- 1Competence Center of Geriatric Medicine, Helios Medical Center Bonn/Rhein-Sieg, Academic Teaching Hospital, University Bonn, Bonn, Germany
- 2Department of Orthopedics and Trauma Surgery, University Hospital Bonn, Bonn, Germany
- 3Institute of Epidemiology and Medical Biometry, University Ulm, Ulm, Germany
- 4Helios Medical Center Bonn/Rhein-Sieg, Palliative Medicine, Academic Teaching Hospital, University Bonn, Bonn, Germany
- 5Department of Palliative Medicine, University Hospital Bonn, Bonn, Germany
- 6Paracelsus Medical University, Institute for Nursing Science and Practice, Salzburg, Austria
Purpose: Responsive behavior, often referred to as behavioral and psychological symptoms of dementia (BPSD), is among the most critical disorders in dementia whereby nursing personnel in hospitals are increasingly confronted with such symptoms. The purpose was to reduce the level of BPSD in an acute hospital environment through a stepwise procedure followed by the initiation of a needs-oriented treatment.
Methods: An open, prospective, interventional study with before-after comparisons was used to implement “Serial Trial Intervention” (STI) in three hospital wards (internal medicine, surgery, geriatric) after its adaption for hospital setting which was supplemented with a detailed pain assessment. Participants were 65 years and older. Potential causes of BPSD were clarified in a stepwise procedure and, if possible, eliminated. The primary outcome was the reduction in BPSD measured by the Neuropsychiatric Inventory (NPI-Q-12) while secondary outcomes were through the use of non-pharmacological and pharmacological interventions.
Results: No significant reduction in NPI-Q-12 could be found. However, significantly more mobilizations and changes of position were carried out. Higher antipsychotic use was seen in the after-groups presumably due to the higher rates of delirium and cognitive impairment. Furthermore, the data showed no increase in analgesic use.
Conclusion: No significant reduction in NPI-Q-12 was observed in the before-after study. The use of antipsychotics even increased most probably due to a higher incidence of deliriousness in the after-group. However, STI seemed to improve attention to underlying causes of BPSD as well as pain. Proof that STI leads to NPI-Q-12 reduction in hospitals is still pending.
Key summary points
• Aim To reduce the level of behavioral and psychological symptoms of dementia (BPSD) in an acute hospital environment through a stepwise procedure following by the initiation of a needs-oriented treatment.
• Findings No significant reduction in Neurospychiatric Inventory (NPI-Q-12) could be found. However, significantly more mobilizations and changes of position were carried out. Higher antipsychotic use was seen in the after-implementation groups presumably due to the higher rates of delirium and cognitive impairment.
• Message Although final proof of the successful use of Serial Trial Intervention (STI) in the hospital setting is still pending, STI appears to be at least a tool for raising awareness regarding possible underlying causes of BPSD and pain as well as offering needs-based therapies.
Introduction
Dementia-related diseases is one of the greatest challenges facing our aging population. They cause considerable stress for the affected person, caregivers and staff members, and lead to considerable economic burdens; for 2019, global costs have been estimated at $1 trillion (1). Up to 40% of people admitted to hospital live with dementia which often leads to a prolonged hospital stay (2). Responsive behavior in the course of in-patient treatment is frequently responsible for prolonging the stay. They lead to a considerable strain on affected patients and healthcare professionals, and disturb the daily scope of pratice in an acute care unit (3).
Responsive behavior, also known as challenging behavior as well as being a subset of BPSD (“Behavioral and Psychological Symptoms of Dementia) (4), is a heterogeneous group of symptoms and behaviors that can be broadly divided into affective symptoms, psychosis, hyperactivity, and euphoria (5). Examples of responsive behaviors include wandering, yelling, kicking and aggression. Up to 76% of people with dementia treated in hospitals suffer from BPSD (2). While the term “BPSD” reflects a more clinically diagnostic language, the term “responsive behavior” reflects a more person-centered language that emphasizes the dignity of the affected person. Furthermore, the term “challenging behavior” has a negative and misleading connotation and suggest that a problem is caused by an individual as an intrinsic deficit of a dementia (6–8).
The incidence of BPSD is influenced by patient-related factors such as the severity of cognitive impairment (9) as well as factors from a patient's environment and interaction with the caregiver (10). In this context, Algase et al. provide a possible explanation for responsive behavior in dementia in their needs-driven, dementia-compromised behavior (NDB) model (11). Thus, BPSD arises in the context of unsatisfied needs such as undetected pain (12–14). Affected people try to express their needs through words, movements or actions (6). Due to their inability to verbally express pain, pain recognition in people with advanced dementia is far more difficult, often leading to under-treatment of pain and increased BPSD (14). Consequently, it has been reported that optimization of analgesic treatment may lead to a reduction in BPSD (15).
Considering the increasing number of affected patients with BPSD in hospital settings, the task is to improve the diagnosis and therapy of responsive behaviors. One possibility to significantly improve the situation is seen in a structured clarification of the causes of BPSD followed by a specific therapy tailored to meet the needs. For this purpose, Kovach et al. (16) developed the so-called Serial Trial Intervention (STI), a structured step-by-step clarification process for apparently unexplainable behavior in people with dementia (PWD). According to the trial-and-error principle, possible reasons for responsive behavior are clarified and, if possible, eliminated through appropriate interventions. While, in the meantime, STI has been successfully used in nursing homes (17), a recent review revealed that none of the 24 projects studied had yet been implemented in an acute care hospital setting (18). To enable its use in an acute care hospital setting, an adapted version of STI for a hospital setting was developed in the present study with particular attention paid to pain as an important potential cause of responsive behavior which was subsequently applied in an acute care hospital. The effect on STI was measured through changes in BPSD using the Neuropsychiatric Inventory Questionnaire (NPI-Q-12) (19, 20), a validated instrument covering 12 neuropsychiatric domains. As not each of the 12 items are equally influenced by the STI, subscales for agitation/aggression, mood and frontal behavior were additionally used (21).
Hence, the aim of the present study was to test the following hypothesis:
A structured stepwise clarification of possible causes for apparently unexplainable behavior in people with dementia (PWD), as represented by the STI, also leads to a more cause-related therapy in an acute hospital environment, such as increased pain therapy in cases of pain and lower use of antipsychotics.
The following questions should be answered in greater detail.
1) In terms of effectiveness, does the use of the adopted version of STI lead to a significant reduction in responsive behavior in the after-implementation group compared to the before-implementation group within a four-day observation period, measured as a score reduction of the Neuropsychiatric Inventory Questionnaire (NPI-Q-12)? (Primary outcome)
2) Are there any significant reductions in the NPI-Q subscales “Agitation/Aggression,” “Mood,” or “Frontal” using the adopted version of STI under the assumption that not all NPI-Q items are equally affected by STI?
3) Can the increased use of non-pharmacological interventions to treat responsive behavior be observed through the use of STI?
4) Is there an increased use of analgesics in the sense of a more cause-related treatment of responsive behavior in the case of underlying pain through the application of STI within the four-day observation period?
5) Can the administration of non-specific psychotropic drugs be reduced at the same time and what is the situation regarding sedatives and antidepressants?
6) Generally speaking, is an adapted version of STI basically feasible and applicable in a hospital setting?
Methods
Study Design
An open, prospective, interventional study in form of an inter-period comparative design with a before-after comparison was conducted in a general hospital in Germany. One study group was recruited before and another after introduction of a stepwise cause assessment for BPSD. The before-implementation phase was designed to gain knowledge about the incidence and severity of BPSD, especially concerning responsive behavior on three different wards (internal medicine, surgery, and geriatric) while the after-implementation phase was used to evaluate the effect of a structured clarification of causes and initiation of a specific treatment.
