- 1Department of Biology, Utah State University, Logan, UT, United States
- 2Interdisciplinary Neuroscience Program, Utah State University, Logan, UT, United States
Chronic pain is a growing public health crisis that requires exigent and efficacious therapeutics. GPR171 is a promising therapeutic target that is widely expressed through the brain, including within the descending pain modulatory regions. Here, we explore the therapeutic potential of the GPR171 agonist, MS15203, in its ability to alleviate chronic pain in male and female mice using a once-daily systemic dose (10 mg/kg, i.p.) of MS15203 over the course of 5 days. We found that in our models of Complete Freund's Adjuvant (CFA)-induced inflammatory pain and chemotherapy-induced peripheral neuropathy (CIPN), MS15203 did not alleviate thermal hypersensitivity and allodynia, respectively, in female mice. On the other hand, MS15203 treatment decreased the duration of thermal hypersensitivity in CFA-treated male mice following 3 days of once-daily administration. MS15203 treatment also produced an improvement in allodynia in male mice, but not female mice, in neuropathic pain after 5 days of treatment. Gene expression of GPR171 and that of its endogenous ligand BigLEN, encoded by the gene PCSK1N, were unaltered within the periaqueductal gray (PAG) in both male and female mice following inflammatory and neuropathic pain. However, following neuropathic pain in male mice, the protein levels of GPR171 were decreased in the PAG. Treatment with MS15203 then rescued the protein levels of GPR171 in the PAG of these mice. Taken together, our results identify GPR171 as a GPCR that displays sexual dimorphism in alleviation of chronic pain. Further, our results suggest that GPR171 and MS15203 have demonstrable therapeutic potential in the treatment of chronic pain.
Introduction
Chronic pain is growing public health concern with over 50 million adults in the United States having suffered from the condition since 2016 (1). Globally, nearly 2 billion people have been affected by chronic pain conditions, emphasizing the need for extensive research, and development of efficacious therapeutics (2). Despite the progress made in studying the causal and therapeutic mechanisms of chronic pain, the inclusion of female cohorts in pain studies is limited (3, 4). Several reports have highlighted the existence of sex differences in the pathology of chronic pain (5–8). Indeed, females have been reported to experience more severe and persistent pain than males in postoperative settings leading to reduced levels of physical activity (9). In addition, the estrus stage of females has also been implicated in their pain sensitivity and their responsiveness to opioid analgesics (10, 11). However, the long-term use of opioid analgesics for the treatment of chronic pain has profound negative side effects and has been shown to have limited effectiveness in the daily management of chronic pain (12). Given these substantial issues with usage of opioids, we sought to look beyond this class of drugs to identify efficacious therapeutics for chronic pain.
G-protein coupled receptors (GPCRs) and their dysregulation is implicated in a wide range of pathologies, including chronic pain (13–15). However, only 34% of FDA-approved drugs target GPCRs, highlighting the yet untapped potential of the GPCR family to be therapeutic targets (16, 17). One particularly promising target of interest for novel pain therapeutics is the GPCRs, GPR171, and its endogenous ligand, BigLEN. The peptide BigLEN is derived from the neuropeptide precursor ProSAAS. The precursor peptide, in turn, has been shown to be upregulated in the cerebrospinal fluid of patients with fibromyalgia and within the periaqueductal gray (PAG) in a rodent model of opioid-induced hyperalgesia (18, 19). The receptor, GPR171, and its endogenous ligand, BigLEN, are widely expressed through the brain including the PAG (20, 21). This particular brain region is situated in the descending pain modulatory pathway and is a site of action for a range of antinociceptive drugs including opioids and cannabinoids (22, 23). Previous studies exploring the role of GPR171 in acute pain showed that agonism of the receptor via systemic administration of the synthetic agonist, compound MS15203, led to an increase in the antinociceptive effect of systemic morphine administration (20).
We explored the role of GPR171 in chronic neuropathic and inflammatory pain in male and female mice. The therapeutic potential of the GPR171 agonist compound, MS15203, was assessed via repeated once-daily systemic (10 mg/kg i.p.) injections. We found that the compound MS15203 reduced the duration of chronic neuropathic and inflammatory pain in male mice, but not female mice. While we found no alterations in gene expression levels of ProSAAS or GPR171 in the PAG of male or female mice, we found that chemotherapy-induced peripheral neuropathy (CIPN) produces a decrease in GPR171 protein levels in vlPAG of male mice. We note that following MS15203 treatment, the GPR171 protein levels in male mice with neuropathic pain recovered and were indeed elevated compared to untreated controls. Our findings demonstrate a sexually dimorphic receptor system in chronic pain and establish a role for the recently deorphanized receptor GPR171 in the reduction of chronic pain in male mice.
Materials and Methods
Animals
One hundred and two adult male and female C57BL/6CS mice (Charles River Laboratories, CA), 6–8 weeks old and weighing 18–26 g at the start of the study were used in the study. Food and water were available ad libitum, except during testing. Mice were housed (four to five per cage) in a humidity and temperature-controlled room with a 12-h light/dark cycle (off at 1900). All behavior testing took place during the light cycle. For the female cohorts, estrus stage was determined by obtaining a vaginal lavage daily starting 5 days prior to the start of the study. A lavage was also obtained daily over the course of the study. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals adopted by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Utah State University (Protocol 2775).
CFA-Induced Inflammatory Pain
Animals were very briefly anesthetized with isofluorane (99.9%, inhaled; Fluriso, VetOne) and restrained in order to access the plantar surface of the hind paw. Complete Freund's Adjuvant (CFA, Cat.F5881, Sigma-Aldrich) was injected under the epidermis into the plantar surface of both hind paws using a 27G needle (20 μl/paw) as described previously (24). Animals in the control group received light isofluorane anesthesia alone without manipulation of their hind paws to prevent inflammation from the injection procedure. Drug administration commenced 24 h following CFA injection.
Assessment of Inflammatory Pain in Mice
A plantar test was performed as previously described (25) to assess inflammatory pain. Briefly, the subjects were placed in plexiglass enclosures on top of a glass platform. The animals were acclimatized to the testing chamber for 3 days prior to the start of the study (1 h/session). On each testing day, the animals were acclimatized in the testing chamber for 20 min, or until cessation of exploratory behavior. A radiant heat source (IITC, Cat. 390) was applied to the plantar surface of the hind paw and the time to a nocifensive response was recorded. A cut-off time of 20 s was enforced to avoid potential injury due to tissue damage. Two trials were performed on each hindpaw to obtain the average reaction time per paw and a third reaction time was obtained if the preceding two values differed by 2 s or more. Both left and right hindpaws were tested and their average thermal latency was considered for statistical analysis. Baseline latencies were established prior to injection of CFA. Following CFA injection on Day 0, the plantar test took place on Days 1, 3, and 5, where Day 0 marks the injection of CFA and Day 1 is 24 h following CFA injection.
Chemotherapy-Induced Peripheral Neuropathy
Paclitaxel (Sigma-Aldrich, MO, USA) was diluted in a vehicle comprising of Cremphor (Sigma-Aldrich, MO, USA)/90% ethanol/0.9% saline in a 1:1:18 ratio, and administered intraperitoneally (i.p., 4 mg/kg) to mice on four alternate days (cumulative dose 16 mg/kg) to induce neuropathy as described previously (26, 27). The control group received four injections of the vehicle (10 ml/kg). As paclitaxel can be present in animal excreta, bedding of mice used in this study was treated as a biohazard and disposed according to University guidelines.
