
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oral. Health , 31 January 2025
Sec. Preventive Dentistry
Volume 6 - 2025 | https://doi.org/10.3389/froh.2025.1535233
Introduction: Dental caries is a prevalent oral disease with a multifactorial etiology. Lactobacillus has been implicated in caries progression on account of its acidogenic properties; On the other hand, they constitute one of the potential probiotic strategies for preventing dental caries. This complex relationship renders the relationship between Lactobacillus and dental caries remains ambiguous.
Methods: The Web of Science core collections (WoSCC) were searched to acquire articles relevant to Lactobacillus and dental caries. After retrieval and manual screening, publications were analyzed by VOSviewer.
Results: Sweden, the US, and China, which have been the center of international cooperation, have produced the most publications in the research area. Caries Research is the main counterpart journal in the field. “Dental caries”, “Streptococcus mutans”, “Lactobacilli”, “Probiotics”, and “Children” have been commonly used as keywords.
Discussion: Based on bibliometric analysis, this study reviews the relationship between lactobacilli and dental caries, emphasizing their dual roles. The detection rate of lactobacilli is closely associated with the incidence and severity of dental caries. However, under specific environmental conditions, these bacteria also exhibit potential probiotic properties that may aid in the prevention of dental caries. Additionally, Lactobacillus is strongly associated with early childhood caries, a specific type of caries.
As a prevalent oral disease with a multifactorial etiology, dental caries have become a significant burden on the public health prevention system (1, 2). According to the World Health Organization, it is estimated that approximately 2.3 billion people worldwide are afflicted with dental caries of permanent teeth and 530 million children have caries of primary teeth (3, 4). Dental plaque refers to the bacterial biofilm attached to the surface of teeth, which is one of the causes of caries and one of the main goals of caries prevention and treatment.
It is postulated that the bacteria of the genus Lactobacillus play a crucial role in the further development of caries, particularly in dentin (5). They have consistently been identified at caries sites, whether in the past through traditional clinical isolation and cultivation technology (6, 7), later with 16S sequence analysis (8, 9), or more recently with deep sequencing (10). In recent years, research on Lactobacillus has shifted from its cariogenicity to the potential of anti-cariogenic probiotics. They constitute one of the potential probiotic approaches for the prevention of dental caries, which has been demonstrated both in vitro (11) and in clinical studies (12).
This complex relationship renders the study of Lactobacillus and dental caries less clear than that of Streptococcus mutans. The pathogenic mechanisms and specific contributions of lactobacilli are still subjects of debate. Against this backdrop, conducting a review of existing literature to elucidate the current state of research, and identifying hot topics is essential. This review aims to comprehensively analyze the research landscape of Lactobacillus in the context of dental caries, emphasizing its pathogenesis, epidemiology, and potential therapeutic applications. By critically evaluating existing findings, the review seeks to identify knowledge gaps, inspire innovative experimental approaches, and optimize research resource allocation.
Bibliometrics, a crucial research methodology, provides a comprehensive and systematic perspective through the analysis of the quantity and trends within a considerable body of relevant literature (13). Via statistical and quantitative analysis, bibliometrics facilitates the identification of trending topics, clarification of the trajectory of disciplinary development, and provision of crucial reference points for the academic community and decision-makers (14). In the field of dentistry, bibliometrics has been applied to research areas such as root caries (15), orthodontics (16), and oral oncology (17). To the best of our knowledge, there is currently no bibliometric analysis conducted in the research field related to lactobacilli and dental caries.
The search was performed on March 18, 2024, on the Web of Science Core Collections (WoSCC) database. When performing a bibliometric analysis, WoSCC is one of the most popular scientific source databases for the superiority of supplying data for reference analysis. The retrieval was Topic = (dental caries) AND Title = (Lactobacilli OR Lactobacillus). The inclusion criteria for this analysis were original research articles that explored the role of Lactobacillus in dental caries, either in terms of prevention or progression. Exclusion criteria included studies that did not involve Lactobacillus or dental caries, those that did not report caries-related outcomes, and studies that were non-peer-reviewed or just abstracts or reviews. The full record and cited references of the retrieved articles were exported in plain text format for subsequent analyses.
The article selection process followed a previously reported methodology (18). In brief, two independent reviewers (D.F and L.Y) conducted an autonomous screening of titles and abstracts, and when required, evaluated full texts to identify pertinent studies. Disagreements between the two reviewers were resolved through discussions facilitated by a third reviewer with substantial experience (L.Z).
The final included literature was exported and analyzed using the professional bibliometric analysis software VOSviewer, owing to its capability to generate clear and easily interpretable visualizations (19).
A total of 274 articles related to Lactobacillus and dental caries were retrieved. After screening, 21 articles were excluded (unrelated to dental caries = 16, non-article = 5). A total of 253 articles on Lactobacillus and dental caries were published and indexed in WoSCC, and the number of publications was generally on the increase (Figure 1).
Table 1 shows the top 10 most productive countries in the Lactobacillus and dental caries research area. Sweden emerged as the country with the highest publications (n = 34), followed by China (24) and the USA (22). Figure 2 illustrates the collaboration among 17 countries with more than 4 publications. Each node in the graph represents a country. Nodes of the same color indicate countries belonging to the same cluster. The lines between nodes represent collaborative relationships between the corresponding countries.
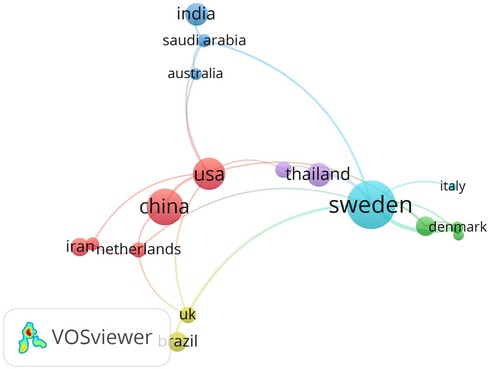
Figure 2. The cooperative network of countries in the Lactobacillus and dental caries research area.
