- 1National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 2Department of Acupuncture and Moxibustion, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3Evidence-based Medicine Center, Tianjin University of Traditional Chinese Medicine, Tianjin, China
Objective: To evaluate the reporting quality of randomized controlled trials (RCTs) of acupuncture for labor pain, and to explore relevant factors for facilitating reporting transparency and integrity for future RCTs.
Method: Eight Chinese and English databases were systematically searched from their inception until August 31, 2021. General characteristics and methodological quality of the included reports were evaluated based on the CONSORT statement and the STRICTA guidelines. Descriptive statistical analysis was performed. Cohen's κ-statistics were calculated to assess the agreement of all items between two reviewers.
Results: A total of 84 RCTs were included. Based on the CONSORT statement, a positive reporting rate (greater than 80%) was evident for the items “trial design” “participants” “intervention” “outcomes” “numbers analyzed” and “generalizability”. The quality of reporting for the items “randomized in the title or abstract” “sample size” “allocation concealment” “implementation” “blinding” “recruitment” “ancillary analyses” “harms” “interpretation” “registration” and “protocol” was poor with positive rates less than 10%. Based on the STRICTA guidelines, the items “extent to which treatment varied” “number of needle insertions per subject per session” and “control or comparator interventions” had poor reporting quality with positive rates of less than 10%. Substantial agreement was observed for most items and excellent agreement for some items.
Conclusion: The reporting quality of RCTs of acupuncture for labor pain is suboptimal generally. Rigorous adherence to the CONSORT statement and the STRICTA guidelines should be emphasized in future studies to improve the quality of acupuncture RCT reports.
Introduction
Labor pain is one of the severe pains caused by uterine contractions, cervical dilatation, and vaginal and pelvic floor stretching (1). The pain of labor is not as well localized as somatic pain. It is diffuse and may reach the iliac crests, buttocks, or thighs. This pain adversely affects uterine oxygen consumption as well as uterine contractility, and it increases peripheral resistance, cardiac output, and blood pressure (2). Pain, anxiety, and stress during delivery increase the release of catecholamines and cortisol into circulation. Elevated cortisol levels will lead to decreased uterine blood flow and delayed contractions even the death of the newborn (3–5). Due to the increased demand for pain management, neuraxial labor analgesia is regarded as the most effective treatment for labor pain (6, 7). However, drugs used for epidural analgesia may temporarily lower blood pressure, thus reducing the blood flow to the fetus and slowing its heart rate.
Acupuncture is effective in treating various pains and has been valued and recognized in Western countries in recent years. Acupuncture is the most used treatment for pain, and it is the most widely covered by health insurance in the World Health Organization (WHO) traditional medicine strategy 2014–2023 (8). The literature on acupuncture for labor pain has grown year by year over the past two decades. Ramnero et al. (9) conducted a randomized trial of acupuncture during delivery and found that acupuncture significantly reduced the need for epidural analgesia and resulted in greater maternal relaxation when compared to a control group. However, no studies have explored the quality of the RCT reports, especially the details of the acupuncture intervention. The number of RCT reports has increased because many clinical studies on acupuncture for labor pain have been conducted both nationally and internationally (10). The distinction between Chinese and Western acupuncture results in disparate acupuncture reports. Chinese acupuncture is guided by the basic theories of Traditional Chinese Medicine, using acupuncture points as stimulation points, and emphasizing “de qi” to treat various pains such as labor pain, and regulate the functions of the whole body and internal organs. On the other hand, Western acupuncture is based on modern neuroscience knowledge and determines the trigger point and the depth of stimulation based on theories such as local axonal reflex, dorsal root reflex, homo- and cross-segmental neuromodulation, and central regulation. Western acupuncture also focuses on quantifying stimulation parameters, such as stimulation duration and the number of needles used to treat painful diseases (11, 12).
The CONSORT statement is an evidence-based, minimum set of recommendations for reporting randomized trials (13). The STRICTA recommendations comprised a checklist that expanded the generic content of item 4 of the CONSORT statement, which is set for clinical trials with controlled groups of acupuncture to report interventions. They offered a standard method for facilitating transparent and more complete reporting of acupuncture RCTs and assisting authors' critical appraisal and interpretation (14). The release of the CONSORT statement and the STRICTA guidelines positively impacted the quality of reporting (15). Both have been widely accepted as the standard for clinical trial reporting, with statistically significant increases in citations (16, 17).
To our knowledge, no study has previously investigated the quality of reporting of trials in this area against these standards. Therefore, the CONSORT statement and the STRICTA guidelines were used to evaluate the quality of reporting of RCTs of acupuncture for labor pain. We also aimed to provide authors with more favorable information for high-quality RCT designs for reference.
Methods
Search strategy
The following eight databases were systematically searched: Chinese National Knowledge Infrastructure (CNKI), VIP information (VIP), Wanfang Data, SinoMed, PubMed, Web of Science, Cochrane Library, and Embase, from their inception until August 31, 2021. The details of the search strategy can be found in the online Supplementary File 1. No language restrictions were applied in the search strategy.
Inclusion and exclusion criteria
RCTs that examined the effects of acupuncture interventions for labor pain were included. The inclusion criteria are as follows.
Types of reports
Only RCTs were included in our research. Non-randomized trials, cross-over clinical trials, case-control studies, retrospective studies, animal experiments, case reports, and reviews were excluded. RCT test reports without available data or results were also excluded.
