
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pain Res. , 16 November 2021
Sec. Pain Mechanisms
Volume 2 - 2021 | https://doi.org/10.3389/fpain.2021.737961
This article is part of the Research Topic Highlights in Pain Mechanisms 2021/22 View all 9 articles
 Jude P. J. Savarraj1*
Jude P. J. Savarraj1* Angela B. Burkett1
Angela B. Burkett1 Sarah N. Hinds1
Sarah N. Hinds1 Atzhiry S. Paz1
Atzhiry S. Paz1 Andres Assing1
Andres Assing1 Shivanki Juneja2
Shivanki Juneja2 Gabriela D. Colpo2
Gabriela D. Colpo2 Luis F. Torres1
Luis F. Torres1 Sung-Min Cho3
Sung-Min Cho3 Aaron M. Gusdon1
Aaron M. Gusdon1 Louise D. McCullough2
Louise D. McCullough2 H. Alex Choi1*
H. Alex Choi1*COVID-19 is an ongoing pandemic with a devastating impact on public health. Acute neurological symptoms have been reported after a COVID-19 diagnosis, however, the long-term neurological symptoms including pain is not well established. Using a prospective registry of hospitalized COVID-19 patients, we assessed pain and neurological function (including functional, cognitive and psychiatric assessments) of several hospitalized patients at 3 months. Our main finding is that 60% of the patients report pain symptoms. 71% of the patients still experienced neurological symptoms at 3 months and the most common symptoms being fatigue (42%) and PTSD (25%). Cognitive symptoms were found in 12%. Our preliminary findings suggests the importance of investigating long-term outcomes and rationalizes the need for further studies investigating the neurologic outcomes and symptoms of pain after COVID-19.
To date over 75 million people have been infected with the severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2), which causes the coronavirus disease 2019 (COVID-19). While the vast majority will survive, many may be left with residual effects. One of the earliest studies reported acute neurological manifestations in hospitalized patients with COVID-19 that include encephalitis, acute myopathic quadriplegia, strokes and seizure (1). Some neurologic symptoms that these hospitalized patients experienced include headache, impaired consciousness, taste and smell impairment (2). Anecdotal reports of long-term neurologic symptoms are emerging as well (3). These reports have emphasized the importance of studying long-term neurologic outcomes, also referred as the “Long-Haul COVID” (4). Long-haul COVID (or post-COVID conditions) is defined by the Center for Disease Control as a wide range of new conditions or returning ones, or ongoing health problems that can persist for more than 4 weeks after the COVID-19 infection (5). Short- and long-term neurologic symptoms were reported during the SARS and MERS outbreaks (6) and it is increasingly evident that long-term symptoms will persist after COVID-19 as well (7). To characterize long-term neurologic outcomes after COVID-19 we followed a cohort of hospitalized patients and assessed 3 months outcomes.
This is a prospective study of COVID-19 patients admitted and hospitalized at the Memorial Herman Hospital System in Houston, Texas, USA. Inclusion criteria were laboratory-confirmed SARS-CoV-2 infection by real-time polymerase chain reaction, written informed consent from the patient or surrogate, and age ≥ 18 years. Exclusion criteria were inability to complete long-term follow-up, severe functional disabilities before hospital admission for COVID-19 [defined by pre-admission modified Rankin Score (mRS) >1] (8), history of pulmonary complications (including resection and transplant), pre-existing systemic diseases which would impact long term outcomes (including stroke, myocardial infarction, pulmonary disease requiring home oxygen, chronic renal failure necessitating hemodialysis and malignancy), documented neurologic and psychiatric disorders, prisoners and pregnant women. Patients were categorized as mild (nasal cannula with <5 liters of O2 and <5 days of hospitalization), moderate (nasal cannula with >5 liters of O2 or heat high flow cannula and > 5 days of hospitalization) and severe (on ventilator or expired). This study was approved by the “Institutional Review Board” (IRB No: HSC –MH-17-0452) at The University of Texas Health Science Center at Houston, Houston, Texas.
