- 1Department of Prosthetics Dentistry with Implantology, Poltava State Medical University, Poltava, Ukraine
- 2Department of Microbiology, Virology and Immunology, Poltava State Medical University, Poltava, Ukraine
Today, about 15.0% of odontogenic pathology is caused by Staphylococcus aureus (S. aureus). The aim of the study was to predict the development of antimicrobial resistance of S. aureus based on retrospective data.
Methods: A total of 425 patients undergoing treatment for odontogenic infectious diseases of the facial area during 2019–2023 were involved in the study. The object of the study was 106 clinical isolates of S. aureus that were isolated and identified from patients. Determining the sensitivity of the obtained isolates to antimicrobial drugs was carried out using Vitek antimicrobial susceptibility testing (Biomerioux, France) and analyzed according to the breackpoint tables of the EUCAST. Prediction of the development of antimicrobial resistance of S. aureus to various antibiotics was carried out on the basis of the received sensitivity data of the studied isolates in 2019–2023 using the exponential smoothing method.
Results: The antimicrobial resistance of S. aureus isolates to various antibiotics changed annually during 2019–2023. The level of resistance of S. aureus isolates to benzylpenicillin wavered between 40%–50% from 2019 to 2023 with the trend of an 18.0% increase over the next five years. A uniform plateau of antimicrobial resistance of S. aureus to cefoxitin is predicted at the level of 32.0% during 2024–2028. We recorded the highest portions of S. aureus resistant to norfloxacin (33.3%) and ciprofloxacin (16.7%) in 2023 with prediction of its increasing in the next five years within the range of 20.0%. It was established that S. aureus may reach 100.0% resistance to gentamicin in 2027. According to exponential smoothing, the level of S. aureus resistance to amikacin will increase by 22.7% over the next five-year period. Moreover, representatives of this species of bacteria can develop complete (100.0%) resistance to tetracycline as early as 2027.
Conclusions: Mathematical prediction of the development of antimicrobial sensitivity of S. aureus isolates showed a high probability of its development to antibiotics of all groups in the next five years.
1 Introduction
Almost a quarter of surgical pathology in the world is due to odontogenic infections (1, 2). Considering the fact that they develop during the spread of microorganisms through the destroyed tissues of the teeth into the underlying tissues, the bacterial etiological factor is obvious and indisputable (2, 3). The development of laboratory and microbiological methods of research contributes to the revision of the patterns of the microbiota of foci of odontogenic infection recently (4). Thus, more and more often there are scientific works indicating the role of gram-negative rods and spirochetes with a predominantly anaerobic type of respiration in the development of this pathology (5–8). However, along with this, the participation of gram-positive cocci, although somewhat reduced in recent years, continues to hold a leading position in odontogenic infections (9, 10).
Today, about 15.0% of odontogenic pathology is caused by Staphylococcus aureus (S. aureus) (2). It is worth noting that this microorganism is one of the so-called ESKAPE pathogens, which are characterized by high virulence and multiple resistance to various antimicrobial agents (11). According to literature data, the frequency of development of methicillin resistance among strains of S. aureus in the world varies, but in general it is not less than 20.0% for highly developed countries (12–14). Research in 2022 shows that the proportion of multi-resistant S. aureus in Ukraine exceeds 35.0%. Taking into account an ineffective state measures to counter the development of resistance and the war on the territory of Ukraine, it can be assumed that this indicator is not final and may increase in the following years (15, 16).
The aim of the study was to predict the development of antimicrobial resistance of S. aureus based on retrospective data.
2 Methods
2.1 Ethics
Written informed consent was obtained from each subject after a detailed explanation of the aim and protocol of the study, which was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki for Ethical Principles for Medical Research Involving Human Subjects. The study was approved by the commission on biomedical ethics of the Poltava State Medical University (minutes #188 dated November 25, 2020).
2.2 Study population and specimens’ collection
A total of 425 patients undergoing treatment for odontogenic infectious diseases of the facial area (abscesses and phlegmons) during 2019–2023 were involved in the study.
The criteria for the inclusion of patients in the study was the confirmed diagnosis of odontogenic infectious diseases of the skin and subcutaneous tissue (abscesses and phlegmons) of the facial area, subject to consent to participate in the study. Exclusion criteria were non-compliance with the diagnosis, pregnancy, diabetes, presence of congenital or acquired immunodeficiency, mental disorders, taking antibiotics before collecting specimens, and refusal to participate in the study.
Along with the general clinical methods of research, specimens were collected from each patient for further microbiological research in order to isolate the etiologically significant pathogen and determine its sensitivity to antibiotics. Purulent exudate was collected with a sterile probe-tampon immediately after dissection of the focus of infection, followed by transfer to Amies transport nutrient medium. Cultivation of the material was carried out using a standard culture method on blood agar, meat-peptone agar and yolk-salt agar at a temperature of 37℃. The final identification of microorganisms was carried out with the automatic bacteriological analyzer Vitek compact (Biomerioux, France).
The object of the study was 106 clinical isolates of S. aureus that were isolated and identified from patients. Determining the sensitivity of the obtained isolates to antimicrobial drugs was carried out using Vitek antimicrobial susceptibility testing with AST-GP67 cards (Biomerioux, France) and analyzed according to the breackpoint tables of the EUCAST (current version for every year of study). The contents of AST-GP67 cards for Vitek 2 Systems is provided in Supplementary Table S1.
2.3 Statistical analysis
Mean, standard deviation, median, minimum, maximum value of frequency and percentage were used for descriptive statistics.
Prediction of the development of antimicrobial resistance of S. aureus to various antibiotics was carried out on the basis of the received sensitivity data of the studied isolates in 2019–2023 using the Holt's exponential smoothing (HES) method. This is a method of mathematical transformation for forecasting time series, in which each subsequent iteration takes into account all previous values of the series, but the degree of consideration decreases according to the exponential law (17). HES also called double Exponential Smoothing performs the forecasting of data with the trend formation not counting seasonality (18). It involves a forecast equation and two smoothing equations (one for the level and one for the trend). The standard exponential smoothing formulas (1–3) were used for mathematical processing of the results:
where: lt - an estimate of the level of the series at time t; bt- an estimate of the trend of the series at time t; α - smoothing parameter for the level, 0 ≤ α ≤ 1; β* - smoothing parameter for the trend, 0 ≤ β* ≤ 1.
Mathematical analysis was carried out using the license package of the program StatPlus:macPro license program, AnalystSoft Inc. 2024 (USA).
3 Results
In general, the obtained results indicated the average similarity of the frequency of the development of antimicrobial resistance to different groups of antibiotics among isolates of S. aureus isolated from patients with odontogenic phlegmons and abscesses (Table 1). This provided the basis for further combined statistical processing of the results.

Table 1. The frequency of the development of antimicrobial resistance of S. aureus under conditions of odontogenic infections of the soft tissues of the facial area.
As a result of a retrospective study of the sensitivity to antibiotics of pathogens that cause infectious and inflammatory diseases of the face, it was established that the resistance of S. aureus isolates to various antibiotics changed annually during 2019–2023.
