- 1Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy
- 2Department of Pharmacy, University “G. d’Annunzio” Chieti-Pescara, Chieti, Italy
- 3Department of Innovative Technologies in Medicine & Dentistry, University of Chieti-Pescara, Chieti, Italy
- 4Center for Advanced Studies and Technology (CAST), “G. d’Annunzio” University of Chieti-Pescara, Chieti, Italy
- 5Plasma Bioscience Research Center, Department of Electrical and Biological Physics, Kwangwoon University, Seoul, Republic of Korea
- 6CNR- Istituto di Nanotecnologia (CNR-NANOTEC) UoS Bari, c/o Dipartimento di Chimica, Università Degli Studi di Bari Aldo Moro, Bari, Italy
- 7UdA-TechLab, Research Center, University “G. d’Annunzio” of Chieti-Pescara, Chieti, Italy
Aim: The intricate structure of the tooth root canals has a role in the colonization and biofilm formation in hidden areas that are hardly reached by standard endodontic treatments. This review aims at summarizing data from in vitro and ex vivo studies for a better understanding of the application of cold atmospheric plasma (CAP) for the disinfection of dental root canals.
Methods: PubMed, Scopus, and Web of Science databases were screened. Characteristics of the included studies were extracted, and a meta-analysis on ex vivo studies was carried out to evaluate the effect of CAP on colony forming unit assay of Enterococcus faecalis (E. faecalis). The study was performed following the PRISMA 2020 guidelines.
Results: A total of 31 studies fulfilled the selection criteria. Only 2 investigations reported an indirect plasma treatment, 28 trials used direct CAP administration, while 1 study applied both methods. Most of the studies were conducted on E. faecalis using as carrier gas Helium or Argon alone or in combination with Oxygen as well air. A considerable heterogeneity among studies was found regarding treatments which varied for source type, settings, and protocols of application. Despite this, CAP showed effectiveness in reducing E. faecalis colony forming unit with a standardized mean difference of 4.51, 95% C.I. = 2.55–6.48, p-value < 0.001.
Conclusion: The data demonstrated the antimicrobial effect of direct CAP application against microorganisms. In-vitro studies showed an effect that depended on the time and distance of treatment, while the meta-analysis performed on ex vivo studies showed that the effect of CAP was independent of time and distance.
Systematic Review Registration: https://doi.org/10.17605/OSF.IO/BJ59V, identifier OSF registries.
1 Introduction
Dental pulp and periapical infections are due to the bacterial colonization of the root canals; therefore, primary objective of endodontic treatment is to kill/remove bacteria (1, 2). In the clinical practice, different treatments such as mechanical debridement, chemical irrigation, and ultrasound activation are used (3–6). The complexity of the root canals system makes access to some areas through mechanical instruments almost impossible during endodontic practices. The chemical irrigation on biofilm debridement is inevitable and almost the only solution for cleaning these locations (7). Sodium hypochlorite (NaOCl) is the most employed root canal irrigant to dissolve the pulp tissue, while displaying a wide spectrum of action on bacteria, viruses, and spores (8).
In general, an effective endodontic decontamination protocol provides an 85%–94% success rate (9), decreasing to 65.5%−87.5% in the case of re-treatment (10–14). Many factors are associated to endodontic treatment failure, such as root canal overfilling, root fractures, broken instruments, mechanical perforation, missed or unfilled canals. However, the main cause is the inadequate instrumentation and decontamination of the root canal system with a lack of apical seal, leading to the persistence of intra or extra-radicular bacteria (15–18), in particular Aggregatibacter actinomycetemcomitans, Propionibacterium propionicum, and Enterococcus faecalis (19). Therefore, to overcome the limits of the above-mentioned treatments, especially in demanding clinical conditions such as narrow and tortuous root canals, more effective decontamination methods are required.
Currently, an innovative research field based on the use of Cold Atmospheric Plasma (CAP) has shown a great potential for root canal disinfection (20). Plasma is generated by partial ionization of a gas by supplying enough energy. Interaction between plasma and oxygen and/or nitrogen leads to the formation of reactive chemical species, with antibacterial, antifungal and antiviral properties (21). The main chemical reactive oxygen and nitrogen species (RONS) include hydrogen peroxide (H2O2), ozone (O3), hydroxyl radicals (OH), superoxide (O2−), singlet oxygen (1O2), nitric oxide (NO), nitrite (NO2−), and peroxynitrite (ONOO−), among others (22). The first clinical trial on the therapeutical use of plasma for the treatment of human diseases dates back to 2013 (23). Since then, two types of plasma application modalities have been developed:
(1) Direct treatment involving direct exposure of the biological target (e.g., cells in vitro) in the presence of a liquid or not.
