
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Oral. Health, 10 February 2025
Sec. Cardiometabolic Health
Volume 6 - 2025 | https://doi.org/10.3389/froh.2025.1488941
This article is part of the Research TopicOral Inflammation as an Emerging Risk Factor for Cardiovascular HealthView all 3 articles
 Ana Molina1*
Ana Molina1* Nagore Ambrosio1
Nagore Ambrosio1 María Molina2
María Molina2 Eduardo Montero1
Eduardo Montero1 Leire Virto1
Leire Virto1 David Herrera1
David Herrera1 Elena Figuero1
Elena Figuero1 Mariano Sanz1
Mariano Sanz1
Aim: To investigate the effect of periodontal therapy on endothelial function of subjects with periodontitis in stages III or IV and established cardiovascular disease.
Materials and methods: A triple-blinded, parallel groups, randomized clinical trial of 6 months duration, on patients with history of coronary heart disease and periodontitis in stages III or IV was performed. Intervention consisted of steps 1 (oral hygiene instructions and professional mechanical plaque removal) and 2 (subgingival instrumentation) of periodontal therapy, including an antiseptic mouth rinse for 7 days. Patients in the control group received only step 1, with the adjunctive use of a fluoride-containing mouth rinse. Endothelial function (flow-mediated dilation [FMD]) and carotid intima-media thickness (cIMT) at baseline, 3 and 6 months, and serum markers of inflammation and cell adhesion at 3 days, 10 days, 3 and 6 months after therapy, were evaluated. Demographic characteristics, cardiovascular risk factors, history of cardiovascular diseases, medication intake, lipids profile, blood pressure, and periodontal outcomes were also evaluated. Student T, Mann–Whitney U, Chi-square and Fisher-exact tests were performed along with repeated measures ANOVA with post hoc Bonferroni's corrections.
Results: Thirty-five patients were included. In the test group, improvements in pocket depth, bleeding on probing and suppuration at 6 months were significantly better than in control patients. Reductions in mean FMD [test group −3.43%; 95% confidence interval—CI [−2.68; 9.54], p = 0.487; control group −6.75%; 95% CI [1.29; 12.22], p = 0.012] and cIMT (test group −0.05 mm; 95% CI [0.01; 0.10], p = 0.014; control group −0.01 mm; 95% CI [−0.03; 0.05], p = 1.000) were observed in both groups from baseline to 6 months, without significant intergroup differences at any time-point. Differences between groups in serum inflammatory markers were detected at baseline and 3 days for interleukin (IL)-18, and at 10 days for IL-8.
Conclusion: Preliminary results from the present pilot study showed that steps 1 and 2 of periodontal treatment in subjects with periodontitis in stages III–IV and established cardiovascular disease induced improvements in cIMT and periodontal outcomes, although changes in FMD were not observed.
Clinical Trial Registration: clinicaltrials.gov, Identifier, database (NCT02716259).
Cardiovascular diseases (CVD) are non-communicable conditions affecting the heart and vessels, that include ischemic heart disease, cerebrovascular disease, peripheral arterial disease, and rheumatic heart disease, among others. CVD constitute the leading cause of death worldwide (1). The majority of these diseases have in common the pathobiological process of atherosclerosis, resulting in the formation of extracellular lipid deposits in the arterial walls. Although its aetiology is not fully elucidated, atherosclerosis onset and progression is influenced by the effect of various factors, including a predisposing genetic background, hypercholesterolemia, modified lipoproteins, hypertension, diabetes mellitus, smoking and certain infections (2, 3). Furthermore, since atheroma formation is a chronic inflammatory process within the vessel wall, it is influenced by systemic chronic inflammation (2, 4).
Periodontitis is a multifactorial chronic inflammatory disease, triggered by dysbiotic changes in the subgingival biofilm, and characterized by destruction of the tooth attachment apparatus (5), coupled with a dysregulated and non-resolving inflammatory response (6). Furthermore, this localized chronic inflammation results in a low-grade systemic inflammatory status, mainly in the cases of severe periodontitis (stages III–IV) (7). The impact of severe periodontitis on systemic inflammation, resulting in increased levels of inflammatory and pro-coagulation markers in serum, has been hypothesized as a plausible mechanism to explain the association of periodontitis with the pathobiology of atherosclerosis (7–10). Furthermore, certain oral bacteria, including some well-known periodontal pathogens, have shown capability to access the bloodstream and invade the inner walls of the vessels, thus hypothetically inducing and promoting the atherosclerotic lesion, as it has been demonstrated in mechanistic investigations in animal models (9, 11). Evidence from epidemiological studies have linked periodontitis with a higher risk of future CVD (12), subclinical atherosclerosis (13–15), established CVD (including coronary heart disease, stroke, and peripheral arterial disease) (16) and premature death from all causes and from CVD (17).
Current treatment of periodontitis is based, not only on removal of the subgingival biofilm deposits and associated bacteria, but also on risk factor control mainly through smoking cessation interventions (18). Beyond its effects in arresting periodontal inflammation, periodontal therapy has proven to be efficient in reducing systemic inflammation (7) and in improving certain surrogate measures of CVD on healthy subjects and in patients with high risk of CVD (9, 19). However, similar evidence on subjects with established CVD is limited and inconclusive (20–22).
It was, therefore, the objective of this pilot clinical trial to investigate the effect of steps 1 and 2 of periodontal therapy on the endothelial function (measured by flow-mediated dilation) in patients with periodontitis in stages III or IV and established CVD. As secondary objectives, the effect of periodontal therapy on periodontal parameters, carotid intima-media thickness, and serum levels of coagulation and systemic inflammation markers were evaluated.
