
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oral. Health , 15 January 2025
Sec. Oral Cancers
Volume 5 - 2024 | https://doi.org/10.3389/froh.2024.1506407
The morbidity of oral disorders, including gingivitis, caries, endodontic-periodontal diseases, and oral cancer, is relatively high globally. Pathogenic cells are the root cause of many oral disorders, and oral therapies depend on eradicating them. Photodynamic therapy (PDT) has been established as a potential and non-invasive local adjuvant treatment for oral disorders. PDT consists of three essential components: photosensitizer (PS), a light source with a certain wavelength, and oxygen dissolved in the cells. These three components can interact to cause damage to proteins, lipids, nucleic acids, and other biological components within diseased tissues. Herein, we aimed to provide a detailed understanding of PDT and how it can treat oral diseases. Concerns about PDT and potential remedies are also a factor. PDT has been shown in numerous clinical studies to be an efficient supplementary therapy that can reduce pathogenic cells. The PDT has great potential for dental applications, including treating bacterial and fungal infections during root canal therapy and preventing oral cancer, potentially malignant disorders, periodontitis, dental caries, and peri-implant disorders. Although PDT has been promoted as having significant potential and utility in dentistry, more clinical research must be conducted before being used broadly.
Millions of individuals worldwide are affected by oral disorders, including gingivitis, caries, endodontic-periodontal diseases, and oral cancer, negatively impacting life quality and placing a massive financial burden on society. Besides affecting daily activities, poor oral health greatly increases several systemic disease risks, including diabetes and cardiovascular disease; therefore, developing more effective therapies to preserve oral and overall health is crucial.
Pathogenic cells, including bacteria, fungal, viral and cancer cells, are the leading causes of oral disorders. Accordingly, oral therapies aim to eliminate these cells instantly. For example, treatments for microbial infectious diseases rely primarily on antibiotic therapy. However, antimicrobial agents abuse has increased the concerns about bacterial drug resistance. Although surgery is the most frequent treatment for resectable tumors, incomplete or ineffective resection of lesions could result in local recurrence and a poor prognosis (1). Radiotherapy and chemotherapy can potentially result in serious systemic adverse effects and permanent damage to normal tissue due to toxicity (2).
Photodynamic therapy (PDT) is a non-invasive and secure therapeutic alternative for treating cancer and many other non-oncological diseases. Oscar Raab, who discovered that paramecia cultured with fluorescent dyes was killed upon exposure to light, first proposed the PDT idea in 1898 (3). The PDT has demonstrated beneficial application in various medical specialties, including dermatology, cancer, gynecology, and urology (4, 5). Using PDT in dentistry for conditions including oral cancer, potentially malignant disorders, periodontitis, dental caries, peri-implant disorders, and bacterial and fungal infections during root canal therapy is expanding rapidly (6, 7). The PDT offers better patient compliance than conventional surgery or drug therapy since it is non-invasive, safe, practical, and drug-resistant. Furthermore, PDT might be easily controlled by changing the parameters as needed to provide an accurate and personalized treatment. This review seeks to present a comprehensive overview of the applications of photodynamic therapy in the treatment of various oral diseases, including oral cancer, potentially malignant disorders, periodontitis, dental caries, peri-implant diseases, endodontic infections, and oral fungal infections (Figure 1).
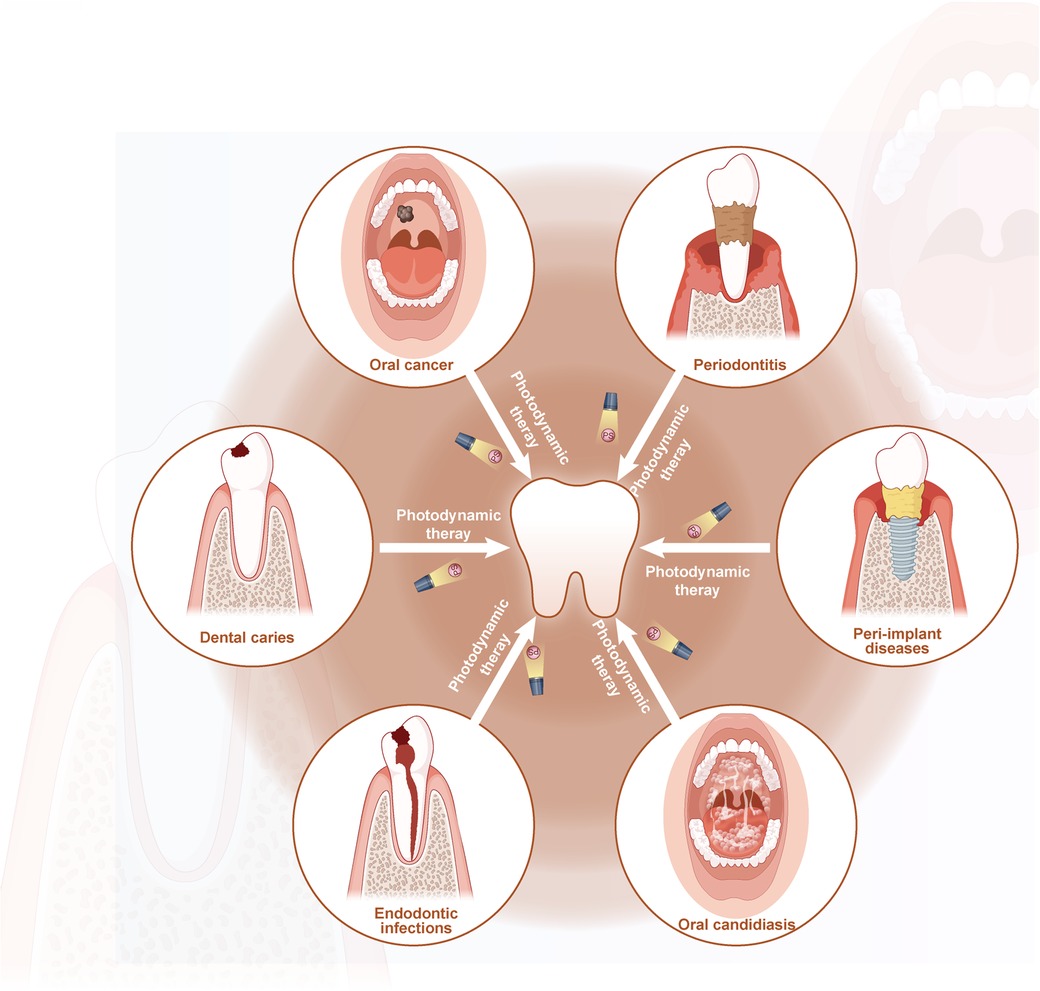
Figure 1. Schematic description of the application of PDT in treatment of oral diseases (by Figdraw).
