- 1Department of Oncology, St. Jude Children's Research Hospital, Memphis, TN, United States
- 2Department of Medicine, University of Puerto Rico, San Juan, Puerto Rico
Background: Pain management at the end of life is a fundamental aspect of care and can improve patients' quality of life. Interventional approaches may be underutilized for pediatric cancer patients.
Objective: To describe a single institution's 10 years of experience with regional pain management at the end of life in pediatric oncology.
Methods: A retrospective cohort study of 27 patients with pediatric cancer who died between April 2011 and December 2021 and received continuous nerve block (CNB) catheters or single-shot nerve blocks (SSBs) during their last three months of life. The type of blocks, analgesic efficacy, and palliative care involvement were evaluated.
Results: Twenty-two patients (81.5%) had solid tumor diagnoses, including carcinomas, sarcomas, and neuroblastoma. Most (59%) patients received CNB catheters, and 12 patients (44%) received SSBs for pain control. The mean pain score decreases for CNB catheters and SSBs after interventions were −2.5 and −2.8, respectively, on an 11-point scale. Decreases in opioid patient-controlled analgesia dosing requirements were noted in 56% of patients with CNB catheters; likewise, in 25% of patients with SSBs at 24 h and in 8% at 5 days after interventions. Nearly all patients had PC involvement and received care from pain specialists (96% and 93%, respectively). Twenty-three (85%) had physician orders for scope of treatment orders completed before death.
Conclusion: Regional pain control interventions can be effective and safe for relieving regional pain and suffering in dying children and young adults. The collaboration between palliative care and pain management specialists at the end of life can help alleviate suffering and improve quality of life.
Introduction
Pain management during end-of-life (EoL) care remains important to optimize a patient's overall quality of life (QoL). Up to 50% of patients undergoing cancer treatment experience pain secondary to their disease. This increases to 76% during the EoL period and can be as high as 90% for pediatric patients with advanced cancer (1, 2). In a study of 185 pediatric patients, pain was reported in 91.5%, being highest in those patients with solid tumors (2, 3). Pain can significantly affect both patient and family QoL, and studies show that relief of suffering, including pain symptoms, is at the forefront of patient, family, and healthcare providers' goals (4). Using a holistic approach, palliative care (PC) is a subspecialty that can provide symptom relief and improve a patient's QoL (5, 6). In larger institutions, PC physicians often work with the primary oncology team and others, including pain medicine specialists and integrative medicine, to provide targeted pain relief interventions (7), with a focus on improving EoL care.
Strategies to enhance QoL and relieve suffering due to pain are multimodal and multidisciplinary and include the following: (1) pharmacological therapy including but not limited to NSAIDs, acetaminophen, opioids, methadone, and gabapentinoids, (2) physical and occupational therapy, (3) advanced infusions such as low-dose ketamine or lidocaine infusions, and (4) interventional approaches such as peripheral nerve blocks and central neuraxial blocks (intrathecal or epidural) (8). Additionally, integrative medicine approaches including acupuncture and massage are being incorporated more frequently for pain and other distressing symptoms (9, 10). Occasionally, traditional symptom management strategies are insufficient to alleviate suffering, so consideration of palliative sedation therapy may be needed (11).
Morphine is the most commonly used drug for pain control in the EoL period (reported in 60%–90% of patients) (12); opioids and other pharmacological therapies can cause significant side effects such as constipation, sedation, and nausea (13), increasing patient distress. Therefore, care providers must optimize pain management regimens and incorporate interventional approaches, when appropriate, to reduce pain and systemic opioid exposure and related side effects. Regional pain interventions such as continuous nerve block (CNB) catheters have been used to decrease pain in both pediatric (8) and adult patients (14); however, the efficacy of single-shot nerve blocks (SSBs) and the use of interventional pain management modalities during EoL care in pediatric cancer patients are not well described. Here, we evaluate the use of regional pain control interventions for children with cancer-related pain during the EoL period.
Patients and methods
In this Institutional Review Board–exempt retrospective review, we evaluated pediatric patients from a single academic institution who were treated with CNB catheters or SSBs as part of regional pain control regimens during the EoL period [defined as the three months before the documented date of death (DOD)]. The study institution is a 78-bed facility that cares for over 500 new pediatric patients with oncology diagnoses each year, comprising patients from the Mid-South as well as national and international patient referrals. Pediatric patients were defined as those who received their primary diagnosis before the age of 21 years or received a diagnosis of pediatric primary cancer. Patient data were obtained from the electronic medical record database PowerChart P134 from CernerWorks. The study review period included patients who died between April 2011 and December 2021, a timeframe during which patients would have all pertinent data for this study placed into the electronic medical record system. Demographic information [age, primary diagnosis, DOD, and location of death (LOD)] was collected. The following pain characteristics were collected at the interventional procedure for pain: (1) location of pain; (2) type of pain (nociceptive, neuropathic, visceral, or somatic); (3) medications for pain management (opioids, anticonvulsant, non-steroidal anti-inflammatory drugs, tricyclic antidepressants, acetaminophen, methadone, ketamine, and corticosteroids); and (4) pain scores (PS) collected 24 h before and 24 h after the interventional procedure, and, as applicable, 5 days after the procedure. For the PS assessment, age-appropriate pain assessment tools were used, including the FACES Revised pain scale (15), FLACC score pain scale (16), and the numeric rating scale (17). A follow up period of 5 days was chosen to limit data inconsistencies that may arise from patients moving between inpatient and outpatient settings, and therefore possibility of missing data. as well as to mitigate the effects that active dying may have on symptom management needs. PC data collected included the (1) preferred death location (if stated), (2) number of PC team visits, (2) number of pain management specialist visits, (3) physician orders for scope of treatment (POST), and (4) patient lifespan following the regional pain block intervention.
