- 1Division of Clinical Psychology and Psychotherapy, Faculty of Psychology, University of Basel, Basel, Switzerland
- 2Department of Anesthesiology, Critical Care and Pain Medicine, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States
- 3Faculty of Health, University of Plymouth, Plymouth, United Kingdom
- 4Clinic for Psychotherapy and Psychosomatics “Hohenegg”, Meilen, Switzerland
- 5Department of Consultation-Liaison Psychiatry and Psychosomatic Medicine, University Hospital Zurich, Zurich, Switzerland
Background: Fibromyalgia (FM) is a chronic primary pain condition, associated with widespread musculoskeletal pain, disturbed sleep, fatigue, cognitive dysfunction, and a range of comorbid conditions such as irritable bowel syndrome, and depression. Despite its high prevalence of 2% in the general population, FM continues to pose scientific and clinical challenges in definition, etiology, and day-to-day management. In terms of treatment, FM can be treated with selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs).
Objective: Patients with FM and other chronic primary pain syndromes are known to experience substantial and clinically relevant placebo effects. An update of the placebo responses for various outcomes in the FM population and especially a discussion about clinical implications is therefore needed.
Methods: We used data from a large data pool that includes randomized controlled trials (RCTs) examining within-placebo mean change scores of baseline vs. follow-up assessments in FM trials of SSRIs and SNRIs. The primary outcomes were pain, functional disability, and depression and using different scales. We assessed heterogeneity of included trials.
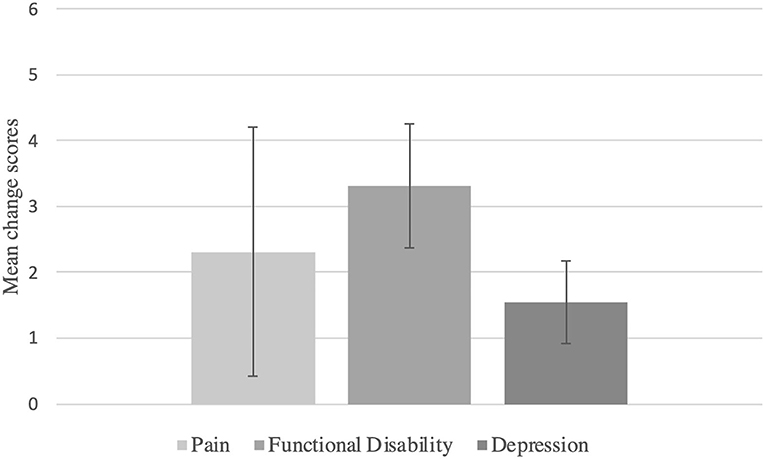
Results: A total of 29 RCTs with N = 8,453 patients suffering from FM were included in our analysis. Within-placebo mean change scores of baseline vs. follow-up assessments were large for pain (mean change = 2.31, 95% CI: 0.42–4.21, p = 0.017), functional disability (mean change = 3.31, 95% CI: 2.37–4.26, p < 0.000), and depression (mean change = 1.55, 95% CI: 0.92–2.18, p < 0.000). Heterogeneity was found to be large for all outcomes.
Impact: Our results provide preliminary evidence that placebo responses, which also consist of non-specific effects, might play a role in the treatment of FM. Furthermore, we highlight limitations of our analyses and make suggestions for future studies.
Introduction
Fibromyalgia (FM) is a Chronic Primary Pain condition classified under MG30.01 Chronic Widespread Pain in the ICD-11. The cardinal markers of FM include non-specific musculoskeletal pain, fatigue, chronically disturbed sleep, and mild cognitive dysfunction (1). FM is common, with estimated prevalence in the general population to be between 2 and 10% (2), and a majority of patients being female (3). Significant challenges, however, in the diagnosis and long-term management of the syndrome persist. On paper, since 1990 reaching a diagnosis has relied on the American College of Rheumatology (ACR) criteria, which are regularly updated to better the quantification of the central FM symptoms and comorbidities (4–6). In clinical practice, both poor knowledge of (7) and poor adherence (8) to the ACR criteria has been observed. Instead, in line with newer recommendations, differentiation (9) from symptomatically similar conditions such as such as somatic or rheumatic diseases and a comprehensive review of patient history drive diagnosis (10).
It remains undetermined what causes FM but separate mechanisms have been suggested for the individual symptoms. Chronic pain, for example, has been linked to central sensitization, a physiological process, in which nociceptive input is abnormally amplified in dorsal horn neurons (11). This leads to both allodynia, perception of otherwise innocuous stimuli as painful, and hyperalgesia, the heightened sensitivity to painful stimuli. A more comprehensive explanation of FM is offered by the biopsychosocial framework, which acknowledges the interactive contribution of biological, psychological and social factors to the syndrome (12). Importantly, it proposes that concurrent management of affective distress such as depression is an integral part of managing FM (13). Still, long-term treatment strategies are effectively reduced to management of individual symptoms, guided by patient treatment preferences (14) and thus lack global standardization (15). Central healthcare goal is pain management (16) and it is commonly addressed through pharmacological interventions.