Participants of included RCTs
All study subjects were women aged 21 to 31 years old, including primipara and multipara, with a normal singleton pregnancy and a fetus in a cephalic presentation at a gestational age of 37 to 42 weeks, at 3–6 cm cervical dilation of labor, and without any obstetrical complications for vaginal delivery. Patients must give verbal or written consent to participate in the study. Patients with hypertension, diabetes mellitus, and coronary heart disease were excluded because these diseases can have an impact on delivery, with a significantly higher incidence of surgical delivery, birth injuries, and postpartum hemorrhage which would affect the trial.
Types of interventions
Acupuncture is manual or electronic stimulation due to filiform needle penetration of the body, scalp, or auricular acupoints regardless of diameter, length, manufacturer, or material. The study group included any type of invasive acupuncture, including manual acupuncture, electroacupuncture, or auricular (ear) acupuncture, or in combination with other interventions (e.g., acupuncture-related treatment, drugs, physical therapy) were included. Trials that compared the effectiveness of acupuncture therapy with sham acupuncture or active control procedures were included. Studies testing non-filiform-needle-penetration (e.g., acupoint injection, bleeding with plum-blossom needles) as a primary intervention were excluded. Our study did not include studies comparing non-invasive techniques such as laser acupuncture or acupressure or moxibustion trials. The control groups included the use of placebo, treatment, as usual, no treatment, or other active interventions. Regarding the control interventions, RCTs using Chinese herbal medicine were excluded because of the ambiguous pharmacological mechanism and curative effect. Moreover, trials that only compared different forms of acupuncture were also excluded since we did not intend to investigate whether one type of acupuncture was more effective than another.
Types of outcome measures
In our review, the assessment of acupuncture for labor pain focused on authoritative indicators are as follows: (1) CONSORT score status (2) STRICTA score status (3) Cohen's κ-statistical analysis, and 95% CIs for each report.
Document screening
Two researchers independently searched the Chinese and English databases (Shi-Yi Jiang for PubMed, EMBASE, Cochrane, and Web of Science; Ying Cui for CNKI, VIP, Wanfang, and SinoMed), and the third reviewer (Tao Jiang) organized the searched articles using the EndNote X9 software. Duplicate records were initially identified and removed by the reviewer (Li Jing) using EndNote X9.
After removing duplicates, two reviewers (Shi-Yi Jiang and Ying Cui) independently evaluated the articles for relevance by title and abstract to look for potentially eligible studies. Subsequently, read the full texts for potential inclusion. If an article did not meet the inclusion criteria and/or met one or more exclusion criteria, it was moved to the folder marked “excluded” in EndNote X9. Some controversial articles were marked into the “questionable” folder, in which case they were resolved through discussion and consensus among the three reviewers. The third reviewer (Tao Jiang) also verified all the information and contacted the primary authors for unavailable articles if needed.
Data extraction
Three reviewers (Shi-Yi Jiang, Ying Cui, and Ji-Peng Yang) extracted the information from each included trial into predefined data collection forms meeting the Cochrane standard. In the “characteristics of trials” form, studies were described in terms of author, country, participants, interventions, control types, frequency and treatment course, duration of one session, and main outcomes. The two reviewers resolved the situation through discussion and negotiation if the data could not be determined during the extraction process. If needed, we contacted the trial's lead author by email to provide incomplete data. In addition, “data extraction” forms were used to record and calculate relevant data for the outcomes.
Assessment of reporting quality
Two reviewers (Shi-Yi Jiang and Ying Cui) used the CONSORT statement and STRICTA guidelines to assess the reporting quality of the included RCTs independently. Each item was scored 1 if it was reported and 0 if it was not clearly stated. Any difficulties or disagreements in the process were solved by the third reviewer (Tao Jiang).
Cohen's κ-statistic was calculated to evaluate the degree of agreement between the two evaluators. A κ of 0.40 or lower, between 0.40 and 0.60, between 0.60 and 0.80, and from 0.80 to 1.00 were considered poor, moderate, substantial, and perfect agreements, respectively. The number, percentage, Cohen's κ-statistical analysis, and 95% CIs of each variable were summarized using the Statistical Package for the Social Sciences (SPSS) V 26.0.
Results
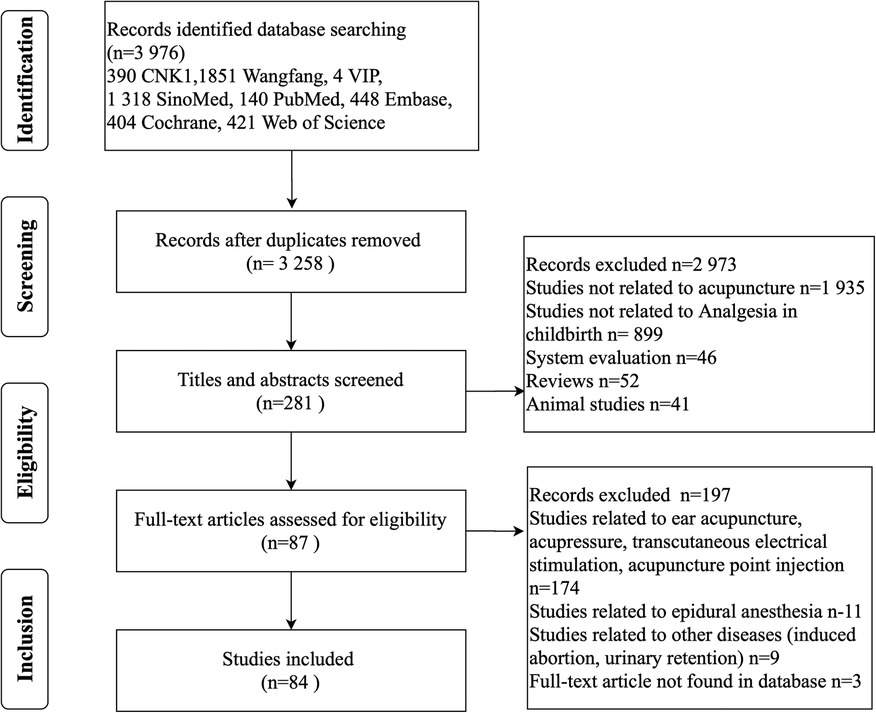
The initial search identified a total of 3, 976 relevant reports, of which 2, 563 were published in Chinese and 1, 413 were published in English. After eliminating duplicates, 3, 258 research articles were left for further consideration. After screening titles and abstracts, 2, 977 records were excluded due to relevance to the topic of interest, leaving 281 studies to be evaluated for inclusion. Articles involving acupressure, patching, transcutaneous electrical stimulation, acupoint injections, epidural anesthesia, and ear acupuncture were excluded. Articles, where the original article could not be found, were also excluded. The final 84 eligible RCTs were extracted for analysis. The search and selection process is outlined in Figure 1.