The level of resistance of S. aureus isolates to benzylpenicillin, by which resistance to all penicillins is assessed, wavered between 40%–50% from 2019 to 2023 (Figure 1). However, the exponential smoothing method clearly outlined the trend of an 18.0% increase in the proportion of S. aureus resistant to benzylpenicillin over the next five years. Thus, according to mathematical forecasting, the frequency of detection of antimicrobial resistance of S. aureus to benzylpenicillin, and hence to all penicillins, will reach 64.3% in 2028.
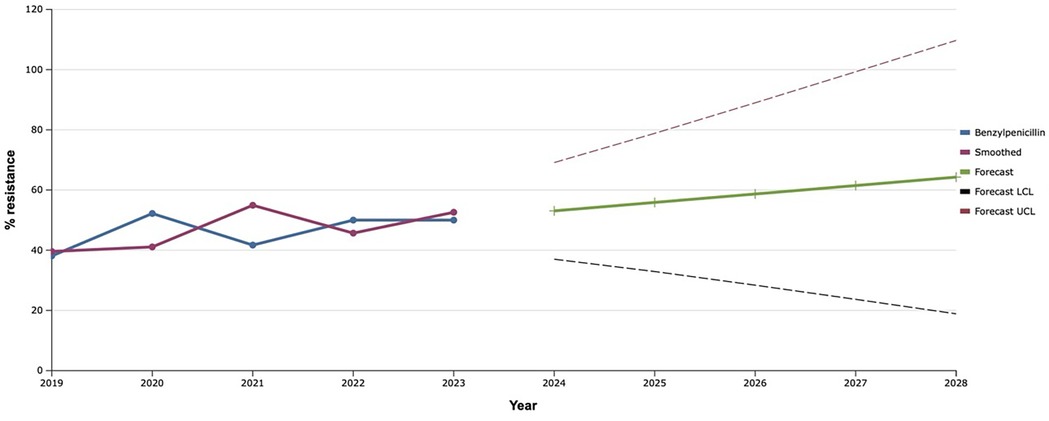
Figure 1. Forecasting the development of S. aureus resistance to benzylpenicillin based on retrospective data from 2019 to 2023 (screenshot of StatPlus: macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
Throughout the study period, the resistance of S. aureus isolates to oxacillin varied within 14%–39.0%. That is why, based on such retrospective data, it was mathematically established that the level of staphylococci resistant to this antibiotic over the next five years would increase by 17.0% compared to the level in 2023 (Figure 2).
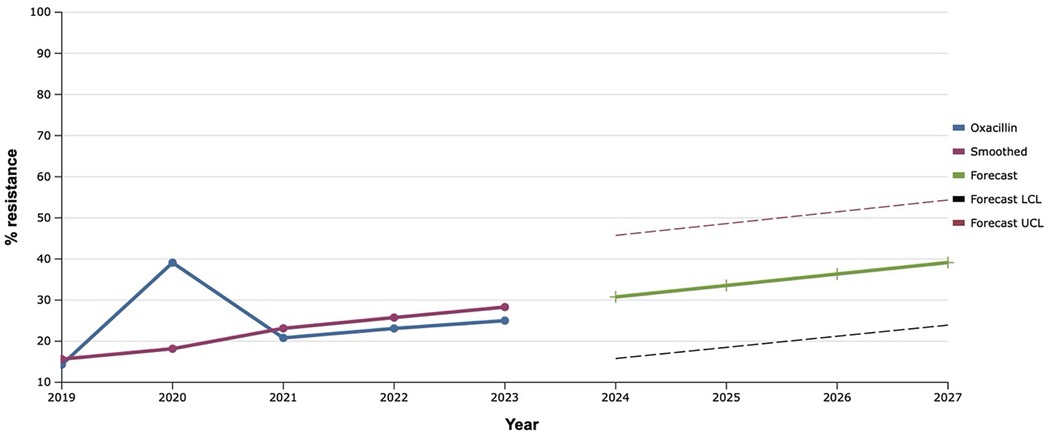
Figure 2. Forecasting the development of S. aureus resistance to oxacillin based on retrospective data from 2019 to 2023 (screenshot of StatPlus:macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
In the course of the study, a slight decrease in the level of resistance of investigated isolates to cefoxitin was observed in 2021–2022 to almost 20.0% with a 1.4-fold increase in the following year 2023 (Figure 3). Based on these data, a uniform plateau of antimicrobial resistance of S. aureus to cefoxitin is predicted at the level of 32.0% during 2024–2028.
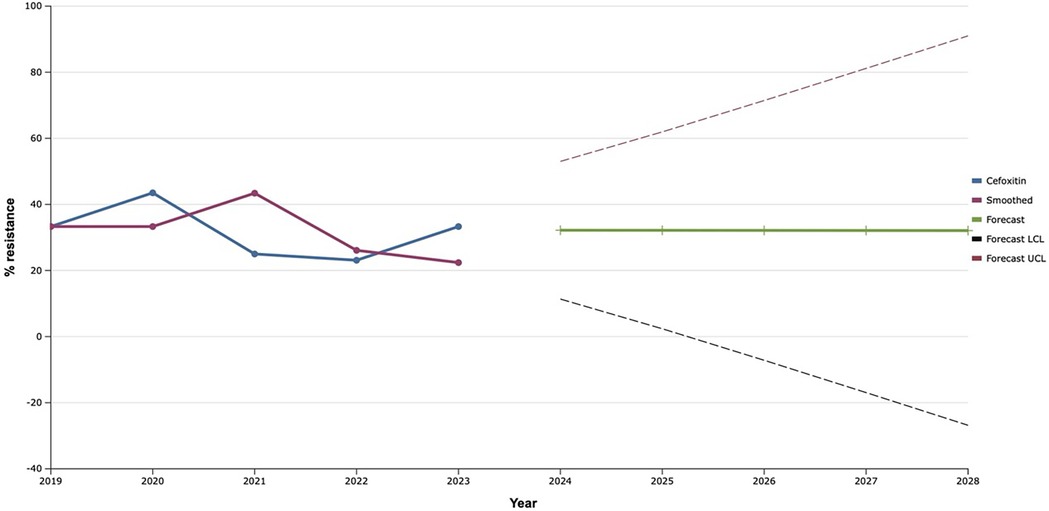
Figure 3. Forecasting the development of S. aureus resistance to cefoxitin based on retrospective data from 2019 to 2023 (screenshot of StatPlus: macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
The level of antimicrobial resistance of clinical strains of S. aureus, isolated from patients with infectious inflammatory diseases of soft tissues of facial area, to fluoroquinolones gradually increased during 2019–2021 (Figure 4). Despite the slight decrease in this indicator in 2022, in the following year 2023, we recorded the highest portions of S. aureus resistant to norfloxacin (33.3%) and ciprofloxacin (16.7%). That is why, after the exponential smoothing analysis, a clear trend towards an increase in the level of resistance of S. aureus to fluoroquinolones was revealed. Thus, according to the results of the analysis, the proportions of ciprofloxacin-resistant isolates of this species will increase from 18.5% to 25.3% during 2024–2028. Norfloxacin showed a similar trend: the predicted proportion of S. aureus resistant to it increased steadily from 37.4% in 2024 to 55.6% in 2028. Taking into account the fact that the level of fluoroquinolone resistance is established based on the result of the sensitivity of staphylococci to norfloxacin, we can assume a predicted increase of the last one in the next five years within the range of 20.0%.