There are several options for pain management through pharmacotherapy in FM. The most common include non-steroidal anti-inflammatory drugs (NSAIDs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs) and gabapentinoids (17). None of these, however, have shown universally beneficial effects in FM patients. NSAIDs, for example, have not been found to be significantly better than the placebos (18). Research on SSRIs and SNRIs, which were deemed promising as they target the typical for FM low serotonin levels, has shown mixed results, with some finding strong evidence for their analgesic efficacy compared to placebo (19), but others failing to find the same (20). Other antidepressants such as tricyclic antidepressants follow a similar pattern with most promising benefits being in terms of sleep quality (21). Opioids, which are not indicated by clinical guidelines but remain common in clinical practice, have been repeatedly rejected in research as a long-term pain management solution due to their lesser effectiveness compared to other medication, but their high incidence of misuse (22). The mixed success rate raises the question if non-specific factors, reflected by the placebo response, have an impact on symptom improvement.
The placebo response is well-established effect across various pharmacological interventions (23). The placebo response is defined as the improvement of patients randomly assigned to the placebo group (24), thus is determined not only by the placebo effect, but also by the natural course of the disease (e.g., spontaneous remission) and statistical artifacts (e.g., regression to the mean) (25). Patients with Chronic Primary Pain (CPP) diagnoses (23), which includes FM, and affective disorders (particularly depression) (26, 27) have been found to be particularly susceptible to placebo (23). Patients with FM have also shown clinically relevant and statistically significant placebo effects (28). However, clinical implications of these findings in the field of FM have only rarely been discussed. A comprehensive assessment of the impact on both pain and concurrent affective distress is needed to reflect the interaction of biopsychological manifestations of FM and the new diagnostic criteria for CPP as stated in the International Classification of Diseases, 11th Edition (ICD-11) (29, 30). Therefore, an updated meta-analysis that takes clinical considerations into account is needed. The main aim of this meta-analysis was to analyze the placebo response in pain, functional disability, and depression in trials examining SSRIs and SNRIs in patients living with FM.
Methods
Search Strategy and Study Selection
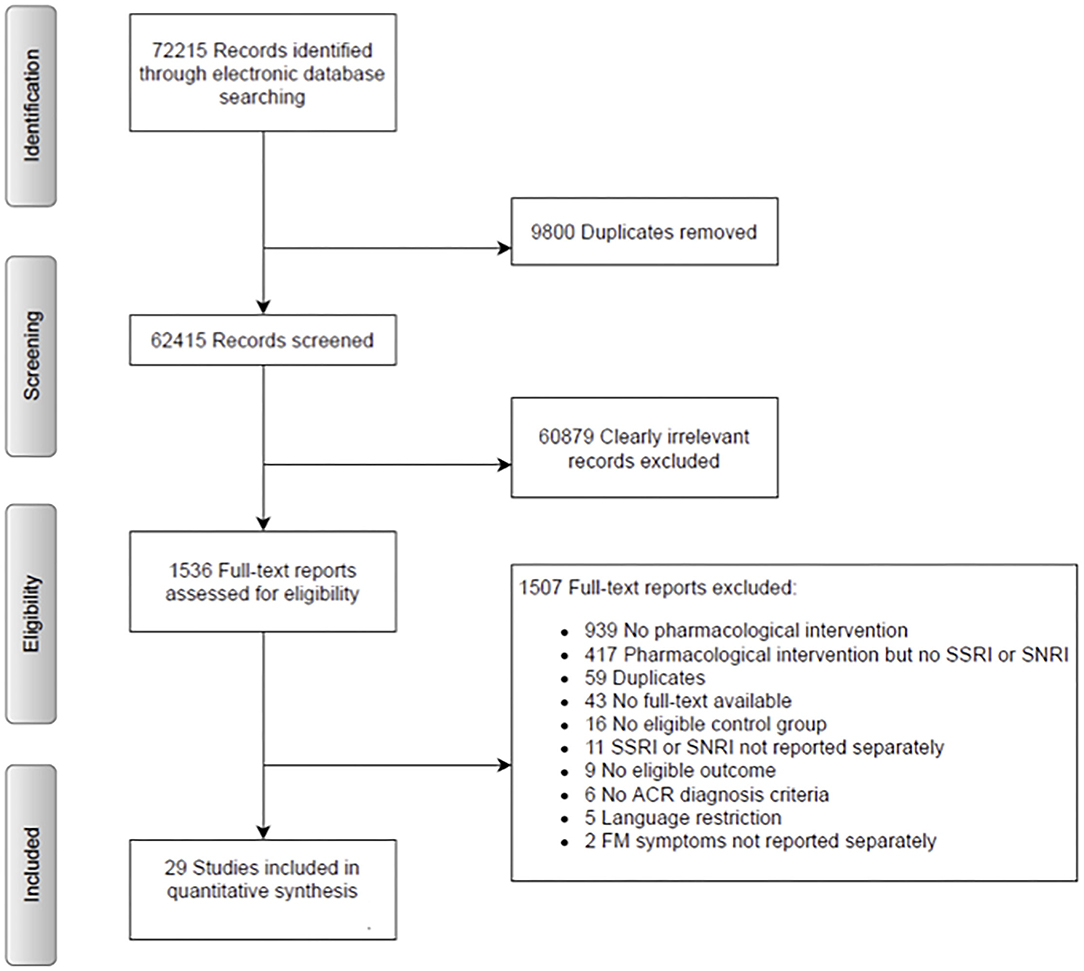
A systematic literature search of RCTs was undertaken in the following electronic databases: MEDLINE, Embase, PsycINFO, Cochrane Central, and Web of Science, without applying restrictions to language or date of publication. A first search was conducted until April 5, 2018 and was updated in October 2019. The search revealed a total of 72215 records. After removing 9,800 duplicates, 62,415 records remained. Note that the search strategy also included all other categories of CPP (i.e., chronic primary musculoskeletal pain, chronic widespread pain, complex regional pain syndrome, chronic primary headache and orofacial pain, and chronic primary visceral pain), as this analysis is part of a larger project (31). See Appendix 1 for the search strategy for the larger project. Within this larger pool of included RCTs, we went through all full-texts and specifically tagged FM papers, which were then included in the presented analysis. We included RCTs that compare an SSRI and/or an SNRI to a placebo control group or another SSRI and/or SNRI in the treatment of FM. Parallel and crossover trials were included. Protocols and conference papers, randomized single control studies, prophylactic interventions, as well as case-control studies, post-hoc analyses or secondary analyses, and results reported solely on clinical trials were excluded. RCTs had to be either in English or German. Patients of both sexes from the age of 18 up, with a primary diagnosis of FM diagnosed by the American College of Rheumatology (ACR) 1990, 2010, or 2016 were included.