(2) Indirect treatment involving the treatment of a liquid (e.g., saline solution, and bacterial culture medium) or hydrogels (plasma treated hydrogels, PTH), and the subsequent application of these plasma treated products onto the biological target, in vitro or in vivo, allowing treatment of internal and less accessible target sites.
Due to the promising anti-microbial and regenerative effects, CAP may find different applications in dental medicine, such the treatment of periodontal and peri-implant diseases (24, 25), caries (26) and dental bleaching (27), and endodontics (20). However, only few studies evaluated root canal disinfection using CAP-based approaches showing contrasting results and underling the lack of standardization in the protocols (i.e., direct vs. indirect approach, exposure time, feeding gas, plasma sources and treatment distance), which can make unfruitful the efforts towards a clinical translation of CAP application.
Therefore, the present study aimed to systematically review the literature published up to now on the use of CAP for root canal decontamination with the purpose to shed light on the definition of a proper protocol of application to guarantee a successful decontamination of the target site (i.e., limited damage to the surrounding biological tissues and reduced incidence of endodontic failures and reinfections).
2 Materials and methods
This systematic review was performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (28). The review was registered on OSF registries https://doi.org/10.17605/OSF.IO/BJ59V.
2.1 Eligibility criteria
2.1.1 Inclusion criteria
The search was limited to studies published in English without any year restriction. To be eligible for inclusion, these criteria had to be satisfied:
1. Case-control design of in vitro or ex vivo studies on dental root canal treatment by CAP.
2. Biological, microbiological, and physic-chemical effects of CAP on dental root canal treatment.
2.1.2 Exclusion criteria
The exclusion criteria were as follows:
1. Proceedings, abstracts;
2. Short communications;
3. Systematic reviews and meta-analyses;
4. Ecologic studies;
5. in vivo studies.
2.2 Search strategy
An electronic search on PubMed, Scopus, and Web of Science was performed to identify suitable studies, using the following terms and keywords alone or in combination:
(“cold atmospheric plasma” OR “cold plasma” OR “low temperature plasma” OR “kinpen med” OR “non-thermal atmospheric pressure plasma” OR “non thermal atmospheric pressure plasma” OR “cold physical plasma” OR “plasma medicine” OR pam OR cap OR “plasma activated medium” OR “cold atmospheric-pressure plasma” OR “plasma gases” OR “plasma activated liquid” OR “cold argon plasma” OR “plasma jet” OR “air plasma”) AND (biofilm OR biofilms OR “oral biofilm” OR “oral microorganism*” OR “biofilm colonization*” OR “dental plaque” OR “dental deposit*” OR “materia alba” OR “endodontic disease*” OR “dental canal” OR endodontics OR “conservative dentistry” OR “dental root”).
The first search was performed on October 16th, 2022. The last electronic search was performed on January 12th, 2024. In addition to the electronic search, a hand search was undertaken by checking the references of the included studies to identify further eligible studies. A reference manager software program (Mendeley Software Manager) was used, and the duplicates were discarded first electronically, then by checking the resulting list manually. The search strategy, adapted for PubMed, Web of Science, and Scopus is shown in Supplementary Table S1.
2.3 Focused PICO question
Based on the methodology of the study, and specifically if ex vivo or in vitro, the question is formulated:
Focused question: which were the biological, microbiological, and chemical-physical effects of CAP on in vitro and ex vivo bacterial cultivation of species involved in endodontic pathology?
2.3.1 In vitro articles
Participants: monospecies and multispecies biofilms commonly isolated from the endodontic space.
Intervention: direct or indirect CAP treatment.
Comparison: traditional endodontic treatments or different CAP treatment protocols.
Outcomes: microbiological and physic-chemical changes of biofilm.
2.3.2 Ex vivo articles
Participants: extracted teeth eligible for endodontic treatment.
Intervention: direct or indirect CAP treatment.
Comparison: traditional endodontic treatment or different CAP treatment protocols.
Outcomes: Disinfection and physicochemical changes of dental root tissues.
2.4 Selection of studies
Retrieved citations were independently screened by two authors (LS and GB), and relevant studies were identified based on title and abstract. If those did not provide sufficient information about the inclusion criteria, the full texts were evaluated to assess eligibility. Any disagreement was solved by discussion, and a third reviewer was consulted to make final decisions (VP). This author also calculated a kappa-statistic value to ascertain the level of reviewers’ agreement.
2.5 Data extraction and method of analysis
Two reviewers (LS and GB) independently extracted data from all the included studies using a predesigned extraction form (Microsoft Excel 2020, Microsoft Corporation, Redmond, WA, USA) for data collection and descriptive analysis. Extraction sheets were organized to collect:
1. CAP device features with the following items: type of plasma device and developer, CAP settings (i.e., gas flow rate, feeding gas, pulse frequency, pulse, temperature, application distance, application time, total energy, and power).
