- 1Danish Headache Center, Department of Neurology, Rigshospitalet Glostrup, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
- 2Department of Biotechnological and Applied Clinical Sciences, Neurological Institute, University of L'Aquila, L'Aquila, Italy
- 3Hospital da Luz Headache Center, Neurology Department, Hospital da Luz, Lisboa, Portugal
- 4Universidade Católica Portuguesa, Institute of Health Sciences, Center for Interdisciplinary Research in Health, Lisboa, Portugal
- 5Neurology Department, Istanbul University Cerrahpaşa School of Medicine, Istanbul, Turkey
- 6Danish Knowledge Center on Headache Disorders, Rigshospitalet Glostrup, Glostrup, Denmark
- 7Department of Neurorehabilitation/Traumatic Brain Injury, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
Introduction: Accessibility of treatment with monoclonal antibodies targeting the calcitonin gene-related peptide (CGRP) signaling pathway is impeded by regulatory restrictions. Affected individuals may seek out other services including non-pharmacological therapies. Thus, we found it timely to ascertain the use of non-pharmacological therapies in individuals with treatment-resistant migraine eligible for and naïve to treatment with CGRP-signaling targeting monoclonal antibodies.
Methods: We conducted a single-center cross-sectional observational study of patients eligible for and naïve to treatment with monoclonal antibodies targeting CGRP or its receptor. We recorded demographical information (gender, age, educational level, employment status, and income), disease burden (frequency of headache days and migraine days), previous use of preventive pharmacological medications for migraine, and use of non-pharmacological therapies over the past 3 months including frequency of interventions, costs, and patient-reported assessment of efficacy on a 6-point scale (0: no efficacy, 5: best possible efficacy).
Results: We included 122 patients between 17 June 2019 and 6 January 2020; 101 (83%) were women and the mean age was 45.2 ± 13.3 years. One-third (n = 41 [34%]) had used non-pharmacological therapy within the past 3 months. Among these participants, the median frequency of different interventions was 1 (IQR: 1–2), the median number of monthly visits was 2.3 (IQR: 1.3–4), mean and median monthly costs were 1,086 ± 1471, and 600 (IQR: 0–1200) DKK (1 EUR = ~7.5 DKK), respectively, and median patient-reported assessment of the efficacy of interventions was 2 (IQR: 0–3).
Conclusion: Even in a high-income country with freely accessible headache services and universal healthcare coverage, there was a non-negligible direct cost in parallel with low satisfaction for non-pharmacological therapies among patients at a tertiary headache center.
Introduction
Headache disorders constitute a major public health problem as they lead to significant disability worldwide, impair quality of life, reduce productivity, and incur a substantial financial burden on both individuals and economies (1–3). Migraine, specifically, directly affects more than 1 billion people across the world and constitutes a leading cause of disability (1, 2). In Europe, the financial burden of migraine has been estimated at €50 to €111 billion in 2011 (3). Assumingly, these costs are the highest for those with the highest disease burden, i.e., individuals with chronic migraine, and evidence suggests that these individuals often require referral to specialist care (4). These services include treatment with migraine-specific medications that target the calcitonin gene-related peptide (CGRP) signaling pathway and are efficacious and tolerable in patients with treatment-resistant migraine (4, 5). In Europe, monoclonal antibodies (mAbs) against the CGRP ligand or its receptor are the drugs available in this class, but their accessibility is low as regulatory restrictions often limit their use due to high costs (4). Even though some countries provide free access to these therapies if pre-specified eligibility criteria are fulfilled, accessibility is often impeded as these medications are available at a limited number of specialist services. In clinical practice, severely affected individuals often seek out other services including non-pharmacological therapies (5), but data on this perspective is sparse. Thus, we found it timely to ascertain the use of non-pharmacological therapies in individuals with treatment-resistant migraine eligible for and naïve to treatment with CGRP-signaling targeting mAbs in a single-center cross-sectional observational study. In particular, we investigated the associated direct costs and patient satisfaction of non-pharmacological treatments.
Methods
Study Overview
We conducted a single-center, cross-sectional observational study. The present study was approved by the regional ethics committee and the Danish Data Protection Agency. All participants provided written informed consent before any assessments. We conducted the study in accordance with the Declaration of Helsinki (6).