Data Assessment
The following information was extracted from all included studies: study characteristics (lead author, publication year, sponsor, country of study conductance, setting, number of clinical sites), participant characteristics (such as diagnostic criteria, duration of diagnosis, age, sex, duration of symptoms, age of onset, comorbidities), study design (type of study such as parallel or crossover design, special population (if 80% or more of the sample share a particular characteristic), special inclusion criteria, special exclusion criteria, emergency medicine, co-intervention), intervention details (such as a description of the intervention by the authors, provider, treatment duration, dose intended, dose delivered, number of randomized people in the treatment arm, timeframe for post [measured at the time point closest to the end of treatment], timeframe for follow-up 1 [at least 3 months/12 weeks but less or equal to 6 months/24 weeks after randomization], timeframe for follow-up 2 [more than 6 months/25 weeks but less or equal to 12 months/52 weeks after randomization]). If several assessments were reported, we chose the one with the longest timeframe since randomization (i.e., FU2 > FU1 > post). For the continuous outcomes, sample sizes (N), means (M), standard deviations (SD), CIs, and changes from baseline were noted for each extracted treatment arm of the respective study. If the study reported different doses of either an SSRI or an SNRI, Ms, SDs, and changes were averaged, and N was merged. If N was reported as a total of all treatment arms, it was divided through the number of treatment arms. Additionally, intention to treat was prioritized over the completer analysis.
As recommended by the Cochrane Handbook for systematic reviews, we always tried to calculate Ms and SDs before imputing them, as imputation methods are based on making assumptions about the trial (32). If a study did not report the mean values numerically, data was extracted from figures using the software DigitizeIt version 2.5 (33). If SDs were not provided, they were calculated from standard errors (SE), N, Ms, and/or p-values. If SDs could not be calculated, the mean of SDs from studies using the same outcome measure was imputed (34).
Primary Outcomes
Global pain intensity and the global measurement of pain were our primary outcomes. We extracted both outcomes where both were reported. Additional primary outcomes were a generic measure of functional disability and depression. For all outcomes, we used a pre-defined hierarchy of validated and standardized measurements. For global pain intensity, the hierarchy was as follows: Visual Analog Scale (VAS) > Fibromyalgia Impact Questionnaire (FIQ) (35) > Numeric Rating Scale (NRS) (36); for the global measurement of pain: Brief Pain Inventory (BPI) (37) > Short-Form McGill Pain Questionnaire (SF-MPQ) (38). For emotional distress, we applied the following hierarchy: Beck Depression Inventory (BDI) (39) > FIQ depression subscale (35) > Patient Health Questionnaire 8 (PHQ-8) (40) > Hamilton Depression Rating Scale (HMD) (41) > Montgomery Åsberg Depression Rating Scale (MADRS) (42). For the generic measures of functional disability, studies applied the BPI (37), the Health Assessment Questionnaire (HAQ) (43), or the FIQ (total score or subscale). If different primary outcomes were given in an individual study, the measurement highest in our hierarchy was extracted. The choice of our primary outcomes is in line with recommendations for clinical trials studying chronic pain (IMMPACT initiative) (44). Furthermore, we decided to focus on self-reported measures. Finally, we intend to prioritize global scores over syndrome-specific scores since the definition of CPMP includes various syndromes (45).
Statistical Analyses
The placebo responses was assessed as the mean change scores of baseline vs. follow-up assessments. A bar chart was created in order to visualize the mean change scores for the placebo group. Analyses were applied within a frequentist framework. We chose to use random-effects models rather than fixed-effects models because the studies that we included were assumed to be heterogenous and the number of included studies was relatively small. Heterogeneity was assessed by calculating the Q statistic (46), the τ2 (47), and the I2 (48). An I2 value of 0% indicates no heterogeneity, a value of 25% is classified as low, 50% as moderate and 75% as high (48).
Results
Study Selection
There were a total of 72,215 identified records for the large project. After removing 9,800 duplicates, 62,415 records were taken into consideration for potential inclusion. For this analysis, 1,536 full texts were screened (see Figure 1). Abstracts and full texts were screened by two independent researchers, consensus was reached in consultation with the first and last author (HK and CL). Finally, 29 RCTs were included in this analysis.