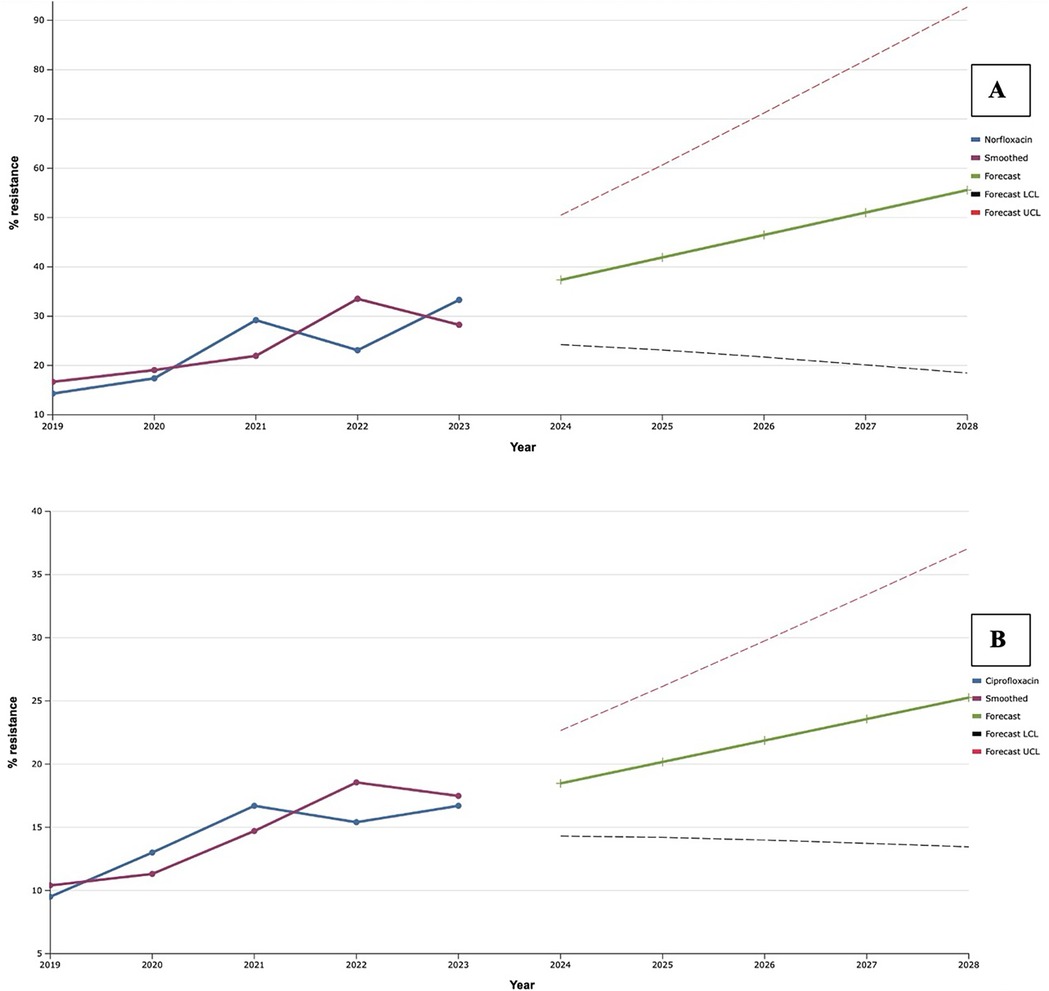
Figure 4. Forecasting the development of S. aureus resistance to norfloxacin (A) and ciprofloxacin (B) based on retrospective data from 2019 to 2023 (screenshot of the StatPlus:macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
As a result of the study, a rapid increase in the level of antimicrobial resistance of S. aureus to aminoglycosides was established during 2019–2021 (Figure 5), when record resistance to gentamicin was established (70.8%). Along with this, resistance to amikacin continued to increase and was maximal (61.5%) in 2022. Despite the fact that in 2022–2023, resistance to gentamicin decreased by 24.0% compared to the maximum indicator, mathematical forecasting indicated a potential increase in the level of resistance to this antibiotic among isolates of S. aureus in the next five years by 33.3%, compared to an indicator of 2023. At the same time, S. aureus may reach 100.0% resistance to gentamicin in 2027.
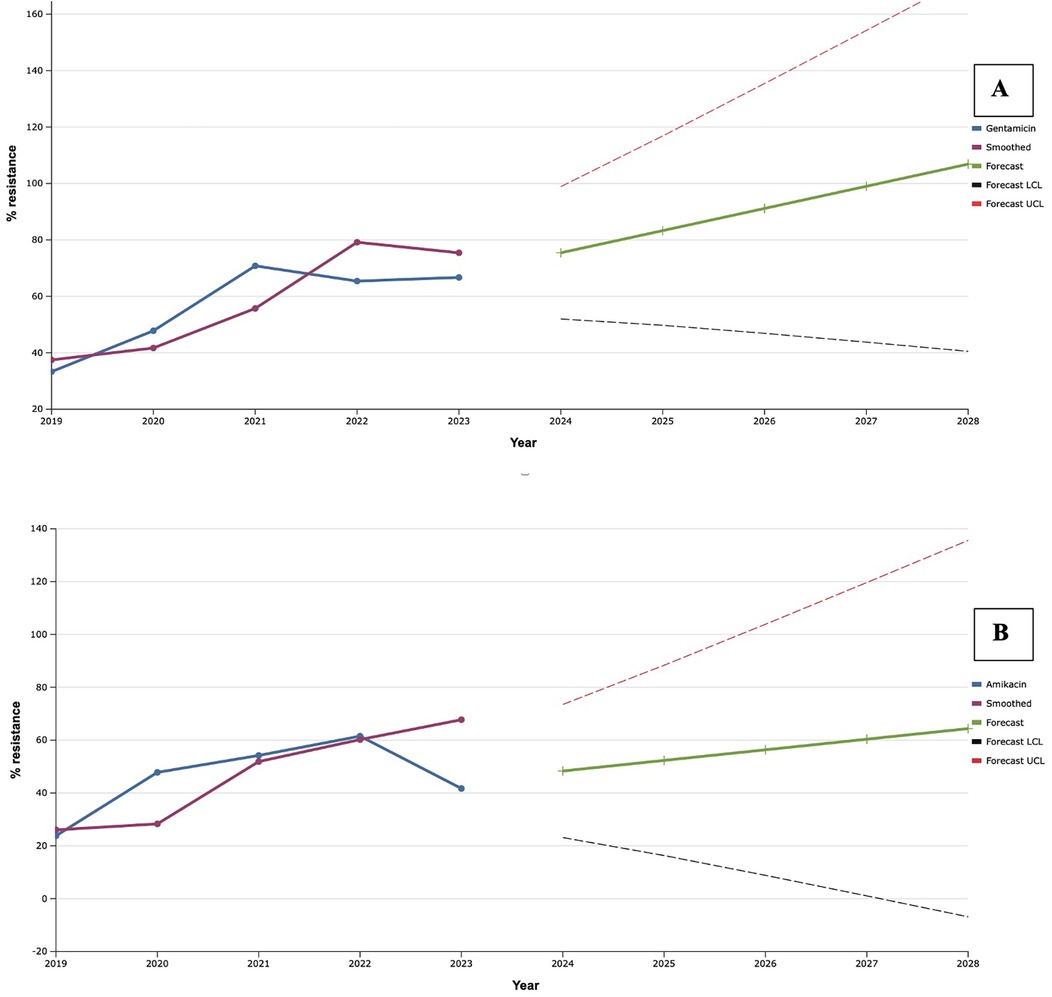
Figure 5. Forecasting the development of S. aureus resistance to gentamicin (A) and amikacin (B) based on retrospective data from 2019 to 2023 (screenshot of the StatPlus: macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
A 20.2% decrease in the level of resistance of the studied isolates to amikacin in 2023, compared to the previous year, contributed to the formation of a less rapid trend of the predicted results. Thus, according to exponential smoothing, the level of S. aureus resistance to amikacin will increase by 22.7% over the next five-year period (from 48.3% in 2024 to 64.4% in 2028).