Intervention
The intervention consisted of the introduction of the Serial Trial Intervention (STI) with special attention being paid to pain. STI works according to the trial-and-error principle. After recognizing BPSD, the possible cause is investigated in a structured step-by-step process (five steps: physical assessment, affective assessment, non-drug treatment, analgesic and antipsychotic treatment). If a supposed cause is found, an attempt is made to eliminate it (e.g., emptying the full bladder). If there is no significant improvement (50% reduction of the BPSD is required), the next step is taken. In the final step, training also included convening a case conference for the decision to administer antipsychotics. The intervention checklist was created and developed in the context of an anonymous survey of nurses from three different wards (internal, geriatric, surgery) by means of a semi-structured interview with open and closed questions about BPSD and possible therapeutic countermeasures as part of a kick-off meeting on dealing with BPSD and especially concerning responsive behavior (3). The list was supplemented by further training materials provided by the nursing experts involved which was recently published (22). The observation period of each PWD in the before and after-phases, respectively, was set at 4 days. Usually, it takes some time until a stable improvement in BPSD can be achieved. In addition, STI is characterized by a stepwise approach. It is also not consistently expected that the first intervention chosen will definitely lead to an improvement in BPSD. In this respect, we chose a follow-up period during whereby STI was applied daily. This daily application served, on the one hand, to control whether the applied intervention showed the effect in BPSD and, on the other, to determine the necessity for a necessary modification of the therapy. Thus, the 4-day observation period was guided by the consideration of providing sufficient time for STI to show possible effects, but also by clinically practical considerations, based on the experience that, within this time, the majority of those affected should show signs of improvement in their BPSD. To highlight a possible difference in terms of BPSD in the before-after comparison, the first and fourth days of the intervention were defined as the main measurement time points of the analysis.
STI-Training
Scientific experts in the field of STI and pain (I.G, J.N., C.S, and A.L.) supported the development of a curriculum. Between the before- and after-implementation phase, a specific trainee programme based on STI and including pain assessment, conducted by a registered nurse and a physician who are both qualified in gerontology, took place. As basic training, all healthcare professionals (registered nurses, physicians, and therapists) on the three wards received a 90-min training course. In addition, so-called 1-day mentor training sessions (8 h) were held for individual employees as support for the briefly trained employees. The training content included, in particular, the recognition, importance and background of BPSD, STI as a clarification process with its five steps (physical assessment, affective assessment, non-drug treatment, tentative administration of an analgesic, and tentative administration of psychotropic drugs), the influence of pain in BPSD, intensive STI application training and pain assessment for PWD as well as a checklist of potential non-pharmacological and pharmacological treatments.
STI Adoption to Accommodate the Hospital Setting
Originally developed as a systematic process to proactively reduce BPSD in nursing homes (16, 17)—whereby the original text was translated into German (23)—STI first had to be adapted to suit the specific requirements of the hospital setting. For example, it can be assumed that in contrast to the original version for a nursing home environment, more injuries, post-operative conditions or overall conditions associated with pain can be expected in a clinic. To make this clear, the nurses were made aware of this fact during the training. Likewise, attention was paid to whether the behavior occurred during specific activities (e.g., nursing procedures).
STI Supplements “Structured Pain Assessment”
Particular emphasis was placed on pain assessment. The original STI only hints at considering pain as a possible cause for an unexplainable behavior in addition to physical and affective disorders and to initiate pain therapy if necessary. Details, such as how pain should be measured, especially in light of the fact that many people on whom the STI is to be used are cognitively impaired, are missing. STI was initially supplemented by the Verbal Rating Scale (VRS) as a self-assessment instrument for pain intensity—for people with mild to moderate cognitive impairment—and, secondly, by a proxy-assessment for pain—the PAINAD scale—for people with advanced dementia (24).
Primary Outcome
The primary outcome was defined as the reduction in BPSD, as measured using the Neuropsychiatric Inventory Questionnaire (NPI-Q-12), in the after-implementation group compared to the before-implementation group within the observation period of 4 days.
Secondary Outcomes
Secondary outcomes were defined as a reduction in BPSD, measured using NPI-Q subscales, by the use of non-pharmacological treatments as well as consumption of analgesics, antipsychotics, sedatives and antidepressants before and after the introduction of STI, and the feasibility of STI in a hospital setting.
Inclusion and Exclusion Criteria
Suitable patients were 65 years and older who had a Mini Mental State Examination (MMSE) ≤ 24, assigned a minimum assumed 4-day length of stay (a decision based on the principal diagnosis which can be assigned to a specific mean length of stay in Germany) at one of the three intervention wards and who showed signs of BPSD (at least one of the 12 possible symptoms according to NPI-Q). All suitable patients received written information and were asked for participation through informed consent. Where informed consent was not possible, this was provided by their legal guardian or authorized person.
Measurements
Neuropsychiatric Inventory Questionnaire
BPSD was measured using the Neuropsychiatric Inventory Questionnaire (NPI-Q-12), a validated instrument for evaluating psychopathology in dementia. The NPI-Q-12 is a brief clinical form of the Neuropsychiatric Inventory (NPI) (19, 20). The questionnaire covers 12 neuropsychiatric areas (Delusion, Hallucination, Agitation/Aggression, Depression/Dysphoria, Anxiety, Elation/Euphoria, Apathy/Indifference, Disinhibition, Irritability/Lability, Motor Disturbance, Night-time Behavior, and Appetite/Eating). The NPI-Q-12 has good test-retest reliability and convergent validity, correlating with the full NPI at 0.9 (20). Due to the inability of many participants to use the NPI-Q as a self-administered questionnaire, we used the instrument with a proxy rating. Each of the 12 areas was assessed by using a screening question. The severity of the symptoms was determined using a three-level Likert scale (1-mild, 2-moderate, 3-severe). The total NPI-Q-12 severity score represents the sum of individual symptom scores ranging from 0 to 36. In order to detect significant changes regarding BPSD, the NPI-Q-12 was applied once a day over the four observation days. The NPI-Q-12 was determined by the study nurse together with the responsible nurses on the wards.
In recent years, there have been recurrent efforts to identify clusters or syndromes of NPI that represent significant subgroups of patients with different neuropsychiatric syndrome constellations. The goal of these efforts has been to give researchers and clinicians more options for individualized care of specific NPI clusters and to better control their therapeutic interventions. Thus, Trzepacz et al. (21) developed and validated three different NPI-Q subscales for agitation/aggression, mood, and frontal syndromes. The compositions were determined based on publications on descriptive population data, and from exploratory factor analyses as well as known phenotypic and neuroanatomical relationships among symptoms drawn from larger neuropsychiatric publications (21). The subscales can be generated from the NPI-Q-12, with the NPI-Q-4-Agitation/Aggression subscale comprised of four items: Agitation/Aggression, Disinhibition, Irritability/Lability, and Motor Disturbance. The NPI-Q-3-Mood subscale consists of three items: Depression/Dysphoria, Anxiety, and Irritability/Lability while the NPI-Q-4-Frontal Subscale comprises Elation/Euphoria, Apathy/Indifference, Disinhibition, and Irritability/Lability. It is conceivable that not all items of the NPI-Q-12 are equally influenced by STI. For example, the NPI-Q-12 includes behavior such as appetite/eating and elation/euphoria that STI would presumably not impact. Thus, in an extended analysis, NPI-Q subscales would also be considered.