Sweden, as the leading contributor in terms of publications, focuses its research primarily on two key areas: (1) the potential anti-caries properties of Lactobacillus as probiotics, including their effects on cariogenic pathogens such as S. mutans (20, 21) and their preventive efficacy in various populations, such as orthodontic patients, children with early caries lesions, high-caries-risk schoolchildren, and elderly individuals with root caries (22–25); (2) the load, and genotypes of Lactobacillus species in the oral environment or carious sites (26–29). The United States, the second-largest contributor to the publications, has placed particular emphasis on the interactions between various Lactobacillus species and other oral microorganisms, such as S. mutans, Candida albicans biofilms, and even multispecies biofilms (30–35).
Table 1 includes the top 10 institutions with the largest number of publications, with the University of Gothenburg being the only institution having more than 15 publications followed by Prince of Songkla University (15) and University of Turku (11).
The top 10 most productive authors are shown in Table 2. Teanpaisan R (15) emerge as the author with the highest publications, followed by Piwat S (11) and Twetman S (10). Besides, Twetman S acquires the highest 51 h-index. Teanpaisan R and Piwat S are affiliated with the same research institution, Prince Songkla University. Their research primarily focuses on the probiotic potential of Lactobacillus, particularly strains like L. rhamnosus SD11 and L. paracasei SD1 (36–41). Twetman's work has involved the use of L. reuteri and L. rhamnosus in the prevention of caries (23, 42, 43). In addition, his research has explored the potential of probiotics to bind fluoride (25, 44).
A total of 133 journals in the WoSCC database published studies relevant to Lactobacillus and dental caries. Table 3 shows the top 10 journals by the number of publications. Caries Research is the number one source with 24 articles, followed by Archives of Oral Biology with 20 articles and Journal of Dental Research with 10 articles.
The top 20 occurrent keywords are listed in Table 4. The “dental caries”, “Streptococcus mutans”, “lactobacilli”, “probiotics”, and “children” are the most used keywords. In VOSviewer, setting the minimum of occurrences of a keyword as 8, 45 keywords that met the requirement were used to depict the co-occurrence map (Figure 3). Nodes of the color correspond to the average time of the keywords appear. The lines between nodes represent the occurrence relationship. Table 4; Figure 3 shows the hot topics in the field of Lactobacillus and dental caries. The keywords “colonization,” “adherence,” “virulence,” and “pH” highlight a significant research focus on the pathogenic mechanisms of Lactobacillus in dental caries, while terms like “prevalence,” “strains,” and “risk” underscore efforts to elucidate the relationship between the abundance and distribution of Lactobacillus and the epidemiological characteristics of dental caries; (2) The frequent mention of the keyword “probiotics”, “prevention” and “milk” suggests a growing interest in exploring the potential probiotic effects of Lactobacillus in caries prevention and management; (3) early childhood caries (ECC).
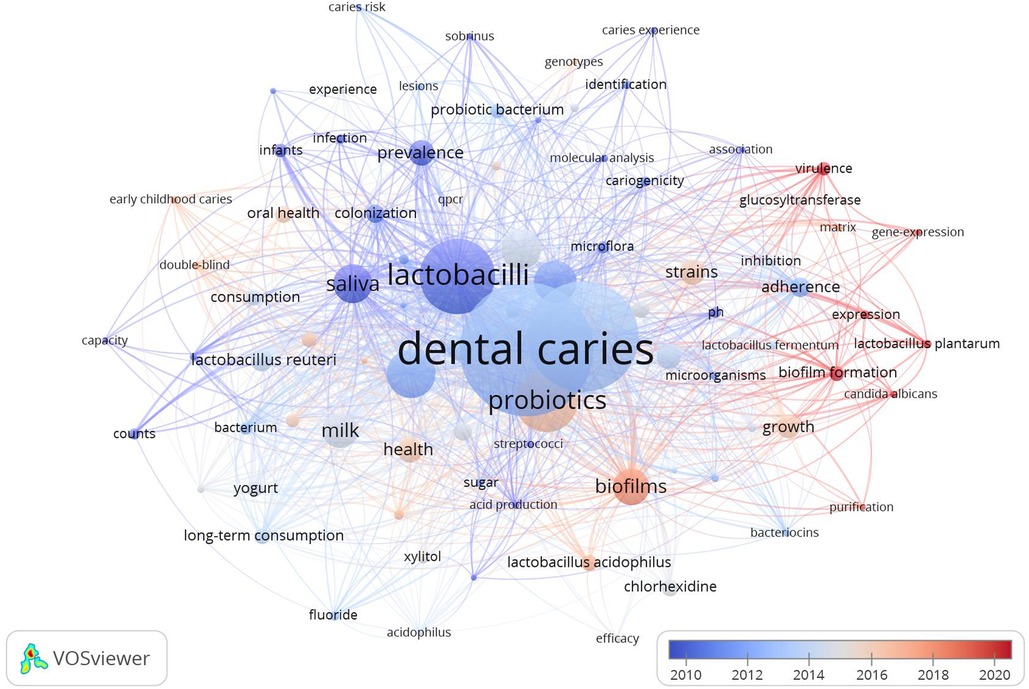
Figure 3. The co-occurrence analysis of keywords in the Lactobacillus and dental caries research area.
Information on the top ten highly cited articles is presented in Table 5. The most frequently cited article, with 376 citations, was a long-term clinical follow-up study of L. rhamnoses used in children with caries or high caries risk (45). The second most cited article, with 269 citations, was a study published in 2004 by Byun et al., which explored the genetic diversity of lactobacilli at advanced dental caries based on 16S ribosomal DNA technology (8).