During 2019–2022 erythromycin demonstrated mostly stable effectiveness against clinical isolates of S. aureus with a frequency of resistance at the level of 40.0%, with the exception of 2020, when a decrease in the proportion of resistant strains to 26.1% was noted (Figure 6). However, in 2023, the level of antimicrobial resistance of S. aureus isolates to erythromycin increased to almost 60.0%. Therefore, as a result of mathematical forecasting, a clear tendency to increase the frequency of isolation of S. aureus resistant to it was established in the future. Taking into account that resistance to erythromycin is evaluated for the presence of macrolide resistance, this forecast indicated a probable increase in the level of the it to 77.4% in 2028, what is 35.0% higher than the average indicator of resistance of S. aureus to macrolides in 2019–2023.
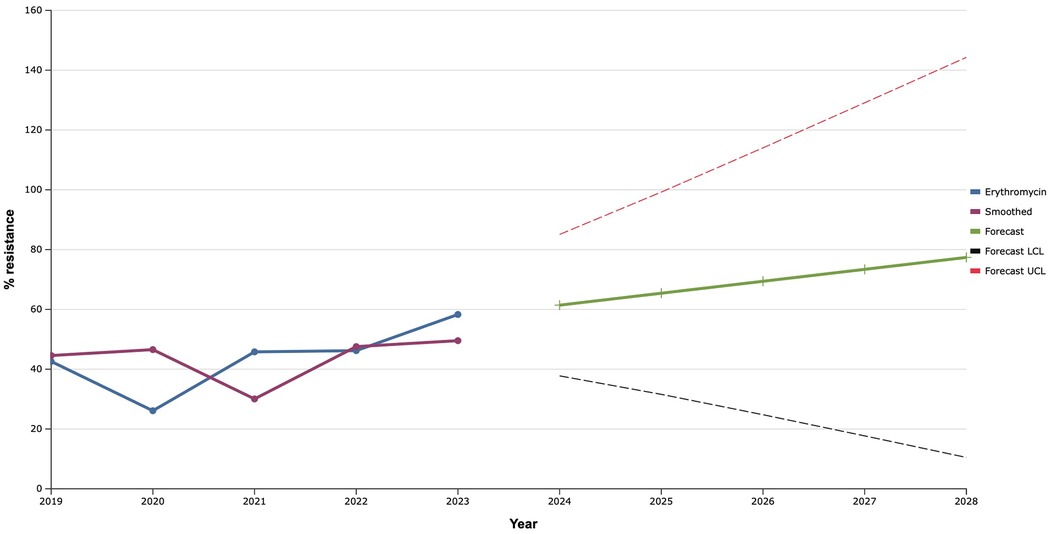
Figure 6. Forecasting the development of S. aureus resistance to erythromycin based on retrospective data from 2019 to 2023 (screenshot of StatPlus:macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
Among lincosamides, the iinvestigated bacterial cultures were tested for clindamycin, the level of resistance to which almost did not change during 2019–2023. Therefore, the fact of the predicted plateau (around 43.0%) in the further development of S. aureus resistance to clindamycin over the next five years was natural (Figure 7).
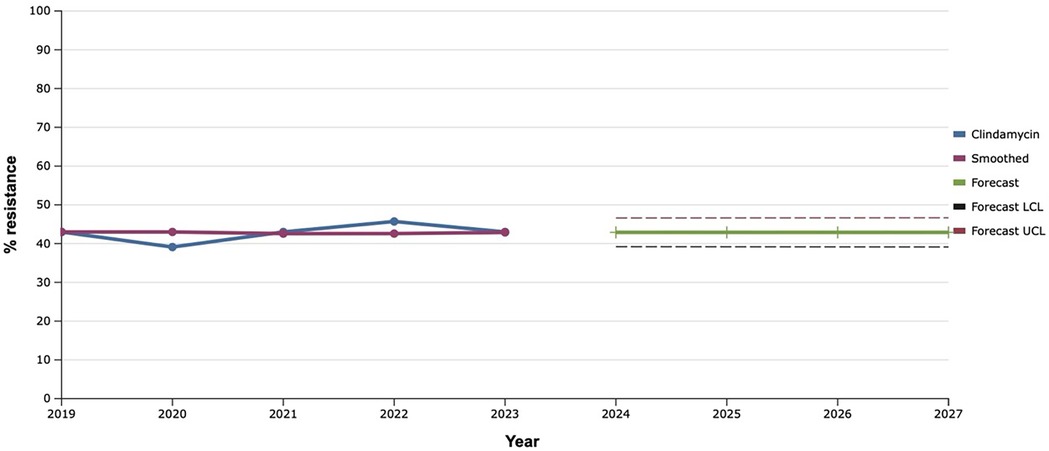
Figure 7. Forecasting the development of S. aureus resistance to clindamycin based on retrospective data from 2019 to 2023 (screenshot of StatPlus:macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
The sensitivity of S. aureus isolates to rifampicin has been gradually increasing since 2019, reaching a maximum value of 38.5% in 2022. That is why, using the mathematical prediction method, it was established that the level of rifampicin-resistant S. aureus would increase to 46.7% in 2027 (Figure 8).
