
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pain Res. , 13 January 2023
Sec. Musculoskeletal Pain
Volume 3 - 2022 | https://doi.org/10.3389/fpain.2022.1082252
This article is part of the Research Topic Insights in Musculoskeletal Pain: 2022 View all 5 articles
Knee Osteoarthritis (OA) is a prevalent musculoskeletal condition, commonly resulting in pain and disability. However, pain and disability in this population are poorly related with the degree of structural joint damage. Underlying pain mechanisms, including activity-related pain and sensitization assessed via Quantitative Sensory Testing (QST), may better predict pain and functional outcomes of those with knee OA. Therefore, the aim of this study was to explore whether activity-related pain and sensitization assessed via QST predict future pain, function, fatigue, physical performance and quality of life outcomes in those living in the community with knee OA. Eighty-six participants with knee OA were recruited in Dunedin, New Zealand. Those eligible to participate underwent baseline testing including QST as well as measures of activity-related pain including Movement-evoked Pain (MEP) and Sensitivity to Physical Activity (SPA). Outcome measures exploring pain, function, fatigue and quality of life outcomes were collected at baseline, and two follow-up periods (two and nine weeks). Univariable linear regression models were developed followed by multivariable linear regression models for each prognostic marker adjusting for age, gender, BMI, OA duration, baseline pain intensity and socioeconomic status. Activity-related measures of pain, including MEP and SPA, demonstrated predictive associations with pain and functional outcomes prospectively in those with knee OA. Therefore, those demonstrating activity-related pain are at future risk of greater pain, disability and reduced quality of life. Larger, externally validated longitudinal studies are required which include individuals with more severe knee OA.
Knee osteoarthritis (OA) is among the most prevalent conditions globally, contributing to significant societal costs and years lived with disability (1–3). Chronic pain is a common symptom of knee OA which is often attributed to changes in joint structures (4). However, recent literature highlights an apparent discordance between pain, disability and the degree of structural changes observed in the knee joint (4, 5). Underlying pain mechanisms including nervous system sensitization have emerged as important considerations in musculoskeletal and pain research over recent decades. Sensitization, which is defined as “increased responsiveness of nociceptive neurons to their normal input, and/or recruitment of a response to normally subthreshold inputs,” (6) has been shown to play a significant role in contributing to pain and disability in the knee OA population (7–9).
Assessment of sensitization in knee OA through Quantitative Sensory Testing (QST) has demonstrated a distinct subgroup of individuals with peripheral and central nervous system sensitization (10, 11). In a study investigating sensitization in those with knee OA, 71% were found to have at least one abnormality on QST, with many displaying widespread hyperalgesia and heightened mechanical temporal summation in comparison to healthy individuals (10, 12). Recent prospective studies have explored whether these QST abnormalities predict future outcomes in people with knee OA. These studies found that those with higher levels of sensitization demonstrated on QST were less responsive to analgesic medications and at risk of poorer surgical outcomes across a range of timeframes (13–21). Fewer observational studies have used QST measures to predict knee OA symptoms. One prospective study reported that greater temporal summation of pain predicted the severity of knee OA pain over the ensuing month among non-Hispanic White but not non-Hispanic Black individuals. Notably, this study only included a single QST measure and had a short follow-up period (22). Studies that explore a greater range of sensitization measures as predictors of pain and functional outcomes in a community sample of those self-managing their knee OA are yet to be performed. Better identifying those at risk of poorer outcomes in the community could assist in deciding who would best benefit from treatments specifically targeting the underlying pain mechanisms.
People with knee OA often experience pain during functional activities such as walking, crouching and climbing stairs (23). Activity-related pain measures, such as Movement-evoked Pain (MEP) and Sensitivity to Physical Activity (SPA) have been used to capture information on distinct aspects of activity-related pain (23–25). Furthermore, as pain information is captured during functional tasks, these measures likely provide greater ecological validity to living and functioning with knee OA in comparison to lab- and clinic-based tests (24, 26–28). Recent studies have confirmed that activity-related pain demonstrates predictive associations with important outcomes. These showed that SPA cross-sectionally predicts levels of function in those with knee OA (29, 30). Additionally, a recent study showed that greater MEP prior to a total knee arthroplasty (TKA), predicted greater post-operative pain at one year (31).
Studies exploring mechanisms of knee OA pain have assessed the relationship between SPA and MEP, and measures of underlying pain mechanisms assessed via QST. Both MEP and SPA have demonstrated statistically significant relationships with temporal summation, a marker of ascending facilitation and sensitization processes within the central nervous system (30, 32). Therefore, activity-related pain measures such as MEP and SPA may be more clinically feasible measures of sensitization that do not rely on specialist equipment or training, unlike QST. However, prior to activity-related pain measures being routinely adopted in clinical practice, studies are required to explore the predictive capacity of SPA and MEP in a community sample of those with knee OA. By identifying those at greater risk of worse outcomes, tailored and timely multidisciplinary interventions could be implemented for those in greatest need.
To the best of our knowledge, this is the first study to explore whether assessments of nervous system sensitivity, including comprehensive QST and activity-related pain measures, prospectively predict pain and functional outcomes in people with knee OA who live in the community. Exploring whether sensitization predicts pain outcomes may contribute to developing a better understanding of the different factors contributing to the pain experience in those with knee OA. Therefore, the current study aims to explore whether QST and activity-related measures of pain, predict future pain, fatigue, disability, physical performance, and quality of life outcomes in people with knee OA in New Zealand. It is anticipated that those demonstrating greater pain sensitization on QST and activity-related pain measures, will demonstrate worse pain and functional outcomes prospectively.
Understanding Knee Osteoarthritis Pain Experiences (U-KOPE) is a prospective longitudinal study including smartphone Ecological Momentary Assessment (EMA) completed in Dunedin, New Zealand. This investigation reports predictive associations between baseline measures of sensitization and validated patient-reported outcome measures collected at two-week and nine-week follow-ups. A summary of the current study is presented in Figure 1. This study was developed in consultation with the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS) (33, 34). Ethical approval was obtained through the New Zealand Central Health and Disability Ethics Committee (21/CEN/89). Cultural consultation was sought through the Ngāi Tahu Research Consultation Committee.
Participants were eligible for inclusion if aged 45–85 years, reported a diagnosis of knee OA and experienced knee pain on most days for at least three months. Participants fulfilling NICE guidelines for a clinical diagnosis of knee OA were also included (>45 years of age, activity-related pain and morning stiffness lasting no longer than 30 min) (35).
Participants were excluded if they were non-English speaking, had an autoimmune condition or other forms of inflammatory arthritis, had uncontrolled hypertension, skin conditions, lower limb sensory loss, were pregnant or within six months postpartum, had undergone or were scheduled for total knee arthroplasty, were recovering from a separate lower limb injury, had a neurological condition, impaired cognition or psychiatric illness (excluding stress, anxiety or depression).
Participants were recruited from Dunedin, New Zealand within hospital outpatient settings and the community through advertisements in local newspapers, health practices and online. Participants were given a $100 voucher to recognise any costs involved with participating.
Eligible participants attended a 90-minute baseline assessment and two 30-minute follow-up assessments (weeks two and nine following baseline assessment) at the University of Otago. All participants completed reliable and validated questionnaires (Table 1) for measuring biopsychosocial constructs involved in pain and disability in the knee OA population (69, 74, 94). Selected outcome measures are recommended by Outcome Measures in Rheumatology (OMERACT), Osteoarthritis Research Society International (OARSI) and the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) for assessing those with painful knee OA (96, 97).
Participant characteristics, including demographic information (age, sex, ethnicity, educational level, residential address, and work status), and anthropometrics (height, weight, hip and waist circumference) were recorded to calculate Body Mass Index (BMI) (kg/m2) and hip-waist ratio (36, 37). The Montreal Cognitive Assessment (MoCA), a highly reliable and valid tool for detecting mild cognitive impairment, was administered to participants at the beginning of their baseline assessment (41). In those scoring less than 16 on the MoCA, testing was discontinued and these participants were excluded from the study (98). Quadricep strength testing was performed as described in previous studies using a handheld dynamometer (Lafayette Hand-held Dynamometer, Lafayette Instrument Evaluation, Lafayette, Indiana, USA) (38, 39). This method demonstrates excellent test-retest reliability and validity in people with knee OA (38–40).
Pain intensity and interference were determined for the affected knee using the short-form Brief Pain Inventory (BPI) (99). The short-form BPI has been validated for use in the arthritis population (42, 43). The BPI body chart asked participants to indicate body regions where they experienced pain. Selected regions were summed to calculate the number of widespread pain sites (44, 45). Widespread pain has been linked with alterations in central pain mechanisms (100, 101).
A Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP): KNEE, was used to determine constant and intermittent pain experiences which have been associated with sensitization in knee OA (46, 102). Preliminary psychometric testing suggests the ICOAP is a valid and reliable pain measure for OA when compared with the Knee Injury and Osteoarthritis Outcome Score (KOOS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (46). The KOOS was also used to measure pain, symptoms and functioning associated with knee OA (94). The KOOS demonstrates adequate measurement properties in the knee OA population (47, 48).
The following procedures were used to quantify aspects of sensitization.
1. Quantitative Sensory Testing. Established and standardised QST assessment procedures were completed and are described in more detail below. These QST procedures demonstrated acceptable psychometric properties when used to assess abnormal somatosensory processing in those with knee pain (103). “Bedside” QST procedures were also completed, with these measures being highly correlated with laboratory-based QST (104). The following QST measures were performed:
- Cold pain intensity (CPI) was assessed using an ice cube which was placed on the participant's non-dominant wrist followed by the affected knee for 10 s each. Immediately following each trial, participants reported their greatest pain intensity on a 101-point NPRS (Numeric Pain Rating Scale) (0 = no pain, 100 = worst pain imaginable) (51). Two trials at each site were performed with the average being calculated. This bedside QST procedure is valid and reliable, significantly correlating with laboratory-based testing (49, 51, 52).
- Pressure Pain Threshold (PPT) was measured at the affected medial knee, tibialis anterior (TA) (5 cm below the tibial tuberosity) and at the non-dominant wrist (9, 11, 53–66). A handheld pressure algometer with a probe area of 1 cm2 was used at a ramp of 50 kilopascals (kPa) per second (53, 54, 66). Participants were asked to indicate the moment that deep pressure became painful. The average across three trials was calculated (54, 66, 105). Up to five trials were completed at each site if outlier readings were collected.
- Punctate Pain Intensity (PPI): PPI was assessed using a 300-gram nylon monofilament over the patella of the affected knee and at the non-dominant wrist as performed by previous studies (49–51). The nylon monofilament was applied perpendicular to the skin at each testing site with enough force to bend the filament. Immediately following each trial, participants reported their pain intensity on a 101-point NRPS. The average across three separate trials was calculated.
- Mechanical Temporal Summation (MTS) was assessed over the patella of the affected knee and at the non-dominant wrist (55). Pain ratings using a 101-point NPRS were recorded following a single 300-gram nylon monofilament stimulus. Subsequently, 10 consecutive stimuli were applied at a rate of one stimulus per second within a 1 cm2 area of skin. After the final stimuli, participants reported their peak pain intensity. The difference between the first pain rating and the peak pain rating was used to calculate a “wind-up” ratio. Three separate trials were performed at each site. This method has been used to calculate MTS in previous studies with greater MTS representing a marker of sensitization within the central nervous system and associated with greater pain intensity (22, 55, 67). Those reporting a ≥20/100 NPRS change, representing the minimally clinically important difference for pain intensity in those with knee OA would be classified as demonstrating MTS (106). Continuous MTS scores were used in statistical models.
- Conditioned Pain Modulation (CPM), a measure of descending pain modulation, was examined as per previous studies (11, 57, 61, 66–68, 107):
○ Conditioning stimulus: Participants were asked to submerge their dominant hand in a manually circulated 10-degree Celsius ice bath for two minutes or until intolerance (67, 68).
○ Test stimulus: PPT40 (PPT with participants indicating when their pain reaches an intensity of 40/100 on the NPRS) was assessed at the non-dominant forearm before and at 30, 60 and 90 s following the conditioning stimulus.
For descriptive purposes, participants were classified as being facilitators, inhibitors or non-responders based on the calculated standard error of measurement (108). A change >2 SEM was interpreted as inhibition and <2 SEM as facilitation. Those with change scores <±2 SEM were categorized as non-responders (108). Continuous CPM scores were used in statistical models.
2. Activity-related pain: Performance-based tests included a Six-Minute Walk Test and a 30-second Chair Test which have been used in previous studies exploring activity-related pain (30, 69). Discomfort ratings were collected on a 101-point discomfort rating scale (0 = no discomfort, 100 = extreme discomfort) before, during and after each test (30). Temporal summation related to Sensitivity to Physical Activity (SPA) was calculated as the difference between prior and peak discomfort ratings to provide a “wind-up ratio.” Those reporting a 20-point increase in discomfort ratings were deemed to have SPA (109). SPA provides a more ecologically valid measure of temporal summation and has been linked with central sensitization and predicting greater pain intensity and reduced functioning (29, 30, 70, 71). Movement-evoked pain (MEP), representing the average level of pain experienced while undergoing performance-based testing was calculated by taking the average of the discomfort ratings across the 6-Minute Walk Test (28, 32). MEP, therefore, represents the average pain experienced during testing, while SPA represents the change in pain during testing, each potentially having distinct mechanisms.
Valid and reliable measures used for assessing psychosocial as well as health and lifestyle-related factors are presented in Table 1.
The primary analysis in the present study included multivariable regression models adjusting for age, sex, socioeconomic status, BMI, baseline pain intensity and disease duration. A sample size of 81 was calculated using G*Power 3.1 based on a 0.25 effect size, power of 0.9 and error of 0.05 with seven predictors (110). A final sample of 101 participants was recruited to account for a 20% dropout rate.
Sensitization data analysis and questionnaire scoring were not performed until all participants completed the study.
All statistical analyses were conducted using SPSS (Version 28.0.1.0). Both dependent and independent variables were assessed for normality by exploring Kolmogorov-Smirnov tests, Shapiro-Wilk tests as well as skew and kurtosis. Non-normally distributed data underwent logarithmic, square root and inverse transformation, with most unable to be transformed to the normal distribution.
Independent variables included PPT's, PPI, MTS, SPA, MEP, CPM, CPI and number of pain sites. Baseline dependent variables included the BPI, KOOS, ICOAP, 6 MWT distance, 30 sCST number and SF-12 outcomes. Longitudinal dependent variables included the BPI, KOOS, Brief Fatigue Inventory (BFI), SF-12 and Keele Assessment of Participation (KAP). Models were developed for each dependent variable including one predictor variable alongside covariates. Univariable and multivariable regression analyses were completed to explore the cross-sectional and prospective predictive associations between measures of sensitization and knee OA outcomes. Non-transformed raw data was used as indicated by the Gauss-Markov theorem (111, 112).
Univariable regression was first completed with p-values ≤0.2 allowing predictors entry into the multivariable regression analysis. Bivariate correlations were also collected within univariable regression analyses. The following ranges were used to classify effect sizes to interpret the strength of the relationship: zero to 0.25 – small; 0.25 to 0.5 – fair; 0.5 to 0.75 – moderate to good; and >0.75 – good to excellent (113).
Multiple adjusted linear regressions each including one sensitization predictor were then completed for each of the dependent variables. Due to the exploratory nature of the study, models were adjusted for covariates which were selected a priori based on pain and knee OA literature. These included age, gender, BMI, OA symptom duration, baseline pain intensity and socioeconomic status (20, 114, 115). Baseline pain intensity was not included as a covariate in multivariable models at baseline where pain intensity measures were the dependent variables. Multicollinearity and heteroskedasticity were assessed for each analysis by examining multicollinearity statistics as well as scatterplots of residuals. The Durbin-Watson test was used to assess autocorrelation in the residuals with 1.5–2.5 being deemed acceptable (116). The level of error considered acceptable for statistical significance was set at p ≤ 0.05.
One hundred and twenty-three individuals registered to participate in the study. Twenty-six (21.1%) did not meet the inclusion criteria and were excluded. A further 11 participants (8.9%) did not respond to initial contact attempts. A total of 86 participants met the inclusion criteria and there was no loss to follow-up. This can be seen in the participant flow diagram in Figure 2.
The study sample is described in Table 2. Table 2 also provides means and standard deviations for baseline measures of included participants including participant characteristics, knee pain and functioning measures, psychosocial measures and health and lifestyle measures respectively.
Table 3 provides means and standard deviations for sensitization measures.
Table 2 provides means and standard deviations for outcome measures collected at two-week and nine-week follow-up.
Results from the univariable regression and correlation analyses with p-values equal to or less than 0.2 are presented in Table 4.