To the best of our knowledge, this is the inaugural study to apply bibliometric analysis to the research domains of Lactobacillus and dental caries. Our review analyzes the relationship between lactobacilli and dental caries through the lens of visualized bibliometrics, evaluating the current state and future directions of this field based on empirical evidence rather than subjective opinions.
The increasing number of publications on the relationship between dental caries and lactobacilli over the years indicates a growing interest in this topic, suggesting that it is emerging as a significant research focus. The rising importance of dental caries prevention in clinical practice and public health may stimulate the research area. Cooperation between countries in Lactobacillus and dental caries research area predominantly concentrated among a few scientifically advanced nations, such as the United States, Sweden, which occupy central positions in the collaboration network. Authors such as Teanpaisan R, Piwat S, and Twetman S, with a high yield, may be leading figures in the research field of lactobacilli and dental caries, playing a significant role in driving progress and fostering innovation in this domain.
As mentioned in the results, the hot topics in the field of Lactobacillus and dental caries include the following three (Figure 4).
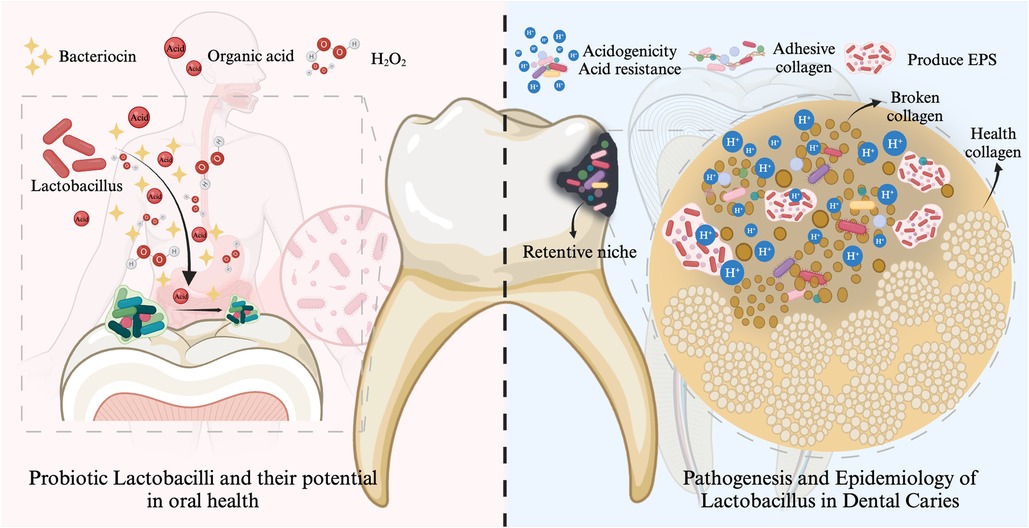
Figure 4. Major research hotspots in the field of Lactobacillus and dental caries. The cariogenic potential of Lactobacillus is primarily attributed to its acidogenicity, aciduricity, collagen adhesion properties, and biofilm-forming capacity. However, certain Lactobacillus strains may exhibit protective effects against dental caries by producing bacteriocins, H₂O₂, and organic acids, facilitating competitive adhesion and colonization, inhibiting cariogenic biofilms, and modulating the host immune response. Created with Biorender.com.
The cariogenic virulence of Lactobacillus has been summarized in the literature (46–48). The cariogenic potential of Lactobacillus is primarily attributed to its acidogenicity, aciduricity, adhesion collagen properties, and biofilm-forming capacity (Figure 4). Lactobacilli possess a diverse array of enzymes, including glycoside hydrolases, glycosyltransferases, and isomerases, which enable them to metabolize a wide range of carbohydrates, particularly the oligosaccharides and starches abundant in the oral cavity, resulting in acid production (46, 49). Various Lactobacillus species, including L. plantarum, L.salivarius, L. rhamnosus, and L. casei/paracasei, have demonstrated the ability to reduce the pH to the critical threshold for enamel demineralization (pH 5.5) within 2.5–3 h (50). Lactobacillus species have evolved complex physiological and molecular mechanisms to survive and adapt to acid stress. These mechanisms include modifications to the cell membrane, activation of ATPase proton pumps, metabolic regulation, and the production of macromolecular protective and repair proteins (51). Lactobacillus species exhibit relatively limited biofilm formation capabilities and weak adhesion to healthy tooth surfaces (31, 52, 53). However, it is noteworthy that when S. mutans and/or other early colonizers establish retention sites, the biofilm formation of Lactobacillus significantly increases (31, 54).
From an epidemiological point of view, the abundance and diversity of Lactobacillus are closely related to caries risk. Based on previous literature (46, 54, 55), we further summarized the species of Lactobacillus detected at caries sites (Table 6). It can be seen that the types of Lactobacillus detected in different populations/individuals are different.
Although Lactobacillus is frequently detected in the mouths of children with dental caries, it has not yet been definitively proven that the high detection rate of Lactobacillus directly leads to the development of caries. Some views suggest that Lactobacillus does not possess the conditions necessary to cause caries. On the contrary, the high detection rate may be more of a passive result of Lactobacillus being retained at the site of the lesion after caries develops (53, 54, 56). Future research should further explore the causal relationship between Lactobacillus and caries, considering its potential multiple mechanisms of action.
In recent years, probiotics have gained increasing attention in the prevention and treatment of dental caries biofilms. Numerous in vitro, animal, and clinical studies have demonstrated the significant potential of Lactobacillus in anti-caries activity. We have summarized these studies in Table 7 for the reader's reference. Certain Lactobacillus strains might demonstrate protective effects against caries through the production of bacteriocin (21, 32, 70), hydrogen peroxide (H2O2) and organic acid (71), competitive adhesion and colonization (72), inhibition of cariogenic biofilms (32, 73), and regulation of the host immune system (32, 41, 74) (Figure 4).