PDT mechanism consists of three crucial elements: photosensitizer (PS), specified wavelength light source, and oxygen dissolved in the cells (8), which exclusively generates the desired outcomes through mutual interactions within pathological tissues. Figure 2 shows that the photodynamic reaction has two basic mechanisms, wholly dependent on oxygen molecules inside cells. Upon exposure to light, the photosensitizer transitions from a singlet basic energy level (S0) to an excited singlet state (S1). The excited singlet state could then transition to a triplet state (T1) or revert to the ground state with fluorescence emission. Reactive oxygen species (ROS) are created when the PS in the triplet state directly interacts with nearby biomolecules through hydrogen or electron transfer (Type I reaction); when this occurs, molecular oxygen is converted by the PS to highly reactive singlet oxygen (1O2) (Type II reaction) (4). Free radicals, including superoxide, hydroxyl, and lipid-derived radicals, are released due to type I reaction and attack cellular targets, resulting in direct cellular damage (9). Singlet excited-state oxygen is released in a type II reaction, which oxidizes lipids, proteins, and nucleic acids to cause cytotoxicity (10). Unsaturated lipids, comprising most cell and nuclear membranes, could react with the singlet oxygen. Consequently, these reaction byproducts may impede cell growth, trigger apoptosis in oral carcinoma cells, and significantly harm microorganisms.
PS development has progressed in recent decades of PDT research; a desirable PS should possess numerous important characteristics, including nontoxicity to surrounding tissues, hydrophilicity, high affinity and selectivity to target cells or microbes, and high quantum yield of photodynamic reaction (PDR). The first-generation typical photosensitizers, the hematoporphyrin derivative (HpD), and photofrin could be absorbed into the body for a long time but penetrate only into the tissue to a limited depth (<0.5 cm) (11). Porfifimer sodium, marketed under photofrin, was authorized for treating Barrett's esophagus in 2003 and early-stage lung cancer in 1998. The absorbance spectrum, tissue selectivity, and ROS production efficiency of the second-generation PSs have been enhanced. A naturally occurring pro-photosensitizer and a precursor to hemoglobin manufacture is 5-aminolaevulinic acid (ALA). A surplus of ALA leads tumor cells to absorb ALA rapidly but slowly degrade protoporphyrin IX (PpIX), causing PpIX photosensitizer to accumulate. Numerous second-generation photosensitizers are available, including phthalocyanines, verteporfin (VP), meta-tetrahydroxyphenylchlorin (m-THPC), and palladium bacteriopheophorbide. A light bleaching capability, antibody conjugates, or a protein/receptor system were added to photosensitizers in the third generation, improving the ability of a photosensitizer to target tumor tissues more effectively. The antibody affinity for tumor cells increases when conjugates to photosensitizers, decreasing healthy cell location. Furtherer more, toluidine blue (TB), methylene blue (MB), and erythrosine have been widely used for antimicrobial photodynamic therapy (12, 13). Besides photosensitizers, certain inorganic salts (such as potassium iodide and potassium bromide) the inhibition or deterioration of target cells and effectively inhibited or eradicated bacterial and fungal biofilms (14–16).
The selection of an appropriate photosensitizer for PDT is a crucial decision influenced by several factors, including the specific type of oral disease being addressed (whether antitumor or antimicrobial), the chemical properties of the photosensitizer, its capacity to generate reactive oxygen species, as well as its toxicity, selectivity, and availability. In addition to these intrinsic properties, the formulation and delivery method of the photosensitizer can significantly impact its therapeutic performance. Utilizing a molecular carrier, including a liposome nano species, is an alternative strategy. To maintain a high concentration in the targeted tissues, these modified photosensitizers with poor solubility in aqueous solutions are prevented from being delivered into the bloodstream.
Lamps, light-emitting diodes (LEDs), and lasers are the main light sources in PDT that are chosen based on the target tissue location, photosensitizer type, and administration dose. Lasers produce a coherent, focused, monochromatic light intensity, widely used to perform superficial and interstitial PDT; its limited clinical applicability is the monochromatic nature and high cost. The LED is becoming increasingly popular for PDT as a viable alternative to lasers because of their low cost, easy manipulation, and access to tissue surfaces. The LED semiconductor device uses electron-hole recombination to produce light: large beam divergence and wide spectral breadth enable LEDs to excite numerous PSs in their emission spectrum simultaneously. Lamps were the first artificial light sources utilized in PDT studies (17). Theoretically, lamps could couple to light guides to concentrate the light on particular therapy lesions; however, the coupling losses are substantial. Consequently, lamps are better suited to treating superficial malignancies such as skin or oral cavities. Compared with lasers or LEDs, the lamps (300–1,200 nm) with appropriate optical filtering could match any photosensitizer, but they have poor monochromaticity, insufficient intensity, and low energy.
The properties of the light source, whether it is a laser or a LED, play a pivotal role in the effectiveness of PDT. Primarily, each photosensitizer requires activation by light at a wavelength that aligns with its specific absorption characteristics. For example, certain photosensitizers, such as mTHPC, porfimer sodium, and ALA, are activated by red light, which typically spans a wavelength range of 600–700 nm. This wavelength range is advantageous for deeper tissue penetration, rendering it suitable for the treatment of oral cavity cancers. Another critical attribute is the intensity of the light source. The light must possess sufficient intensity to activate the photosensitizer while avoiding thermal damage to adjacent tissues. Low-power light sources are often favored as they reduce the risk of heat-induced damage while still effectively activating the photosensitizer. Additionally, the coherence and collimation of the light are important considerations. Lasers, which emit coherent and collimated light, are frequently employed in PDT due to their ability to deliver light precisely to the target area, thereby enabling controlled activation of the photosensitizer. LEDs, which emit non-coherent light, are increasingly utilized due to their cost-effectiveness and ease of use. LEDs are advantageous for treating larger surface areas as they provide a broader area of illumination. Critical parameters in this context include the duration of exposure and the total energy delivered, known as fluence. The light source must deliver an adequate dose of energy to effectively activate the photosensitizer. The optimal fluence is contingent upon the specific application and the photosensitizer employed. In conclusion, the ideal light source for PDT should possess a wavelength that aligns with the absorption spectrum of the photosensitizer, sufficient intensity to activate the photosensitizer without inducing thermal damage, and the capability to deliver the appropriate fluence to the target tissue.
