
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oral. Health, 22 January 2025
Sec. Oral Cancers
Volume 5 - 2024 | https://doi.org/10.3389/froh.2024.1524313
This article is part of the Research TopicImmuno-Oncologic Biomarker Signatures for Personalized Immunotherapy and Immunoprevention in Oral Squamous Cell CarcinomaView all 5 articles
Introduction: Upper aerodigestive tract cancers are prevalent, with a global incidence surpassing 500,000 new cases in 2018. Among these, oral squamous cell carcinomas (OSCC) constitute the majority. OSCC has a low 5-year survival rate due to late-stage diagnosis. Risk factors include alcohol and tobacco use. However, non-smokers and non-drinkers are also affected, especially young patients with tongue cancer. The impact of tumor microenvironment (TME) and tumor-infiltrating lymphocytes (TILs) on OSCC prognosis remains debated. Remarkably, Tertiary Lymphoid Structures (TLS) identified in solid tumors have shown associations with favorable outcomes, yet their prognostic significance in OSCC remains understudied.
Objective: Thus, this systematic review aims to explore the value of TLS in OSCC reported in the literature.
Method: A scoping review was conducted and six retrospective cohort studies involving 1,203 patients met the inclusion criteria.
Results: Predominantly male patients, with an average age of 49.3 years were included. Immunohistochemistry was the primary method to identify TLS, present in 21% up to 100% of cases. TLS were predominantly located in the peri-tumoral area (75.4%–84.8%) compared to the intra-tumoral area (33.8%–33.9%). Our review shows that the presence of TLS is associated with improved survival in OSCC.
Discussion: However, variations in TLS detection and classification methods across studies introduce potential biases, hindering direct comparisons between findings. For instance, reports that are based solely on examining HES-stained slides for TLS identification may raise reliability concerns. Standardization of methodologies is imperative to ensure consistency in criteria utilization, thereby facilitating meaningful data comparisons.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023428010, PROSPERO (CRD42023428010).
The incidence of upper aerodigestive tract cancers in 2018 was over 500,000 new cases per year in the world (1–4). They are the 18th most common cancer in the world (2) and the 6th in France and are two to three times more common in men than in women (5). More than 90% of these cancers are squamous cell carcinomas grouping different locations (mainly the oropharynx and oral cavity, then the cavum and larynx). In a quarter of the cases, they are oral squamous cell carcinomas (OSCC). Mortality is estimated at 170,000 patients/year (6). OSCC has a low 5-year survival rate, below 50%, mainly due to late diagnosis (stages III/IV), which makes management difficult. Survival also depends on gender (better in women than in men) and tumor location (higher for lip cancer and lower for tongue cancer) (7). The main risk factors for OSCC are alcohol and tobacco consumption, and smokeless tobacco too (8), especially when combined (9). However, these cancers can also occur in patients who do not have these risk factors, particularly in young patients under 45 years of age (10, 11), and especially in tongue cancers in young women (12). These patients, with no “classic” risk factors (non-drinkers and non-smokers), present epidemiological and clinical characteristics distinct from smoker-drinker patients (10). The causes of cancer are not identified in these patients. Strikingly, the incidence of cases in young patients has been increasing in the last decade. Unlike oropharyngeal carcinomas (13, 14), human papillomavirus (HPV) is not a risk factor for OSCC.
Since the early 2000's, the management of cancer patients has been considering the immune tumor microenvironment (TME) as well as the immune response in addition to tumor-specific parameters (size and extension). TME varies from patient to patient and is now considered as a key parameter for cancer prognosis and patient response to immunotherapy. In various solid cancers, in particular in colorectal cancer, an “immunoscore” of immune cells present in TME is considered to have a prognostic value superior to that provided by the TNM classification (15–17). Studies of the OSCC TME have indicated that the influence of tumor-infiltrating lymphocytes (TILs), including regulatory T cells (Treg) and, for non-smoker and non-drinker patients, CD8+ T lymphocytes, is linked with improved overall survival (OS) (18). By contrast, It has been shown that a significant accumulation of peripheral IL-17+ T lymphocytes and higher IL-17 production in patients with head and neck cancer are negatively correlated with OS (19). A higher proportion of circulating T helper 17 (Th17) lymphocytes is found in patients with OSCC, with an increase in Th17/Tregs ratio in early stages and a decrease in this ratio in higher stages of oral cancer (20).
When the architecture and organization of TME in solid tumors were examined, the presence of tertiary lymphoid structures (TLS) was first evidenced and associated with more favorable prognosis in non-small cell lung carcinoma (NSCLC) (21). TLS are ectopic and transitory structures appearing within non-lymphoid tissues in a context of inflammation, especially when the latter is chronic. The term “tertiary lymphoid tissue” was first used in 1992 by Picker and Butcher, who described the formation of lymphocyte aggregates outside of lymphoid tissue, bringing together memory lymphocytes and/or their precursors (22). In 1996, Schröder and her colleagues described B cell differentiation in ectopic lymphoid structures organized in the same way as lymph nodes, possibly mimicking their function in rheumatoid arthritis (23). The same year, Ruddle and her colleagues showed in a murine model that chronic inflammatory lesions caused by lymphotoxin resembled lymph nodes with regard to cellular composition, T and B cell zones, primary and secondary follicles, presence of high endothelial venules, and ability to respond to antigenic challenge (24). Many reports have now shown that tumor-associated TLS participate in the mounting of adaptive immune responses resulting in the differentiation of cytotoxic effector T cells and the production of anti-tumor antibodies (25). The non-encapsulation of TLS, unlike lymph nodes, is likely to allow for easier migration of immune cells, resulting in improved antigens addressing and the accumulation of new antigen specificities, which in turn promotes the persistence of these structures (26).
A recent bibliographic study on global trends in TLS research highlighted the growing interest in these structures, with a significant increase in publications between 2017 and 2023. The main contributing countries were China (231 publications), the United States of America (212 publications), and France (89 publications). The most productive institutions were French, including Inserm, followed by Université Paris Cité and Sorbonne Université. Key topics encompassed the links between TLS and cancers, immunotherapy, the tumor microenvironment, prognosis, and the responsiveness to immune checkpoint inhibitors (27).
TLS presence is mostly synonymous with a better survival prognosis in various solid tumors such as NSCLC, melanoma, breast cancer, and many digestive cancers (21, 25, 28), except for a rare subtype of hepatocellular carcinoma (29), although the latter has not been confirmed by other studies so far. Remarkably, it has been demonstrated that CD8+ lymphocytes are associated with more favorable prognosis only when TLS are present, in NSCLC (30) and ovarian cancers (31). Furthermore, better responses to immunotherapy have been observed when stimulating TLS formation (32) or when TLS are present in cancer patients receiving anti-immune checkpoint antibodies (anti-PD1, anti-PD-L1, and anti-CTLA-4) (33–35).