Assessment of Mechanical Allodynia in Mice
The von Frey filament test was performed as previously described (26) to assess allodynia in mice. Briefly, all mice (vehicle- and paclitaxel-treated) were placed in plexiglass enclosures mounted onto a testing platform containing a metal, perforated floor (Stoelting Co., Wood Dale, IL, USA). Animals were acclimatized to the testing chamber for 3 days prior to the start of the study (1 h/session). On each testing day, the animals were acclimatized in the testing chamber for 20 min, or until cessation of exploratory behavior. Mechanical allodynia was assessed in male mice by applying von Frey filaments to the midplantar region of both hindpaws for approximately 2 s per stimulus using calibrated filaments (Touch Test kit, Cat. No. NC12775-99, North Coast Medical). All trials began with the 1 g filament and proceeded using an up–down trial design. Both right and left hindpaws were tested and their average mechanical threshold was considered for statistical analysis. A sudden paw withdrawal, flinching, or paw licking was regarded as a nocifensive response. A negative response was followed by the use of a larger filament. For assessment of mechanical allodynia in females, an electronic von Frey device (Ugo Basile, Italy; Cat.38450) was used as the allodynic females' mechanical thresholds were lower than the detectable range of the manual filaments and the up-down method could not be used (Supplementary Figure 1). As the pain behaviors are stable across our experimental timeline and there are no paw deformities, both electronic and manual von Frey can be assumed to provide a comparable result (28). The effect of MS15203 treatment on paclitaxel-induced neuropathy was evaluated on a secondary cohort of male mice and their mechanical thresholds were assessed on the electronic von Frey. Paw mechanical withdrawal thresholds are expressed as % of baseline values, where the baseline represents von Frey thresholds prior to paclitaxel or vehicle treatment.
Drug Treatment
GPR171 agonist (MS15203, a gift from Dr. Sanjai Pathak) was dissolved in sterile 0.9% saline (1 mg/ml). Mice in the Paclitaxel- and CFA-induced chronic pain studies were randomly assigned, by block randomization, into four treatment groups: pain + MS15203, pain + saline, no pain + MS15203, and no pain + saline. Mice in the MS15203 treatment group received 10 mg/kg i.p. as this dose was previously shown to produce an increase in hot plate thermal latency when co-administered with morphine (20). To evaluate the effect of MS15203 on chronic neuropathic pain, the animals were administered with one dose of either MS15203 or saline, once daily for 5 days starting on Day 15. To evaluate the effect of MS15203 on chronic inflammatory pain, the animals were administered with one dose of either MS15203 or saline, once daily for 5 days starting 24 h following induction of inflammatory pain.
Immunofluorescence Staining and Microscopy
Immediately following the final behavioral testing, a subset of the subjects (~3/group) were deeply anesthetized using isofluorane and transcardially perfused with 4% paraformaldehyde (PFA). Their brains were post-fixed in 4% PFA for 1 h and then were stored in 1x PBS at 4°C until further processing. Sectioning was performed on a vibratome (Leica Biosystems, Germany) and 50 μm sections containing the vlPAG were selected for immunofluorescence analysis. The sections were first incubated in sodium borohydride (1% in PBS) to expose the epitopes and decrease autofluorescence from aldehydes. Subsequently, they were permeabilized with Triton-X 100 (3%, Sigma-Aldrich), blocked with normal goat serum (5% in PBS), and incubated overnight in primary antibodies in their appropriate buffer (1% BSA (Sigma-Aldrich) in PBS). The following day, the slices were washed in PBS and incubated for 2 h in diluted secondary antibodies in 1% BSA in PBS. The antibodies were sourced from GeneTex (Rabbit anti-GPR171, Cat.GTX108131, 1:400) and Life Technologies (Goat anti-Rabbit A594, Cat.A11037, 1:1,000). Following subsequent PBS washes, the slices were briefly (5 min) incubated in DAPI, washed with PBS, and mounted with an anti-fade (ProLong Diamond Anti-Fade, Cat.P36961, Invitrogen) on a glass slide. Representative images of coronal sections containing the vlPAG, as defined by the mouse brain atlas (29), were acquired on a Zeiss LSM 710 confocal microscope (Carl Zeiss Microscopy, Germany) at Utah State University's microscopy core facility.
Quantification of Immunofluorescence
Quantification of GPR171-fluorescence was performed using ImageJ (NIH) on automatically thresholded images as previously described (30, 31). Up to seven images of the vlPAG representative of both left and right hemispheres and across different rostro-caudal positions were analyzed per animal per group. They were analyzed as independent measurements to account for the structural and functional heterogeneity of the PAG (32–35). Ten cells in the field of view were randomly selected along with three areas representing background fluorescence. The fluorescence from GPR171 signal was calculated using the formula for Corrected Total Cell Fluorescence (CTCF) [Integrated Density – (area of selection × mean background fluorescence)]. The measurements have been presented in arbitrary units (AU).
Quantitative RT-PCR
Immediately following the final behavioral testing, a subset of the subjects (4–5/group) were euthanized by decapitation and the PAG was dissected and snap-frozen on dry ice. The tissue samples were stored at −80°C until further processing. RNA was extracted from the tissues using Trizol (Cat. 15596026, Invitrogen) and RNeasy Plus Mini Kit (Cat. 74136, Qiagen) following the manufacturer's instructions. The eluted RNA was quantified using a Qubit 4 fluorometer (Invitrogen). cDNA was prepared using the Maxima first-strand synthesis kit for qRT-PCT (Cat. K1642, Thermo-Fisher) following the manufacturer's instructions. Samples were prepared using SYBR green (iTaq Universal SYBR Green Supermix, Cat. 1725121, Bio-Rad, CA) for gene expression analysis on a real-time thermocycler (CFX384 Touch, Bio-Rad). Custom primers as described previously (36) were used for GAPDH, GPR171, and ProSAAS (Integrated DNA Technologies). The synthesized cDNA was assayed in triplicate and analyzed using the 2−ΔΔCt method. Here, Ct indicates the cycle number at which the fluorescence signal crosses an arbitrary threshold within the exponential phase of the amplification curve. To calculate ΔΔCt, we used the formula ΔΔCt = {(Cttarget : treatedsample – CtGAPDH : treatedsample) – (Cttarget : controlsample – CtGAPDH : controlsample)}. The value of the control sample was set to 100% and all samples were evaluated with respect to the control. Negative control reactions were performed to ascertain contaminant-free cDNA synthesis and primer specificity was evaluated using melt curve analyses. The primer sequence information can be found in the Supplementary Material (Supplementary Table 1).
Statistical Analysis
Results are presented as mean ± standard error of mean (SEM). Statistical analyses of behavioral studies were performed using a repeated measures two-way analysis of variance (ANOVA) combined with Bonferroni's post-hoc test. Normality of data was evaluated using the D-Agostino and Pearson test. Statistical analyses of qRT-PCR data and fluorescence quantification were performed using a one-way ANOVA combined with a Tukey's post-hoc test. The ROUT method was used to identify and exclude outliers in the fluorescence quantification analysis only (<5% of images). Experimenters were blinded to drug treatment (saline and MS15203), but not to the pain state during behavioral studies and statistical analysis. All graphing and statistical analyses were performed using Prism 9.0 (GraphPad, San Diego, CA).
Results
GPR171 Agonist Does Not Reduce Chronic Inflammatory Pain in Female Mice
We used a model of CFA-induced inflammatory pain to study the effect of GPR171 agonism on pain relief (Figure 1A). In females, (n = 6–8/group), intra-plantar CFA injection produced inflammatory pain and associated thermal hypersensitivity as measured by a plantar test. The estrus stage did not impact the subjects' thermal thresholds (Supplementary Figure 2A). A repeated measures two-way ANOVA indicated significant differences in thermal thresholds over the course of the study {Time [F(3.159, 88.44) = 36.78, p < 0.0001]; Treatment [F(3, 38) = 14.57, p < 0.0001]; Time × Treatment [F(12, 112) = 8.211, p < 0.0001]}. A Bonferroni's post-hoc test revealed that the female mice developed thermal hypersensitivity 24 h following the CFA insult (Figure 1B: Day 1, Control + Saline vs. CFA + Saline or CFA + MS15203, p < 0.001) which persisted through the duration of the study (Figure 1B: Day 5 post-drug, Control + Saline vs. CFA + Saline, p < 0.0001). However, the Bonferroni's post-hoc analysis revealed that despite chronic (once-daily) treatment with MS15203, there was no change in the thermal latency of female mice with inflammation-induced hypersensitivity (Figure 1B: Day 5 post-drug, CFA + Saline vs. CFA + MS15203, p > 0.05). MS15203 treatment alone did not alter the thermal latencies of females in the Control group.