Oral cavity cancers are prevalent globally, accounting for nearly 202,000 new cases yearly (18). Additionally, oral squamous cell carcinoma (OSCC) accounts for nearly 90% of oral cavity malignancies, with a global incidence anticipated at 377,713 new cases and 177,757 deaths in 2020 (19). Oral cancer oncogenesis is influenced by several variables and developmental stages. The malignant development of oral potentially malignant disorders (OPMDs) including actinic cheilitis (AC), oral leukoplakia (OL), oral lichen planus (OLP), oral erythroleukoplakia (OEL), oral erythroplakia (OE), oral submucosal fibrosis, proliferative verrucous leukoplakia (PVL), and epithelial dysplasia can be viewed through oral carcinogenesis studies. Recently, it has become widely accepted. Patients with OPMDs have a 5–100 fold higher risk of developing malignant transformation than the general population (20); OL, OE, and PVL exhibited a 15%–90% malignant transformation rate (21). The most popular forms of oral cancer treatment include surgery, chemotherapy, and radiation therapy, all of which have clear drawbacks. Oral cancer surgery could alter the facial appearance, and radiation therapy, chemotherapy, and surgery all have severe functional side effects that can affect the ability of a patient to chew, speak, swallow, and taste (22). PDT may be appropriate for patients with significant lesions in areas with high cosmetic value or who refuse standard invasive surgery.
Hopper et al. (23) conducted a study that was open-label and multicenter in nature. The purpose of the study was to investigate the effectiveness of administering 0.15 mg/kg of mTHPC through intravenous injection in conjunction with a 20 J/cm2 red laser (652 nm) for the treatment of early-stage OSCC at stages Tis, T1, or T2, N0M0. The results indicated that 85% of the patients who followed the treatment (97 out of 114) experienced full tumor responses. The response rate remained constant at 85% (95% CI: 77%–93%) after one year and 77% (95% CI: 66%–87%) after two years. The actuarial survival rates at one and two years were 89% (95% CI: 83%–95%) and 75% (95% CI: 66%–84%), respectively. Researchers also recorded remarkable aesthetic and functional outcomes, with no significant adverse effects on tissue integrity, speech, or swallowing ability.
A study conducted by Han et al. showcased the therapeutic effectiveness of ALA-PDT in the treatment of oral leukoplakia in Chinese patients (24). The therapy consisted of applying a 20% ALA gel and exposing the area to a 632 nm laser with an intensity of 500 mW/cm2 and a 90–180 J/cm2 dose. The overall response rate was 86.2%, with 55.2% of participants achieving complete remission. In a separate investigation, Yao et al. (25) conducted a comparison between the impacts of ablative fractional laser-assisted photodynamic treatment (AFL-PDT) and ablative fractional laser (AFL) alone for oral leukoplakia. The findings revealed a notably greater percentage of successful treatment in the AFL-PDT group (100%) than in the AFL group (80.9%), with a difference of 19.1% (95% CI: 0.7%–40.0%). In addition, the AFL-PDT group demonstrated decreased recurrence rates at 6 and 12 months after therapy. Neither group experienced any serious adverse events or systemic effects.
Schuch et al. (26) extensively examined the effects of PDT on treating OPMD and OSCC. Fourteen different types of photosensitizing agents (ALA, chlorine-e6, foscan, and so on) with a light source irradiation at 417–670 nm, 10–500 mW/cm2, 1.5–200 J/cm2, and 0.5–143 min was used. The analysis included 9,245 people with OPMD (n = 7,487) or OSCC (n = 1,758). OEL (93%; 100 cases) and AC (67.6%; 448 cases) had the highest complete response rates among the informed cases; conversely, OLP had a 42.9% complete response rate. Eight studies were conducted to investigate the OL, OE, OEL, or both, revealing that OL responded less strongly than OE and OEL. Less than half of OL cases receive a complete answer, compared with over 90% of instances for OE and OEL. Four investigations (n = 411) on OSCC demonstrated complete resolution in 83.4% of the cases and nonresolution in 16.6% of instances; a follow-up of 28.4 months showed a 12% recurrence rate.
PDT is commonly used to treat OPMD or early-stage oral cavity cancers without nodal metastases (Cis, T1, T2), either as the main treatment or as an alternate option (Tables 1, 2). The greatest positive reactions are seen in surface tumors that fall within the light source's permeability region, which is between 5 and 10 mm (27). Although PDT provides favorable cosmetic results and preserves functionality with low invasiveness and no toxicity (28), it is crucial to acknowledge that its efficacy is restricted to treating superficial early-stage lesions and does not affect lymph nodes. Moreover, PDT can serve as an adjunctive therapy for treating remaining margins following surgical procedures or in conjunction with other therapies.
Periodontitis is a chronic multi-factorial inflammatory disease brought on by bacteria and is characterized by the loss of alveolar bone and loosening of the teeth (29, 30); it is related to the formation of the dental plaque biofilm. Mechanical debridement (MD) and antibiotic treatment are frequently utilized in clinics. However, mechanical debridement frequently leaves dental plaque in periodontal pockets, furcations, and uneven root surface areas, ultimately leading to treatment failure. Porphyromonas gingivalis (P. gingivalis) and Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) are primarily responsible for periodontitis (31). The effectiveness of many systemic antimicrobials is constrained because they cannot continuously suppress P. gingivalis and A. actinomycetemcomitans.
Antimicrobial resistance, dysbacteriosis, and gastrointestinal disorders are increasingly common due to antibiotic abuse, resulting in antibiotic failures; therefore, a more effective method for treating periodontitis is needed. PDT may be helpful in hard-to-reach places like periodontal pockets or furcation sites for microbial reduction without bacterial resistance, demonstrating its potential as an adjuvant treatment for periodontitis (Table 3). Besides having a bactericidal effect on the periodontal tissues, PDT has an anti-inflammatory effect by lowering inflammatory mediator levels, creating a more favorable healing environment, and re-establishing the cellular biological balance (32).
In order to evaluate the clinical and radiographic outcomes of periodontitis patients treated with conventional mechanical debridement along with those treated with methylene blue-mediated adjunctive photodynamic treatment (MB-PDT), Alasqah et al. (33) conducted a meta-analysis of randomized controlled trials. The findings showed that when MB-PDT was used in conjunction with MD, as opposed to only MD, periodontal plaque index (PI), probing depth (PD), as well as bleeding on probing (BOP) scores exhibited significant improvements. Nevertheless, there was no statistically significant disparity in clinical attachment level (CAL) values seen between the control group (treated with MD alone) and the experimental group (treated with supplementary MB-PDT). The long-term effects of PDT and antibiotic therapy (amoxicillin 500 mg and metronidazole 400 mg for seven days) combined with traditional nonsurgical therapy in individuals with advanced periodontitis were compared in another investigation (34), proving that PDT and antibiotic therapy significantly improve clinical parameters (PD, CAL, and BOP) three months after treatment, and that stays also decreased six, nine, and twelve months later.