The data collected regarding pain management interventions included intervention type (i.e., epidural catheter, intrathecal catheter, peripheral nerve block catheter, or single-shot nerve block); device location; tunneling technique (i.e., tunneled or not tunneled); local anesthetic and/or ablative agent, concentration, and rate of infusion at day of insertion and by day 5 after insertion (if applicable); duration of the device in relation to the patient's lifespan (measured in days); outpatient status with device; and reason for device removal (if applicable). Potential limiting factors for assessing a patient's candidacy for the interventional pain blocks were collected, specifically the absolute neutrophil count (ANC) (×103·1−1) and the platelet count (×10−1·1−1). Adverse effects following the pain blocks were evaluated.
The change in PS before and 24 h after the pain intervention, as well as the change in opioid patient-controlled analgesia (PCA) requirement before intervention, 24 h post, and at day 5 after intervention were used to analyze the effectiveness of the pain block interventions. Patients who did not receive PCA before or after the intervention were evaluated using only the change in PS before and 24 h after the intervention. When PS were not documented within a 24 h interval, this was counted as missing data. To facilitate comparison of different intravenous opioids used for PCA, the doses were calculated as mean intravenous morphine equivalent (mg·kg−1·day−1), using the following equianalgesic rations: 100:1 for fentanyl:morphine and 5:1 for hydromorphone:morphine.
Results
Patient demographics
Altogether, 2,151 patients died during the study period; 415 of them (19%) engaged with the pain medicine team. Of those who received subspecialty pain care, 27 (6.5%) received interventional pain management procedures during the EoL period and were reviewed for this study; of them, 4 patients had multiple interventional procedures reported. Table 1 summarizes the demographic data collected. Briefly, the mean age was 14 years (range: 2 to 26 years), and the predominant primary diagnosis was osteosarcoma (18.5%, n = 5), with most patients (81.5%, n = 22) having solid tumor diagnoses. The most common site of pain was the abdominal region (48%, n = 13), and most patients (74%, n = 20) had a documented nociceptive type of pain.