It is important to note that while lactobacilli have been extensively studied and applied in the prevention of dental caries, the individual variability in its probiotic efficacy remains incompletely understood. Individual differences in oral microbiota, dietary habits, and genetic factors significantly influence the efficacy of lactobacilli (75), with notable variability observed across different age groups, regions, and health conditions, posing challenges for its clinical application. Moreover, the long-term safety of lactobacilli remains an unresolved concern. The acidogenic properties of Lactobacillus may exacerbate the acidic environment in the oral cavity under certain conditions, thereby promoting the occurrence and progression of dental caries (76). Additionally, the adhesive properties and biofilm-forming ability of Lactobacillus may, in some cases, synergize with other cariogenic bacteria, increasing the risk of oral microbial dysbiosis (31, 77). Therefore, although Lactobacillus exhibits probiotic effects in promoting oral health, its safety in clinical applications requires further evaluation, particularly in high-risk populations such as individuals with a history of dental caries or those with compromised immune function (78).
It is notable that a particular type of caries emerged in the keyword analysis: early childhood caries (ECC). ECC, defined as the existence of one or more decayed teeth that have been extracted or filled in children under the age of 6, is one of the most prevalent diseases worldwide among children of this age group (79). Research on the relationship between ECC and Lactobacillus can be divided into two main categories: one group explores the anti-caries effects of Lactobacillus on children with ECC, while the other investigates the load of Lactobacillus in the oral environment of children with ECC, such as in saliva and dental plaque (Table 8). ECC progresses rapidly and, similar to rampant caries, can progress rapidly to dentin (90). Lactobacillus has a high affinity for dentin collagen (91–93). We speculate that this may be the reason why more lactobacilli tend to be detected at ECC lesion sites.
This study is founded on a bibliometric analysis of the included studies, which inherently possesses several limitations. Firstly, there exists the potential for publication bias. Although the utilization of the WoSCC database contributes to ensuring the credibility and authority of the included data, it might also constrain the comprehensiveness of the information. Additionally, the publications selected for the bibliometric analysis were initially identified through pre-searching with a predefined search formula and then manually filtered. However, the process of data exportation and filtering might result in data loss, misidentification of articles, or other problems. Moreover, analysis software VOSviewer still has scope for improvement. For instance, synonymous entities, such as authors, institutions, and keywords, might fail to be precisely identified or discriminated, giving rise to circumstances where the same institution, author, or synonym is not accurately merged. Furthermore, only English-language studies were encompassed within this analysis, which could have resulted in an underrepresentation of research capabilities from non-English-speaking countries.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
DF: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing. XS: Data curation, Formal Analysis, Project administration, Validation, Visualization, Writing – review & editing. LY: Data curation, Writing – original draft, Formal Analysis. GZ: Writing – review & editing. MJ: Data curation, Supervision, Writing – review & editing. GL: Methodology, Supervision, Writing – review & editing. YZ: Supervision, Writing – review & editing. LZ: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grant from the National Natural Science Foundation of China (grant no. 82071111).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Primers. (2017) 3:17030. doi: 10.1038/nrdp.2017.30
2. Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: a global public health challenge. Lancet. (2019) 394(10194):249–60. doi: 10.1016/S0140-6736(19)31146-8
3. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1789–858. doi: 10.1016/S0140-6736(18)32279-7
4. World Health Organization. Ending childhood dental caries: WHO implementation manual. Geneva: World Health Organization (2020). Available online at: https://www.who.int/publications/i/item/ending-childhood-dental-caries-who-implementation-manual (Accessed January 16, 2025).
6. Ellen R, Banting D, Fillery E. Streptococcus mutans and Lactobacillus detection in the assessment of dental root surface caries risk. J Dent Res. (1985) 64(10):1245–9. doi: 10.1177/00220345850640101301
7. Arneberg P, Ogaard B, Scheie AA, Rölla G. Selection of Streptococcus mutans and Lactobacilli in an intra-oral human caries model. J Dent Res. (1984) 63(10):1197–200. doi: 10.1177/00220345840630100501