2. study characteristics: first author and year, study type (i.e.: ex vivo, in vitro), biofilm cultivation characteristics, sample size, outcomes, and results.
2.6 Risk of bias assessment
Two reviewers (LS and GB) evaluated the quality of studies by risk of bias assessment. Diverse scales were employed because of the heterogeneity of study designs of the included trials.
For in vitro studies, criteria based on the guidelines for creating bacterial culture studies were modified for the methodological quality of in vitro research. Six items were considered: 1. condition of bacterial culture before experimentation; 2. condition of bacterial culture during treatment; 3. description of methodology to evaluate outcomes; 4. case-control description; 5. multiple experiments performed; 6. descriptions of plasma settings and devices. Studies that satisfied at least 5 over 6 of the stated criteria were deemed to have a low risk of bias (Supplementary Table S2).
For ex vivo studies, the following criteria were used: 1. randomization of teeth in the different groups; 2. presence or absence of caries or restoration in teeth considered; 3. use of materials according to the manufacturer's instructions; 4. use of teeth with similar dimensions; 5. endodontic treatment performed by the same operator; 6. description of characteristic of bacterial cultivation; 7. blinding of the operator of the testing machine and the accuracy of plasma protocol description (29, 30).
The article received a “✓” (yes), fulfilling the investigated parameter; otherwise, it received a “–” (no) in case of negative response, U (Unclear) if the information was not clear in the article and N/A (not applicable) if it was not possible to obtain the information. Studies were identified as having a low risk of bias if they met at least 5 over 6 of the specified criteria, whereas studies that did not satisfy 2 or more than 3 of the reported elements were categorized as having a medium or high risk of bias (Supplementary Table S3).
2.7 Meta-analysis
Meta-analysis was carried out on ex vivo studies. Changes in standardized mean difference (SMD) of colony forming unit (CFU) counts were pooled for the meta-analysis of the included studies. Data were input as log (CFU/ml) and standard deviations (SDs) for both test (CAP treatment) and control group. Estimation of overall SMD and relative 95% confidence intervals (95% C.I.) was performed as Hedges’ g weighted data. These results were graphically represented as forest plots. Heterogeneity assessment was inspected to set a random or fixed-effect meta-analysis. Cochran's Q test was used to evaluate study heterogeneity and the I2 index was used to measure it. A random model was set for I2 values more than 50%, otherwise a fixed effect model was used (31).
To further assess heterogeneity, subgroup analysis was performed for the different feeding gas employed in CAP treatment; relation between effect size and respectively time of exposure treatment, application distance and publication year was inspected with meta-regression.
3 Results
3.1 Study selection
For this review 3,699 potentially relevant records from the databases were analysed following PRISMA statement. After the removal of duplicates, the articles were screened based on their titles and abstracts, and 3,520 articles were excluded. A total of 31 full-text articles were evaluated, and 2 were subsequently excluded for the reasons listed in Supplementary Table S4. The reference list of included studies was also reviewed to identify any further study missing in the electronic research and 2 articles were selected for inclusion.
Therefore, a total of 31 studies fulfilled the selection criteria of the present review and were included in qualitative analyses (Figure 1). The value of the kappa-statistic was 0.83 which indicated a moderate agreement between the 2 reviewers, following the formula provided by the Cochrane Handbook for Systematic Reviews of Interventions.
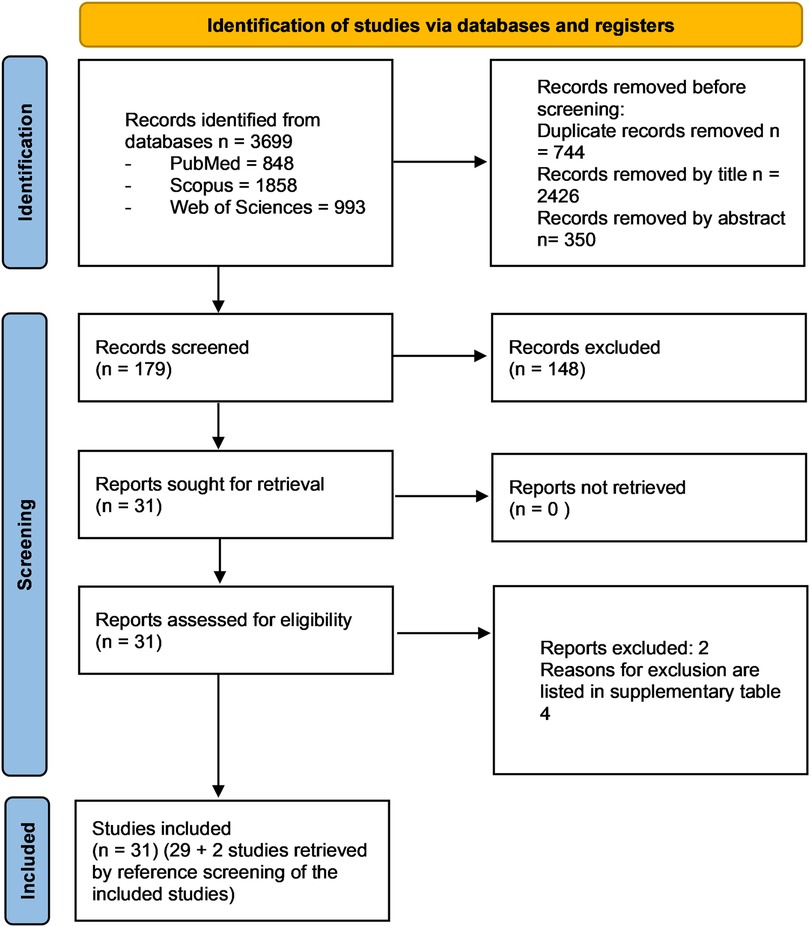
Figure 1. PRISMA 2020 flow diagram of the screening process. In total, 31 studies were included in the present systematic review.