Ivanaga et al. (35) compared the clinical effectiveness of PDT with 100 mg/L curcumin (CUR) solution and LED irradiation (465–485 nm, 100 mW/cm2, 60 s) in treating residual pockets in type 2 diabetics, indicating that PDT group (SRP, irrigation with CUR solution and LED irradiation) showed a notable CAL gain at three months in comparison to baseline data compared with SRP group, CUR group (SRP and irrigation with CUR solution), and LED group (SRP and LED irradiation). All treatment groups showed reductions in PD and BOP in the intragroup comparison at three and six months. The result showed that the addition of PDT to SRP may yield short-term benefits in CAL gain among the type 2 diabetics with residual pockets.
In vitro experiments, Al-Ahmad et al. (36) tested the antimicrobial effects of PDT against a variety of periodontal pathogens, including subgingival dental biofilm and Eikenella corrodens, Actinomyces odontolyticus, A. actinomycetemcomitans, Fusobacterium nucleatum, P. gingivalis, Atopobium rimae, Slackia exigua and Parvimonas micra. It is showed that all tested periodontal pathogens and the PDT-treated subgingival biofilm were eliminated over the ranges of 3.43–8.34 and 3.91–4.28 log10 colony forming units (CFU), respectively.
Tooth caries is a multi-factorial, non-transmissible, biofilm-mediated disease that affects sensitive tooth hard tissues and is characterized by a phasic demineralization and remineralization phase. Dental caries is mostly caused by Lactobacilli and Streptococcus mutans, which produce acidity from carbohydrate metabolism, leading to an acidic pH and enamel demineralization (37). Dental caries has historically been frequently treated with fillings, eliminating carious tissue and cariogenic germs. However, insufficient clearance leads to a significant recurrence risk (secondary caries). PDT prevents cariogenic bacterial biofilm growth by damaging bacterial structure and decreasing acid production. Therefore, PDT is regarded as a successful adjuvant therapy for dental caries.
PDT can prevent dental caries by reducing microbial colonization and removing biofilm from the enamel surface. Alsaif et al. (38) studied the effects of erythrosine-PDT on in vivo-formed dental plaque biofilms using dental plaque samples from 18 healthy adult participants wearing intraoral appliances, showing that 220 M erythrosine-B (2 min or 15 min incubation times) with green light source (500–550 nm, 22.7 mW/cm2, 15 min continuous light or the fractionated light with 30 s light pulses ×5) reduced total bacterial counts (93%–95%) of in vivo-formed biofilms. Faria et al. (39) conducted a randomized controlled experiment to assess the effectiveness of composite restorations after selective caries removal (SCR) coupled with PDT. Following 12 months of observation, the group that had PDT treatment demonstrated a notably superior level of marginal adaption of the restoration in comparison to the control group.
Nie et al. (40) demonstrated that photodynamic inactivation based on Ce6 or methylene blue could be useful for preventing caries by controlling biofilms. Additionally, Ce6-mediated PDT produces more bactericidal activity (with an excess of 3 log10) than MB-PDT at the same PS concentration and light dose. Furthermore, PDT could impact the virulence characteristics of Streptococcus mutans as well (41); however, applying PDT to caries has only been studied in limited clinical trials, necessitating further in vivo proof to be considered valid (Table 4).
Dental implants are crucial components of oral rehabilitation to restore missing teeth and enhance the quality of life of those with such therapeutic needs. Depending on the degree of the peri-implant tissue inflammatory pathological state, peri-implantitis can be subdivided into peri-implant mucositis and peri-implantitis. While peri-implantitis is an inflammatory condition resulting in cracked and absorbed alveolar bone, loosening of implants, and other risks, peri-implant mucositis is an inflammation of the mucous membrane around the implant (12). This element is a significant contributor to implant failure. The etiopathogenesis of peri-implant inflammatory disorders is related to A. Actinomycetemcomitans and Treponema denticola (T. denticola) (42). These microorganisms increase probing depth, plaque index, and gingival index (GI) surrounding implants, promoting crestal bone loss (CBL) as well as soft tissue inflammation (43, 44). The primary objective of peri-implantitis treatment is to eradicate the deleterious constituents of bacterial plaque in the vicinity of the implant. In most cases, peri-implantitis is treated with mechanical debridement using ultrasonic scalers, air-powered abrasives, and polishing brushes. However, these techniques have not entirely eradicated or effective inactivated peri-implant infections. This is primarily attributed to the complex nature of implant surfaces, which possess rough and microporous characteristics on macro- and microscopic scales (45). PDT has demonstrated successful infection cures from Staphylococcus aureus, Pseudomonas aeruginosa, P. gingivalis, and multidrug-resistant bacteria (46). And PDT, used with surgery, is more effective while preventing medication resistance and harm to nearby tissues (Table 5).
For nonsurgical peri-implantitis treatment, adjunctive use of PDT has been investigated in four randomized controlled studies (47–50), and all studies have shown significant improvements for PD, BOP, and CBL. As for bacteria count measurement, Labban et al. (51) found that PDT application significantly reduced peri-implant pathogens than mechanical debridement. Oral yeasts are also related to the etiopathogeneses of peri-implant disorders besides bacteria (52). In patients with peri-implant mucositis, Shetty et al. (53) investigated the effectiveness of 0.005% methylene-blue with 660 nm diode laser at the energy density of 16.8 J/cm2 in lowering subgingival oral yeast colonization (OYC). After a 3-month follow-up, the modified plaque index (mPI), modified bleeding index (mBI), PD, and OYC scores in the PDT group were lower than those in the control group.
The in vitro investigation proved that T. Forsythia and P. gingivalis grown on titanium specimens subjected to PDT mediated by 312 µM methylene blue for 1 min under a 685 nm diode laser with a dosage of 7.9 J/cm2 could significantly lower T. forsythia and P. gingivalis biofilm compared with neodymium-doped yttrium aluminum garnet (Nd: YAG) laser, H2O2, and chlorhexidine groups (54). The study also revealed that surface roughness remained constant while the contact angle decreased in the PDT group. In another vitro investigation, Anil et al. (55) discovered that methylene blue-PDT significantly reduced P. gingivalis and T. Forsythia viability over Zirconia specimens in comparison with other disinfection groups (the hydrogen peroxide group, the Nd: YAG laser, and the chlorhexidine group). The PDT approach decreased contact angles from zirconia specimens, suggesting increased hydrophilicity. PDT had the highest surface free energy (SFE) score (41.68) across all decontamination methods, followed by chlorhexidine (39.83), Nd: YAG (34.52), and H2O2 (29.88).