Study characteristics
Years of publication
All 84 articles were RCTs published from 1974 to 2021. 31(36.9%) RCTs were published in the early period (before 2010), and 53 (63.1%) RCTs were in the late period (after 2010). All included articles were in Chinese (73.8%) or English (26.2%). We can find the number of publications of RCTs after 2010 is about double the number before 2010. The trend graph for the year of publication is outlined in Figure 2.
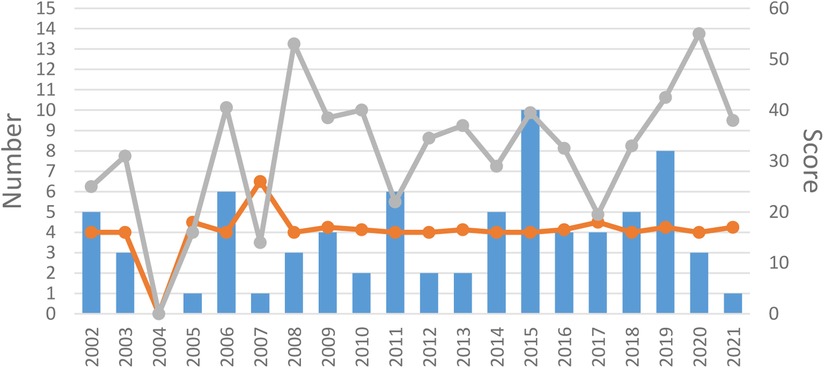
Figure 2. Year of publication. The blue bar is the number of RCTs published each year. The orange line is the median OQS with CONSORT statement of each year. The gray line is the median OQS with STRICTA statement of each year.
Journals and language
These 84 papers were published in 63 different journals. 3 (3.5%) were published in the Shanghai Journal of Acupuncture and Moxibustion, 2 (2.3%) in the Chinese Journal of Acupuncture and Moxibustion, and 3 (3.5%) in the Journal of Clinical Acupuncture and Moxibustion. Among the 87 RCT papers, 62 (73.8%) were written in Chinese. The remaining 22 (26.2%) were published in English and appeared in 18 different journals. In terms of journal type, 58 (69%) were published in general medical journals, 13 (15.5%) in specialty medical journals, and 13 (15.5%) in traditional or alternative medical journals.
Participants
12, 014 full-term deliveries including primipara and multipara, were recruited in 84 RCTs, with sample sizes ranging from 12 to 500. Participants were recruited as outpatients or inpatients in a hospital setting. All participants had to meet the required delivery conditions, with no contraindications to obstetric delivery, no serious comorbidities, complications, etc. All trials restricted the age range and medication use of participants. In addition, all the included trials excluded structural abnormalities.
Intervention/controls and comparison
Interventions include manual acupuncture or electroacupuncture alone, acupuncture combined with medication, or other interventions such as relaxation techniques. The most used acupuncture points are LI-4 and SP-6 mainly. 61 of these trials required the presence of a “De qi” sensation after stimulation, which is the key factor in the effectiveness of acupuncture treatment. The controls in the trials were generally used as blank controls, and only eight trials used sham acupuncture as a control group.
Outcome measures
Of the trials of acupuncture for labor pain, 41 assessed pain using the visual analog scale (VAS), 11 trials used the WHO pain scale for assessment, 38 trials measured labor time, 28 trials assessed bleeding at 2 h postpartum, and 27 trials used the Apgar scores.