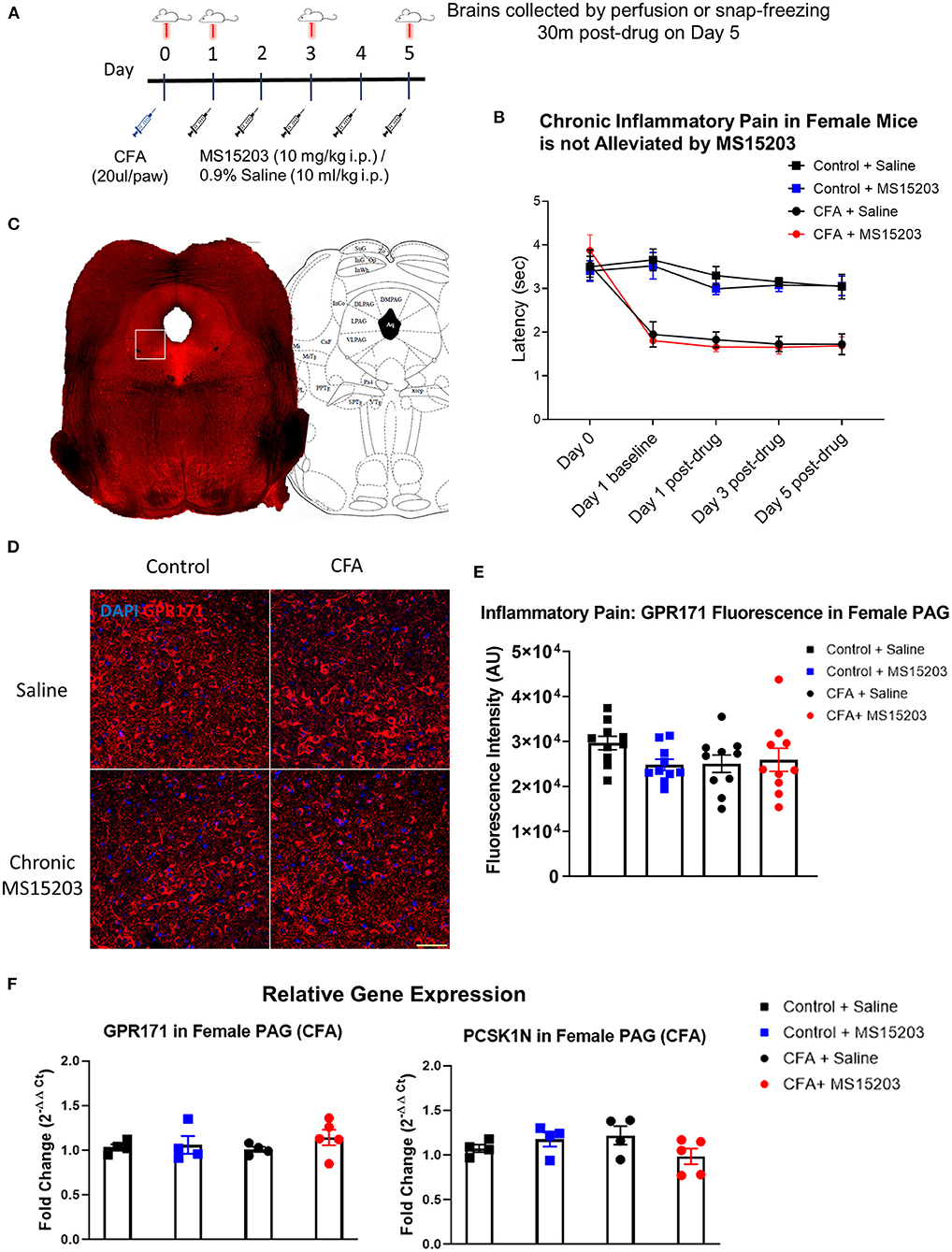
Figure 1. MS15203 does not reduce chronic inflammatory pain in female mice. (A) An illustrative timeline describing the experimental design of CFA-induced inflammatory pain followed by MS15203 treatment. (B) Female mice (n = 6–8/group) injected with CFA in their hind paws developed rapid thermal hypersensitivity as measured by a plantar test. Chronic treatment with MS15203 failed to alleviate inflammatory pain as noted by the persistence of thermal hypersensitivity through Day 5. Repeated measured two-way ANOVA with Bonferroni's post-hoc test. (C) A representative image of a coronal section of a control female mouse stained for GPR171. The white box represents the approximate boundary of the 20x images of the vlPAG that were analyzed for quantitative immunofluorescence. The coronal section is juxtaposed against a corresponding image from the mouse brain atlas at 4.72 mm caudal to the bregma. (D) Fluorescence immunostaining and (E) quantification of GPR171 signal show that protein levels of GPR171 are unchanged in the ventrolateral PAG (vlPAG) of female mice (n = 10–12 images each from 2 mice/group). (F) Analysis of gene expression changes within the periaqueductal gray (PAG) reveals that the transcript levels of GPR171 and its endogenous ligand, PCSK1N, are unchanged in female mice (n = 4–5/group) irrespective of inflammatory pain status or MS15203 treatment. One-way ANOVA with Tukey's post-hoc test. Scale bar = 50 μm.
We performed immunofluorescence staining and quantification of GPR171 in the PAG of female mice as shown in Figure 1C. Ten confocal images of the ventrolateral PAG (vlPAG) from 2 mice/group were analyzed to visualize receptor localization and evaluate GPR171 protein levels within the vlPAG (Figures 1D,E). A one-way ANOVA of fluorescence intensities indicated no significant differences between treatment groups on quantification of GPR171 protein levels in the vlPAG [Treatment, F(3, 36) = 1.44, p > 0.05].
We then performed qRT-PCR on dissected whole PAG from a subset of females (n = 4–5/group) to evaluate gene expression changes in GPR171 or its endogenous ligand, PCSK1N (Figure 1F). The PAG is a critical modulator of antinociception within the descending pain pathway and is a site of action of endogenous opioid activity (33, 37). A one-way ANOVA indicated no significant differences between treatment groups on evaluation of GPR171 [Treatment, F(3, 13) = 0.71, p > 0.05] or PCSK1N [Treatment, F(3, 13) = 1.68, p > 0.05] expression in the PAG.
GPR171 Agonist Reduces Chronic Inflammatory Pain in Male Mice
We performed a similar experimental paradigm as Figure 1A to evaluate the analgesic properties of GPR171 in male mice. In males, (n = 5–6/group), intra-plantar CFA injection produced inflammatory pain and thermal hypersensitivity on the plantar test. A repeated measures two-way ANOVA indicated significant differences in thermal thresholds over the course of the study {Time [F(3.025, 57.48) = 19.48, p < 0.0001]; Treatment [F(3, 19) = 27.08, p < 0.0001]; Time × Treatment [F(12, 76) = 7.417, p < 0.0001]}. Similar to the females, the male mice injected with CFA developed thermal hypersensitivity 24 h later as revealed by a Bonferroni's post-hoc test (Figure 2A: Day 1, Control + Saline vs. CFA + Saline or CFA + MS15203, p < 0.0001). The Bonferroni's post-hoc analysis also revealed that acute MS15203 did not improve thermal latencies in CFA-treated male mice (Figure 2A: Day 1 post-drug, Control + Saline vs. CFA + MS15203, p < 0.01). However, 3 days of once-daily MS15203 treatment increased thermal latencies of male mice and this improvement was sustained through the 5-day chronic treatment paradigm (Figure 2A: Day 3 post-drug, CFA + Saline vs. CFA + MS15203, p < 0.05 and Day 5 post-drug, CFA + Saline vs. CFA + MS15203, p < 0.001).