Pulpitis and periapical periodontitis are prevalent oral disorders mostly attributed to anaerobic bacteria, including P. gingivalis and Enterococcus faecalis (E. faecalis). E. faecalis is a frequent species of re-infection after root canal therapy, owing to factors such as antibiotic resistance, microbial biofilm formation, and dentinal penetration, whose eradication poses significant challenges (56). Although the main treatment for pulpitis and periapical periodontitis is root canal therapy, the complexity of the root canal system (communicating branches, lateral branches, and an apical bifurcation) and the multispecies biofilm communities have made the microbial biofilm complete removal more challenging. Clinical studies on this topic recommend that PDT could be a promising technique to eliminate root canal bacteria after standard chemo mechanical debridement (Table 6).
Alves-Silva et al. (57) evaluated the effectiveness of PDT as an additional treatment for improving bacterial clearance and reducing lipopolysaccharide (LPS) and lipoteichoic acid (LTA) levels. The study involved two groups: one group received endodontic therapy with chemo-mechanical preparation (CMP) alone, while the other group received PDT (using a 9 J/cm2 660 nm red laser with 0.005% methylene blue for 3 min) following CMP. The results revealed that root canals had samples with pulp necrosis and periapical lesions. Additionally, LPS and LTA levels were significantly reduced in the PDT group, with a higher reduction in the CMP group.
Pourhajibagher et al. performed a comprehensive study and synthesis of existing studies to examine the effectiveness of combining PDT with standard chemo-mechanical debridement in treating infected root canal systems in endodontic diseases (58). Their research revealed a substantial decrease in the amount of microorganisms when PDT was used in addition to other treatments.
Candida species are the most common fungi indigenous to human mucosal surfaces, which is mainly caused by Candida albicans (C. albicans). Although C. albicans is a commensal organism in healthy individuals, it could convert into a pathogenic one following changes in the host environment. Oral candidiasis (OC) is the most commonly encountered oral manifestation, which is mainly caused by C. albicans infection (59). Traditional antifungal treatment applied in OC include amphotericin B, nystatin, clotrimazole and ketoconazole (60, 61). However, the limitation of antifungal drugs, high resistance and host defense mechanisms made antifungal treatment difficult. Hence, alternative strategies such as PDT against the emergence of drug-resistant C. albicans are being considered (Table 7).
Ma et al. (62) conducted investigations on the impact of curcumin on biofilms of C. albicans. The researchers carried out experimental analyses on a standard strain as well as two clinical isolates obtained from individuals with HIV and oral lichen planus. The findings of their study indicated that a 20 min pre-irradiation of 60 µM curcumin and a 6 min exposure to LED with a dosage of 7.92 J/cm2 resulted in a reduction of C. albicans biofilms. Furthermore, expression of efg1, ume6, hgc1, and ece1 genes expression of C. albicans was decreased after PDT treatment. Additionally, ALA–PDT (using a 635 nm red laser at 300 J/cm2) showed potent inhibition of the metabolic activity of C. albicans (63). Pereira et al. (64) evaluated potential effects of 200 µM erythrosine plus a 532 ± 10 nm green LED (237 mW/cm2, 42.63 J/cm2) in planktonic culture, biofilms and virulence factors of Candida strains. The results indicated that the addition of PDT could significantly reduce Candida species growth as well as lower the virulence and pathogenicity of certain Candida species, however, there was a greater resistance to PDT in biofilm structures compared to planktonic cultures.
Hu et al. (65) conducted a meta-analysis of 11 trials to assess the impact of PDT as a supplementary or substitute treatment for oral candidiasis compared to conventional antifungal medications such as nystatin, fluconazole, and miconazole. The results demonstrated that PDT outperformed nystatin in reducing the number of oral candida colonies in the palates of patients. However, no statistically significant difference was observed in the denture location. Fluconazole and PDT had similar efficiency in the treatment of oral candidiasis. However, miconazole was shown to be more effective than PDT. However, the use of PDT in conjunction with nystatin has been found to be a more effective treatment for oral candidiasis, with improved effectiveness and a lower chance of the condition recurrence.
PDT development direction mainly focuses on development of photosensitizer and laser development and utilization to enhance its targeting and light penetration ability. Combining functionalized nano-materials, radiosensitizers, and hyperthermia with PDT can enhance the synergistic effect of photosensitizers and increase the curative effect on deep tissues. Numerous functional nano-materials have been created due to the quick development of nanotechnology for better medication delivery and anticancer and antibacterial effects. Many different nanoparticles have been created, including 5-ALA-loaded chitosan-tripolyphosphate nanoparticles (CS-TPP NPs) (66), MPP (Polymers) combined with Ce6 (67), and rose bengal (RB) in silver nanoclusters (AgNCs) (68), among others (69). Sonodynamic therapy employs ultrasonic waves to activate photosensitizers, inducing singlet oxygen production. This mechanism serves as a compensatory strategy for the limited tissue penetration capabilities of light-based therapies (70). Photochemical internalization (PCI) is a new method wherein photosensitizers and cytotoxic substances (including bleomycin) are injected into tissues. Subsequently, the cytotoxic molecules exert their effects on the cytoplasm through light internalization. Two-photon absorption PDT uses two wavelengths of photons to reach the photosensitizer simultaneously to increase the absorption power and intensity to act on cells. This brings a new research direction for PDT, makes up for the shortcomings of traditional PDT, and hopefully becomes a new method widely used in clinical treatment. Aiming at increasing the therapeutic efficiency, combination regimens through multiple photosensitizers with multiple certain wavelengths of light sources either sequentially or simultaneously, is needed in the future.
PDT is ROS-dependent, non-invading and convenient. However, all three crucial elements for PDT (PS, light, and oxygen) could contribute to the limitation of ROS generation. Poor tissue penetration occurs with short wavelength lights. Due to the hypoxic tumor environment caused by extensive areas of tumoral necrosis and local hypoxia, which results in a poor response to PDT, the absolute demand for oxygen may be insufficient. The severe limitations of conventional PS, including poor solubility, low stability, and inadequate tissue penetration, continue to be a barrier to PDT, requiring innovative solutions to improve PDT clinical results.
Before PDT becomes widely used in therapeutic settings, various questions about its utilization must be cleared up; PDT toxicity, oral ecosystem balance, and impact of complicated infection should be investigated in vitro and in vivo research. Photosensitizers, including MB, toluidine blue O (TBO), or malachite green, can alter tooth structure shade. More PS molecules can permeate the internal composition of the tooth with the increased PS incubation time.
Although PDT has demonstrated much promise and usefulness in dentistry, it is important to acknowledge several limitations. These factors encompass the restricted capacity of the PS to deeply enter dentinal tubules, difficulties in the transmission of light, and the lack of oxygen in deep periodontal pockets. Moreover, there is a lack of consensus about the most effective treatment strategy for oral problems. Therefore, further thorough and carefully controlled study is necessary to determine the most efficient PS, the suitable irradiation protocols, and the optimal wavelengths for activating the PS. This will enable healthcare practitioners to achieve the intended outcomes.