Studies have also focused on the regulation of TLS by Tregs. Using a murine model, Joshi and his colleagues have reported that the formation of TLS can be inhibited by Tregs (36). It has been also recently shown that Tregs infiltrate TLS in a number of NSCLC patients and are then associated with a poor clinical outcome (37).
However, to our knowledge, no published study has comprehensively synthesized data regarding the protective role of TLS in OSCC. To gain deeper insights into the potential significance of TLS as a prognostic marker in OSCC, we undertook this systematic review to compile all available data on the subject. The primary aim of this review was to investigate the potential link between TLS presence and OSCC prognosis. Additionally, the secondary objective was to evaluate whether the organization and/or tissue localization of TLS influence the prognosis of OSCC patients.
A systematic review was conducted between January 2022 and May 2023. The research protocol was registered on the International Prospective Register Of Systematic Reviews PROSPERO on number CRD42023428010.
This is a scoping review of the literature according to the Joanna Briggs Institute guide (38). The methodology of the Scoping Review met PRISMA requirements (39). We have used the following databases: Pubmed, Embase, Scopus, and Web of Science. The same research was performed on the grey literature (OpenGrey and Google Scholar). The complete search strategy is available in Supplementary Appendix 1. No time restriction was selected.
An additional search was conducted by replacing the OSSC keywords with head and neck squamous cell carcinoma (HNSCC) keywords to find articles that mainly focus on OSCC patients. Two authors of the present study independently performed data extraction, with a third author consulted in case of disagreement. However, it is important to note that HNSCC included both OSCC and oropharyngeal cancers, which have distinct immunological characteristics (40). Therefore, we only included articles that provided detailed data specifically related to OSCC or where OSCC comprised the majority of HNSCC cases (more than 75%).
OSCC refers to cancers of the oral cavity derived from the epithelial cells of the oral mucosa. These epithelial neoplasms exhibit varying degrees of differentiation and tend to local and regional invasion. Typically, squamous carcinoma cells are detected by a pan-cytokeratin marker (41). As stressed in the Introduction section, TLS are ectopic and transitory structures that appear within non-lymphoid tissues in the context of inflammation, particularly in cases of chronic inflammation. Histologically, TLS consist of clusters of B and T lymphocytes with dendritic cells, organized as in secondary lymphoid organs (24). Their organization can vary widely, from loosely aggregated lymphocytes to well-formed structures featuring a T-cell and a B-cell zones. Within the T-cell zone, diverse T-cell subsets can be detected, marked by the presence of follicular helper T-cells (Tfh) (42), alongside with mature dendritic cells (mDC). The B-cell zone includes a network of follicular dendritic cells (FDC) and a germinal center where class switch recombination (CSR) and hypersomatic mutations (HSM) occur. This organizational framework is complemented by high endothelial venules (HEV), supplying the necessary cellular components and chemokines essential for the formation of mature TLS (25).
To be selected, the articles needed to focus on studying the presence of TLS in human OSCC samples as the primary outcome and evaluate the difference in prognosis between samples with and without TLS (classified by “yes” or “not” or “high” or “low” or “classic” and “non-classic” or “mature” or “immature”) and using survival index like overall survival (OS). Studies were eligible for inclusion with no period restriction. We also made no language restrictions when an English translation was available. We therefore included experimental and observational studies.
We excluded review articles, editorial notes, and studies involving only animal or in vitro models from our selection.
The titles and abstracts were first selected by screening various databases (see « Search Strategy ») to extract articles that respected the inclusion criteria. A full-text reading of these articles was then performed to select those to be included in the systematic review. Full-texts of articles with a degree of uncertainty with regard to inclusion criteria were also read. The references listed in the selected articles were then analyzed to include additional papers not identified by our initial search. Details of each study, design, participant characteristics, intervention and comparator, and outcomes were also extracted. Softwares used to perform this reference-based search were EndNote (version 21.4, London, United Kingdom) and Zotero (version 7.0.8, Virginia, United States of America (USA)) for ranking and referencing articles, and Rayyan (version 2023, Cambridge, Massachusetts, USA) for the screening.
We have assessed the levels of evidence of the articles obtained by applying the Haute Autorité de Santé (HAS) literature analysis guide (43), which categorizes each article according to its rigor and reliability as providing low, intermediate, or high levels of proof. This guide helps to standardize the evaluation of scientific literature, ensuring that conclusions drawn are based on evidence of varying strengths. Articles with a high level of proof typically have more rigorous methodologies and stronger outcomes, while those with lower levels may have limitations or less robust methodologies. By grading each article, authors can better interpret the reliability and validity of the collective findings, making informed decisions based on the weight of the evidence provided.
After completing the literature search using various databases and relevant keywords, fifty-five publications were identified: we obtained eighteen articles from the PubMed database and, using the same keywords, also eighteen from the Scopus transdisciplinary database, ten from EMBASE, and nine from the Web of Science. The Cochrane database did not allow us to include any articles on the topic. Finally, after removing duplicates, thirty-three articles were selected (Figure 1). Of these 33 papers, only ten were eligible for abstract screening based on title selection. The others did not meet our inclusion criteria as their titles and/or abstracts did not mention a prognostic study related to the presence of TLS. All the ten articles were eligible for full-text screening. It led to the selection of six articles (44–49), the four others being excluded for different reasons (some studies did not include a survival analysis related to the presence of TLS, and others did not include a study of TLS). Three other articles (50–52) were added as the result of the complementary research as indicated in Figure 1.