Study Population
Participants eligible for and naïve to treatment with CGRP-mAbs followed at a tertiary headache center (the Danish Headache Center). According to national practice guidelines, participants eligible for treatment with and reimbursement for CGRP-targeting mAbs had a diagnosis of chronic migraine in accordance with the International Classification of Headache Disorders, 3rd edition (ICHD-3) (7) and documented failure based on lack of efficacy or tolerability of at least one antihypertensive and one anticonvulsant that is used for migraine prevention. Exclusion criteria were medication-overuse headache (MOH), as defined in ICHD-3, as this is a criterion for reimbursement for treatment with CGRP-targeting mAbs in Denmark (7). Participants underwent a semi-structured interview on demographical information (gender, age, educational level, employment status, and income), disease burden (frequency of headache days and migraine days), previous use of preventive pharmacological medications for migraine (with no time restrictions), and use of non-pharmacological therapies the past 3 months (both self-referral and by prescription), including frequency of interventions, costs, and patient-reported assessment of efficacy on a 6-point scale (0: no efficacy, 5: best possible efficacy). Non-pharmacological therapies were assessed for the past 3 months to determine current active use.
Statistical Methods
Continuous and count outcomes are presented using means with SDs. Binary and multinomial outcomes are presented with absolute numbers and percentages. All other data are presented as reported.
Results
Sociodemographic and Migraine-Related Characteristics
A total of 122 patients were included in the study between 17 June 2019 and 6 January 2020. As shown in Table 1, 101 (83%) were women, 21 (17%) were men, the mean age was 45.2 ± 13.3 years, 34 (28%) had a university degree, 75 (61%) were employed full time, part-time, manager or self-employed, and 25 (20%) had an annual income >400,000 DKK (~53,000 EUR). The baseline means monthly headache days was 22.3 ± 5 days, and the baseline mean monthly migraine days was 17.2 ± 6.3 days. One-fourth (31 [25%]) of the population was using any pharmacological preventive medication at the time. All patients had discontinued the use of at least two preventive medications for migraine due to lack of efficacy or tolerability; the median number of previous preventive medications was 7 (IQR: 5–8).
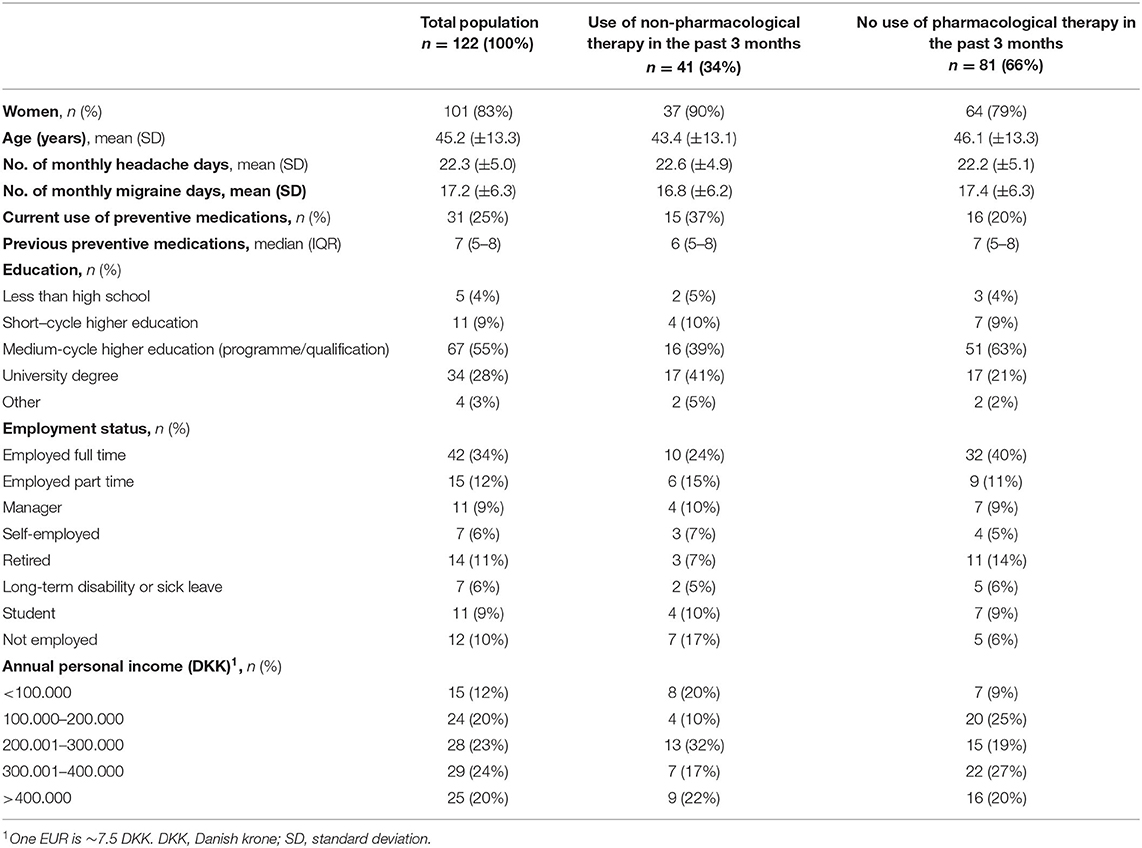
Table 1. Sociodemographic and migraine-related characteristics of participants using and not using non-pharmacological therapies.
Use of Non-pharmacological Therapies