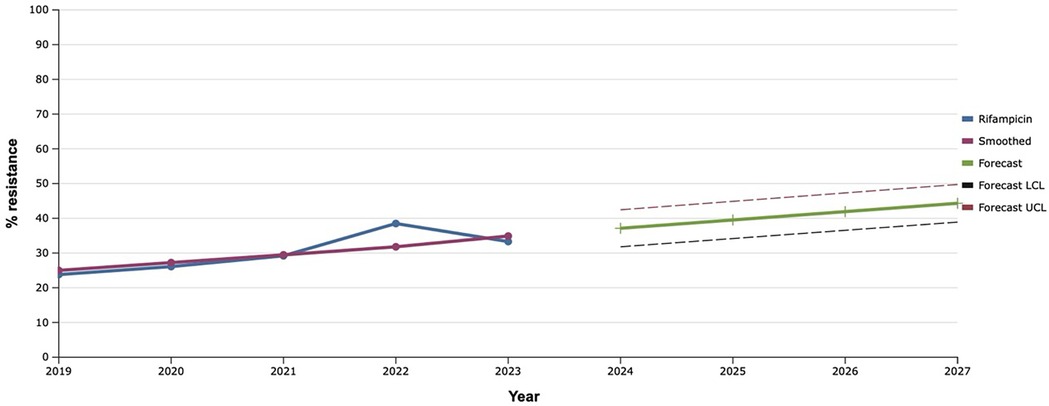
Figure 8. Forecasting the development of S. aureus resistance to rifampicin based on retrospective data from 2019 to 2023 (screenshot of StatPlus:macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
Among all the antibiotics tested, vancomycin, linezolid, and tigecycline showed the best results, as the level of resistance to them over the past five years did not exceed 20.0% (Figures 9–11). However, the detection of 16.7% vancomycin-resistant staphylococci significantly influenced the outcome of further prediction of the development of resistance among S. aureus. Thus, there is a mathematical probability of S. aureus acquiring vancomycin resistance at the level of 37.6% by 2027. The only antibiotic that did not show any signs of increasing the proportion of resistant S. aureus to it, according to the results of the retrospective analysis, was linezolid. The predictive trend for the next five years was close to zero, which confirmed the promising potential of linezolid in the fight against antibiotic-resistant strains of S. aureus.
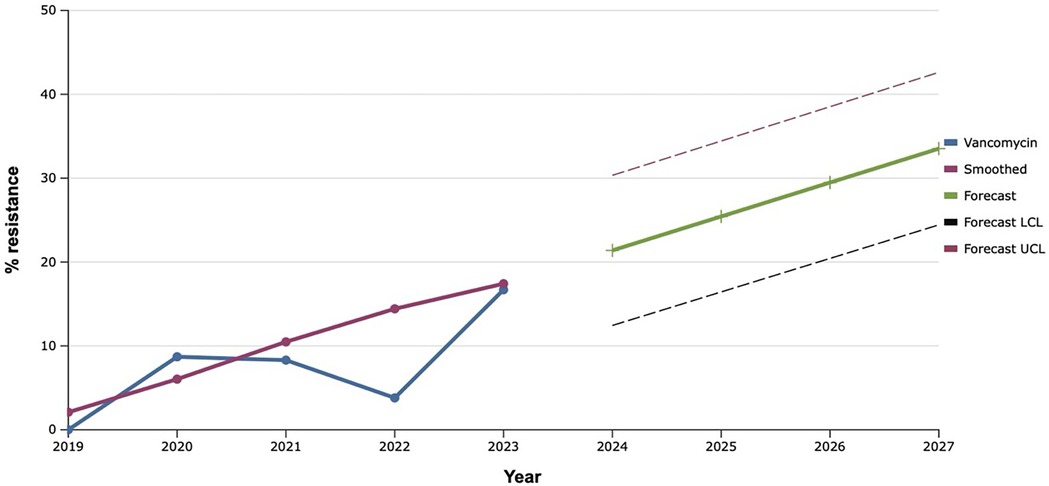
Figure 9. Forecasting the development of S. aureus resistance to vancomycin, based on retrospective data from 2019 to 2023 (screenshot of StatPlus:macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
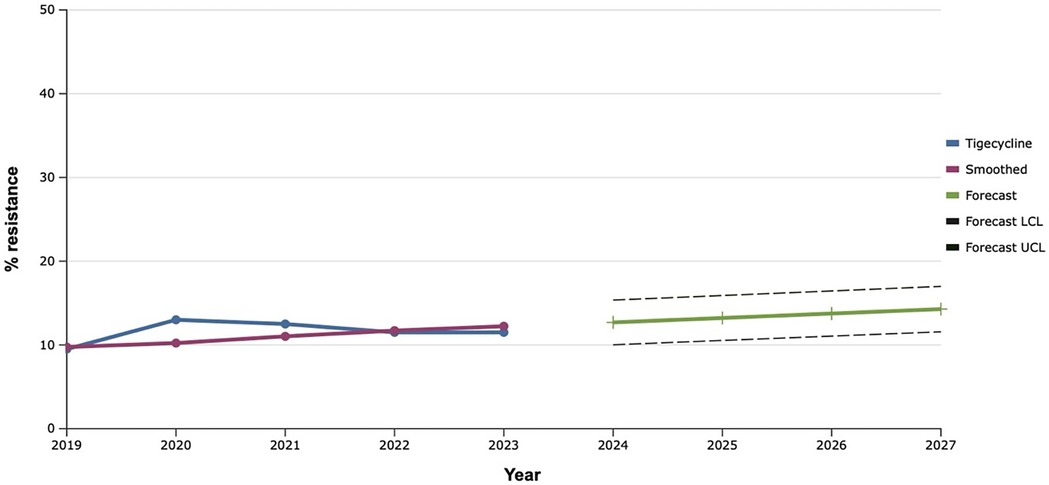
Figure 10. Forecasting the development of S. aureus resistance to linezolid, based on retrospective data from 2019 to 2023 (screenshot of StatPlus:macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
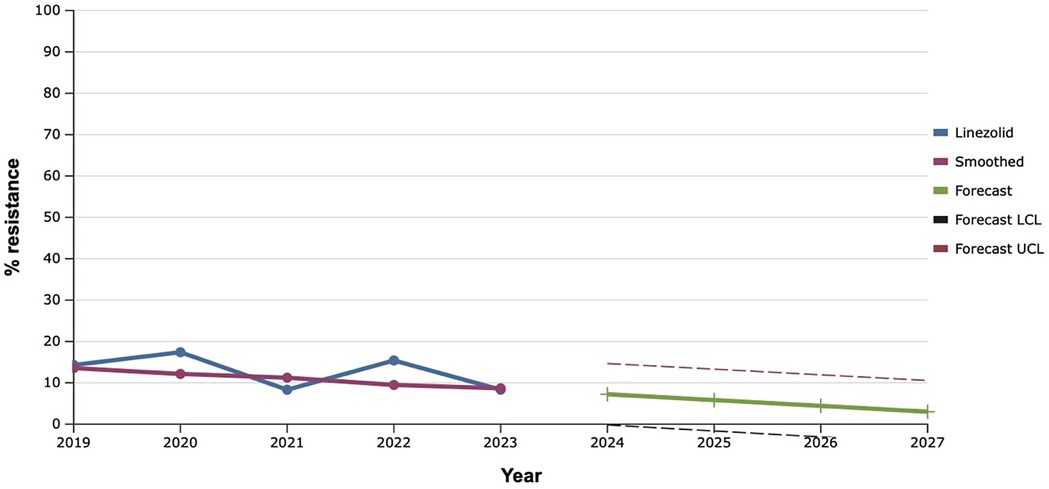
Figure 11. Forecasting the development of S. aureus resistance to tigecycline, based on retrospective data from 2019 to 2023 (screenshot of StatPlus:macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
As a result of the study, it was established that the proportion of tetracycline-resistant S. aureus isolated from patients with IID of soft-tissue FA increased from 2019 and reached a recorded maximum (66.7%) in 2023 (Figure 12). Based on this, the predicted probability of rapid acquisition of antimicrobial resistance to tetracycline by S. aureus strains during the next five years was determined using the exponential smoothing method. Moreover, representatives of this species of bacteria can develop complete (100.0%) resistance to tetracycline as early as 2027.