We assessed the functional, cognitive and psychiatric symptoms at 3 months after hospitalization. Pain, fatigue and sleepiness were evaluated using the Pain, Enjoyment of life and General activity (PEG) (9), the Fatigue Severity Scale (FSS) (10) and Epworth Sleepiness Scale (ESS) (11). Post-traumatic stress disorder was evaluated using the Primary Care PTSD Screen for DSM-5 (PC-PTSD-5) (12). Functional outcome was evaluated using the modified Rankin Score (mRS) (13). Cognitive status was evaluated using the brief neurocognitive screening test (BNST). Depression symptoms were evaluated using the Patient Health Questionnaire (PHQ-9) (14). Anxiety symptoms were assessed using the Generalized Anxiety Disorder (GAD-7). The battery of assessments, the range of the values, the cutoff and the interpretation are listed in Table 1. The primary rationale for the selection of the assessments include the ease of administering them over the phone and the limited time required to get the results. The entire battery of tests typically takes about 30–45 min to administer over the phone by a trained research personnel.
Descriptive statistics were calculated for demographic variables in COVID-19 subjects. To describe differences in demographics, χ2-test, Fisher's exact test, student's t-test, and the Mann-Whitney U test were used where appropriate. The Mann-Whitney U test was used to test for significance across different groups A p-value of ≤ 0.05 was considered statistically significant (two-tailed). All statistical analyses were performed using open-source software packages in R (v3.1.3).
One Hundred and forty Subjects Were Enrolled. 55 Were Lost to Follow-up and 27 Were Dead. 3 Month Outcomes Were Determined in 58 Subjects Using Telephone Questionnaires. The Average age of the Patients Were 49.2 ± 16 and 46% of the Subjects Were Female. 72% Were Hispanic-Reflective of the Large Hispanic Population at Houston. A Majority of the Patients Has co-Morbidities With 53% Being Obese, 38% With Diabetes and 46% With a History of Hypertension. The COVID-19 Symptoms of 44% of the patients were considered severe.
71% had continued neurologic symptoms highlighting the importance of considering the long-haul COVID phenomena. The most common symptom was pain (60%) followed by fatigue (41%) and PTSD symptoms (25%) (Table 2). There were no significant associations between PTSD and, ethnicity and race (p>0.05). People with long-term neurological symptoms were significantly older [mean, years (SD): 54 (16) vs. 41 (16); p = 0.01]. The persistence of long-term symptoms was not associated with the severity of acute COVID-19 symptoms. Neither the maximum C-reactive protein levels [(137 (73) vs. 153 (92); p = 0.59] nor the clinical severity [WHO ≤ 4 vs. WHO > 5, p=0.58] were associated with 3 month symptoms. In fact, we found that even subjects with mild course of hospitalization had a high incidence of symptoms, especially fatigue (58%).
In our cohort of hospitalized COVID-19 survivors, 60% of the subjects reported persistent pain symptoms. The most common location of pains were back, chest and head and 31% reported pain in two or more locations (Table 3). People who reported headache also reported the highest PEG score [median (IQR): 7.6(5–8.3)] compared to others (Table 3). Next, we compared the clinical characteristics and the assessments across subjects who did and did not report pain (Table 4). Interestingly, subjects who reported pain symptoms were significantly younger than those who did not report pain (mean ± SD: 45 ± 16 vs. 53 ± 11, p < 0.05). Women were also more likely to report pain symptoms-though this relationship was not statistically significant (p = 0.08). There were no differences in ethnicity and past medical history (including obesity, diabetes and smoking status) across the pain and no-pain subjects. Subjects who reported pain were also likely to experience other neurological and psychiatric symptoms at 3 months. Patients with pain were twice as likely to report symptoms of fatigue (53 vs. 23%, p < 0.05) and were seven times likely to be assessed with PTSD symptoms (37 vs. 4.7%, p < 0.01) than those who reported no pain. There was a strong and significant and positive correlation between pain and PTSD symptoms (r = 0.6, p < 0.01, Figure 1A). Anxiety and depression symptoms were positively and significantly correlated with pain symptoms (r = 0.59, p < 0.01 and r = 0.63, p < 0.01 respectively, Figures 1B,C). The pain subjects were also likely to have poor neurological outcomes and interestingly all patients who reported no pain had good neurological outcomes at 3 months. Subjects who reported pain were also more likely to report sleepiness, cognitive deficits, and, depression and anxiety symptoms, though these associations were not statistically significant.