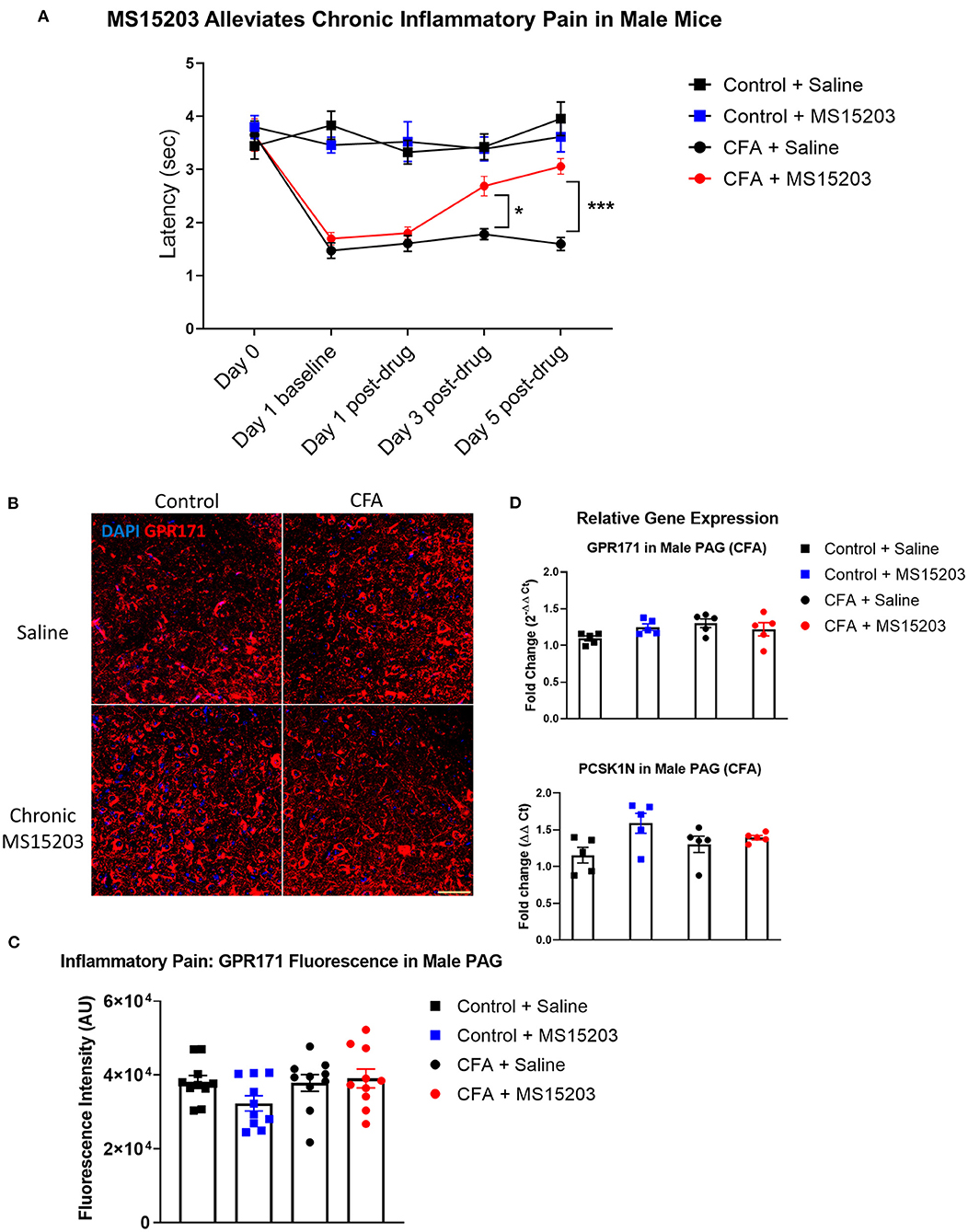
Figure 2. MS15203 reduces chronic inflammatory pain in male mice. (A) Male mice (n = 5–6/group) injected with CFA in their hind paws developed rapid thermal hypersensitivity as measured by a plantar test. Chronic treatment with MS15203 alleviated inflammatory pain by Day 3 of the treatment and the antinociception was ongoing through Day 5 as measured by an increase in thermal latencies on the plantar test. Repeated measured two-way ANOVA with Bonferroni's post-hoc test. (B) Fluorescence immunostaining and (C) quantification of GPR171 signal show that the protein levels of GPR171 are unchanged in the vlPAG of male mice (n = 10–12 images each from 2 mice/group). (D) Analysis of gene expression changes within the PAG reveals that the transcript levels of GPR171 and its endogenous ligand, PCSK1N, are unchanged in male mice (n = 4–5/group). One-way ANOVA with Tukey's post-hoc test. *p < 0.05, ***p < 0.0001. Scale bar = 50 μm.
We performed immunofluorescence staining and quantification of GPR171 in the PAG of male mice (10 images from 2 mice/group; Figures 2C,D). A one-way ANOVA of fluorescence intensities indicated no significant differences between treatment groups on quantification of GPR171 protein levels in the vlPAG [Treatment, F(3, 36) = 1.97, p > 0.05].
We then performed qRT-PCR on dissected whole PAG from males (4–5/group) to evaluate gene expression changes in GPR171 and PCSK1N (Figure 2B). A one-way ANOVA indicated no significant differences between treatment groups on evaluation of GPR171 [Treatment F(3, 16) = 2.06, p > 0.05] or PCSK1N [Treatment, F(3, 16) = 3.07, p > 0.05] expression in the PAG.
GPR171 Agonist Does Not Reduce Chronic Neuropathic Pain in Female Mice
We employed a model of CIPN to study the effects of GPR171 agonist treatment on chronic pain (Figure 3A). In females (n = 6–7/group), paclitaxel produced allodynia as assessed by an electronic von Frey sensor. The estrus stage did not impact the subjects' mechanical thresholds (Supplementary Figure 2B). A repeated measures two-way ANOVA indicated significant differences in mechanical thresholds over the course of the study {Time [F(3.77, 98) = 63.87, p < 0.0001]; Treatment [F(3, 26) = 44.36, p < 0.0001]; Time × Treatment [F(15, 130) = 12.54, p < 0.0001]}. A Bonferroni's post-hoc test revealed that the female mice developed allodynia by Day 5 following the first dose of paclitaxel (Figure 3B: Day-5, Vehicle + Saline vs. Paclitaxel + Saline or Paclitaxel + MS15203, p < 0.001). Allodynia was maintained through the duration of the study and was ongoing on Day 15 prior to the initiation of pharmacological intervention (Figure 3B, Day-15, Vehicle + Saline vs. Paclitaxel + Saline or Paclitaxel + MS15203, p < 0.0001). We then tested the effect of MS15203 at both acute (30 min) and chronic (5 days) dosing paradigms. A Bonferroni's post-hoc analysis revealed that neither acute nor chronic MS15203 treatment increased the mechanical thresholds of Paclitaxel + MS15203-treated females (Figure 3B: Day 15 post-test and Day 20, Vehicle + Saline vs. Paclitaxel + MS15203, p < 0.0001). The compound MS15203 alone did not have any effect in vehicle-treated female mice.