Study Characteristics
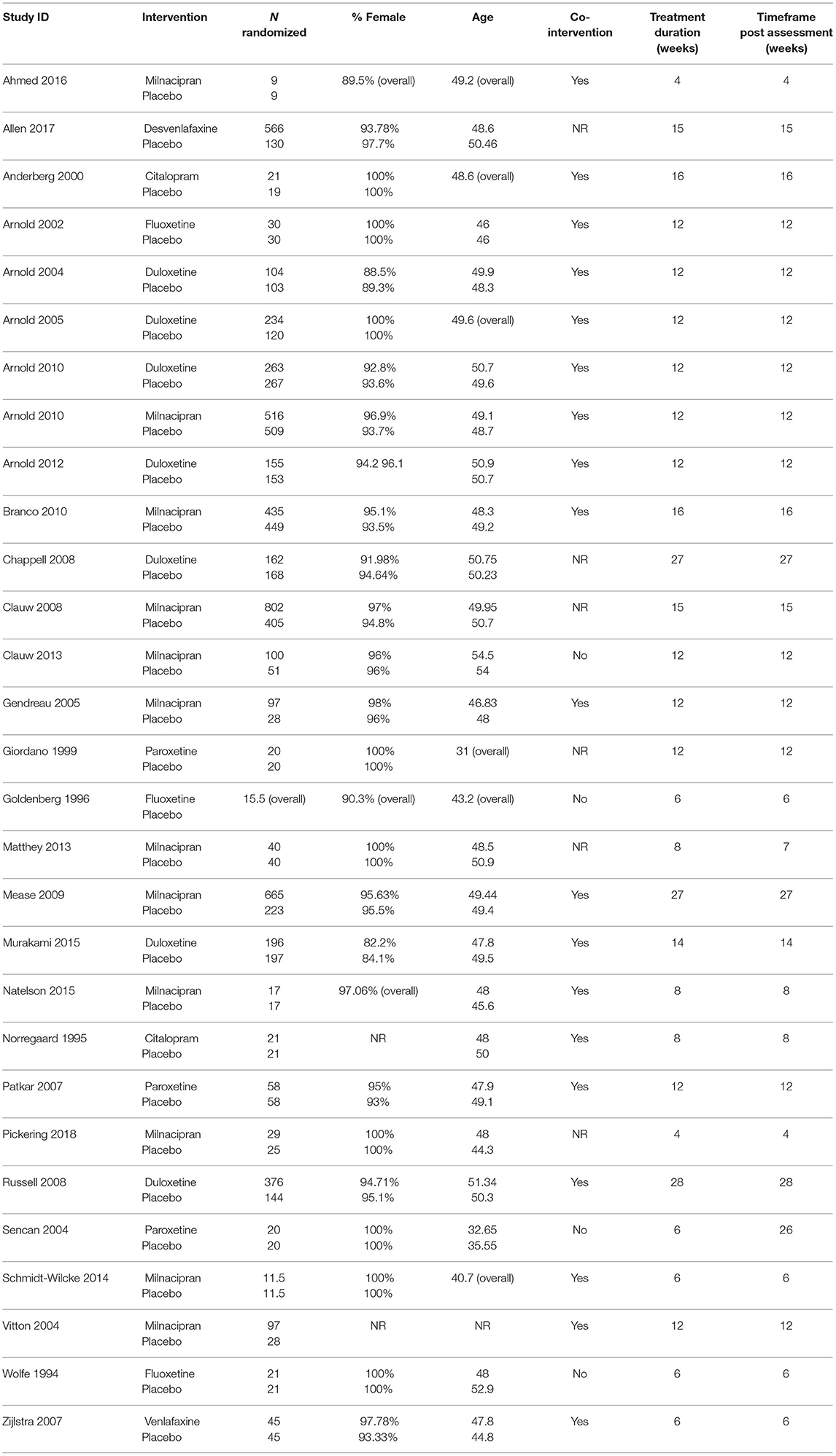
A total of N = 8,453 patients were included in the analysis. RCTs were conducted between 1994 and 2018 and compared seven SSRIs and SNRIs with placebo. No study compared two or more pharmacological interventions. Mean sample size was N = 146 (SD = 184.90). In total, 5,126 (M sample size = 176.76, SD = 220.42) participants were randomly assigned to pharmacological treatments and 3,327 (M sample size = 114.73, SD = 137.92) were randomly assigned to placebo. Weighted mean age was 49.15 years. In those studies that reported sex, 94.40% of patients were female. Seventeen of 29 trials (58.62%) recruited patients from the USA, eight from Europe (27.59%), three recruited patients cross continental (10.34%), and one from Asia (3.45%). On average 21.91% of patients suffered from Major Depressive Disorder (MDD). Mean treatment duration was 12.5 weeks (range 4–28 weeks). More detailed information and individual characteristics of the included studies can be found in Table 1. Table 2 shows individual measurements including mean, mean change, and standard deviation for all outcomes across studies.
Within-Placebo Mean Change Scores of Baseline vs. Follow-Up Assessments
The mean change score for pain reduction in the placebo group was large and statistically significant (mean change score of baseline vs. follow-up = 2.31, 95% CI: 0.42 to 4.21, p = 0.017; see Figure 2). Heterogeneity was large with τ2 = 25.49, I2 = 99.9%, and Q = 23,728.42 (p < 0.000).