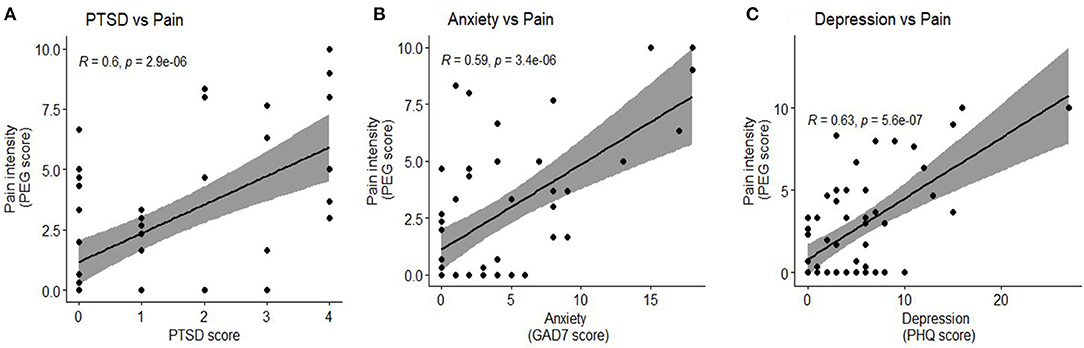
Figure 1. Association of Pain with PTSD, anxiety and depression. (A) Pain intensity had a significant positive correlation with PTSD score (r = 0.6, p < 0.01) (B) Pain intensity had a significant positive correlation with anxiety, quantified by the GAD7 score (r = 0.59, p < 0.01) (C) Pain intensity had a significant positive correlation with depression, quantified by the PHQ score (r = 0.63, p < 0.01).
It is know that human coronaviruses (HCoVs) are neuro-invasive and neurotropic and have been shown to contribute to both short- and long-term neurological symptoms (6, 16, 17). Early studies have reported acute neurologic and psychiatric complications at COVID-19 hospitalization and these symptoms (18) include ischemic stroke, intracerebral hemorrhage, hallucinations, encephalopathy, anosmia and ageusia (19–24). Autopsy cases after COVID-19 (2) have shown brain injury after death. However, studies are underway to investigate the whether there are persistent long-term symptoms.
We undertook a study to investigate persistent symptoms at 3 months after COVID-19 hospitalization. Our main finding is that 60% of the patients experienced pain at 3 months after hospitalization. 71% of the patients still experienced neurological symptoms at 3 months and the most common symptoms being pain (60%), fatigue (41%) and PTSD (25%). The symptoms of pain were also associated with other symptoms like PTSD, anxiety and depression (Figure 1, Table 4) suggesting a relationship between these symptoms in recovering subjects. This relationship is consistent with reports in other conditions as well (25, 26) and also in survivors of the SARS pandemic of 2005 (27). Cognitive symptoms were found in 11.7% of the subjects. Our findings support the anecdotal reports of long-term symptoms even in those with mild acute pulmonary symptoms. The prevalence of cognitive symptoms (11.7%) were relatively lower than generalized symptoms. However, 11.7% of survivors represents a large number of people altogether, and gives credence to the reports of “brain fog” that survivors have experienced. Although symptoms of pain were frequent (60%), there is no standardized cut off for pain measurements so was not included in our analysis of combined neurologic symptoms. In those subjects who described pain, the PEG score was 4.42 ± 2.8 (mean ± SD), a score similar to ones reported in other diseases in which pain is prevalent after hospital discharge (28).
Interestingly, subjects who reported pain at 3 months were younger and tend to be female compared to those subjects who did not report pain. This demographic trend i.e., younger female and pain has been previously reported in fibromyalgia (29). Additionally, the subjects who reported pain in our cohort were also likely to be screened with psychiatric and neurologic comorbidities-a relationship that has been observed in fibromyalgia as well (29). Other studies in fibromyalgia have shown that women are at a greater risk at developing pain and that this sex dependent disparity could be due to factors including hormonal influences and gender expectations (30). Besides the incidence of pain being high in women compared to men, among those who reported pain, the degree of reported pain was also higher in women compared to men (4.63 ± 2.7 vs. 4.16 ± 2.94, p = 0.08, not shown in table). It is known that COVID-19 disproportionately affects men compared to women and some studies have shown that while the prevalence of infection is the same in men and women, men are more likely to be hospitalized and have a severe course of disease and death with a case fatality rate of 1.5–1.8 times that of women (31, 32). While gender-related behaviors such as smoking, drinking, the propensity to seek hospital care and presence of co-morbidities could affect the outcome of COVID-19, the presence of innate biological risk determinants could impact the outcomes as (33, 34). A number of reasons have been postulated for the differential response across sex to COVID-19. Differences in hormone levels and the immune response may underlie the differential effects by sex of infection (31). Sex differences are well-known in innate and adaptive immunity with resulting sex-specific responses to vaccines and infections (33). The fluctuation of sex steroid hormones that occurs over the life span also contributes to different disease susceptibility at different ages and to changes in immune profiles. In fact, the ACE2 receptor, a main SARS-CoV-2 (the virus that induces COVID-19) viral entry receptor, is X chromosome encoded, and is downregulated by estrogens (35). Higher levels of innate immune cytokines were associated with worse disease progression in women, but not in men. These early studies on sex differences in immunity to SARS-CoV-2 demonstrate that the immune landscape is different in males and females.