Table 1. Demographic data of the 27 patients who received an interventional pain modality during the end of life.
Continuous regional pain control interventions
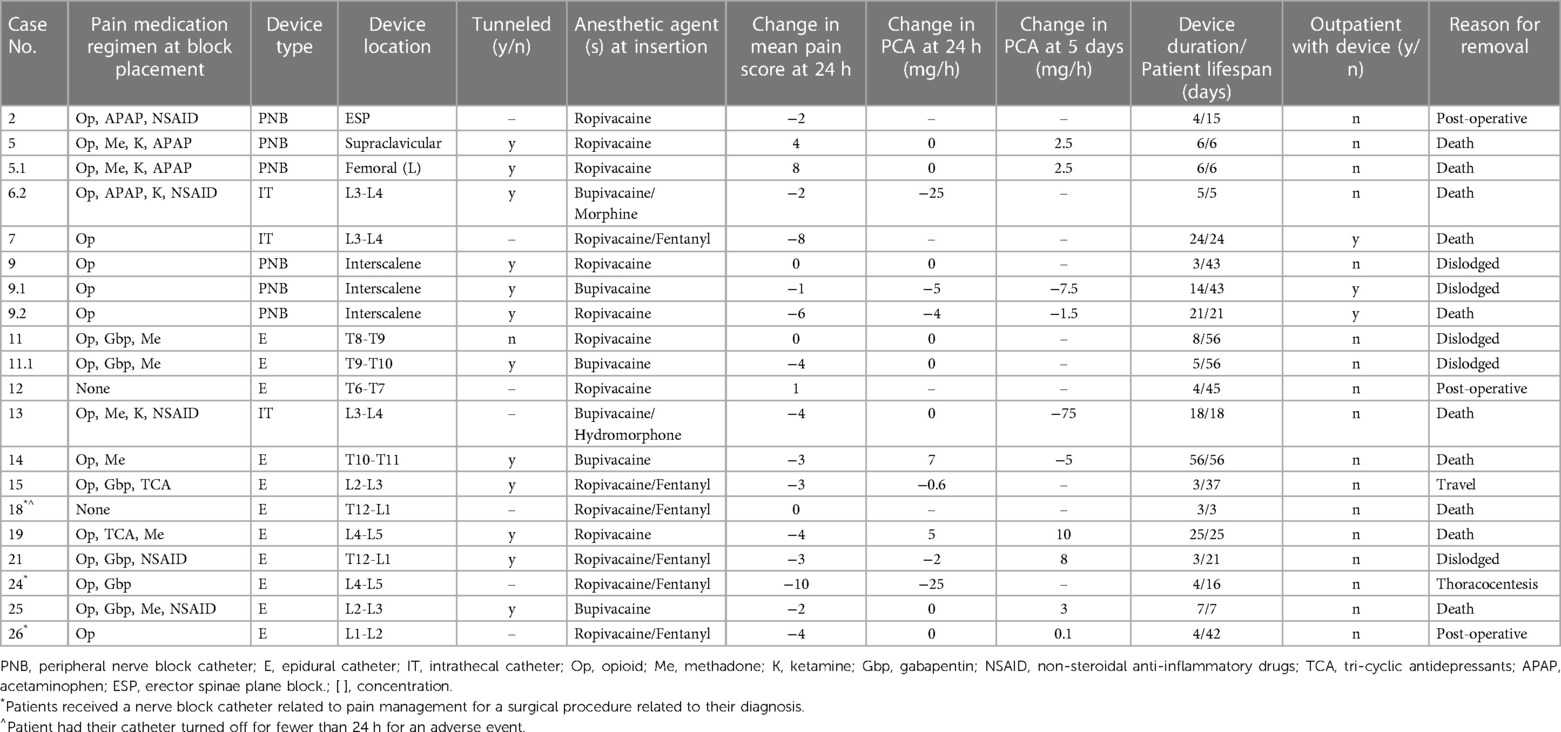
Most patients (59%, n = 16) received CNB catheters, 62% (n = 10) of which were epidural catheters (Table 2). Additionally, 19% (n = 3) received multiple CNB catheter infusions: one patient had two peripheral nerve block catheters placed on the same day in two different locations, and two patients had dislodged catheters replaced. The average use of a CNB was 6 days (range, 4–15 days) for peripheral nerve block, 16 days (range 5–24 days) for intrathecal catheters, and 11 days (range 2–56 days) for epidural catheters (Table 2). Three-quarters (n = 12) of the catheters were placed using a tunneling technique, and 62% (n = 10) remained in place on the DOD. Most patients (88%, n = 14) using CNB catheters were receiving opioids (excluding methadone) prior to catheter insertion.
The mean PS was 5 (range, 0–10) before catheter insertion and 3 (range, 0–10) 24 h after insertion. Nearly all catheter-based interventions (88%, n = 14) resulted in decreasing PS, with a mean decrease of 2.15 (Table 2). Three-quarters of patients receiving CNB intervention (n = 12) had an opioid PCA as part of their pain management regimen. After CNB intervention, 37% (n = 6) had decreased PCA dosing requirements 24 h after catheter placement; 37% had decreased PCA dosing requirements 5 days after catheter placement.
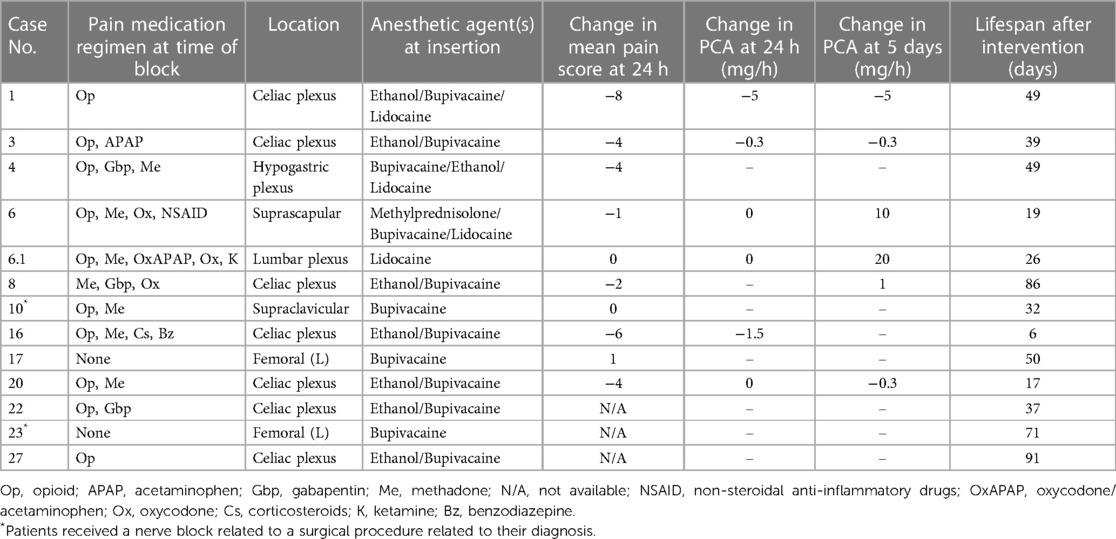
Single-shot regional pain control interventions
Nearly half (44%, n = 12) of patients received a SSB intervention for pain control, mainly celiac plexus blocks (n = 7, 58%; Table 3). One patient (patient 6) had both a suprascapular and lumbar plexus SSB performed one week apart to address pain from the primary tumor site and diffuse pain from worsening metastatic disease, respectively. Before intervention, opioids (excluding methadone) were used for pain control by most patients (83%, n = 10).