8. Byun R, Nadkarni MA, Chhour K-L, Martin FE, Jacques NA, Hunter N. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J Clin Microbiol. (2004) 42(7):3128–36. doi: 10.1128/JCM.42.7.3128-3136.2004
9. Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, Janies DA, et al. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol. (2010) 48(11):4121–8. doi: 10.1128/JCM.01232-10
10. Liu G, Wu C, Abrams W, Li Y. Structural and functional characteristics of the microbiome in deep-dentin caries. J Dent Res. (2020) 99(6):713–20. doi: 10.1177/0022034520913248
11. Wu CC, Lin CT, Wu CY, Peng WS, Lee MJ, Tsai YC. Inhibitory effect of Lactobacillus salivarius on Streptococcus mutans biofilm formation. Mol Oral Microbiol. (2015) 30(1):16–26. doi: 10.1111/omi.12063
12. Stensson M, Koch G, Coric S, Abrahamsson T, Jenmalm M, Birkhed D, et al. Oral administration of Lactobacillus reuteri during the first year of life reduces caries prevalence in the primary dentition at 9 years of age. Caries Res. (2014) 48(2):111–7. doi: 10.1159/000354412
13. Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. (2021) 133:285–96. doi: 10.1016/j.jbusres.2021.04.070
14. Mejia C, Wu M, Zhang Y, Kajikawa Y. Exploring topics in bibliometric research through citation networks and semantic analysis. Front Res Metr Anal. (2021) 6:742311. doi: 10.3389/frma.2021.742311
15. Ji M, Fu D, Liao G, Zou L. A bibliometric analysis of studies on root caries. Caries Res. (2023) 57(1):32–42. doi: 10.1159/000529050
16. Tarazona B, Lucas-Dominguez R, Paredes-Gallardo V, Alonso-Arroyo A, Vidal-Infer A. The 100 most-cited articles in orthodontics: a bibliometric study. Angle Orthod. (2018) 88(6):785–96. doi: 10.2319/012418-65.1
17. Yang X, Yang X, Ji T, Zhou Q, Liu W. A bibliometric analysis of the papers on oral potentially malignant disorder in oral oncology. Oral Oncol. (2022) 132:105996. doi: 10.1016/j.oraloncology.2022.105996
18. Liu F, Wu TT, Lei G, Fadlelseed AFA, Xie N, Wang DY, et al. Worldwide tendency and perspectives in traumatic dental injuries: a bibliometric analysis over two decades (1999–2018). Dental Traumatol. (2020) 36(5):489–97. doi: 10.1111/edt.12555
19. Van Eck N, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3
20. Keller MK, Hasslöf P, Stecksén-Blicks C, Twetman S. Co-aggregation and growth inhibition of probiotic lactobacilli and clinical isolates of mutans streptococci: an in vitro study. Acta Odontol Scand. (2011) 69(5):263–8. doi: 10.3109/00016357.2011.554863
21. Teanpaisan R, Piwat S, Dahlén G. Inhibitory effect of oral Lactobacillus against oral pathogens. Lett Appl Microbiol. (2011) 53(4):452–9. doi: 10.1111/j.1472-765X.2011.03132.x
22. Alforaidi S, Bresin A, Almosa N, Lehrkinder A, Lingström P. Effect of drops containing Lactobacillus reuteri (DSM 17938 and ATCC PTA 5289) on plaque acidogenicity and other caries-related variables in orthodontic patients. BMC Microbiol. (2021) 21:1–10. doi: 10.1186/s12866-021-02310-2
23. Keller M, Larsen IN, Karlsson I, Twetman S. Effect of tablets containing probiotic bacteria (Lactobacillus reuteri) on early caries lesions in adolescents: a pilot study. Benef Microbes. (2014) 5(4):403–7. doi: 10.3920/bm2013.0089
24. Campus G, Cocco F, Carta G, Cagetti MG, Simark-Mattson C, Strohmenger L, et al. Effect of a daily dose of Lactobacillus brevis CD2 lozenges in high caries risk schoolchildren. Clin Oral Investig. (2014) 18:555–61. doi: 10.1007/s00784-013-0980-9
25. Petersson LG, Magnusson K, Hakestam U, Baigi A, Twetman SJAOS. Reversal of primary root caries lesions after daily intake of milk supplemented with fluoride and probiotic lactobacilli in older adults. Acta Odontol Scand. (2011) 69(6):321–7. doi: 10.3109/00016357.2011.568962
26. Piwat S, Teanpaisan R, Thitasomakul S, Thearmontree A, Dahlén G. Lactobacillus species and genotypes associated with dental caries in Thai preschool children. Mol Oral Microbiol. (2010) 25(2):157–64. doi: 10.1111/j.2041-1014.2009.00556.x
27. Teanpaisan R, Hintao J, Dahlén GJA. Oral Lactobacillus species in type 2 diabetic patients living in southern Thailand. Anaerobe. (2009) 15(4):160–3. doi: 10.1016/j.anaerobe.2009.01.004
28. Sullivan A, Borgström MK, Granath L, Nilsson G. Number of mutans streptococci or lactobacilli in a total dental plaque sample does not explain the variation in caries better than the numbers in stimulated whole saliva. Community Dent Oral Epidemiol. (1996) 24(3):159–63. doi: 10.1111/j.1600-0528.1996.tb00834.x
29. Köhler B, Bjarnason S, Finnbogason SY, Holbrook WP. Mutans streptococci, lactobacilli and caries experience in 12-year-old Icelandic urban children, 1984 and 1991. Community Dent Oral Epidemiol. (1995) 23(2):65–8. doi: 10.1111/j.1600-0528.1995.tb00202.x
30. Bao J, Huang X, Zeng Y, Wu TT, Lu X, Meng G, et al. Dose-dependent inhibitory effect of probiotic Lactobacillus plantarum on Streptococcus mutans-Candida albicans cross-kingdom microorganisms. Pathogens (Basel, Switzerland). (2023) 12(6):848. doi: 10.3390/pathogens12060848
31. Wen ZT, Liao S, Bitoun JP, De A, Jorgensen A, Feng S, et al. Streptococcus mutans displays altered stress responses while enhancing biofilm formation by Lactobacillus casei in mixed-species consortium. Front Cell Infect Microbiol. (2017) 7:524. doi: 10.3389/fcimb.2017.00524