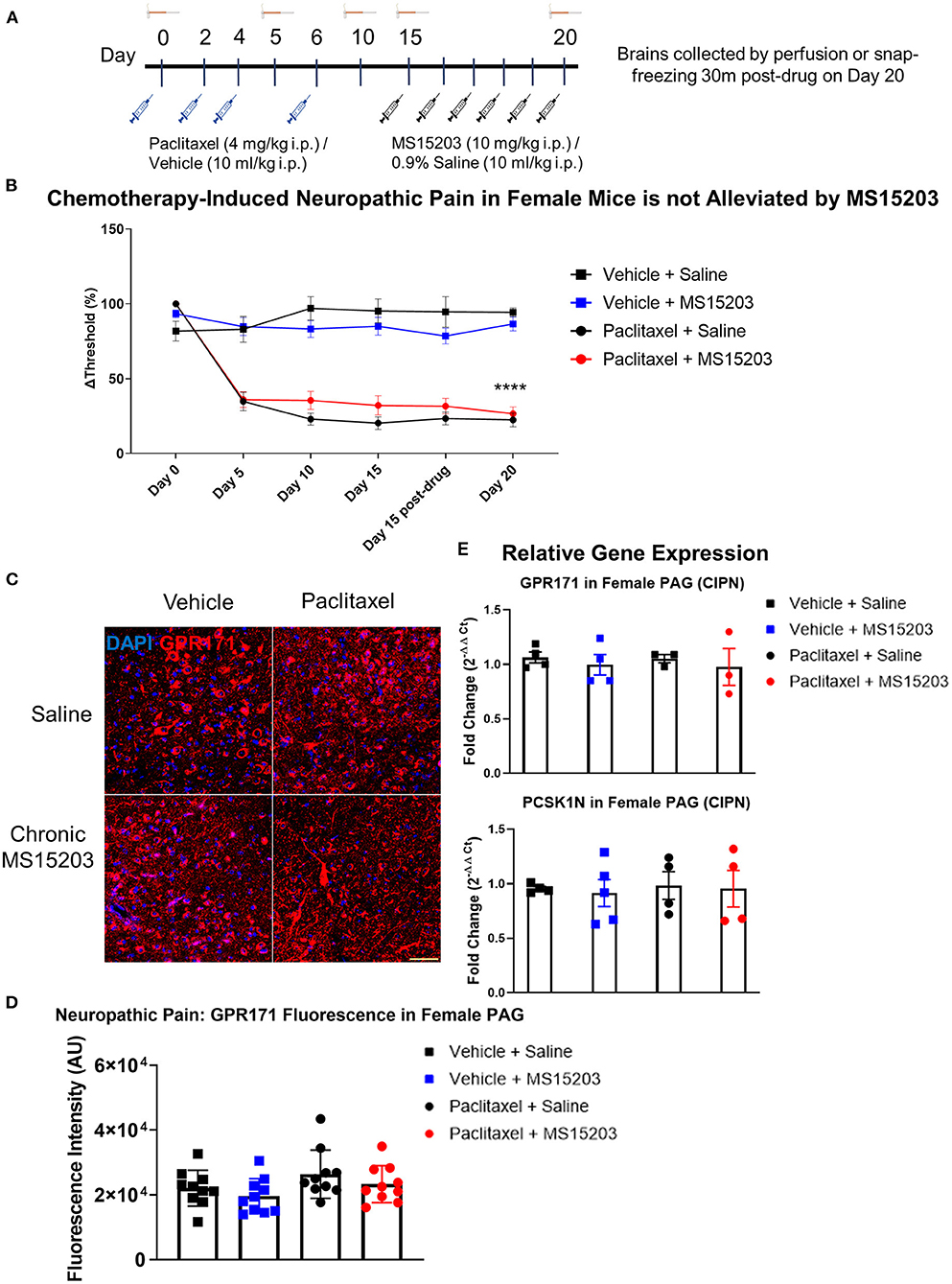
Figure 3. MS15203 does not reduce chronic neuropathic pain in female mice. (A) An illustrative timeline describing the experimental design of chemotherapy-induced peripheral neuropathy followed by MS15203 treatment. (B) Female mice (n = 6–7/group) treated with paclitaxel (16 mg/kg cumulative, i.p.) developed allodynia by Day 5 of the study as measured by the von Frey test. Chronic treatment with MS15203 from Day 15 through Day 20 failed to alleviate allodynia as noted by the persistence of mechanical hypersensitivity through Day 20. Repeated measures two-way ANOVA with Bonferroni's post-hoc test. (C) Fluorescence immunostaining and (D) quantification of GPR171 signal show that protein levels of GPR171 are unchanged in the vlPAG of female mice (n = 10–12 images each from 2 mice/group). (E) Analysis of gene expression changes within the periaqueductal gray (PAG) reveals that the transcript levels of GPR171 and its endogenous ligand, PCSK1N, are unchanged in female mice (n = 4–5/group) irrespective of neuropathic pain status or MS15203 treatment. One-way ANOVA with Tukey's post-hoc test. ****indicates significant difference between Paclitaxel + MS15203 vs. Vehicle + Saline, p < 0.0001. Scale bar = 50 μm.
We performed immunofluorescence staining and quantification of GPR171 in the vlPAG of female mice (10 images from 2 mice/group; Figures 3C,D). A one-way ANOVA of fluorescence intensities indicated no significant differences between treatment groups on quantification of GPR171 protein levels in the vlPAG [Treatment, F(3, 36) = 2.17, p > 0.05].
We proceeded to perform qRT-PCR on dissected PAG from female mice (4–5/group) to assess whether gene expression levels of GPR171 and PCSK1N are altered following neuropathic pain and MS15203 treatment (Figure 3E). A one-way ANOVA revealed that the expression levels of GPR171 are unchanged in the PAG of female mice irrespective of pain condition or MS15203 treatment [F(3, 13) = 0.572, p > 0.05]. Similarly, the expression levels of PCSK1N were also unchanged in the PAG of female mice across pain conditions or MS15203 treatment [F(3, 13) = 0.076, p > 0.05].
GPR171 Agonist Reduces Chronic Neuropathic Pain in Male Mice
We performed a similar experimental paradigm as Figure 3A using male mice to assess the anti-allodynic effect of MS15203. In males (n = 6–7/group), paclitaxel produced allodynia as assessed by manual von Frey filaments using the up-down method. A repeated measures two-way ANOVA indicated significant differences in mechanical thresholds over the course of the study {Time [F(3.31, 92.71) = 38, p < 0.0001]; Treatment [F(3, 28) = 82.53, p < 0.0001]; Time × Treatment [F(15, 140) = 13.36, p < 0.0001]}. A Bonferroni's post-hoc test revealed that the male mice developed allodynia by Day 5 following the first dose of paclitaxel (Figure 4A: Day-5, Vehicle + Saline vs. Paclitaxel + Saline or Paclitaxel + MS15203, p < 0.001). Allodynia was maintained through the duration of the study and was ongoing on Day 15 prior to the initiation of pharmacological intervention (Figure 4A, Day-15, Vehicle + Saline vs. Paclitaxel + Saline or Paclitaxel + MS15203, p < 0.0001). We then tested the effect of MS15203 at both acute (30 min) and chronic (5 days) dosing paradigms. A Bonferroni's post-hoc analysis revealed that acute MS15203 treatment did not alter the mechanical thresholds of male mice in neuropathic pain (Figure 4A: Paclitaxel + MS15203 vs. Paclitaxel + Saline, Day 15 baseline vs. post-test, Bonferroni p > 0.05). However, following 5 days of repeated dosing, MS15203 treatment increased the mechanical thresholds of mice in neuropathic pain compared to their saline-treated counterparts (Figure 4A: Paclitaxel + MS15203 vs. Paclitaxel + Saline, Day 20, Bonferroni p < 0.05). The compound MS15203 alone did not have any effect in vehicle-treated male mice. Evaluation of mechanical thresholds using an electronic von Frey test on male mice showed outcomes comparable with the manual filament testing (Supplementary Figure 4).