Pain Assessment
The original STI was supplemented by a stepwise pain assessment with a self-assessment tool (VRS) and proxy-assessment tool (PAINAD). The VRS, a four-level Likert scale (none, mild, moderate, severe pain), is one of the preferred self-report pain assessments for detecting pain in older people and is easy to use with reliable measurements even more so in people with moderate cognitive impairment (25). The PAINAD scale is also one of the most frequently recommended pain assessment scales in advanced dementia, clinically useful, easy to perform and with sufficiently good psychometric values (26). This proxy-assessment instrument consists of five observable items (breathing, negative vocalization, facial expression, body language, and reaction to consolation), where each item can be rated between 0 and 2 thus resulting in a maximum score of 10 points. The pain cut-off level in PAINAD is a controversial issue in publications. Zwakhalen et al. suggested a value of ≥2 (27). Other authors were able to determine a value of ≥4 as the cut-off for pain in a comparison with a self-report pain assessment (28). In the present work, we opted for a more conservative estimate (cut off ≥ 4), because we assumed a greater probability of relevant pain being present at a higher value. Both pain assessments were integrated parts of STI and applied by the respective ward nurses once daily over the four observation days. The selection of the appropriate pain assessment was decided by a nurse according to the patient's cognitive abilities at the time of assessment. In accordance with the general recommendations of pain measurement, a self-assessment was always initially attempted. Only in those cases where this was not reliably possible was the PAINAD scale used. Pain was measured both during rest and movement. In each case, the highest pain intensity measured over the four-day observation period was included in the analysis. This was usually a pain measurement during movement whereby transfer situations were mostly used for this purpose (26).
Pain was also measured in the “before” group but was not as structured as with the modified STI in the “after” group. In addition to VRS, the Numerical Rating Scale (NRS) was used in this group. NRS is a pain scale with numerical values from 0 (= no pain) to 10 (= maximum imaginable pain). It is known from publications that NRS is less suitable for cognitively impaired older people in relation to VRS (25). However, in the “before” group we did not want to influence the choice of pain assessment.
Treatment Documentation
Non-pharmacological treatment of BPSD was recorded daily within the 4 days of observation. A checklist including different procedures such as pacifying talk, providing assistance with mobilization, offering food/drinks or other activities like listening to music, was used.
Permanent pharmacological treatment was documented on each of the four observation days, focusing on tranquilizers, antidepressants, psychotropics, and analgesics. Analgesics were classified according to their WHO level and psychotropic drugs according to their neuroleptic potency.
Nurses applied non-pharmacologic treatments or initiated pharmacologic interventions through consultation with physicians. The treatments were documented by the study nurses who were in close contact with the nurses on site. According to STI steps, priority was given to non-pharmacological interventions.
Assessments to Describe the Study Population
A wealth of assessments served to describe the study population in more detail and were collected by a member of the research team (study nurse).
Cognition was assessed by means of MMSE (29). It measures cognitive function especially in terms of orientation and memory. The score ranged from 0 to 30. MMSE scores < 24 indicate the presence of dementia (30).
Functional Assessment Staging (FAST) reflects functional deterioration throughout the course of dementia (31, 32). FAST is an interview-based global severity scale for clinicians, measuring progression of dementia on the basis of cognition-based functioning (33). It is comprised of seven major functional levels (from I to VII) and an additional 11 sub-stages corresponding to Stage VI (a-e = moderately severe dementia) and VII (a-f = severe dementia). The higher the score, the more severe functional deterioration and cognitive impairment is seen. MMSE and FAST were determined exclusively on the first day.
The Delirium Observation Scale (DOS) is a valid instrument used to detect delirium with high sensitivity (90%) and specificity (91%) (34, 35). For this study, the shortened 13-item version was used. Item scores equal to or higher than 3 points (out of 13) indicate a state of delirium. The DOS was assessed on the first and on the fourth observation day. Changes during the observation period were calculated using these two measurement points.
The four-item Geriatric Depression Scale (GDS-4) is suitable as a screening tool for detecting the risk of depression in older people. It is easy to administer and has a high degree of sensitivity and specificity. A score equal to or higher than 2 indicates a risk of depression (36, 37).
Patient functionality was assessed by using Barthel's Index (ADL). This 10-point scale measures a patient's degree of autonomy in daily living activities with a total score ranging from 0 to 100. Lower scores indicate higher levels of dependency (38). GDS as well as Barthel's Index were determined exclusively on the first day.
Ethical Considerations
Ethical approval for the study was obtained by the Ethics Committee, University Bonn, Germany (No. 252/15). An ongoing informed consensus enabled the participants to communicate their non-participation at any time.
Statistical Analysis
The analysis was designed as a before-after comparison. Regarding the description of the study population, absolute and relative frequencies have been given. Differences between the before and after-implementation group were tested using a chi-squared test or the Fisher exact test for categorical variables and for normally distributed continuous variables with the t-test for independent samples or the Mann-Whitney U-test in case for skewed distributions. The analysis included the first and fourth observation days of the patients from the two study groups. For the primary outcome, evaluation was designed to take into account the specificity of STI with its wide range of possible intervention methods, and the fact that STI is individually tailored to meet the needs of those affected which inevitably leads to heterogeneity in the measured “intervention effect.” Thus, in addition to a restricted look at NPI-Q-12 on specific days by determining median NPI-Q-12 values for treatment Days 1 and 4, the “individual treatment effect” by means of a difference in NPI-Q-12 (d1-d4 difference) in order to form categories from improved to deteriorated was determined. Median NPI-Q-12 values together with their range are reported for each assessment day. In addition, “changes in the NPI-Q-12 score between Day 1 and 4,” e.g., due to a pharmacological intervention, were categorized (improvement, deterioration, unchanged findings) per patient in each examination group. For the comparison of both cohorts, a chi-square test was applied to this categorized assessment of NPI-Q-12 change from Day 1 to 4. All analyses were performed identically for both the NPI-Q-12 version and its three subscales: NPI-Q-4-Agitation/Aggression, NPI-Q-3-Mood and NPI-Q-4-Frontal.
Furthermore, in order to address the possibility of a confounded result, linear mixed model analysis was used to assess the potential impact of unequally distributed variables in the before and after cohorts on NPI-Q-12 scores using repeated measures data assessed on Days 1 and 4. In general, a p-value < 0.05 was considered to be statistically significant whereas the study design did not allow for a confirmatory interpretation of the findings.
All analyses were performed using IBM SPSS® Statistics for Windows version 26.0, Armonk, New York, USA. Microsoft Excel® 2019 was used for the creation of graphs.