For functional disability, the mean reduction was large and statistically significant with a mean change score of baseline vs. follow-up = 3.31, 95% CI: 2.37–4.26, p < 0.0001 (see Figure 2). Heterogeneity was found to be large with τ2 = 4.02, I2 = 99.4%, and Q = 2,561.9 (p < 0.000).
Finally, for depression, the mean change score of baseline vs. follow-up assessment was large and statistically significant again, with a mean change = 1.55, 95% CI: 0.92–2.18, p < 0.0001 (see Figure 2). Heterogeneity was large with τ2 = 1.32, I2 = 87.7%, and Q = 146.38 (p < 0.0001).
Discussion
The present meta-analysis intended to examine the placebo response in the baseline vs. follow-up comparison in pain, functional disability, and depression in trials examining SSRIs and SNRIs in patients with FM. In total, 29 RCTs were included with a mean treatment duration of 12.5 weeks, which is longer than in previous meta-analyses on antidepressants for FMS (19). We found large and statistically significant within-placebo mean change scores of baseline vs. follow-up assessments.
Notably, the placebo response was highest for the outcome functional disability with a mean change score of baseline vs. follow-up = 3.31 (95% CI: 2.37–4.26, p < 0.0001). In the included studies, functional disability measures assessed the impact of FM on a broad range of activities: from mundane everyday tasks, such as self-care and mobility, to general well-being, and engagement in vocational tasks. It has been long-recognized that FM can disrupt these common actions and thus considerably disable patients (49). For many patients maintaining work ability is a primary health concern (50). This is understandable as up to 46% of patients point to their FM as the reason for losing their jobs (51). However, no single treatment option has been established as best to address all the challenges encompassed by functional disability.
Our results indicate that not only a change in pain intensity is possible, but also in other important domains, namely functional disability and depression. These results support the claim that, in many cases, chronic primary pain disorders require a multidisciplinary treatment approach, also referring to the biopsychosocial framework (52–54). Given that placebo responses consists of non-specific effects (besides statistical artifacts and the natural course of the disease), and FM presents as a complex condition, a single-component treatment such as SSRIs and SNRIs falls short (55). From a patients' perspective, however, a reduction in pain intensity is frequently declared to be the most desired treatment outcome (56). Importantly, improvements in different outcome domains do not necessarily correlate with each other, as has been shown in a study that analyzed within-treatment trajectories of patients with chronic pain (57).
Our findings reveal preliminary suggestions for clinical implications. Considering the large placebo response on the different outcome domains, the question arises how these effects can be harnessed in clinical practice. First of all, it is important to clearly define what a placebo is. In research, placebos in randomized controlled trials are used to control for confounders associated with clinical trials, such as spontaneous remission and regression toward the mean (58). In clinical practice, however, placebos can be utilized to enhance positive outcomes by means of well-known placebo mechanisms. These include positive treatment expectations, a patient-physician relationship that is built on trust, and a plausible treatment narrative (59). With the aim to actively harness these mechanisms, the following suggestions might be taken into account when treating patients living with FM: (1) to address key ethical principles such as autonomy and transparency during the administration of SSRIs and SNRIs, i.e., by talking about the empirical evidence for the intervention, including placebo responses and their underlying processes (60); (2) to foster a patient-physician relationship that is based on trust, i.e., by ensuring that patients feel understood and cared for (61); and (3) to address and discuss patients' expectations, i.e., by asking what they expect about the treatment, what wishes and fears are associated with the prospect of receiving SSRIs and SNRIs (62).
Two additional approaches that have been studied in the past and enable to harness placebo effects in the clinical practice are the following: First, placebos could be used as dose extenders. By pairing placebo pills with a physiologically active drug, studies have revealed that medication dosages can be substantially lowered without decreasing the efficacy of the drug (63, 64). A second strategy is known as open-label placebo administration, i.e., the placebo treatment with full disclosure. Open-label placebos are administered with a scientific rationale, i.e., patients are told that ‘we know that placebos have powerful effects' (65). Two meta-analyses reveal that the open-label placebo therapy shows statistically significant and clinically meaningful effects in pain and non-pain conditions (66, 67).
Our analysis has several limitations. First and foremost, within-group analyses have limited validity (68): Mean change scores of baseline vs. follow-up assessments are not independent of each other, since baseline and follow-up scores are correlated. Furthermore, they are affected the natural course and characteristics of the patients and settings, and these cannot be disentangled from the effects of the intervention. However, we were especially interested to research preliminary indication for the potential of placebo in this population and to focus on first recommendations for the clinical routine. Second, since included studies span more than two decades, it cannot be ruled out that a change in the diagnostic criteria over time may have influenced the findings. Third, due to small sample sizes in some SSRI/SNRI treatments, these results might be statistically underpowered. Therefore, some effects might be due to the so-called small-study effect. This means that smaller trials show different, sometimes larger, treatment effects than bigger studies (69). Fourth, treatment duration of included interventions varied largely between 4 and 28 weeks. The optimal duration of treatment therefore remains unclear, and the short duration of several studies leads to open questions with regard to long-term beneficial effects of SSRI/SNRI treatments on FMS symptoms. In a similar fashion, the time points for follow-up assessments varied, which might have contributed to heterogeneity in our results. Finally, the systematic literature search was conducted 2 years ago, hence we cannot rule out that the inclusion of newer studies would have changed the results of our analyses.