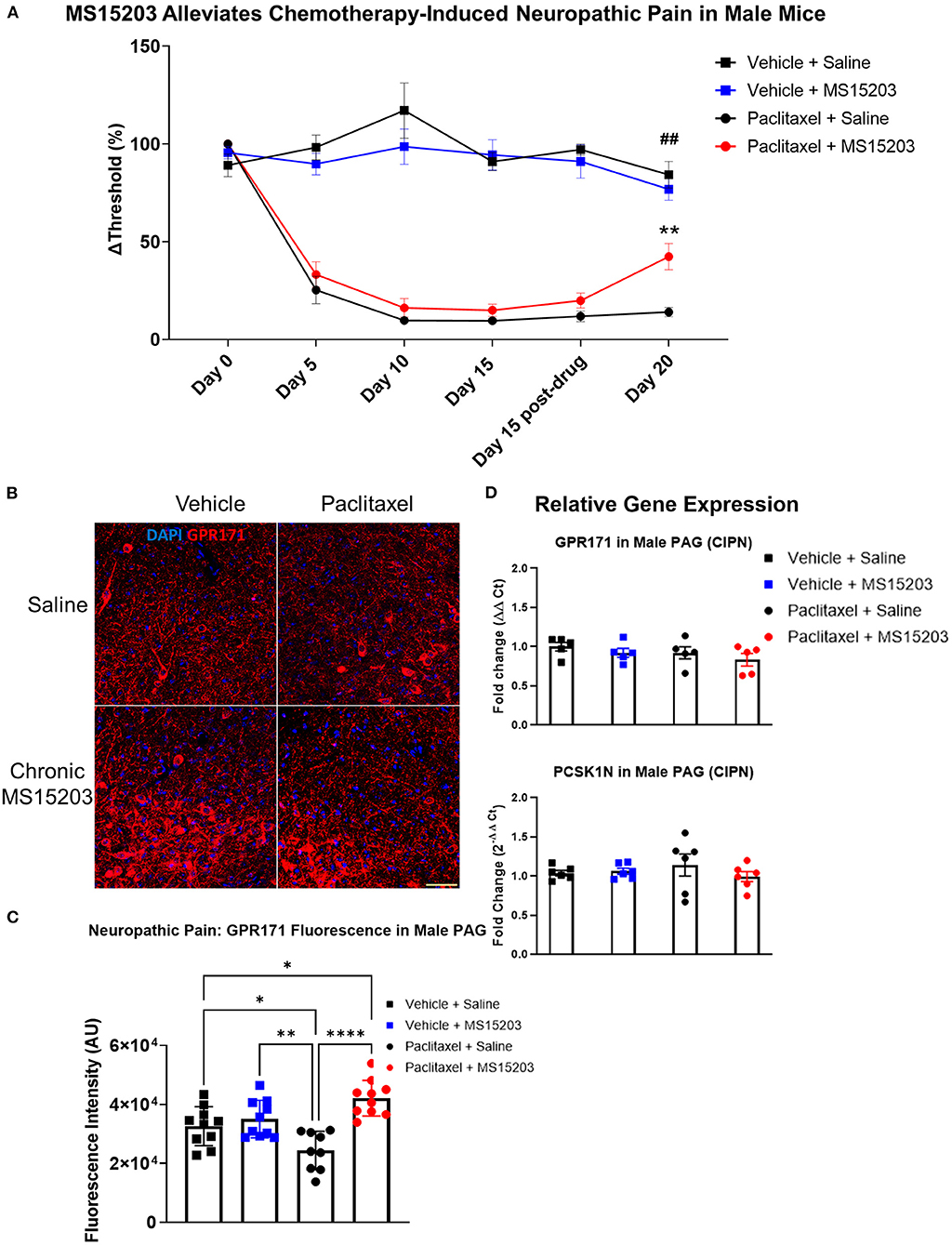
Figure 4. MS15203 reduces chronic neuropathic pain in male mice. (A) Male mice (n = 6–7/group) treated with paclitaxel (16 mg/kg cumulative, i.p.) developed allodynia by Day 5 of the study as measured by the von Frey test. Chronic, but not acute, treatment with MS15203 alleviated allodynia as noted by the significant increase in mechanical thresholds compared to Paclitaxel + Saline treated mice. Repeated measures two-way ANOVA with Bonferroni's post-hoc test. (B) Fluorescence immunostaining and (C) quantification of GPR171 signal show that protein levels of GPR171 are decreased following neuropathic pain and chronic treatment with MS15203 results in an increase of GPR171 in the vlPAG (10–12 images each from 2 mice/group). (D) Analysis of gene expression changes within the PAG reveals that the transcript levels of GPR171 and its endogenous ligand, PCSK1N, are unchanged in male mice (n = 4–5/group) irrespective of neuropathic pain status or MS15203 treatment. One-way ANOVA with Tukey's post-hoc test. *p < 0.05, **p < 0.01, ****p < 0.0001. In (A) ##indicates significant differences between Vehicle + Saline and Paclitaxel + MS15203 groups (p < 0.01) and **indicates significant differences between Paclitaxel + MS15203 and Paclitaxel + Saline groups. Scale bar = 50 μm.
We performed immunostaining for the receptor GPR171 and quantified fluorescence intensity within the vlPAG of male mice (10–12 images each from 2 mice/group) (Figures 4B,C). Intriguingly, a one-way ANOVA of the fluorescence intensities revealed significant differences between the groups [F(3, 35) = 10.15, p < 0.0001]. A Tukey's post-hoc test revealed that neuropathic pain resulted in a significant decrease of GPR171 immunostaining compared to vehicle-treated controls (Figure 4D: Vehicle + Saline vs. Paclitaxel + Saline, Tukey's p < 0.05). Further, the post-hoc analysis revealed that following chronic MS15203 treatment, there was a rescue effect and, in fact, the levels of GPR171 were elevated compared to the vehicle-treated controls (Figure 4D: Vehicle + Saline vs. Paclitaxel + MS15203, Tukey's p < 0.05).
We proceeded to perform qRT-PCR on dissected PAG from male mice (4–5/group) to assess whether gene expression levels of GPR171 and PCSK1N are altered following neuropathic pain and MS15203 treatment (Figure 4D). A one-way ANOVA revealed that the expression levels of GPR171 are unchanged in the PAG of male mice irrespective of pain condition or MS15203 treatment [F(3, 13) = 0.572, p > 0.05]. Similarly, the expression levels of PCSK1N were also unchanged in the PAG of male mice across pain conditions or MS15203 treatment [F(3, 13) = 0.076, p > 0.05].
Discussion
The current study establishes that the receptor GPR171 is a promising target for the treatment of chronic pain in males. A synthetic agonist for the receptor, MS15203, decreases the duration of both allodynia caused by neuropathic pain and thermal hypersensitivity caused by inflammatory pain in male mice. Interestingly, MS15203 does not reduce allodynia nor thermal pain in female mice using the same dose administered in males. Further, although GPR171 receptor immunostaining is unaltered in males after chronic inflammation, neuropathic pain induces a decrease in GPR171 protein levels which is rescued following chronic MS15203 treatment. The gene expression levels of GPR171 and its endogenous ligand, PCSK1, are unaltered in the PAG. Further, while GPR171 activation is recognized to promote food intake (38), we note that our 5-day treatment did not result in significant alterations in the subjects' weights (Supplementary Figures 3A–D).
The development of CFA-induced inflammatory pain occurs over a biphasic response time. The initial course of inflammation occurs over the first 24 h, followed by persistent pain lasting over the course of a week. We report here that systemic administration of the compound MS15203 decreases the duration of CFA-induced inflammatory pain, in a sex-dependent manner, after 3 days of treatment following the initial inflammatory phase. We also note that while this reduction of chronic pain is sustained over the course of the study in male mice, female mice do not display any reduction of thermal hypersensitivity over the course of the study. In previous studies assessing therapeutic options for chemotherapy-induced neuropathic pain, we noted that mechanical allodynia is most pronounced by Day 15 of the study and persists through 30 days (26). We assessed allodynia following 5 days of once-daily MS15203 treatment (10 mg/kg i.p.). We report here that systemic administration of the compound MS15203 decreases the duration of paclitaxel-induced peripheral neuropathy and associated allodynia after 5 days of treatment in male mice. While the pathophysiology of sex differences in neuropathic pain induced by chemotherapy is unclear, it is indeed a remarkable observation that the GPR171 activation did not promote alleviation of allodynia in females in chronic neuropathic pain.
The absence of changes in ProSAAS or GPR171 mRNA indicate that a 5-day, once-daily treatment regimen does not alter receptor or ligand gene expression in the brain although the drug does exert a physiological effect at this timescale. A recent study showed that peripheral sensory neurons contribute to the pain relief seen with GPR171 activation (39). This study provided mechanistic evidence of the involvement of peripheral nociceptors and the dorsal root ganglion in the alleviation of allodynia and hyperalgesia following localized activation of GPR171. While this observation explains alterations at the peripheral level in addition to spinal sensitization to chronic pain, the systemic GPR171 activation paradigm used here can probe both peripheral and central contributions to the alleviation of chronic pain. Our data implicate that GPR171 within the descending pain pathway is reduced in chronic pain states and may contribute to the antinociceptive effect of MS15203. The role of alterations in PAG connectivity and excitability is well established in the context of chronic pain paradigms (40–42). The absence of gene expression changes of GPR171 or PCSK1N within the PAG, while observing a modulation of protein levels is indeed a remarkable observation. We postulate that the transcript levels obtained from whole dissected PAG are not representative of the protein levels within the local vlPAG region, a highly variable relationship that has been reviewed previously (43). The decrease in GPR171 protein levels in the vlPAG following neuropathic pain is comparable to reports of decreased mu opioid receptor availability following chronic pain (44). We previously found synergistic antinociceptive effects of MS15203 with morphine, indicating the receptors could also be similar in their physiological modulation.