Results
Study Sample
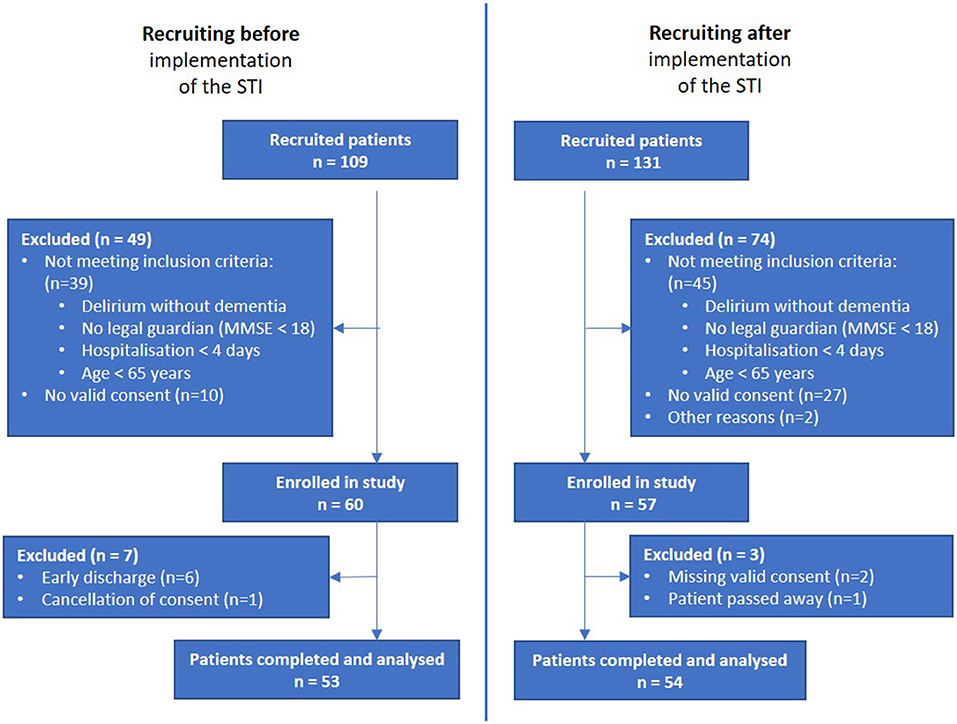
From the initial 109 patients recruited from the before-implementation group, those not meeting inclusion criteria (n = 39), with no consent given (n = 10), with early discharge (n = 6) and cancellation of consent (n = 1) were excluded, leading to a before-interventional study population of 53 participants. From the initial 131 patients taken from the after-implementation group, those not meeting the inclusion criteria (n = 74), with no valid consent (n = 27), other reasons (n = 2), missing valid consent (n = 2) and deceased patient (n = 1) were excluded, leading to an after-interventional study population of 54 participants. In total, the recruitment process took 21 months. Recruitment for the “before” group was set at 6 months in advance. Training sessions then took place, followed by the introduction of the STI. In the “after” group, the recruitment process for a comparable number of patients took a significantly longer 15 months. The final analysis included 107 participants, 53 in the before-implementation group and 54 in the after-implementation group (Figure 1).
Sample Characteristics
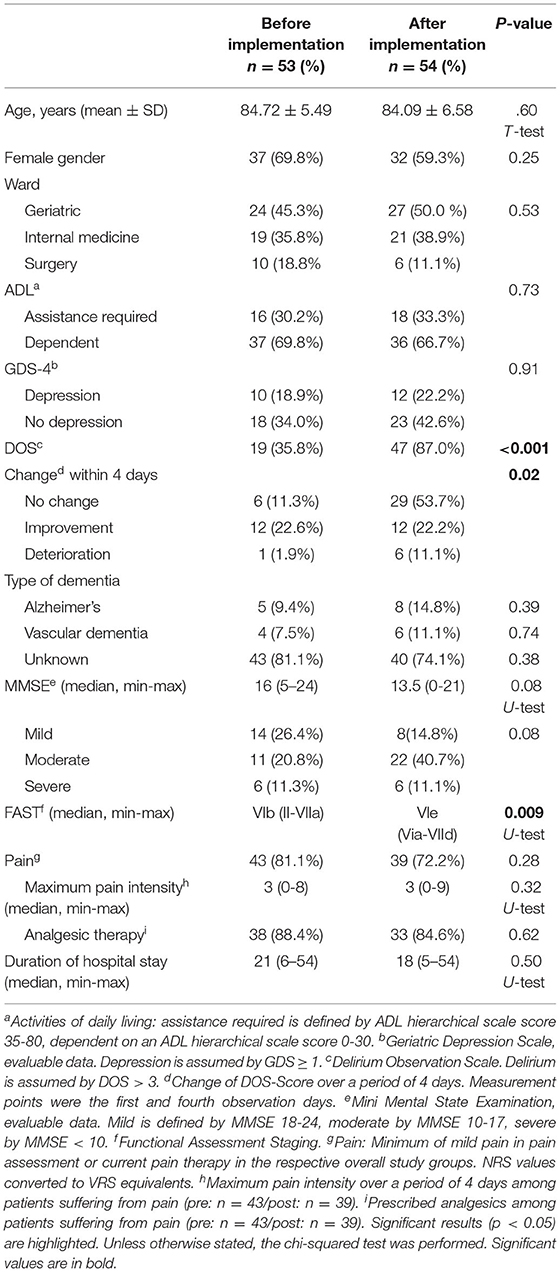
No significant difference between the two study groups was found regarding age, gender, distribution of the participants in the three wards, ADL functions and MMSE (Table 1). A relevant difference could however be seen in the Functional Assessment Staging (FAST) with considerably higher functional deterioration in the after-group [VIb (II-VIIa) vs. Vie (Via-VIId), p =.009]. The after-implementation group showed significantly more frequent indications of delirium [19 (35.8%) vs. 47 (87.0%), p < 0.001) and had a higher proportion of participants who did not experience any change in their delirium among the interventions initiated. There was no significant difference regarding the type of dementia, signs of depression, pain, maximum pain intensity, analgesic therapy, and duration of hospital stay.
Neuropsychiatric Symptoms
Regarding the primary outcome—a reduction in BPSD in the after-implementation group compared to the before-implementation group within the observation period of 4 days in each case—no significant reduction was found (Table 2). Neither the direct comparison of the two study groups using the categorized change assessment between Days 1 and 4, nor the findings based on a linear mixed model adjusted for FAST and DOS values indicated a significant effect of STI on the NPI-Q. Although DOS, in contrast to FAST, showed a statistically significant effect on the NPI-Q and must therefore be treated as an important confounder, its plain effect was negligible (only half of a point on the NPI-Q-12 scale). Based on the results of the linear mixed model analysis there is an additional non-significant tendency of slightly decreased NPI-Q-12 values in the post-cohort when compared to the pre-cohort, but the plain effect was also only about half of a point on the NPI-Q-12 scale. Summing up, the results confirm that no meaningful effect of STI on the NPI-Q-12 was detectable. In the extended subgroup analysis (NPI-Q-4-Agitation/Aggression, NPI-Q-3-Mood, and NPI-Q-4-Frontal) no significant differences were found between the study groups as well.
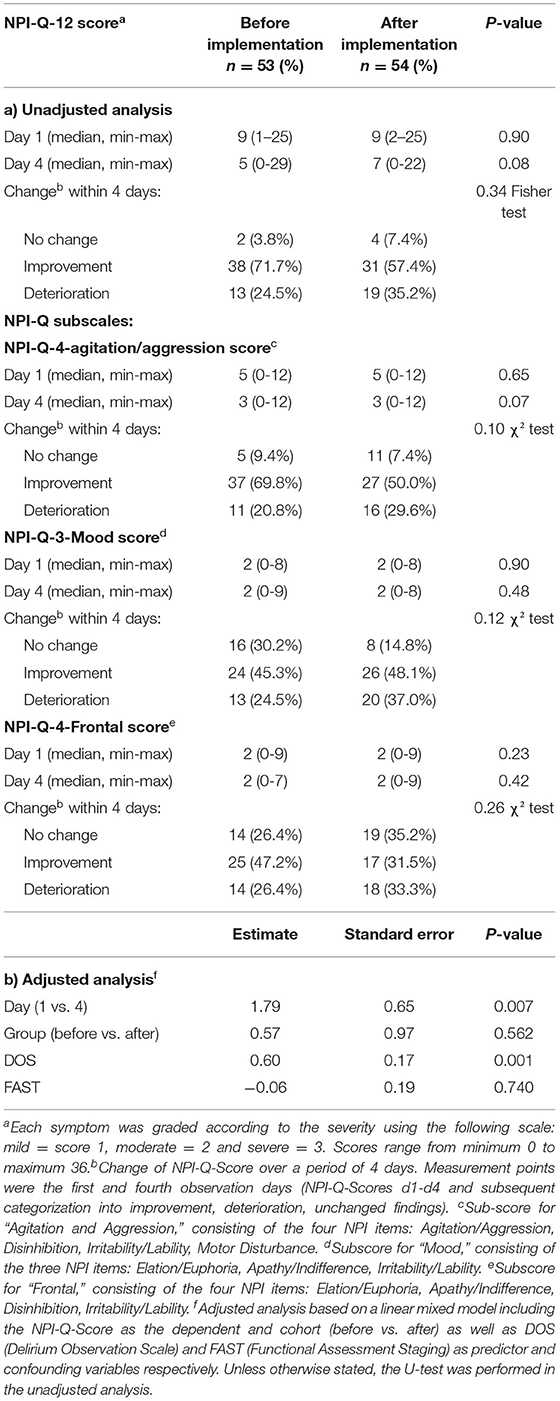
Table 2. Severity of NPI-Q symptoms and changes within the study population on Days 1 and 4 before and after STI implementation, for NPI-Q-12 and NPI-subscores.