LW: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. QC: Conceptualization, Investigation, Supervision, Visualization, Writing – review & editing. DL: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Aaboubout Y, Ten Hove I, Smits RWH, Hardillo JA, Puppels GJ, Koljenovic S. Specimen-driven intraoperative assessment of resection margins should be standard of care for oral cancer patients. Oral Dis. (2021) 27(1):111–6. doi: 10.1111/odi.13619
2. Spijkervet FKL, Schuurhuis JM, Stokman MA, Witjes MJH, Vissink A. Should oral foci of infection be removed before the onset of radiotherapy or chemotherapy? Oral Dis. (2021) 27(1):7–13. doi: 10.1111/odi.13329
3. Malik R, Manocha A, Suresh DK. Photodynamic therapy–a strategic review. Indian J Dent Res. (2010) 21(2):285–91. doi: 10.4103/0970-9290.66659
4. Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, et al. Photodynamic therapy—mechanisms, photosensitizers and combinations. Biomed Pharmacother. (2018) 106:1098–107. doi: 10.1016/j.biopha.2018.07.049
5. Aghajanzadeh M, Zamani M, Rajabi Kouchi F, Eixenberger J, Shirini D, Estrada D, et al. Synergic antitumor effect of photodynamic therapy and chemotherapy mediated by nano drug delivery systems. Pharmaceutics. (2022) 14(2):322. doi: 10.3390/pharmaceutics14020322
6. Qi M, Chi M, Sun X, Xie X, Weir MD, Oates TW, et al. Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. Int J Nanomedicine. (2019) 14:6937–56. doi: 10.2147/IJN.S212807
7. Li Y, Jiao J, Qi Y, Yu W, Yang S, Zhang J, et al. Curcumin: a review of experimental studies and mechanisms related to periodontitis treatment. J Periodontal Res. (2021) 56(5):837–47. doi: 10.1111/jre.12914
8. Allison RR, Moghissi K. Photodynamic therapy (PDT): PDT mechanisms. Clin Endosc. (2013) 46(1):24–9. doi: 10.5946/ce.2013.46.1.24
9. Plotino G, Grande NM, Mercade M. Photodynamic therapy in endodontics. Int Endod J. (2019) 52(6):760–74. doi: 10.1111/iej.13057
10. Redmond RW, Gamlin JN. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem Photobiol. (1999) 70(4):391–475. doi: 10.1111/j.1751-1097.1999.tb08240.x
11. Gomer CJ. Preclinical examination of first and second generation photosensitizers used in photodynamic therapy. Photochem Photobiol. (1991) 54(6):1093–107. doi: 10.1111/j.1751-1097.1991.tb02133.x
12. Li Y, Sun G, Xie J, Xiao S, Lin C. Antimicrobial photodynamic therapy against oral biofilm: influencing factors, mechanisms, and combined actions with other strategies. Front Microbiol. (2023) 14:1192955. doi: 10.3389/fmicb.2023.1192955
13. Gonçalves MLL, Sobral APT, Gallo J, Gimenez T, Ferri EP, Ianello S, et al. Antimicrobial photodynamic therapy with erythrosine and blue light on dental biofilm bacteria: study protocol for randomised clinical trial. BMJ Open. (2023) 13(9):e075084. doi: 10.1136/bmjopen-2023-075084
14. Hamblin MR, Abrahamse H. Inorganic salts and antimicrobial photodynamic therapy: mechanistic conundrums? Molecules. (2018) 23(12):3190. doi: 10.3390/molecules23123190
15. Pitaksanurat P, Mayeah N, Saithong P, Pimha S, Sirikarn P, Damrongrungruang T. Anticandidal effect of multiple sessions of erythrosine and potassium iodide-mediated photodynamic therapy. J Oral Microbiol. (2024) 16(1):2369357. doi: 10.1080/20002297.2024.2369357
16. Alves F, Nakada PJT, Marques M, Rea LDC, Cortez AA, Pellegrini VOA, et al. Complete photodynamic inactivation of Pseudomonas aeruginosa biofilm with use of potassium iodide and its comparison with enzymatic pretreatment. J Photochem Photobiol B. (2024) 257:112974. doi: 10.1016/j.jphotobiol.2024.112974
17. Kim MM, Darafsheh A. Light sources and dosimetry techniques for photodynamic therapy. Photochem Photobiol. (2020) 96(2):280–94. doi: 10.1111/php.13219
18. Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. (2017) 67(1):51–64. doi: 10.3322/caac.21384
19. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
20. Warnakulasuriya S. Oral potentially malignant disorders: a comprehensive review on clinical aspects and management. Oral Oncol. (2020) 102:104550. doi: 10.1016/j.oraloncology.2019.104550
21. Sakthivel P, Raveendran S, Panda S, Singh CA. Oral potential malignant disorders—a long list not to be forgotten. Oral Oncol. (2021) 116:105244. doi: 10.1016/j.oraloncology.2021.105244
22. Patil VM, Noronha V, Joshi A, Abhyankar A, Menon N, Dhumal S, et al. Beyond conventional chemotherapy, targeted therapy and immunotherapy in squamous cell cancer of the oral cavity. Oral Oncol. (2020) 105:104673. doi: 10.1016/j.oraloncology.2020.104673
23. Hopper C, Kübler A, Lewis H, Tan IB, Putnam G. mTHPC-mediated photodynamic therapy for early oral squamous cell carcinoma. Int J Cancer. (2004) 111(1):138–46. doi: 10.1002/ijc.20209
24. Han Y, Xu S, Jin J, Wang X, Liu X, Hua H, et al. Primary clinical evaluation of photodynamic therapy with oral leukoplakia in Chinese patients. Front Physiol. (2018) 9:1911. doi: 10.3389/fphys.2018.01911
25. Yao Y, Shi L, Wang Y, Shen X, Ye S, Tang G, et al. Ablative fractional laser-assisted photodynamic therapy vs. ablative fractional laser for oral leukoplakia treatment: a randomized, controlled pilot study. Photodiagnosis Photodyn Ther. (2021) 36:102523. doi: 10.1016/j.pdpdt.2021.102523
26. Schuch LF, Schmidt TR, Kirschnick LB, de Arruda JAA, Campagnol D, Martins MAT, et al. Revisiting the evidence of photodynamic therapy for oral potentially malignant disorders and oral squamous cell carcinoma: an overview of systematic reviews. Photodiagnosis Photodyn Ther. (2023) 42:103531. doi: 10.1016/j.pdpdt.2023.103531
27. Saini R, Lee NV, Liu KY, Poh CF. Prospects in the application of photodynamic therapy in oral cancer and premalignant lesions. Cancers. (2016) 8(9):83. doi: 10.3390/cancers8090083