Reporting quality evaluation
Reporting quality based on CONSORT
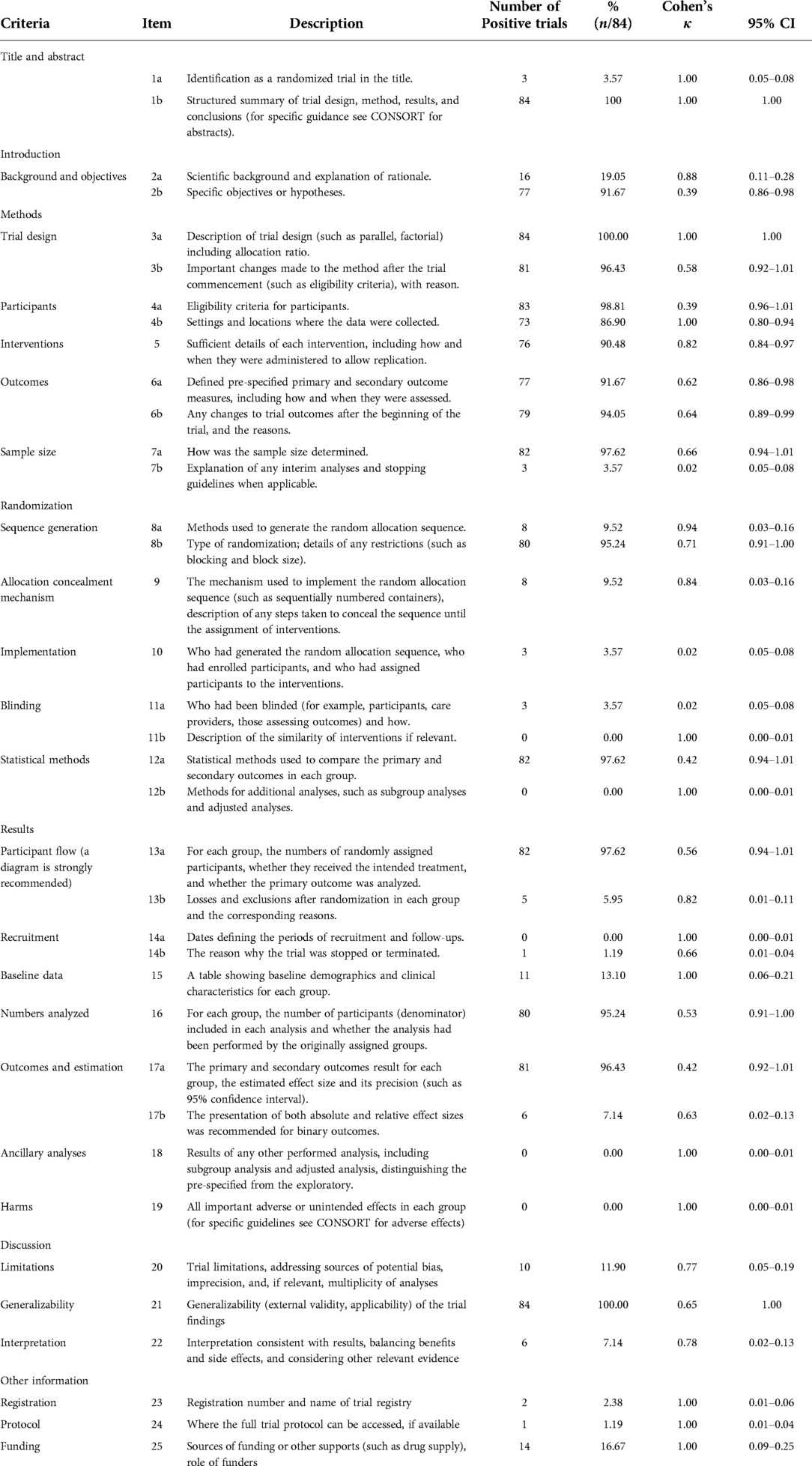
The ratings of reporting quality based on the CONSORT statements are listed in Table 1. The positive reporting rates of items such as “trial design” “participants” “intervention” “outcomes” and “outcomes and estimation” were above 80%. However, the quality of reporting in items “randomized in the title or abstract” “sample size” “allocation concealment” “implementation” “blinding” “recruitment” “intent-to-treat analysis” “ancillary analyses” “limits” and “trial protocol” was very poor with positive rates <10%. According to the statistical results, there were no reports describing the following relevant items: “description of the similarity of interventions if relevant” “methods for additional analyses, such as subgroup analyses and adjusted analyses” “dates defining the periods of recruitment and follow-ups” “subgroup analysis and adjusted analysis, distinguishing the pre-specified from the exploratory” “all important adverse or unintended effects in each group”. In this study, Cohen's κ-statistic showed that both reviewers agreed on all items. Consistency was judged to be moderate, substantial, or perfect for most items.