32. Wasfi R, Abd El-Rahman OA, Zafer MM, Ashour HM. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J Cell Mol Med. (2018) 22(3):1972–83. doi: 10.1111/jcmm.13496
33. Zeng Y, Fadaak A, Alomeir N, Wu TT, Rustchenko E, Qing S, et al. Lactobacillus plantarum disrupts S. mutans-C. albicans cross-kingdom biofilms. Front Cell Infect Microbiol. (2022) 12:872012. doi: 10.3389/fcimb.2022.872012
34. Zeng Y, Fadaak A, Alomeir N, Wu Y, Wu TT, Qing S, et al. Effect of probiotic Lactobacillus plantarum on Streptococcus mutans and Candida albicans clinical isolates from children with early childhood caries. Int J Mol Sci. (2023) 24(3):2991. doi: 10.3390/ijms24032991
35. Srivastava N, Ellepola K, Venkiteswaran N, Chai LYA, Ohshima T, Seneviratne CJ. Lactobacillus Plantarum 108 inhibits Streptococcus mutans and Candida albicans mixed-Species biofilm formation. Antibiotics. (2020) 9(8):478. doi: 10.3390/antibiotics9080478
36. Piwat S, Pahumunto N, Srisommai P, Mapaisansin C, Teanpaisan R. Effect of probiotic delivery vehicles for probiotic Lactobacillus rhamnosus SD11 in caries prevention: a clinical study. J Food Process Preserv. (2019) 43(10):e14147. doi: 10.1111/jfpp.14147
37. Ritthagol W, Saetang C, Teanpaisan R. Effect of probiotics containing Lactobacillus paracasei SD1 on salivary mutans streptococci and lactobacilli in orthodontic cleft patients: a double-blinded, randomized, placebo-controlled study. Cleft Palate Craniofac J. (2014) 51(3):257–63. doi: 10.1597/12-243
38. Teanpaisan R, Piwat S. Lactobacillus paracasei SD1, a novel probiotic, reduces mutans streptococci in human volunteers: a randomized placebo-controlled trial. Clin Oral Investig. (2014) 18:857–62. doi: 10.1007/s00784-013-1057-5
39. Pahumunto N, Piwat S, Chankanka O, Akkarachaneeyakorn N, Rangsitsathian K, Teanpaisan R. Reducing mutans streptococci and caries development by Lactobacillus paracasei SD1 in preschool children: a randomized placebo-controlled trial. Acta Odontol Scand. (2018) 76(5):331–7. doi: 10.1080/00016357.2018.1453083
40. Pahumunto N, Piwat S, Chanvitan S, Ongwande W, Uraipan S, Teanpaisan R. Fermented milk containing a potential probiotic Lactobacillus rhamnosus SD11 with maltitol reduces Streptococcus mutans: a double-blind, randomized, controlled study. J Dent Sci. (2020) 15(4):403–10. doi: 10.1016/j.jds.2020.03.003
41. Pahumunto N, Sophatha B, Piwat S, Teanpaisan R. Increasing salivary IgA and reducing Streptococcus mutans by probiotic Lactobacillus paracasei SD1: a double-blind, randomized, controlled study. J Dent Sci. (2019) 14(2):178–84. doi: 10.1016/j.jds.2019.01.008
42. Gizani S, Petsi G, Twetman S, Caroni C, Makou M, Papagianoulis L. Effect of the probiotic bacterium Lactobacillus reuteri on white spot lesion development in orthodontic patients. Eur J Orthod. (2016) 38(1):85–9. doi: 10.1093/ejo/cjv015
43. Marttinen A, Haukioja A, Karjalainen S, Nylund L, Satokari R, Öhman C, et al. Short-term consumption of probiotic lactobacilli has no effect on acid production of supragingival plaque. Clin Oral Investig. (2012) 16:797–803. doi: 10.1007/s00784-011-0584-1
44. Stecksén-Blicks C, Sjöström I, Twetman S. Effect of long-term consumption of milk supplemented with probiotic lactobacilli and fluoride on dental caries and general health in preschool children: a cluster-randomized study. Caries Res. (2009) 43(5):374–81. doi: 10.1159/000235581
45. Näse L, Hatakka K, Savilahti E, Saxelin M, Pönkä A, Poussa T, et al. Effect of long–term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. (2001) 35(6):412–20. doi: 10.1159/000047484
46. Ahirwar S, Gupta M, Snehi SK. Dental caries and Lactobacillus: role and ecology in the oral cavity. Int J Pharm Sci Res. (2019) 10:4818–29. doi: 10.13040/IJPSR.0975-8232.10(11).4818-29
47. Wen ZT, Huang X, Ellepola K, Liao S, Li Y. Lactobacilli and human dental caries: more than mechanical retention. Microbiology. (2022) 168(6):001196. doi: 10.1099/mic.0.001196
48. Jin Z, Xin X. Research progress in the relationship between lactobacillus and dental caries. Sichuan Da Xue Xue Bao Yi Xue Ban. (2022) 53(5):929–34. doi: 10.12182/20220960103
49. Abriouel H, Pérez Montoro B, Casimiro-Soriguer CS, Pérez Pulido AJ, Knapp CW, Caballero Gómez N, et al. Insight into potential probiotic markers predicted in Lactobacillus pentosus MP-10 genome sequence. Front Microbiol. (2017) 8:891. doi: 10.3389/fmicb.2017.00891
50. Piwat S, Teanpaisan R, Dahlén G, Thitasomakul S, Douglas CW. Acid production and growth by oral Lactobacillus species in vitro. J Investig Clin Dent. (2012) 3(1):56–61. doi: 10.1111/j.2041-1626.2011.00098.x
51. Guan N, Liu L. Microbial response to acid stress: mechanisms and applications. Appl Microbiol Biotechnol. (2020) 104(1):51–65. doi: 10.1007/s00253-019-10226-1
52. Wen ZT, Yates D, Ahn SJ, Burne RA. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. (2010) 10:111. doi: 10.1186/1471-2180-10-111
53. Van Houte J, Gibbons RJ, Pulkkinen AJ. Ecology of human oral lactobacilli. Infect Immun. (1972) 6(5):723–9. doi: 10.1128/iai.6.5.723-729.1972