28. Mosaddad SA, Mahootchi P, Rastegar Z, Abbasi B, Alam M, Abbasi K, et al. Photodynamic therapy in oral cancer: a narrative review. Photobiomodul Photomed Laser Surg. (2023) 41(6):248–64. doi: 10.1089/photob.2023.0030
29. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. (2017) 3:17038. doi: 10.1038/nrdp.2017.38
30. Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. (2018) 45(20):S162–70. doi: 10.1111/jcpe.12946
31. Afrasiabi S, Partoazar A, Chiniforush N, Goudarzi R. The potential application of natural photosensitizers used in antimicrobial photodynamic therapy against oral infections. Pharmaceuticals. (2022) 15(6):767. doi: 10.3390/ph15060767
32. Braham P, Herron C, Street C, Darveau R. Antimicrobial photodynamic therapy may promote periodontal healing through multiple mechanisms. J Periodontol. (2009) 80(11):1790–8. doi: 10.1902/jop.2009.090214
33. Alasqah MN. Efficacy of methylene blue-mediated antimicrobial photodynamic therapy on clinical and radiographic outcomes among patients with periodontal diseases: a systematic review and meta-analysis of randomized controlled trials. Photodiagnosis Photodyn Ther. (2024) 46:104000. doi: 10.1016/j.pdpdt.2024.104000
34. Skalerič E, Petelin M, Gašpirc B. Antimicrobial photodynamic therapy in treatment of aggressive periodontitis (stage III, grade C periodontitis): a comparison between photodynamic therapy and antibiotic therapy as an adjunct to non-surgical periodontal treatment. Photodiagnosis Photodyn Ther. (2023) 41:103251. doi: 10.1016/j.pdpdt.2022.103251
35. Ivanaga CA, Miessi DMJ, Nuernberg MAA, Claudio MM, Garcia VG, Theodoro LH. Antimicrobial photodynamic therapy (aPDT) with curcumin and LED, as an enhancement to scaling and root planing in the treatment of residual pockets in diabetic patients: a randomized and controlled split-mouth clinical trial. Photodiagnosis Photodyn Ther. (2019) 27:388–95. doi: 10.1016/j.pdpdt.2019.07.005
36. Al-Ahmad A, Walankiewicz A, Hellwig E, Follo M, Tennert C, Wittmer A, et al. Photoinactivation using visible light plus water-filtered infrared-A (vis+wIRA) and chlorine e6 (Ce6) eradicates planktonic periodontal pathogens and subgingival biofilms. Front Microbiol. (2016) 7:1900. doi: 10.3389/fmicb.2016.01900
37. Xue X, Sztajer H, Buddruhs N, Petersen J, Rohde M, Talay SR, et al. Lack of the delta subunit of RNA polymerase increases virulence related traits of Streptococcus mutans. PLoS One. (2011) 6(5):e20075. doi: 10.1371/journal.pone.0020075
38. Alsaif A, Tahmassebi JF, Wood SR. Treatment of dental plaque biofilms using photodynamic therapy: a randomised controlled study. Eur Arch Paediatr Dent. (2021) 22(5):791–800. doi: 10.1007/s40368-021-00637-y
39. Faria LV, Antunes LS, Pio LRR, Dias JC, Pinheiro LHM, Reis CLB, et al. Evaluation of composite restorations in primary molars subjected to selective caries removal associated with antimicrobial photodynamic therapy: a randomized controlled trial. Int J Paediatr Dent. (2022) 32(4):585–97. doi: 10.1111/ipd.12937
40. Nie M, Deng DM, Wu Y, de Oliveira KT, Bagnato VS, Crielaard W, et al. Photodynamic inactivation mediated by methylene blue or chlorin e6 against Streptococcus mutans biofilm. Photodiagnosis Photodyn Ther. (2020) 31:101817. doi: 10.1016/j.pdpdt.2020.101817
41. Pourhajibagher M, Keshavarz Valian N, Bahador A. Theranostic nanoplatforms of emodin-chitosan with blue laser light on enhancing the anti-biofilm activity of photodynamic therapy against Streptococcus mutans biofilms on the enamel surface. BMC Microbiol. (2022) 22(1):68. doi: 10.1186/s12866-022-02481-6
42. Ibraheem WI, Fageeh HI, Preethanath RS, Alzahrani FA, Al-Zawawi AS, Divakar DD, et al. Comparison of RANKL and osteoprotegerin levels in the gingival crevicular fluid of young cigarette- and waterpipe-smokers and individuals using electronic nicotine delivery systems. Arch Oral Biol. (2020) 115:104714. doi: 10.1016/j.archoralbio.2020.104714
43. Khammissa RA, Feller L, Meyerov R, Lemmer J. Peri-implant mucositis and peri-implantitis: bacterial infection. Sadj. (2012) 67(2):70; 2–4.23189895
44. Khammissa RA, Feller L, Meyerov R, Lemmer J. Peri-implant mucositis and peri-implantitis: clinical and histopathological characteristics and treatment. SADJ. (2012) 67(3):122; 4–6.23198360
45. Renvert S, Lessem J, Dahlén G, Lindahl C, Svensson M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: a randomized clinical trial. J Clin Periodontol. (2006) 33(5):362–9. doi: 10.1111/j.1600-051X.2006.00919.x
46. Vieira GCS, Antunes HS, Pérez AR, Gonçalves LS, Antunes FE, Siqueira JF Jr., et al. Molecular analysis of the antibacterial effects of photodynamic therapy in endodontic surgery: a case series. J Endod. (2018) 44(10):1593–7. doi: 10.1016/j.joen.2018.06.012
47. Bassetti M, Schär D, Wicki B, Eick S, Ramseier CA, Arweiler NB, et al. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res. (2014) 25(3):279–87. doi: 10.1111/clr.12155
48. Javed F, BinShabaib MS, Alharthi SS, Qadri T. Role of mechanical curettage with and without adjunct antimicrobial photodynamic therapy in the treatment of peri-implant mucositis in cigarette smokers: a randomized controlled clinical trial. Photodiagnosis Photodyn Ther. (2017) 18:331–4. doi: 10.1016/j.pdpdt.2017.04.015
49. Ahmed P, Bukhari IA, Albaijan R, Sheikh SA, Vohra F. The effectiveness of photodynamic and antibiotic gel therapy as an adjunct to mechanical debridement in the treatment of peri-implantitis among diabetic patients. Photodiagnosis Photodyn Ther. (2020) 32:102077. doi: 10.1016/j.pdpdt.2020.102077