This study was designed as a parallel-group, triple-blinded (patient, examiner and statistician) randomized clinical trial (RCT), with a 6-month follow-up, in patients with periodontitis in stages III or IV, and established CVD. The study protocol was designed in accordance with the Helsinki Declaration (2008) and it was approved by the Ethics Committee of Complutense University (Madrid, Spain) and Severo Ochoa University Hospital (Leganés, Madrid, Spain). The study protocol was registered at clinicaltrials.gov database (NCT02716259).
Participants were screened among patients attending the Unit of Cardiac Rehabilitation, at the Department of Cardiology of Severo Ochoa University Hospital in Leganés, Spain. All patients gave written informed consent prior to inclusion in the trial.
Patients had to be ≥18 years old and to present established CVD, i.e., history of a coronary acute syndrome (myocardial infarction or angina) in the past 3–12 months and a left ventricular ejection fraction ≥50%. Patients included had a diagnosis of periodontitis in stages III or IV according to the 2017 World Workshop on the Classification for Periodontal and Peri-implant Diseases and Conditions (5), with probing depths (PD) >5 mm and marginal bone loss >30% in ≥50% of their teeth, and a minimum of three teeth per quadrant.
Exclusion criteria were:
• Periodontal treatment in the previous 12 months.
• Antibiotic intake in the previous 3 months.
• Smokers of ≥10 cigarettes per day.
• Pregnant or nursing women.
• Diabetes mellitus type 1, or type 2 with HbA1c >7.
• HIV infection.
• Chronic use of non-steroid anti-inflammatory drugs.
• Necrotizing periodontal diseases.
Figure 1 depicts the study outline, with the intervention and measures recorded at every time point at Severo Ochoa University Hospital and at the Postgraduate Clinic in Periodontology at Complutense University.
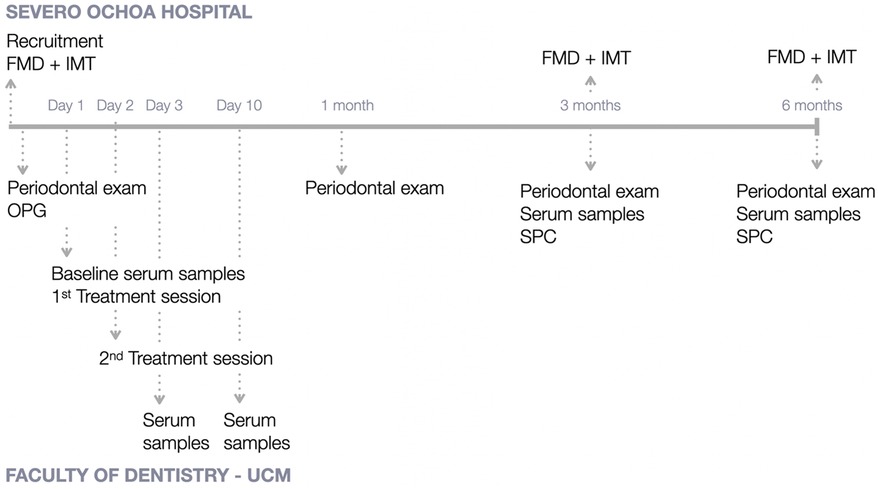
Figure 1. Study outline. FMD, flow-mediated dilation; IMT, intima media thickness; OPG, orthopantomography; SPC, supportive periodontal care; UCM, University Complutense of Madrid.
After being included in the trial and once the baseline examinations were performed, patients were randomized to receive either subgingival instrumentation with ultrasonic devices, curettes and prophylaxis paste (within step 2 of periodontal therapy) with adjunctive use of a 0.12% chlorhexidine (CHX) and 0.05% cetylpyridinium chloride (CPC) mouth rinse (PerioAid® Tratamiento, Dentaid, Barcelona, Spain) for 7 days (test group), or professional mechanical plaque removal (PMPR) with ultrasonic devices, curettes and prophylaxis paste (within step 1 of periodontal therapy) with adjunctive use of a fluoride mouth rinse (FluorAid®, Dentaid, Barcelona, Spain) for 7 days (control group), in two consecutive sessions under local anaesthesia.
Randomization was prepared by an independent researcher using block randomization (size of the block = 6). Allocation concealment was kept by means of opaque sealed envelopes, and randomization was revealed to the operator immediately before starting the intervention.
All procedures were performed by a single trained periodontist (AM). Subjects in both groups received step 1 of periodontal therapy, including oral hygiene instructions (modified Bass brushing technique and the use of interdental brushes). In follow-up visits (3- and 6-month), supportive periodontal care (SPC) was provided by the same operator, consisting of oral hygiene reinforcement and PMPR with ultrasonic devices, curettes and prophylaxis paste and subgingival instrumentation, if appropriate, in the test group, and oral hygiene reinforcement and PMPR alone in the control group.
Sociodemographic characteristics were collected at baseline by patient interview and included age, gender, smoking habit (never smoker, ex-smoker, current smoker), high level of stress (yes/no), sedentarism (yes/no), postmenopausal (yes/no), and family history of premature CVD (yes/no). Medical parameters were obtained from the hospital records of the participants, at baseline and at 6 months follow-up, including: allergies, history of other systemic diseases (besides CVD), medication intake, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG). Blood pressure (BP) measurements with a digital BP machine (Omron X2 Basic, Omron Healthcare Co. Ltd., Kyoto, Japan) and anthropometric measurements [height, weight, abdominal circumference, and body mass index (BMI)] were recorded by the researchers at baseline and 6 months.