Over half of these patients (58%, n = 7) had a documented decrease in PS at 24 h after the SSB intervention; the mean change in PS was −2.8, with a median change of −3 (Table 3). Of the 5 patients who were on PCA management before SSB intervention, 25% (n = 3) had a decreased PCA requirement at 24 h, and 17% (n = 2) had no change in the PCA requirement. Five days after the intervention, 17% (n = 2) had decreased PCA requirements, and another 17% had an increased or new PCA requirement (Table 3). Three patients (25%) did not have PS recorded before or after the procedure, so their analgesic efficacy could not be evaluated.
Palliative care characteristics
Nearly all patients in this study (96%, n = 26) had PC involvement, with an average of 40 visits, not limited to the EOL period, throughout their care journey (range, 2–120). Similarly, 93% (n = 25) of patients received care from a pain medicine specialist, with an average of 37 visits spanning the course of their clinical care (range, 1–223). Of the patients who received a CNB catheter, 2 (7.4%) used their devices in the outpatient setting. The mean lifespan following regional pain control intervention was 34 days (median, 32 days; range, 3–91).
Most patients (85%, n = 23) had POST orders completed before the DOD: 70% (n = 16) indicated limited additional interventions, and 22% (n = 5) specified comfort measures only (Table 4). Over half (59%, n = 16) died in a hospital setting although only 5 of the 11 patients who specified a preferred LOD chose a hospital setting. Of the patients who set a preferred LOD, 64% (n = 7) died at their preferred location (Table 4).
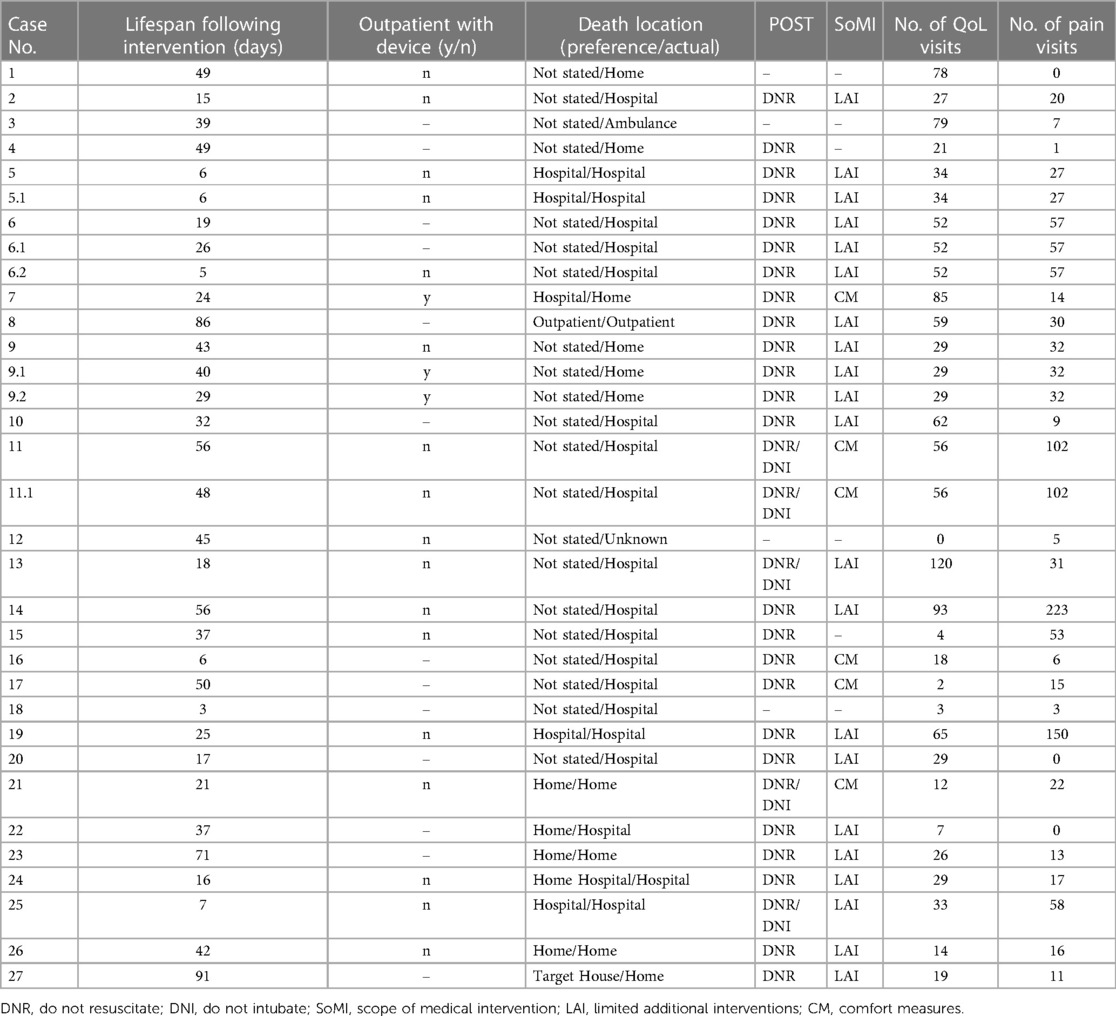
Table 4. Palliative care characteristics of patients who received regional pain control intervention.