3.2 General characteristics of included studies
Based on the study design, the studies were categorized into 2 different groups:
- 15 in vitro studies that investigated the antibacterial activity of CAP against E. faecalis (32–38), Candida albicans (C. albicans) (39–43), Escherichia coli (E. coli) (44), Streptococcus mutans (S. mutans) (36, 45, 46) and Streptococcus sanguinis (S. sanguinis) (45). Studies were carried out on biofilms on: agar (33, 35, 40, 42), PMMA disc (39, 43), simulated root canals in plastic blocks (34, 37), on cover glass (32), 96 well plate (41), 48 well plate (45), hydroxyapatite blocks (46), dentin blocks (38) porous sterile cellulose (33). Only 2 studies are carried out in microorganism in suspension (36, 44). Among papers showing the antibacterial properties of CAP, 4 studies were conducted in Germany (35, 37, 40, 43), 3 in China (33, 34, 38) and in Brazil (41, 42), 1 study was performed in Turkey (39), Taiwan (32), Japan (36), Ireland (44), USA (45), and South Korea (46), respectively.
- 16 ex vivo studies investigated the decontamination efficacy of CAP in root canals of human-extracted teeth, mainly on E. faecalis (1, 47–59), followed by 1 study using a human-derived microbial culture (60), and C. albicans (61), respectively. The sample size (teeth) in the articles ranged from 24 to 200, and the articles were published between 2011 and 2023. Among papers on decontamination efficacy on ex vivo experiments, five of them were conducted in Germany (48, 49, 60, 61), 6 articles were conducted in China (1, 38, 50, 51, 56, 59), 2 studies in Iran (47, 55), 1 in Mexico (54), 1 in USA (57), 1 in India (58) and 1 in Turkey (52).
3.3 Results of risk of bias assessment
Analysing risk of bias in in vitro studies, 14 presented a low risk of bias (32, 33, 35–46); while 1 presented a moderate risk (34) (Supplementary Table S2).
Referring to ex-vivo studies, none was rated as at a high risk of bias; 5 studies presented a moderate risk of bias (1, 47, 49, 50, 60), while 11 studies showed a low risk of bias (48, 51–59, 61) (Supplementary Table S3).
3.4 in vitro studies on relevant pathogens
Ten studies applied CFU count to evaluate the biofilm viability (32–36, 40, 41, 43, 44, 46). Two studies used MTT to evaluate viability and biofilm formation (39, 45). Scanning Electron Microscopy (SEM) was used in 5 articles to evaluate the bacterial surface morphologies (32, 33, 38, 39, 46). Delben et al. (42), Zhu et al. (38) and Yoo et al. (46) showed the bacterial viability via confocal laser microscopy (CLSM) analysis. Moreover, Delben et al. (42) evaluated the growth inhibition zone. All results are summarized in Supplementary Table S5.
3.4.1 Biofilm development
Most studies have utilized E. faecalis (32, 33, 35, 36, 38) and C. albicans (39–43). The reported cultivation times for E. faecalis in biofilms are highly variable, ranging from 18 h (36) to 7 days (38). Similarly, the cultivation time for C. albicans varies significantly across studies, from 24 h (39, 41, 43) to 16 days (43). S. mutans was cultured for 24 h by Hirano et al. (36) and Yoo et al. (46), while Liu et al. (45) employed a 7-day biofilm. Charoux et al. (44) and Liu et al. (45) applied E. coli cultured for 72 h and S. sanguinis cultured for 7 days, respectively.