GPR171 is an inhibitory Gαi/o coupled receptor which inhibits cAMP production (38). Based on our study showing GPR171 in GABA neurons in the PAG, we hypothesize that activation of GPR171 leads to a decrease in GABA release (20). This in turn leads to antinociception by excitation of output neurons in the medulla. In addition, GPR171 is found in the dorsal root ganglion and alleviates inflammatory pain following intrathecal administration of a GPR171 agonist, presumably by inhibiting TRP ion channels (39). Since we administered the agonist systemically, it is likely that it is working within the central and peripheral nervous system to alleviate pain. However, this approach limits the ability to investigate the mechanistic actions of GPR171 localized to particular regions of the nervous system. In addition, the increase in the endogenous ligand ProSAAS, seen in circulating CSF of fibromyalgia patients, is likely a consequence of adaptations toward restoring GPR171 signaling as we have observed a decrease in brain-specific GPR171 receptor expression in males with neuropathic pain (18).
The lack of alleviation of neuropathic or inflammatory pain in females following chronic MS15203 treatment warrants further investigation. Previous studies have indicated that C57BL/6 mice display stable pain behaviors across estrus stages (45, 46). It is plausible that the dose of agonist administered (10 mg/kg, i.p.) was insufficient to produce a physiological response in females. Further, there is considerable evidence of heightened immune activation in females at both central and peripheral sensory processing regions, which contribute to mechanical and thermal hypersensitivity (11, 47–49). The heightened microglial activation in the PAG in females has been shown to reduce morphine-induced antinociception (50). While there is a paucity of studies examining immune interactions with GPR171 activity, the possibility of the immune microenvironment modulating GPR171-dependent analgesic activity cannot be ruled out. Further, studies with female mice have reported that the sex-specific differences in chronic pain are driven in part by the action of hormones and are peripherally regulated by TRP channels (45, 51). As GPR171 exerts its peripheral effects via TRP channels on sensory afferents in male mice, the microenvironment in this region can be postulated to contribute to the observed sex differences (39). Further, as the gene encoding GPR171 is located on chromosome 3 of the mouse, the observed differences in behavior cannot be attributed to sex chromosome-linked causes. Indeed, sex differences in antinociception have been found in other systems such as the fatty acid-derived resolvin D5, that was able to produce antinociception in males with neuropathic or inflammatory pain, but not in females (52). Our findings thus identify GPR171 not only as a novel target for the treatment of chronic inflammatory and neuropathic pain, but also one that displays sexual dimorphism in pain regulation.
The behavioral effects of MS15203 treatment in the reduction of chronic pain can be strengthened by the further evaluation of multiple chronic pain modalities and testing paradigms. The persistent and severe allodynia in female mice precluded the use of manual von Frey filaments and necessitated the use of an electronic probe. While the electronic method may not capture the extent of the manual filament's sensitivity, absence of differences in GPR171 protein levels and GPR171 and PCSK1N RNA levels in female mice indicate an agreement with the behavioral response where MS15203 did not reduce the duration of mechanical allodynia.
Chronic inflammatory and neuropathic pain present public health concerns without efficacious treatment options. Our findings suggest that the receptor GPR171 is a therapeutic target for the treatment of multiple modalities of chronic pain in a sex-dependent manner and its agonist, MS15203, can be used to treat chronic pain.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Utah State University IACUC.
Author Contributions
AR and EB designed the study. AR conducted the experiments and acquired data. TE, AM, LA, and MM acquired data. AR and EB analyzed the data and wrote the manuscript. EB supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by startup funds from the Department of Biology at Utah State University, a Pharmacology and Toxicology startup grant from the PhRMA Foundation, Young Investigator Grant from the Brain and Behavior Research Foundation, and by National Institute of Health (TR003667 to EB).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Dr. Mona Buhusi and Valerie Martin (Utah State University) for timely assistance during the COVID-19 pandemic with supplies essential for the completion of the study. The authors thank Dr. Sanjai Pathak (Queen's College, New York) for the kind gift of MS15203.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2021.695396/full#supplementary-material
References
1. Dahlhamer J, Lucas J, Zelaya, C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults. Morb Mortal Wkly Rep. (2018) 67:1001–6. doi: 10.15585/mmwr.mm6736a2
2. Burma NE, Leduc-Pessah H, Fan CY, Trang T. Animal models of chronic pain: advances and challenges for clinical translation. J Neurosci Res. (2017) 95:1242–56. doi: 10.1002/jnr.23768
3. Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. (2012) 13:859–66. doi: 10.1038/nrn3360
4. Shansky RM, Murphy AZ. Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci. (2021) 24:457–64. doi: 10.1038/s41593-021-00806-8
5. Tonsfeldt KJ, Suchland KL, Beeson KA, Lowe JD, Li M-H, Ingram SL. Sex differences in GABAA signaling in the periaqueductal gray induced by persistent inflammation. J Neurosci. (2016) 36:1669–81. doi: 10.1523/JNEUROSCI.1928-15.2016
6. de Mos M, de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BHC, Sturkenboom MCJM. The incidence of complex regional pain syndrome: a population-based study. Pain. (2007) 129:12–20. doi: 10.1016/j.pain.2006.09.008
7. Barnabe C, Bessette L, Flanagan C, LeClercq S, Steiman A, Kalache F, et al. Sex differences in pain scores and localization in inflammatory arthritis: a systematic review and metaanalysis. J Rheumatol. (2012) 39:1221–30. doi: 10.3899/jrheum.111393
8. Hannan MT. Epidemiologic perspectives on women and arthritis: an overview. Arthritis Care Res (Hoboken). (1996) 9:424–34. doi: 10.1002/art.1790090603
9. Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. (2009) 10:447–85. doi: 10.1016/j.jpain.2008.12.001
10. Shekunova E V., Bespalov AY. Estrous cycle stage-dependent expression of acute tolerance to morphine analgesia in rats. Eur J Pharmacol. (2004). 486:259–64. doi: 10.1016/j.ejphar.2004.01.012
11. Doyle HH, Eidson LN, Sinkiewicz DM, Murphy AZ. Sex differences in microglia activity within the periaqueductal gray of the rat: a potential mechanism driving the dimorphic effects of morphine. J Neurosci. (2017) 37:3202–14. doi: 10.1523/JNEUROSCI.2906-16.2017
12. Volkow N, Benveniste H, McLellan AT. Use and misuse of opioids in chronic pain. Annu Rev Med. (2018) 69:451–65. doi: 10.1146/annurev-med-011817-044739
13. Gottesman-Katz L, Latorre R, Vanner S, Schmidt BL, Bunnett NW. Targeting G protein-coupled receptors for the treatment of chronic pain in the digestive system. Gut. (2020) 0:1–12. doi: 10.1136/gutjnl-2020-321193
14. Cabral-Marques O, Marques A, Giil LM, De Vito R, Rademacher J, Günther J, et al. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat Commun. (2018) 9:5224. doi: 10.1038/s41467-018-07598-9
15. Jacobson KA, Giancotti LA, Lauro F, Mufti F, Salvemini D. Treatment of chronic neuropathic pain: purine receptor modulation. Pain. (2020) 161:1425–41. doi: 10.1097/j.pain.0000000000001857