Contraindications and limitations to regional pain control interventions
One patient experienced moderate neutropenia (ANC <1,500 and ≥1,000) and two patients had platelet counts <50k. Four adverse events were reported in our patient cohort: ventricular tachycardia, bleeding, leaking of the catheter, and a temporary discontinuation of catheter infusion due to a surgical procedure. Of note, the reporting of potential complications spans the time period of date of regional pain intervention until the day of death, to ensure potential procedural complications were not inadvertently missed.
Discussion
This single-institution retrospective study reports on a decade of regional pain management interventions for pediatric oncology patients during the EoL period and represents the largest known cohort to date. Historically, regional pain management interventions have been underutilized during the EoL period because of concerns about procedural complications, such as infection and bleeding, patient distress/discomfort during the procedure, and the time requirements for patient assessment (18). Likewise, our study reports a low utilization rate of interventional approaches in only 6.5% of the patients followed by the pain specialists in this series. Underutilization of interventional approaches for pain is of concern, especially in view of our findings that highlight their analgesic efficacy as reflected by decreased PS and opioid utilization, safety, and benefits of these regional pain management interventions as efforts toward increasing QoL during EoL care for children with cancer. Our findings suggestive of underutilization of interventional pain management in pediatric oncology are consistent with those of a recent review article in pediatric oncology (19), which supported the fact that interventional approaches are typically reserved for refractory pain unresponsive to noninvasive treatment. Similarly, a review of literature from 1980 to 2012 indicated that although regional techniques are usually considered only in the limited context of failure of systemic treatments and/or intolerable medication side effects, their associated risks are often acceptable when the potential benefits are consistent with the overall goals of care (20).
Despite leukemias remaining the most prevalent pediatric cancer type (21), 81% of patients in our study cohort had a solid tumor primary diagnosis. This was unsurprising as our study focused on pain in the EoL period, and patients with solid tumor diagnoses often have an increased tumor burden, contributing to increased pain (3, 22). Solid tumors are thought to release neuroimmune mediators that activate nociceptive nerve terminals, leading to increased nociceptive pain (23). Almost two-thirds of our cohort reported nociceptive pain, as expected given the predominance of solid tumor diagnoses reported. The various locations of reported pain were directly correlated with tumor location, as expected, and influenced the location of CNB or SSB. Regardless of which regional pain control intervention was used, opioids were a mainstay of pharmacological pain regimen. In many patients, opioids were administered as intravenous PCA. This was an expected finding as opioids are frequently prescribed for EoL symptom management and often require massive and rapid dose escalation in this setting (12, 24, 25).
Case reports and case series in adults and pediatrics emphasize that CNB catheters can mitigate pain for patients with advanced cancer pain (8, 14, 26); in our study, CNB catheters were used for most patients. Of these patients, 25% required less opioid medication to achieve pain relief at 24 h and 5 days after placement, and 88% had a lower PS. It may appear from our data that celiac plexus CNB were less effective in this series despite prior case series reporting analgesic efficacy (27). We attribute this potential correlation with cancer progression in the EoL period. CNB can sometimes be used for prolonged periods, with the literature demonstrating duration of use of 81–240 days (8, 28, 29). Our study cohort demonstrated catheter duration of 4–56 days. Additionally, 10 patients kept their catheters in place until the DOD, with two continuing this intervention in the home setting. Together, our findings show that CNB can be important in EoL pain management, can be tolerated for prolonged periods, and may serve patients in the outpatient and home/hospice setting.
Similar to CNB, little has been published about the role of SSBs as pain management for EoL care. One study reported SSB of the brachial plexus to be useful for adults (22), with pain relief sometimes lasting up to 10 months (30). In our patient cohort, nearly half of patients received a SSB, with 58% reporting a decrease in PS intensity. In patients who had an opioid PCA regimen before their SSB, four had a decrease in their opioid PCA dosing requirements after the intervention was performed, suggesting improved pain relief secondary to the SS nerve block. Notably, two patients (16%) reported no change in PS after the procedure, and one patient (8%) reported an increase in PS. We hypothesize that this may be partially due to incomplete optimization of adjuvant pain control such as oral pharmacological regimen or PCA, technical difficulties in performing the blocks, or disease progression and extension outside the dermatomal levels blocked. Additionally, one patient (patient 6) did not have successful pain relief after two SSBs performed in two different anatomical locations due to progressive tumor burden, and thus underwent escalation of their pain management regimen to include a continuous intrathecal catheter, which did result in improved PS and decreased PCA opioid requirements. These findings establish the potential benefits of regional pain control interventions and should encourage physicians to partner with their interdisciplinary colleagues to offer these modalities for relief of suffering whenever appropriate.