An experienced cardiologist (MM) performed the recording of vascular parameters at baseline, 3- and 6-month of follow-up, at the Severo Ochoa University Hospital, with an ultrasound (Vivid E9, General Electric HealthCare, Horton, Norway) and a linear array transducer of 7 MHz (9l Ultrasound probe, General Electric HealthCare, Horton, Norway). Flow mediated dilation (FMD) was automatically measured (Brachial Tools, version 3.2.6, Medical Imaging Applications, Coralville, IA, USA) at the brachial artery in response to 250 mmHg arterial pressure exerted with a manual sphygmomanometer for 5 min. Dilation was calculated as the change, expressed in percentage, between baseline to the maximum dilation peak measured between 45 and 75 s after releasing the cuff pressure. Carotid intima-media thickness (cIMT) was evaluated by ultrasounds. With the patient in dorsal decubitus, images were taken from the dorsal walls of the common carotid at 1 cm from the carotid bifurcation. Maximal values of cIMT were calculated with an automatic border detection software for cIMT measurement (EchoPAC®, General Electric HealthCare, Horton, Norway).
Periodontal examinations were performed by a single trained periodontist (EM), blinded to the allocated treatment, at baseline, one month, three months and six months after therapy. PD, gingival recession (Rec), clinical attachment level (CAL), plaque index (PlI), bleeding on probing (BoP), and suppuration (Sup) were recorded at 6 sites in all teeth except third molars with a UNC-15 manual periodontal probe. Tooth mobility and furcation involvement were also assessed. Mean values of each periodontal variable were calculated per patient.
Serum samples were taken at baseline, 3-days, 10-days, 3-months and 6-months for the quantification of serum levels of inflammatory mediators (interleukin [IL]-1β, IL-6, IL-8, IL-10, IL-18 and tumour necrosis factor [TNF]-α) and endothelium activation markers (soluble intercellular adhesion molecule-1 [sICAM-1] and soluble vascular adhesion molecule 1 [sVCAM-1]). Peripheral venous blood samples were taken from the antecubital fossa or the dorsum of the hand using an intravenous catheter (VacutainerTM, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) with a conventional technique, according to the recommendations from the Spanish Society of Infectious Diseases and Clinical Microbiology (23). Samples were processed at the Microbiology Laboratory of the Faculty of Odontology at Complutense University of Madrid using high-sensitivity multiplex map human immunoassays. Laboratory tests are further explained in the Supplementary (Section 1.1).
The occurrence of adverse events, either cardiovascular or treatment-related, were recorded at every study visit.
Sample size was calculated to detect a 1% difference in FMD between groups, with a standard deviation (SD) of 1.67% (19) at a two-sided alpha level of 5% and 90% power. A total of 96 patients, 48 per group were required. To compensate for possible dropouts and adjustment for confounders, a final sample of 120 was established (20% more). Since the intended sample was not achieved, a post-hoc power and sample size calculation was performed.
The primary outcome variable was the change in FMD (baseline-6 months). Secondary outcomes included all other previously described. Changes between baseline and 6 months, baseline to 3 months and 3- to 6-month visits were calculated. A subject-level analysis was performed for each study parameter. Data were expressed by means and SD (for quantitative variables), prevalence and proportions (%) (for qualitative variables). Normality of the distribution of quantitative variables were assessed by means of Shapiro-Wilk normality test and box plots. Differences between groups in quantitative variables at baseline, 3- and 6-month visits and their changes were determined by the Student t test or Mann–Whitney U test for quantitative outcomes. Additionally, repeated measures ANOVA with post hoc Bonferroni's correction considering the visit for the intra-group comparisons, the group (intervention or control) for the inter-group comparisons, and the interaction between time and group, was performed. Categorical data were compared by means of the Chi-square test or Fisher-exact test. The level of statistical significance was set at p < 0.05. A statistical software package IBM®SPSS Statistics 29.0 (IBM Corporation, Armonk, NY, USA) was used for all data analysis.
Figure 2 depicts the flow chart of the study. A total of 440 subjects with history of coronary acute syndrome in the past 3–12 months, attending the Unit of Cardiac Rehabilitation of Severo Ochoa Hospital, were considered for inclusion. The screening period lasted from February 2016 to February 2020. Thirty-nine patients fulfilled the periodontal inclusion criteria and were invited to enter the trial; three of them refused to participate due to inability to travel to Madrid and attend the visits at the Faculty of Odontology. Finally, 36 patients were recruited and randomized; one of them could not receive the intervention assigned due to the onset of the Covid-19 pandemic, so data from 35 patients were included in the present study. Sixteen patients were randomized to test group and 19 to control group. One subject from control group was excluded at 3 months of follow-up due to having receive further periodontal treatment in a private clinic (Figure 2).
Baseline characteristics of the study sample are shown in Table 1. The mean age was 61.05 years in test group and 55.55 years in control group, and three participants were women (one test, two controls). Most of the participants were former smokers (75% test group, 73.7% control group), and three subjects (one test, two controls) were still smokers of <10 cigarettes per day. High stress levels were reported by 19 subjects (62.5% test group, 47.4% control group) and five subjects in each group referred to have family history of CVD (31.3% test group, 26.3% control group). Mean BMI in both treatment groups was in the over-weight category (>25).
Regarding cardiovascular disease diagnosis, all subjects had suffered a coronary acute syndrome 3–12 months prior to study inclusion and presented with left ventricular ejection fraction ≥50%. Most of the participants (87.5% test group, 84.2% control group) had received reperfusion therapy (stent placement) in one or more coronary arteries, and three participants had undergone bypass surgery (12.5% test group, 5.3% control group). ST-segment elevation acute coronary syndrome (STEACS) had occurred in seven (43.8%) subjects in the test and six (31.6%) subjects in the control group; one subject in the test group had also been diagnosed with peripheral arterial disease, and none had suffered a cerebrovascular accident. Other comorbidities, diagnosed prior to the onset of the CVD event, such as dyslipidaemia and hypertension, were frequent.