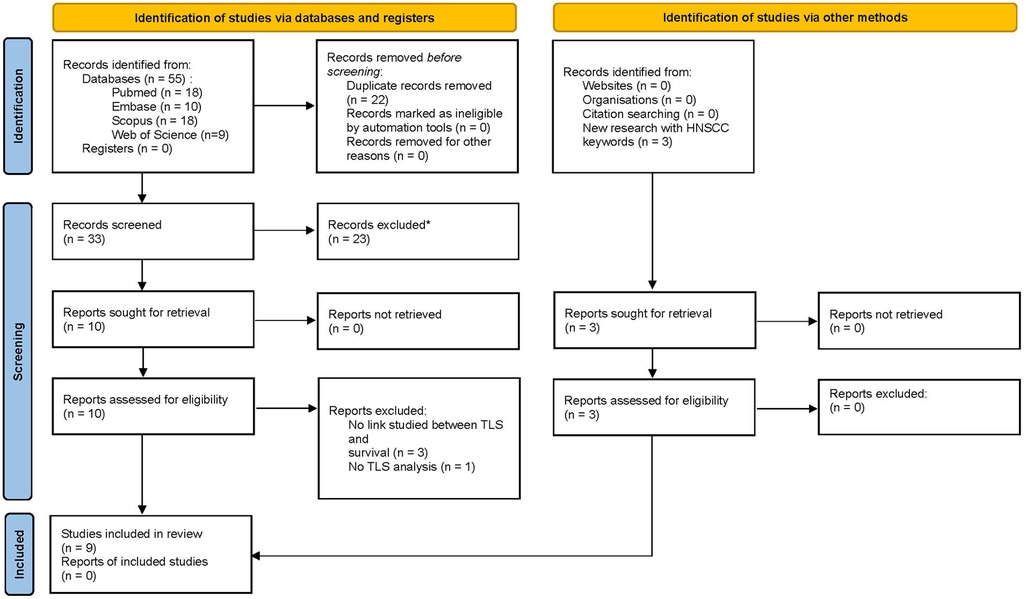
Figure 1. PRISMA 2020 flow for new systematic reviews which included searches of databases, registers and other sources.
Thus, the bibliographic search identified nine articles (44–52) focusing on the study of TLS presence in human OSCC samples and evaluating the prognostic differences between samples with and without TLS. Eight of them were retrospective cohort studies and one was a prospective cohort study. These nine articles presented an intermediate level of proof, with a grade of 4C, except for one study, which was graded 2B, according to the HAS (43) which means that, although the studies have a certain reliability and provide relevant results, they present an acceptable but not optimal level of rigor, which may imply methodological limitations. Nevertheless, they provided a valuable contribution to our overall conclusions.
Our review included a total of 1,203 patients, with each study having a cohort size ranging from n = 9 to n = 310 individuals. Among them, 745 (61.93%) patients were male and 458 (38.07%) were female. The mean age was 49.3 years (ranging from 23–83 years). The other clinicopathological and demographic data of the patients are presented in Table 1. Most of the samples analyzed consisted of early-stage tongue OSCCs, with proportions ranging from 32.98%–85.85% (Table 1). In one study, 11 out of 65 samples were not OSCC, comprising four adenocarcinomas, four classified as “other,” and three unknown cases (45). Regarding tumor location, some studies focused specifically on tongue OSCCs (47, 49), whereas others examined tumors involving the lips, gums, floor of the mouth, palate, or oral mucosa.
The presence of TLS was reported in 21.0%–100% of cases across studies, considering both intra-tumoral and peri-tumoral locations (Table 2). The methods used to identify TLS varied significantly among the nine studies analyzed, as summarized in Table 3. One study exclusively employed hematoxylin-eosin-safranin (HES) staining to detect and quantify TLS, whereas the others used immunohistochemical (IHC) labeling to confirm their presence (49). Common markers utilized included CD20 for B cells, CD3 for T cells, CD21 to highlight follicular dendritic cell (FDC) networks, DC-Lamp to detect mature dendritic cells, and PNAd for high endothelial venules (HEVs). Some studies also employed the Bcl-6 marker to confirm the presence of germinal centers (44, 47). For studies using HES staining, evaluations were performed by trained pathologists or researchers (46, 47, 49). However, one study described only the presence of TLS within (intra-tumoral) or outside (peri-tumoral) tumor region, which limited the ability to evaluate the overall prevalence of these structures. This study found intra-tumoral TLS in 33.8% of cases compared to 75.4% for peri-tumoral TLS (45).
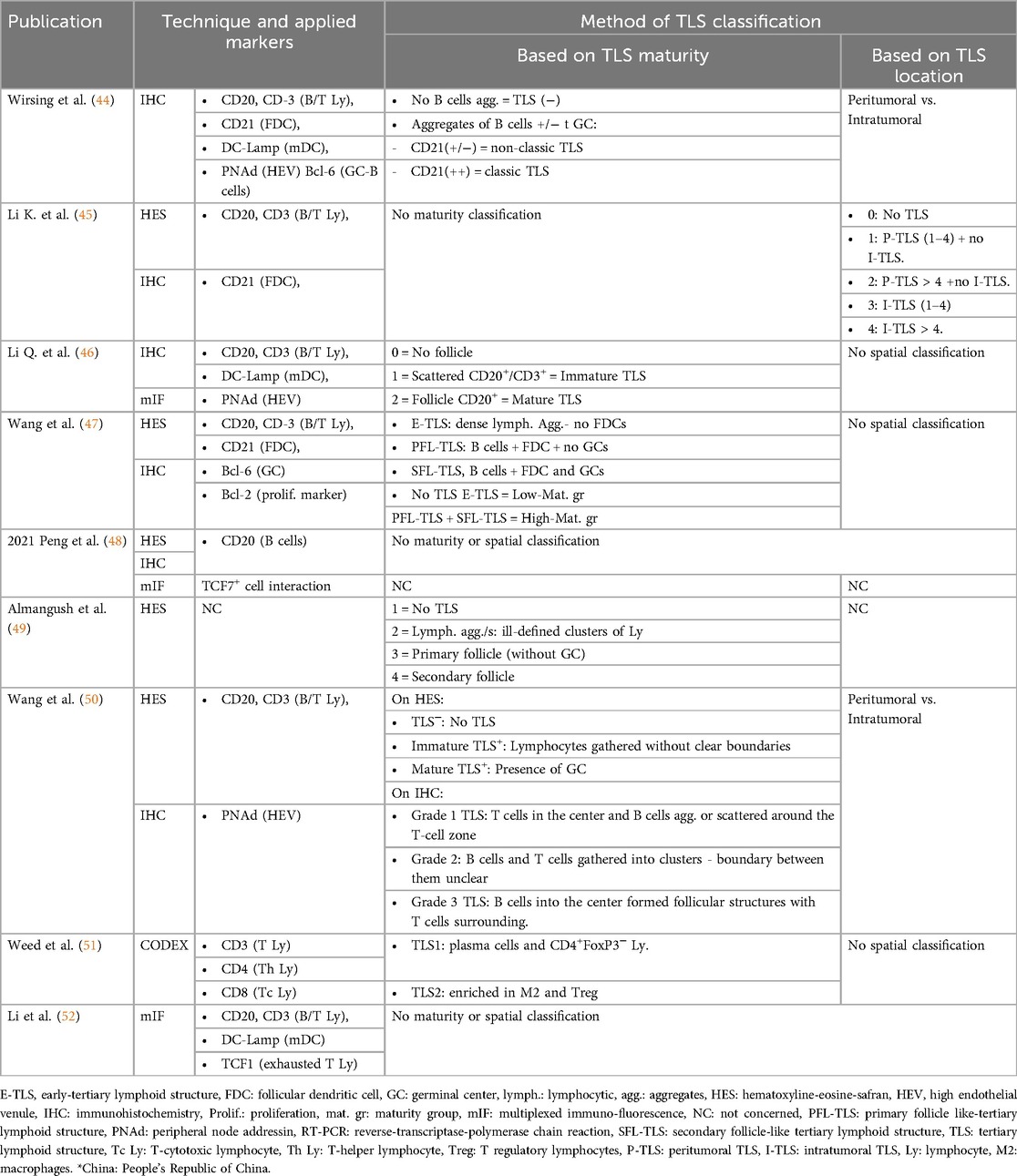
Table 3. Method of identification, labeling, and ranking of TLS (Tertiary Lymphoid Structures) among the different studies.