Clinicians may avoid regional pain control interventions at the EoL for fear of procedural complications such as bleeding and infection or for worries that it may interfere with patient and family EoL preferences, such as dying at home (8, 31). Few of our patients had complications related to CNB or SSB procedures, with one patient experiencing mild bleeding that did not result in catheter discontinuation, another experiencing ventricular tachycardia that was thought to be related to sedation medications administered during the procedure, and another having a leaky catheter, which is an equipment malfunction rather than a procedural complication. Most patients had platelet counts >50k and absolute neutrophil count (ANC) values >1,000, minimizing bleeding and infection risks, respectively. The absence of any infectious complications in our study could be related to the high percentage of tunneled catheter use (75%). When catheters are placed using other techniques, prolonged use can increase the risk of inflammation and infection (32), but when the tunneling technique is used, extended use does not correlate with increased risk of infection (33).
Finally, the importance of collaboration between interdisciplinary teams to ensure the needs of the patient and family are being met is evident. Nearly all patients in this study received consultation from the PC and pain medicine specialists. This is significant because delayed PC involvement is linked to a greater chance of dying in the intensive care unit and more intensive interventions that may contribute to increased suffering during the EoL (34–37). Early involvement of the PC team often results in conversations of advanced care planning, including preferences for location of death, DNR/DNI status, and assessment of symptom management needs (31, 38–40). Most of our patients (85%) had POST orders in place before the DOD, 11 patients (41%) stated their preferred LOD, and 63% died in their preferred LOD. More importantly, for patients whose preference was to be in the outpatient setting, SSB interventions may have helped to achieve more-suitable outpatient regimens for adequate pain control; continuing CNB catheters as part of a patient's pain management regimen was a viable option, as seen in 2 study patients. This observation is highly valuable for clinicians caring for children during the EoL as it supports an intervention that can relieve suffering and allow a death at home, if preferred. More than half of our patients died in a hospital setting. We hypothesize that this could be related to other factors such as complex medical comorbidities, not being candidates for outpatient settings, adverse effects of the advanced cancer diagnosis, and being far from their preferred place of death.
The limitations of this study are those inherent to retrospective studies. First, this was a single-institution study with a relatively small cohort of eligible patients. To our knowledge, this is the largest sample size describing regional pain control interventions at the EoL in pediatric oncology. Additionally, retrospective data collection prohibits the procurement of dynamic information related to pain, so we ascertained pain intensity and relief through objective PS documentation and opioid dosing requirements. Given the overall sample size, intercomparison analyses between the types of blocks performed were not powered accordingly and thus not performed and reported. Lastly, our comparison of opioid doses before and after regional pain intervention excluded the use of sustained-release oral or transdermal opioids. We analyzed PCA-delivered opioids exclusively because it is the most “titratable” component of a pain management regimen and likely reflects the change in opioid need in our clinical practice; catheter placements were unaltered during the remaining pain treatments. One may also argue that the lack of evaluation of non-pharmacological interventions for pain and psychology support represents a limitation of this study. Indeed, in the difficult and complex context of EOL, this type of data may have been a valuable component to reflect the multidisciplinary approach to pain management in view of the bio-psycho-social model of pain.
Conclusion
Our study highlights the analgesic efficacy and safety of regional interventions for relieving pain-related suffering in children dying with progressive cancer and suggests that interventional approaches remain underutilized in this clinical context. The coordinated efforts of the primary oncology service, pain and PC services, and home hospice agencies enabled the implementation of regional pain interventions and their continuation in the outpatient setting. This collaboration is important to help relieve suffering during EoL care and enable preferred location of death. Future prospective studies to investigate the analgesic efficacy and the safety of CNB and SSB in children at the end of life are necessary.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the conceptualization, methodology, data curation, formal analysis and writing of this study. All authors contributed to the article and approved the submitted version.