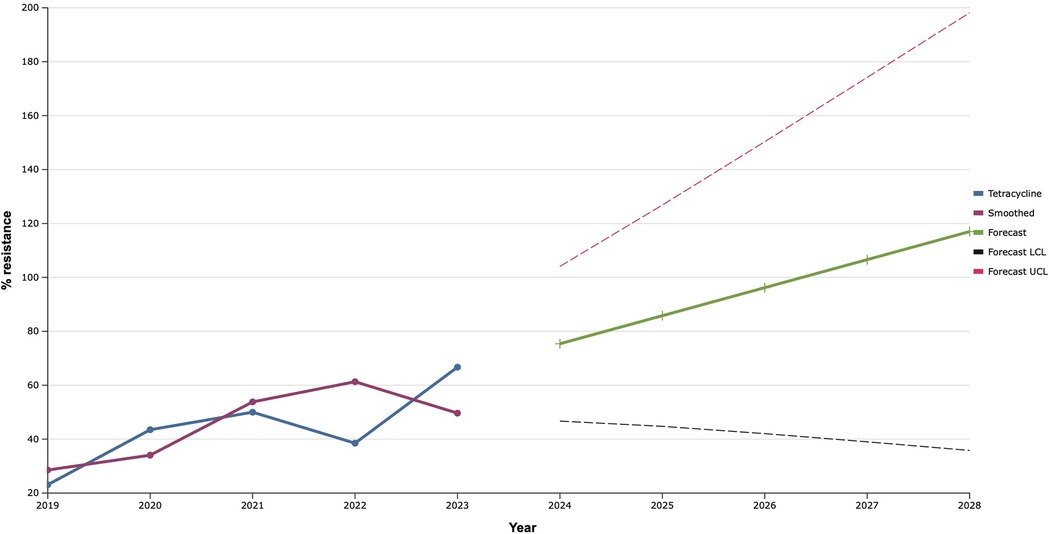
Figure 12. Forecasting the development of S. aureus resistance to tetracycline based on retrospective data from 2019 to 2023 (screenshot of StatPlus:macPro license program, AnalystSoft Inc. 2024); Smoothed, the smoothed value at time; Forecast, the predicted value at time; Forecast LCL, forecast lower control limit; Forecast UCL, forecast upper control limit.
4 Discussion
Today, there are a number of possibilities and methods for forecasting various indicators, both short-term, medium-term, and long-term. This makes it possible to have an idea of the likely course of certain processes in the future, from several days to several years (19). Therefore, it is obvious that the correct choice of forecasting method should be selected in accordance with the needs of the study, the characteristics of the variational data series with the possibility of obtaining the minimum error rate (18). A time series forecasting method that is often used is the exponential smoothing method, which we chose in this study (18, 20). For such forecasts, a set of retrospective values is used to calculate components in the future step by step and are widely used in science, engineering, telecommunications, finance, and economics. Exponential smoothing methods have several advantages over other methods, namely: power, simplicity, and the small number of variables required (19, 21). Among these exponential smoothing methods, the three most commonly used are: Holt's linear method single exponential smoothing, and double or triple exponential smoothing. While the single exponential method is known as simple, Holt's linear method provides trend data forecasts with no seasonality and is of the highest quality (19, 22).
The rapid acquisition of antimicrobial resistance by strains of S. aureus is of great concern to scientists and doctors. After all, the level of resistance of representatives of this species to penicillins in China and UK during the last 10 years varied in the range of 17%–33% (23–25). The results obtained by us in Ukraine slightly exceeded these indicators. Along with this, the data of American scientists indicate the development of resistance of S.aureus to cefoxitin in 62.8% of cases, which is 19.3% higher than the maximum indicator obtained by us in 2020 (26). It is worth noting that the peaks of resistance to benzylpenicillin and cefoxitin coincided with the beginning of the COVID-19 pandemic and the war in the country, what were probably related. In general, this is obvious, since the frequency of antibiotic prescriptions for the treatment of complicated pneumonias increased significantly during the epidemic. In addition, the fact of increasing antimicrobial resistance during wartime is confirmed not only by the experience of Ukraine (16, 27–29). Of particular concern is the fact that we predict the level of resistance of S. aureus strains to β-lactams at the level of 32%–64%. Moreover, according to the recommendations of EUCAST, it is possible to make conclusions about the sensitivity of staphylococci to all β-lactam antibiotics based on sensitivity to benzylpenicillin and cefrxitin. This may indirectly indicate such a level of development of methicillin resistance. Because studies have proven that testing of S. aureus for cefoxitin is an experimentally confirmed marker of methicillin resistance (30).
For more than 30 years, scientists around the world have been reporting the acquisition of resistance to fluoroquinolones by S. aureus isolates. Moreover, almost a quarter of these are polyresistant species, especially in combination with methicillin resistance (31). The main reasons for such a rapid acquisition of resistance among gram-positive cocci are modification of fluoroquinolone targets, inhibition of permeability due to changes in the outer membrane, overexpression of efflux pumps, and plasmid-mediated resistance. However, more attention has recently been paid to the study of genetic determinants of resistance of staphylococci to quinolone antibiotics, gene mutations and changes in gene regulation. That is why we observed an almost annual increase in the parts of S. aureus resistant to fluoroquinolones with a natural predicted increase in the future (32). The results we obtained over the past five years demonstrated a similar global trend. Thus, according to research results in Nigeria, S. aureus develop resistance to fluoroquinolones in 31%–33.0% of cases, in Saudi Arabia—33.8%, which fully correlates with similar indicators in Ukraine (33, 34).
The disappointing prognosis for the resistance of S. aureus to aminoglycosides, in particular gentamicin, can probably be explained by their widespread use in clinical practice. It is worth noting that the most common mechanism of development of bacterial resistance to aminoglycosides is the synthesis of transferase enzymes, which, by modifying the drug, reduce its effect. However, from the point of view of S. aureus as a film-forming bacteria, more attention should be paid to another mechanism of resistance, such as a decrease in enzymatic activity in low-oxygen metabolism during its living in biofilms (35, 36).