Reporting quality based on STRICTA
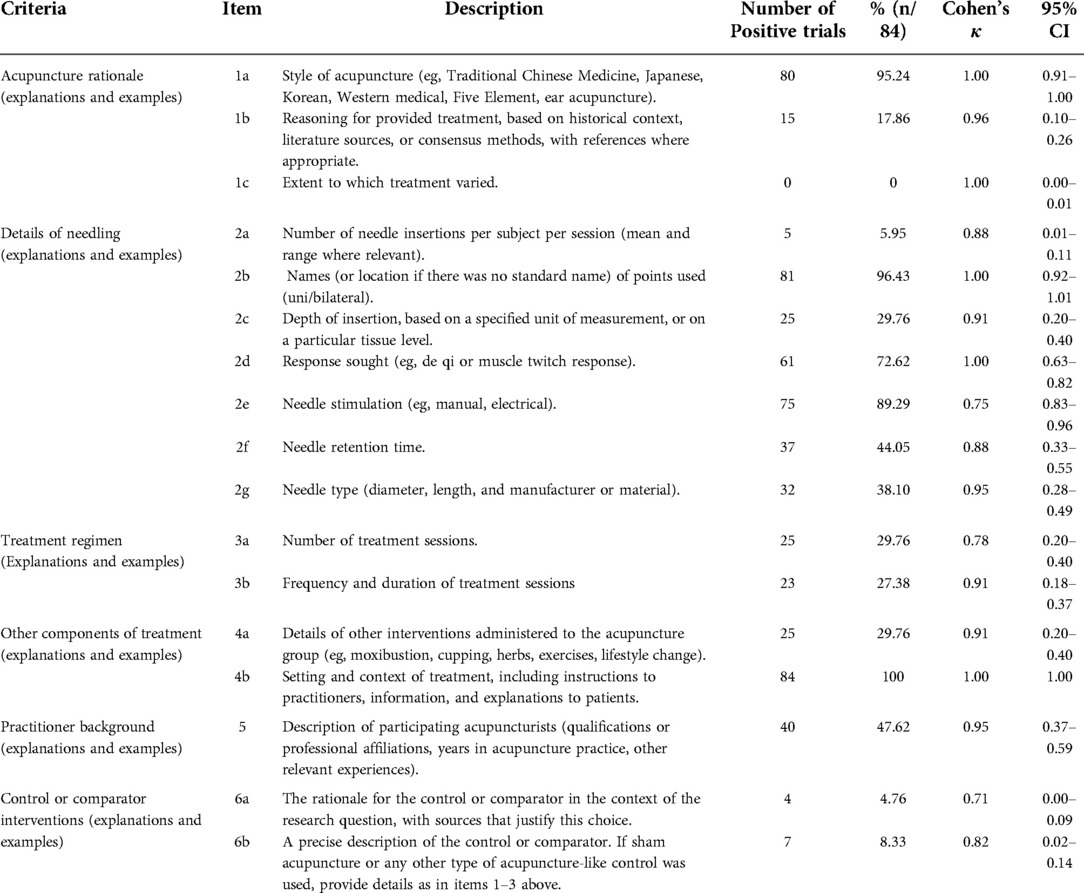
The reporting quality ratings for the needling details based on the STRICTA guidelines are listed in Table 2. Good reporting existed for the items “style of acupuncture” “names of points used” “needle stimulation” and “explanation to patients”, with positive ratings >80%. However, the reporting quality for items “extent to which treatment varied” “number of needle insertions per subject per session” and “control or comparator interventions” was poor, with positive rates <10%. Cohen's κ-statistic showed that good agreement was observed for the items “needle stimulation” “number of treatment sessions” and “control or comparator interventions”. Perfect agreement was observed for the other remaining items.
Discussion
This study first systematically assessed and analyzed the quality of reported RCTs of acupuncture for labor pain by statistical methods, adhering strictly to the CONSORT statement and STRICTA guidelines. It has been demonstrated that RCTs of acupuncture for labor pain were generally varied in reporting quality in the past two decades, with a considerable number of essential items incomplete or omitted. This may substantially bias readers' judgment of the actual and verifiable results of research, as well as their external validity (18, 19).
CONSORT statement
Generally, scores of reporting quality were not satisfactory enough in the 84 trials in accordance with the CONSORT statement, with only 16 of the total 37 items (including sub-items of each section) showing relatively sufficient reporting rates (above 85%). The under-reported items are particularly those concerned methodological sections such as methods of generating random sequence, allocation concealment and implementation of randomization, as well as blinding. This is consistent with results of our previous review on the annual evidence of reported RCTs using acupuncture and moxibustion therapy during 2019 and 2020, which found an insufficiency in reporting of randomization and blinding (20).
Regarding the randomization section, it is suggested that authors should provide sufficient information so that the reader can evaluate the likelihood of bias in the process of generating random allocation sequence and grouping (item 8a) (13). However, it was merely reported by eight trials, of which only one trial fully reported methods of sequence generation and allocation concealment. Besides, for the description of the type of randomization (item 8b), though reported in 95.24% of the research, most trials only referred to “random” “random number tables” and “opaque envelopes”; Some studies, despite the use of the word “random”, have used non-random or quasi-random methods, such as “alternating grouping” “grouping by hospital number”. Moreover, how the random sequence is implemented is important when the subject enters a trial (item 9), and the ideal approach in this case is to use allocation (21), aiming to prevent selection bias (22). While only eight of the included trials mentioned in their reports of using sequentially numbered, opaque, sealed envelopes, none had reported if envelopes are opened in order and other details. Such flaws in reporting of detailed randomization process will impact the bias risk assessment and subsequently, the judgment of research reliability and adaptability (18, 23).
Blinding, additionally, is used for preventing bias in implementation and outcome determination (21), for the purpose of protecting random sequences after the allocation occurs. However, blinding is not always possible. Since the late 1970s, clinical trials have been using a “real vs. sham” model to evaluate the effects of acupuncture (24), with much effort devoted to the development and validation of “sham” (or designated as “placebo” by some researchers) needling, for the points of view of minimizing therapeutic effects and successful blinding (25). To date, however, there still lacks consensus on the complete inertness of either non-penetrating needling appliance (such as Streitberger needle, Park sham device, Takakura sham device) or “sham” penetrating process (e.g., needling at non-specific points, shallow insertion, minimal stimuli), as well as their reliability for blinding of acupuncturists or patients (26). The true effect size of acupuncture may be greatly underestimated when compared to that of the current sham controls (26, 27). Besides, the recognition rate of two types of sham needling procedures (either shallow penetrating or needling at non-specific points) has been shown as high as 50% to 83% among health volunteers (28); the blinded effect of non-penetrating devices was also not ideal, especially among subjects with acupuncture experience (29). According to our findings, only three included trials briefly mentioned the use of blinding—all performed on the patients. In general, such issues of blinding and sham controlling in acupuncture trials still need to be addressed to promote their feasibility and reporting quality.