Funding
This work was funded, in part, by ALSAC. As a participant in the Pediatric Oncology Education program at St. Jude Children's Research Hospital, Ashley Cianchini de la Sota was supported in part by NIH grant R25CA23944 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
We thank our pediatric palliative care and pain management experts for their care of our patients and Cherise Guess, PhD, ELS, for scientific editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lesage P, Portenoy RK. Trends in cancer pain management: treating severe pain in cancer patients requires knowledge of the etiology of pain, the patient's medical status, and the goals of care. Cancer Control. (1999) 6(2):136–45. doi: 10.1177/107327489900600202
2. Wolfe J, Grier HE, Klar N, Levin SB, Ellenbogen JM, Salem-Schatz S, et al. Symptoms and suffering at the End of life in children with cancer. N Engl J Med. (2000) 342(5):326–33. doi: 10.1056/NEJM200002033420506
3. Goldman A, Hewitt M, Collins GS, Childs M, Hain R. Symptoms in children/young people with progressive malignant disease: united Kingdom children's cancer study group/paediatric oncology nurses forum survey. Pediatrics. (2006) 117(6):e1179–86. doi: 10.1542/peds.2005-0683
4. Meier EA, Gallegos JV, Thomas LP, Depp CA, Irwin SA, Jeste DV. Defining a good death (successful dying): literature review and a call for research and public dialogue. Am J Geriatr Psychiatry. (2016) 24(4):261–71. doi: 10.1016/j.jagp.2016.01.135
5. Wolfe J, Friebert S, Hilden J. Caring for children with advanced cancer: integrating palliative care. Pediatric Clinics. (2002) 49(5):1043–62. doi: 10.1016/s0031-3955(02)00034-2
6. Mack JW, Wolfe J. Early integration of pediatric palliative care: for some children, palliative care starts at diagnosis. Curr Opin Pediatr. (2006) 18(1):10–4. doi: 10.1097/01.mop.0000193266.86129.47
7. Anghelescu DL, Knapp E, Johnson LM, Baker JN. The role of the pediatric anesthesiologist in relieving suffering at the end of life: when is palliative sedation appropriate in pediatrics? Paediatr Anaesth. (2017) 27(4):443–4. doi: 10.1111/pan.13103
8. Anghelescu DL, Faughnan LG, Baker JN, Yang J, Kane JR. Use of epidural and peripheral nerve blocks at the end of life in children and young adults with cancer: the collaboration between a pain service and a palliative care service. Paediatr Anaesth. (2010) 20(12):1070–7. doi: 10.1111/j.1460-9592.2010.03449.x
9. Golianu B, Yeh AM, Brooks M. Acupuncture for pediatric pain. Children. (2014) 1(2):134–48. doi: 10.3390/children1020134
10. Lin YC, Lee AC, Kemper KJ, Berde CB. Use of complementary and alternative medicine in pediatric pain management service: a survey. Pain Med. (2005) 6(6):452–8. doi: 10.1111/j.1526-4637.2005.00071.x
11. Cuviello A, Anghelescu D, Johnson LM, Baker J. Dexmedetomidine and propofol use for palliative sedation therapy (PST) in pediatric oncology: a ten-year review (GP750). J Pain Symptom Manage. (2022) 63(6):1139–40. doi: 10.1016/j.jpainsymman.2022.04.141
12. Zernikow B, Michel E, Craig F, Anderson BJ. Pediatric palliative care. Pediatric Drugs. (2009) 11(2):129–51. doi: 10.2165/00148581-200911020-00004
13. Benyamin R. Opioid complications and Side effects. Pain Physician. (2008) 11(3;2s):S105–120. doi: 10.36076/ppj.2008/11/S105
14. Pacenta HL, Kaddoum RN, Pereiras LA, Chidiac EJ, Burgoyne LL. Continuous tunnelled femoral nerve block for palliative care of a patient with metastatic osteosarcoma. Anaesth Intensive Care. (2010) 38(3):563–5. doi: 10.1177/0310057X1003800324
15. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The faces pain scale–revised: toward a common metric in pediatric pain measurement. Pain. (2001) 93(2):173–83. doi: 10.1016/S0304-3959(01)00314-1
16. Merkel S, Voepel-Lewis T, Malviya S. Pain control: pain assessment in infants and young children: the FLACC scale. Am J Nurs. (2002) 102(10):55–8. doi: 10.1097/00000446-200210000-00024
17. Von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the numerical rating scale (NRS-11) for children’s self-reports of pain intensity. PAIN®. (2009) 143(3):223–7. doi: 10.1016/j.pain.2009.03.002
18. Chambers W. Nerve blocks in palliative care. Br J Anaesth. (2008) 101(1):95–100. doi: 10.1093/bja/aen105
19. Le-Short C, Katragadda K, Nagda N, Farris D, Gelter MH. Interventional pain management for the pediatric cancer patient: a literature review. Children. (2022) 9(3):389. doi: 10.3390/children9030389
20. Rork JF, Berde CB, Goldstein RD. Regional anesthesia approaches to pain management in pediatric palliative care: a review of current knowledge. J Pain Symptom Manage. (2013) 46(6):859–73. doi: 10.1016/j.jpainsymman.2013.01.004