While hormonal differences and early immune response to COVID-19 between men and women can plausibly account for the acute disease severity and long-term outcomes, the underlying mechanisms of the higher incidence and degree of pain in women compared to men is unclear and warrants a detailed investigation.
The battery of questionnaires used in the study, while useful in screening patients with probable risk, are not conclusive in determining the status of the subject. Detailed and thorough in-person assessments are required to conclusively ascertain whether a clinical diagnosis of the condition is present. One aspect that governed the choice of the chosen assessments in the relative ease in which they can be administered–over the phone with relatively less training for the personnel who administer the assessments. Since COVID-19 is a relatively new disease, it not known a priori what symptoms that recovering subjects are experiencing. Therefore a broad range of assessments–pain, cognitive, functional, QoL and psychiatric–were administered. Additionally, these assessments take about 30–45 min to administer over the phone. We limited the assessment period to this time window (30–45 min) as we observed that some subjects were experienced “respondent fatigue” when the assessments took longer than that.
The findings of this study is limited to a cohort of hospitalized patient and results cannot be extrapolated to patients who experienced milder forms of COVID-19 that did not require hospitalization. Hospitalized Covid-19 patients, besides a higher degree of disease severity, are afflicted with a host of secondary complications including organ damage, invasive procedures and complications due to increased hospitalization. The disease pathophysiology is different from that of non-hospitalized patients. Despite the small sample size, this is one of the first study to prospectively evaluate long-term neurologic effects seen in COVID-19 patients. All the assessments were performed using phone questionnaires instead of in-person assessments. However, remote contactless assessments are necessary as the pandemic has changed the care paradigm (causing disruption in routine health care services) and many recovering patients are reluctant to participate in an in-person follow-up due to health limitations. The influence of age and co-morbidities were not analyzed due to the small sample size. Furthermore, this study does not include a suitable control cohort.
Although studies have reported acute neurological symptoms after COVID-19, our study is one of the first to examine the persistence of neurologic symptoms and pain at 3 months after hospitalization. Studies examining pathophysiology and the time course of persistent symptoms after COVID-19 are needed. Our findings emphasize the importance of continued evaluation and focused rehabilitation for pain, functional, cognitive and neurobehavioral consequences in COVID-19 survivors.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board, The University of Texas Health Science Center at Houston. The patients/participants provided their written informed consent to participate in this study.
JS, LT, AG, LM, and HC were involved in the conception and design of the study. AB, SH, AP, AA, SJ, and GC were involved in the acquisition and analysis of data. JS, LT, AG, LM, S-MC, and HC were involved in the analysis of data. JS, S-MC, LT, AG, LM, and HC contributed substantially in drafting the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank all the health care workers and the research personnel who were involved in the care and the follow-up of the patients.
1. García-Moncó J, Muras A, Iriarte M, Armenteros P, Fernández A, Arranz-Martínez J, et al. Neurological manifestations in a prospective unselected series of hospitalized COVID-19 patients. Neurol Clin Pract. (2020) 11. doi: 10.1212/CPJ.0000000000000913
2. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
3. Rubin R. As their numbers grow, COVID-19 “Long Haulers” stump experts. JAMA. (2020) 324:1381–3. doi: 10.1001/jama.2020.17709
5. CDC. COVID-19 and Your Health. Centers for Disease Control and Prevention. (2020) Available online at: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed September 22, 2021).
6. Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. (2019) 12:14. doi: 10.3390/v12010014
7. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatr. (2021) 8:416–27. doi: 10.1016/S2215-0366(21)00084-5
8. Modified Rankin Score (mRS). Available online at: https://manual.jointcommission.org/releases/TJC2018A/DataElem0569.html (accessed January 14, 2021).
9. Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. (2009) 24:733–8. doi: 10.1007/s11606-009-0981-1
10. Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep. (2008) 31:1601–7. doi: 10.1093/sleep/31.11.1601
11. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
12. Prins A, Bovin MJ, Smolenski DJ, Marx BP, Kimerling R, Jenkins-Guarnieri MA, et al. The primary care PTSD screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. (2016) 31:1206–11. doi: 10.1007/s11606-016-3703-5
13. Stroke. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials. Available online at: https://www.ahajournals.org/doi/full/10.1161/01.str.0000258355.23810.c6 (accessed September 21, 2021).
14. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
15. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
16. Lau K-K, Yu W-C, Chu C-M, Lau S-T, Sheng B, Yuen K-Y. Possible central nervous system infection by SARS Coronavirus. Emerg Infect Dis. (2004) 10:342–4. doi: 10.3201/eid1002.030638
17. Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. (2004) 113:e73–6. doi: 10.1542/peds.113.1.e73
18. Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatr. (2020) 7:875–82. doi: 10.2139/ssrn.3601761
19. Lee Y, Min P, Lee S, Kim S-W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. (2020) 35:e174. doi: 10.3346/jkms.2020.35.e174
20. Russell B, Moss C, Rigg A, Hopkins C, Papa S, Hemelrijck MV. Anosmia and ageusia are emerging as symptoms in patients with COVID-19: what does the current evidence say? Ecancermedicalscience. (2020) 14:ed98. doi: 10.3332/ecancer.2020.ed98
21. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 Patients. Laryngoscope. (2020) 130:E695. doi: 10.1002/lary.28753
22. NEJM. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. Available online at: https://www.nejm.org/doi/full/10.1056/NEJMc2009787 (accessed September 20, 2020).
23. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. (2020) 194:105921. doi: 10.1016/j.clineuro.2020.105921
24. Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. (2020) 413:116832. doi: 10.1016/j.jns.2020.116832
25. Morasco BJ, Lovejoy TI, Lu M, Turk DC, Lewis L, Dobscha SK. The relationship between PTSD and chronic pain: mediating role of coping strategies and depression. Pain. (2013) 154:609–16. doi: 10.1016/j.pain.2013.01.001
26. Woo AK. Depression and anxiety in pain. Rev Pain. (2010) 4:8–12. doi: 10.1177/204946371000400103
27. Wu KK, Chan SK, Ma TM. Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS). J Trauma Stress. (2005) 18:39–42. doi: 10.1002/jts.20004
28. Kroenke K, Theobald D, Wu J, Tu W, Krebs EE. Comparative responsiveness of pain measures in cancer patients. J Pain. (2012) 13:764–72. doi: 10.1016/j.jpain.2012.05.004
29. Arout CA, Sofuoglu M, Bastian LA, Rosenheck RA. Gender differences in the prevalence of fibromyalgia and in concomitant medical and psychiatric disorders: a national veterans health administration study. J Womens Health. (2018) 27:1035–44. doi: 10.1089/jwh.2017.6622
30. Epstein SA, Kay G, Clauw D, Heaton R, Klein D, Krupp L, et al. Psychiatric disorders in patients with fibromyalgia. a multicenter investigation. Psychosomatics. (1999) 40:57–63. doi: 10.1016/S0033-3182(99)71272-7
31. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. (2020) 11:29. doi: 10.1186/s13293-020-00304-9
32. Jin J-M, Bai P, He W, Wu F, Liu X-F, Han D-M, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. (2020) 8:152. doi: 10.3389/fpubh.2020.00152
33. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. (2020) 20:442–7. doi: 10.1038/s41577-020-0348-8
34. Global Health 50/50. The Sex, Gender and COVID-19 Project. Available online at: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/ (accessed December 9, 2020).
Keywords: pain, COVID-19, long-haul, neurological symptoms, fatigue
Citation: Savarraj JPJ, Burkett AB, Hinds SN, Paz AS, Assing A, Juneja S, Colpo GD, Torres LF, Cho S-M, Gusdon AM, McCullough LD and Choi HA (2021) Pain and Other Neurological Symptoms Are Present at 3 Months After Hospitalization in COVID-19 Patients. Front. Pain Res. 2:737961. doi: 10.3389/fpain.2021.737961
Received: 07 July 2021; Accepted: 20 October 2021;
Published: 16 November 2021.
Edited by:
Guillaume Leonard, Université de Sherbrooke, CanadaReviewed by:
Grisell Vargas-Schaffer, Université de Montréal, CanadaCopyright © 2021 Savarraj, Burkett, Hinds, Paz, Assing, Juneja, Colpo, Torres, Cho, Gusdon, McCullough and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jude P. J. Savarraj, SnVkZS5QLlNhdmFycmFqQHV0aC50bWMuZWR1; H. Alex Choi, SHVpbWFobi5BLkNob2lAdXRoLnRtYy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.