The incidence of the twelve NPI-Q symptoms vary widely. Agitation/aggression (in both study groups ~90%), night-time behavior disturbances (~80%), irritability (~75%) and anxiety (~57%, ~78%, respectively) were the most common. Anxiety and aberrant motor behaviors were significantly more common in the after-implementation group [30 (56.6%) vs. 42 (77.8%), p = 0.02, 35 (66.0%) vs. 45 (83.3%), p = 0.04, respectively] while apathy was observed more often in the before-implementation group [34 (64.2%) vs. 24 (44.4%), p = 0.04]. All other symptoms (delusion, hallucination, agitation/aggression, depression/dysphoria, euphoria, disinhibition, irritability, night-time behavior disturbances as well as appetite and eating abnormalities) occurred with equal frequency in the two study groups.
Non-pharmacological Treatment
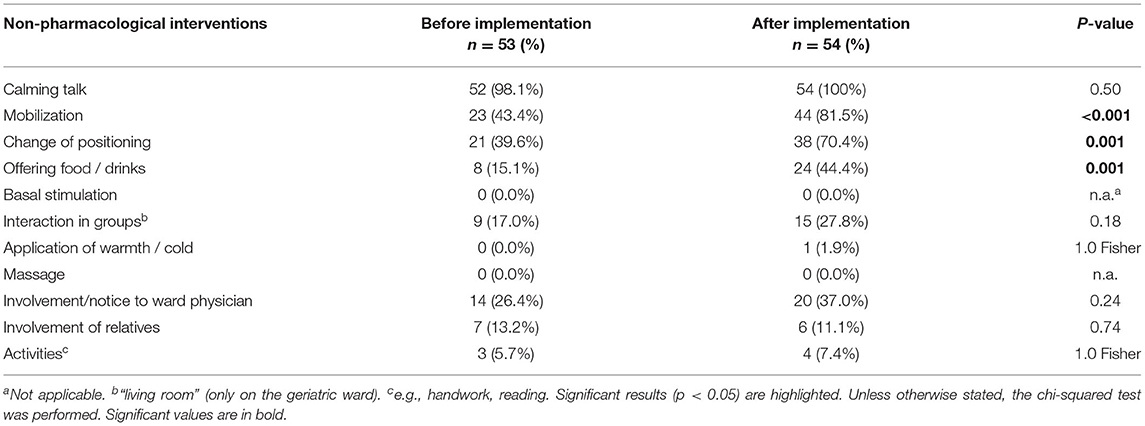
With regard to non-pharmacological measures, the most frequent was a calming conversation with the PWD. Mobilization was used significantly more often in the after-implementation group [23 (43.4%) vs. 44 (81.5%), p < 0.001] similar to change of position in bedridden PWD [21 (39.6%) vs. 38 (70.4%), p = 0.001] and offering drinks or food to overcome needs-driven behavior [8 (15.1%) vs. 24 (44.4%), p = 0.001] (Table 3).
Pharmacological Treatment
The presumed additional use of analgesics with STI including pain assessment tools did not occur. There were no significant differences in the before-after comparison of the analgesics used on the first or the fourth observation day (Table 4). Similarly, the before-after comparison of antipsychotics or sedatives on the first and fourth observation days showed no significant difference. There was no lower usage of antipsychotics on the fourth observation day as had been expected. Antidepressants were used significantly more often in the before-implementation group both on the first and fourth day of the study [19 (35.8%) vs. 8 (14.8%), p = 0.02 vs. 20 (37.7%) vs. 9 (16.7%), p = 0.02].
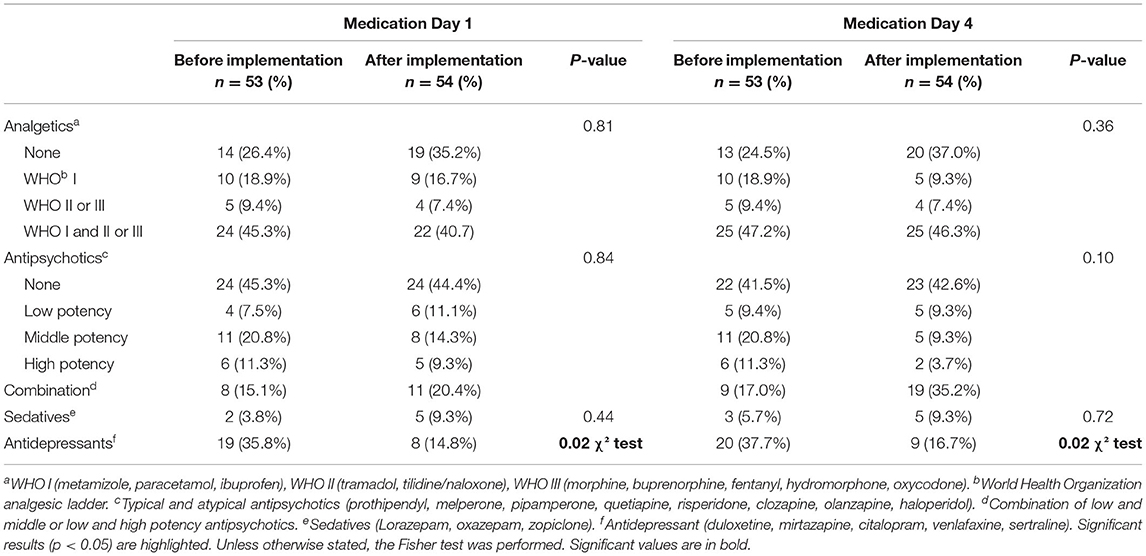
Table 4. Pharmacological intervention of study participants before and after STI implementation, Day 1 and Day 4.
Changes in pharmacological intervention (increase and decrease) during the observation period are listed in Table 5. The summary of pharmacological intervention over the 4 days of observation showed no significant differences regarding analgesics and sedatives. Antidepressants did show a significant p-value [19 (35.8%) vs. 8 (14.8%), p = 0.02] but this can be primarily explained by the differences in antidepressant prescribing that already existed on the first day of the study in both study groups. This was different for antipsychotics. Although the comparison of antipsychotics on the first as well as on the fourth day showed no difference in before-after comparisons, the summary of pharmacological interventions showed a significant increase in antipsychotic drug use in the after-implementation group during the observation period [2 (3.8%) vs. 12 (22.2%), p = 0.02].