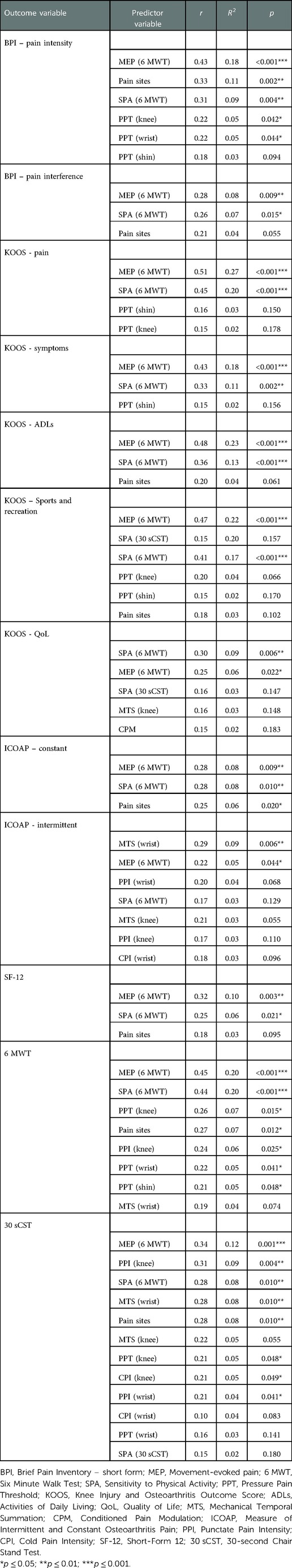
Table 4. Unadjusted univariable regression and correlation outcomes between measures of sensitization and knee OA outcomes at baseline
Bivariate correlations showed that MEP was significantly related with a number of outcomes. Similarly, Sensitivity to Physical Activity was also related with several outcomes. QST measures of sensitization demonstrated small to fair statistically significant correlations.
Outcomes from the multivariable regression analyses between various measures of sensitization and outcomes presenting statistically significant at the 0.05 level cross-sectionally and longitudinally are presented in Tables 5, 6, respectively. Separate multivariable models were conducted for each predictor variable with adjustment for covariates including age, gender, BMI, knee OA duration, baseline pain intensity as well as socioeconomic status.
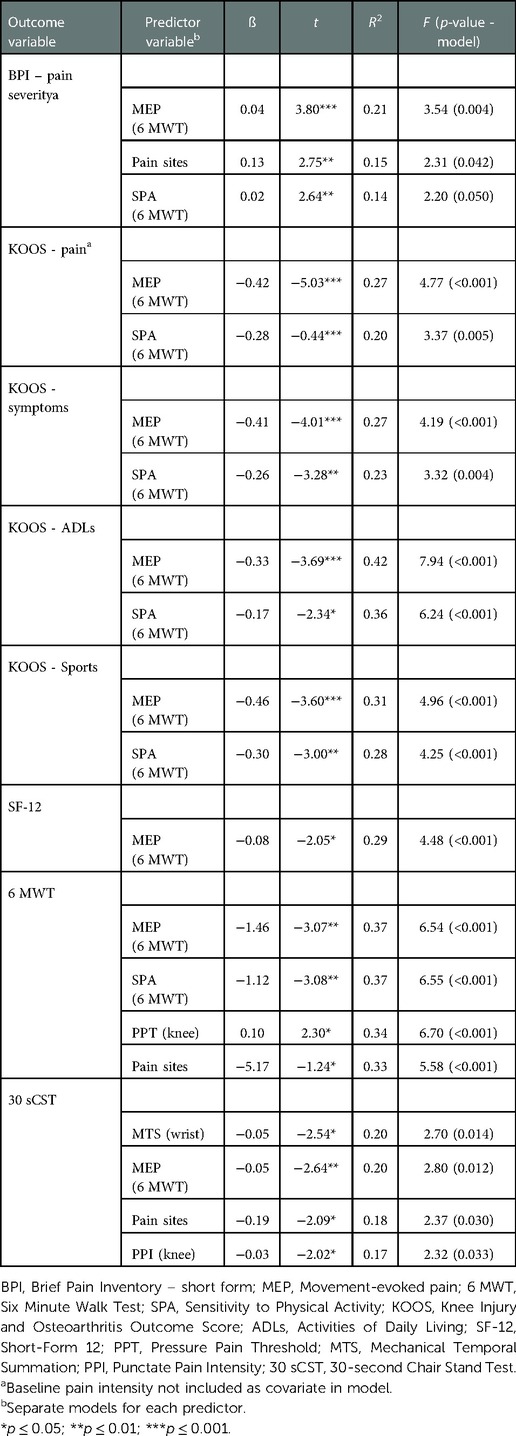
Table 5. Adjusted models of cross-sectional associations between measures of sensitization and clinical outcomes at baseline
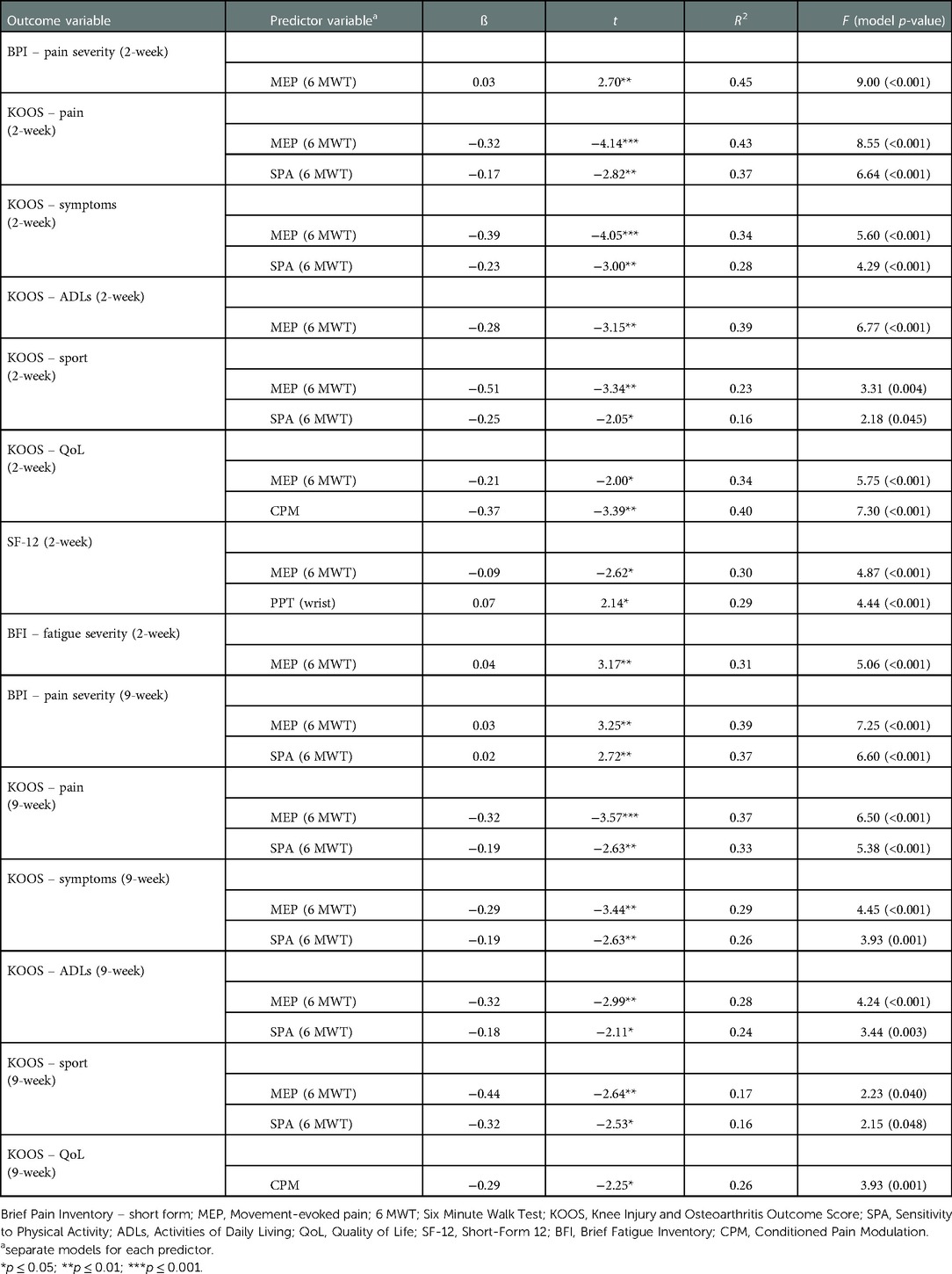
Table 6. Adjusted models of associations between measures of sensitization and clinical outcomes at two- and nine-week follow-ups
At baseline, adjusted models including MEP explained a significant degree of variance across several outcomes including BPI-pain intensity (ß 0.04, R2 21%), KOOS pain (ß −-0.42, R2 27%), KOOS sport and recreation (ß −0.46, R2 31%), 6 MWT performance (ß −1.46, R2 37%) and health-related quality of life (ß −0.08, R2 29%). SPA also explained a significant degree of variance in outcomes including KOOS pain (ß −0.28, R2 20%), KOOS ADLs (ß −0.17, R2 36%), KOOS sport and recreation (ß −0.30, R2 28%) and 6 MWT performance (ß −1.12, R2 37%). Adjusted models including pressure pain thresholds at the knee explained 34% of the variance in 6 MWT performance (ß 0.10) while MTS at the wrist explained 20% of 30 sCST performance (ß −0.05).
Adjusted models each including MEP and SPA continued to explain outcome variance at the two-week follow-up. MEP was shown responsible for variance in BPI – pain severity (ß 0.03, R2 45%), KOOS pain (ß −0.32, R2 43%), KOOS ADLs (ß −0.28, R2 39%), health-related quality of life (ß −0.21, R2 30%) and fatigue severity (ß 0.04, R2 31%). SPA also explained outcome variance in KOOS pain (ß −0.17, R2 37%), KOOS symptoms (ß −0.23, R2 28%) and KOOS sports (ß −0.25, R2 16%). At the two-week follow-up, CPM was shown to predict KOOS QoL outcomes explaining 40% of the variance (ß −0.37).
At the 9-week follow-up, adjusted models including MEP remained significant in explaining variance in BPI - pain severity (ß 0.03, R2 39%), KOOS pain (ß −0.32, R2 37%), KOOS ADLs (ß −0.32, R2 28%) and KOOS sports (ß −0.44, R2 17%). SPA continued to explain 37% of the variance in pain intensity at the 9-week follow-up (ß 0.02) as well as KOOS pain (ß −019, R2 33%), KOOS symptoms (ß −0.19, R2 26%), KOOS ADLs (ß −0.18, R2 24%) and KOOS sports (ß −0.32, R2 16%). Adjusted models including CPM continued to explain 26% of the variance in KOOS QoL outcomes (ß −0.29).
Underlying pain mechanisms including nervous system sensitization have emerged as important considerations in musculoskeletal and pain research over recent decades. Activity-related pain measures, with links to nervous system sensitization, are also being more widely investigated (24, 29). This study explored whether these measures prospectively predict outcomes in those with knee OA. Findings included activity-related pain measures, such as MEP and SPA, demonstrated predictive associations with pain, function and health-related quality of life outcomes cross-sectionally as well as longitudinally, even after controlling for age, gender, BMI, symptom duration, socioeconomic status and baseline pain intensity. Therefore, people with knee OA who demonstrate greater activity-related pain may be at risk of higher pain, disability and reduced quality of life in a limited prospective period.
Both pain and disability are commonly reported by those suffering from knee OA (23). Therefore, measuring pain during functioning is highly relevant to the knee OA pain experience (24, 28). MEP is an emerging measure of activity-related pain which is represented by the average pain intensity reported during a standardized physical task. Therefore, MEP likely provides greater ecological validity to living and functioning with pain related to knee OA (24, 28, 117). Mechanisms of MEP are proposed to include peripheral mechanical factors including activation of silent nociceptors, as well as central nervous system changes resulting in lowered nociceptive thresholds (24, 28). This measure demonstrated predictive associations with distinct outcomes in the current study (i.e., health-related quality of life). Mechanisms of MEP may therefore be different from SPA and potentially include other related factors, such as psychosocial status which have been shown as important contributors towards health-related quality of life outcomes (118).
A recent study, which compared MEP with QST measures found that 12% of the variance of MEP was explained by TS, a marker of central sensitization (32). Therefore, at least some part of the underlying pain mechanisms of MEP involves central nervous system nociceptive changes reflective of central sensitization. Additionally, psychosocial factors, genetic and environmental factors are proposed to influence MEP (24, 28). Recent cross-sectional studies have confirmed that higher fear avoidance, pain catastrophizing and stress were related to greater MEP (23, 117). Interestingly, positive psychosocial factors, such as resilience have been found to demonstrate a protective buffering effect (23, 117). Therefore, addressing psychosocial factors could influence MEP and potentially improve outcomes.
MEP also has clinical importance with this activity-related measure potentially helping health professionals better understand the relationship between pain and functioning in those with knee OA (24). A recent prediction study assessed MEP using weight-bearing items from the Western Ontario and McMaster Universities Arthritis Index (WOMAC). This found that those with greater MEP prior to TKA had greater post-operative pain at one year (31). The current study also highlights that MEP importantly predicts future pain and disability outcomes in those living with knee OA in the community. Therefore, MEP may be an ecologically valid and clinically feasible measure of activity-related pain in those with knee OA.
Another activity-related measure of pain which captures the change in pain intensity during a physical task is SPA (30). Previous studies have highlighted the potential importance of SPA, with SPA cross-sectionally predicting pain and functional outcomes in those with knee OA (29, 30, 71, 119). Similar to MEP, underlying mechanisms of SPA are reported to include temporal summation as a result of the repetitive mechanical demands of physical tasks resulting in “wind-up” of nociception at the dorsal horn of the spinal cord (30, 70, 119–121). Studies have highlighted the impact that psychosocial factors, such as fear and pain catastrophising, have on SPA in those with knee OA (29, 30, 71, 119). Therefore, complex interactions between mechanical loading, pain mechanisms and psychosocial factors, and SPA are present in those with knee OA (29, 30, 71, 119).
Activity-related pain in the current study was assessed during OARSI-recommended physical performance measures including the 6 MWT and 30 sCST (69). The 6 MWT is a valid and reliable assessment of submaximal cardiovascular capacity (30, 122). An additional mechanism of activity-related pain could involve submaximal cardiovascular demands, which have been linked to the reactivity of the autonomic nervous system (123). The autonomic nervous system has been shown to have extensive interactions with nociceptive processing meaning that activation may result in greater nociception, reduced modulation and increased pain (124). This may explain why SPA and MEP during the 30 sCST, which arguably involves greater loading of the affected knee, had a limited predictive capacity in comparison to the 6 MWT in the current study. An impaired endogenous analgesia response to exercise (exercise-induced hypoalgesia), may also contribute to activity-related pain (125). As noted previously, psychosocial factors including pain-related fear, low mood and self-efficacy as well as pain catastrophizing have been independently linked to abnormal nociceptive processing and may influence pain experienced during physical activities (126–129). Therefore, mechanisms underlying activity-related pain are likely to be multifactorial and individual, including but not limited to mechanical, cardiovascular, autonomic, neural as well as psychological. Activity-related pain warrants consideration in clinical practice as it may act as a barrier to recommended exercise-based treatments (130), placing those with knee OA at risk of a negative cascade of sedentariness, disability and ongoing pain (29, 119). Further research is required to explore the mechanisms of activity-related pain and whether interventions such as pain self-management support including pain education, strategy implementation and exercise may be beneficial for this population (131–133).
QST is commonly used in clinical pain studies as a way of quantifying nervous system processing of noxious information (53). Previous studies have demonstrated the ability of QST to predict treatment outcomes in those suffering from musculoskeletal pain, highlighting that underlying pain mechanisms are an important consideration (20). Interestingly, in the current study, QST demonstrated variable relationships with pain outcomes. Cross-sectionally, PPTs at the wrist and knee demonstrated small correlations with pain intensity and 6 MWT performance. While CPI at the affected knee was not related with any outcomes. MTS, a lab-based psychophysical measure of central sensitization, was related with intermittent pain experiences, health-related quality of life and performance on the 30 sCST test. However, none of these relationships were maintained longitudinally.
When looking at the predictive capacity of QST, many of the measures did not meet the criteria for inclusion in multivariable models. However, in those that did, PPTs were shown to uniquely predict walking distance on the 6 MWT while greater MTS uniquely predicted worse performance on the 30 sCST, but not the 6 MWT. These findings potentially highlight some task-specific variability. Interestingly, CPM a measure of central nervous system pain modulation, demonstrated predictive associations with KOOS QoL outcomes longitudinally. These variable findings may be due to the range of different pain mechanisms presenting across the knee OA population, the susceptibility of QST being influenced by demographic and other factors, as well as the community sample which was made up by a majority experiencing a mild pain intensity (134). Further research exploring the predictive utility of QST measures in community samples of those with knee OA are needed to better support the use of these sensory measures in clinical practice.
Better understanding pain mechanisms and their ability to inform prognosis in those with knee OA could guide mechanism-based care and improve outcomes. By better understanding mechanisms involved in knee pain, targeted therapies could be provided that specifically focus on addressing the underlying cause of symptoms. A core recommended treatment for those with knee OA includes the prescription of physical activity (130). However, clinicians should be mindful that 57% of people with knee OA have SPA as demonstrated by this study. A more refined approach to activity prescription which considers activity-related pain and central pain mechanisms could aid in improving pain and functioning outcomes in the knee OA population while reducing the risk of symptom flares and subsequent fear avoidance and disability. Additionally, future research which considers mediating and moderating factors to better understand neurophysiological and biopsychosocial factors involved in knee OA pain would also provide useful information on targets for treatment (i.e., psychological interventions). Studies which use more ecologically valid biopsychosocial pain-related outcome variables with reduced recall bias, such as Ecological Momentary Assessment are warranted.
Strengths of the current study include both the cross-sectional and longitudinal design with follow-up data collected at two and nine weeks in a community sample of those living with knee OA. In addition, a range of measures assessing for sensitization were performed including static and dynamic QST as well as activity-related pain measures. Considering all of the above, this study provides a unique contribution to the pain mechanisms and knee OA literature, highlighting important prognostic markers in the community sample. Limitations include the majority of the sample reporting mild knee OA pain. Participants also lacked ethnic diversity with most being New Zealand European. Although one of the recruitment strategies was to recruit patients from an outpatient tertiary hospital setting, no participants were recruited via this route. The follow-up window of the current study was only nine weeks. Although this provides a brief longitudinal indicator as used by other knee OA studies (135), pain outcomes explored over at least 1–2 years would be useful to determine the medium- and long-term trajectories and outcomes. Participants within the current study may present as less sensitized compared to other samples (average remote PPTs were 447.4 ± 219 kPa compared to 146–369 kPa in the literature) (11). Scores on psychological measures were also low in the current sample meaning the included sample may not fully represent the wider knee OA population. Psychometric properties of MEP and SPA measures also need to be established including exploration of MEP and SPA during unstandardized, free-living functional tasks to maximise ecological validity. Variable selection methods for including variables in the multivariable analysis may have wrongly rejected potentially important candidate variables, however, a larger p-value threshold was used to reduce the risk of this. Furthermore, multivariable model checks were completed for candidate variables from the univariable analysis with no additional predictor variables meeting statistical significance (Appendix A). Overall, larger, multi-centred studies of people with moderate and severe knee OA with longer follow-up durations are required. External validation of the models exploring potentially important moderators and mediators of these relationships is warranted.
Pain and reduced functioning remain a major problem, contributing to disability and reduced quality of life in those with knee OA. Alterations in underlying nervous system pain mechanisms have been highlighted in this population which may inform prognosis. This was confirmed by the current study which highlights that activity-related pain measures including MEP and SPA, demonstrate predictive associations with pain intensity, function and quality of life outcomes prospectively in those with knee OA. Pain mechanisms assessed via QST demonstrated variable relationships with outcomes which were often not maintained longitudinally.
Therefore, consideration of clinically feasible, ecologically valid measures of activity-related pain and sensitization is recommended when assessing and treating those with knee OA. This could provide important clinical information and assist in identifying those at greatest risk of pain and disability. By identifying those at higher risk, treatments can be provided to those in greatest need and potentially target mechanisms of activity-related pain to improve knee OA outcomes. However, before these measures are routinely implemented into clinical practice, further studies are required that externally validate findings as well as explore measures of activity-related pain over longer durations, in different samples and use a range of standardized physical tests.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by New Zealand Central Health and Disability Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
All authors (MO, NS, DGJ, CF, RF, RM) provided equal contribution to the original idea, design, carrying out data collection, data analysis, writing and proofing of the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by the Otago Medical Research Foundation (grant number: JT-387). This funding enabled participant recruitment advertising online and in local newspapers as well as $100 supermarket vouchers to recognise participant contribution. Some of this funding is also being used for open access publication fees.
Thank you to the Otago Medical Research Foundation who provided funding to carry out this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hooper G, Lee AJ, Rothwell A, Frampton C. Current trends and projections in the utilisation rates of hip and knee replacement in New Zealand from 2001 to 2026. N Z Med J. (2014) 127(1401):82. http://www.nzma.org.nz/journal/read-the-journal/all-issues/2010-2019/2014/vol-127-no-1401/627625225759
2. Whittaker JL, Runhaar J, Bierma-Zeinstra S, Roos EM. A lifespan approach to osteoarthritis prevention. Osteoarthritis Cartilage. (2021) 29(12):1638–53. doi: 10.1016/j.joca.2021.06.015
3. Leifer V, Katz J, Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage. (2022) 30(1):10–6. doi: 10.1016/j.joca.2021.05.007
4. Perrot S, Cohen M, Barke A, Korwisi B, Rief W, Treede RD, et al. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain. (2019) 160(1):77–82. doi: 10.1097/j.pain.0000000000001389
5. Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. (2013) 65(2):363–72. doi: 10.1002/art.34646
6. International Association for the Study of Pain. Terminology (2021). Available from: https://www.iasp-pain.org/resources/terminology/
7. Skou ST, Graven-Nielsen T, Rasmussen S, Simonsen O, Laursen M, Arendt-Nielsen L. Facilitation of pain sensitization in knee osteoarthritis and persistent post-operative pain: a cross-sectional study. Eur J Pain. (2014) 18(7):1024–31. doi: 10.1002/j.1532-2149.2013.00447.x
8. Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. (2015) 67(5):1386–94. doi: 10.1002/art.39051
9. Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, De Souza LPM, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Care Res. (2008) 59(10):1424–31. doi: 10.1002/art.24120
10. Dell’Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord. (2016) 17(1):425. doi: 10.1186/s12891-016-1286-2
11. Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. (2015) 23(7):1043–56. doi: 10.1016/j.joca.2015.02.163
12. Wylde V, Palmer S, Learmonth ID, Dieppe P. Somatosensory abnormalities in knee OA. Rheumatology. (2012) 51(3):535–43. doi: 10.1093/rheumatology/ker343
13. Edwards RR, Dolman AJ, Martel MO, Finan PH, Lazaridou A, Cornelius M, et al. Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMC Musculoskelet Disord. (2016) 17(1):1–9. doi: 10.1186/s12891-016-1124-6
14. Luna I, Kehlet H, Petersen M, Aasvang E. Clinical, nociceptive and psychological profiling to predict acute pain after total knee arthroplasty. Acta Anaesthesiol Scand. (2017) 61(6):676–87. doi: 10.1111/aas.12899
15. Noiseux NO, Callaghan JJ, Clark CR, Zimmerman MB, Sluka KA, Rakel BA. Preoperative predictors of pain following total knee arthroplasty. J Arthroplasty. (2014) 29(7):1383–7. doi: 10.1016/j.arth.2014.01.034
16. Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain. (2015) 156(1):55–61. doi: 10.1016/j.pain.0000000000000022
17. Vaegter HB, Handberg G, Emmeluth C, Graven-Nielsen T. Preoperative hypoalgesia after cold pressor test and aerobic exercise is associated with pain relief 6 months after total knee replacement. Clin J Pain. (2017) 33(6):475–84. doi: 10.1097/AJP.0000000000000428
18. Wylde V, Palmer S, Learmonth I, Dieppe P. The association between pre-operative pain sensitisation and chronic pain after knee replacement: an exploratory study. Osteoarthritis Cartilage. (2013) 21(9):1253–6. doi: 10.1016/j.joca.2013.05.008
19. Ziv YB, Shemesh S, Agar G, Benedict S, Heller S, Kosashvili Y. The sphygmomanometer pain test: a simple method for identifying patients at risk of excessive pain after total knee arthroplasty. J Arthroplasty. (2016) 31(4):798–801. doi: 10.1016/j.arth.2015.10.027
20. O’Leary H, Smart KM, Moloney NA, Doody CM. Nervous system sensitization as a predictor of outcome in the treatment of peripheral musculoskeletal conditions: a systematic review. Pain Pract. (2017) 17(2):249–66. doi: 10.1111/papr.12484
21. Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain. (2019) 160(9):1920–32. doi: 10.1097/j.pain.0000000000001590
22. Goodin BR, Bulls HW, Herbert MS, Schmidt J, King CD, Glover TL, et al. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and above with knee osteoarthritis: ethnic differences. Psychosom Med. (2014) 76(4):302. doi: 10.1097/PSY.0000000000000058
23. Booker S, Cardoso J, Cruz-Almeida Y, Sibille KT, Terry EL, Powell-Roach KL, et al. Movement-evoked pain, physical function, and perceived stress: an observational study of ethnic/racial differences in aging non-hispanic blacks and non-hispanic whites with knee osteoarthritis. Exp Gerontol. (2019) 124:110622. doi: 10.1016/j.exger.2019.05.011
24. Corbett DB, Simon CB, Manini TM, George SZ, Riley JL III, Fillingim RB. Movement-evoked pain: transforming the way we understand and measure pain. Pain. (2019) 160(4):757. doi: 10.1097/j.pain.0000000000001431
25. Mankovsky-Arnold T, Wideman TH, Thibault P, Larivière C, Rainville P, Sullivan MJ. Sensitivity to movement-evoked pain and multi-site pain are associated with work-disability following whiplash injury: a cross-sectional study. J Occup Rehabil. (2017) 27(3):413–21. doi: 10.1007/s10926-016-9672-z
26. Wallis JA, Taylor NF, Bunzli S, Shields N. Experience of living with knee osteoarthritis: a systematic review of qualitative studies. BMJ Open. (2019) 9(9):e030060. doi: 10.1136/bmjopen-2019-030060
27. Gay C, Eschalier B, Levyckyj C, Bonnin A, Coudeyre E. Motivators for and barriers to physical activity in people with knee osteoarthritis: a qualitative study. Joint Bone Spine. (2018) 85(4):481–6. doi: 10.1016/j.jbspin.2017.07.007
28. Fullwood D, Means S, Merriwether EN, Chimenti RL, Ahluwalia S, Booker SQ. Toward understanding movement-evoked pain (MEP) and its measurement: a scoping review. Clin J Pain. (2021) 37(1):61–78. doi: 10.1097/AJP.0000000000000891
29. Wideman TH, Edwards RR, Finan PH, Haythornthwaite JA, Smith MT. Comparing the predictive value of task performance and task-specific sensitivity during physical function testing among people with knee osteoarthritis. J Orthop Sports Phys Ther. (2016) 46(5):346–56. doi: 10.2519/jospt.2016.6311
30. Wideman TH, Finan PH, Edwards RR, Quartana PJ, Buenaver LF, Haythornthwaite JA, et al. Increased sensitivity to physical activity among individuals with knee osteoarthritis: relation to pain outcomes, psychological factors, and responses to quantitative sensory testing. PAIN®. (2014) 155(4):703–11. doi: 10.1016/j.pain.2013.12.028
31. Sayers A, Wylde V, Lenguerrand E, Beswick AD, Gooberman-Hill R, Pyke M, et al. Rest pain and movement-evoked pain as unique constructs in hip and knee replacements. Arthritis Care Res (Hoboken). (2016) 68(2):237–45. doi: 10.1002/acr.22656
32. Simon CB, Lentz TA, Ellis L, Bishop MD, Fillingim RB, Riley JL III, et al. Static and dynamic pain sensitivity in adults with persistent low back pain: comparison to healthy controls and associations with movement-evoked pain versus traditional clinical pain measures. Clin J Pain. (2021) 37(7):494–503. doi: 10.1097/AJP.0000000000000945
33. Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. (2014) 11(10):e1001744. doi: 10.1371/journal.pmed.1001744
34. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158(4):280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
35. National Clinical Guideline C. National institute for health and clinical excellence: Guidance. Osteoarthritis: care and management in adults. London: National Institute for Health and Care Excellence (UK) (2014). Copyright © National Clinical Guideline Centre, 2014.
36. Abbott JH, Wilson R, Pinto D, Chapple CM, Wright AA. Incremental clinical effectiveness and cost effectiveness of providing supervised physiotherapy in addition to usual medical care in patients with osteoarthritis of the hip or knee: 2-year results of the MOA randomised controlled trial. Osteoarthritis Cartilage. (2019) 27(3):424–34. doi: 10.1016/j.joca.2018.12.004
37. Medicine ACoS. ACSM’s health-related physical fitness assessment manual. Philadelphia: Lippincott Williams & Wilkins (2013).
38. Kittelson AJ, Christensen JC, Loyd BJ, Burrows KL, Iannitto J, Stevens-Lapsley JE. Reliability, responsiveness, and validity of handheld dynamometry for assessing quadriceps strength in total knee arthroplasty. Disabil Rehabil. (2020) 43(21):1–8. doi: 10.1080/09638288.2020.1730454
39. Arnold CM, Warkentin KD, Chilibeck PD, Magnus CR. The reliability and validity of handheld dynamometry for the measurement of lower-extremity muscle strength in older adults. J Strength Cond Res. (2010) 24(3):815–24. doi: 10.1519/JSC.0b013e3181aa36b8
40. Chopp-Hurley JN, Wiebenga EG, Gatti AA, Maly MR. Investigating the test–retest reliability and validity of hand-held dynamometry for measuring knee strength in older women with knee osteoarthritis. Physiother Can. (2019) 71(3):231–8. doi: 10.3138/ptc-2018-0051
41. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x
42. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. (2004) 5(2):133–7. doi: 10.1016/j.jpain.2003.12.005
43. Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. (2004) 20(5):309–18. doi: 10.1097/00002508-200409000-00005
44. Lacey RJ, Lewis M, Jordan K, Jinks C, Sim J. Interrater reliability of scoring of pain drawings in a self-report health survey. Spine (Phila Pa 1976). (2005) 30(16):E455–E8. doi: 10.1097/01.brs.0000174274.38485.ee
45. Ohlund C, Eek C, Palmbald S, Areskoug B, Nachemson A. Quantified pain drawing in subacute low back pain. Validation in a nonselected outpatient industrial sample. Spine (Phila Pa 1976). (1996) 21(9):1021–30; discussion 31. doi: 10.1097/00007632-199605010-00005
46. Hawker G, Davis A, French M, Cibere J, Jordan J, March L, et al. Development and preliminary psychometric testing of a new OA pain measure–an OARSI/OMERACT initiative. Osteoarthritis Cartilage. (2008) 16(4):409–14. doi: 10.1016/j.joca.2007.12.015
47. Collins NJ, Prinsen CAC, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee injury and osteoarthritis outcome score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage. (2016) 24(8):1317–29. doi: 10.1016/j.joca.2016.03.010
48. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS) – validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. (2003) 1(1):17. doi: 10.1186/1477-7525-1-17
49. Timmerman H, Wilder-Smith OH, Steegers MA, Vissers KC, Wolff AP. The added value of bedside examination and screening QST to improve neuropathic pain identification in patients with chronic pain. J Pain Res. (2018) 11:1307. doi: 10.2147/JPR.S154698
50. Reimer M, Forstenpointner J, Hartmann A, Otto JC, Vollert J, Gierthmühlen J, et al. Sensory bedside testing: a simple stratification approach for sensory phenotyping. Pain Rep. (2020) 5(3):e820. doi: 10.1097/PR9.0000000000000820
51. Zhu GC, Böttger K, Slater H, Cook C, Farrell SF, Hailey L, et al. Concurrent validity of a low-cost and time-efficient clinical sensory test battery to evaluate somatosensory dysfunction. Eur J Pain. (2019) 23(10):1826–38. doi: 10.1002/ejp.1456
52. Osgood E, Trudeau JJ, Eaton TA, Jensen MP, Gammaitoni A, Simon LS, et al. Development of a bedside pain assessment kit for the classification of patients with osteoarthritis. Rheumatol Int. (2015) 35(6):1005–13. doi: 10.1007/s00296-014-3191-z
53. Rolke R, Baron R, Ca M, Tölle T, Treede R-D, Beyer A, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): standardized protocol and reference values. Pain. (2006) 123(3):231–43. doi: 10.1016/j.pain.2006.01.041
54. Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. (2006) 10(1):77–88. doi: 10.1016/j.ejpain.2005.02.003
55. Skou ST, Graven-Nielsen T, Lengsoe L, Simonsen O, Laursen MB, Arendt-Nielsen L. Relating clinical measures of pain with experimentally assessed pain mechanisms in patients with knee osteoarthritis. Scand J Pain. (2013) 4(2):111–7. doi: 10.1016/j.sjpain.2012.07.001
56. Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. (2012) 20(10):1075–85. doi: 10.1016/j.joca.2012.06.009
57. Arendt-Nielsen L. Pain sensitisation in osteoarthritis. Clin Exp Rheumatol. (2017) 35(Suppl 107):68–74. PMID: 28967356
58. Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. (2010) 149(3):573–81. doi: 10.1016/j.pain.2010.04.003
59. Akinci A, Al Shaker M, Chang MH, Cheung CW, Danilov A, Duenas HJ, et al. Predictive factors and clinical biomarkers for treatment in patients with chronic pain caused by osteoarthritis with a central sensitisation component. Int J Clin Pract. (2016) 70(1):31–44. doi: 10.1111/ijcp.12749
60. Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. (2015) 74(4):682–8. doi: 10.1136/annrheumdis-2013-204191
61. Graven-Nielsen T, Wodehouse T, Langford R, Arendt-Nielsen L, Kidd B. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. (2012) 64(9):2907–16. doi: 10.1002/art.34466
62. Guérard O, Dufort S, Besnard LF, Gougeon A, Carlesso L. Comparing the association of widespread pain, multi-joint pain and low back pain with measures of pain sensitization and function in people with knee osteoarthritis. Clin Rheumatol. (2020) 39(3):873–9. doi: 10.1007/s10067-019-04828-3
63. Cohen E, Lee YC. A mechanism-based approach to the management of osteoarthritis pain. Curr Osteoporos Rep. (2015) 13(6):399–406. doi: 10.1007/s11914-015-0291-y
64. Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J Pain. (2014) 18(10):1367–75. doi: 10.1002/j.1532-2149.2014.499.x
65. Rakel B, Vance C, Zimmerman MB, Petsas-Blodgett N, Amendola A, Sluka KA. Mechanical hyperalgesia and reduced quality of life occur in people with mild knee osteoarthritis pain. Clin J Pain. (2015) 31(4):315–22. doi: 10.1097/AJP.0000000000000116
66. Foucher KC, Chmell SJ, Courtney CA. Duration of symptoms is associated with conditioned pain modulation and somatosensory measures in knee osteoarthritis. J Orthop Res. (2019) 37(1):136–42. doi: 10.1002/jor.24159
67. Mani R, Adhia DB, Leong SL, Vanneste S, De Ridder D. Sedentary behaviour facilitates conditioned pain modulation in middle-aged and older adults with persistent musculoskeletal pain: a cross-sectional investigation. Pain Rep. (2019) 4(5):e773. doi: 10.1097/PR9.0000000000000773
68. Yarnitsky D, Bouhassira D, Drewes A, Fillingim R, Granot M, Hansson P, et al. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain. (2015) 19(6):805–6. doi: 10.1002/ejp.605
69. Dobson F, Hinman RS, Roos EM, Abbott JH, Stratford P, Davis AM, et al. OARSI Recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. (2013) 21(8):1042–52. doi: 10.1016/j.joca.2013.05.002
70. Woznowski-Vu A, Uddin Z, Flegg D, Aternali A, Wickens R, Sullivan MJ, et al. Comparing novel and existing measures of sensitivity to physical activity among people with chronic musculoskeletal pain. Clin J Pain. (2019) 35(8):656–67. doi: 10.1097/AJP.0000000000000732
71. Lambin DI, Thibault P, Simmonds M, Lariviere C, Sullivan MJ. Repetition-induced activity-related summation of pain in patients with fibromyalgia. Pain. (2011) 152(6):1424–30. doi: 10.1016/j.pain.2011.02.030
72. Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42-item and 21-item versions of the depression anxiety stress scales in clinical groups and a community sample. Psychol Assess. (1998) 10(2):176. doi: 10.1037/1040-3590.10.2.176
73. Wood BM, Nicholas MK, Blyth F, Asghari A, Gibson S. The utility of the short version of the depression anxiety stress scales (DASS-21) in elderly patients with persistent pain: does age make a difference? Pain Med. (2010) 11(12):1780–90. doi: 10.1111/j.1526-4637.2010.01005.x
74. Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, et al. Patient phenotyping in clinical trials of chronic pain treatments: iMMPACT recommendations. Pain. (2016) 157(9):1851. doi: 10.1097/j.pain.0000000000000602
75. Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The pain catastrophizing scale: further psychometric evaluation with adult samples. J Behav Med. (2000) 23(4):351–65. doi: 10.1023/A:1005548801037
76. Yakobov E, Stanish W, Tanzer M, Dunbar M, Richardson G, Sullivan MJ. The prognostic value of pain catastrophizing in health-related quality of life judgments after total knee arthroplasty. Health Qual Life Outcomes. (2018) 16(1):126. doi: 10.1186/s12955-018-0955-2
77. Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the pain catastrophizing scale. J Behav Med. (1997) 20(6):589–605. doi: 10.1023/A:1025570508954
78. Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7(4):524. doi: 10.1037/1040-3590.7.4.524
79. Nicholas MK, McGuire BE, Asghari A. A 2-item short form of the pain self-efficacy questionnaire: development and psychometric evaluation of PSEQ-2. J Pain. (2015) 16(2):153–63. doi: 10.1016/j.jpain.2014.11.002
80. Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. (2007) 11(2):153–63. doi: 10.1016/j.ejpain.2005.12.008
81. Benhamou M, Baron G, Dalichampt M, Boutron I, Alami S, Rannou F, et al. Development and validation of a questionnaire assessing fears and beliefs of patients with knee osteoarthritis: the knee osteoarthritis fears and beliefs questionnaire (KOFBeQ). PLoS One. (2013) 8(1):e53886. doi: 10.1371/journal.pone.0053886
82. Riddle DL, Jensen MP. Construct and criterion-based validity of brief pain coping scales in persons with chronic knee osteoarthritis pain. Pain Med. (2013) 14(2):265–75. doi: 10.1111/pme.12007
83. Lugt CMC VD, Rollman A, Naeije M, Lobbezoo F, Visscher CM. Social support in chronic pain: development and preliminary psychometric assessment of a new instrument. J Oral Rehabil. (2012) 39(4):270–6. doi: 10.1111/j.1365-2842.2011.02269.x
84. Harden RN, Weinland SR, Remble TA, Houle TT, Colio S, Steedman S, et al. Medication quantification scale version III: update in medication classes and revised detriment weights by survey of American pain society physicians. J Pain. (2005) 6(6):364–71. doi: 10.1016/j.jpain.2005.01.350
85. Nishigami T, Tanaka K, Mibu A, Manfuku M, Yono S, Tanabe A. Development and psychometric properties of short form of central sensitization inventory in participants with musculoskeletal pain: a cross-sectional study. PLoS One. (2018) 13(7):e0200152. -e. doi: 10.1371/journal.pone.0200152
86. Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. (2008) 61(12):1234–40. doi: 10.1016/j.jclinepi.2008.01.006
87. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28(2):193–213. doi: 10.1016/0165-1781(89)90047-4
88. Scerbo T, Colasurdo J, Dunn S, Unger J, Nijs J, Cook C. Measurement properties of the central sensitization inventory: a systematic review. Pain Pract. (2018) 18(4):544–54. doi: 10.1111/papr.12636
89. Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. (2012) 12(4):276–85. doi: 10.1111/j.1533-2500.2011.00493.x
90. Neblett R. The central sensitization inventory: a user’s manual. J Appl Biobehav Res. (2018) 23(2):e12123. doi: 10.1111/jabr.12123
91. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
92. Cleland C, Ferguson S, Ellis G, Hunter RF. Validity of the international physical activity questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med Res Methodol. (2018) 18(1):176. doi: 10.1186/s12874-018-0642-3
93. Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. (2011) 8(1):115. doi: 10.1186/1479-5868-8-115
94. Taylor AM, Phillips K, Patel KV, Turk DC, Dworkin RH, Beaton D, et al. Assessment of physical function and participation in chronic pain clinical trials: iMMPACT/OMERACT recommendations. Pain. (2016) 157(9):1836–50. doi: 10.1097/j.pain.0000000000000577
95. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item short-form health survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. (2001) 23(7):1080–98. doi: 10.1016/S0149-2918(01)80093-X
96. Smith TO, Hawker GA, Hunter DJ, March LM, Boers M, Shea BJ, et al. The OMERACT-OARSI core domain set for measurement in clinical trials of hip and/or knee osteoarthritis. J Rheumatol. (2019) 46(8):981–9. doi: 10.3899/jrheum.181194
97. Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: iMMPACT recommendations. Pain. (2005) 113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012
98. Milani SA, Marsiske M, Cottler LB, Chen X, Striley CW. Optimal cutoffs for the Montreal cognitive assessment vary by race and ethnicity. Alzheimers Dement (Amst). (2018) 10:773–81. doi: 10.1016/j.dadm.2018.09.003
99. Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: iMMPACT recommendations. J Pain. (2008) 9(2):105–21. doi: 10.1016/j.jpain.2007.09.005
100. Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol. (2007) 26(4):465–73. doi: 10.1007/s10067-006-0433-9
101. Ji R-R, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. (2018) 129(2):343–66. doi: 10.1097/ALN.0000000000002130
102. Carlesso LC, Frey Law L, Wang N, Nevitt M, Lewis CE, Neogi T, et al. The association of pain sensitization and conditioned pain modulation to pain patterns in knee osteoarthritis. Arthritis Care Res (Hoboken). (2020) 74(1):107–12. doi: 10.1002/acr.24437
103. Alqarni AM, Manlapaz D, Baxter D, Tumilty S, Mani R. Test procedures to assess somatosensory abnormalities in individuals with peripheral joint pain: a systematic review of psychometric properties. Pain Pract. (2018) 18(7):895–924. doi: 10.1111/papr.12680
104. Koulouris AE, Edwards RR, Dorado K, Schreiber KL, Lazaridou A, Rajan S, et al. Reliability and validity of the Boston bedside quantitative sensory testing battery for neuropathic pain. Pain Med. (2020) 21(10):2336–47. doi: 10.1093/pm/pnaa192
105. Cruz-Almeida Y, Sibille KT, Goodin BR, Petrov ME, Bartley EJ, Riley JL III, et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol. (2014) 66(7):1800–10. doi: 10.1002/art.38620
106. Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. (2005) 64(1):29. doi: 10.1136/ard.2004.022905
107. Skou ST, Graven-Nielsen T, Rasmussen S, Simonsen OH, Laursen MB, Arendt-Nielsen L. Widespread sensitization in patients with chronic pain after revision total knee arthroplasty. PAIN®. (2013) 154(9):1588–94. doi: 10.1016/j.pain.2013.04.033
108. Kennedy DL, Kemp HI, Wu C, Ridout DA, Rice AS. Determining real change in conditioned pain modulation: a repeated measures study in healthy volunteers. J Pain. (2020) 21(5–6):708–21. doi: 10.1016/j.jpain.2019.09.010
109. Teoli A, Robbins S, Wideman T. The relationship between baseline sensitivity to physical activity with clinical outcomes following an 8-week rehabilitation program in patients with knee osteoarthritis. Osteoarthritis Cartilage. (2021) 29:S391. doi: 10.1016/j.joca.2021.02.507
110. Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G* power 3.1: tests for correlation and regression analyses. Behav Res Methods. (2009) 41(4):1149–60. doi: 10.3758/BRM.41.4.1149
111. Eaton ML. Gauss-Markov estimation for multivariate linear models: a coordinate free approach. Ann Math Stat. (1970) 1:528–38. doi: 10.1214/aoms/1177697093
112. Schmidt AF, Finan C. Linear regression and the normality assumption. J Clin Epidemiol. (2018) 98:146–51. doi: 10.1016/j.jclinepi.2017.12.006
113. Portney LG, Watkins MP. Foundations of clinical research: applications to practice. Upper Saddle River, NJ: Pearson/prentice Hall (2009).
114. Sullivan MJL. Controlling for “confounders” in psychosocial pain research. Pain. (2016) 157(4):775–6. doi: 10.1097/j.pain.0000000000000493
115. Greenland S, Morgenstern H. Confounding in health research. Annu Rev Public Health. (2001) 22(1):189–212. doi: 10.1146/annurev.publhealth.22.1.189
116. Ahsan N, Abdullah Z, Fie DYG, Alam SS. A study of job stress on job satisfaction among university staff in Malaysia: empirical study. Eur J Soc Sci. (2009) 8(1):121–31. doi: 10.1108/19852510780001575
117. Palit S, Fillingim RB, Bartley EJ. Pain resilience moderates the influence of negative pain beliefs on movement-evoked pain in older adults. J Behav Med. (2020) 43(5):754–63. doi: 10.1007/s10865-019-00110-8
118. Lowry V, Ouellet P, Vendittoli P-A, Carlesso LC, Wideman TH, Desmeules F. Determinants of pain, disability, health-related quality of life and physical performance in patients with knee osteoarthritis awaiting total joint arthroplasty. Disabil Rehabil. (2018) 40(23):2734–44. doi: 10.1080/09638288.2017.1355412
119. Miller LK, Naugle KM. Increased sensitivity to physical activity in healthy older adults predicts worse pain and functional outcomes. Med Sci Sports Exerc. (2016) 48(5S):691. doi: 10.1249/01.mss.0000487074.56420.1a
120. Sullivan MJ, Larivière C, Simmonds M. Activity-related summation of pain and functional disability in patients with whiplash injuries. PAIN®. (2010) 151(2):440–6. doi: 10.1016/j.pain.2010.08.005
121. Sullivan MJ, Thibault P, Andrikonyte J, Butler H, Catchlove R, Larivière C. Psychological influences on repetition-induced summation of activity-related pain in patients with chronic low back pain. PAIN®. (2009) 141(1–2):70–8. doi: 10.1016/j.pain.2008.10.017
122. Rikli RE, Jones CJ. The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J Aging Phys Act. (1998) 6(4):363–75. doi: 10.1123/japa.6.4.363
123. Kulshreshtha P, Deepak KK. Autonomic nervous system profile in fibromyalgia patients and its modulation by exercise: a mini review. Clin Physiol Funct Imaging. (2013) 33(2):83–91. doi: 10.1111/cpf.12000
124. Benarroch EE. Pain-autonomic interactions. Neurol Sci. (2006) 27(2):s130–s3. doi: 10.1007/s10072-006-0587-x
125. Fingleton C, Smart KM, Doody CM. Exercise-induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. Clin J Pain. (2017) 33(5):395–404. doi: 10.1097/AJP.0000000000000418
126. Bishop MD, Horn ME, George SZ. Exercise-induced pain intensity predicted by pre-exercise fear of pain and pain sensitivity. Clin J Pain. (2011) 27(5):398–404. doi: 10.1097/AJP.0b013e31820d9bbf
127. Skidmore JR, Koenig AL, Dyson SJ, Kupper AE, Garner MJ, Keller CJ. Pain self-efficacy mediates the relationship between depressive symptoms and pain severity. Clin J Pain. (2015) 31(2):137–44. doi: 10.1097/AJP.0000000000000094
128. Parr JJ, Borsa PA, Fillingim RB, Tillman MD, Manini TM, Gregory CM, et al. Pain-Related fear and catastrophizing predict pain intensity and disability independently using an induced muscle injury model. J Pain. (2012) 13(4):370–8. doi: 10.1016/j.jpain.2011.12.011
129. Sullivan MJL, Rodgers WM, Wilson PM, Bell GJ, Murray TC, Fraser SN. An experimental investigation of the relation between catastrophizing and activity intolerance. Pain. (2002) 100(1):47–53. doi: 10.1016/S0304-3959(02)00206-3
130. Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI Guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. (2019) 27(11):1578–89. doi: 10.1016/j.joca.2019.06.011
131. Woznowski-Vu A, Aternali A, Gervais A, Pavilanis AD, Nijs J, Sullivan MJ, et al. The prospective prognostic value of biopsychosocial indices of sensitivity to physical activity among people with back pain. Clin J Pain. (2021) 37(10):719–29. doi: 10.1097/AJP.0000000000000965
132. Miller J, MacDermid JC, Walton DM, Richardson J. Chronic pain self-management support with pain science education and exercise (COMMENCE) for people with chronic pain and multiple comorbidities: a randomized controlled trial. Arch Phys Med Rehabil. (2020) 101(5):750–61. doi: 10.1016/j.apmr.2019.12.016
133. Toomey E, Currie-Murphy L, Matthews J, Hurley DA. Implementation fidelity of physiotherapist-delivered group education and exercise interventions to promote self-management in people with osteoarthritis and chronic low back pain: a rapid review part II. Man Ther. (2015) 20(2):287–94. doi: 10.1016/j.math.2014.10.012
134. Othman R, Jayakaran P, Swain N, Dassanayake S, Tumilty S, Mani R. Relationships between psychological, sleep, and physical activity measures and somatosensory function in people with peripheral joint pain: a systematic review and meta-analysis. Pain Pract. (2020). 21(2):226–61. doi: 10.1111/papr.12943
135. Abbott J, Robertson M, Chapple C, Pinto D, Wright A, De la Barra SL, et al. Manual therapy, exercise therapy, or both, in addition to usual care, for osteoarthritis of the hip or knee: a randomized controlled trial. 1: clinical effectiveness. Osteoarthritis Cartilage. (2013) 21(4):525–34. doi: 10.1016/j.joca.2012.12.014
Keywords: knee osteoarthritis, pain, sensitization, activity-related pain, quantitative sensory testing, longitudinal
Citation: Overton M, Swain N, Falling C, Gwynne-Jones D, Fillingim R and Mani R (2023) Activity-related pain predicts pain and functional outcomes in people with knee osteoarthritis: A longitudinal study. Front. Pain Res. 3:1082252. doi: 10.3389/fpain.2022.1082252
Received: 28 October 2022; Accepted: 19 December 2022;
Published: 13 January 2023.
Edited by:
Brian E Cairns, University of British Columbia, CanadaReviewed by:
Henrik Bjarke Vægter, Odense University Hospital, Denmark© 2023 Overton, Swain, Falling, Gwynne-Jones, Fillingim and Mani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark Overton bWFyay5vdmVydG9uQG90YWdvLmFjLm56
Specialty Section: This article was submitted to Musculoskeletal Pain, a section of the journal Frontiers in Pain Research
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.