Future studies should have an in-depth examination of the placebo response by using individual patient data instead of aggregate data. This would allow to determine patient-related and trial-related placebo moderators and would therefore be in line with the personalized medicine approach (70). This is also strengthened by our data that showed substantial heterogeneity across outcomes. Furthermore, and in order to disentangle placebo effects from the natural course and statistical artifacts, it would be advantageable to compare a placebo arm with a no-treatment arm in SSRI and SNRI trials (25).
In conclusion, our results provide preliminary evidence that placebo responses, which also consist of non-specific effects, might play a role in the treatment of FM.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Author Contributions
HK and CL initiated the study concept, conducted the analyses, and drafted the paper. HK, CL, and AK designed the extraction template. TP and JP extracted the data. HK, CL, TP, JP, AK, and SB wrote the final paper, critically revised the manuscript, and gave important intellectual contribution to it. All authors have read and approved the manuscript.
Funding
CL received funding from the Swiss National Science Foundation (SNSF): P4P4PS_194536. HK is sponsored by the Swiss National Science Foundation (SNSF) fellowship P400PS_186658. AK received funding from the EPIC (eHealth Productivity and Innovation in Cornwall and the Isles of Scilly) project, which was in part funded by the University of Plymouth and the European Regional Development Fund (ERDF), Grant Number [05R18P02814]. The funding bodies have no role in the design of the study and collection, analysis, and interpretation of data and manuscript writing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2021.750523/full#supplementary-material
References
2. Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The prevalence and characteristics of Fibromyalgia in the 2012 National Health Interview Survey. PLoS ONE. (2015) 10:e0138024. doi: 10.1371/journal.pone.0138024
3. Heidari F, Afshari M, Moosazadeh M. Prevalence of Fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int. (2017) 37:1527–39. doi: 10.1007/s00296-017-3725-2
4. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. (1990) 33:160s Rheumultice1002/art.1780330203
5. Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. (2010) 62:600n)Care Resleg1002/acr.20140
6. Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RL, et al. Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. (2016) 46:319thritis Rheum1016/j.semarthrit.2016.08.012
7. Perrot S, Choy E, Petersel D, Ginovker A, Kramer E. Survey of physician experiences and perceptions about the diagnosis and treatment of fibromyalgia. BMC Health Serv Res. (2012) 12:356. doi: 10.1186/1472-6963-12-356
8. Kumbhare D, Ahmed S, Sander T, Grosman-Rimon L, Srbely J. A survey of physicians' knowledge and adherence to the diagnostic criteria for fibromyalgia. Pain Med. (2018) 19:1254–64. doi: 10.1093/pm/pnx271
9. Heymann RE, Paiva ES, Martinez JE, Helfenstein M, Rezende MC, Provenza JR, et al. New guidelines for the diagnosis of fibromyalgia. Rev Bras Reumatol. (2017) 57:s46757:7as Reumat1016/j.rbre.2017.07.002
10. Silverman SL, Harnett J, Zlateva G, Mardekian J. Identifying fibromyalgia-associated symptoms and conditions from a clinical perspective: a step toward evaluating healthcare resource utilization in fibromyalgia. Pain Pract. (2010) 10:520ctSL, Harnet1111/j.1533-2500.2010.00383.x
11. Nielsen LA, Henriksson KG. Pathophysiological mechanisms in chronic musculoskeletal pain (fibromyalgia): the role of central and peripheral sensitization and pain disinhibition. Best Pract Res Clin Rheumatol. (2007) 21:465ct Res Clin R1016/j.berh.2007.03.007
12. Adams LM, Turk DC. Central sensitization and the biopsychosocial approach to understanding pain. J Appl Biobehav Res. (2018) 23:e12125. doi: 10.1111/jabr.12125
13. Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom Med. (2004) 66:837m Medo somati1097/01.psy.0000146329.63158.40
14. Valentini E, Fetter E, Orbell S. Treatment preferences in Fibromyalgia patients: a cross-sectional web-based survey. Eur J Pain. (2020) 24:1290–290:0Pained s1002/ejp.1570
15. Kia S, Choy E. Update on treatment guideline in Fibromyalgia syndrome with focus on pharmacology. Biomedicines. (2017) 5:20. doi: 10.3390/biomedicines5020020
16. Pastor-Mira M-A, López-Roig S, Martínez-Zaragoza F, León E, Abad E, Lledó A, et al. Goal preferences, affect, activity patterns and health outcomes in women with Fibromyalgia. Front Psychol. (2019) 10:1912. doi: 10.3389/fpsyg.2019.01912
17. Okifuji A, Gao J, Bokat C, Hare BD. Management of Fibromyalgia syndrome in 2016. Pain Manag. (2016) 6:383–400. doi: 10.2217/pmt-2016-0006