3.4.2 Biofilm viability
Thirteen studies (32–36, 38–43, 45, 46) applied a direct plasma treatment, while only 1 study (44) used an indirect approach. Five studies (32, 33, 35, 36, 38) analysed the effect of plasma treatment on E. faecalis. All these studies except Zhu et al. (38) found a decrease in CFU increasing with the employment of different treatment times. Chang et al. (32) observed a significant decrease in CFU starting from 2 min, and after 15 min of treatment a complete eradication was observed. Cao et al. (33) and Theinkom et al. (35) observed a significant decrease in CFU starting from 5 min, while Hirano et al. (36) found a significative reductions at 3 min and eradication was observed in half of the time compared to Chang et al. (32) study (15 min). This difference could be determined by a different approach used in the two papers: planar DBD on dry biofilm (32) vs. plasma Jet on bacteria suspensions (36). The plasma jet in the paper of Hirano et al. (36) worked in contact with the biofilm suspension (i.e., direct contact of the ROS/UV and electric field of the plasma with the biofilm) while the planar DBD of the paper of Chang et al. (32) is remotely switched on (diffusion of the plasma produced species toward the biofilm with limited role of electric field and UV-radiation against biofilm). Zhu et al. (38) did not measure CFU but observed a decrease in live/dead microorganism ratio by CLSM analysis following 5 min. treatment with plasma loaded microbubbles (PMBs) and PMBs + ultrasound (US) compared to control group. The two PMBs groups showed no significant differences. Finally, when a bacterial suspension is used the contemporary effect of stable reactive species produced in the liquid could positively contribute to the efficacy of plasma processing.
Five studies (39–43) analysed the effect of plasma on C. albicans. Leite et al. (41) showed a decrease in CFU after 5 min of treatment, Maisch et al. (40) and Matthes et al. (43) observed respectively a diminution of CFU after 7 min and after 10 min of treatment. Avukat et al. (39) and Delben et al. (42) reported similar results at 2 min.
Avukat et al. (39) differently from the other paper used a RF sputtering under vacuum thus increasing dramatically the effect of ion bombardment does not present in atmospheric pressure plasmas. Surprisingly Delben et al. (42) obtained the same results using a plasma jet by demonstrating how the growth inhibition zone increases with the proximity of the plasma application and with an increase in exposure time.
S. mutans and S. sanguinis resulted also sensitive to direct CAP treatment, and a significant reduction in CFU was achieved in treatment time ranging from 1 to 2 min (45, 46). The efficacy was tested for both single- and dual-species biofilms (45). In summary, direct treatment with plasma shows an effective action against endodontic microorganisms with an action comparable to the traditional methods that have been compared. Furthermore, the application time and distance can influence the effectiveness against the microorganism.
The only study on indirect CAP application (plasma-activated water) was performed on E. coli and a CFU reduction was observed after 15 min treatment in combination with acoustic ultrasounds (44). The paper of Hirano et al. (36) shows experiments in which the plasma jet was faced to a suspension of bacteria in PBS showing efficacy on biofilm suspension after 7 min of plasma exposure, an approach that compared with that one of Charoux et al. (44) in which plasma jet was faced with distilled water. The major difference between the two approaches lies in the fact that when the plasma interfaces with a suspension the bacteria are affected by both the less stable reactive species (ex. NO, ONOO) and the more stable ones (ex. H2O2, NO2−, NO3−) while in case of an indirect approach like that one proposed by Charoux et al. (44) the bacteria are affected only by long-living species.
3.4.3 Chemical characteristics
Only Chang et al. (32) evaluated reactive species using Optical Emission Spectroscopy (OES) produced by an air based CAP. The result revealed emission lines corresponding to He, neutral O2 atom radiation, hydroxyl radicals, and neutral and atomic species of N (32), which might partially explain the anti-microbial activity. Charoux et al. (44) evaluated the concentration of nitrites and H2O2 produced other than the pH and electrical conductivity of produced plasma activated water (PAW). The pH values of the PAW showed a significant decrease after both cold and thermal plasma treatment. Plasma treatment also led to an increase in nitrate and nitrites, and higher levels of reactive nitrogen species (RNS) are founded in cold PAW than thermal PAW. Moreover, both plasma treatment methods increased the oxidation–reduction potential (ORP) in the treated PAW compared to the control. Finally, the conductivity of PAW generated by the cold plasma jet was higher than thermal plasma jet. Zhu et al. (38) observed an increase of NO and H2O2 in PMBs group and PMBs + US compared to control group due to the plasma dissolved in solution.
3.5 Preclinical models: in vitro model in resin
Only 2 studies (34, 37) included in this review used a simulated root-canal model in resin infected by E. faecalis.
3.5.1 Biofilm development
Both studies described the contamination protocol. In general, the bacterial suspension of E. faecalis was obtained by preliminary growth on agar plates and subsequently, the bacterial solution was inoculated into the simulated root canal. Subsequently, the simulated root canals were incubated aerobically at 37°C for 2 h (37) and 72 h (34).