Another issue is the determination of the sample size, which was only 3.57% of the included trials. The lack of a basis for determining the sample size calculation is an important factor restricting clinical research; inaccurate calculation will also cause the waste of effort and resources and impact the quality of research (30). Moreover, in terms of additional information, the International Committee of Medical Journal Editors (ICMJE) requires all clinical trials to be registered to improve transparency and accountability (31). However, only two trials reported the clinical trial registration numbers on ClinicalTrials.gov, and one published the full protocol. However, most RCTs did not provide a complete trial protocol and did not mention funding sources and other support. This is not conducive to the standardized management of clinical studies and the quality of clinical trials. Both authors and editors need to pay attention to it (32).
STRICTA guidelines
The STRICTA guidelines was developed in 2010 as a formal extension of the CONSORT statement and had become an independent guideline for regulating the details of the acupuncture procedure (33). It has been found that half of the published articles reviewed lacked intervention elements and insufficient detail, and journals have increased their requirements for the use of this guideline in recent years (34). However, contradictions exposed between trials largely reflected the discrepant processing relevant to ethnic and environmental differences, culture, policies, as well as physician's skills, and correspondingly, the variant “therapeutical habits” in acupuncture procedure (frequency, course, needling site, manipulation, time for needle retention, et al.) (35, 36). The STRICTA checklist assessment in our study indicated moderate or low reporting quality with most items, especially explanations of needling, treatment regimen and controls.
First, reporting rates for the number of needles and the needling depth in Chinese articles were much lower than English publications. Chinese acupuncturists tend to judge the therapeutic effect by patients' status or the “deqi” responses and apply individual regimen via targeted point selection and manipulation. While Western (or called modern) acupuncture relatively focuses on the trigger-point theory and quantitative parameters during stimulation (37, 38). Despite the difference, inadequate reporting of items or details of the manipulation is a barrier to future replication. Related studies have shown that the number of needles inserted and the depth of needle insertion, as well as the correct acupuncture point, can be crucial to the effectiveness of acupuncture treatment (34), providing a valuable reference for clinicians. Moreover, items related to contextual factors and practitioner qualifications were also severely lacking, which will impact the validity and reliability of those interventions.
In addition, there showed a difference in the use of controls between trials. Most of studies conducted in China applied no-treatment or drug controls, while in Western studies, up to 36% used sham acupuncture controls. Control groups for acupuncture should also be rigorously described to facilitate other researchers seeking to replicate acupuncture control interventions, assess the internal validity of clinical outcomes, and promote the use of effective interventions in clinical practice (39, 40). This is particularly important for empirical medicine like acupuncture, as different acupuncturists treating different study subjects may affect the generalizability of trial results. Therefore, details of the interventions for the experimental and control groups need to be explained in a structured format. In practice, acupuncture is a practitioner-dependent, experience-requiring intervention. These items meant that under-reporting of items or details related to manipulation and technique was a significant barrier to future replication studies. Therefore, full compliance with STRICTA guidelines is emphasized in the design, implementation, and reporting of acupuncture RCTs to ensure the generalizability and reliability of studies.
In the evaluated RCTs, no articles reported adverse effects, possibly because the adverse effects were not taken seriously or were not documented. The reporting of adverse events, especially for invasive interventions, is critical to understanding safety issues. The human body can have varying degrees of adverse reactions to any intervention, ranging from nausea, vomiting, bruising, and bleeding in mild cases to pneumothorax and even death in severe cases.
Despite the strict inclusion criteria and methodology, this study still has some limitations. Due to language limitations, we only included Chinese and English reports of RCTs in this area; countries and regions with high rates of acupuncture use, like Japan and Korea, are not included. Both researchers inserted subjective opinions in the CONSORT and STRICTA quality assessment of each included RCT, and although we used Cohen's κ-statistic to reduce inconsistency between evaluators, this still does not exclude bias in the results due to subjective evaluations. Publication bias caused by journal editors, authors, funding sources, and literature inclusion is a serious problem in systematic reviews and meta-analyses, which can affect the validity and generalization of conclusions. Most of the articles included in the journal were dominated by positive results, while negative results may be delayed in publication or ignored, resulting in publication bias (41).
Conclusion
In conclusion, this study indicates that the quality of RCTs of acupuncture for maternal labor pain was inadequate and needs further improvement, especially in terms of some key methodological entries and acupuncture details. Future RCTs still need further refinement under the CONSORT statement and STRICTA guidelines as both have become important references for journals and editors in evaluating and selecting manuscripts. Researchers are recommended to adopt such guidelines when designing or reporting acupuncture RCTs to enhance the reporting quality and transparency of their studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval and written informed consent were not required for this study in accordance with the local legislation and institutional requirements since all included data were obtained from published articles.
Author contributions
TJ, BL, and Y-HD conceptualized and designed the study. S-YJ, YC, and JL acquired the data and extracted the data. TJ, S-YJ, and J-PY performed statistical analysis and assessed the reporting quality. TJ and S-YJ wrote the manuscript. BP revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the project from National Science Foundation of China (No. 81904093) and Foundation of State Key Laboratory of Component-based Chinese Medicine (grant QMJ 202101).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2022.999162/full#supplementary-material.