It is worth noting that the best results, both retrospectively and prospectively, regarding the development of resistance among S. aureus were demonstrated by vancomycin, linezolid, and tigecycline. A similar trend was observed in Latin America, where researchers found almost 99.0% susceptibility to these antibiotics among multidrug-resistant S. aureus (37).
Recent research by French scientists through detailed sequencing has determined that isolates of S. aureus have a much larger range of genetic determinants responsible for resistance to β-lactams, fluoroquinolones and macrolides, compared to antibiotics of other groups (38). There, it is possible to explain the similarly stable trend of increasing resistance to them in the temporal aspect.
The challenges that have befallen the health care system of Ukraine in recent years significantly affect the spectrum of pathogens of the most common infections and their pattern of antimicrobial resistance (28, 39–41). Therefore, it is important to take into account these changes and develop adequate methods of responding to the perspectives that are expected to develop in the near future.
5 Conclusions
The exponential smoothing method clearly outlined the trend of an 18.0% increase in the proportion of S. aureus resistant to benzylpenicillin over the next five years. Fluoroquinolone resistance is established based on the result of the sensitivity of staphylococci to norfloxacin, what predicts an increase of the last one among S. aureus in the next five years within the range of 20.0%. Mathematical forecasting indicates a potential increase in the level of resistance to aminoglycosides and macrolides among isolates of S. aureus in the next five years by 22.7%–33.3% and 35.0% respectively. S. aureus can develop complete (100.0%) resistance to tetracycline as early as 2027. There is a mathematical probability of S. aureus acquiring vancomycin resistance at the level of 37.6% by 2027. The only antibiotic that did not show any signs of increasing the proportion of resistant S. aureus to it, according to the results of the retrospective analysis, was linezolid.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Commission on biomedical ethics of the Poltava State Medical University (minutes #210 dated November 23, 2022). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
OS: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft. MF: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. TP: Data curation, Methodology, Resources, Writing – review & editing. HB: Formal Analysis, Resources, Supervision, Validation, Writing – review & editing. IP: Investigation, Resources, Software, Validation, Writing – review & editing. GL: Conceptualization, Investigation, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2024.1514070/full#supplementary-material
References
1. Fu B, McGowan K, Sun JH, Batstone M. Increasing frequency and severity of odontogenic infection requiring hospital admission and surgical management. Br J Oral Maxillofac Surg. (2020) 58(4):409–15. doi: 10.1016/j.bjoms.2020.01.011
2. Faustova M, Nazarchuk O, Loban’ G, Avetikov D, Ananieva M, Chumak Y, et al. Microbiological aspects concerning the etiology of acute odontogenic inflammatory diseases in the soft tissues of the head and neck region. Open Access Maced J Med Sci. (2022) 10(F):636–40. doi: 10.3889/oamjms.2022.10535
3. Faustova MO, Ananieva MM, Basarab YO, Loban’ GA. Neutrophil bactericidal activity through the stages of placement of different dental implants depending on their chemical composition. Wiad Lek. (2017) 70(5):921–4.29203742
4. Abdullaeva SA. Modern condition of the problem of etiology, pathogenesis and treatment of the flegmon of the lower part of the mouth and neck (review of literature). Izvestiia OshTU. (2018) 3:168–72.
5. Böttger S, Zechel-Gran S, Schmermund D, Streckbein P, Wilbrand JF, Knitschke M, et al. Microbiome of odontogenic abscesses. Microorganisms. (2021) 9(6):1307. doi: 10.3390/microorganisms9061307
6. Ogle OE. Odontogenic infections. Dent Clin North Am. (2017) 61(2):235–52. doi: 10.1016/j.cden.2016.11.004
7. Böttger S, Zechel-Gran S, Schmermund D, Streckbein P, Wilbrand JF, Knitschke M, et al. Clinical relevance of the microbiome in odontogenic abscesses. Biology. (2021) 10(9):916. doi: 10.3390/biology10090916
8. Loban’ GA, Faustova MO, Chereda VV, Ananieva MM. Epidemiological and etiological aspects of dental caries development. Acta Facult Med Naissensis. (2021) 38(1):27–34. doi: 10.5937/afmnai38-27564
9. Chen J, Wu X, Zhu D, Xu M, Yu Y, Yu L, et al. Microbiota in human periodontal abscess revealed by 16S rDNA sequencing. Front Microbiol. (2019) 10:1723. doi: 10.3389/fmicb.2019.01723
10. Demkovych A, Kalashnikov D, Hasiuk P, Zubchenko S, Vorobets A. The influence of microbiota on the development and course of inflammatory diseases of periodontal tissues. Front Oral Health. (2023) 4:1237448. doi: 10.3389/froh.2023.1237448
11. Denissen J, Reyneke B, Waso-Reyneke M, Havenga B, Barnard T, Khan S, et al. Prevalence of ESKAPE pathogens in the environment: antibiotic resistance status, community-acquired infection and risk to human health. Int J Hyg Environ Health. (2022) 244:114006. doi: 10.1016/j.ijheh.2022.114006
12. Lim WW, Wu P, Bond HS, Wong JY, Ni KW, Seto WH, et al. Determinants of methicillin-resistant Staphylococcus aureus(MRSA) prevalence in the Asia-pacific region: a systematic review and meta-analysis. J Glob Antimicrob Resist. (2019) 16:17–27. doi: 10.1016/j.jgar.2018.08.014
13. De Oliveira DMP, Forde BM, Kidd TJ, Harris PNA, Schembri MA, Beatson SA, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. (2020) 33(3):e00181–19. doi: 10.1128/CMR.00181-19
14. Ardila CM, Bedoya-García JA. Antimicrobial resistance in patients with odontogenic infections: a systematic scoping review of prospective and experimental studies. J Clin Exp Dent. (2022) 14(10):e834–45. doi: 10.4317/jced.59830
15. Ludvigsson JF, Loboda A. Systematic review of health and disease in Ukrainian children highlights poor child health and challenges for those treating refugees. Acta Paediatr. (2022) 111(7):1341–53. doi: 10.1111/apa.16370
16. Loban’ G, Faustova M, Dobrovolska O, Tkachenko P. War in Ukraine: incursion of antimicrobial resistance. Ir J Med Sci. (2023) 192:2905–7. doi: 10.1007/s11845-023-03401-x
17. Goodwin P. The holt-winters approach to exponential smoothing: 50 years old and going strong. Foresight. (2010) 19:30–3.