Classification of TLS and their degree of maturity also varied considerably between studies. Some classifications were based on structural organization, while others relied on the location of TLS relative to the tumor or their functional maturity. Certain works distinguished between mature TLS, characterized by a well-organized structure with a B-cell follicle containing a germinal center surrounded by a T-cell zone, and immature TLS, which consisted of less organized aggregates of lymphocytes and dendritic cells (44, 46). Other studies refined this classification by introducing three levels of maturity: dense lymphocyte aggregates without FDCs, TLS with a germinal center, and TLS containing a B-cell follicle and dense FDC network. The frequency of these TLS types was inversely proportional to their degree of maturity, with immature structures being the most common (47). Another approach followed a similar methodology, initially detecting TLS via HES staining before classifying their organization based on IHC observations (49).
Some of the studies focused on the location of TLS as a key classification criterion. One study categorized TLS according to their presence within, adjacent to, or in both regions relative to the tumor. Tumors were then graded based on TLS density and distribution, with grades ranging from 0 (absence of TLS) to 4 (high density and complexity) (45). Another work, in contrast, did not consider TLS maturity but rather categorized tumors as TLS-positive or TLS-negative, based on IHC and immunofluorescence findings. TLS density was also quantified by normalizing their number to the tumor surface area, with TLS-positive tumors reported in 32.08% of cases (48). A different approach investigated the relationship between TLS and TME. Two distinct TME profiles associated with TLS were identified. The first was enriched in plasma cells and CD4+ FoxP3− T lymphocytes, alongside monocytes, neutrophils, and M1 macrophages. The second was characterized by an abundance of M2 macrophages and Treg cells, accompanied by other immune cell populations, including CD4+ and CD8+ T cells, NK cells, and neutrophils (51).
Clearly, this variability in methodologies and classification systems underscores the need for standardization in TLS evaluation. Differences in detection techniques, markers, and definitions of maturity or location may significantly impact the interpretation of TLS prevalence, organization, and their functional relevance within tumor microenvironments.
The presence of TLS was shown to significantly correlate with better OS in several studies. One study demonstrated that patients with TLS had an 88.2% 5-year survival rate compared to 60.3% for TLS-negative patients. However, this association was statistically significant only when TLS were observed at multiple slice levels (p = 0.039), whereas a single slice level showed only a non-significant trend towards improved prognosis (44). Another study reported a significant association between TLS and long-term survival (p = 0.006), with survival curves showing longer OS in patients with more advanced TLS maturation stages (p = 0.031). The 5-year OS rates for patients without TLS, with peri-tumoral TLS, and with intra-tumoral TLS were 45.9 ± 12.4 months, 59.2 ± 3.9 months, and 76.1 ± 3.1 months, respectively (45). Finally, another analysis found a significant difference in the 5-year OS rates between TLS-positive and TLS-negative groups (88.9% vs. 56.1%; p < 0.001), confirmed by multivariate analysis (HR = 3.784, 95% CI, 1.498–9.562) (46).
The maturity of TLS also appears to play a role, although its influence varies across studies. “Classic” (mature) and “non-classic” (immature) TLS were both associated with better 5-year OS, but patients with mature TLS tended to have lower disease-specific mortality, though this was not statistically significant (p = 0.304) (44). In contrast, another study found no significant impact of TLS maturity on OS (94.1% vs. 85.7%, p = 0.411) (46). A further analysis showed a statistically significant difference in 5-year OS between patients with “low-maturity” TLS (63.4%) and those with “high-maturity” TLS (96%) (p = 0.0176) (47).
The association between TLS and better OS was also linked to molecular and immunological features. One study identified the overexpression of IL-7, CXCL-13, and lymphotoxin beta (LTβ) in patients with advanced TLS maturation stages, supporting their positive impact on prognosis (45). Another study highlighted the presence of TCF7+ T cells within TLS, associated with improved prognosis (48). Gene expression analyses in a large TCGA database cohort further confirmed TLS as a protective factor for OS (HR = 0.142, 95% CI 0.024–0.858, p = 0.033) (50).
Multiple studies confirmed the independent prognostic value of TLS. One analysis showed that TLS presence remained a protective factor for OS (HR = 0.434, 95% CI, 0.212–0.886, p = 0.022) in early-stage tongue OSCC (47). Another study reaffirmed that TLS positivity correlated with better OS (p < 0.001) and DFS (p = 0.019), with multivariate analysis identifying TLS score as an independent factor for OS (HR = 0.874, 95% CI, 0.765–0.997, p = 0.045) (50).
Our work has highlighted several key points. Only a total of nine articles investigating the prognostic value of TLS in OSCC met our inclusion criteria. The reported presence of TLS ranged from 21.0%–100% of cases across the studies, with most research teams utilizing HES or IF for their detection. In all included studies, TLS were consistently associated with improved survival outcomes. We specifically focused on OSCC due to the scarcity of reviews addressing this topic and because OSCC represent the majority of cancers within the oral cavity (53). Within the broader category of HNSCC, there is considerable variability among cancer subtypes, including salivary gland cancers (54, 55) and oropharyngeal cancers, some of which are HPV-related (56–58). This diversity, combined with our emphasis on TLS, prompted us to narrow the scope of the study to OSCC. By doing so, we were able to concentrate on survival outcomes, detection techniques, and TLS classifications in this specific context.