16. Insel PA, Sriram K, Gorr MW, Wiley SZ, Michkov A, Salmerón C, et al. GPCRomics: an approach to discover GPCR drug targets. Trends Pharmacol Sci. (2019). doi: 10.1016/j.tips.2019.04.001
17. Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. (2017) 16:829–42. doi: 10.1038/nrd.2017.178
18. Khoonsari PE, Musunri S, Herman S, Svensson CI, Tanum L, Gordh T, et al. Systematic analysis of the cerebrospinal fluid proteome of fibromyalgia patients. J Proteomics. (2019) 190:35–43. doi: 10.1016/j.jprot.2018.04.014
19. Anapindi KDB, Yang N, Romanova E V, Rubakhin SS, Tipton A, Dripps I, et al. PACAP and other neuropeptide targets link chronic migraine and opioid-induced hyperalgesia in mouse models. Mol Cell Proteomics. (2019) 18:2447–58. doi: 10.1074/mcp.RA119.001767
20. McDermott M V, Afrose L, Gomes I, Devi LA, Bobeck EN. Opioid-induced signaling and antinociception are modulated by the recently deorphanized receptor, GPR171. J Pharmacol Exp Ther. (2019) 371:56–62. doi: 10.1124/jpet.119.259242
21. Mack SM, Gomes I, Devi LA. Neuropeptide PEN and its receptor GPR83: distribution, signaling, and regulation. ACS Chem Neurosci. (2019) 10:1884–91. doi: 10.1021/acschemneuro.8b00559
22. Wilson-Poe AR, Morgan MM, Aicher SA, Hegarty DM. Distribution of CB1 cannabinoid receptors and their relationship with mu-opioid receptors in the rat periaqueductal gray. Neuroscience. (2012) 213:191–200. doi: 10.1016/j.neuroscience.2012.03.038
23. Wang H, Wessendorf MW. Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience. (2002) 109:619–34. doi: 10.1016/S0306-4522(01)00328-1
24. Chen Y, Boettger MK, Reif A, Schmitt A, Üçeyler N, Sommer C. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol Pain. (2010) 6:1–11. doi: 10.1186/1744-8069-6-13
25. Cheah M, Fawcett J, Andrews M. Assessment of thermal pain sensation in rats and mice using the hargreaves test. Bio-Protocol. (2017) 7:e2506. doi: 10.21769/BioProtoc.2506
26. Sierra S, Gupta A, Gomes I, Fowkes M, Ram A, Bobeck EN, et al. Targeting cannabinoid 1 and delta opioid receptor heteromers alleviates chemotherapy-induced neuropathic pain. ACS Pharmacol Transl Sci. (2019) 2:219–29. doi: 10.1021/acsptsci.9b00008
27. Deng L, Cornett BL, Mackie K, Hohmann AG. CB1 knockout mice unveil sustained CB2-mediated antiallodynic effects of the mixed CB1/CB2 agonist CP55,940 in a mouse model of paclitaxel-induced neuropathic pain. Mol Pharmacol. (2015) 88:64–74. doi: 10.1124/mol.115.098483
28. Nirogi R, Goura V, Shanmuganathan D, Jayarajan P, Abraham R. Comparison of manual and automated filaments for evaluation of neuropathic pain behavior in rats. J Pharmacol Toxicol Methods. (2012) 66:8–13. doi: 10.1016/j.vascn.2012.04.006
29. Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates, 2nd Edn. Cambridge, MA: Academic Press (2001). p. 39–44.
30. Lemos Duarte M, Trimbake NA, Gupta A, Tumanut C, Fan X, Woods C, et al. High-throughput screening and validation of antibodies against synaptic proteins to explore opioid signaling dynamics. Commun Biol. (2021) 4:238. doi: 10.1038/s42003-021-01744-8
31. McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A. Partial inhibition of Cdk1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle. (2014) 13:1400–12. doi: 10.4161/cc.28401
32. Morgan MM, Whittier KL, Hegarty DM, Aicher SA. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain. (2008) 140:376–86. doi: 10.1016/j.pain.2008.09.009
33. Bagley EE, Ingram SL. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology. (2020) 173:108131. doi: 10.1016/j.neuropharm.2020.108131
34. Levine R, Morgan MM, Cannon JT, Liebeskind JC. Stimulation of the periaqueductal gray matter of the rat produces a preferential ipsilateral antinociception. Brain Res. (1991) 567:140–4. doi: 10.1016/0006-8993(91)91446-8
35. Marek P, Yirmiya R, Liebeskind JC. Stimulation-produced analgesia in the mouse: evidence for laterality of opioid mediation. Brain Res. (1991) 541:154–6. doi: 10.1016/0006-8993(91)91090-N
36. Wardman JH, Gomes I, Bobeck EN, Stockert JA, Kapoor A, Bisignano P, et al. Identification of a small-molecule ligand that activates the neuropeptide receptor GPR171 and increases food intake. Sci Signal. (2016) 9:ra55. doi: 10.1126/scisignal.aac8035
37. Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray–rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci. (2008) 27:1517–24. doi: 10.1111/j.1460-9568.2008.06100.x
38. Gomes I, Aryal DK, Wardman JH, Gupta A, Gagnidze K, Rodriguiz RM, et al. GPR171 is a hypothalamic G protein-coupled receptor for BigLEN, a neuropeptide involved in feeding. Proc Natl Acad Sci USA. (2013) 110:16211–6. doi: 10.1073/pnas.1312938110
39. Cho PS, Lee HK, Choi YI, Choi SI, Lim JY, Kim M, et al. GPR171 activation modulates nociceptor functions, alleviating pathologic pain. Biomedicines. (2021) 9:256. doi: 10.3390/biomedicines9030256
40. Cheriyan J, Sheets PL. Altered excitability and local connectivity of mPFC-PAG neurons in a mouse model of neuropathic pain. J Neurosci. (2018) 38:4829–39. doi: 10.1523/JNEUROSCI.2731-17.2018
41. Knerlich-Lukoschus F, Noack M, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J. Spinal cord injuries induce changes in CB [[sb]]1[[/s]] cannabinoid receptor and C-C chemokine expression in brain areas underlying circuitry of chronic pain conditions. J Neurotrauma. (2011) 28:619–34. doi: 10.1089/neu.2010.1652
42. Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. (2016) 18:20–30. doi: 10.1038/nrn.2016.162
43. Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. (2016) 165:535–50. doi: 10.1016/j.cell.2016.03.014
44. Thompson SJ, Pitcher MH, Stone LS, Tarum F, Niu G, Chen X, et al. Chronic neuropathic pain reduces opioid receptor availability with associated anhedonia in rat. Pain. (2018) 159:1856–66. doi: 10.1097/j.pain.0000000000001282
45. Chakrabarti S, Pattison LA, Singhal K, Hockley JRF, Callejo G, Smith ESJ. Acute inflammation sensitizes knee-innervating sensory neurons and decreases mouse digging behavior in a TRPV1-dependent manner. Neuropharmacology. (2018) 143:49–62. doi: 10.1016/j.neuropharm.2018.09.014
46. Meziane H, Ouagazzal A-M, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes, Brain Behav. (2007) 6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x
47. Loyd DR, Wang X, Murphy AZ. Sex differences in mu-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. (2008) 28:14007–17. doi: 10.1523/JNEUROSCI.4123-08.2008
48. Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin J-S, Ritchie J, et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. (2011) 31:15450–4. doi: 10.1523/JNEUROSCI.3859-11.2011
49. Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. (2015) 18:1081–3. doi: 10.1038/nn.4053
50. Eidson LN, Murphy AZ. Blockade of toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci. (2013) 33:15952–63. doi: 10.1523/JNEUROSCI.1609-13.2013
51. Patil MJ, Ruparel SB, Henry MA, Akopian AN. Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: contribution of prolactin receptor to inflammatory pain. Am J Physiol Metab. (2013) 305:E1154–64. doi: 10.1152/ajpendo.00187.2013
Keywords: G-protein coupled receptor, sex difference, neuropathy, inflammation, orphan receptor
Citation: Ram A, Edwards T, McCarty A, Afrose L, McDermott MV and Bobeck EN (2021) GPR171 Agonist Reduces Chronic Neuropathic and Inflammatory Pain in Male, But Not Female Mice. Front. Pain Res. 2:695396. doi: 10.3389/fpain.2021.695396
Received: 14 April 2021; Accepted: 17 August 2021;
Published: 10 September 2021.
Edited by:
George Latimer Wilcox, University of Minnesota Twin Cities, United StatesReviewed by:
Temugin Berta, University of Cincinnati, United StatesSufang Liu, Texas A&M University, United States
Copyright © 2021 Ram, Edwards, McCarty, Afrose, McDermott and Bobeck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erin N. Bobeck, ZXJpbi5ib2JlY2tAdXN1LmVkdQ==
 Akila Ram
Akila Ram Taylor Edwards1
Taylor Edwards1 Erin N. Bobeck
Erin N. Bobeck