The proportion of patients who had at least one non-pharmacological treatment for migraine in the previous 3 months was one-third (n = 41 [34%]) (Table 1). Among this one-third who had used a non-pharmacological treatment, an intervention with physical therapy (n = 17 [41%]) or massage (n = 17 [41%]) was most common. Other interventions included reflexology, (n = 7 [17%]), acupuncture (n = 6 [15%]), chiropractic (n = 4 [10%]), craniosacral therapy (n = 3), osteopathy (n = 3 [12%]), and others (n = 4 [12%]). Others included Body Self Development (Body SDS, n = 1), freeze gel (n = 1), mindfulness (n = 1), and neuromodulation device (n = 1). The median frequency of different non-pharmacological interventions was 1 (IQR: 1–2), the median number of monthly visits was 2 (IQR: 1–4) visits, and the mean and median monthly costs were 1,086 ± 1,471 and 600 (IQR: 0–1200) DKK (1 EUR = ~7.5 DKK), respectively, and the median patient-reported assessment of the efficacy of interventions was 2 (IQR: 0–3). Twelve patients (29%) out of 41 patients who used non-pharmacological therapies did not pay for their inventions.
Discussion
In a population of individuals with chronic migraine eligible for and naïve to treatment with mAbs targeting CGRP or its receptor at a tertiary headache center, one-third had used non-pharmacological therapies within the past 3 months.
Several non-pharmacological therapies are used in clinical practice for migraine, but there is limited evidence of the clinical benefits of these interventions for chronic migraine (5). While there is some evidence for neuromodulation and biobehavioral therapies, e.g., cognitive behavioral therapy, there is less evidence for the use of physical therapy for chronic migraine (5). Yet, physical therapy (alongside massage) was the most common intervention among users of non-pharmacological therapies. Musculoskeletal symptoms are common both outside and during migraine attacks in patients (8–11). Consequently, it has been suggested that interventions targeting these factors may provide clinical benefits, which provide a possible explanation for its popularity in this cohort. Another possible explanation is that physical therapy is often suggested as an adjunct therapy (5). However, a randomized clinical trial did not report any further gains from physical therapy as an adjunct to standard care (12), and a meta-analysis of controlled trials found that these interventions did not affect the frequency and intensity of attacks, albeit there was possibly a reduced duration of migraine attacks (13). Similarly, there is a low to very low level of evidence for other popular interventions in this cohort (5, 14).