Most cohorts were dominated by tongue OSCC cases, aligning with literature trends showing an increasing proportion of tongue cancers in OSCC cohorts (59–62). Studies also differed significantly in terms of tumor staging, which impacts survival (63, 64). Some studies included mostly advanced-stage cancers (18, 59), while others focused on early-stage cancers (60), likely due to recruitment bias. TME composition, influenced by tobacco and alcohol exposure, may also differ by tumor stage (18). Since the included studies did not stratify patients based on risk factors, this adds complexity to evaluating the prognostic value of TLS in OSCC. In one study, tumor staging was determined based on histological grades rather than the TNM classification system (45). Additionally, 11 specimens were not confirmed as OSCC, including four adenocarcinomas, four cases of “other tumors,” and three unidentified tumors. Despite the lack of stratified data on survival and TLS presence by cancer type, the study was included as over 83% of the samples were OSCC. Similarly, other included articles reported non-OSCC tumor types in 2.78%–22.22% of cases but were included because their cohorts were predominantly including OSCC patients.
HES staining was the most commonly used technique to detect TLS. Among five studies using HES, only two relied on qualified pathologists, while the other ones used trained investigators. Two studies clearly defined TLS presence on HES sections, classifying samples with at least one ill-defined lymphocyte aggregate (which means that samples containing at least one ill-defined lymphocyte aggregate are classified as positive), while others did not specify histological criteria. Immunostaining methods, including immunohistochemistry (IHC) and multiplexed immunofluorescence (mIF), were also employed, with consistent marker use across studies. These markers are part of a quintet found in several articles attempting to highlight the different cell populations found in TLS (30, 65–71). Similarly to Wirsing et al., some authors also used the Bcl-6 marker to highlight germinal centers and confirm the presence of an active B-cell follicle. This methodology appears to be the most accurate for detecting histologically the presence of TLS with an active germinal center, as it allows for the assessment of TLS maturity, as explained in further detail below.
When examining the detection methods employed in the literature, it becomes evident that HES staining is the most used approach, sometimes combined with validation through IHC labeling. Although HES staining cannot differentiate between distinct lymphocyte populations, it remains a simple, rapid, and improved technique (71, 72). Moreover, its accuracy is maintained, particularly when performed by a qualified pathologist or trained examiner. One study used CODEX, a complex imaging technique that better reflects cellular interactions within the TME. This technique makes it possible to highlight many markers on the same slide, enabling more precise analysis of cell neighborhoods to identify different histological profiles. Some studies also analyzed immune signatures in the TCGA database to assess TLS presence and prognostic value in OSCC and HNSCC (47, 52). Regardless of the method used, concerning OSCC, the results are consistent, TLS correlated with a better prognosis.
TLS classification also varied among the studies, focusing on location, maturity, and specific cell populations involved. Maturity-based classification often distinguished between immature and mature TLS, with some studies going further by subtyping TLS based on various levels of maturity. Studies examining spatial distribution found higher TLS proportions in peritumoral stroma or at invasion margins, which is consistent with recent reviews (73, 74). However, one study contradicts this by associating intra-tumoral TLS with better prognosis (46). These discrepancies may be attributed to methodological and classification differences as well as patient cohort characteristics. The lack of standardized criteria complicates comparisons between studies, highlighting the need for unified classification systems to enable consistent evaluations across cancer types.
In terms of survival, all the studies demonstrated a positive association between the presence of TLS and improved OS, as well as better Recurrence-Free Survival (RFS) or Progression-Free Survival (PFS). However, variations in survival indicators used among the studies make direct comparisons challenging. For example, one study defined disease-specific survival (DSS) as the time between diagnosis and death due to OSCC or at the last follow-up (49), while another used DFS, which was defined as the time from surgery to disease relapse or death due to disease (47). Li et al. (45) also employed this survival index but defined it as the time from surgery to the onset of local or metastatic recurrence. Thus, standardizing survival metrics would greatly improve data comparability and reliability. However, survival results overwhelmingly indicated that OSCC patients with organized local immune responses in TLS have better survival outcomes. This finding is consistent with data reported in the literature for other solid tumors, including breast cancer (42, 67, 68, 75, 76), non-small cell lung cancer (21, 30, 66, 77), melanoma (35, 78, 79), hepatocellular carcinoma (80), pancreatic cancer (81), sarcoma (34), clear cell renal cancer (82) and colorectal cancer (83–90). Similarly, to the identification methods, standardizing patient survival assessments would facilitate more accurate comparisons of the data. These nine studies exhibit significant limitations. Notably, not all of them accounted for risk factors like tobacco and alcohol exposure, treatment history, or patient comorbidities. These gaps further complicate the analysis of TLS as a prognostic factor.
Last, immune checkpoint inhibitors targeting PD-1 (programmed cell death protein 1), such as nivolumab and pembrolizumab, are currently utilized for OSCC treatment (91, 92). Additional PD-1/PD-L1 axis inhibitory antibodies are currently under clinical investigation, such as cemiplimab (NCT04398524), sintilimab (NCT05000892), or toripalimab (NCT04825938). However, the clinical effectiveness of these therapies remains relatively low, ranging from 20%–40% depending on the cancer type. The causes of resistance to immunomodulators remain largely unknown. Among various factors, mature TLS may forecast the efficacy of immune checkpoint inhibitors in solid tumors (65). In soft tissue sarcomas, an immune classification rooted in the analysis of the TME has enabled the classification of tumors into immune-desert, immune-rich, or highly vascularized immune classes. The most immune-rich class is distinguished by a high expression of a B lymphocyte signature and the presence of TLS, correlating with enhanced survival (93). Beyond mere identification and prognostication of patient response capabilities, it might be feasible to induce TLS, allowing patients without these structures to develop them and thus benefit from improved therapeutic responses. This concept has been explored in murine models (32, 94, 95). It could potentially represent the future of personalized anti-cancer therapies. This underscores the importance of studying these structures.