18. Gustriansyah R, Alie J, Suhandi N. Modeling the number of unemployed in south sumatra province using the exponential smoothing methods. Qual Quant. (2023) 57(2):1725–37. doi: 10.1007/s11135-022-01445-2
19. Burinskiene A. Forecasting model: the case of the pharmaceutical retail. Front Med (Lausanne). (2022) 9:582186. doi: 10.3389/fmed.2022.582186
20. Ahmar AS, Fitmayanti F, Ruliana R. Modeling of inflation cases in South Sulawesi Province using single exponential smoothing and double exponential smoothing methods. Qual Quant. (2022) 56(1):227–37. doi: 10.1007/s11135-021-01132-8
21. Billah B, King ML, Snyder RD, Koehler AB. Exponential smoothing model selection for forecasting. Int J Forecast. (2006) 22:239–47. doi: 10.1016/j.ijforecast.2005.08.002
22. Holt CC. Forecasting seasonals and trends by exponentially weighted moving averages. Int J Forecast. (2004) 20:5–13. doi: 10.1016/j.ijforecast.2003.09.015
23. Gimza BD, Cassat JE. Mechanisms of antibiotic failure during Staphylococcus aureusosteomyelitis. Front. Immunol. (2021) 12:638085. doi: 10.3389/fimmu.2021.638085
24. Wu H, Jia C, Wang X, Shen J, Tan J, Wei Z, et al. The impact of methicillin resistance on clinical outcome among patients with Staphylococcus aureus osteomyelitis: a retrospective cohort study of 482 cases. Sci Rep. (2023) 13(1):7990. doi: 10.1038/s41598-023-35111-w
25. Ronga L, Abbasciano A, Calia C, Mosca A, Battista M, Sparapano E, et al. Trends in the antibiotic resistance of S. aureus clinical isolates: a 4 years retrospective study in a teaching hospital in south Italy. Infez Med. (2019) 27(3):266–73.31545770
26. Schulte RH, Munson E. Staphylococcus aureus resistance patterns in Wisconsin: 2018 surveillance of Wisconsin organisms for trends in antimicrobial resistance and epidemiology (SWOTARE) program report. Clin Med Res. (2019) 17(3-4):72–81. doi: 10.3121/cmr.2019.1503
27. Bousquet J, Agache I, Blain H, Jutel M, Ventura MT, Worm M, et al. Management of anaphylaxis due to COVID-19 vaccines in the elderly. Allergy. (2021) 76(10):2952–64. doi: 10.1111/all.14838
28. Kovalchuk V, Riesbeck K, Nazarchuk O, Faustova M, Dmytriiev D, Nazarchuk H, et al. A current view on the phenotypic antibiotic resistance of leading pathogens in wounded patients during the war in Ukraine. Acta Biomed. (2024) 95(2):e2024030. doi: 10.23750/abm.v95i2.15395
29. Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB. Trauma system development in a theater of war: experiences from operation Iraqi freedom and operation enduring freedom. J Trauma. (2006) 61(6):1366–72. discussion. doi: 10.1097/01.ta.0000245894.78941.90
30. Fernandes CJ, Fernandes LA, Collignon P, Australian group on antimicrobial resistance. Cefoxitin resistance as a surrogate marker for the detection of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. (2005) 55(4):506–10. doi: 10.1093/jac/dki052
31. Maple P, Hamilton-Miller J, Brumfitt W. Ciprofloxacin resistance in methicillin- and gentamicin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. (1989) 8(7):622–4. doi: 10.1007/BF01968141
32. Huynh TQ, Tran VN, Thai VC, Nguyen HA, Nguyen NTG, Tran MK, et al. Genomic alterations involved in fluoroquinolone resistance development in Staphylococcus aureus. PLoS One. (2023) 18(7):e0287973. doi: 10.1371/journal.pone.0287973
33. Ezeh CK, Eze CN, Dibua MEU, Emencheta SC. A meta-analysis on the prevalence of resistance of Staphylococcus aureus to different antibiotics in Nigeria. Antimicrob Resist Infect Control. (2023) 12(1):40. doi: 10.1186/s13756-023-01243-x
34. Alkuraythi DM, Alkhulaifi MM, Binjomah AZ, Alarwi M, Mujallad MI, Alharbi SA, et al. Comparative genomic analysis of antibiotic resistance and virulence genes in Staphylococcus aureus isolates from patients and retail meat. Front Cell Infect Microbiol. (2024) 13:1339339. doi: 10.3389/fcimb.2023.1339339
35. Clark JA, Burgess DS. Plazomicin: a new aminoglycoside in the fight against antimicrobial resistance. Ther Adv Infect Dis. (2020) 7:2049936120952604. doi: 10.1177/2049936120952604
36. Mlynarczyk-Bonikowska B, Kowalewski C, Krolak-Ulinska A, Marusza W. Molecular mechanisms of drug resistance in Staphylococcus aureus. Int J Mol Sci. (2022) 23(15):8088. doi: 10.3390/ijms23158088
37. Vega S, Dowzicky MJ. Antimicrobial susceptibility among gram-positive and gram-negative organisms collected from the Latin American region between 2004 and 2015 as part of the tigecycline evaluation and surveillance trial. Ann Clin Microbiol Antimicrob. (2017) 16(1):50. doi: 10.1186/s12941-017-0222-0
38. Pikalyova K, Orlov A, Horvath D, Marcou G, Varnek A. Predicting S. aureus antimicrobial resistance with interpretable genomic space maps. Mol Inform. (2024) 43(5):e202300263. doi: 10.1002/minf.202300263
39. Ljungquist O, Nazarchuk O, Kahlmeter G, Andrews V, Koithan T, Wasserstrom L, et al. Highly multidrug-resistant Gram-negative bacterial infections in war victims in Ukraine, 2022. Lancet Infect Dis. (2023) 23(7):784–6. doi: 10.1016/S1473-3099(23)00291-8
40. Kondratiuk V, Jones BT, Kovalchuk V, Kovalenko I, Ganiuk V, Kondratiuk O, et al. Phenotypic and genotypic characterization of antibiotic resistance in military hospital-associated bacteria from war injuries in the Eastern Ukraine conflict between 2014 and 2020. J Hosp Infect. (2021) 112:69–76. doi: 10.1016/j.jhin.2021.03.020
Keywords: antimicrobial resistance, S. aureus, antibiotics, odontogenic infections, phlegmons, forecasting
Citation: Shemetov O, Faustova M, Perepelova T, Balia H, Pavlish I and Loban' G (2025) Forecasting the development of antimicrobial resistance of S. aureus. Front. Oral. Health 5:1514070. doi: 10.3389/froh.2024.1514070
Received: 19 October 2024; Accepted: 18 December 2024;
Published: 9 January 2025.
Edited by:
Katarzyna Garbacz, Medical University of Gdansk, PolandReviewed by:
Tomasz M. Karpiński, Poznan University of Medical Sciences, PolandMarta Katkowska, Medical University of Gdansk, Poland
Copyright: © 2025 Shemetov, Faustova, Perepelova, Balia, Pavlish and Loban'. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariia Faustova, bS5mYXVzdG92YUBwZG11LmVkdS51YQ==
 Oleh Shemetov1
Oleh Shemetov1 Mariia Faustova
Mariia Faustova Galina Loban'
Galina Loban'