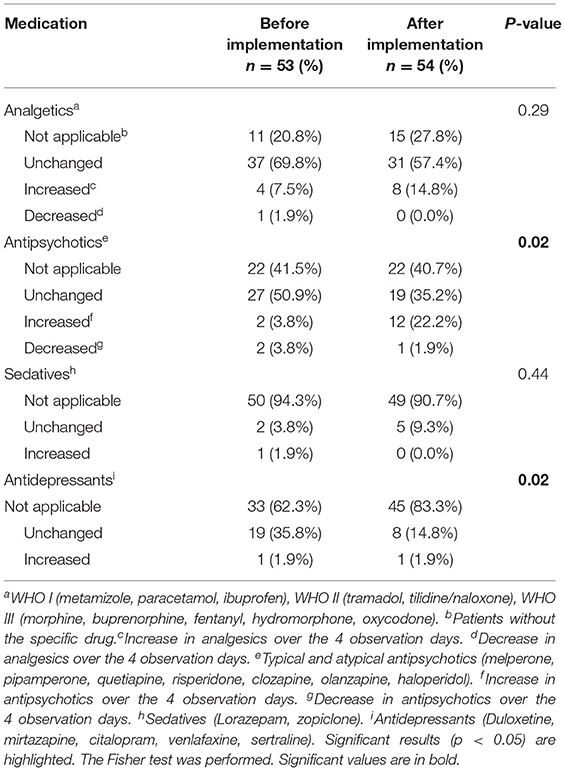
Table 5. Changes in pharmacological intervention (increases and decreases) over the four observation days, before and after STI implementation.
Discussion
Neuropsychiatric Inventory
The basic idea of the project was that an apparently inexplicable behavior in a person with dementia very often has an identifiable reason. Evaluation of potential causes and needs-oriented treatment along the Serial Trial Intervention (STI) may help to eliminate these causes and reduce responsive behavior of people with dementia in an acute care hospital.
We expected a reduction in BPSD, measured as NPI-Q-12 as the primary outcome. However, no significant improvement in neuropsychiatric symptoms was found in the after vs. before-implementation group during the four-day observation periods, neither by using the NPI-Q-12 version nor by using NPI-Q subscales for agitation/aggression, mood or frontal symptoms. The reason for this observation could be the fact that both study groups were not as equivalent as initially expected. In this context, it may also be relevant that it was significantly more difficult to recruit patients for the “after” group. It is possible that milder cases of responsive behavior with knowledge of STI training in this group were already “resolved” on the ward without being reported to the study nurses thus resulting in the differences in the two groups. The incidental higher BPSD burden in the after-implementation group, represented by higher delirium rate and increased cognitive impairment, may have masked the positive effects of STI on NPI-Q-12 to some extent. However, in a linear mixed model analysis, an effect of delirium could also be detected but this effect seems to be very small.
In addition, even in the before-implementation group, there was already a substantial NPI-Q-12 improvement in 69% of the patients which can be interpreted as an indication that also in the before-group the interventions used in dealing with BPSD were already quite effective.
This result is in marked contrast to previous studies with STI which, unlike the present study, were conducted exclusively in nursing homes. A double-blinded randomized interventional study in nursing homes found significantly less discomfort in the STI group and more frequently behavioral symptoms returned to baseline (17). A cluster randomized controlled trial with 288 residents suffering from advanced dementia also showed an improvement in their behavior under strict application of the STI (39). Numerous publications in recent years favor such structured investigations of causes including non-pharmacological and pharmacological treatment strategies (40, 41).
Non-pharmacological Treatment
In general, non-pharmacological interventions are considered as a first-line treatment option whenever possible with effect sizes that are similar to pharmacological approaches but with a lower risk of adverse events and often simpler application (40–42). However, the quality of evidence for such non-pharmacological interventions must be assessed as low (43).
In the study presented here, significantly more mobilizations and changes of position were performed for the patients in the after-implementation group. Likewise, nurses offered drinks and food more frequently in the context of responsive behavior. These changes can be seen as a possible success of the intervention. Successful increases of non-pharmacological interventions have also been described by other authors. Kovach et al. saw a clear increase in non-pharmacological treatments from pre- to post-testing through the use of STI (44). The most frequently used non-pharmacological comfort intervention at both pre- and post-testing times were soothing verbal communication and touch, movement, and sensory stimulation, respectively. However, this was not confirmed in a subsequent study (17).
Pharmacological Treatment
We evaluated medications most commonly used in responsive behavior such as antipsychotics, antidepressants, sedatives, and anxiolytics (45).
No increase in analgesic use was demonstrated. The reason for the absence of a significant increase in analgesics could have been the low level of pain (median = 3) in both study groups. Given this low pain intensity, analgesic administration may not be as critical as originally expected. Our hypothesis was, among other things, that pain is one of the most important triggers for BPSD and an increase in analgesic consumption had been predicted when recognizing the problem. However, this could not be demonstrated which was possibly due to the relatively low pain intensity. The higher number of mobilizations, positioning, offering food or drink according to STI could be more useful and effective interventions. However, it should be noted that this statement is made against the background of existing adequate pain therapy. A non-structured pain assessment might have yielded different results. In both groups, almost 50% of the patients had already initially received a WHO step III analgesic and the proportion of patients treated with analgesics was over 80% thus possibly representing a special feature of hospital setting compared to nursing home environments. For example, Pieper et al. saw a significantly lower proportion of pain patients (50%) in nursing homes whereby the initial use of opiates was only 8% (46).
In contrast, Kovach et al. found significantly more use of analgesics in STI intervention compared to the control group (17) thus confirming an older study (44). A systematic clarification of the cause of BPSD improved pain management associated with responsive behavior more effectively than a non-stepwise approach (46). A recent consensus statement clearly prioritizes treatment with an analgesic over treatment with an antipsychotic (40).
Use of antipsychotics was even higher in the after-implementation group when considering the changes over the whole 4-day observation period in our study. One might assume that this increase is related to the random higher incidence of delirium and cognitive impairment in the after-implementation group. A linear mixed model analysis then also showed an influence of delirium on NPI-Q-12 values but its plain effect was ultimately negligible. However, this fact must be taken into account when interpreting the results. No correlation was found for cognitive impairment. In contrast, Pieper et al. found that gradual interventions in nursing home residents were successful without increasing psychotropic drug use (47).
Feasibility
The study was able to show that introduction and application of an adapted version of STI is feasible in a hospital setting too. Almost 96% of the nursing staff in the selected sample wards were trained in the use of STI and its application. STI adaptations, especially regarding pain and its assessment but also regarding possible interventions in the hospital environment, were successfully implemented in the clinical routine. As part of the study, a curriculum and a pocket guide with the most important information regarding STI were developed and handed out to each nurse. In addition, a supporting e-version of STI with intervention suggestions was developed for use in everyday clinical practice.
Strengths and Weaknesses
To the best of our knowledge, this is the first time that STI, originally developed for nursing home residents, has been applied and evaluated in an acute hospital setting (3). The transfer of STI into a new “acute hospital” setting and the implementation in a hospital routine characterized by time pressure and a high workload, where nurses are particularly affected by PWD with BPSD in their daily work (3) are certainly among the strengths of the study.
However, our study has some limitations which should not be left unmentioned. Due to the introduction of a new approach to BPSD with a before-after comparison, we decided against conducting a randomized controlled trial for several reasons. As the study was planned monocentrically, mixing effects, such as an exchange of relevant study information between the groups, could not be excluded with sufficient certainty. Furthermore, blinding for interventions was not possible. RCTs usually stand for a strictly defined equality of treatment which was not the case in the present study. Instead, the therapy was oriented to meet the individual needs of those affected and was formed from a plethora of possible interventions. Furthermore, it was never planned to conduct a confirmatory study as this would only be achievable with an RCT. On the other hand, the lack of randomization could bias the results in an unrepresentative direction. Possible confounders may have been overlooked and could explain the differences found between the groups. In order to provide an estimate of this effect in terms of delirium and cognition, we performed a linear mixed model analysis. However, we also know from Sessler and Imrey (48) that an inter-period comparative design, as in the current study, uses temporally proximal controls which are highly similar to those exposed to the intervention which may somewhat mitigate this effect.
