. 65% of early researchers. That there was a portion of respondents that attempted research and have dropped out, or are interested in research but haven't pursued it, suggests that more formal research training is needed. Adams and Memtsoudis suggest that having a more reproducible research training infrastructure would be a greater benefit to more younger scientists and could help avoid issues such as mismatches between mentors and trainees or conflicts over prioritizing the mentor's own research over that of the trainee.
Across career stages, there are several factors that appear most important to help clinicians continue their careers with pain research: support and funding from their home institutions (53% considered important), and protected time to enable them to develop applications and conduct research (40% considered important). The finding regarding protected time echoes Meador's findings (17) on academic medicine more generally, with financial pressures seeing faculty increasing their clinical activities (which bring in money) at the expense of time to conduct research (which costs money).
Among our survey respondents, 28% reported receiving NIH awards for training or career development. This proportion is consistent with the 28% average success rate in recent years for applicants for NIH Fellowships (“F” awards), and is similar to the 33% average rate in recent years for NIH career development grants (“K” awards). It is lower, however, than the 52% average rate for NIH research training grants (“T” awards) (18).
Support from the NIH itself was more important for established investigators, who were more likely to have received NIH funding than those earlier in their careers (25% of established researchers considered it important, compared to 6% of early-career researchers). Previous publications on challenges facing clinical researchers have noted that it takes time for younger faculty members to support themselves through grants, suggesting the need for bridge awards until independence can be established (19). In combination, these findings from the literature and the present survey's findings suggest that research funders and institutions should consider the importance of providing protected research time across career stages if they want to promote a research environment that is accessible to a greater number of interested clinicians.
If there are efforts to attract former investigators back to research, those might pose a different kind of challenge. The survey found that those who have dropped out of research were significantly less likely to say that any of the most important factors that facilitate research (protected time, NIH funding, or institutional support) would be helpful to return to that career trajectory.
4.1. Limitations
As this was an anonymous survey, it is possible that respondents may have taken the survey multiple times, or that they may have been misrepresenting themselves in their responses. Similarly, since this was an anonymous survey, we do not have demographic data of the participants who completed it. Given that the survey was distributed through channels that tend to be more research-focused, the survey was also biased toward those who are already tapped into the research community in some way and may have biased the results towards those with interest and experience in research rather than those who are exclusively in clinical practice. The survey was administered approximately one year into the beginning of widespread SARS-Cov2 transmission in the U.S. With the numerous implications that restrictions to limit the spread of the virus had for both healthcare settings and other workplaces, and at people's own homes, it is likely that some of the responses reflected increased challenges and difficulties caused by the pandemic.
4.2. Implications/next steps
The results of the survey suggest that there are system level and individual level interventions that can help expand the clinical pain research workforce. In order to address these factors, however, a diverse group of stakeholders (e.g., funding organizations, universities and institutions, and research organizations) should consider how they can help enhance the workforce.
The NIH, one of the primary funding organizations within the United States, have already reviewed the survey results presented in this article and have begun to release Notice of Funding Opportunity (NOFO)s to address some of the concerns that clinical researchers reported in the survey. For example, in 2021 Midcareer Investigator Awards in Patient-Oriented Research (K24) (20), which are meant include support for mentoring, were awarded to HEAL clinical pain grantees so that they can devote additional time to mentoring earlier-stage members of their research teams. In 2022, a Clinical Scientist Institutional Career Development Award (K12) (21), grant was awarded to help promising pain researchers across the country access a rigorous research training program with mentors outside of their home institutions. Also in 2022, a grant was awarded to help enhance research infrastructure (R24) (22) established a pain research coordinating center to help increase the access to mentorship, training, and multidisciplinary collaboration among pain investigators.
However, the survey's findings suggest that the NIH alone cannot meet the needs of aspiring clinical pain researchers. There are numerous ways that the broader pain research community, including universities, hospitals and organizations representing researchers and practitioners, could contribute to enhancing the clinical research workforce.
Universities and institutions can play a role in enhancing the clinical pain research workforce. The survey's results suggest that a person is more likely to continue a career in research if they receive research training and mentoring in pain research. Thus, universities and institutions that have offer graduate training, fellowship, or post-doctoral training, in disciplines that treat pain to enhance their pain education, mentorship, and research training earlier in a person's career to help encourage a career in research.