18. Derry S, Wiffen PJ, Häuser W, Mücke M, Tölle TR, Bell RF, et al. Oral nonsteroidal anti-inflammatory drugs for Fibromyalgia in adults. Cochrane Database Syst Rev. (2017) 3:CD012332 doi: 10.1002/14651858.CD012332
19. H2332r W, Bernardy K ilable from: https://www.cochranelibrary.com/cdsr/doi/10. with Fibromyalgia.: a meta-analysis. JAMA. (2009) 301:198. doi: 10.1001/jama.2008.944
20. Walitt B, Urrútia G, Nishishinya MB, Cantrell SE, Häuser W. Selective serotonin reuptake inhibitors for Fibromyalgia syndrome. Cochrane Database Syst Rev. (2015) 2015:CD011735. doi: 10.1002/14651858.CD011735
21. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with Fibromyalgia. Pain Med. (2007) 8:S63–74. doi: 10.1111/j.1526-4637.2006.00178.x
22. Goldenberg DL, Clauw DJ, Palmer RE, Clair AG. Opioid use in fibromyalgia: a cautionary tale. Mayo Clin Proc. (2016) 91:640–8. doi: 10.1016/j.mayocp.2016.02.002
23. Vase L, Skyt I, Laue Petersen G, Price DD. Placebo and nocebo effects in chronic pain patients. Zeitschrift für Psychol. (2014) 222:135–9. doi: 10.1027/2151-2604/a000181
24. Gomeni R, Merlo-Pich E. Bayesian modelling and ROC analysis to predict placebo responders using clinical score measured in the initial weeks of treatment in depression trials. Br J Clin Pharmacol. (2007) 63:595n Pharmacolpre1111/j.1365-2125.2006.02815.x
25. Häuser W, Bartram-Wunn E, Bartram C, Reinecke H, TOC analysis to predict pl: placebo response in drug trials of Fibromyalgia syndrome and painful peripheral diabetic neuropathying clinical score measured in the initial Pain. (2011) 152:1709–7092:nse doi: 10.1016/j.pain.2011.01.050
26. Li F, Nasir M, Olten B, Bloch MH. Meta-analysis of placebo response in adult antidepressant trials. CNS Drugs. (2019) 33:971sof placebo r1007/s40263-019-00662-y
27. Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. JAMA Psychiatry. (2017) 74:1011–20. doi: 10.1001/jamapsychiatry.2017.2432
28. Chen X, Zou K, Abdullah N, Whiteside N, Sarmanova A, Doherty M, et al. The placebo effect and its determinants in Fibromyalgia: Meta-analysis of randomised controlled trials. Clin Rheumatol. (2017) 36:1623–30. doi: 10.1007/s10067-017-3595-8
29. Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. (2015) 156:1003–0036:assifi1097/j.pain.0000000000000160
30. Barke A, Koechlin H, Korwisi B, Locher C. Emotional distress: specifying a neglected part of chronic pain. Eur J Pain. (2020) 24:477fed part of c1002/ejp.1525
31. Koechlin H, Whalley B, Welton NJ, Locher C. The best treatment option(s) for adult and elderly patients with chronic primary musculoskeletal pain: a protocol for a systematic review and network meta-analysis. Syst Rev. (2019) 8:269. doi: 10.1186/s13643-019-1174-6
32. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. (2020). 726 p. USA: John Wiley & Sons. doi: 10.1002/9781119536604
33. !!!DigitizeIt—Plot Digitizer Software. Digitize Graphs, Charts and Math Data. I. Bormann (editor) (2021). Available online at: https://www.digitizeit.xyz/ (accessed July 26, 2021).
34. Higgins JPT, Green S, (editors),. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available online at: www.handbook.cochrane.org
35. Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. (1991) 18:728t33.
36. von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for childrenelopment and vs of pain intensity. Pain. (2009) 143:223ets supporti1016/j.pain.2009.03.002
37. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. (1994) 23:129.
38. Med Melzack R. The short-form McGill pain questionnaire. Pain. (1987) 30:191–7. doi: 10.1016/0304-3959(87)91074-8
39. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. (1961) 4:561n Psychiatrye1001/archpsyc.1961.01710120031004
40. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606tern Med. RL1046/j.1525-1497.2001.016009606.x
41. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56l Neurosurg P1136/jnnp.23.1.56
42. Montgomery SA, Asberg M A. new depression scale designed to be sensitive to change. Br J Psychiatry. (1979) 134:382hiatryAsberg1192/bjp.134.4.382
43. Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ). Clin Exp Rheumatol. (2005) 23:S14–18.
44. Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. (2005) 113:9recommendatio1016/j.pain.2004.09.012
45. Melidis C, Denham SL, Hyland ME. A test of the adaptive network explanation of functional disorders using a machine learning analysis of symptoms. BioSystems. (2018) 165:22–30. doi: 10.1016/j.biosystems.2017.12.010
46. Cochran WG. The comparison of percentages in matched samples. Biometrika. (1950) 37:256ka. The compa1093/biomet/37.3-4.256
47. Higgins JPT. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. (2008) 37:1158–60. doi: 10.1093/ije/dyn204
48. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
49. Grodman I, Buskila D, Arnson Y, Altaman A, Amital D, Amital H. Understanding Fibromyalgia and its resultant disability. Isr Med Assoc J. (2011) 13:769.