References
1. Mackenzie IZ, Xu J, Cusick C, Midwinter-Morten H, Meacher H, Mollison J, et al. Acupuncture for pain relief during induced labour in nulliparae: a randomised controlled study. BJOG Int J Obstet Gynaecol. (2011) 118(4):440–7. doi: 10.1111/j.1471-0528.2010.02825.x
2. Brownridge P. The nature and consequences of childbirth pain. Eur J Obstet Gynecol Reprod Biol. (1995) 59:S9–S15. doi: 10.1016/0028-2243(95)02058-Z
3. Huntley AL, Coon JT, Ernst E. Complementary and alternative medicine for labor pain: a systematic review. Am J Obstet Gynecol. (2004) 191(1):36–44. doi: 10.1016/j.ajog.2003.12.008
4. Stocche RM, Klamt JG, Antunes-Rodrigues J, Garcia LV, Moreira AC. Effects of intrathecal sufentanil on plasma oxytocin and cortisol concentrations in women during the first stage of labor. Reg Anesth Pain Med. (2001) 26(6):545–50. doi: 10.1053/rapm.2001.27851
5. Vogl SE, Worda C, Egarter C, Bieglmayer C, Szekeres T, Huber J, et al. Mode of delivery is associated with maternal and fetal endocrine stress response. BJOG Int J Obstet Gynaecol. (2006) 113(4):441–5. doi: 10.1111/j.1471-0528.2006.00865.x
6. Traynor AJ, Aragon M, Ghosh D, Choi RS, Dingmann C, Vu Tran Z, et al. Obstetric anesthesia workforce survey: a 30-year update. Anesth Analg. (2016) 122(6):1939–46. doi: 10.1213/ANE.0000000000001204
7. Nanji JA, Carvalho B. Pain management during labor and vaginal birth. Best Pract Res Clin Obstet Gynaecol. (2020) 67:100–12. doi: 10.1016/j.bpobgyn.2020.03.002
8. Fan AY, Miller DW, Bolash B, Bauer M, McDonald J, Faggert S, et al. Acupuncture's role in solving the opioid epidemic: evidence, cost-effectiveness, and care availability for acupuncture as a primary, non-pharmacologic method for pain relief and management–white paper 2017. J Integr Med. (2017) 15(6):411–25. doi: 10.1016/S2095-4964(17)60378-9
9. Ramnerö A, Hanson U, Kihlgren M. Acupuncture treatment during labour–a randomised controlled trial. BJOG Int J Obstet Gynaecol. (2002) 109(6):637–44. doi: 10.1111/j.1471-0528.2002.01212.x
10. Schlaeger JM, Gabzdyl EM, Bussell JL, Takakura N, Yajima H, Takayama M, et al. Acupuncture and acupressure in labor. J Midwifery Womens Health. (2017) 62(1):12–28. doi: 10.1111/jmwh.12545
11. Lin WL, Chen B, Dai XQ. Comparative study and reflection on the development of Chinese and western acupuncture [in Chinese]. Hunan J Tradit Chin Med. (2022) 38(01):111–4. doi: 10.16808/j.cnki.issn1003-7705.2022.01.033
12. Gao LL, Chen B, Guo Y. Comparative analysis of western acupuncture and Chinese acupuncture [in Chinese]. Liaoning J Tradit Chin Med. (2015) 42(12):2407–9. doi: 10.13192/j.issn.1000-1719.2015.12.052
13. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Br Med J. (2010) 340:c869. doi: 10.1136/bmj.c869
14. MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, et al. Revised STandards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. (2010) 7(6):e1000261. doi: 10.1371/journal.pmed.1000261
15. Ma B, Chen ZM, Xu JK, Wang YN, Chen KY, Ke FY, et al. Do the CONSORT and STRICTA checklists improve the reporting quality of acupuncture and moxibustion randomized controlled trials published in Chinese journals? A systematic review and analysis of trends. PLoS One. (2016) 11(1):e0147244. doi: 10.1371/journal.pone.0147244
16. Svenkerud S, MacPherson H. The impact of STRICTA and CONSORT on reporting of randomised control trials of acupuncture: a systematic methodological evaluation. Acupunct Med. (2018) 36(6):349–57. doi: 10.1136/acupmed-2017-011519
17. Prady SL, Macpherson H. Assessing the utility of the standards for reporting trials of acupuncture (STRICTA): a survey of authors. J Altern Complement Med. (2007) 13(9):939–43. doi: 10.1089/acm.2007.7186
18. Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. (2005) 20(2):187–91; discussion 191–3. doi: 10.1016/j.jcrc.2005.04.005
19. He J, Du L, Liu G, Fu J, He X, Yu J, et al. Quality assessment of reporting of randomization, allocation concealment, and blinding in traditional Chinese medicine RCTs: a review of 3159 RCTs identified from 260 systematic reviews. Trials. (2011) 12:122. doi: 10.1186/1745-6215-12-122
20. Pang B, Ji ZC, Zhang JH, Du YZ, Li L, Ou Y. 2019–2020 Annual evidence analysis of randomized controlled clinical trials in acupuncture [in Chinese]. Tianjin Chin Med. (2021) 38(11):1408–13. doi: 10.11656/j.issn.1672-1519.2021.11.11
21. Schulz KF, Chalmers I, Grimes DA, Altman DG. Assessing the quality of randomization from reports of controlled trials published in obstetrics and gynecology journals. JAMA. (1994) 272(2):125–8. doi: 10.1001/jama.1994.03520020051014
22. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. (1995) 273(5):408–12. doi: 10.1001/jama.1995.03520290060030
23. Pandis N, Polychronopoulou A, Eliades T. Randomization in clinical trials in orthodontics: its significance in research design and methods to achieve it. Eur J Orthod. (2011) 33(6):684–90. doi: 10.1093/ejo/cjq141
24. Lewith GT, Machin D. On the evaluation of the clinical effects of acupuncture. Pain. (1983) 16(2):111–27. doi: 10.1016/0304-3959(83)90202-6
25. Yang HY, Wang DY, Dong X, He L, Lu SY, Jiao ML. Status of placebo acupuncture control research [in Chinese]. Chin Acupunct Moxibustion. (2020) 40(03):337–41. doi: 10.13703/j.0255-2930.20190311-0002