There was a non-negligible direct cost of ~1,000 DKK (~133 EUR) per month in patients who had used non-pharmacological treatments within the past 3 months. With a median hourly wage of 218 DDK (~29 EUR) in 2012, this represents a net cost of ~5 h or 14% of a standardized Danish 37-h workweek (15). Of note, the majority of the cohort had an annual income lower than average (<400 DKK; ~53,000 EUR) (Table 1). (15) and the relative cost is, therefore, higher for this population. This is despite the welfare system of Denmark providing free access to healthcare providers, subsidization of patient fees for both pharmacological and non-pharmacological therapies, and, potentially, universal coverage for all its residents. Consequently, the true direct (and indirect) costs are almost certain to be higher – and perhaps much higher – than in our sample (many patients did not pay for their non-pharmacological treatments), and direct costs have been estimated to constitute 7% of the overall financial burden of migraine in Europe (3). When inquired to rate the efficacy of their non-pharmacological treatments, patients in this cohort ranked the efficacy of interventions in the lower end of the scale, and these findings may reflect that patients refractory to treatment with standard care are more likely to seek out and pay for other services despite the marginal benefits. However, the number of previous preventive medications between users and non-users of these services appear comparable. In addition, there does not appear to be a clear pattern related to the reported sociodemographic factors, but further data are needed to clarify this aspect.
The present study has some limitations. First, we did not inquire about the lifetime use of non-pharmacological interventions, which could have provided further insight into the non-users in our cohort, e.g., this sub-population may not have had any active use of other popular non-pharmacological treatments, e.g., dietary interventions, due to previous failed attempts in the past. Second, the design of the study was a priori descriptive with a relatively modest sample size in combination with averaging patient-reported outcomes and costs of different interventions, which limits regression modeling; therefore, more detailed investigations are merited to determine potential influencing factors.
Conclusion
Even in a high-income country with freely accessible headache services and universal healthcare coverage, there is a non-negligible direct cost for non-pharmacological therapies combined with overall low satisfaction with these therapies amongst patients treated at a tertiary headache center. These findings can be used to support policy-making decisions and incentivize stakeholders to make sensible healthcare policies to improve headache services. It is imperative that we further assess and address factors that influence treatment patterns.
Data Availability Statement
Data supporting the conclusions of this article is available upon reasonable request and acquisition of necessary permissions.
Ethics Statement
The studies involving human participants were reviewed and approved by Regional Committee on Health Research Ethics (RegionH). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
FA and TD contributed to conception and design of the work. LR and CC contributed to acquisition of data for the work. LR, CC, TD, and FA contributed to analysis of data for the work and wrote the first draft of the manuscript. All authors contributed to interpretation of data for the work, contributed to critical revision of the work for important intellectual content, and read and approved the final manuscript.
Conflict of Interest
SS reports grants, personal fees and/or non-financial support from Allergan, Novartis, Teva, Eli Lilly, AstraZeneca, Abbott, Medscape, Pfizer, Bayer, Medtronic, Starmed, Bristol-Meyer-Squibb, Daiichi-Sankyo, Lundbeck, Uriach and Neurodiem Ology Medical Education. RG-G reports Honoraria for conferences, consulting or educational activities: Novartis, Allergan/ Abbvie, Teva, Lilly, Lundbeck, Tecnifar, Pfizer, FLOAT, CMBE. Research grants: Fundação para a Ciência e Tecnologia (project 29675, MigN2Treat, 02/SAICT/2017), Novartis-Sociedade Portuguesa de Cefaleias and Learning-Health, Luz Saúde (Research group LiON, Luz Innovation on Neurosciences). DU reports grants, personal fees and/or non-financial support from Allergan, Novartis, Teva, Eli Lilly. FA has received honoraria and personal fees from Teva, Lundbeck, Novartis, Eli Lilly for lecturing or participating in advisory boards.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CGRP, calcitonin gene-related peptide; DKK, Danish krone; ICHD-3, International Classification of Headache Disorders, third edition; IQR, interquartile range; mAb, monoclonal antibody; SD, standard deviation.