Research organizations could also help enhance the pain research workforce, based on the results of this survey. Some research organizations are providing courses, training, mentoring to their early-stage investigators (ESI), as well as provide small research training grants to help initiate the careers of ESI. For example, the Foundation for Anesthesia Education and Research has several programs to promote younger investigators, including one to bring undergraduates into anesthesiology research labs, and grants to early-career clinicians that require their departments to provide protected time for research (8). Similarly, the U.S. Association for the Study of Pain provides either (1) the Rita Allen Foundation Award for chronic pain research to early-career leaders in basic pain research whose work has the potential to uncover new pathways to treat chronic pain, and (2) the MAYDAY Fund Award to support innovative projects to close the gap between knowledge and practice in the treatment of pain.
The survey suggests that when institutions provide protected time to their ESI, it is more likely that the researcher will stay in the research field. Thus, it may be helpful to provide more protected time for ESI as they are launching their careers. It could also be helpful to provide support to ESI as they are applying for their first federally supported grant, vs. requiring a professor to have a grant before they can be hired at a university, hospital, and/or institution.
4.3. Conclusion
There are many clinicians and medical professionals with an interest in treating pain who could help discover innovative and effective pain management techniques, but their ability to conduct the research to generate evidence in support of innovative pain management is hindered by many factors beyond their control, such as the need to prioritize day-to-day patient care, a lack of protected time to pursue research, and a challenging financial environment in terms of winning a research grant as well as institutional pressures. While additional research funding would help interested clinicians pursue research, funding alone won't fix other issues that hold aspiring researchers back, such as the need for a more formal research training paradigm. Creating a sustainable clinical pain research workforce will require a coordinated effort by research funders, training institutions, pain management professional organizations and other parts of the health care system.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://figshare.com/projects/Pain_research_workforce_survey/163009.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirement. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
LP and LW conceived the survey and developed initial survey questions. AS refined the questions and programmed the surveys. LW and AS conducted outreach to potential survey respondents. AS managed the survey data and conducted data analysis. AS wrote the first draft of the manuscript and LW contributed substantive revisions. All authors contributed to the article and approved the submitted version.
Funding
This research was conducted as part of the authors' employment with the National Institutes of Health and N4 Solutions, LLC.
Acknowledgments
Giulia Bova and Kaleen Ly of the NIH/NINDS Office of Pain Policy and Planning provided data management and analysis assistance.
Conflict of interest
AS is employed by the company N4 Solutions, LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Institute of Medicine Committee on Advancing Pain Research and Education. The national academies collection: Reports funded by national institutes of health. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US) Copyright © 2011, National Academy of Sciences (2011).
2. Interagency Pain Research Coordinating Committee. IPRCC Meeting - 11/23/2020 2020. Available at: https://www.iprcc.nih.gov/meetings/iprcc-meeting-11232020
3. Feldman AM. The national institutes of health physician-scientist workforce working group report: a roadmap for preserving the physician-scientist. Clin Transl Sci. (2014) 7(4):289–90. doi: 10.1111/cts.12209
4. Vassie C, Smith S, Leedham-Green K. Factors impacting on retention, success and equitable participation in clinical care academic careers: a scoping review and meta-thematic synthesis. MetaAnalysis. (2020) 10(3):1–13. doi: 10.1136/bmjopen-2019-033480