26. Pang B, Du YH, Jiang T, LI J. The “super placebo” debate [in Chinese]. Chin J Tradit Chin Med. (2017) 32(05):2174–7.
27. Linde K, Niemann K, Schneider A, Meissner K. How large are the nonspecific effects of acupuncture? A meta-analysis of randomized controlled trials. BMC Med. (2010) 8:75. doi: 10.1186/1741-7015-8-75
28. Wong EL, Leung PC, Zhang L. Placebo acupuncture in an acupuncture clinical trial. How good is the blinding effect? J Acupunct Meridian Stud. (2015) 8(1):40–3. doi: 10.1016/j.jams.2014.10.010
29. Tsukayama H, Yamashita H, Kimura T, Otsuki K. Factors that influence the applicability of sham needle in acupuncture trials: two randomized, single blind, crossover trials with acupuncture experienced subjects. Clin J Pain. (2006) 22(4):346–9. doi: 10.1097/01.ajp.0000176359.94644.mL
30. Noordzij M, Dekker FW, Zoccali C, Jager KJ. Sample size calculations. Nephron Clin Pract. (2011) 118(4):c319–23. doi: 10.1159/000322830
31. DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the international committee of medical journal editors. JAMA. (2004) 292(11):1363–4. doi: 10.1001/jama.292.11.1363
32. MacPherson H, Altman DG, Hammerschlag R, Li Y, Wu T, White A, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. Acupunct Related Ther. (2015) 3(4):35–46. doi: 10.1016/j.arthe.2016.03.001
33. Kane RL, Wang J, Garrard J. Reporting in randomized clinical trials improved after adoption of the CONSORT statement. J Clin Epidemiol. (2007) 60(3):241–9. doi: 10.1016/j.jclinepi.2006.06.016
34. Vickers AJ, Vertosick EA, Lewith G, MacPherson H, Foster NE, Sherman KJ, et al. Acupuncture for chronic pain: update of an individual patient data meta-analysis. J Pain. (2018) 19(5):455–74. doi: 10.1016/j.jpain.2017.11.005
35. Fregni F, Imamura M, Chien HF, Lew HL, Boggio P, Kaptchuk TJ, et al. Challenges and recommendations for placebo controls in randomized trials in physical and rehabilitation medicine: a report of the international placebo symposium working group. Am J Phys Med Rehabil. (2010) 89(2):160–72. doi: 10.1097/PHM.0b013e3181bc0bbd
36. Yang Y, Shen Z, Wu Z, Luo L, Liu J, Liu B. [Strategy programming for acupuncture development along one belt-one-road countries]. Zhongguo Zhen Jiu. (2017) 37(4):343–8. doi: 10.13703/j.0255-2930.2017.04.001
37. Huang Q, Zhang Y, Ma Y, Hou B, Fei Q, Tan S, et al. Understanding of the trigger points of myalgia: acupuncture and dry needling exploration and modern acupuncture mechanism. Zhongguo Zhen Jiu. (2018) 38(7):779–84. doi: 10.13703/j.0255-2930.2018.07.027
38. Zhu J, Li J, Yang L, Liu S. Acupuncture, from the ancient to the current. Anat Rec (Hoboken). (2021) 304(11):2365–71. doi: 10.1002/ar.24625
39. Long Y, Chen R, Guo Q, Luo S, Huang J, Du L. Do acupuncture trials have lower risk of bias over the last five decades? A methodological study of 4 715 randomized controlled trials. PLoS One. (2020) 15(6):e0234491. doi: 10.1371/journal.pone.0234491
40. Guo Y, Zhao H, Wang F, Li SN, Sun YX, Han MJ, et al. Recommendations for acupuncture in clinical practice guidelines of the national guideline clearinghouse. Chin J Integr Med. (2017) 23(11):864–70. doi: 10.1007/s11655-016-2750-4
Keywords: labor pain, RCTs, acupuncture, CONSORT, STRICTA
Citation: Jiang T, Jiang S, Cui Y, Yang J, Du Y, Li J, Pang B and Li B (2022) Assessment of reporting quality in randomized controlled trials of acupuncture for labor pain. Front. Pain Res. 3:999162. doi: 10.3389/fpain.2022.999162
Received: 20 July 2022; Accepted: 3 November 2022;
Published: 21 November 2022.
Edited by:
John Farrar, University of Pennsylvania, United StatesReviewed by:
Lisa Witkin, Weill Cornell Medical Center, NewYork-Presbyterian, United StatesCarole Anne Paley, University of Leeds, United Kingdom
© 2022 Jiang, Jiang, Cui, Yang, Du, Li, Pang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Pang cGFuZ2JvMjAyMUAxNjMuY29t Bo Li aWJvdTExOUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Clinical Trials, Methods, and Evidence Synthesis, a section of the journal Frontiers in Pain Research
 Tao Jiang1,2,†
Tao Jiang1,2,† ShiYi Jiang
ShiYi Jiang Bo Pang
Bo Pang