References
1. Ashina M, Katsarava Z, Do TP, Buse DC, Pozo-Rosich P, Özge A, et al. Migraine: epidemiology and systems of care. Lancet. (2021) 397:1485–95. doi: 10.1016/S0140-6736(20)32160-7
3. Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. (2012) 19:703–11. doi: 10.1111/j.1468-1331.2011.03612.x
4. Eigenbrodt AK, Ashina H, Khan S, Diener HC, Mitsikostas DD, Sinclair AJ, et al. Diagnosis and management of migraine in ten steps. Nat Rev Neurol. (2021) 17:501–14. doi: 10.1038/s41582-021-00509-5
5. Ashina M, Buse DC, Ashina H, Pozo-Rosich P, Peres MF, Lee MJ, et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet. (2021) 397:1505–18. doi: 10.1016/S0140-6736(20)32342-4
6. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4 doi: 10.1001/jama.2013.281053
7. Arnold M. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
8. Al-Khazali HM, Younis S, Al-Sayegh Z, Ashina S, Ashina M, Schytz HW. Prevalence of neck pain in migraine: a systematic review and meta-analysis. Cephalalgia. (2022) 42:663–73. doi: 10.1177/03331024211068073
9. Hvedstrup J, Kolding LT, Ashina M, Schytz HW. Increased neck muscle stiffness in migraine patients with ictal neck pain: a shear wave elastography study. Cephalalgia. (2020) 40:565–74. doi: 10.1177/0333102420919998
10. Hvedstrup J, Kolding LT, Younis S, Ashina M, Schytz HW. Ictal neck pain investigated in the interictal state – a search for the origin of pain. Cephalalgia. (2020) 40:614–24. doi: 10.1177/0333102419896369
11. Do TP, Heldarskard GF, Kolding LT, Hvedstrup J, Schytz HW. Myofascial trigger points in migraine and tension-type headache. J Headache Pain. (2018) 19:84. doi: 10.1186/s10194-018-0913-8
12. Bevilaqua-Grossi D, Gonçalves MC, Carvalho GF, Florencio LL, Dach F, et al. Additional effects of a physical therapy protocol on headache frequency, pressure pain threshold, and improvement perception in patients with migraine and associated neck pain: a randomized controlled trial. Arch Phys Med Rehabil. (2016) 97:866–74. doi: 10.1016/j.apmr.2015.12.006
13. Luedtke K, Allers A, Schulte LH, May A. Efficacy of interventions used by physiotherapists for patients with headache and migraine—systematic review and meta-analysis. Cephalalgia. (2016) 36:474–92. doi: 10.1177/0333102415597889
14. Krøll LS, Callesen HE, Carlsen LN, Birkefoss K, Beier D, Christensen HW, et al. Manual joint mobilisation techniques, supervised physical activity, psychological treatment, acupuncture and patient education in migraine treatment, A systematic review and meta-analysis. J Headache Pain. (2021) 22:1–2. doi: 10.1186/s10194-021-01298-4
15. Danmarks Statistik. Befolkningens løn. (2013). Available online at: https://www.dst.dk/Site/Dst/Udgivelser/GetPubFile.aspx?id=19581&sid=befloen (accessed April 22, 2022).
Keywords: acupuncture, chiropractic, complementary and alternative medicine, headache, migraine, osteopathy, physical therapy, reflexology
Citation: Rundblad L, Cullum CK, Sacco S, Gil-Gouveia R, Uludüz D, Do TP and Amin FM (2022) Use of Non-pharmacological Therapies in Individuals With Migraine Eligible for Treatment With Monoclonal Antibodies Targeting Calcitonin Gene-Related Peptide (CGRP)-Signaling: A Single-Center Cross-Sectional Observational Study. Front. Pain Res. 3:935183. doi: 10.3389/fpain.2022.935183
Received: 03 May 2022; Accepted: 07 June 2022;
Published: 13 July 2022.
Edited by:
Cherubino Di Lorenzo, Sapienza University of Rome, ItalyReviewed by:
Roberta Messina, Vita-Salute San Raffaele University, ItalyEloisa Rubio-Beltran, King's College London, United Kingdom
Copyright © 2022 Rundblad, Cullum, Sacco, Gil-Gouveia, Uludüz, Do and Amin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faisal Mohammad Amin, ZmFpc2FsQGRhZGxuZXQuZGs=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
 Lucas Rundblad1†
Lucas Rundblad1† Simona Sacco
Simona Sacco Raquel Gil-Gouveia
Raquel Gil-Gouveia Derya Uludüz
Derya Uludüz Thien Phu Do
Thien Phu Do