3.5.2 Biofilm viability
Both studies evaluated antimicrobial effects via the CFU count. Zhou et al. (34) applied plasma jet treatment (He-1%O2) for 2, 4, 6, 8, 10 and 12 min. A significant decrease in CFU was observed starting from the 2-minute treatment. Jablonowski et al. (37) compared plasma (Ar) treatment for 3 min with chlorhexidine digluconate (CHX) and sodium hypochlorite (NaOCL). All three treatments showed a significant decrease in CFU compared to the untreated control.
3.5.3 Cell morphology
SEM was used to examine the morphological changes of bacteria located on the simulated root canal wall in the study by Jablonowski et al. (37), while Zhou et al. (34) applied transmission electron microscopy (TEM) and they observed also nuclear chromatin condensation and cell-division arrest.
3.6 Preclinical model: ex vivo humans
Four-teen studies (1, 47–59) evaluated the CAP effect on E. faecalis in ex vivo models of extracted root canals. Only 1 study (61) used a strain of C. albicans. In 1 study biofilm was not specified (60). Most studies employed direct CAP treatment compared to conventional therapies, such as NaOCl (42, 48, 49, 52, 57, 58, 60, 61), CHX (50, 53, 56, 61), photodynamic therapy (PDT) (47, 48), gaseous ozone delivery system with 2.5% NaOCl (52) and Ca(OH)2 (1, 50, 51, 55).
Different assays were performed to assess the biological and chemical reactions. Most articles (1, 47–50, 52–61) investigated the efficacy of CAP by CFU count and live/dead staining, and variations in the bacteria morphology via SEM and CLSM analysis. Reactive species generated by plasma were identified by OES for the plasma phase, and Electron Spin Resonance (ESR) spectroscopy in distilled water (50, 51). All the results are summarized in Supplementary Table S6.
3.6.1 Biofilm development
All the studies (1, 47–53, 60, 61) described the contamination protocol. In general, the bacterial suspension was obtained by a preliminary growth on agar plates and subsequently in brain-heart infusion broth (BHI). Subsequently, the sterilized tooth was immersed in a tube with 2 ml of the bacterial suspension. The tooth is incubated to allow biofilm formation under aerobic conditions and at 37°C. During the incubation period the culture medium was replaced every 2 days. Only 1 study (61) used C. albicans to infect root canals; in particular, the operator inoculated fungal suspension in the canals and incubated them aerobically for 7 days at 37°C.
3.6.2 Biofilm viability
Most of the studies (1, 47–50, 52–61), the efficacy of CAP treatment was evaluated by CFU counting. Live/dead staining and CLSM observation were also carried out in 4 studies (1, 50, 53, 60). In general, plasma treatment has been confirmed as a valid option compared to conventional disinfection treatments, such as NaOCl, CHX, Octenidine (OCT), or PDT. A common observation regarding the efficacy of plasma resulted the time-dependent disinfecting ability. The plasma treatment time range varied from 2 min to 15 min, moreover, longer exposure time led to a greater reduction of CFU (1, 47, 49–51, 56). Wang et al. (51) evaluates the rate of bacterial in the root canal after treatment with Plasma microjet (PMJ). Reinfection decreases with the increase of PMJ treatment time. After 40 min, the samples showed no reinfection.
Concerning the fed gas, in Armand et al. (47), the He + 0.5% O2 plasma treatment group showed better results in reducing CFU compared to the He plasma alone, especially at 8 min of treatment. Other papers use He/O2 or Ar/O2 mixtures as gas feed with variable percentage of O2 up to 20%. The observed results in these papers suggest a role of O2 in the microbial activity of plasma. In few articles (49, 53, 61), in addition to the normal comparison of plasma monotherapy vs. conventional therapies, the effect of possible combination therapies was tested. Specifically, in Kerlikowski et al. (61) a higher disinfecting power was observed when combining O2 based CAP with NaOCl. In contrast, Ballout et al. (48) showed that traditional therapies, such as NaOCl, had better disinfecting capacity than plasma treatment. This could be determined by a shorter plasma treatment time compared to the other studies. Though, Schaudinn et al. (60) instead observed how both NaOCl and plasma treatment were effective for biofilm removal although NaOCl was more effective during the same exposure time. Similarly, Arguello-Sanchez et al. (54) and Kumar et al. (58) showed a decrease of CFU starting from 1 to 2 min with NaOCl treatment respectively, while to obtain a similar reduction with CAP treatment it is necessary to treat root canals for at least 5 min (58). Moreover, Arguello-Sanchez et al. (54) treated E. faecalis samples for 5 min. using PAW and a direct plasma jet. The authors observed a significant decrease in CFU following direct plasma jet treatment, whereas no such reduction was observed with PAW treatment.