5. Sonstein SA, Jones CT. Joint task force for clinical trial competency and clinical research professional workforce development. Front Pharmacol. (2018) 9:1148. doi: 10.3389/fphar.2018.01148
6. Schwinn DA, Balser JR. Anesthesiology physician scientists in academic medicine: a wakeup call. Anesthesiology. (2006) 104(1):170–8. doi: 10.1097/00000542-200601000-00023
7. Adams MCB, Memtsoudis SG. The world needs our science: broadening the research piepline in anesthesiology. Reg Anesth Pain Med. (2021) 46(2):164–8. doi: 10.1136/rapm-2020-102029
8. Lane-Fall MB, Bedell VM, Eckenhoff RG. The future of research in anesthesiology. Int Anesthesiol Clin. (2020) 58(4):41–5. doi: 10.1097/AIA.0000000000000291
9. Adams MCB, Bicket MC, Murphy JD, Wu CL, Hurley RW. Opportunities and challenges for junior investigators conducting pain clinical trials. Pain Rep. (2019) 4(3):e639. doi: 10.1097/PR9.0000000000000639.31372584
10. BroadcastMed’s. Acquisition of Digitell. Available at: https://pages.broadcastmed.com/digitell-acquisition.html
11. Hall DA, Ramos AR, Gelfand JM, Videnovic A, Benatar M, Cahill C, et al. The state of clinical research in neurology. Neurology. (2018) 90(1):1347–54. doi: 10.1212/WNL.0000000000005295
12. DiBiase RM, Beach MC, Carrese JA, Haythornthwaite JA, Wheelan SJ, Atkinson MA, et al. A medical student scholarly concentrations program: scholarly self-efficacy and impact on future research activties. Med Educ Online. (2020) 25(1):1–9. doi: 10.1080/10872981.2020.1786210
13. Estrada M, Hernandez PR, Schultz PW. A longitudinal study of how quality mentorship and research experience integrate underrepresented minorities into STEM careers. CBE Life Sci Educ. (2018) 17(1):1–13. doi: 10.1187/cbe.17-04-0066
14. Kolber BJ, Janjic JM, Pollock JA, Tidgewell KJ. Summer undergraduate research: a new pipeline for pain clinical practice and research. BMC Med Educ. (2016) 16:1–11. doi: 10.1186/s12909-016-0648-7
15. von Baeyer CL, Stevens BJ, Craig KD, Finley GA, Johnston CC, Grunau RVE, et al. Pain in child health from 2002 to 2015: the early years of an international research training initiative. Can J Pain. (2019) 3(1):1–7. doi: 10.1080/24740527.2018.1562844
16. Burns LJ, Clayton C, George JN, Mitchell BS, Gitlin SD. The effect of an intense mentoring program on junior investigators’ preparation for a patient-orientated clinical research career. Acad Med. (2015) 90(8):1061–6. doi: 10.1097/ACM.0000000000000742
17. Meador KJ. Decline of clinical research in academic medical centers. Neurology. (2015) 85(13):1171–6. doi: 10.1212/WNL.0000000000001818
18. National Institutes of Health RePORT Success Rates, Training and Research Career Development Programs. (accessed 05/05/2023). Available at: https://report.nih.gov/funding/nih-budget-and-spending-data-past-fiscal-years/success-rates
19. Kubiak NT, Guidot DM, Trimm RF, Kamen DL, Roman J. Recruitment and retention in academic medicine–what junior faculty and trainees want department chairs to know. Am J Med Sci. (2012) 344(1):24–7. doi: 10.1016/S0002-9629(15)30914-9
20. National Institutes of Health Division of Biomedical Research Workforce. Research Career Development Awards- Midcareer Investigator Award in Patient-Oriented Research 2017 (updated 05/23/2017). Available at: https://researchtraining.nih.gov/programs/career-development/K24
21. National Institutes of Health Division of Biomedical Research Workforce. Research Career Development Awards—Clinical Scientist Institutional Career Development Program Award 2017 (updated 05/23/2017). Available at: https://researchtraining.nih.gov/programs/careerdevelopment/K12
22. National Institutes of Health. Grants & Funding—Types of Grant Programs 2022 (updated 12/14/2022). Available at: https://grants.nih.gov/grants/funding/funding_program.htm
Keywords: pain, research training, workforce development, mentorship, clinical research
Citation: Siddons A, Wandner LD and Porter LL (2023) Challenges and opportunities for research clinicians interested in pain: results of a survey. Front. Pain Res. 4:1194818. doi: 10.3389/fpain.2023.1194818
Received: 27 March 2023; Accepted: 24 July 2023;
Published: 24 August 2023.
Edited by:
John Farrar, University of Pennsylvania, United StatesReviewed by:
Michael Perloff, Boston University, United StatesEllen Air, Henry Ford Health System, United States
© 2023 Siddons, Wandner and Porter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew Siddons YW5kcmV3LnNpZGRvbnNAbmloLmdvdg==
 Andrew Siddons
Andrew Siddons Laura Dover Wandner2
Laura Dover Wandner2