Medication intake at baseline and 6 months is summarised in Supplementary Table S1. In the selected sample population, all participants were under treatment with a minimum of three drugs: one antithrombotic, one hypolipidemic and one antihypertensive drug. At baseline, most subjects were under the combination of two antiplatelet drugs (81.3% test group, 89.5% control group), and four of them were also prescribed anticoagulant medication (acenocumarol) (18.8% test group, 5.3% control group). Patients were prescribed one to four antihypertensive medications, and eight subjects used vasodilators (25% test group, 21.1% control group). Significant differences between groups were detected for the number of subjects taking stomach protectors and their prescription at baseline, and for the number of antihypertensive drugs prescribed at 6 months.
Twenty-three patients were diagnosed of generalized periodontitis in stage III and 12 of periodontitis in stage IV, with grades B or C.
At baseline, no statistically significant differences between groups for any demographic characteristics, CVD status or periodontitis diagnosis were observed.
At baseline, no significant differences between groups were observed for any periodontal variable (Table 2). Periodontal therapy improved all periodontal parameters in both treatment groups, with significant differences for mean PD and BoP reductions between baseline and 3 months, and baseline and 6 months (Supplementary Tables S2, S3).
Periodontal parameters were significantly better in the test group, at 3 months, for PD and BoP, and at 6 months, for PD, BoP, and Sup. Frequency distribution of periodontal pockets showed a marked reduction of periodontal pockets ≥4 mm in test group at 3 and 6 months. Significantly differences were observed between groups in the frequency distribution of all categories of PD at 3 and 6 months, with greater percentage of periodontal pockets ≥4 mm in control group.
Mean values and differences between groups in FMD and cIMT values are depicted in Table 3 and Figure 3.
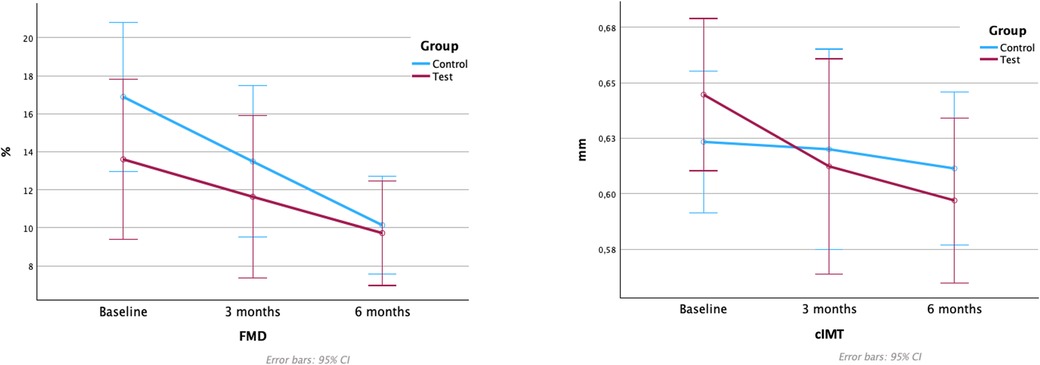
Figure 3. Flow-mediated dilation and carotid intima-media thickness values. FMD, flow-mediated dilation; cIMT, carotid intima-media thickness. Note: Figure based on data from the repeated measures ANOVA with post hoc Bonferroni's correction analysis.
A reduction in FMD was observed in both treatment groups, with statistically significant differences between baseline to 6 months in the control group [−6.75%, 95% CI (1.286; 12.217); p = 0.012] (Supplementary Table S4). At 3 months, the test group showed lower FMD, and at 6 months, the results were similar, with no significant differences between groups at any time-point.
No statistically significant differences in cIMT were detected between groups at any time point, with a tendency towards reductions in cIMT in both groups. The test group demonstrated significant reductions in cIMT from baseline to 6 months [−0.053 mm, 95% CI (0.009; 0.096); p = 0.014], while the corresponding changes were not statistically significant in the control group (Supplementary Table S5).
Table 4 shows the results of the lipid profile and blood pressure per visit. There were no statistically significant differences between groups at baseline or after 6 months. Subjects in the test group showed marked reductions in total cholesterol, triglycerides, HDL-C and LDL-C from baseline to 6 months, while in the control group these reductions were only observed for total cholesterol and LCL-C levels. There were no significant differences in BP between groups, with systolic and diastolic BP showing stable values in both groups during the 6 months follow-up.
One hundred and fifty-nine samples were collected, and two of them could not be processed due to technical problems, thus a total of 157 samples were analyzed. Values under the detection limit of the kit were not included in the analysis. Results are shown in Table 5 and Figure 4.