Overall, the results of the literature review suggest that the presence of TLS is associated with better survival outcomes in oral squamous cell carcinoma. The variability in classification criteria and survival indicators highlights the need for standardized approaches in future research. Further studies with larger sample sizes and standardized methodologies are warranted to validate these findings and explore the underlying mechanisms of TLS in oral cancer.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
VR: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft. JLT: Supervision, Writing – review & editing. MCDN: Supervision, Writing – review & editing. GL: Supervision, Writing – review & editing. JR: Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2024.1524313/full#supplementary-material
1. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. (2016) 91:386–96. doi: 10.1016/j.mayocp.2015.12.017
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Kaur G, Singh M, Kaur M, Singh B, Gupta RK. A clinicopathological study of upper aerodigestive tract cancers. Niger J Clin Pract. (2019) 22:1208–12. doi: 10.4103/njcp.njcp_131_19
4. Renou A, Guizard A-V, Chabrillac E, Defossez G, Grosclaude P, Deneuve S, et al. Evolution of the incidence of oral cavity cancers in the elderly from 1990–2018. J Clin Med. (2023) 12:1071. doi: 10.3390/jcm12031071
6. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018 : GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
7. Seoane JS, Takkouche B, Varela-Centelles P, Tomás I, Seoane-Romero J. Impact of delay in diagnosis on survival to head and neck carcinomas: a systematic review with meta-analysis. Clin Otolaryngol. (2012) 37:99–106. doi: 10.1111/j.1749-4486.2012.02464.x
8. Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. (2008) 9:667–75. doi: 10.1016/S1470-2045(08)70173-6
9. Boccia S, Hashibe M, Gallì P, De Feo E, Asakage T, Hashimoto T, et al. Aldehyde dehydrogenase 2 and head and neck cancer: a meta-analysis implementing a Mendelian randomization approach. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. (2009) 18:248–54. doi: 10.1158/1055-9965.EPI-08-0462
10. Mneimneh WS, Xu B, Ghossein C, Alzumaili B, Sethi S, Ganly I, et al. Clinicopathologic characteristics of young patients with oral squamous cell carcinoma. Head Neck Pathol. (2021) 15:1099–108. doi: 10.1007/s12105-021-01320-w
11. Hussein AA, Helder MN, de Visscher JG, Leemans CR, Braakhuis BJ, de Vet HCW, et al. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: a systematic review. Eur J Cancer Oxf Engl. (2017) 82:115–27. doi: 10.1016/j.ejca.2017.05.026
12. Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18–44 years. J Clin Oncol Off J Am Soc Clin Oncol. (2011) 29:1488–94. doi: 10.1200/JCO.2010.31.7883
13. Ak C. Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol. (2012) 6(Suppl 1):16–24. doi: 10.1007/s12105-012-0377-0
14. Li G, Sturgis EM. The role of human papillomavirus in squamous carcinoma of the head and neck. Curr Oncol Rep. (2006) 8:130–9. doi: 10.1007/s11912-006-0048-y
15. Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. (2011) 29:610–8. doi: 10.1200/JCO.2010.30.5425
16. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. (2006) 313:1960–4. doi: 10.1126/science.1129139
17. Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. (2005) 353:2654–66. doi: 10.1056/NEJMoa051424
18. Rochefort J, Karagiannidis I, Baillou C, Belin L, Guillot-Delost M, Macedo R, et al. Defining biomarkers in oral cancer according to smoking and drinking status. Front Oncol. (2023) 12:1068979. doi: 10.3389/fonc.2022.1068979
19. Lee S, Kim DW, Kwon S, Kim HJ, Cha I-H, Nam W. Prognostic value of systemic inflammatory markers for oral cancer patients based on the 8th edition of AJCC staging system. Sci Rep. (2020) 10:12111. doi: 10.1038/s41598-020-68991-3
20. Gaur P, Qadir GA, Upadhyay S, Singh AK, Shukla NK, Das SN. Skewed immunological balance between Th17 (CD4(+)IL17A (+)) and Treg (CD4 (+)CD25 (+)FOXP3 (+)) cells in human oral squamous cell carcinoma. Cell Oncol Dordr. (2012) 35:335–43. doi: 10.1007/s13402-012-0093-5
21. Dieu-Nosjean M-C, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non–small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. (2008) 26:4410–7. doi: 10.1200/JCO.2007.15.0284
22. Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. (1992) 10:561–91. doi: 10.1146/annurev.iy.10.040192.003021
23. Schröder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci. (1996) 93:221–5. doi: 10.1073/pnas.93.1.221
24. Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. (1996) 183:1461–72. doi: 10.1084/jem.183.4.1461
25. Teillaud J-L, Houel A, Panouillot M, Riffard C, Dieu-Nosjean M-C. Tertiary lymphoid structures in anticancer immunity. Nat Rev Cancer. (2024) 24:629–46. doi: 10.1038/s41568-024-00728-0
26. Pipi E, Nayar S, Gardner DH, Colafrancesco S, Smith C, Barone F. Tertiary lymphoid structures: autoimmunity goes local. Front Immunol. (2018) 9:1952. doi: 10.3389/fimmu.2018.01952
27. Bao Y, Mo Z, Wang S, Long J, Zhang H, Xu Y, et al. Global trends in tertiary lymphoid structures: a bibliometric analysis from 2014–2023. Front Immunol. (2024) 15: 1475062. doi: 10.3389/fimmu.2024.1475062
28. Domblides C, Rochefort J, Riffard C, Panouillot M, Lescaille G, Teillaud J-L, et al. Tumor-associated tertiary lymphoid structures : from basic and clinical knowledge to therapeutic manipulation. Front Immunol. (2021) 12:2611. doi: 10.3389/fimmu.2021.698604
29. Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, Unger K, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol. (2015) 16:1235–44. doi: 10.1038/ni.3290
30. Goc J, Germain C, Vo-Bourgais TKD, Lupo A, Klein C, Knockaert S, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. (2014) 74:705–15. doi: 10.1158/0008-5472.CAN-13-1342
31. Truxova I, Kasikova L, Hensler M, Skapa P, Laco J, Pecen L, et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer. (2018) 6:139. doi: 10.1186/s40425-018-0446-3
32. Johansson-Percival A, He B, Li Z-J, Kjellén A, Russell K, Li J, et al. de novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol. (2017) 18:1207–17. doi: 10.1038/ni.3836
33. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. (2020) 577:549–55. doi: 10.1038/s41586-019-1922-8
34. Petitprez F, de Reyniès A, Keung EZ, Chen TW-W, Sun C-M, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. (2020) 577:556–60. doi: 10.1038/s41586-019-1906-8
35. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. (2020) 577:561–5. doi: 10.1038/s41586-019-1914-8
36. Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity. (2015) 43:579–90. doi: 10.1016/j.immuni.2015.08.006
37. Devi-Marulkar P, Fastenackels S, Karapentiantz P, Goc J, Germain C, Kaplon H, et al. Regulatory T cells infiltrate the tumor-induced tertiary lymphoïd structures and are associated with poor clinical outcome in NSCLC. Commun Biol. (2022) 5:1416. doi: 10.1038/s42003-022-04356-y
38. Santos WMD, Secoli SR, Püschel VADA. The Joanna Briggs institute approach for systematic reviews. Rev Lat Am Enfermagem. (2018) 26:e3074. doi: 10.1590/1518-8345.2885.3074
39. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Br Med J. (2021) 372:n160. doi: 10.1136/bmj.n160
40. Wallis SP, Stafford ND, Greenman J. Clinical relevance of immune parameters in the tumor microenvironment of head and neck cancers. Head Neck. (2015) 37:449–59. doi: 10.1002/hed.23736
41. Barnes L, Eveson JW, Reichart P, Sidransky D. Pathology and Genetics of Head and Neck Tumours. World Health Organ. Classif. Tumours. Lyon: IARC Press (2005).
42. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4+ Follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. (2013) 123:2873–92. doi: 10.1172/JCI67428
43. Haute Autorité de Santé. Niveau de preuve et gradation des recommandations de bonne pratique. Recommandations et leurs synthèse. Saint-Denis: Haute Autorité de Santé (2013). ISBN: 978-2-11-138037-0. https://www.has-sante.fr
44. Wirsing AM, Rikardsen OG, Steigen SE, Uhlin-Hansen L, Hadler-Olsen E. Characterisation and prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma. BMC Clin Pathol. (2014) 14:38. doi: 10.1186/1472-6890-14-38
45. Li K, Guo Q, Zhang X, Dong X, Liu W, Zhang A, et al. Oral cancer-associated tertiary lymphoid structures: gene expression profile and prognostic value. Clin Exp Immunol. (2020) 199:172–81. doi: 10.1111/cei.13389
46. Li Q, Liu X, Wang D, Wang Y, Lu H, Wen S, et al. Prognostic value of tertiary lymphoid structure and tumour infiltrating lymphocytes in oral squamous cell carcinoma. Int J Oral Sci. (2020) 12:24. doi: 10.1038/s41368-020-00092-3
47. Wang C, Huang Z, Zhang M, Xiong G, Chen X, Xie N. Prognostic value of tertiary lymphoid structures in early clinical stage oral tongue squamous cell carcinoma. J Oral Pathol Med. (2021) 50:776–84. doi: 10.1111/jop.13215
48. Peng Y, Xiao L, Rong H, Ou Z, Cai T, Liu N, et al. Single-cell profiling of tumor-infiltrating TCF1/TCF7+ T cells reveals a T lymphocyte subset associated with tertiary lymphoid structures/organs and a superior prognosis in oral cancer. Oral Oncol. (2021) 119:105348. doi: 10.1016/j.oraloncology.2021.105348
49. Almangush A, Bello IO, Elseragy A, Hagström J, Haglund C, Kowalski LP, et al. Tertiary lymphoid structures associate with improved survival in early oral tongue cancer. BMC Cancer. (2022) 22:1–6. doi: 10.1186/s12885-022-10208-z
50. Wang M, Zhai R, Wang M, Zhu W, Zhang J, Yu M, et al. Tertiary lymphoid structures in head and neck squamous cell carcinoma improve prognosis by recruiting CD8+ T cells. Mol Oncol. (2023) 17:1514–30. doi: 10.1002/1878-0261.13403
51. Weed DT, Zilio S, McGee C, Marnissi B, Sargi Z, Franzmann E, et al. The tumor immune microenvironment architecture correlates with risk of recurrence in head and neck squamous cell carcinoma. Cancer Res. (2023) 83:3886–900. doi: 10.1158/0008-5472.CAN-23-0379
52. Li H, Zhu S-W, Zhou J-J, Chen D-R, Liu J, Wu Z-Z, et al. Tertiary lymphoid structure raises survival and immunotherapy in HPV- HNSCC. J Dent Res. (2023) 102:678–88. doi: 10.1177/00220345231151685
53. Louredo B-V, Vargas P-A, Pérez-de-Oliveira M-E, Lopes M-A, Kowalski L-P, Curado M-P. Epidemiology and survival outcomes of lip, oral cavity, and oropharyngeal squamous cell carcinoma in a Southeast Brazilian population. Med Oral Patol Oral Cirugia Bucal. (2022) 27:e274–84. doi: 10.4317/medoral.25147
55. Skálová A, Hyrcza MD, Leivo I. Update from the 5th edition of the world health organization classification of head and neck tumors: salivary glands. Head Neck Pathol. (2022) 16:40–53. doi: 10.1007/s12105-022-01420-1
56. Cillo AR, Kürten CHL, Tabib T, Qi Z, Onkar S, Wang T, et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity. (2020) 52:183–99.e9. doi: 10.1016/j.immuni.2019.11.014
57. Alotaibi M, Valova V, HÄnsel T, Stromberger C, Kofla G, Olze H, et al. Impact of smoking on the survival of patients with high-risk HPV-positive HNSCC: a meta-analysis. Vivo Athens Greece. (2021) 35:1017–26. doi: 10.21873/invivo.12345
58. Arenz A, Patze J, Kornmann E, Wilhelm J, Ziemann F, Wagner S, et al. HPV-negative and HPV-positive HNSCC cell lines show similar numerical but different structural chromosomal aberrations. Head Neck. (2019) 41:3869–79. doi: 10.1002/hed.25924
59. Dahlstrom KR, Little JA, Zafereo ME, Lung M, Wei Q, Sturgis EM. Squamous cell carcinoma of the head and neck in never smoker-never drinkers: a descriptive epidemiologic study. Head Neck. (2008) 30:75–84. doi: 10.1002/hed.20664
60. Koo K, Barrowman R, McCullough M, Iseli T, Wiesenfeld D. Non-smoking non-drinking elderly females: a clinically distinct subgroup of oral squamous cell carcinoma patients. Int J Oral Maxillofac Surg. (2013) 42:929–33. doi: 10.1016/j.ijom.2013.04.010
61. Durr ML, Li D, Wang SJ. Oral cavity squamous cell carcinoma in never smokers: analysis of clinicopathologic characteristics and survival. Am J Otolaryngol. (2013) 34:388–93. doi: 10.1016/j.amjoto.2013.01.017
62. Müller S, Nelson BL, Thompson LDR, Wenig BM. Diagnostic Pathology: Head and Neck. 2nd ed. Philadelphia: Elsevier (2017).