In addition, the following limitations can be discussed: although the proportion of trained nurses was very high (95.8%), it is conceivable that nurses who were not trained were also used in each of the four STI application days. However, given the high training rate, we believe that any resulting effects from lack of training are negligible. It is also conceivable that the observation period of 4 days, based purely on clinical experience, may not have been long enough to observe any significant differences between the two observation groups regarding BPSD. The measured pain scores of the participants were possibly too low to expect significant changes regarding analgesics. There were no specifications such as a minimum pain score as an entry criterion. To sum up, the present study only considered patient-related factors such as pain, cognition, and physical function. BPSD can, however, be influenced by many other factors such as a patient's environment (e.g., living situation), socioeconomic factors (e.g., school education) and by reactions to the interaction with the caregiver (e.g., caregiver burden), which were not considered in the current study (10, 49).
Conclusion
Even though the study ultimately failed to show a significant reduction in BPSD, the introduction of stepwise clarification of possible causes of BPSD, especially pain, demonstrates an attractive approach to BPSD management. STI seems to at least raise awareness of underlying causes of BPSD and increase the use of non-pharmacological interventions. In this respect, STI seems applicable and beneficial in the treatment of BPSD, also for hospitalized patients. Nevertheless, further studies need to follow to conclusively clarify the value of STI in an acute hospital setting.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee, University Bonn, Germany (No. 252/15). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AL initiated and conceptualized the study idea, formulated the hypothesis, carried out the study planning, conducted the training, supervised the data collection, analyzed the data, and drafted and revised the manuscript. MB was involved conceptualized the study idea, formulated the hypothesis, carried out the assessment and collected data, analyzed the data, and drafted and revised the manuscript. BM was extensively involved in the statistical planning and analyses and also drafted and revised the manuscript. LR initiated and conceptualized the study idea, was involved in the study planning, and drafted and revised the manuscript. IG initiated and conceptualized the study idea, supported in the context of pain issues, designed the curriculum, conducted the training, and drafted and revised the manuscript. All authors participated in critical revision of the article for intellectual content, meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals, and have read and approved the final manuscript.
Funding
The study was funded by the Robert Bosch Foundation within the framework Dementia in acute hospitals (Grant number: 32.4.1365.0103.0). The sponsor had no role in the design, methods, subject recruitment, data collection, analysis or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Charité – represented by Dr. Johanna Nordheim – for providing the German translated version of the STI for use in the project. We also thank Mrs. Claudia Spahn, who shared her great experience in training the STI with us. With her experience and the materials, she provided, she made a decisive contribution to the success of the project. The authors would like to thank Paul East for intensively reviewing the manuscript.
References
1. Alzheimer's Disease International. World Alzheimer Report 2019: Attitudes to Dementia (London: Alzheimer's Disease International), (2019).
2. Hessler JB, Schäufele M, Hendlmeier I, Junge MN, Leonhardt S, Weber J, et al. Behavioural and psychological symptoms in general hospital patients with dementia, distress for nursing staff and complications in care: results of the general hospital study. Epidemiol Psychiatr Sci. (2018) 27:278-87. doi: 10.1017/S2045796016001098
3. Bienas M, Gnass I, Mayer B, Radbruch L, Lukas A. [Challenging behavior and pain in dementia : experiences of nursing staff in an acute care hospital]. Schmerz. (2019) 33:212-9. doi: 10.1007/s00482-019-0360-8
4. Finkel SI, Costa e Silva J, Cohen G, Miller S, Sartorius N. Behavioral and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Int Psychogeriatr. (1996) 8(Suppl 3):497-500. doi: 10.1017/S1041610297003943
5. van der Linde RM, Dening T, Matthews FE, Brayne C. Grouping of behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry. (2014) 29:562-8. doi: 10.1002/gps.4037
6. Yous M-L, Ploeg J, Kaasalainen S, Martin LS. Nurses' experiences in caring for older adults with responsive behaviors of dementia in acute care. SAGE Open Nurs. (2019) 5:2377960819834127. doi: 10.1177/2377960819834127
7. Alzheimer Society of Canada. Person-Centred Language Guidlines. Toronto: Alzheimer Society of Canada (2017).
8. O'Neill D, Kennelly S. Responding to responsive behaviour in Alzheimer's disease. Lancet. (2021) 398:842. doi: 10.1016/S0140-6736(21)01452-5
9. Hashimoto M, Yatabe Y, Ishikawa T, Fukuhara R, Kaneda K, Honda K, et al. Relationship between dementia severity and behavioral and psychological symptoms of dementia in dementia with lewy bodies and Alzheimer's disease patients. Dement Geriatr Cogn Disord Extra. (2015) 5:244-52. doi: 10.1159/000381800
10. Hughes TB, Black BS, Albert M, Gitlin LN, Johnson DM, Lyketsos CG, et al. Correlates of objective and subjective measures of caregiver burden among dementia caregivers: influence of unmet patient and caregiver dementia-related care needs. Int Psychogeriatr. (2014) 26:1875-83. doi: 10.1017/S1041610214001240
11. Algase DL, Beck C, Kolanowski A, Whall A, Berent S, Richards K, et al. Need-driven dementia-compromised behavior: an alternative view of disruptive behavior. Am J Alzheimers Dis. (1996) 5:10-9. doi: 10.1177/153331759601100603
12. Tosato M, Lukas A, van der Roest HG, Danese P, Antocicco M, Finne-Soveri H, et al. Association of pain with behavioral and psychiatric symptoms among nursing home residents with cognitive impairment: results from the shelter study. Pain. (2012) 153:305-10. doi: 10.1016/j.pain.2011.10.007
13. Pieper MJ, van Dalen-Kok AH, Francke AL, van der Steen JT, Scherder EJ, Husebo BS, et al. Interventions targeting pain or behaviour in dementia: a systematic review. Ageing Res Rev. (2013) 12:1042-55. doi: 10.1016/j.arr.2013.05.002
14. Atee M, Morris T, Macfarlane S, Cunningham C. Pain in dementia: prevalence and association with neuropsychiatric behaviours. J Pain Symptom Manage. (2020) 61:1215-26. doi: 10.1016/j.jpainsymman.2020.10.011
15. Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. (2011) 343:d4065. doi: 10.1136/bmj.d4065
16. Kovach CR, Noonan PE, Schlidt AM, Reynolds S, Wells T. The serial trial intervention: an innovative approach to meeting needs of individuals with dementia. J Gerontol Nurs. (2006) 32:18-25; quiz 6-7. doi: 10.3928/00989134-20060401-05
17. Kovach CR, Logan BR, Noonan PE, Schlidt AM, Smerz J, Simpson M, et al. Effects of the serial trial intervention on discomfort and behavior of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. (2006) 21:147-55. doi: 10.1177/1533317506288949
18. Feast AR, White N, Candy B, Kupeli N, Sampson EL. The effectiveness of interventions to improve the care and management of people with dementia in general hospitals: a systematic review. Int J Geriatr Psychiatry. (2020) 35:463-88. doi: 10.1002/gps.5280
19. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. (1994) 44:2308-14. doi: 10.1212/WNL.44.12.2308
20. Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the Npi-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. (2000) 12:233-9. doi: 10.1176/jnp.12.2.233
21. Trzepacz PT, Saykin A, Yu P, Bhamditipati P, Sun J, Dennehy EB, et al. Subscale validation of the neuropsychiatric inventory questionnaire: comparison of Alzheimer's disease neuroimaging initiative and National Alzheimer's Coordinating Center Cohorts. Am J Geriatr Psychiatry. (2013) 21:607-22. doi: 10.1016/j.jagp.2012.10.027
22. Fischer T, Nordheim J, Spahn C. Individuelle Pflege Von Menschen Mit Demenz: Ein Praxishandbuch Zum Umgang Mit Herausforderndem Verhalten. Stuttgart: Kohlhammer W., GmbH (2019).