50. Annie P, Kaisa M. Work ability in Fibromyalgia: an update in the 21st century. Curr Rheumatol Rev. (2017) 13:180umatol RevWo2174/1573397113666170502152955
51. Al-Allaf AW. Work disability and health system utilization in patients with Fibromyalgia syndrome. Clin Rheumatol. (2007) 13:199umatolork disa1097/RHU.0b013e31812e6b0c
52. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. (2007) 133:581ullvances and 1037/0033-2909.133.4.581
53. Hechler T, Dobe M, Zernikow B. Commentary: a worldwide call for multimodal inpatient treatment for children and adolescents suffering from chronic pain and pain-related disability. J Pediatr Psychol. (2010) 35:138–40. doi: 10.1093/jpepsy/jsp066
54. Miller-Matero LR, Saulino C, Clark S, Bugenski M, Eshelman A, Eisenstein D. When treating the pain is not enough: a multidisciplinary approach for chronic pelvic pain. Archives of Women's Mental Health. (2016) 19:349 of Women's M1007/s00737-015-0537-9
55. Rutherford BR, Roose SP. A model of placebo response in antidepressant clinical trials. AJP. (2013) 170:723–33. doi: 10.1176/appi.ajp.2012.12040474
56. Goudman L, De Smedt A, Linderoth B, Eldabe S, Witkam R, Henssen D, et al. Identifying goals in patients with chronic pain: a European survey. Eur J Pain. (2021) 25:1959–959:1 ofsurv1002/ejp.1814
57. Vowles KE, Witkiewitz K, Levell J, Sowden G, Ashworth J. Are reductions in pain intensity and pain-related distress necessary? An analysis of within-treatment change trajectories in relation to improved functioning following interdisciplinary acceptance and commitment therapy for adults with chronic pain. J Consult Clin Psychol. (2017) 85:87lfis of withi1037/ccp0000159
58. Locher C, Gaab J, Blease C. When a placebo is not a placebo: problems and solutions to the gold standard in psychotherapy research. Front Psychol. (2018) 0:2317. doi: 10.3389/fpsyg.2018.02317
59. Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. (2010) 375:686G, Kaptchuk T1016/S0140–6736(09)61706-2
60. Trachsel M, Gaab J. Disclosure of incidental constituents of psychotherapy as a moral obligation for psychiatrists and psychotherapists. J Med Ethics. (2016) 42:493–5. doi: 10.1136/medethics-2015-102986
61. Bystad M, Wynn R, Bystad C. How can placebo effects best be applied in clinical practice? A narrative review. PRBM. (2015) 8:41–5. doi: 10.2147/PRBM.S75670
62. McKay KM, Imel ZE, Wampold BE. Psychiatrist effects in the psychopharmacological treatment of depression. J Affect Disord. (2006) 92:287–90. doi: 10.1016/j.jad.2006.01.020
63. Perlis M, Grandner M, Zee J, Bremer E, Whinnery J, Barilla H, et al. Durability of treatment response to Zolpidem with three different maintenance regimens: a preliminary study. Sleep Med. (2015) 16:1160–8. doi: 10.1016/j.sleep.2015.06.015
64. Sandler AD, Glesne CE, Bodfish JW. Conditioned placebo dose reduction: a new treatment in attention-deficit hyperactivity disorder? J Dev Behav Pediatr. (2010) 31:369hav Pediatrte1097/DBP.0b013e3181e121ed
65. Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, Kirsch I. Open-label placebo treatmentvadjust in chronic low back pain: a randomized controlled trial. Pain. (2016) 157:2766–7667:ized co1097/j.pain.0000000000000700
66. Charlesworth JEG, Petkovic G, Kelley JM, Hunter M, Onakpoya I, Roberts N, et al. Effects of placebos without deception compared with no treatment: a systematic review and meta-analysis. J Evid Based Med. (2017) 10:97Based Medw and1111/jebm.12251
67. von Wernsdorff M, Loef M, Tuschen-Caffier B, Schmidt S. Effects of open-label placebos in clinical trials: a systematic review and meta-analysis. Sci Rep. (2021) 11:3855. doi: 10.1038/s41598-021-83148-6
68. Cuijpers P, Weitz E, Cristea IA, Twisk J. Pre-post effect sizes should be avoided in meta-analyses. Epidemiol Psychiatr Sci. (2017) 26:364l Psychiatr 1017/S2045796016000809
69. Ioannidis JPA, Munafo MR, Fusar-Poli P, Nosek BA, David SP. Publication and other reporting biases in cognitive sciences: detection, prevalence, and prevention. Trends Cogn Sci. (2014) 18:235–41. doi: 10.1016/j.tics.2014.02.010
Keywords: placebo, fibromyalgia, antidepressants, SSRIs, SNRIs, meta-analysis
Citation: Koechlin H, Kharko A, Probst T, Pradela J, Buechi S and Locher C (2021) Placebo Responses and Their Clinical Implications in Fibromyalgia: A Meta-Analysis Using SSRI and SNRI Trials. Front. Pain Res. 2:750523. doi: 10.3389/fpain.2021.750523
Received: 30 July 2021; Accepted: 11 November 2021;
Published: 07 December 2021.
Edited by:
Lene Vase, Aarhus University, DenmarkReviewed by:
Robert Gyula Almasi, University of Pécs, HungaryIrving Kirsch, Harvard Medical School, United States
Copyright © 2021 Koechlin, Kharko, Probst, Pradela, Buechi and Locher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cosima Locher, Y29zaW1hYW50b2luZXR0ZS5sb2NoZXJAdXpoLmNo
 Helen Koechlin
Helen Koechlin Anna Kharko
Anna Kharko Tamara Probst1
Tamara Probst1 Cosima Locher
Cosima Locher