63. Almangush A, Mäkitie AA, Triantafyllou A, de Bree R, Strojan P, Rinaldo A, et al. Staging and grading of oral squamous cell carcinoma: an update. Oral Oncol. (2020) 107:104799. doi: 10.1016/j.oraloncology.2020.104799
64. Caldeira PC, Soto AML, de Aguiar MCF, Martins CC. Tumor depth of invasion and prognosis of early-stage oral squamous cell carcinoma: a meta-analysis. Oral Dis. (2020) 26:1357–65. doi: 10.1111/odi.13194
65. Vanhersecke L, Brunet M, Guégan J-P, Rey C, Bougouin A, Cousin S, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. (2021) 2:794–802. doi: 10.1038/s43018-021-00232-6
66. Siliņa K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. (2018) 78:1308–20. doi: 10.1158/0008-5472.CAN-17-1987
67. Martinet L, Filleron T, Le Guellec S, Rochaix P, Garrido I, Girard J-P. High endothelial venule blood vessels for tumor-infiltrating lymphocytes are associated with lymphotoxin β-producing dendritic cells in human breast cancer. J Immunol Baltim Md. (2013) 191:2001–8. doi: 10.4049/jimmunol.1300872
68. Lee HJ, Park IA, Song IH, Shin S-J, Kim JY, Yu JH, et al. Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol. (2016) 69:422–30. doi: 10.1136/jclinpath-2015-203089
69. Phanthunane C, Wijers R, de Herdt M, Langeveld TPM, Koljenovic S, Dasgupta S, et al. B-cell clusters at the invasive margin associate with longer survival in early-stage oral-tongue cancer patients. OncoImmunology. (2021) 10:1882743. doi: 10.1080/2162402X.2021.1882743
70. Phanthunane C, Wijers R, De Herdt MJ, Koljenović S, Sleijfer S, de Baatenburg Jong RJ, et al. Intratumoral niches of B cells and follicular helper T cells, and the absence of regulatory T cells, associate with longer survival in early-stage oral tongue cancer patients. Cancers (Basel). (2022) 14:4298. doi: 10.3390/cancers14174298
71. Vanhersecke L, Bougouin A, Crombé A, Brunet M, Sofeu C, Parrens M, et al. Standardized pathology screening of mature tertiary lymphoid structures in cancers. Lab Investig J Tech Methods Pathol. (2023) 103:100063. doi: 10.1016/j.labinv.2023.100063
72. Almangush A, Mäkitie AA, Leivo I. Back to basics: hematoxylin and eosin staining is the principal tool for histopathological risk assessment of oral cancer. Oral Oncol. (2021) 115:105134. doi: 10.1016/j.oraloncology.2020.105134
73. Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. (2022) 375:eabf9419. doi: 10.1126/science.abf9419
74. Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. (2019) 19:307–25. doi: 10.1038/s41568-019-0144-6
75. Lee HJ, Kim JY, Park IA, Song IH, Yu JH, Ahn J-H, et al. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol. (2015) 144:278–88. doi: 10.1309/AJCPIXUYDVZ0RZ3G
76. Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie J-J, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. (2011) 71:5678–87. doi: 10.1158/0008-5472.CAN-11-0431
77. Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. (2014) 189:832–44. doi: 10.1164/rccm.201309-1611OC
78. Ladányi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, et al. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother CII. (2007) 56:1459–69. doi: 10.1007/s00262-007-0286-3
79. Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. (2012) 2:765. doi: 10.1038/srep00765
80. Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. (2019) 70:58–65. doi: 10.1016/j.jhep.2018.09.003
81. Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. (2015) 112:1782–90. doi: 10.1038/bjc.2015.145
82. Giraldo NA, Becht E, Pagès F, Skliris G, Verkarre V, Vano Y, et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res Off J Am Assoc Cancer Res. (2015) 21:3031–40. doi: 10.1158/1078-0432.CCR-14-2926
83. Prabhakaran S, Rizk VT, Ma Z, Cheng C-H, Berglund AE, Coppola D, et al. Evaluation of invasive breast cancer samples using a 12-chemokine gene expression score: correlation with clinical outcomes. Breast Cancer Res BCR. (2017) 19:71. doi: 10.1186/s13058-017-0864-z
84. Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. (2011) 179:37–45. doi: 10.1016/j.ajpath.2011.03.007
85. Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean M-C, Riquet M, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases : influence of tumor origin. Clin Cancer Res Off J Am Assoc Cancer Res. (2013) 19:4079–91. doi: 10.1158/1078-0432.CCR-12-3847
86. Väyrynen JP, Sajanti SA, Klintrup K, Mäkelä J, Herzig K-H, Karttunen TJ, et al. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer. (2014) 134:2126–35. doi: 10.1002/ijc.28533
87. McMullen TPW, Lai R, Dabbagh L, Wallace TM, de Gara CJ. Survival in rectal cancer is predicted by T cell infiltration of tumour-associated lymphoid nodules. Clin Exp Immunol. (2010) 161:81–8. doi: 10.1111/j.1365-2249.2010.04147.x
88. Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res Off J Am Assoc Cancer Res. (2014) 20:2147–58. doi: 10.1158/1078-0432.CCR-13-2590
89. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. (2013) 39:782–95. doi: 10.1016/j.immuni.2013.10.003
90. Meshcheryakova A, Tamandl D, Bajna E, Stift J, Mittlboeck M, Svoboda M, et al. B cells and ectopic follicular structures: novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer. PLoS One. (2014) 9:e99008. doi: 10.1371/journal.pone.0099008
91. Rochefort J, Radoi L, Campana F, Fricain J-C, Lescaille G. Oral cavity cancer: a distinct entity. Med Sci MS. (2024) 40:57–63. doi: 10.1051/medsci/2023196
92. Mello FW, Miguel AFP, Dutra KL, Porporatti AL, Warnakulasuriya S, Guerra ENS, et al. Prevalence of oral potentially malignant disorders: a systematic review and meta-analysis. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. (2018) 47:633–40. doi: 10.1111/jop.12726
93. Fridman WH, Petitprez F, Sautes-Fridman C. Intratumoral B cells and tertiary lymphoid structures are biomarkers of survival and immunotherapy responses. Bull Cancer (Paris). (2020) 107:403–4. doi: 10.1016/j.bulcan.2020.02.008
94. Chelvanambi M, Fecek RJ, Taylor JL, Storkus WJ. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J Immunother Cancer. (2021) 9:e001906. doi: 10.1136/jitc-2020-001906
Keywords: oral cancer, oral oncoimmunology, oral squamous cell carcinoma, prognostic biomarker, tertiary lymphoid structures
Citation: Ribeiro V, Teillaud J-L, Dieu-Nosjean M-C, Lescaille G and Rochefort J (2025) The prognostic significance of tertiary lymphoid structures in oral squamous cell carcinomas: a systematic review. Front. Oral. Health 5:1524313. doi: 10.3389/froh.2024.1524313
Received: 7 November 2024; Accepted: 31 December 2024;
Published: 22 January 2025.
Edited by:
Nikolaos Nikitakis, National and Kapodistrian University of Athens, GreeceReviewed by:
Vishal Gupta, University at Buffalo, United StatesCopyright: © 2025 Ribeiro, Teillaud, Dieu-Nosjean, Lescaille and Rochefort. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: V. Ribeiro, dmlhbm5leS5yaWJlaXJvQGFwaHAuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.