23. Fischer A, Kuhlmei A, Sibbel R, Nordheim J. Die Deutsche Fassung Der “serial trial intervention” (Sti-D) - Entwicklung Und Testung Eines Pflegerischen Ansatzes Zur Reduktion Von Herausfordernden Verhalten Bei Menschen Mit Demenz. Zeitschrift für Gerontopsychologie Psychiatrie. (2008) 21:199-203. doi: 10.1024/1011-6877.21.3.199
24. Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the pain assessment in advanced dementia (painad) scale. J Am Med Dir Assoc. (2003) 4:9-15. doi: 10.1097/01.JAM.0000043422.31640.F7
25. Lukas A, Niederecker T, Gunther I, Mayer B, Nikolaus T. Self- and proxy report for the assessment of pain in patients with and without cognitive impairment: experiences gained in a geriatric hospital. Z Gerontol Geriatr. (2013) 46:214-21. doi: 10.1007/s00391-013-0475-y
26. Hadjistavropoulos T, Herr K, Prkachin KM, Craig KD, Gibson SJ, Lukas A, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. (2014) 13:1216-27. doi: 10.1016/S1474-4422(14)70103-6
27. Zwakhalen SM, van der Steen JT, Najim MD. Which score most likely represents pain on the observational painad pain scale for patients with dementia? J Am Med Dir Assoc. (2012) 13:384-9. doi: 10.1016/j.jamda.2011.04.002
28. Lukas A, Barber JB, Johnson P, Gibson SJ. Observer-rated pain assessment instruments improve both the detection of pain and the evaluation of pain intensity in people with dementia. Eur J Pain. (2013) 17:1558-68. doi: 10.1002/j.1532-2149.2013.00336.x
29. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189-98. doi: 10.1016/0022-3956(75)90026-6
30. Creavin ST, Wisniewski S, Noel-Storr AH, Trevelyan CM, Hampton T, Rayment D, et al. Mini-mental state examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochr Database Syst Rev. (2016)2016:CD011145. doi: 10.1002/14651858.CD011145.pub2
31. Sclan SG, Reisberg B. Functional assessment staging (Fast) in Alzheimer's disease: reliability, validity, and ordinality. Int Psychogeriatr. (1992) 4(Suppl 1):55-69. doi: 10.1017/S1041610292001157
32. Reisberg B. Functional assessment staging (Fast). Psychopharmacol Bull. (1988) 24:653-9. doi: 10.1037/t08620-000
33. Reisberg B. Global Measures: utility in defining and measuring treatment response in dementia. Int Psychoger. (2007) 19:421-56. doi: 10.1017/S1041610207005261
34. Gavinski K, Carnahan R, Weckmann M. Validation of the delirium observation screening scale in a hospitalized older population. J Hosp Med. (2016) 11:494-7. doi: 10.1002/jhm.2580
35. Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The delirium observation screening scale: a screening instrument for delirium. Res Theory Nurs Pract. (2003) 17:31-50. doi: 10.1891/rtnp.17.1.31.53169
36. Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to Icd-10 and Dsm-Iv. Int J Geriatr Psychiatry. (1999) 14:858-65.
37. Mitchell AJ, Bird V, Rizzo M, Meader N. Which version of the geriatric depression scale is most useful in medical settings and nursing homes? Diagnostic validity meta-analysis. Am J Geriatr Psychiatry. (2010) 18:1066-77. doi: 10.1097/JGP.0b013e3181f60f81
38. Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. (1965) 14:61-5. doi: 10.1037/t02366-000
39. Pieper MJC, Francke AL, van der Steen JT, Scherder EJA, Twisk JWR, Kovach CR, et al. Effects of a stepwise multidisciplinary intervention for challenging behavior in advanced dementia: a cluster randomized controlled trial. J Am Geriatr Soc. (2016) 64:261-9. doi: 10.1111/jgs.13868
40. Kales HC, Lyketsos CG, Miller EM, Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer's disease: an international delphi consensus. Int Psychogeriatr. (2019) 31:83-90. doi: 10.1017/S1041610218000534
41. Bessey LJ, Walaszek A. Management of behavioral and psychological symptoms of dementia. Curr Psychiatry Rep. (2019) 21:66. doi: 10.1007/s11920-019-1049-5
42. Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr. (2018) 30:295-309. doi: 10.1017/S1041610217002344
43. Wang G, Albayrak A, Molenbroek J, van der Cammen T editors. Non-Pharmacological Interventions for People with Dementia: Design Recommendations from an Ergonomics Perspective. Cham: Springer International Publishing (2019). doi: 10.1007/978-3-319-96065-4_15
44. Kovach CR, Weissman DE, Griffie J, Matson S, Muchka S. Assessment and treatment of discomfort for people with late-stage dementia. J Pain Symptom Manage. (1999) 18:412-9. doi: 10.1016/S0885-3924(99)00094-9
45. Magierski R, Sobow T, Schwertner E, Religa D. Pharmacotherapy of behavioral and psychological symptoms of dementia: state of the art and future progress. Front Pharmacol. (2020) 11:1168. doi: 10.3389/fphar.2020.01168
46. Pieper MJ, van der Steen JT, Francke AL, Scherder EJ, Twisk JW, Achterberg WP. Effects on pain of a stepwise multidisciplinary intervention (Sta Op!) that targets pain and behavior in advanced dementia: a cluster randomized controlled trial. Palliat Med. (2017) 2017:269216316689237. doi: 10.1177/0269216316689237
47. Ballard CG, Gauthier S, Cummings JL, Brodaty H, Grossberg GT, Robert P, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol. (2009) 5:245-55. doi: 10.1038/nrneurol.2009.39
48. Sessler DI, Imrey PB. Clinical research methodology 2: observational clinical research. Anesth Analg. (2015) 121:1043-51. doi: 10.1213/ANE.0000000000000861
Keywords: pain, dementia, behavioral and psychological symptoms of dementia (BPSD), non-pharmacological and pharmacological intervention, acute care hospital, responsive behavior management
Citation: Lukas A, Bienas M, Mayer B, Radbruch L and Gnass I (2022) Responsive Behaviors and Pain Management in Hospital Dementia Care: A Before and After Comparison of the “Serial Trial Intervention”. Front. Pain Res. 3:810804. doi: 10.3389/fpain.2022.810804
Received: 07 November 2021; Accepted: 21 March 2022;
Published: 04 May 2022.
Edited by:
Keela Herr, The University of Iowa, United StatesReviewed by:
Clarissa Shaw, The University of Iowa, United StatesAnn Horgas, University of Florida, United States
Copyright © 2022 Lukas, Bienas, Mayer, Radbruch and Gnass. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Albert Lukas, YWxiZXJ0Lmx1a2FzQGdteC5kZQ==
 Albert Lukas
Albert Lukas Melanie Bienas1
Melanie Bienas1 Benjamin Mayer
Benjamin Mayer