22. Vern-Gross TZ, Lam CG, Graff Z, Singhal S, Levine DR, Gibson D, et al. Patterns of End-of-life care in children with advanced solid tumor malignancies enrolled on a palliative care service. J Pain Symptom Manage. (2015) 50(3):305–12. doi: 10.1016/j.jpainsymman.2015.03.008
23. Schmidt BL, Hamamoto DT, Simone DA, Wilcox GL. Mechanism of cancer pain. Mol Interv. (2010) 10(3):164. doi: 10.1124/mi.10.3.7
24. Hewitt M, Goldman A, Collins GS, Childs M, Hain R. Opioid use in palliative care of children and young people with cancer. J Pediatr. (2008) 152(1):39–44. doi: 10.1016/j.jpeds.2007.07.005
25. Baker JN, Anghelescu DL, Kane JR. Pain still lords over children. J Pediatr. (2008) 152(1):6–8. doi: 10.1016/j.jpeds.2007.08.019
26. Rouhento EAS, Lehto JT, Kalliomäki ML. Peripheral nerve blocks in advanced cancer pain: retrospective case series. BMJ Support Palliat Care. (2021) 0:1–14. doi: 10.1136/bmjspcare-2021-003293
27. Anghelescu DL, Guo A, Morgan KJ, Frett M, Prajapati H, Gold R, et al. Pain outcomes after celiac Plexus block in children and young adults with cancer. J Adolesc Young Adult Oncol. (2018) 7(6):666–72. doi: 10.1089/jayao.2018.0035
28. Podgorski E, Driver L, Gulati A, Abdi S. Catheter-based techniques for terminal cancer pain: a review of nonneuraxial interventions with clinical implications for End-of-life pain management. Pain Physician. (2021) 24(7):E1137.34704723
29. Aram L, Krane EJ, Kozloski LJ, Yaster M. Tunneled epidural catheters for prolonged analgesia in pediatric patients. Anesth Analg. (2001) 92(6):1432–8. doi: 10.1097/00000539-200106000-00016
30. Zinboonyahgoon N, Vlassakov K, Abrecht CR, Srinivasan S, Narang S. Brachial plexus block for cancer-related pain: a case series. Pain Physician. (2015) 18(5):E917–24. doi: 10.36076/ppj.2015/18/E917
31. Kaye EC, DeMarsh S, Gushue CA, Jerkins J, Sykes A, Lu Z, et al. Predictors of location of death for children with cancer enrolled on a palliative care service. Oncologist. (2018) 23(12):1525–32. doi: 10.1634/theoncologist.2017-0650
32. Neuburger M, Büttner J, Blumenthal S, Breitbarth J, Borgeat A. Inflammation and infection complications of 2285 perineural catheters: a prospective study. Acta Anaesthesiol Scand. (2007) 51(1):108–14. doi: 10.1111/j.1399-6576.2006.01173.x
33. Compère V, Legrand JF, Guitard PG, Azougagh K, Baert O, Ouennich A, et al. Bacterial colonization after tunneling in 402 perineural catheters: a prospective study. Anesth Analg. (2009) 108(4):1326–30. doi: 10.1213/ane.0b013e31819673aa
34. Kaye EC, Gushue CA, DeMarsh S, Jerkins J, Sykes A, Lu Z, et al. Illness and end-of-life experiences of children with cancer who receive palliative care. Pediatr Blood Cancer. (2018) 65(4):1550–6. doi: 10.1002/pbc.26895
35. Brock KE, Steineck A, Twist CJ. Trends in End-of-life care in pediatric hematology, oncology, and stem cell transplant patients. Pediatr Blood Cancer. (2016) 63(3):516–22. doi: 10.1002/pbc.25822
36. Burns JP, Rushton CH. End-of-life care in the pediatric intensive care unit: research review and recommendations. Crit Care Clin. (2004) 20(3):467–85, x. doi: 10.1016/j.ccc.2004.03.004
37. Garros D, Rosychuk RJ, Cox PN. Circumstances surrounding end of life in a pediatric intensive care unit. Pediatrics. (2003) 112(5):e371. doi: 10.1542/peds.112.5.e371
38. Kaye EC, Friebert S, Baker JN. Early integration of palliative care for children with high-risk cancer and their families. Pediatr Blood Cancer. (2016) 63(4):593–7. doi: 10.1002/pbc.25848
39. Cheng BT, Rost M, De Clercq E, Arnold L, Elger BS, Wangmo T. Palliative care initiation in pediatric oncology patients: a systematic review. Cancer Med. (2019) 8(1):3–12. doi: 10.1002/cam4.1907
Keywords: palliative care, pain management, end of life, pediatric oncology, continuous nerve block, single-shot nerve block
Citation: Cuviello A, Cianchini de la Sota A, Baker J and Anghelescu D (2023) Regional blocks for pain control at the end of life in pediatric oncology. Front. Pain Res. 4:1127800. doi: 10.3389/fpain.2023.1127800
Received: 20 December 2022; Accepted: 6 March 2023;
Published: 21 March 2023.
Edited by:
Kenneth Craig, University of British Columbia, CanadaReviewed by:
Caridad Velazquez Cardona, Grey's Hospital, South AfricaDupoiron Denis, Institut de Cancérologie de l'Ouest (ICO), France
© 2023 Cuviello, Cianchini de la Sota, Baker and Anghelescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Cuviello YWpjdXZpZWxsb0BnbWFpbC5jb20=
Specialty Section: This article was submitted to Pediatric Pain, a section of the journal Frontiers in Pain Research
 Andrea Cuviello
Andrea Cuviello Ashley Cianchini de la Sota
Ashley Cianchini de la Sota Justin Baker
Justin Baker Doralina Anghelescu
Doralina Anghelescu