3.6.3 Cell morphology
SEM analysis was used in 7 studies on E. faecalis (1, 47–51, 53) and 1 on C. albicans (61). A change in the morphology of bacteria was observed. Specifically, a reduced cell size, ruptures, and damaged structures of E. faecalis biofilm were observed in the treated samples compared to the control group. In particular Wen et al. (53) observed how the integrity of the bacterial outer membrane was severely damaged and the normal spherical structure dissociated.
3.6.4 Mechanical safety evaluation
Only one study (50) evaluated the values of pulpal dentin microhardness (HV) and roughness (Ra). In this study, plasma treatment was able to remove E. faecalis biofilm, without affecting pulpal dentin HV and Ra after different treatment times (3, 6, 9, and 12 min), indicating that CAP did not change the mechanical properties of pulpal dentin.
3.6.5 Chemical characteristics
The development of ROS and RNS species after treatment, was demonstrated via two different assays, ESR and OES, in 1 (51) and 3 studies (47, 50, 51), respectively. Armand et al. (47) analysis observed the presence of reactive O2 radicals that oxidized the outer membrane of the microorganism. They observe excited O lines at 285 and 777 nm, OH line at 309 nm, O+ line at 427 nm. Li et al. (50) and Wang et al. (51) observed that Ar emission was dominant because Ar is the major component (98%) of the working gas, but also identified a strong atomic O2 emission at 777 nm and at 844 nm. O2 emission at 777 nm is determined by dissociative excitation by electron collisions from the ground state O2 molecules, while O2 emission at 844 nm, on the other hand, is determined mainly by direct electron impact with ground state O2 atoms.
3.7 Types of CAP devices and CAP treatment parameters
All in vitro studies (32, 43, 45, 46) used direct plasma treatment except one (44). CAP treatment was delivered directly on the in vitro model by means of different devices. 10 studies used plasma jet (33, 34, 36, 37, 39, 41, 42, 44–46), in 2 studies applied Dielectric Barrier Discharge Plasma (DBD) plasma (32, 43), 2 studies used Surface Micro Discharge Plasma (SMDP) (35, 40), and only 1 study applied PMB (38). Five studies (32, 35, 36, 40, 44) used ambient air to produce plasma, 3 studies (33, 34, 39) employed a mixture of O2 and He, in 3 studies Ar was the feeding gas (37, 42, 43), 2 studies used a mixture of Ar and O2 (43, 45), and finally 1 study N (46), and 1 study He (41). One study applied plasma activated water treatment (44). This technique employs a plasma technology to modify the properties of water, with consecutive generation of reactive species such as radicals, ions, and other highly reactive molecules with documented antimicrobial activity (44). The plasma application protocol varied greatly among studies. The application distance ranged between 2- and 20-mm. Plasma application time varied from 20 s to 30 min. Most of articles did not report the power either the temperature as experimental variables. The plasma sources used in the plasma jet and DBD studies applied a gas flow that is suitable for root canal treatment, while the studies that used SMDP, did not generate a strong gas flow necessary to thoroughly treat complex dental root canal systems.
All ex vivo (1, 47–53, 55–58, 60, 61) studies except one (59) used direct plasma treatment. Only Arguello-Sanchez et al. (54) applied both direct and indirect methods. One study (53) shows a direct approach in which root canals are filled with a liquid before plasma treatment. 16 studies used plasma jet (1, 47–61), and 1 study applied DBD plasma (48). Combination of Ar with 1 or 2% of O2 was employed in 6 studies (1, 49–51, 57, 61), 5 studies employed He and O2 (47, 52, 55, 56, 60), 4 studies used He (47, 54, 58, 59) and 1 study used Ar (48). Indirect plasma treatment was used in two studies (54, 59). Zhou et al. (59) applied plasma jet fed with He and vapours of H2O2, while Arguello-Sanchez et al. used PAW (54). The application protocol differed widely among studies, including pulse frequency, pulse, flow rate, plasma temperature, source application distance and time, total emitted energy, and power. The pulse frequency ranged between 0.05 to 100 kHz, and the application distance and time ranged between 1 and 10 mm and 1 to 15 min respectively. The power varied between 0.5 and 30 W, with the most commonly used device, Kinpen, which was typically set to a power of 8 W. All the parameters used in these different protocols are summarized and outlined below (Supplementary Table S7).