50. Ahmed AR, Kamran MA, Suleman G, Sharif RA, Alamrey AAM, Sulaiman SA. Novel use of chloro-aluminum phthalocyanine assisted photodynamic therapy helps in periimplant healing among smoking patients. Photodiagnosis Photodyn Ther. (2023) 41:103193. doi: 10.1016/j.pdpdt.2022.103193
51. Labban N, Shibani NA, Al-Kattan R, Alfouzan AF, Binrayes A, Assery MK. Clinical, bacterial, and inflammatory outcomes of indocyanine green-mediated photodynamic therapy for treating periimplantitis among diabetic patients: a randomized controlled clinical trial. Photodiagnosis Photodyn Ther. (2021) 35:102350. doi: 10.1016/j.pdpdt.2021.102350
52. Alqahtani F. Role of oral yeasts in the etiopathogenesis of peri-implantitis: an evidence-based literature review of clinical studies. Arch Oral Biol. (2020) 111:104650. doi: 10.1016/j.archoralbio.2020.104650
53. Shetty B, Ali D, Ahmed S, Ibraheem WI, Preethanath RS, Vellappally S, et al. Role of antimicrobial photodynamic therapy in reducing subgingival oral yeasts colonization in patients with peri-implant mucositis. Photodiagnosis Photodyn Ther. (2022) 38:102803. doi: 10.1016/j.pdpdt.2022.102803
54. Anil S, Alageel O, Alsadon O, Alaqeel SM, Alsarani MM, Hashem M, et al. Topographical changes and bactericidal efficacy of antimicrobial photodynamic therapy on titanium implant surface. Photodiagnosis Photodyn Ther. (2022) 39:102882. doi: 10.1016/j.pdpdt.2022.102882
55. Anil S, Yahia ME, Alsarani MM, Alolayani BM, Alsadon O, Vellappally S, et al. Antimicrobial efficacy and topographical alterations of photodynamic therapy versus conventional antimicrobials on contaminated zirconia ceramic in vitro. Photodiagnosis Photodyn Ther. (2022) 38:102804. doi: 10.1016/j.pdpdt.2022.102804
56. Parolia A, Kumar H, Ramamurthy S, Madheswaran T, Davamani F, Pichika MR, et al. Effect of propolis nanoparticles against Enterococcus faecalis biofilm in the root canal. Molecules. (2021) 26(3):715. doi: 10.3390/molecules26030715
57. Alves-Silva EG, Arruda-Vasconcelos R, Louzada LM, de-Jesus-Soares A, Ferraz CCR, Almeida JFA, et al. Effect of antimicrobial photodynamic therapy on the reduction of bacteria and virulence factors in teeth with primary endodontic infection. Photodiagnosis Photodyn Ther. (2023) 41:103292. doi: 10.1016/j.pdpdt.2023.103292
58. Pourhajibagher M, Bahador A. Adjunctive antimicrobial photodynamic therapy to conventional chemo-mechanical debridement of infected root canal systems: a systematic review and meta-analysis. Photodiagnosis Photodyn Ther. (2019) 26:19–26. doi: 10.1016/j.pdpdt.2019.02.009
59. Villar CC, Dongari-Bagtzoglou A. Fungal diseases: oral dysbiosis in susceptible hosts. Periodontol 2000. (2021) 87(1):166–80. doi: 10.1111/prd.12378
60. Lee Y, Puumala E, Robbins N, Cowen LE. Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chem Rev. (2021) 121(6):3390–411. doi: 10.1021/acs.chemrev.0c00199
61. Van Daele R, Spriet I, Wauters J, Maertens J, Mercier T, Van Hecke S, et al. Antifungal drugs: what brings the future? Med Mycol. (2019) 57(3):S328–43. doi: 10.1093/mmy/myz012
62. Ma J, Shi H, Sun H, Li J, Bai Y. Antifungal effect of photodynamic therapy mediated by curcumin on Candida albicans biofilms in vitro. Photodiagnosis Photodyn Ther. (2019) 27:280–7. doi: 10.1016/j.pdpdt.2019.06.015
63. Shi H, Li J, Zhang H, Zhang J, Sun H. Effect of 5-aminolevulinic acid photodynamic therapy on Candida albicans biofilms: an in vitro study. Photodiagnosis Photodyn Ther. (2016) 15:40–5. doi: 10.1016/j.pdpdt.2016.04.011
64. Pereira CA, Domingues N, Silva MP, Costa AC, Junqueira JC, Jorge AO. Photodynamic inactivation of virulence factors of Candida strains isolated from patients with denture stomatitis. J Photochem Photobiol B. (2015) 153:82–9. doi: 10.1016/j.jphotobiol.2015.08.029
65. Hu Q, Li T, Yang J, Peng Y, Liu Q, Liu N. Efficacy of photodynamic therapy in the treatment of oral candidiasis: a systematic review and meta-analysis. BMC Oral Health. (2023) 23(1):802. doi: 10.1186/s12903-023-03484-z
66. Wang J, Wang K, Liang J, Jin J, Wang X, Yan S. Chitosan-tripolyphosphate nanoparticles-mediated co-delivery of MTHFD1l shRNA and 5-aminolevulinic acid for combination photodynamic-gene therapy in oral cancer. Photodiagnosis Photodyn Ther. (2021) 36:102581. doi: 10.1016/j.pdpdt.2021.102581
67. Liu D, Ma X, Ji Y, Chen R, Zhou S, Yao H, et al. Bioresponsive nanotherapy for preventing dental caries by inhibiting multispecies cariogenic biofilms. Bioact Mater. (2022) 14:1–14. doi: 10.1016/j.bioactmat.2021.12.016
68. Shitomi K, Miyaji H, Miyata S, Sugaya T, Ushijima N, Akasaka T, et al. Photodynamic inactivation of oral bacteria with silver nanoclusters/rose bengal nanocomposite. Photodiagnosis Photodyn Ther. (2020) 30:101647. doi: 10.1016/j.pdpdt.2019.101647
69. Yan R, Liu J, Dong Z, Peng Q. Nanomaterials-mediated photodynamic therapy and its applications in treating oral diseases. Biomater Adv. (2023) 144:213218. doi: 10.1016/j.bioadv.2022.213218
Keywords: photodynamic therapy, oral oncology, periodontitis, caries, peri-implant infection(s), candidiasis
Citation: Wang L, Chen Q and Liu D (2025) Development of photodynamic therapy in treating oral diseases. Front. Oral. Health 5:1506407. doi: 10.3389/froh.2024.1506407
Received: 5 October 2024; Accepted: 27 December 2024;
Published: 15 January 2025.
Edited by:
Rogelio González-González, Juárez University of the State of Durango, MexicoReviewed by:
Sven Eric Niklander, Universidad Andres Bello, ChileCopyright: © 2025 Wang, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Wang, MTAyMzA5MDQxNUBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.