54. Caufield P, Schön C, Saraithong P, Li Y, Argimón S. Oral Lactobacilli and dental caries. J Dent Res. (2015) 94(9_suppl):110S–8. doi: 10.1177/0022034515576052
55. Badet C, Thebaud NB. Ecology of lactobacilli in the oral cavity: a review of literature. Open Microbiol J. (2008) 2:38–48. doi: 10.2174/1874285800802010038
56. McGrady JA, Butcher WG, Beighton D, Switalski LM. Specific and charge interactions mediate collagen recognition by oral lactobacilli. J Dent Res. (1995) 74(2):649–57. doi: 10.1177/00220345950740020501
57. Banakar M, Pourhajibagher M, Etemad-Moghadam S, Mehran M, Yazdi MH, Haghgoo R, et al. Antimicrobial effects of postbiotic mediators derived from Lactobacillus rhamnosus GG and Lactobacillus reuteri on Streptococcus mutans. Front Biosci. (2023) 28(5):88. doi: 10.31083/j.fbl2805088
58. Liang J, Zhou Y, Tang G, Wu R, Lin H. Exploration of the main antibiofilm substance of Lactobacillus plantarum ATCC 14917 and its effect against Streptococcus mutans. Int J Mol Sci. (2023) 24(3):1986. doi: 10.3390/ijms24031986
59. Gu M, Cheng J, Lee YG, Cho JH, Suh JW. Discovery of novel iminosugar compounds produced by Lactobacillus paragasseri MJM60645 and their anti-biofilm activity against Streptococcus mutans. Microbiol Spectr. (2022) 10(4):e0112222. doi: 10.1128/spectrum.01122-22
60. Luan C, Jiang N, Zhou X, Zhang C, Zhao Y, Li Z, et al. Antibacterial and anti-biofilm activities of probiotic Lactobacillus curvatus BSF206 and Pediococcus pentosaceus AC1-2 against Streptococcus mutans. Microb Pathog. (2022) 164:105446. doi: 10.1016/j.micpath.2022.105446
61. Jung HY, Cai JN, Yoo SC, Kim SH, Jeon JG, Kim D. Collagen peptide in a combinatorial treatment with Lactobacillus rhamnosus inhibits the cariogenic properties of Streptococcus mutans: an in vitro study. Int J Mol Sci. (2022) 23(3):1860. doi: 10.3390/ijms23031860
62. Noda M, Sugihara N, Sugimoto Y, Hayashi I, Sugimoto S, Danshiitsoodol N, et al. Lactobacillus reuteri BM53-1 produces a compound that inhibits sticky glucan synthesis by Streptococcus mutans. Microorganisms. (2021) 9(7):1390. doi: 10.3390/microorganisms9071390
63. Jang HJ, Kim JH, Lee NK, Paik HD. Inhibitory effects of Lactobacillus brevis KU15153 against Streptococcus mutans KCTC 5316 causing dental caries. Microb Pathog. (2021) 157:104938. doi: 10.1016/j.micpath.2021.104938
64. Guo M, Wu J, Hung W, Sun Z, Zhao W, Lan H, et al. Lactobacillus paracasei ET-22 suppresses dental caries by regulating Microbiota of dental plaques and inhibiting biofilm formation. Nutrients. (2023) 15(15). doi: 10.3390/nu15153316
65. Zhang Q, Qin S, Xu X, Zhao J, Zhang H, Liu Z, et al. Inhibitory effect of Lactobacillus plantarum CCFM8724 towards Streptococcus mutans- and Candida albicans-induced caries in rats. Oxid Med Cell Longevity. (2020) 2020:1. doi: 10.1155/2020/4345804
66. Weng L, Wu L, Guo R, Ye J, Liang W, Wu W, et al. Lactobacillus cell envelope-coated nanoparticles for antibiotic delivery against cariogenic biofilm and dental caries. J Nanobiotechnology. (2022) 20(1):356. doi: 10.1186/s12951-022-01563-x
67. Zhang G, Lu M, Liu R, Tian Y, Vu VH, Li Y, et al. Inhibition of Streptococcus mutans biofilm formation and virulence by Lactobacillus plantarum K41 isolated from traditional sichuan pickles. Front Microbiol. (2020) 11:774. doi: 10.3389/fmicb.2020.00774
68. Lin T-H, Pan T-M. Inhibitory effect of Lactobacillus paracasei subsp. paracasei NTU 101 on rat dental caries. J Funct Foods. (2014) 10:223–31. doi: 10.1016/j.jff.2014.06.015
69. Staszczyk M, Jamka-Kasprzyk M, Kościelniak D, Cienkosz-Stepańczak B, Krzyściak W, Jurczak A. Effect of a short-term intervention with Lactobacillus salivarius probiotic on early childhood caries-an open label randomized controlled trial. Int J Environ Res Public Health. (2022) 19(19):12447. doi: 10.3390/ijerph191912447
70. Wannun P, Piwat S, Teanpaisan R. Purification and characterization of bacteriocin produced by oral Lactobacillus paracasei SD1. Anaerobe. (2014) 27:17–21. doi: 10.1016/j.anaerobe.2014.03.001
71. Kang M-S, Oh J-S, Lee H-C, Lim H-S, Lee S-W, Yang K-H, et al. Inhibitory effect of Lactobacillus reuteri on periodontopathic and cariogenic bacteria. J Microbiol. (2011) 49:193–9. doi: 10.1007/s12275-011-0252-9
72. Haukioja A, Loimaranta V, Tenovuo J. Probiotic bacteria affect the composition of salivary pellicle and streptococcal adhesion in vitro. Oral Microbiol Immunol. (2008) 23(4):336–43. doi: 10.1111/j.1399-302X.2008.00435.x
73. Jeong D, Kim D-H, Song K-Y, Seo K-H. Antimicrobial and anti-biofilm activities of Lactobacillus kefiranofaciens DD2 against oral pathogens. J Oral Microbiol. (2018) 10(1):1472985. doi: 10.1080/20002297.2018.1472985
74. Wattanarat O, Nirunsittirat A, Piwat S, Manmontri C, Teanpaisan R, Pahumunto N, et al. Significant elevation of salivary human neutrophil peptides 1–3 levels by probiotic milk in preschool children with severe early childhood caries: a randomized controlled trial. Clin Oral Investig. (2021) 25:2891–903. doi: 10.1007/s00784-020-03606-9
75. Lundtorp-Olsen C, Markvart M, Twetman S, Belstrøm D. Effect of probiotic supplements on the oral Microbiota-a narrative review. Pathogens. (2024) 13(5):419. doi: 10.3390/pathogens13050419
76. Homayouni Rad A, Pourjafar H, Mirzakhani E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front Cell Infect Microbiol. (2023) 13:1120995. doi: 10.3389/fcimb.2023.1120995
77. Filoche SK, Anderson SA, Sissons CH. Biofilm growth of Lactobacillus species is promoted by Actinomyces species and Streptococcus mutans. Oral Microbiol Immunol. (2004) 19(5):322–6. doi: 10.1111/j.1399-302x.2004.00164.x
78. Chugh P, Dutt R, Sharma A, Bhagat N, Dhar MS. A critical appraisal of the effects of probiotics on oral health. J Funct Foods. (2020) 70:103985. doi: 10.1016/j.jff.2020.103985
79. Phantumvanit P, Makino Y, Ogawa H, Rugg-Gunn A, Moynihan P, Petersen PE, et al. WHO global consultation on public health intervention against early childhood caries. Community Dent Oral Epidemiol. (2018) 46(3):280–7. doi: 10.1111/cdoe.12362
80. Leme L, Rizzardi KF, Santos IB, Parisotto TM. Exploring the relationship between salivary levels of TNF-α, Lactobacillus acidophilus, Lactobacillus gasseri, obesity, and caries in early childhood. Pathogens. (2022) 11(5):579. doi: 10.3390/pathogens11050579
81. Indiani C, Rizzardi KF, Crescente CL, Steiner-Oliveira C, Nobre-Dos-Santos M, Parisotto TM. Relationship between mutans streptococci and lactobacilli in the oral cavity and intestine of obese and eutrophic children with early childhood caries-preliminary findings of a cross-sectional study. Front Pediatr. (2020) 8:588965. doi: 10.3389/fped.2020.588965
82. Reis ACM, Bezerra DDS, Hart-Chú ENS, Stipp RN, Guedes SFF, Neves BG, et al. Quantification and gene expression of Lactobacillus casei group species associated with dentinal lesions in early childhood caries. Saudi Dent J. (2021) 33(2):69–77. doi: 10.1016/j.sdentj.2020.01.006
83. Liu JF, Hsu CL, Chen LR. Correlation between salivary mutans streptococci, lactobacilli and the severity of early childhood caries. J Dent Sci. (2019) 14(4):389–94. doi: 10.1016/j.jds.2019.06.003
84. Klinke T, Urban M, Lück C, Hannig C, Kuhn M, Krämer N. Changes in Candida spp., mutans streptococci and lactobacilli following treatment of early childhood caries: a 1-year follow-up. Caries Res. (2014) 48(1):24–31. doi: 10.1159/000351673
85. Ramamurthy PH, Swamy HS, Bennete F, Rohini M, Nagarathnamma T. Relationship between severe-early childhood caries, salivary mutans streptococci, and lactobacilli in preschool children of low socioeconomic status in Bengaluru city. J Indian Soc Pedod Prev Dent. (2014) 32(1):44–7. doi: 10.4103/0970-4388.127054
86. Mitrakul K, Chanvitan S, Jeamset A, Vongsawan K. Quantitative analysis of S. mutans, Lactobacillus and Bifidobacterium found in initial and mature plaques in Thai children with early childhood caries. Eur Arch Paediatr Dent. (2017) 18(4):251–61. doi: 10.1007/s40368-017-0295-7
87. Hasslöf P, West CE, Videhult FK, Brandelius C, Stecksén-Blicks C. Early intervention with probiotic Lactobacillus paracasei F19 has no long-term effect on caries experience. Caries Res. (2013) 47(6):559–65. doi: 10.1159/000350524
88. Krzyściak W, Kościelniak D, Papież M, Vyhouskaya P, Zagórska-Świeży K, Kołodziej I, et al. Effect of a Lactobacillus salivarius probiotic on a double-species Streptococcus mutans and Candida albicans caries biofilm. Nutrients. (2017) 9(11):1242. doi: 10.3390/nu9111242
89. Plonka KA, Pukallus ML, Barnett AG, Walsh LJ, Holcombe TF, Seow WK. A longitudinal study comparing mutans streptococci and lactobacilli colonisation in dentate children aged 6 to 24 months. Caries Res. (2012) 46(4):385–93. doi: 10.1159/000339089
90. Agim B, Merita B, Shefqet M, Blerta Xhemajli L, Prokshi , Haliti , et al. Chapter 2. Early childhood caries (ECC) — etiology, clinical consequences and prevention. In: Mandeep Singh V, editor. Emerging Trends in Oral Health Sciences and Dentistry. Rijeka: IntechOpen (2015). doi: 10.5772/59416
91. Lapirattanakul J, Nomura R, Okawa R, Morimoto S, Tantivitayakul P, Maudcheingka T, et al. Oral lactobacilli related to caries status of children with primary dentition. Caries Res. (2020) 54(2):194–204. doi: 10.1159/000506468
92. Salzillo M, Vastano V, Capri U, Muscariello L, Sacco M, Marasco R. Identification and characterization of enolase as a collagen-binding protein in Lactobacillus plantarum. J Basic Microbiol. (2015) 55(7):890–7. doi: 10.1002/jobm.201400942
Keywords: lactobacilli, dental caries, cariogenicity, probiotics, caries prevention, early childhood caries, bibliometrics
Citation: Fu D, Shu X, Yao L, Zhou G, Ji M, Liao G, Zhu Y and Zou L (2025) Unveiling the dual nature of Lactobacillus: from cariogenic threat to probiotic protector—a critical review with bibliometric analysis. Front. Oral. Health 6:1535233. doi: 10.3389/froh.2025.1535233
Received: 27 November 2024; Accepted: 13 January 2025;
Published: 31 January 2025.
Edited by:
Keke Zhang, Wenzhou Medical University, ChinaCopyright: © 2025 Fu, Shu, Yao, Zhou, Ji, Liao, Zhu and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunwo Zhu, emh1eXVud29AMTYzLmNvbQ==; Ling Zou, em91bGluZ0BzY3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.