3.7.1 Temperature evaluation
Thermal effect is one of the physical factors developed in direct treatment along with UV radiation and electromagnetic fields. A thermal imaging camera was used to measure the working temperature of the plasma device in 2 articles (47, 50). The device used by Armand et al. (47) developed a temperature less than 40°C after 4, 6, and 8 min of plasma exposure, while Li et al. (50) detected mean CAP temperatures near the top of the root canal of 30.1°C and a room temperature of 27.6°C. Other authors reported plasma temperatures. Arguello-Sanchez et al. (54) and Leite et al. (41) reported a working plasma temperature below 40°C. Delben et al. (42) reported a plasma temperature at 3 mm of 30.67 ± 0.58°C and at 10 mm 29.83 ± 0.29°C. Habib et al. reported a temperature of plasma between 30 and 65°C (57). Pan et al. (1) reported a working plasma temperature between 25°C and 31°C. Theinkom et al. (35) reported a plasma temperature of 21°C. Wen et al. in 2022 (53) reported a working plasma temperature between 40°C and 70°C. Jablonowski et al. (37) reported a temperature of 42°C at the tip of the plasma jet. Summarizing the temperature range of gases varying from 21°C reported by Theinkom et al. (35). which use ambient air as gas up to the 65°C reported by Habib et al. which use a mix O2 and Ar gas for direct plasma treatment, while for indirect plasma treatment authors used a range of temperature between 40°C reported by Wen et al. (53) and Charoux et al. (44) up to 70°C reported in Wen et al. (53).
3.8 Meta-analysis
High heterogeneity emerged as I2 resulted 93.82%. For this reason, means and SDs were pooled in a random effects model, and SMD resulted 4.51 (95% C.I. = 2.55–6.48, p-value < 0.001) (Figure 2). Heterogeneity I2 dropped to 0% when considering only those studies in which treatment was based on Ar/O2 while high heterogeneity persisted for He alone and He/O2 subgroup. Fixed effects model meta-analysis for Ar/O2 subgroup showed a SMD of 2.23 (95% C.I. = 1.52–2.94, p-value < 0.001) while random effects model resulted in a SMD of 3.52 (95% C.I. = 0.88–6.16, p-value = 0.009) in He/O2 subgroup and 17.85 when He alone was employed (95% C.I. = −4.89–40.58, p-value = 0.124). Meta-regression showed neither correlation between estimated effect sizes and publication year nor exposure treatment time and distance (respectively, p-value = 0.151, p-value = 0.330, and p-value = 0.590). Characteristics considered for meta-analysis of included studies are summarized in Table 1.
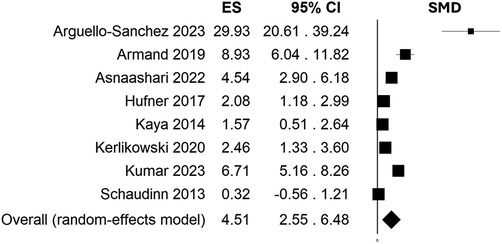
Figure 2. Effect size (ES) estimation by standardized mean difference (SMD) and 95% C.I. showing efficacy of CAP treatment compared to control in reducing overall CFU/ml counts (4.51, 95% C.I. = 2.55-6.48, p-value < 0.001).
4 Discussion
The oral cavity consists of a wide number of microorganisms involved in endodontic and periodontal diseases, as well as dental caries (19). Following the pulp necrosis, oral bacteria colonize the root canals, initially in their planktonic phenotype, free of floating, and arranging in biofilm, attached to root canal walls (62). Biofilms are highly organized and complex communities of microorganisms, often multispecies, able to better resist to adverse conditions, such as nutrient deprivation, the host immune system and antimicrobials.
As expanding application of CAP as therapeutic mean in medicine, results from this systematic review and meta-analysis highlight a successful antimicrobial activity for both planktonic and biofilm phenotype (SMD = 4.51, 95% C.I. = 2.55–6.48, p-value < 0.001).
The complex anatomy of the root canal allows microorganisms to develop biofilm in internal sites, hardly reached by standard endodontic treatments. Therefore, the physical and chemical properties of CAP treatment may enhance the efficacy of traditional root canal disinfection methods (48, 50–52, 61).
The localization of biofilms on the walls of ramifications and isthmuses of the root canal poses difficulties in the removal of these persistent microorganisms, increasing the rate of endodontic treatment failure. Among biofilms, the exchange of resistance genes between different clinically relevant species is facilitated, causing the acceleration of the phenomenon of the antimicrobial resistance (63). Therefore, endodontic infections could be a reservoir for antimicrobial resistance.
The microbial communities associated with endodontic infections are heterogeneous, and their persistence in root canal infections is a major role in endodontic treatment failure. Among microbial oral communities, E. faecalis is one of the most frequent microorganisms isolated from patients with endodontic infections, especially in cases of reinfections (64). E. faecalis has a prolonged survival capacity and a morphology that facilitates its diffusion into dentinal tubules. Its virulence factors, such as surface adhesins, are responsible for the formation of a strong biofilm within the root canals (64). From the moment that the plasma jet could curve within the curved root canals, the plasma could reach any place inside the inner canal, inactivating hidden bacteria in places where current therapies cannot reach (33, 52). