- 1Division of Medicine, Wolfson Institute of Biomedical Research, University College London, London, United Kingdom
- 2William Harvey Research Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom
Personalised and targeted interventions have revolutionised cancer treatment and dramatically improved survival rates in recent decades. Nonetheless, effective pain management remains a problem for patients diagnosed with cancer, who continue to suffer from the painful side effects of cancer itself, as well as treatments for the disease. This problem of cancer pain will continue to grow with an ageing population and the rapid advent of more effective therapeutics to treat the disease. Current pain management guidelines from the World Health Organisation are generalised for different pain severities, but fail to address the heterogeneity of mechanisms in patients with varying cancer types, stages of disease and treatment plans. Pain is the most common complaint leading to emergency unit visits by patients with cancer and over one-third of patients that have been diagnosed with cancer will experience under-treated pain. This review summarises preclinical models of cancer pain states, with a particular focus on cancer-induced bone pain and chemotherapy-associated pain. We provide an overview of how preclinical models can recapitulate aspects of pain and sensory dysfunction that is observed in patients with persistent cancer-induced bone pain or neuropathic pain following chemotherapy. Peripheral and central nervous system mechanisms of cancer pain are discussed, along with key cellular and molecular mediators that have been highlighted in animal models of cancer pain. These include interactions between neuronal cells, cancer cells and non-neuronal cells in the tumour microenvironment. Therapeutic targets beyond opioid-based management are reviewed for the treatment of cancer pain.
The problem of cancer pain
Cancer survival rates have dramatically increased since the turn of the century with the advent of targeted and improved treatment strategies. On the other hand, the annual number of cancer cases worldwide is estimated to rise from 14 million in 2012 to 22 million by 2032, leaving behind the growing problem of effective pain management for side effects of cancer and its treatments. Almost all patients diagnosed with cancer endure chronic pain because of surgery, treatments or side effects like a pathological fracture. Between 30% and 50% of patients receiving curative-intent therapy and 75%–90% of patients with advanced disease endure chronic pain that is strong enough to require opioid therapy (1, 2). More than one-third of patients in disease remission will continue to report pain after curative treatment (3). Cancer-related pain often consists of background pain with acute exacerbations, peaking several times a day that can be either spontaneous or evoked in areas of sensory abnormality. Spontaneous pain may be ongoing (either at a constant or fluctuating pain intensity), or it can be dominated by a juxtaposition of pain paroxysms of short duration interspersed by pain-free intervals or a less intense background pain. Paraesthesia and dysaesthesia are also frequently reported.
The World Health Organization (WHO) Analgesic Ladder is frequently the first step in a paradigm for guiding clinicians to manage pain in a systemic manner, where the selection of pharmacological analgesic treatments is based on the degree of pain. Over-the-counter analgesics comprise Step 1, “weak” opioids (such as codeine) are escalated in Step 2, followed by the use of “strong” opioids in Step 3 to treat moderate-to-severe pain. Clinical professionals are urged to take non-pharmacologic pain management methods in Step 4 into account (4). Non-opioid analgesics include acetaminophen (5) and non-steroidal anti-inflammatory drugs (NSAIDs) (6). The advantage of NSAID/opioid combination therapy is a reduction in overall opioid prescriptions, but data on its efficacy is conflicting (6–8). Adjuvant therapies include anti-depressants [like duloxetine (9) and anticonvulsants like gabapentin and pregabalin (10)], which are first or second-line analgesics for other chronic pain conditions, including painful neuropathies (11). Adjuvant analgesics, integrative therapeutic options [such as acupuncture (12)], as well as interventions [e.g., nerve block (13) and epidural or intrathecal analgesics (7)] can be considered at any stage of pain management even though they are not listed on the WHO ladder.
There remains a continuous debate regarding the suitability of these generalised WHO recommendations to adequately manage pain in a heterogeneous group of patients that have a cancer diagnosis. A meta-analysis of WHO Cancer Pain Relief guideline outcomes indicates that adequate analgesia is achieved in a range of as little as 20% but up to 100% of patients (14). A contentious point has been raised regarding the prescription of weak Step 2 opioids before starting morphine for managing moderate pain; patients with cancer suffering from moderate pain have a higher probability of responding to low-morphine doses than they are to codeine (15). Furthermore, new research indicates that potent analgesics may be more effective if given earlier in the course of the disease prior to a critical transition point when the plasticity of the nociceptive system may become resistant to conventional pharmacological treatment (16).
The need to modify current pain management protocols in patients with cancer is indicated by reports that pain is the most common complaint leading to emergency unit visits by patients with cancer (17–19). Despite well-established findings demonstrating that the experience of pain can impact long-term clinical outcomes, patients with cancer frequently receive insufficient pain management (20). Studies investigating the frequency and effectiveness of pain control suggest areas for improvement, e.g., a systematic analysis reported that despite a 25% drop in under-treated cancer pain between 2007 and 2013, over one-third of patients with cancer still experience under-treated pain (21). Pain has a demonstrable impact on the quality of life of patients with cancer, and the lack of adequate pain management among this growing population underlines the need for novel and targeted approaches to treat pain arising from cancer or its treatment. This review summarises the use of animal models of cancer pain, with a focus on cancer-induced bone pain (CIBP) and chemotherapy associated pain. Peripheral and central nervous system mechanisms involved in cancer pain are also discussed, followed by potential targets for pain relief.
Animal models of cancer pain
Here we describe animal models of cancer pain, including method of induction and pain related outcomes.
Animal models of cancer-induced bone pain (CIBP)
Early models of CIBP relied on the administration of cancer cells into the left ventricle of mice, followed by migration of cancer cells in the general circulation to develop metastases at different tissue sites, including bone marrow (22). The main advantage of models relying on systemic administration of cancer cells models is the replication of the clinical course of disease due to the fact that bone tumours usually develop as metastases rather than as primary tumours. However, significant disadvantages of such models include the poor predictability of metastatic sites and size, leading to insufficient reproducibility of disease progression across a cohort of animals. Another strategy is to directly administer cancer cells into the intramedullary space of a bone (e.g., the femur) in rodents (23). The site of the injection of the cancer cells into the bone is then sealed to restrict tumour growth in the intramedullary space. In comparison to systemic administration of cancer cells, CIBP models that involve direct injection in the bone facilitate the evaluation of tumour growth over time, radiological imaging, observation of bone degradation, examination of histopathologic changes, site-specific behavioural testing, as well as evaluation of neurochemical changes that take place at the tumour site, in the dorsal root ganglion (DRG) and the central nervous system (CNS) (24). Mouse models typically involve the implantation of tumour cells into the femur, and rat models are typically created via percutaneous injection of cancer cells into the tibia (25). The cell lines used will vary across species and studies, e.g., for B6C3-Fe-a/a and C3H/HeJ mice, fibrosarcoma cells have been reported to be administered in the femur (26, 27), humerus (28) and calcaneus bones (29). Lewis lung carcinoma cells are administered into the intramedullary space of the femur in C57BL/6 mice (30). In rats, MRMT1 mammary gland carcinoma cells are administered into the tibias of female Sprague–Dawley rats (31), and MDA-MB231 human breast cancer cells are injected into femoral arteries of nude rats (32) as well as R3327 prostate cancer cells (33). Cancer cells that have metastasised to the bone can be classified as either osteosclerotic or osteolytic based on x-ray images (34). Osteosclerotic cancers are distinguished by increased bone deposition through increased osteoblast activity. Prostate cancer is the protype for osteosclerotic bone metasases, whereas breast cancer is the protype for osteolytic bone metastases because breast cancer cells potentiate osteoclast-induced bone degradation (34, 35).
Rodents exhibit pain-like behaviours within 2–3 weeks of intrafemoral inoculation with cancer cells in a dose-dependent manner (30, 36, 37). Non-evoked pain behaviours include reduced use of the affected limb, which can be quantified using a limb score, as well as a reduction in weight borne on the affected limb when tested by an incapacitance tester (30, 36–38). Loss of bone mineral density or osteolysis can also be reliably detected with the progression of tumour growth (30). The ability to reproduce a chronic pain phenotype, as well as the cancer-induced bone remodelling, is critical to understanding the structural and neuronal mechanisms underlying CIBP (39, 40). One major shortcoming of animal models is the challenge in predicting breakthrough pain, which is an important clinical feature of CIBP. Assessment of breakthrough pain would require continuous monitoring of animals, e.g., with the use of ultrasound vocalisation techniques, but has yet to be an established feature of rodent CIBP models. See Table 1 for a summary of different animal models of CIBP.
Animal models of non-bone cancer pain
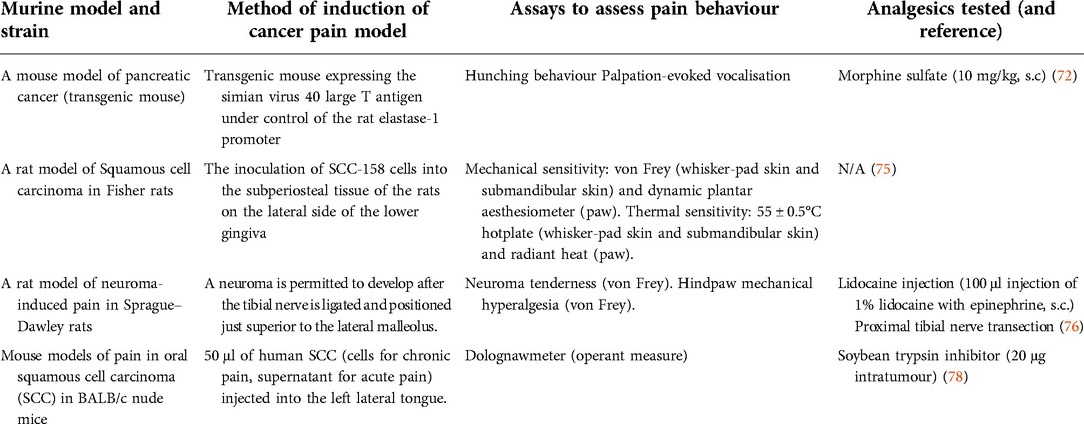
Besides the models of cancer pain arising from bone, other animal models of primary cancers originating from different organs have been described, such as pancreatic cancer, squamous cell carcinoma and neuroma. One mouse model of pancreatic cancer involves the elastase 1 promoter-driven expression of the simian virus 40 large T antigen (72). In this model, pathological effects of these precancerous cells can be observed after 6 weeks, such as elevation in microvasculature density, the number of nerve growth factor (NGF) expressing macrophages and increased density of sensory and sympathetic fibers innervating the pancreas. Although these aforementioned changes would be expected to lead to severe pain in somatic structures like the skin, no apparent changes in pain-related behaviours, like morphine-reversible severe hunching and vocalisation, are observed in this model of pancreatic cancer until an advanced stage of disease after 16 weeks (72). These findings imply that a stereotypical series of pathological changes are present in both humans and mice as pancreatic cancer progresses. Although weight loss typically corresponds to disease progression, there is often a considerable delay from the onset of tumour growth to behaviours suggestive of pancreatic cancer pain (72). Clinical presentation of back and abdominal pain in pancreatic cancer is commonly reported at an advanced stage of disease in more than 80% of cases (73), but the underlying causes of the discrepancy between neuronal innervation and symptomatic pain in pancreatic cancer remain to be investigated.
Another preclinical model of pancreatic cancer that involves the implantation of SW 1990 cells into the pancreas of female BALB/c-nu mice carries several advantages, including reproducible transcriptional changes at the level of the dorsal horn in both mice and humans with pancreatic cancer (74). Pain-like behaviours in pancreatic cancer-bearing mice can be assessed by stimulating the abdomen mechanically (by von Frey hairs) to assess mechanical withdrawal thresholds and score hunching behaviours, as well as electrophysiologically record visceromotor responses from the rectus abdominis (74). An animal model of squamous cell carcinoma involves the injection of cancer cells into the subperiosteal tissue of the lower gingiva, which leads to elevated levels of calcitonin gene-related peptide, Substance P, P2X3 receptors and TRPV1 channels in the trigeminal ganglia, coinciding with the development of mechanical hypersensitivity and thermal hyperalgesia (75). Finally, the tibial neuroma transposition model involves the ligation of the tibial nerve and placement above the lateral malleolus—while not perfectly representative of the natural clinical course of disease progression, this model can be useful for mechanistic investigations of pain due to tumour-induced nerve injury (76, 77).
Patients with head and neck cancer experience pain early in the disease process, and orofacial pain is often the presenting symptom for oral squamous cell carcinoma (SCC). Schmidt and collleagues have pioneered the development of mouse models of oral cancer pain, either through orthotopic oral tissue injection of human SCC to produce operant nocifensive behaviours measured using the Dolognawmeter (to measure gnaw time) (78). Other work has used administration of SCC cells to the hindpaw to investigate mechanisms of mechanical allodynia (79). See Table 2 for a summary of different animal models of non-bone cancer pain.
Animal models of cancer related neuropathic pain
Neuropathic pain is a key feature in some animal models of pain upon either cancer invasion or treatment with chemotherapy (80, 81). For the former, cancer cells are implanted in close proximity to nerves, e.g., sciatic nerve, and standardised behavioural assays measure changes in hindlimb sensitivity (81). Animal models of cancer invasion typically cause more profound neuronal damage than classic rodent models of neuropathic pain, such as the chronic constriction injury model, and the degree and effects of nerve compression after cancer appear mechanistically distinct (81).
Neurotoxicity is one of the key treatment-limiting side effects of chemotherapy, which can manifest as painful peripheral neuropathy or chemotherapy-induced neuropathic pain. Across classes of chemotherapeutic agents, the extent of polyneuropathy depends on the dose and length of treatment but is generally associated with an acute phase of allodynia and pricking dysaesthesia affecting the hands and feet in patients. Acute or persistent neuropathic pain can be reproduced by systemic administration of chemotherapeutic agents in rodents. Some studies also report the development of motor neuropathy, gait abnormalities and impaired rotarod performance (82), which mimic aspects of sensory impairment besides hypersensitivity that develops in chemotherapy-induced peripheral neuropathy (83). For example, daily intravenous administration of vincristine, a vinca alkaloid, elicits dose-dependent mechanical and cold (but not heat) hypersensitivity within 2 days (84–86) that is attenuated 2 weeks after cessation of treatment (84). Paclitaxel is a commonly used taxane in rodent models of chemotherapy-induced peripheral neuropathy to produce both thermal and mechanical hypersensitivity (87, 88). A recent study on the responses of 10 mouse strains to paclitaxel administration revealed that nearly all strains experienced mechanical allodynia and cold allodynia, including DBA/2J and C57BL/6J mice (89). Cisplatin is a platinum derivative that also results in numbness, tingling, as well as painful neuropathy in humans (90, 91). In rats, typically, three doses totaling 15 mg/kg are administered to produce mechanical allodynia and hyperalgesia lasting up to 15 days (92). The fast onset of neuropathic symptoms is a major benefit of this model (92). Additionally, while motor nerve conduction velocity is unaltered in electrophysiological studies, a considerable drop in sensory nerve conduction velocity can be detected (93). Lastly, preclinical studies using oxaliplatin-induced neuropathic pain have highlighted key mechanisms associated with cold allodynia that occur with chemotherapy administration. Mice administered 80 µg intraplantar oxaliplatin show a significant reduction in pain thresholds assessed using the cold plate test. Symptoms of cold allodynia become evident as early as 3 h after oxaliplatin administration (94). An important advantage of this model is its rapid onset resembling the rapid cold allodynia seen in patients with cancer treated using oxaliplatin (95). See Table 3 for a summary of different animal models of chemotherapy associated pain.

Table 3. Some of the animal models used to study chemotherapy-associated pain and targets for analgesia.
Mechanisms of cancer pain
Cancer-induced bone pain (CIBP)
Paget's “seed and soil” hypothesis of the late 19th century was seminal to our current understanding that certain tumours exhibit a predilection for metastasis to specific organs (104). Bone is the optimal “soil” for metastatic cells of primary tumours, most commonly from breast, prostate myeloma, thyroid, lung and bladder cancer. Post-mortem examinations revealed that the incidence of bone metastases is around 70% for patients with breast or prostate cancer and 36% for patients with lung cancer (105). Bone metastases are the most common cause of cancer-related pain (105, 106) and are associated with poor prognosis and survival (107). Metastasis to bone disrupts skeletal homeostasis by disturbing the balance between osteoblastic bone formation and osteoclast-mediated bone destruction (108). Fenestrations in bone marrow sinusoids allow easy trafficking of hematopoietic cells but can also be permissive to metastatic invasion (109). Receptor activator of nuclear factor kappa-Β ligand (RANKL) is a chemotactic factor that promotes bone metastasis (110), and chemokine receptors such as CXCR4 (C-X chemokine receptor 4), which binds the survival chemokine stromal cell-derived factor 1 present on osteoblasts and bone lining cells, promote adhesion and metastasis within the bone microenvironment (111, 112). Within the bone, cancer cells directly compete with hematopoietic stem cells driving their terminal differentiation (113). In this microenvironment, bone-innervating neurons and cancer and tumour-associated stromal cells (including fibroblasts, endothelial cells, lymphocytes and bone marrow-derived cells, such as macrophages, neutrophils, mesenchymal stem cells and mast cells) can all contribute to CIBP through changes in bone homeostasis, structural and neurochemical reorganisation of sensory and sympathetic nerve fibres innervating bone, as well as the neurochemical reorganisation in the spinal cord. These processes highlight the importance of peripheral and central mechanisms driving the complex condition of CIBP that comprises inflammatory, neuropathic and cancer-specific mechanisms of nociceptive signalling. We discuss peripheral and central mechanisms below (see Figure 1).

Figure 1. Cellular interactions in the bone microenvironment in CIBP. Tumour cells release endothelin (ET), which interacts with osteoblasts via their appropriate receptors to stimulate the proliferation of osteoblasts. Activated osteoblasts release receptor activator of nuclear factor-kappa-Β ligand (RANKL), which serves as a signal for osteoclast proliferation and maturation to enhance osteoclast-mediated bone matrix destruction. Osteoclasts generate adenosine triphosphate (ATP) and acidosis by releasing protons, resulting in the activation of various receptors and ligand-gated ion channels (LGICs) like P2X receptors, transient receptor potential V1 receptors and acid-sensing ion channels type 3 expressed on bone innervating sensory neurons. Tumour cells, stromal cells and activated immune cells release a variety of mediators (such as endothelin, the nerve growth factor, protons, and pro-inflammatory cytokines) that activate their respective receptors expressed on sensory neurons and thereby initiate the detection of noxious stimuli. GPCRs can sometimes indirectly sensitise various voltage-gated ion channels (VGICs) expressed on sensory neurons leading to a further potentiation of nociceptive signalling to the spinal cord. Osteolytic cancers (like breast cancer) activate osteoclasts, while osteosclerotic cancers (like prostate cancer) activate osteoblasts leading to a further potentiation of pain signal transmission.
Acidosis is thought to be a key mechanism driving CIBP and is largely driven by disseminated tumour cells that dysregulate bone remodelling through osteomimicry of osteoblast and osteoclasts (114–116). The disruption of skeletal homeostasis and acidic bone microenvironment can contribute to peripheral sensitisation through the activation of acid-sensing ion channels (ASICs) and transient receptor potential (TRP) channels. In the Warburg effect, cancer cells tend to undergo anaerobic respiration even in the abundance of oxygen, unlike normal cells that mainly undergo aerobic respiration in the abundance of oxygen and shift to anaerobic respiration when there is a shortage of oxygen supply (117). Among the products of anaerobic respiration is lactic acid (at physiological pH, lactic acid deprotonates to form lactate and protons), which causes acidosis. Cancer-induced acidosis is enhanced in bone cancers because the bone is a hypoxic tissue (118). In addition, tumour cells activate osteoclasts, which in turn cause bone degradation by releasing protons to solubilise the mineralised bone matrix (119). Upon the physiological activation of osteoclasts, the release of acids by osteoclasts during bone resorption is restricted to the bone matrix due to the tightly sealed sac called the resorption lacuna (120). Typically in bone cancer the number and activity of osteoclasts increase dramatically, thereby disrupting this tight regulation and resulting in the leakage of protons that can amplify acidity levels of the bone marrow. The bone marrow itself is richly innervated with nociceptors expressing ASICs and TRP channels—especially TRPV1—that are important sites of neuronal activation by protons (69, 121–124).
In addition to acidosis, several mediators released by cancer cells and their associated stromal cells contribute to CIBP. These include NGF and interleukin (IL)-1β (125), which can contribute to CIBP both directly and indirectly. For instance, IL-1β enhances the expression of cyclooxygenase (COX)-2 by macrophages, in turn amplifying prostaglandin synthesis. These prostaglandins can sensitise the primary afferent neurons by binding the prostanoid receptors expressed on terminals of bone innervating neurons (126, 127). Similarly, the production of NGF by cancer cells in the bone (e.g., derived from primary tumours of the breast and prostate) can lead to sensitisation of sensory neurons directly by increasing the expression ion channels linked to pain signal transduction and transmission. These include the TRPV1 channel, which is of particular interest in CIBP given the strong acidosis that characterises this painful condition (128). Moreover, in the spontaneous osteosarcoma canine model, TRPV1 blockade attenuates hypersensitivity suggesting it may be a potential analgesic target candidate in CIBP (122). NGF can also enhance the production and release of TNF-α, IL-6, IL-1β and prostaglandin (PG)E2 by macrophages (129). TNF-α is known to exert pro-nociceptive actions in animal models of chronic pain and can is targeted for pain relief in rheumatic disease (130). The peripheral pro-nociceptive actions of TNF-α are evident from the observation that intraplantar, intradermal, endoneurial or intramuscular administration of TNF-α induces thermal hyperalgesia and mechanical allodynia (131–135). In addition, TNF-α affects several ion channels like TRPV1, sodium and potassium channels (136–138) and results in the spontaneous firing of primary sensory neurons (139, 140). In preclinical models of CIBP, the administration of etanercept [a humanised soluble recombinant TNF receptor fusion protein (141)] was found to attenuate the thermal and mechanical allodynia in bone cancer-bearing mice (142). Additionally, blockade of the interaction between NGF and its receptor Tropomyosin receptor kinase A (TrkA) reduces pain-like behaviour in a mouse model of CIBP, with analgesic effects that are superior to morphine (143). Furthermore, several factors at the site of bone metastasis, such as reactive oxygen species (ROS), immune cell infiltration and activation, as well as tumour-induced cytotoxicity, result in the production of adenosine triphosphate (ATP) following the death of bone marrow cells. When ATP is released, it binds its receptor P2X3 on sensory neurons leading to their activation and subsequent sensitization (125). The blockade of P2X3 receptors can attenuate pain behaviours in a rat model of CIBP (144).
The neuropathic pain component of CIBP can be driven by increased intraosseous pressure, nerve sprouting and direct nerve injury. In humans, increased innervation density is also observed at sites of active bone remodelling, supporting the importance of neural regulation of skeletal remodelling and pain (145). Bone cancer pain, similar to intraosseous engorgement syndrome, produces intraosseous pressure within the bone microenvironment, which can sensitise primary afferents through activation of mechanoreceptors (146) and mechanotransducing osteocytes (147). Moreover, CIBP is characterised by the sprouting of sensory and sympathetic fibres, which has been shown to be driven by an NGF-dependent process. The requirement of NGF-TrkA signalling in bone innervating neurons for endochondral ossification and vascularisation, as well as bone formation upon mechanical loading, is well established (148, 149). In preclinical models of CIBP, prophylactic antibody-based blocking of NGF prevents ectopic sprouting and neuroma formation (150). The ablation of capsaicin-sensitive sensory neurons also results in loss of bone mineral density in adult rats (151). At the level of the peripheral somata, biomarkers that are indicative of nerve injury can also be detected, including cyclic activating transcription factor 3 (ATF3) expression. Spinal cord compression occurs in about 5% of patients with metastatic cancer, mainly presenting as back pain (152), and in animal models, spinal cord compression readily triggers mechanical hyperalgesia in the fore and hind limbs (153). Macrophage infiltration into peripheral sensory ganglia is also commonly observed in nerve injury models and studies highlight the importance of this neuro-immune interface in the development of CIBP (154).
At the level of the spinal cord, CIBP modulates synaptic plasticity between the peripheral neurons and second-order neurons, as observed through increased neuronal excitability measured with elevated expression of c-Fos, the internalisation of substance P, the rise in the expression of dynorphin (a pro-nociceptive opioid) and a significant activation and elevation in astrocytes and microglia (26, 155). In addition, CIBP has been shown to increase the proportion of the wide dynamic range to nociceptive-specific neurons in the superficial laminae of the dorsal horn in rats (156). It was reported that gabapentin can re-establish the typical ratio of wide dynamic range neurons to nociceptive-specific neurons, but long-term use of morphine maintains allodynia and fails to correct the pathophysiological phenotype of superficial dorsal horn neurons (157). Descending modulatory circuits also regulate spinal excitability in CIBP; 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists can diminish the hyperexcitability of lamina I neurons in rodents with CIBP through the blockade of a descending serotonergic facilitatory drive (158). Other studies report an important role for amplified excitatory neurotransmission that may underlie enhanced spinal cord plasticity in CIBP; spinal glial cells secrete IL-1 that causes hyperalgesia by phosphorylating the NR1 subunit of N-methyl-D-aspartate receptor (NMDA) receptors (159). Moreover, spinal NR2B expression is increased in CIBP, and its specific inhibition prevents the induction of mechanical and thermal hypersensitivity (160). Due to hypertrophy of astrocytes and the subsequent decline in glutamate reuptake transporters, glutamate concentrations are also elevated in CIBP, which leads to excitotoxicity (161). Mice with CIBP also exhibit elevated amounts of dynorphin in the dorsal horn (26), which causes long-lasting pain through activation of NMDA receptors instead of opioid receptors (162).
Chemotherapy-induced neuropathic pain
Chemotherapy-induced neuropathic pain has a detrimental impact on the quality of life of patients during and after chemotherapy treatment (163). Because chemotherapeutic agents do not normally cross the blood-brain barrier, neuropathies are restricted to peripheral sensory and/or motor neurons, leading to a reduction in two-point discrimination and proprioception, as well as cold allodynia, myalgia and pain in extremities (164). The severity and duration of neuropathy are determined by the class of the chemotherapeutic agent used, the total duration of administration, as well as the cumulative dose administered. If the cell body is spared and a sufficient time-period for recovery is allowed before subsequent drug administration, the peripheral nervous system regenerates rapidly after the cessation of chemotherapy treatment. Still, up to one-third of patients experience damage lasting more than 6 months after cessation of a chemotherapy course (165). How do chemotherapeutic agents predominantly cause sensory neuropathy while leaving the motor neurons unaffected, even though motor neurons have axons that are as long as those of sensory counterparts and have extensive microtubule networks? And why are adult postmitotic sensory neurons preferentially harmed by chemotherapy drugs that are meant to target fast-dividing tumour cells? One explanation for this latter susceptibility with vinca alkaloids and taxanes is that these classes of chemotherapy drugs interfere with the stability of microtubules that are necessary for the axonal transport of chemicals and growth factors required for normal nerve function in sensory neurons. Unfortunately, this rationale does not apply to platinum-based chemotherapy drugs that generate DNA adducts in the nucleus but also injure sensory neurons to trigger chemotherapy-induced neuropathic pain (163, 166).
Chemotherapeutic agents with a high incidence of chemotherapy-induced neuropathic pain include paclitaxel, oxaliplatin and vincristine. Studies from animals and humans indicate that these agents build up in peripheral sensory ganglia and, to a lesser extent, in peripheral nerves (167–169). The neurotoxic effects of these classes of chemotherapeutic agents lead to cell death and neuronal degeneration. Expression of ATF3 in DRG neurons is significantly increased with administration of paclitaxel, and the same neurons also exhibit a deposition of neurofilaments and a translocation of their nuclei towards the periphery. Dorsal roots of rats receiving paclitaxel also show substantial axonal degradation and hypomyelination (170). Moreover, exposure to NGF attenuates some neurotoxic effects triggered by paclitaxel in CD1 mice, including the release of neuropeptides in DRG neurons (98). Apoptosis of DRG neurons is thought to be partially responsible for chemotherapy-induced neuropathy, and a high dose of NGF can prevent DRG apoptosis induced by cisplatin (171, 172). Chemotherapeutic agents have also been shown to increase the generation of ROS, which impairs mitochondrial electron transport chains, and disrupts ATP synthesis in the DRG neurons (173–177). These findings support the role of mitochondrial stress pathways in the neurotoxic effects of chemotherapeutic agents.
Ion channels have also been linked to mechanisms of chemotherapy-induced neuropathic pain. Rat studies demonstrate that paclitaxel and vincristine administration enhance the expression of the calcium channel alpha2delta-1 subunit in the dorsal horn of the spinal cord. Moreover, ensuing mechanical hypersensitivity is susceptible to attenuation by repeated gabapentin-dosing, which is also associated with a decrease in the expression of the spinal alpha2delta-1 subunit (176). Previous work has also shown an important role for tetrodotoxin (TTX)-sensitive sodium channels in chemotherapy-associated pain in mice. Acute subcutaneous administration of TTX at doses as low as 1 or 3 mg/kg diminished mechanical allodynia in paclitaxel-treated mice, while cold allodynia and heat hyperalgesia were attenuated with higher doses (3 or 6 mg/kg) (175). The antagonist of TRPA1 channels HC-030031 partially attenuated paclitaxel-evoked mechanical allodynia (174), whilst co-administration of HC-030031 and HC-067047 (TRPV4 antagonist) reversed paclitaxel-induced mechanical allodynia entirely (173) in rodents. Furthermore, a key mechanism of cold allodynia induced by oxaliplatin administration is the activation of “silent cold-sensing neurons” that express the voltage-gated sodium channel Nav1.8 (94, 178). Oxaliplatin treatment in mice with diphtheria toxin-ablated Nav1.8-positive neurons failed to exhibit cold allodynia compared to wild-type controls (94).
Distinct effects of different classes of chemotherapeutic agents are less studied. However, it is known that paclitaxel and vincristine-induced neuropathy trigger significant inflammatory processes which are less evident with oxaliplatin-induced neuropathy, mainly arising from the enhanced release of proalgesic mediators such as TNF and IL-1β by activated microglia, astrocytes and satellite glial cells within the dorsal horn of the spinal cord (179). The mechanism of action of vinca alkaloids that involves the prevention of microtubule formation by binding tubulin has also been shown to affect micro-tubuli, causing oedema of axons in the peripheral nervous system (163). Paclitaxel administration is also associated with a significant increase in macrophage activation and augmented staining for glial fibrillary acidic protein (GFAP). Following paclitaxel administration, satellite cells are reported to be tightly packed in “Nodules of Nagoette” that serve as a “tombstone” for the DRG neuron whose cell body they formerly encircled (180).
Mechanisms of pain in non-bone cancers
In pancreatic cancer, pain is the third most prevalent complaint among patients diagnosed with pancreatic cancer after weight reduction and jaundice (181). At the time of diagnosis, more than one-third of patients complain of abdominal discomfort and are already at an advanced stage of disease, but with disease progression, pain becomes severe in more than half of patients diagnosed with pancreatic cancer (182). Pain reports in recently diagnosed patients with pancreatic cancer can also predict survival and resectability, where preoperative pain is linked with a poor prognosis and a greater likelihood of recurrence (183). As a consequence of late diagnoses, the typical survival time of patients with pancreatic cancer ranges between 6 and 9 months, with the 5-year survival rate being less than 5% (184). Deciphering mechanisms that suppress nociceptive signalling in the early stages of pancreatic cancer and/or amplify nociceptive signalling in advanced disease is critical for improving the diagnosis, treatment and care of patients with pancreatic cancer.
Perineural invasion is a key feature of pancreatic cancer, which is enabled by the low resistance of the perineural space as well as the ideal milieu possessing chemoattractants and growth factors (such as transforming growth factor-alpha, epidermal growth factor receptor, and neural cell adhesion protein) to recruit cancer cells and promote their proliferation (185–187). A recent study showed that pancreatic cancer cells derived from patients had higher levels of TRPV1 gene expression with a downstream effect of the enhanced release of neuropeptides and augmented neurogenic inflammation (188). In mouse models of pancreatic cancer, extensive sprouting of sensory and sympathetic fibres is also detected (72) and partly attributed to the high levels of NGF released by the cancer cells and/or the inflammatory cells (187). Specifically, mice develop increased microvascular density, NGF-expressing macrophages and sensory and sympathetic innervation to the pancreas at 6 weeks following the induction of the pancreatic cancer model—all pathological events that are linked to precancerous cellular abnormalities (72, 189). Despite early changes in cell and tissue composition of the pancreatic microenvironment, pain-like behaviours are only detected after 16 weeks of age when pancreatic cancer is at an advanced stage, similar to clinical reports of pain in patients at advanced stages of the disease. Some studies suggest that regulation of nociceptive input by the descending opioidergic controls contributes to the slow evolution of tissue injury (190, 191) and that endogenous opioids may mask the full expression of pain at the early stages of disease (192). When naloxone or naltrexone were given subcutaneously to mice with early- and mid-stage pancreatic cancer, behaviours associated with pain were observed in these mice, while the healthy littermate controls failed to demonstrate pain behaviours (189). Unmasking of endogenous opioid regulation of pain behavior has also been observed in a rat model of prostate cancer induced bone pain, as well as a rat model of breast cancer induced bone pain (193, 194). A salient point is that this opioid-based un-masking of pain only occurs with opioid antagonists penetrating the blood-brain barrier, but not with peripherally-restricted opioid antagonists (192).
Visceral pain in pancreatic cancer can be caused by pancreatic neuropathy driven by cancer cells that infiltrate the perineurium of local intrapancreatic nerves (195). It is also thought that the initiation and maintenance of pain after pancreatic cancer is through neurogenic inflammation (187). In addition, several genes shown to be linked to pain are upregulated in the dorsal horn of the spinal cord in murine models of pancreatic cancer, such as Ccl12, Pin1 and Notum (74). The palmitoleoyl-protein carboxylesterase encoded by Notum is implicated in the Wnt signalling-mediated initiation and maintenance of neuropathic pain (196). Moreover, mRNA levels of Ccl12 increase by up to 35-fold in the prostate of mice with experimental autoimmune prostatitis (a model of chronic pelvic pain syndrome) (197).
In oral SCC, patients exhibit high levels of ET-1 in the cancer microenvironment, which has been shown to correlate with functional pain in response to mechanical stimulation (198, 199). Protein and mRNA levels of NGF are also significantly higher in patients with oral cancer and in oral SCC culture (200). Other studies have linked protease-activated receptor 2 (PAR2) to cancer pain (201). For instance, the supernatant of human oral SCC cells contains proteases that can activate and sensitise PAR2 expressing-sensory neurons (201). Injecting this supernatant (without cancer cells) in mice results in a severe and protracted mechanical allodynia. Analgesic approaches like serine protease blockade or mast cell depletion eliminate or lessen this nociceptive effect, respectively. Moreover, PAR2 knockout mice do not display nociceptive behaviours following exposure to SCC cell supernatant. Patients with oral cancer may develop mechanical allodynia due to the constant production of serine proteases from malignant as well as non-malignant cells within the tumour microenvironment. In addition, activating PAR2 sensitises TRPV1 and TRPV4 receptors on nociceptive afferents causing mechanical allodynia and thermal hyperalgesia, respectively (202).
Pharmacological interventions to treat cancer pain
The efficacy of pain management in cancer is limited by the multidimensional aspects of the pathophysiology of cancer, such as widespread localisation of metastases, skeletal-related events (SREs) or due to severe systemic side effects of chemotherapy treatment (203). Gabapentinoids and antidepressants are common first and second line treatments for treatment of neuropathic pain syndromes and indicated also for cancer pain (204), but drugs that act on peripheral tissues would be preferable due to mechanisms involved in the initiation of pain and primary afferent sensitisation. The added attractive benefit of targeting the peripheral nervous system in cancer pain states is the obvious circumvention of central side effects. Here we provide an overview of some conventional treatments and potential new targets for treating cancer pain states.
Opioids
Opioids remain the principal treatment option for intractable malignant pain by acting on peripheral and central nervous system sites, i.e., through inhibition of presynaptic release of neurotransmitters from primary afferent terminals to induce postsynaptic hyperpolarisation of interneurons in the dorsal horn, as well as engaging top down opioidergic controls from higher centres (205, 206). Strong immediate-release opioids are recommended as rescue medication for episodes of breakthrough pain. However, preclinical studies suggest that morphine is less effective in treating bone cancer pain compared to other painful conditions (41, 47), possibly due to decreased expression of μ-opioid receptors (MORs) in both DRG and superficial dorsal horn neurons with disease progression (207, 208). Moreover, opioid treatment in CIBP may also be linked to disease progression, as observed in mice administered morphine that demonstrated accelerated sarcoma-induced bone pain, bone loss and fractures (45). Altered expression of MORs in CIBP can also be reversed with anti-NGF in rats (209).
Agents targeting bone resorption
SREs such as pathological fractures, hypercalcemia of malignancy, and bone marrow failure/leukoerythroblastic anaemia, among others, are common complications of bone metastases. The occurrence of SREs in CIBP provides the rationale for bone targeting agents to manage CIBP and to reduce the occurrence of SREs by reducing bone resorption (210). Besides bone cancer, other painful syndromes such as osteoporosis and fracture repair are also associated with increased bone resorption (37). Bisphosphonates and Denosumab are osteoclast targeting molecules that inhibit bone resorption. Bisphosphonates have a phosphorus-carbon-phosphorus that enable resistance to hydrolysis; nitrogen-containing bisphosphonates prevent prenylation of small guanosine triphosphate binding proteins that are essential for osteoclast function and survival (211), and non-nitrogen containing bisphosphonates are metabolised as ATP analogues to induce osteoclast apoptosis (212). The human monoclonal antibody Denosumab targets RANKL to prevent the development, activation and survival of osteoclasts (213). Moreover, IL-6 and TNF-α can induce osteoclastogenesis and bone erosion through a non-canonical pathway, which is independent of the activation of RANK (214).
Anti-NGF
In animal models of CIBP, administration of anti-NGF neutralising antibodies dramatically reduces cutaneous and skeletal pain (215–217) by preventing ectopic sprouting (150) and reducing loss of bone integrity associated with CIBP (218). In non-cancer skeletal pain models, anti-NGF therapy has shown promising results, e.g., in mice with femoral fracture, anti-NGF therapy reduces pain behaviours without affecting bone healing (219). In a murine model of autoimmune arthritis, anti-NGF neutralising antibodies reduce hyperalgesia and cachexia with no effect on joint destruction and gross inflammation (220). Anti-NGF therapy may reduce hyperalgesia through the re-establishment of homeostatic MOR expression at the level of DRG and dorsal horn, where its anti-nociceptive effects can be reversed with naloxone pre-treatment (209). In humans, several monoclonal antibodies that bind NGF (including tanezumab, fulranumab, and fasinumab) have been used in clinical studies in a range of chronic pain conditions such as osteoarthritis [for a comprehensive review, see (221)]. A phase 2 trial investigating the analgesic efficacy of tanezumab as add-on therapy to opioid medication in patients with metastatic bone pain has recently been completed, and previous randomised control trials showed that tanezumab was shown to have greater analgesic efficacy in patients with lower baseline opioid use and/or higher baseline pain (222). Although tanezumab has been tested as a potential analgesic for patients with osteoarthritis, it did not secure FDA approval due to safety concerns linking use of NGF inhibitors to accelerated joint damage, and publication of NICE guidelines for the use of tanezumab to treat moderate-to-severe osteoarthritis pain is currently suspended.
Endothelins
The family of endothelins (ET) consists of ET-1, ET-2 and ET-3 peptides that act on ETA and ETB G-protein-coupled receptors (223, 224). ET-1 increases intracellular calcium in peripheral sensory neurons and activates PKC-ε, leading to phosphorylation and activation of TRPV1 channels expressed on nociceptive C-fibres (225). In addition to activating TRPV1 channels, ET-1 also modulates the activity of TTX-resistant sodium channels (226), likely through PKC (227). ET-1 increases the release of neuropeptides and glutamate from isolated sensory neurons through an ETA-dependent increase in intracellular calcium (228, 229). ET-1 also modulates N and L-type calcium channels in a biphasic manner; initially, ET-1 slows down the activity of these calcium channels and following that causes long-lasting facilitation (230). The effect of ET-1 on the membrane potential is also biphasic as ET-1 initially causes depolarisation along with non-selective inward cationic currents (mostly calcium-mediated) followed by hyperpolarisation (probably as a result of calcium-activated outward potassium currents) (231). Furthermore, the release of calcium ions from intracellular stores contributes to the ET-1-mediated increase of intra-neuronal calcium ions concentration (229, 232). ETB receptors are primarily expressed in DRG satellite cells and ensheathing Schwann cells (233), where it triggers the production and release of PGE2 (234). ETB receptors also enhance the release of β-endorphin from keratinocytes and accordingly generate a local analgesia (235). ETA receptor activation leads to excitation of small-to-medium diameter DRG neurons (233), partly through the ability of ET-1 to activate voltage-gated sodium channels (226). According to (226), ET-1 predominantly activates TTX-resistant sodium channels on nociceptors by enabling these channels to open at more negative membrane potential. In vivo injection of ET-1 close to nerves enhances their excitability. In contrast, TTX-sensitive sodium channels are not affected by ET-1 (226). In addition, ET-1 represses currents generated by the outward delayed rectifier potassium channels in the vast majority of sensory neurons (236). ET-1-mediated suppression of outward delayed rectifier potassium currents combined with the activation shift that ET-1 causes on TTX-resistant sodium current greatly enhances the excitability of neurons (236). Besides the mechanisms mentioned above, ET-1 is of particular interest in CIBP for its ability to activate osteoblasts. In turn, osteoblasts can release RANKL, which can cause the activation and differentiation of pre-osteoclasts into mature osteoclasts leading to substantial bone degradation and acidosis-mediated pain (237) (see Figure 1). Due to its strong involvement in CIBP, ET-1 was tested as a potential target in preclinical models of CIBP. Another study showed that mice injected with 2472 sarcoma cell lines possess an increased level of ET-1 in the plasma compared to the controls (48). In the same study, antagonising ETA receptors reduced pain-associated behaviour in cancer-bearing mice, including both spontaneous and movement-evoked pain; in contrast, antagonising ETB receptors exacerbated pain-like behaviour in the CIBP mice. Similar promising results were obtained when ETA receptors were targeted in further preclinical investigation CIBP mechanisms (56).
Other excitatory mediators
ATP is readily generated during inflammation and is abundantly expressed in malignant tissues (238). ATP receptors are known as purinergic receptors; P2Y receptors are members of the GPCR superfamily and P2X receptors are ligand-gated ion channels (239). Under physiological settings, it was discovered that cutaneous nerves have high concentrations of P2X3, but the periosteum and mineralised bone are nearly devoid of P2X3 (240). In line with these findings, it was demonstrated in a mouse model of CIBP (characterised by significant skin and skeletal hypersensitivity) that blocking P2X3 receptors with a monoclonal antibody reduces skin hypersensitivity (as measured by the von Frey test) while leaving skeletal pain-like behaviours largely unaffected (215). Another study reported a 5-fold increase in the expression of P2X3 in CGRP-positive epidermal nerve fibres in mice with osteolytic fibrosarcoma compared to control animals (241). Similarly, it was shown that the DRG neurons that innervate the rat tibia express P2X3 de novo in rat models of CIBP and systemic administration of the P2X2/3 antagonist AF-353 alleviates mechanical hypersensitivity without affecting cancer-induced bone deterioration. Not only does ATP contribute to pain peripherally, but it also has central effects demonstrated by in vivo recordings of dorsal horn neuronal excitability where intrathecal AF-353 decreases evoked action potential firing in significantly in a dose-dependent manner (144). For chemotherapy-induced neuropathy, preclinical studies suggest that glutamate can serve a protective function against neurotoxicity caused by cisplatin or paclitaxel, as measured by a reduction in proprioceptive loss and compromised performance in the rotarod test during dark cycles of rodents (82). Other protective strategies for cisplatin-induced neuropathy include ORG 2766 (93) and the recombinant human glial growth factor 2 (242).
Conclusion
Cancer pain arising from disease pathology and/or cancer treatments will increase in prevalence with increasing and prolonged survival rates. Preclinical rodent models of cancer-induced bone pain and of chemotherapy induced neuropathy address prevalent chronic pain syndromes that are experienced by patients with bone metastases or undergoing chemotherapy treatments. Cancer pain is a complex condition driven by inflammatory, neuropathic and cancer-specific mechanisms. Peripheral cross talk between tumour cells, non-neuronal cells and neurons is a key process for the induction and maintenance of cancer pain states. Changes in the tumour microenvironment, such as bone remodelling and acidosis also contribute to the sensitisation of peripheral sensory neurons. Conventional pain management still relies on opioids and standard conventional first- and second-line analgesics that are normally indicated for neuropathic pain states. Improved understanding of mechanisms specific to cancer pain states is necessary to highlight new targets for pain relief. Modelling the heterogeneity of sensory dysfunction across different types of cancer pain is one of the biggest challenges in preclinical investigations. Future studies that use animal models of distinct cancer pain states to investigate the peripheral crosstalk between neuronal and non-neuronal cells will provide valuable insight into pathophysiological mechanisms of cancer pain.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
Authors are supported by the European Commission’s Horizon 2020 Research and Innovation Programme, Marie Skłodowska-Curie grant (814244), the Wellcome Trust (200183/Z/15/Z), Versus Arthritis UK (21734) and Cancer Research UK (185341).
Acknowledgements
Figure 1 was created through BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. (2007) 34(1):94–104. doi: 10.1016/j.jpainsymman.2006.10.015
2. van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain. (2007) 132(3):312–20. doi: 10.1016/j.pain.2007.08.022
3. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. (2007) 18(9):1437–49. doi: 10.1093/annonc/mdm056
4. Organization WH. Cancer pain relief: With a guide to opioid availability. Geneva: World Health Organization (1996).
5. Nabal M, Librada S, Redondo MJ, Pigni A, Brunelli C, Caraceni A. The role of paracetamol and nonsteroidal anti-inflammatory drugs in addition to WHO step III opioids in the control of pain in advanced cancer. A systematic review of the literature. Palliat Med. (2012) 26(4):305–12. doi: 10.1177/0269216311428528
6. Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. (2012) 13(2):e58–68. doi: 10.1016/S1470-2045(12)70040-2
7. Goldstein NE, Morrison RS. Evidence-based practice of palliative medicine. Philadelphia: Elsevier Saunders (2013).
8. McNicol E, Strassels S, Goudas L, Lau J, Carr D. Nonsteroidal anti-inflammatory drugs, alone or combined with opioids, for cancer pain: a systematic review. J Clin Oncol. (2004) 22(10):1975–92. doi: 10.1200/JCO.2004.10.524
9. Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. J Am Med Assoc. (2013) 309(13):1359–67. doi: 10.1001/jama.2013.2813
10. Mishra S, Bhatnagar S, Goyal GN, Rana SP, Upadhya SP. A comparative efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled study. Am J Hosp Palliat Care. (2012) 29(3):177–82. doi: 10.1177/1049909111412539
11. Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, et al. A comprehensive algorithm for management of neuropathic pain. Pain Med. (2019) 20(Suppl 1):S2–s12. doi: 10.1093/pm/pnz075
12. Lu W, Dean-Clower E, Doherty-Gilman A, Rosenthal DS. The value of acupuncture in cancer care. Hematol Oncol Clin North Am. (2008) 22(4):631–48. doi: 10.1016/j.hoc.2008.04.005
13. Amr YM, Makharita MY. Neurolytic sympathectomy in the management of cancer pain-time effect: a prospective, randomized multicenter study. J Pain Symptom Manage. (2014) 48(5):944–56.e2. doi: 10.1016/j.jpainsymman.2014.01.015
14. Carlson CL. Effectiveness of the world health organization cancer pain relief guidelines: an integrative review. J Pain Res. (2016) 9:515–34. doi: 10.2147/JPR.S97759
15. Bandieri E, Romero M, Ripamonti CI, Artioli F, Sichetti D, Fanizza C, et al. Randomized trial of low-dose morphine versus weak opioids in moderate cancer pain. J Clin Oncol. (2016) 34(5):436–42. doi: 10.1200/JCO.2015.61.0733
16. Scarborough BM, Smith CB. Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin. (2018) 68(3):182–96. doi: 10.3322/caac.21453
17. Barbera L, Taylor C, Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? Can Med Assoc J. (2010) 182(6):563–8. doi: 10.1503/cmaj.091187
18. Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J Clin Oncol. (2011) 29(19):2683–8. doi: 10.1200/JCO.2010.34.2816
19. Batalini F, Gomes M, Fábio I, Kuwae F, Macanhan G, Pereira JLB. Cancer complaints: the profile of patients from the emergency department of a Brazilian oncology teaching hospital. F1000Res. (2017) 6:1919. doi: 10.12688/f1000research.12632.1
20. Glare PA, Davies PS, Finlay E, Gulati A, Lemanne D, Moryl N, et al. Pain in cancer survivors. J Clin Oncol. (2014) 32(16):1739–47. doi: 10.1200/JCO.2013.52.4629
21. Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. (2014) 32(36):4149–54. doi: 10.1200/JCO.2014.56.0383
22. Yoneda T, Sasaki A, Mundy GR. Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat. (1994) 32(1):73–84. doi: 10.1007/BF00666208
23. Arguello F, Baggs RB, Frantz CN. A murine model of experimental metastasis to bone and bone marrow. Cancer Res. (1988) 48(23):6876–81.3180096
24. Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. (2005) 103(5):1052–9. doi: 10.1097/00000542-200511000-00020
25. Goblirsch MJ, Zwolak P, Clohisy DR. Advances in understanding bone cancer pain. J Cell Biochem. (2005) 96(4):682–8. doi: 10.1002/jcb.20589
26. Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, et al. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. (1999) 19(24):10886–97. doi: 10.1523/JNEUROSCI.19-24-10886.1999
27. Honoré P, Schwei J, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, et al. Cellular and neurochemical remodeling of the spinal cord in bone cancer pain. Prog Brain Res. (2000) 129:389–97. doi: 10.1016/S0079-6123(00)29030-4
28. Wacnik PW, Kehl LJ, Trempe TM, Ramnaraine ML, Beitz AJ, Wilcox GL. Tumor implantation in mouse humerus evokes movement-related hyperalgesia exceeding that evoked by intramuscular carrageenan. Pain. (2003) 101(1-2):175–86. doi: 10.1016/S0304-3959(02)00312-3
29. Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, et al. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. (2001) 21(23):9355–66. doi: 10.1523/JNEUROSCI.21-23-09355.2001
30. de Clauser L, Luiz AP, Santana-Varela S, Wood JN, Sikandar S. Sensitization of cutaneous primary afferents in bone cancer revealed by in vivo calcium imaging. Cancers (Basel). (2020) 12(12). doi: 10.3390/cancers12123491
31. Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, et al. A rat model of bone cancer pain. Pain. (2002) 96(1-2):129–40. doi: 10.1016/S0304-3959(01)00437-7
32. Bäuerle T, Adwan H, Kiessling F, Hilbig H, Armbruster FP, Berger MR. Characterization of a rat model with site-specific bone metastasis induced by MDA-MB-231 breast cancer cells and its application to the effects of an antibody against bone sialoprotein. Int J Cancer. (2005) 115(2):177–86. doi: 10.1002/ijc.20840
33. Liepe K, Geidel H, Haase M, Hakenberg OW, Runge R, Kotzerke J. New model for the induction of osteoblastic bone metastases in rat. Anticancer Res. (2005) 25(2a):1067–73. PMID: 15868947
34. Coleman RE. Bone cancer in 2011: prevention and treatment of bone metastases. Nat Rev Clin Oncol. (2011) 9(2):76–8. doi: 10.1038/nrclinonc.2011.198
35. Rucci N, Teti A. Osteomimicry: how the seed grows in the soil. Calcif Tissue Int. (2018) 102(2):131–40. doi: 10.1007/s00223-017-0365-1
36. Bangash MA, Alles SRA, Santana-Varela S, Millet Q, Sikandar S, de Clauser L, et al. Distinct transcriptional responses of mouse sensory neurons in models of human chronic pain conditions. Wellcome Open Res. (2018) 3:78. doi: 10.12688/wellcomeopenres.14641.1
37. de Clauser L, Santana-Varela S, Wood JN, Sikandar S. Physiologic osteoclasts are not sufficient to induce skeletal pain in mice. Eur J Pain. (2021) 25(1):199–212. doi: 10.1002/ejp.1662
38. Sabino MA, Luger NM, Mach DB, Rogers SD, Schwei MJ, Mantyh PW. Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int J Cancer. (2003) 104(5):550–8. doi: 10.1002/ijc.10999
39. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. (1987) 55(1):61–6. doi: 10.1038/bjc.1987.13
40. Mercadante S, Fulfaro F. Management of painful bone metastases. Curr Opin Oncol. (2007) 19(4):308–14. doi: 10.1097/CCO.0b013e3281214400
41. El Mouedden M, Meert TF. Evaluation of pain-related behavior, bone destruction and effectiveness of fentanyl, sufentanil, and morphine in a murine model of cancer pain. Pharmacol Biochem Behav. (2005) 82(1):109–19. doi: 10.1016/j.pbb.2005.07.016
42. Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, et al. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. (2005) 65(20):9426. doi: 10.1158/0008-5472.CAN-05-0826
43. Honore P, Luger NM, Sabino MAC, Schwei MJ, Rogers SD, Mach DB, et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med. (2000) 6(5):521–8. doi: 10.1038/74999
44. Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. (2000) 98(3):585–98. doi: 10.1016/S0306-4522(00)00110-X
45. King T, Vardanyan A, Majuta L, Melemedjian O, Nagle R, Cress AE, et al. Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain. (2007) 132(1-2):154–68. doi: 10.1016/j.pain.2007.06.026
46. Luger NM, Honore P, Sabino MAC, Schwei MJ, Rogers SD, Mach DB, et al. Osteoprotegerin diminishes advanced bone cancer pain1. Cancer Res. (2001) 61(10):4038–47. PMID: 11358823
47. Luger NM, Sabino MAC, Schwei MJ, Mach DB, Pomonis JD, Keyser CP, et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs. inflammatory pain. Pain. (2002) 99(3):397–406. doi: 10.1016/S0304-3959(02)00102-1
48. Peters CM, Lindsay TH, Pomonis JD, Luger NM, Ghilardi JR, Sevcik MA, et al. Endothelin and the tumorigenic component of bone cancer pain. Neuroscience. (2004) 126(4):1043–52. doi: 10.1016/j.neuroscience.2004.04.027
49. Vermeirsch H, Nuydens RM, Salmon PL, Meert TF. Bone cancer pain model in mice: evaluation of pain behavior, bone destruction and morphine sensitivity. Pharmacol Biochem Behav. (2004) 79(2):243–51. doi: 10.1016/j.pbb.2004.07.011
50. Khasabov SG, Hamamoto DT, Harding-Rose C, Simone DA. Tumor-evoked hyperalgesia and sensitization of nociceptive dorsal horn neurons in a murine model of cancer pain. Brain Res. (2007) 1180:7–19. doi: 10.1016/j.brainres.2007.08.075
51. Wacnik PW, Eikmeier LJ, Simone DA, Wilcox GL, Beitz AJ. Nociceptive characteristics of tumor necrosis factor-α in naive and tumor-bearing mice. Neuroscience. (2005) 132(2):479–91. doi: 10.1016/j.neuroscience.2004.12.035
52. Cain DM, Wacnik PW, Eikmeier L, Beitz A, Wilcox GL, Simone DA. Functional interactions between tumor and peripheral nerve in a model of cancer pain in the mouse. Pain Med. (2001) 2(1):15–23. doi: 10.1046/j.1526-4637.2001.002001015.x
53. Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, et al. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. (2003) 103(1):175–86. doi: 10.1016/s0304-3959(02)00450-5
54. Vit J-P, Ohara PT, Tien DA, Fike JR, Eikmeier L, Beitz A, et al. The analgesic effect of low dose focal irradiation in a mouse model of bone cancer is associated with spinal changes in neuro-mediators of nociception. Pain. (2006) 120(1-2).16360279
55. Sabino MAC, Ghilardi JR, Jongen JLM, Keyser CP, Luger NM, Mach DB, et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-21. Cancer Res. (2002) 62(24):7343–9. PMID: 12499278
56. Baamonde A, Lastra A, Fresno MF, Llames S, Meana Á, Hidalgo A, et al. Implantation of tumoral XC cells induces chronic, endothelin-dependent, thermal hyperalgesia in mice. Cell Mol Neurobiol. (2004) 24(2):269–81. doi: 10.1023/B:CEMN.0000018621.58328.ea
57. Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. (1977) 4:161–74. doi: 10.1016/0304-3959(77)90130-0
58. Menéndez L, Lastra A, Fresno MF, Llames S, Meana Á, Hidalgo A, et al. Initial thermal heat hypoalgesia and delayed hyperalgesia in a murine model of bone cancer pain. Brain Res. (2003) 969(1):102–9. doi: 10.1016/S0006-8993(03)02284-4
59. Menéndez L, Lastra A, Hidalgo A, Meana Á, García E, Baamonde A. Peripheral opioids act as analgesics in bone cancer pain in mice. NeuroReport. (2003) 14(6):867–9. doi: 10.1097/00001756-200305060-00018
60. Asai H, Ozaki N, Shinoda M, Nagamine K, Tohnai I, Ueda M, et al. Heat and mechanical hyperalgesia in mice model of cancer pain. Pain. (2005) 117(1):19–29. doi: 10.1016/j.pain.2005.05.010
61. Lee BH, Seong J, Kim UJ, Won R, Kim J. Behavioral characteristics of a mouse model of cancer pain. Yonsei Med J. (2005) 46(2):252–9. doi: 10.3349/ymj.2005.46.2.252
62. Park HC, Seong J, An JH, Kim J, Kim UJ, Lee BW. Alteration of cancer pain-related signals by radiation: proteomic analysis in an animal model with cancer bone invasion. Int J Radiat Oncol Biol Phys. (2005) 61(5):1523–34. doi: 10.1016/j.ijrobp.2004.12.070
63. Seong J, Park HC, Kim J, Kim UJ, Lee BW. Radiation-induced alteration of pain-related signals in an animal model with bone invasion from cancer. Ann N Y Acad Sci. (2004) 1030(1):179–86. doi: 10.1196/annals.1329.023
64. Minett MS, Falk S, Santana-Varela S, Bogdanov YD, Nassar MA, Heegaard AM, et al. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep. (2014) 6(2):301–12. doi: 10.1016/j.celrep.2013.12.033
65. Beyreuther BK, Callizot N, Brot MD, Feldman R, Bain SC, Stöhr T. Antinociceptive efficacy of lacosamide in rat models for tumor- and chemotherapy-induced cancer pain. Eur J Pharmacol. (2007) 565(1):98–104. doi: 10.1016/j.ejphar.2007.02.041
66. Brigatte P, Sampaio SC, Gutierrez VP, Guerra JL, Sinhorini IL, Curi R, et al. Walker 256 tumor-bearing rats as a model to study cancer pain. J Pain. (2007) 8(5):412–21. doi: 10.1016/j.jpain.2006.11.006
67. Fox A, Medhurst S, Courade J-P, Glatt M, Dawson J, Urban L, et al. Anti-hyperalgesic activity of the cox-2 inhibitor lumiracoxib in a model of bone cancer pain in the rat. Pain. (2004) 107(1):33–40. doi: 10.1016/j.pain.2003.09.003
68. Mao-Ying Q-L, Zhao J, Dong Z-Q, Wang J, Yu J, Yan M-F, et al. A rat model of bone cancer pain induced by intra-tibia inoculation of walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun. (2006) 345(4):1292–8. doi: 10.1016/j.bbrc.2006.04.186
69. Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab. (2007) 25(2):99–104. doi: 10.1007/s00774-006-0734-8
70. Donovan-Rodriguez T, Dickenson Anthony H, Urch Catherine E. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology. (2005) 102(1):132–40. doi: 10.1097/00000542-200501000-00022
71. Zhang R-X, Liu B, Wang L, Ren K, Qiao J-T, Berman BM, et al. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. (2005) 118(1-2):125–36. doi: 10.1016/j.pain.2005.08.001
72. Lindsay TH, Jonas BM, Sevcik MA, Kubota K, Halvorson KG, Ghilardi JR, et al. Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain. (2005) 119(1-3):233–46. doi: 10.1016/j.pain.2005.10.019
73. Lahoud MJ, Kourie HR, Antoun J, El Osta L, Ghosn M. Road map for pain management in pancreatic cancer: a review. World J Gastrointest Oncol. (2016) 8(8):599–606. doi: 10.4251/wjgo.v8.i8.599
74. Wang L, Xu H, Ge Y, Zhu H, Yu D, Yu W, et al. Establishment of a murine pancreatic cancer pain model and microarray analysis of pain-associated genes in the spinal cord dorsal horn. Mol Med Rep. (2017) 16(4):4429–36. doi: 10.3892/mmr.2017.7173
75. Nagamine K, Ozaki N, Shinoda M, Asai H, Nishiguchi H, Mitsudo K, et al. Mechanical allodynia and thermal hyperalgesia induced by experimental squamous cell carcinoma of the lower gingiva in rats. J Pain. (2006) 7(9):659–70. doi: 10.1016/j.jpain.2006.02.013
76. Dorsi MJ, Chen L, Murinson BB, Pogatzki-Zahn EM, Meyer RA, Belzberg AJ. The tibial neuroma transposition (TNT) model of neuroma pain and hyperalgesia. Pain. (2008) 134(3):320–34. doi: 10.1016/j.pain.2007.06.030
77. Tyner TR, Parks N, Faria S, Simons M, Stapp B, Curtis B, et al. Effects of collagen nerve guide on neuroma formation and neuropathic pain in a rat model. Am J Surg. (2007) 193(1):e1–6. doi: 10.1016/j.amjsurg.2006.08.026
78. Lam DK, Dang D, Zhang J, Dolan JC, Schmidt BL. Novel animal models of acute and chronic cancer pain: a pivotal role for PAR2. J Neurosci. (2012) 32(41):14178–83. doi: 10.1523/JNEUROSCI.2399-12.2012
79. Pickering V, Gupta RJ, Quang P, Jordan RC, Schmidt BL. Effect of peripheral endothelin-1 concentration on carcinoma-induced pain in mice. Eur J Pain. (2008) 12(3):293–300. doi: 10.1016/j.ejpain.2007.06.001
80. Fallon MT. Neuropathic pain in cancer. Br J Anaesth. (2013) 111(1):105–11. doi: 10.1093/bja/aet208
81. Shimoyama M, Tanaka K, Hasue F, Shimoyama N. A mouse model of neuropathic cancer pain. Pain. (2002) 99(1-2):167–74. doi: 10.1016/S0304-3959(02)00073-8
82. Boyle FM, Wheeler HR, Shenfield GM. Amelioration of experimental cisplatin and paclitaxel neuropathy with glutamate. J Neurooncol. (1999) 41(2):107–16. doi: 10.1023/A:1006124917643
83. Wang AB, Housley SN, Flores AM, Kircher SM, Perreault EJ, Cope TC. A review of movement disorders in chemotherapy-induced neurotoxicity. J Neuroeng Rehabil. (2021) 18(1):16. doi: 10.1186/s12984-021-00818-2
84. Aley KO, Reichling DB, Levine JD. Vincristine hyperalgesia in the rat: a model of painful vincristine neuropathy in humans. Neuroscience. (1996) 73(1):259–65. doi: 10.1016/0306-4522(96)00020-6
85. Nozaki-Taguchi N, Chaplan SR, Higuera ES, Ajakwe RC, Yaksh TL. Vincristine-induced allodynia in the rat. Pain. (2001) 93(1):69–76. doi: 10.1016/S0304-3959(01)00294-9
86. Lynch JJ 3rd, Wade CL, Zhong CM, Mikusa JP, Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain. (2004) 110(1-2):56–63. doi: 10.1016/j.pain.2004.03.010
87. Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. (2001) 2(1):8–14. doi: 10.1046/j.1526-4637.2001.002001008.x
88. Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. (2001) 94(3):293–304. doi: 10.1016/S0304-3959(01)00363-3
89. Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci. (2004) 74(21):2593–604. doi: 10.1016/j.lfs.2004.01.002
90. Gispen WH, Hamers FP, Vecht CJ, Jennekens FG, Neyt JP. ACTH/MSH like peptides in the treatment of cisplatin neuropathy. J Steroid Biochem Mol Biol. (1992) 43(1-3):179–83. doi: 10.1016/0960-0760(92)90205-W
91. Strumberg D, Brügge S, Korn MW, Koeppen S, Ranft J, Scheiber G, et al. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol. (2002) 13(2):229–36. doi: 10.1093/annonc/mdf058
92. Authier N, Fialip J, Eschalier A, Coudoré F. Assessment of allodynia and hyperalgesia after cisplatin administration to rats. Neurosci Lett. (2000) 291(2):73–6. doi: 10.1016/S0304-3940(00)01373-2
93. de Koning P, Neijt JP, Jennekens FG, Gispen WH. Org.2766 protects from cisplatin-induced neurotoxicity in rats. Exp Neurol. (1987) 97(3):746–50. doi: 10.1016/0014-4886(87)90132-4
94. MacDonald DI, Luiz AP, Iseppon F, Millet Q, Emery EC, Wood JN. Silent cold-sensing neurons contribute to cold allodynia in neuropathic pain. Brain. (2021) 144(6):1711–26. doi: 10.1093/brain/awab086
95. Deuis JR, Zimmermann K, Romanovsky AA, Possani LD, Cabot PJ, Lewis RJ, et al. An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1.6 in peripheral pain pathways. Pain. (2013) 154(9):1749–57. doi: 10.1016/j.pain.2013.05.032
96. Authier N, Coudore F, Eschalier A, Fialip J. Pain related behaviour during vincristine-induced neuropathy in rats. NeuroReport. (1999) 10(5):965–8. doi: 10.1097/00001756-199904060-00013
97. Joseph EK, Levine JD. Sexual dimorphism for protein kinase c epsilon signaling in a rat model of vincristine-induced painful peripheral neuropathy. Neuroscience. (2003) 119(3):831–8. doi: 10.1016/S0306-4522(03)00203-3
98. Apfel SC, Lipton RB, Arezzo JC, Kessler JA. Nerve growth factor prevents toxic neuropathy in mice. Ann Neurol. (1991) 29(1):87–90. doi: 10.1002/ana.410290115
99. Matsumoto M, Inoue M, Hald A, Xie W, Ueda H. Inhibition of paclitaxel-induced A-fiber hypersensitization by gabapentin. J Pharmacol Exp Ther. (2006) 318(2):735–40. doi: 10.1124/jpet.106.103614
100. Dina OA, Chen X, Reichling D, Levine JD. Role of protein kinase cepsilon and protein kinase A in a model of paclitaxel-induced painful peripheral neuropathy in the rat. Neuroscience. (2001) 108(3):507–15. doi: 10.1016/S0306-4522(01)00425-0
101. Tassler P, Dellon AL, Lesser GJ, Grossman S. Utility of decompressive surgery in the prophylaxis and treatment of cisplatin neuropathy in adult rats. J Reconstr Microsurg. (2000) 16(6):457–63. doi: 10.1055/s-2006-947153
102. Han FY, Wyse BD, Smith MT. Optimization and pharmacological characterization of a refined cisplatin-induced rat model of peripheral neuropathic pain. Behav Pharmacol. (2014) 25(8):732–40. doi: 10.1097/FBP.0000000000000090
103. Furgała A, Sałat R, Sałat K. Acute cold allodynia induced by oxaliplatin is attenuated by amitriptyline. Acta Neurobiol Exp. (2018) 78(4):315–21. doi: 10.21307/ane-2018-030
104. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. (1989) 8(2):98–101. PMID: 2673568
105. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. (2006) 12(20 Pt 2):6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931
106. Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. (1997) 69(1-2):1–18. doi: 10.1016/S0304-3959(96)03267-8
107. Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. (2006) 12(8):895–904. doi: 10.1038/nm1469
108. Lipton A. Pathophysiology of bone metastases: how this knowledge may lead to therapeutic intervention. J Support Oncol. (2004) 2(3):205–13; discussion 13–4, 16–7, 19–20. PMID: 15328823
109. Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology. (2005) 20:349–56. doi: 10.1152/physiol.00025.2005
110. Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. (2006) 440(7084):692–6. doi: 10.1038/nature04524
111. Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. (2003) 3(6):537–49. doi: 10.1016/S1535-6108(03)00132-6
112. Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. (2005) 20(2):318–29. doi: 10.1359/JBMR.041109
113. Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. (2011) 121(4):1298–312. doi: 10.1172/JCI43414
114. Bellahcène A, Bachelier R, Detry C, Lidereau R, Clézardin P, Castronovo V. Transcriptome analysis reveals an osteoblast-like phenotype for human osteotropic breast cancer cells. Breast Cancer Res Treat. (2007) 101(2):135–48. doi: 10.1007/s10549-006-9279-8
115. Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, et al. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. (2010) 29(6):811–21. doi: 10.1038/onc.2009.389
116. Andersen TL, Boissy P, Sondergaard TE, Kupisiewicz K, Plesner T, Rasmussen T, et al. Osteoclast nuclei of myeloma patients show chromosome translocations specific for the myeloma cell clone: a new type of cancer-host partnership? J Pathol. (2007) 211(1):10–7. doi: 10.1002/path.2078
117. Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci. (2016) 41(3):211–8. doi: 10.1016/j.tibs.2015.12.001
118. Eliasson P, Jönsson J-I. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. (2010) 222(1):17–22. doi: 10.1002/jcp.21908
119. Zhang L, Wen C. Osteocyte dysfunction in joint homeostasis and osteoarthritis. Int J Mol Sci. (2021) 22(12):6522. doi: 10.3390/ijms22126522
120. Cappariello A, Maurizi A, Veeriah V, Teti A. The great beauty of the osteoclast. Arch Biochem Biophys. (2014) 558:70–8. doi: 10.1016/j.abb.2014.06.017
121. Pan HL, Zhang YQ, Zhao ZQ. Involvement of lysophosphatidic acid in bone cancer pain by potentiation of TRPV1 via PKCε pathway in dorsal root ganglion neurons. Mol Pain. (2010) 6:85. doi: 10.1186/1744-8069-6-85
122. Ghilardi JR, Röhrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. (2005) 25(12):3126. doi: 10.1523/JNEUROSCI.3815-04.2005
123. Lautner MA, Ruparel SB, Patil MJ, Hargreaves KM. In vitro sarcoma cells release a lipophilic substance that activates the pain transduction system via TRPV1. Ann Surg Oncol. (2011) 18(3):866–71. doi: 10.1245/s10434-010-1328-1
124. Qiu F, Wei X, Zhang S, Yuan W, Mi W. Increased expression of acid-sensing ion channel 3 within dorsal root ganglia in a rat model of bone cancer pain. NeuroReport. (2014) 25(12):887–93. doi: 10.1097/WNR.0000000000000182
125. Aielli F, Ponzetti M, Rucci N. Bone metastasis pain, from the bench to the bedside. Int J Mol Sci. (2019) 20(2):280. doi: 10.3390/ijms20020280
126. Barrios-Rodiles M, Chadee K. Novel regulation of cyclooxygenase-2 expression and prostaglandin E2 production by IFN-gamma in human macrophages. J Immunol. (1998) 161(5):2441–8. PMID: 9725242
127. Sabino MA, Ghilardi JR, Jongen JL, Keyser CP, Luger NM, Mach DB, et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. (2002) 62(24):7343–9. PMID: 12499278
128. Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. (2000) 288(5464):306–13. doi: 10.1126/science.288.5464.306
129. Williams KS, Killebrew DA, Clary GP, Seawell JA, Meeker RB. Differential regulation of macrophage phenotype by mature and pro-nerve growth factor. J Neuroimmunol. (2015) 285:76–93. doi: 10.1016/j.jneuroim.2015.05.016
130. Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. (2004) 363(9410):675–81. doi: 10.1016/S0140-6736(04)15640-7
131. Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor α in the development of inflammatory hyperalgesia. Br J Pharmacol. (1992) 107(3):660–4. doi: 10.1111/j.1476-5381.1992.tb14503.x
132. Schäfers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. (2003) 104(3):579–88. doi: 10.1016/S0304-3959(03)00115-5
133. Wagner R, Myers RR. Endoneurial injection of TNF-α produces neuropathic pain behaviors. NeuroReport. (1996) 7(18):2897-901. doi: 10.1097/00001756-199611250-00018
134. Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. (1997) 121(3):417–24. doi: 10.1038/sj.bjp.0701148
135. Zelenka M, Schäfers M, Sommer C. Intraneural injection of interleukin-1β and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. (2005) 116(3):257–63. doi: 10.1016/j.pain.2005.04.018
136. Czeschik JC, Hagenacker T, Schäfers M, Büsselberg D. TNF-α differentially modulates ion channels of nociceptive neurons. Neurosci Lett. (2008) 434(3):293–8. doi: 10.1016/j.neulet.2008.01.070
137. Liu BG, Dobretsov M, Stimers JR, Zhang JM. Tumor necrosis factor-α suppresses activation of sustained potassium currents in rat small diameter sensory neurons. Open Pain J. (2008) 1:1. doi: 10.2174/1876386300902010001
138. Jin X, Gereau RW 4th. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. (2006) 26(1):246–55. doi: 10.1523/JNEUROSCI.3858-05.2006
139. Schäfers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. (2003) 23(7):3028–38. doi: 10.1523/JNEUROSCI.23-07-03028.2003
140. Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-α induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. (1997) 81(1):255–62. doi: 10.1016/S0306-4522(97)00147-4
141. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. (2008) 117(2):244–79. doi: 10.1016/j.pharmthera.2007.10.001
142. Yang Y, Zhang J, Gao Q, Bo J, Ma Z. Etanercept attenuates thermal and mechanical hyperalgesia induced by bone cancer. Exp Ther Med. (2017) 13(5):2565–9. doi: 10.3892/etm.2017.4260
143. Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. (2005) 115(1-2):128–41. doi: 10.1016/j.pain.2005.02.022
144. Kaan TKY, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, et al. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain. (2010) 133(9):2549–64. doi: 10.1093/brain/awq194
145. Sayilekshmy M, Hansen RB, Delaissé JM, Rolighed L, Andersen TL, Heegaard AM. Innervation is higher above bone remodeling surfaces and in cortical pores in human bone: lessons from patients with primary hyperparathyroidism. Sci Rep. (2019) 9(1):5361. doi: 10.1038/s41598-019-41779-w
146. Nencini S, Ivanusic J. Mechanically sensitive aδ nociceptors that innervate bone marrow respond to changes in intra-osseous pressure. J Physiol. (2017) 595(13):4399–415. doi: 10.1113/JP273877
147. Sottnik JL, Dai J, Zhang H, Campbell B, Keller ET. Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. (2015) 75(11):2151–8. doi: 10.1158/0008-5472.CAN-14-2493
148. Tomlinson RE, Li Z, Li Z, Minichiello L, Riddle RC, Venkatesan A, et al. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc Natl Acad Sci U S A. (2017) 114(18):E3632–e41. doi: 10.1073/pnas.1701054114
149. Tomlinson RE, Li Z, Zhang Q, Goh BC, Li Z, Thorek DLJ, et al. NGF-TrkA signaling by sensory nerves coordinates the vascularization and ossification of developing endochondral bone. Cell Rep. (2016) 16(10):2723–35. doi: 10.1016/j.celrep.2016.08.002
150. Jimenez-Andrade JM, Ghilardi JR, Castañeda-Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. (2011) 152(11):2564–74. doi: 10.1016/j.pain.2011.07.020
151. Offley SC, Guo TZ, Wei T, Clark JD, Vogel H, Lindsey DP, et al. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J Bone Miner Res. (2005) 20(2):257–67. doi: 10.1359/JBMR.041108
152. Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol. (2008) 7(5):459–66. doi: 10.1016/S1474-4422(08)70089-9
153. Yu D, Thakor DK, Han I, Ropper AE, Haragopal H, Sidman RL, et al. Alleviation of chronic pain following rat spinal cord compression injury with multimodal actions of huperzine A. Proc Natl Acad Sci U S A. (2013) 110(8):E746–E55. doi: 10.1073/pnas.1300083110
154. Peters CM, Ghilardi JR, Keyser CP, Kubota K, Lindsay TH, Luger NM, et al. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol. (2005) 193(1):85–100. doi: 10.1016/j.expneurol.2004.11.028
155. Yanagisawa Y, Furue H, Kawamata T, Uta D, Yamamoto J, Furuse S, et al. Bone cancer induces a unique central sensitization through synaptic changes in a wide area of the spinal cord. Mol Pain. (2010) 6:38. doi: 10.1186/1744-8069-6-38
156. Urch EC, Donovan-Rodriguez T, Dickenson HA. Alterations in dorsal horn neurones in a rat model of cancer-induced bone pain. Pain. (2003) 106(3):347–56. doi: 10.1016/j.pain.2003.08.002
157. Urch CE, Donovan-Rodriguez T, Gordon-Williams R, Bee LA, Dickenson AH. Efficacy of chronic morphine in a rat model of cancer-induced bone pain: behavior and in dorsal horn pathophysiology. J Pain. (2005) 6(12):837–45. doi: 10.1016/j.jpain.2005.08.005
158. Donovan-Rodriguez T, Urch CE, Dickenson AH. Evidence of a role for descending serotonergic facilitation in a rat model of cancer-induced bone pain. Neurosci Lett. (2006) 393(2-3):237–42. doi: 10.1016/j.neulet.2005.09.073
159. Zhang RX, Liu B, Li A, Wang L, Ren K, Qiao JT, et al. Interleukin 1beta facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation. Neuroscience. (2008) 154(4):1533–8. doi: 10.1016/j.neuroscience.2008.04.072
160. Gu X, Zhang J, Ma Z, Wang J, Zhou X, Jin Y, et al. The role of N-methyl-D-aspartate receptor subunit NR2B in spinal cord in cancer pain. Eur J Pain. (2010) 14(5):496–502. doi: 10.1016/j.ejpain.2009.09.001
161. Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. (2001) 24(1-3):107–29. doi: 10.1385/MN:24:1-3:107
162. Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, et al. Single intrathecal injections of dynorphin A or des-tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. (1996) 68(2-3):275–81. doi: 10.1016/S0304-3959(96)03225-3
163. Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. (2002) 249(1):9–17. doi: 10.1007/PL00007853
164. Wang Z, Sun H, Yakisich JS. Overcoming the blood-brain barrier for chemotherapy: limitations, challenges and rising problems. Anticancer Agents Med Chem. (2014) 14(8):1085–93. doi: 10.2174/18715206113139990029
165. Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. (2014) 155(12):2461–70. doi: 10.1016/j.pain.2014.09.020
166. Holmes J, Stanko J, Varchenko M, Ding H, Madden VJ, Bagnell CR, et al. Comparative neurotoxicity of oxaliplatin, cisplatin, and ormaplatin in a wistar rat model. Toxicol Sci. (1998) 46(2):342–51. doi: 10.1006/toxs.1998.2558
167. Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D'Incalci M, et al. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology. (2000) 21(3):389–93. PMID: 10894128
168. Cavaletti G, Tredici G, Petruccioli MG, Dondè E, Tredici P, Marmiroli P, et al. Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur J Cancer. (2001) 37(18):2457–63. doi: 10.1016/S0959-8049(01)00300-8
169. Screnci D, McKeage MJ, Galettis P, Hambley TW, Palmer BD, Baguley BC. Relationships between hydrophobicity, reactivity, accumulation and peripheral nerve toxicity of a series of platinum drugs. Br J Cancer. (2000) 82(4):966–72. doi: 10.1054/bjoc.1999.1026
170. Cliffer KD, Siuciak JA, Carson SR, Radley HE, Park JS, Lewis DR, et al. Physiological characterization of taxol-induced large-fiber sensory neuropathy in the rat. Ann Neurol. (1998) 43(1):46–55. doi: 10.1002/ana.410430111
171. Fischer SJ, Podratz JL, Windebank AJ. Nerve growth factor rescue of cisplatin neurotoxicity is mediated through the high affinity receptor: studies in PC12 cells and p75 null mouse dorsal root ganglia. Neurosci Lett. (2001) 308(1):1–4. doi: 10.1016/S0304-3940(01)01956-5
172. McDonald ES, Windebank AJ. Cisplatin-induced apoptosis of DRG neurons involves bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol Dis. (2002) 9(2):220–33. doi: 10.1006/nbdi.2001.0468
173. Materazzi S, Fusi C, Benemei S, Pedretti P, Patacchini R, Nilius B, et al. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch. (2012) 463(4):561–9. doi: 10.1007/s00424-011-1071-x
174. Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. (2011) 152(7):1621–31. doi: 10.1016/j.pain.2011.02.051
175. Nieto FR, Entrena JM, Cendán CM, Del Pozo E, Vela JM, Baeyens JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain. (2008) 137(3):520–31. doi: 10.1016/j.pain.2007.10.012
176. Xiao W, Boroujerdi A, Bennett GJ, Luo ZD. Chemotherapy-evoked painful peripheral neuropathy: analgesic effects of gabapentin and effects on expression of the alpha-2-delta type-1 calcium channel subunit. Neuroscience. (2007) 144(2):714–20. doi: 10.1016/j.neuroscience.2006.09.044
177. Musatov A, Robinson NC. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic Res. (2012) 46(11):1313–26. doi: 10.3109/10715762.2012.717273
178. Luiz AP, MacDonald DI, Santana-Varela S, Millet Q, Sikandar S, Wood JN, et al. Cold sensing by NaV1.8-positive and NaV1.8-negative sensory neurons. Proc Natl Acad Sci U S A. (2019) 116(9):3811–6. doi: 10.1073/pnas.1814545116
179. Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, Mantyh PW. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. (2007) 1168:46–59. doi: 10.1016/j.brainres.2007.06.066
180. Scuteri A, Nicolini G, Miloso M, Bossi M, Cavaletti G, Windebank AJ, et al. Paclitaxel toxicity in post-mitotic dorsal root ganglion (DRG) cells. Anticancer Res. (2006) 26(2a):1065–70. PMID: 16619507
181. Freelove R, Walling AD. Pancreatic cancer: diagnosis and management. Am Fam Physician. (2006) 73(3):485–92. PMID: 16477897
182. Moore JC, Adler DG. Celiac plexus neurolysis for pain relief in pancreatic cancer. J Support Oncol. (2009) 7(3):83–7, 90. PMID: 19507453
183. Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. (2003) 26(4):322–5. doi: 10.1097/00006676-200305000-00002
184. Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. (2004) 101(1):3–27. doi: 10.1002/cncr.20288
185. Seki H, Tanaka J-I, Sato Y, Kato Y, Umezawa A, Koyama K. Neural cell adhesion molecule (NCAM) and perineural invasion in bile duct cancer. J Surg Oncol. (1993) 53(2):78–83. doi: 10.1002/jso.2930530205
186. Zhu Z, Friess H, di Mola FF, Zimmermann A, Graber HU, Korc M, et al. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. (1999) 17(8):2419–28. doi: 10.1200/JCO.1999.17.8.2419
187. di Mola FF, di Sebastiano P. Pain and pain generation in pancreatic cancer. Langenbeck's Arch Surg. (2008) 393(6):919–22. doi: 10.1007/s00423-007-0277-z
188. Hartel M, di Mola FF, Selvaggi F, Mascetta G, Wente MN, Felix K, et al. Vanilloids in pancreatic cancer: potential for chemotherapy and pain management. Gut. (2006) 55(4):519–28. doi: 10.1136/gut.2005.073205
189. Sevcik MA, Jonas BM, Lindsay TH, Halvorson KG, Ghilardi JR, Kuskowski MA, et al. Endogenous opioids inhibit early-stage pancreatic pain in a mouse model of pancreatic cancer. Gastroenterology. (2006) 131(3):900–10. doi: 10.1053/j.gastro.2006.06.021
190. Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. (2002) 25(6):319–25. doi: 10.1016/S0166-2236(02)02157-4
191. Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. (2002) 100(1-2):1–6. doi: 10.1016/S0304-3959(02)00368-8
192. Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. (2006) 7(10):797–809. doi: 10.1038/nrn1914
193. Muralidharan A, Wyse BD, Smith MT. Optimization and characterization of a rat model of prostate cancer-induced bone pain using behavioral, pharmacological, radiological, histological and immunohistochemical methods. Pharmacol Biochem Behav. (2013) 106:33–46. doi: 10.1016/j.pbb.2013.02.020
194. Shenoy P, Kuo A, Vetter I, Smith MT. Optimization and in vivo profiling of a refined rat model of walker 256 breast cancer cell-induced bone pain using behavioral, radiological, histological, immunohistochemical and pharmacological methods. Front Pharmacol. (2017) 8:442. doi: 10.3389/fphar.2017.00442
195. Ceyhan GO, Michalski CW, Demir IE, Müller MW, Friess H. Pancreatic pain. Best Pract Res Clin Gastroenterol. (2008) 22(1):31–44. doi: 10.1016/j.bpg.2007.10.016
196. Feng W, Teng R, Zhao Y, Gao J, Chu H. Epigenetic modulation of wnt signaling contributes to neuropathic pain in rats. Mol Med Rep. (2015) 12(3):4727–33. doi: 10.3892/mmr.2015.3972
197. Quick ML, Mukherjee S, Rudick CN, Done JD, Schaeffer AJ, Thumbikat P. CCL2 and CCL3 are essential mediators of pelvic pain in experimental autoimmune prostatitis. Am J Physiol Regul Integr Comp Physiol. (2012) 303(6):R580–9. doi: 10.1152/ajpregu.00240.2012
198. Pickering V, Jordan RC, Schmidt BL. Elevated salivary endothelin levels in oral cancer patients–a pilot study. Oral Oncol. (2007) 43(1):37–41. doi: 10.1016/j.oraloncology.2005.12.027
199. Schmidt BL, Pickering V, Liu S, Quang P, Dolan J, Connelly ST, et al. Peripheral endothelin A receptor antagonism attenuates carcinoma-induced pain. Eur J Pain. (2007) 11(4):406–14. doi: 10.1016/j.ejpain.2006.05.007
200. Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan JC, et al. Nerve growth factor links oral cancer progression, pain, and cachexiaanti-NGF as a therapy in head and neck cancer. Mol Cancer Ther. (2011) 10(9):1667–76. doi: 10.1158/1535-7163.MCT-11-0123
201. Lam DK, Schmidt BL. Serine proteases and protease-activated receptor 2-dependent allodynia: a novel cancer pain pathway. Pain. (2010) 149(2):263–72. doi: 10.1016/j.pain.2010.02.010
202. Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. (2006) 575(Pt 2):555–71. doi: 10.1113/jphysiol.2006.111534
203. McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain. (2003) 4(5):231–56. doi: 10.1016/S1526-5900(03)00556-X
204. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. (2015) 14(2):162–73. doi: 10.1016/S1474-4422(14)70251-0
205. Glaum SR, Miller RJ, Hammond DL. Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina II neurons of adult rat spinal cord. J Neurosci. (1994) 14(8):4965–71. doi: 10.1523/JNEUROSCI.14-08-04965.1994
206. Trafton JA, Abbadie C, Marek K, Basbaum AI. Postsynaptic signaling via the [mu]-opioid receptor: responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J Neurosci. (2000) 20(23):8578–84. doi: 10.1523/JNEUROSCI.20-23-08578.2000
207. Yamamoto J, Kawamata T, Niiyama Y, Omote K, Namiki A. Down-regulation of mu opioid receptor expression within distinct subpopulations of dorsal root ganglion neurons in a murine model of bone cancer pain. Neuroscience. (2008) 151(3):843–53. doi: 10.1016/j.neuroscience.2007.11.025
208. Zhu C, Tang J, Ding T, Chen L, Wang W, Mei XP, et al. Neuron-restrictive silencer factor-mediated downregulation of μ-opioid receptor contributes to the reduced morphine analgesia in bone cancer pain. Pain. (2017) 158(5):879–90. doi: 10.1097/j.pain.0000000000000848
209. Yao P, Ding Y, Wang Z, Ma J, Hong T, Zhu Y, et al. Impacts of anti-nerve growth factor antibody on pain-related behaviors and expressions of opioid receptor in spinal dorsal horn and dorsal root ganglia of rats with cancer-induced bone pain. Mol Pain. (2016) 12:1744806916644928. doi: 10.1177/1744806916644928
210. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. (2001) 27(3):165–76. doi: 10.1053/ctrv.2000.0210
211. Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including ras. J Bone Miner Res. (1998) 13(4):581–9. doi: 10.1359/jbmr.1998.13.4.581
212. Frith JC, Mönkkönen J, Auriola S, Mönkkönen H, Rogers MJ. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum. (2001) 44(9):2201–10. doi: 10.1002/1529-0131(200109)44:9%3C2201::AID-ART374%3E3.0.CO;2-E
213. Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, Kurahara C, Sun N, et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res. (2009) 24(2):182–95. doi: 10.1359/jbmr.081112
214. O'Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, et al. RANK-independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheumatol. (2016) 68(12):2889–900. doi: 10.1002/art.39837
215. Guedon JG, Longo G, Majuta LA, Thomspon ML, Fealk MN, Mantyh PW. Dissociation between the relief of skeletal pain behaviors and skin hypersensitivity in a model of bone cancer pain. Pain. (2016) 157(6):1239–47. doi: 10.1097/j.pain.0000000000000514
216. Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Mantyh WG, Bloom AP, Kuskowski MA, et al. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain. (2010) 6:87. doi: 10.1186/1744-8069-6-87
217. Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. (2010) 171(2):588–98. doi: 10.1016/j.neuroscience.2010.08.056
218. McCaffrey G, Thompson ML, Majuta L, Fealk MN, Chartier S, Longo G, et al. NGF blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer Res. (2014) 74(23):7014–23. doi: 10.1158/0008-5472.CAN-14-1220
219. Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez-Andrade JM, Ghilardi JR, et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res. (2007) 22(11):1732–42. doi: 10.1359/jbmr.070711
220. Shelton DL, Zeller J, Ho WH, Pons J, Rosenthal A. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain. (2005) 116(1-2):8–16. doi: 10.1016/j.pain.2005.03.039
221. Wise BL, Seidel MF, Lane NE. The evolution of nerve growth factor inhibition in clinical medicine. Nat Rev Rheumatol. (2021) 17(1):34–46. doi: 10.1038/s41584-020-00528-4
222. Sopata M, Katz N, Carey W, Smith MD, Keller D, Verburg KM, et al. Efficacy and safety of tanezumab in the treatment of pain from bone metastases. Pain. (2015) 156(9):1703–13. doi: 10.1097/j.pain.0000000000000211
223. Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. (1990) 348(6303):730–2. doi: 10.1038/348730a0
224. Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. (1990) 348(6303):732–5. doi: 10.1038/348732a0
225. Plant TD, Zöllner C, Kepura F, Mousa SS, Eichhorst J, Schaefer M, et al. Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol Pain. (2007) 3:35. doi: 10.1186/1744-8069-3-35
226. Zhou Z, Davar G, Strichartz G. Endothelin-1 (ET-1) selectively enhances the activation gating of slowly inactivating tetrodotoxin-resistant sodium currents in rat sensory neurons: a mechanism for the pain-inducing actions of ET-1. J Neurosci. (2002) 22(15):6325–30. doi: 10.1523/JNEUROSCI.22-15-06325.2002
227. Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. (1998) 18(24):10345–55. doi: 10.1523/JNEUROSCI.18-24-10345.1998
228. Dymshitz J, Vasko MR. Endothelin-1 enhances capsaicin-induced peptide release and cGMP accumulation in cultures of rat sensory neurons. Neurosci Lett. (1994) 167(1-2):128–32. doi: 10.1016/0304-3940(94)91044-8
229. Zhou QL, Strichartz G, Davar G. Endothelin-1 activates ET(A) receptors to increase intracellular calcium in model sensory neurons. Neuroreport. (2001) 12(17):3853–7. doi: 10.1097/00001756-200112040-00050
230. Nishimura T, Akasu T, Krier J. Endothelin modulates calcium channel current in neurones of rabbit pelvic parasympathetic ganglia. Br J Pharmacol. (1991) 103(1):1242–50. doi: 10.1111/j.1476-5381.1991.tb12331.x
231. Suzuki T. Endothelin-1-induced depolarization and hyperpolarization in submandibular ganglion neurons. Bull Tokyo Dent Coll. (2004) 45(3):189–92. doi: 10.2209/tdcpublication.45.189
232. Yamamoto H, Kawamata T, Ninomiya T, Omote K, Namiki A. Endothelin-1 enhances capsaicin-evoked intracellular Ca2+ response via activation of endothelin a receptor in a protein kinase cepsilon-dependent manner in dorsal root ganglion neurons. Neuroscience. (2006) 137(3):949–60. doi: 10.1016/j.neuroscience.2005.09.036
233. Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. (2001) 21(3):999–1006. doi: 10.1523/JNEUROSCI.21-03-00999.2001
234. Koyama Y, Mizobata T, Yamamoto N, Hashimoto H, Matsuda T, Baba A. Endothelins stimulate expression of cyclooxygenase 2 in rat cultured astrocytes. J Neurochem. (1999) 73(3):1004–11. doi: 10.1046/j.1471-4159.1999.0731004.x
235. Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, et al. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. (2003) 9(8):1055–61. doi: 10.1038/nm885
236. Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain. (2009) 10(1):4–28. doi: 10.1016/j.jpain.2008.09.009
237. Yoneda T, Hiasa M, Nagata Y, Okui T, White FA. Acidic microenvironment and bone pain in cancer-colonized bone. Bonekey Rep. (2015) 4:690. doi: 10.1038/bonekey.2015.58
238. Vultaggio-Poma V, Sarti AC, Di Virgilio F. Extracellular ATP: a feasible target for cancer therapy. Cells. (2020) 9(11):2496. doi: 10.3390/cells9112496
239. Sawynok J. Adenosine and ATP receptors. In: Stein C, editors. Analgesia. Berlin, Heidelberg: Springer Berlin Heidelberg (2007). p. 309–28.
240. Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, et al. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone. (2010) 46(2):306–13. doi: 10.1016/j.bone.2009.09.013
241. Gilchrist LS, Cain DM, Harding-Rose C, Kov AN, Wendelschafer-Crabb G, Kennedy WR, et al. Re-organization of P2X3 receptor localization on epidermal nerve fibers in a murine model of cancer pain. Brain Res. (2005) 1044(2):197–205. doi: 10.1016/j.brainres.2005.02.081
Keywords: pain, dorsal root ganglia, cancer-induced bone pain, chemotherapy associated pain, neuropathy, cancer, sensitisation
Citation: Haroun R, Wood John N and Sikandar S (2023) Mechanisms of cancer pain. Front. Pain Res. 3:1030899. doi: 10.3389/fpain.2022.1030899
Received: 29 August 2022; Accepted: 14 November 2022;
Published: 4 January 2023.
Edited by:
Marta Diaz-delCastillo, University of Copenhagen, DenmarkReviewed by:
Juan M. Jimenez Andrade, Universidad Autónoma de Tamaulipas, MexicoMaree Therese Smith, The University of Queensland, Australia
© 2023 Haroun, Wood and Sikandar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shafaq Sikandar c2hhZmFxLnNpa2FuZGFyQHVjbC5hYy51aw==
Specialty Section: This article was submitted to Cancer Pain, a section of the journal Frontiers in Pain Research
 Rayan Haroun
Rayan Haroun John N Wood
John N Wood Shafaq Sikandar
Shafaq Sikandar