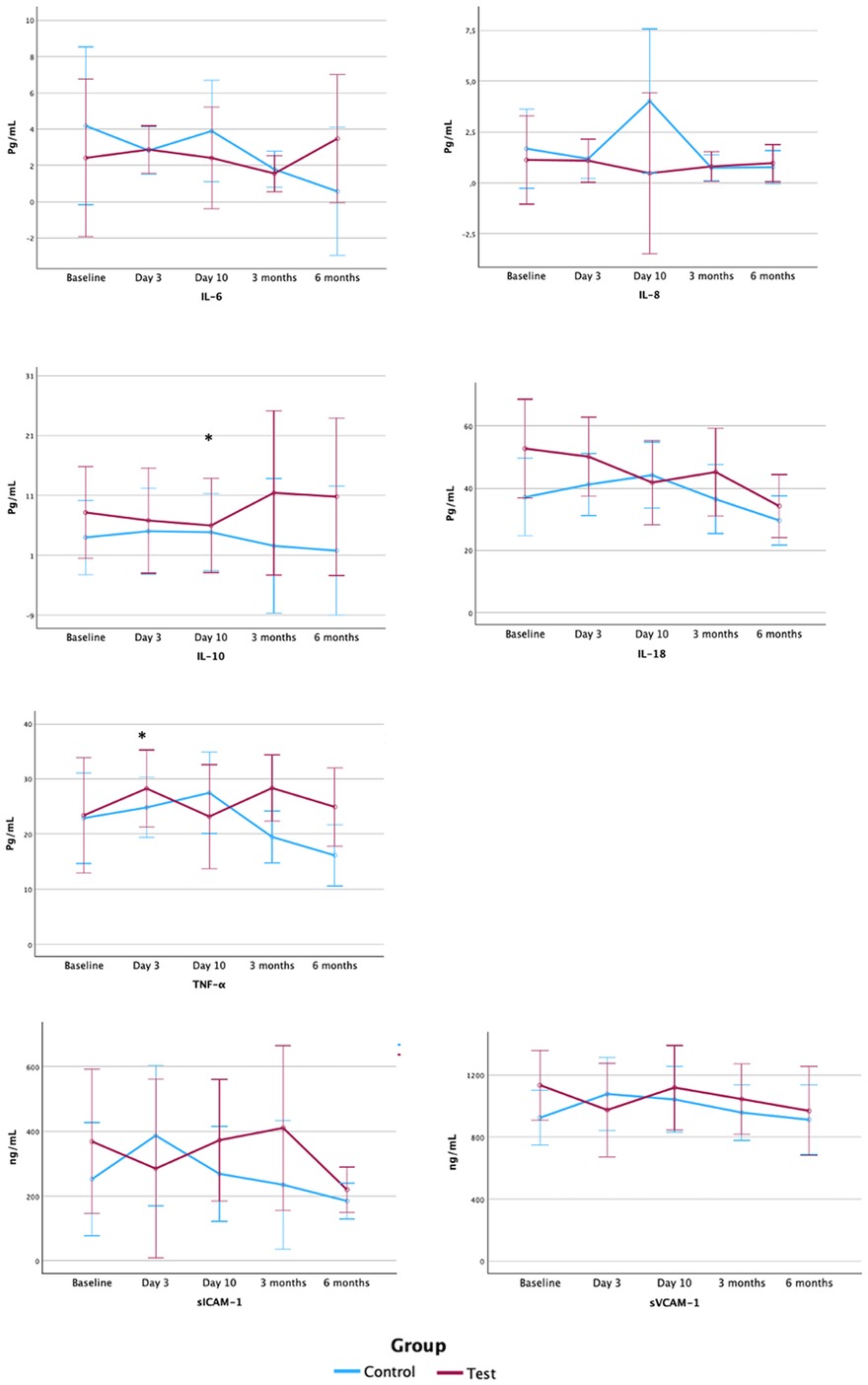
Figure 4. Serum levels of inflammatory mediators and endothelium activation markers. Pg, picogram; ml, millilitre; ng, nanogram; IL, interleukin; TNF, tumour necrosis factor; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule 1; Gr., group. Note: Figure based on data from the repeated measures ANOVA with post hoc Bonferroni's correction analysis.
Since only four samples presented values of IL-1β above the detection limit of the kit, data on this inflammatory mediator is not presented. Statistically significant differences between groups in serum inflammatory markers were detected at baseline and 3 days for IL-18, with lower levels in control group, and at 10 days for IL-8, with lower values in test group. No further differences between groups were observed for the remaining inflammatory mediators or the endothelium activation markers, sICAM-1 and sVCAM-1.
No systemic adverse events were recorded. Minor to moderate bleeding was reported 12 h after periodontal therapy in 4 subjects in the test group, which was controlled by means of local haemostatic measures (gauze pressure, use of aminocaproic acid solution).
Considering the reported results on the primary outcome (effect size of 2.87%, common SD of 8.14%), and assuming a two-sided alpha level of 5%, the power of the study resulted in 36%. Therefore, in order to obtain an 80% power, a total of 254 patients, 127 per group, would have been required (305 patients, considering a 20% more for drop-outs compensation and confounders adjustment).
The results from the present investigation reveal that periodontal therapy (steps 1 and 2, with subgingival instrumentation and adjunctive antiseptics) of stage III–IV periodontitis, in subjects with recent history of acute coronary heart disease, provided some benefits in terms of cardiovascular and periodontal outcomes, although statistically significant differences with the control group were not observed, most probably due to the limited sample size. cIMT experienced a statistically significant reduction in test group from baseline to 6 months follow-up (−0.05 mm), while no statistically significant changes were observed for FMD (−3.45%). On its hand, control subjects experienced a slight worsening in their endothelial function, with statistically significant reductions in FMD from baseline to 6 months (−6.75%), while no changes were observed for cIMT values (−0.01 mm). These results should be interpreted with caution, since this pilot study was underpowered to detect significant differences between groups for the main outcome variable (36% post-hoc power calculation).
Previous investigations have explored the effect of periodontal therapy on endothelial function and systemic inflammation in different populations, with conflicting results. Tonetti and coworkers (19), in a RCT with 120 subjects with periodontitis and no history of CVD, reported improvements in FMD 6 months after “intensive” periodontal treatment (combining non-surgical therapy and systemic antibiotics), with statistically significant greater FMD in the “intensive” treatment group vs. the controls (absolute difference 2.0%). However, in periodontitis patients with established CVD, reported results on the effect of non-surgical periodontal treatment on 69 subjects with coronary artery disease and periodontitis, were not able to detect statistically significant differences in FMD between groups after a 3 months follow-up (test, 1.37%,; control, 1.39%) (24). In this study, similarly to the results from the present investigation, there were no changes in the serum markers of endothelium activation in the test group, while sVCAM-1 and sICAM-1 increased in controls, with significant differences between groups at 3 months.
Flow-mediated dilation (FMD) is a surrogate marker of CVD, widely used in intervention and epidemiological studies, since it is a non-invasive sensitive method to assess endothelial function. However, FMD has certain technical limitations related to its reproducibility and it is not frequently used in the clinical practice (25, 26). In systemically healthy subjects, mean FMD ranges between 0.20%–19.2%, while in coronary heart disease subjects FMD varies from −1.3%–14% (25). A cut-off value for normal endothelial function of 7.1% has been proposed (27), but this figure should be interpreted with caution since has been estimated based on a Japanese population, and variations among different populations have been described (25). Previous RCTs in periodontitis patients have reported mean FMD values of 6.5%–7.1% in cardiovascular healthy subjects (19) and 7.05%–7.10% in coronary heart disease patients (24). Our sample presented baseline values of FMD substantially higher (test, 13.35% vs. control, 15.06%), and despite a reduction in FMD in both groups after periodontal treatment, final values were still above the cut-off value estimated for normal endothelial function (test, 9.72% vs. control, 10.14%). These baseline FMD values in a population with established CVD could be the result of the compliance with multiple medications prescribed as part of their secondary prevention cardiovascular rehabilitation. Under these circumstances, the possible effect of periodontal therapy on FMD, although demonstrating improvements, could be confounded by the high values of FMD at baseline.
Carotid intima-media thickness is also a non-invasive surrogate marker of early detection of atherosclerosis widely used clinical trials in CVD (28), since it has been associated with several forms of CVD (29–31). A RCT on the effect of steps 1 and 2 of periodontal treatment vs. oral hygiene instructions alone on 169 Aboriginal Australians with periodontitis and no previous history of CVD, observed a statistically significant reduction in cIMT at 12 months, in the intervention group, of −0.023 mm, while no statistically significant differences were observed in controls (0.002 mm). However, as reported by the authors, this improvement did not translate in better endothelial function, measured through pulse wave velocity (20). Similarly, a cohort study on systemically healthy subjects, with mild to moderate forms of periodontitis, reported statistically significant reductions of −0.12 mm, 12 months after step 2 of periodontal treatment, but no control group was available (32). In our study, we observed a statistically significant reduction in cIMT of −0.05 mm in the test group after 6 months, with no significant differences between groups at any time point: thus, it was not possible to elucidate whether these changes were due to the periodontal therapy assigned or to the concomitant medical treatments.
Previous publications have reported a transient elevation of systemic inflammation, 24 hours after periodontal therapy, in subjects with and without CVD, that tend to disappear 7 days later, and subsequent improvements in these biomarker's serum levels in the long term (19, 32–36). This effect has been interpreted as a response to the “tissue trauma” induced by subgingival instrumentation, or as a possible systemic response to transient bacteraemia (37). This transient inflammatory response after therapy was observed in our investigation, in the test group, for TNF-α and IL-6, what may be explained by the tissue trauma during subgingival instrumentation, carried out only in the test group. However, we observed the previously reported tendency towards a reduction of serum levels in time for all the biomarkers evaluated in both treatment groups, except for IL-6 in the test group, although differences between groups were not statistically significant. Caution must be taken when interpreting these results, since certain interleukins, specifically IL-1β and IL-10, showed low values, frequently under the detection limit of the kit, and analyses were based on less than 10 samples (see Table 2, Supplementary Table S1). This lack of differences between groups could be due to the anti-inflammatory effects of regular medications taken by all the subjects in this RCT, as part of their CVD rehabilitation program. In fact, statins, which are the first drug of choice for the management of dyslipidaemia (38), due to their proven efficacy in reducing LDL cholesterol (39), have an anti-inflammatory effect. This effect has also been investigated when statins have been applied as adjunct host-modulator agents in the treatment of periodontitis (40). Similarly, certain lipid-lowering drugs have proven to induce significant reductions in cIMT (41–43). Furthermore, aspirin, which was also prescribed in 11% of our sample for its antithrombotic effect, has anti-inflammatory properties, and 23% of our patients used vasodilators, which may have also influenced the FMD results. Within this complex context, of multiple medication protocols combining anti-inflammatory and lipid-lowering drugs, medication intake may have confounded the possible effect of the periodontal interventions on systemic inflammation or endothelial function. Adjusting for the confounding effect of patient's medications to evaluate the effect of periodontal therapy on these patients would require much larger samples than those of this pilot clinical trial.
Our investigation has different limitations, one of them related to the intervention in the test group, since it was limited to steps 1 and 2 of periodontal treatment (18, 44), while participants presented generalised stage III and stage IV periodontitis. It is well documented that, in the presence of deep periodontal pockets (≥6 mm), periodontal treatment consisting of steps 1 and 2 might not be sufficient to arrest periodontal inflammation (18). In fact, even though there was a substantial improvement in the periodontal parameters after treatment, it failed to result in disease resolution and adequate management of inflammation in the test (steps 1 and 2) and control (step 1) patients, since both treatment groups exhibited residual pockets (PD >6 mm: control group 12.6% and test group 3.1%) and high levels of BoP (control group ≈ 47% and test group ≈ 28%), 6 months after therapy. Clinical endpoints of therapy were only achieved in two test patients at 3 months, and in one test patient at 6 months. Therefore, additional surgical therapy (step 3) might have been needed in the treatment of some of these patients. Providing full periodontal treatment, including step 3 of the therapy, would have led to better disease resolution and, hypothetically, greater improvements in endothelial function and serum biomarkers. Another limitation is that oral hygiene instructions and performance by the patients (step 1) could not be considered as a successful intervention in the present study, since both treatment groups exhibited high levels of plaque (test and control groups above 50%). Furthermore, longer follow-ups are desirable, since due to the natural history of atherosclerosis (3), changes in its progression are slow and take long time to be observed. Similarly, the occurrence of hard outcomes of CVD should be ideally investigated, but they require large cohorts of patients and longer follow-ups. However, the main limitation of this study was the sample size, since this trial was underpowered to detect the intended difference between groups. Even though screening was extended for 4 years, the study population was insufficient to provide an adequate pool of subjects for recruitment. Furthermore, the onset of Covid-19 pandemic and the implementation of lockdown and access restrictions to medical facilities, precluded from continuing patient screening and recruitment, therefore the final sample was limited to 35 patients. Additional RCTs, involving multiple research centres to overcome recruiting limitations, with longer follow-ups and reliable measures of CVD are needed, to further clarify the possible impact of periodontal therapy on CVD.
Despite the acknowledged limitations, the findings from the present trial support the recommendations from the European Federation of Periodontology and the World Heart Federation to provide periodontal treatment to patients with stablished cardiovascular disease and periodontitis as soon as its cardiovascular condition allows (45). In our sample, treatment of advanced forms of periodontitis (stages III and IV) three months after an acute cardiovascular event, proved to be safe, with minor post-intervention bleeding reported, and to induce certain improvement in surrogate markers of CVD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee, Hospital Universitario Clínico San Carlos (affiliated committee to Complutense University) and Ethics Committee of University Hospital Severo Ochoa. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AM: Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. NA: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MM: Data curation, Investigation, Methodology, Writing – review & editing. EM: Investigation, Methodology, Writing – review & editing. LV: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. DH: Conceptualization, Funding acquisition, Writing – review & editing. EF: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing. MS: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The present investigation was funded by a research grant from the Ministry of Science, Innovation and Universities of Spain, Research in Health Fund, Instituto de Salud Carlos III (grant number PI11/00542) and by a scholarship granted to Mrs. Ana Molina for Training of University Faculty (FPU, from the Spanish Formación de Profesorado Universitario) from the Ministry of Education and Science of Spain (FPU12/02720).
To Mrs. Rosa Simón, for her assistance collecting the serum samples.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2025.1488941/full#supplementary-material
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. (1999) 340(2):115–26. doi: 10.1056/NEJM199901143400207
3. Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592(7855):524–33. doi: 10.1038/s41586-021-03392-8
4. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. (2002) 105(9):1135–43. doi: 10.1161/hc0902.104353
5. Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. (2018) 45(Suppl 20):S162–70. doi: 10.1111/jcpe.12946
6. Sanz M, Beighton D, Curtis MA, Cury JA, Dige I, Dommisch H, et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. (2017) 44(Suppl 18):S5–11. doi: 10.1111/jcpe.12682
7. Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. (2008) 35(4):277–90. doi: 10.1111/j.1600-051X.2007.01173.x
8. Machado V, Botelho J, Escalda C, Hussain SB, Luthra S, Mascarenhas P, et al. Serum C-reactive protein and periodontitis: a systematic review and meta-analysis. Front Immunol. (2021) 12:706432. doi: 10.3389/fimmu.2021.706432
9. Herrera D, Molina A, Buhlin K, Klinge B. Periodontal diseases and association with atherosclerotic disease. Periodontol 2000. (2020) 83(1):66–89. doi: 10.1111/prd.12302
10. Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 2000. (2020) 83(1):90–106. doi: 10.1111/prd.12304
11. Reyes L, Herrera D, Kozarov E, Roldán S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Clin Periodontol. (2013) 40(Suppl 14):S30–50. doi: 10.1111/jcpe.12079
12. Donders HCM, Veth EO, van ‘t Hof AWJ, de Lange J, Loos BG. The association between periodontitis and cardiovascular risks in asymptomatic healthy patients. Int J Cardiol Cardiovasc Risk Prev. (2021) 11:200110. doi: 10.1016/j.ijcrp.2021.200110
13. Cairo F, Castellani S, Gori AM, Nieri M, Baldelli G, Abbate R, et al. Severe periodontitis in young adults is associated with sub-clinical atherosclerosis. J Clin Periodontol. (2008) 35(6):465–72. doi: 10.1111/j.1600-051X.2008.01228.x
14. Khatri R, Deshmukh J, Shrivastava R, Gupta S, Kawadkar A, Vinaya Kumar K. Is periodontitis an independent risk factor for subclinical atherosclerosis? Singapore Dent J. (2016) 37:9–13. doi: 10.1016/j.sdj.2016.10.004
15. Cairo F, Nieri M, Gori AM, Rotundo R, Castellani S, Abbate R, et al. Periodontal variables may predict sub-clinical atherosclerosis and systemic inflammation in young adults. A cross-sectional study. Eur J Oral Implantol. (2009) 2(2):125–33.20467611
16. Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Clin Periodontol. (2013) 40(Suppl 14):S70–84. doi: 10.1111/jcpe.12062
17. Garcia RI, Krall EA, Vokonas PS. Periodontal disease and mortality from all causes in the VA dental longitudinal study. Ann Periodontol. (1998) 3(1):339–49. doi: 10.1902/annals.1998.3.1.339
18. Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, et al. Treatment of stage I-III periodontitis-the EFP S3 level clinical practice guideline. J Clin Periodontol. (2020) 47(Suppl 22):4–60. doi: 10.1111/jcpe.13290
19. Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. (2007) 356(9):911–20. doi: 10.1056/NEJMoa063186
20. Kapellas K, Maple-Brown LJ, Jamieson LM, Do LG, O'Dea K, Brown A, et al. Effect of periodontal therapy on arterial structure and function among aboriginal Australians: a randomized, controlled trial. Hypertension. (2014) 64(4):702–8. doi: 10.1161/HYPERTENSIONAHA.114.03359
21. D'Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Clin Periodontol. (2013) 40(Suppl 14):S85–105. doi: 10.1111/jcpe.12061
22. Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D'Aiuto F, Bouchard P, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. (2020) 47(3):268–88. doi: 10.1111/jcpe.13189
23. Fernández E L, Planes A, Rodríguez M. Procedimientos en Microbiología Clínica: Hemocultivo. Madrid, Spain: Sociedad Española de Enfermedades Infecciosas y Microbiologia Clínica (2003).
24. Saffi MAL, Rabelo-Silva ER, Polanczyk CA, Furtado MV, Montenegro MM, Ribeiro IWJ, et al. Periodontal therapy and endothelial function in coronary artery disease: a randomized controlled trial. Oral Dis. (2018) 24(7):1349–57. doi: 10.1111/odi.12909
25. Bots ML, Westerink J, Rabelink TJ, de Koning EJ. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. Eur Heart J. (2005) 26(4):363–8. doi: 10.1093/eurheartj/ehi017
26. Arrebola-Moreno AL, Laclaustra M, Kaski JC. Noninvasive assessment of endothelial function in clinical practice. Rev Esp Cardiol. (2012) 65(1):80–90. doi: 10.1016/j.recesp.2011.09.012
27. Maruhashi T, Kajikawa M, Kishimoto S, Hashimoto H, Takaeko Y, Yamaji T, et al. Diagnostic criteria of flow-mediated vasodilation for normal endothelial function and nitroglycerin-induced vasodilation for normal vascular smooth muscle function of the brachial artery. J Am Heart Assoc. (2020) 9(2):e013915. doi: 10.1161/JAHA.119.013915
28. de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. (2004) 109(23 Suppl 1):III33–8. doi: 10.1161/01.CIR.0000131516.65699.ba
29. Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) study, 1987–1993. Am J Epidemiol. (1997) 146(6):483–94. doi: 10.1093/oxfordjournals.aje.a009302
30. Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation. (2020) 142(7):621–42. doi: 10.1161/CIRCULATIONAHA.120.046361
31. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med. (1999) 340(1):14–22. doi: 10.1056/NEJM199901073400103
32. Piconi S, Trabattoni D, Luraghi C, Perilli E, Borelli M, Pacei M, et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J. (2009) 23(4):1196–204. doi: 10.1096/fj.08-119578
33. Eickholz P, Siegelin Y, Scharf S, Schacher B, Oremek GM, Sauer-Eppel H, et al. Non-surgical periodontal therapy decreases serum elastase levels in aggressive but not in chronic periodontitis. J Clin Periodontol. (2013) 40(4):327–33. doi: 10.1111/jcpe.12076
34. Morozumi T, Yashima A, Gomi K, Ujiie Y, Izumi Y, Akizuki T, et al. Increased systemic levels of inflammatory mediators following one-stage full-mouth scaling and root planing. J Periodontal Res. (2018) 53(4):536–44. doi: 10.1111/jre.12543
35. Graziani F, Cei S, Orlandi M, Gennai S, Gabriele M, Filice N, et al. Acute-phase response following full-mouth versus quadrant non-surgical periodontal treatment: a randomized clinical trial. J Clin Periodontol. (2015) 42(9):843–52. doi: 10.1111/jcpe.12451
36. Bokhari SA, Khan AA, Butt AK, Azhar M, Hanif M, Izhar M, et al. Non-surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J Clin Periodontol. (2012) 39(11):1065–74. doi: 10.1111/j.1600-051X.2012.01942.x
37. Orlandi M, Graziani F, D'Aiuto F. Periodontal therapy and cardiovascular risk. Periodontol 2000. (2020) 83(1):107–24. doi: 10.1111/prd.12299
38. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. (2021) 42(34):3227–337. doi: 10.1093/eurheartj/ehab484
39. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. (2013) 34(38):2949–3003. doi: 10.1093/eurheartj/eht296
40. Balta MG, Papathanasiou E, Blix IJ, Van Dyke TE. Host modulation and treatment of periodontal disease. J Dent Res. (2021) 100(8):798–809. doi: 10.1177/0022034521995157
41. Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet. (2001) 357(9256):577–81. doi: 10.1016/S0140-6736(00)04053-8
42. Blankenhorn DH, Selzer RH, Crawford DW, Barth JD, Liu CR, Liu CH, et al. Beneficial effects of colestipol-niacin therapy on the common carotid artery. Two- and four-year reduction of intima-media thickness measured by ultrasound. Circulation. (1993) 88(1):20–8. doi: 10.1161/01.CIR.88.1.20
43. de Groot E, Jukema JW, Montauban van Swijndregt AD, Zwinderman AH, Ackerstaff RG, van der Steen AF, et al. B-mode ultrasound assessment of pravastatin treatment effect on carotid and femoral artery walls and its correlations with coronary arteriographic findings: a report of the regression growth evaluation statin study (REGRESS). J Am Coll Cardiol. (1998) 31(7):1561–7. doi: 10.1016/S0735-1097(98)00170-3
44. Herrera D, Sanz M, Kebschull M, Jepsen S, Sculean A, Berglundh T, et al. Treatment of stage IV periodontitis: the EFP S3 level clinical practice guideline. J Clin Periodontol. (2022) 49(Suppl 24):4–71. doi: 10.1111/jcpe.13639
Keywords: periodontitis, periodontal therapy, cardiovascular diseases, flow-mediated dilation, carotid intima-media thickness
Citation: Molina A, Ambrosio N, Molina M, Montero E, Virto L, Herrera D, Figuero E and Sanz M (2025) Effect of periodontal therapy on endothelial function and serum biomarkers in patients with periodontitis and established cardiovascular disease: a pilot study. Front. Oral. Health 6:1488941. doi: 10.3389/froh.2025.1488941
Received: 30 August 2024; Accepted: 17 January 2025;
Published: 10 February 2025.
Edited by:
Davide Pietropaoli, University of L'Aquila, ItalyReviewed by:
Alejandra Chaparro, University of the Andes, ChileCopyright: © 2025 Molina, Ambrosio, Molina, Montero, Virto, Herrera, Figuero and Sanz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Molina, YXIubW9saW5hQHVjbS5lcw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.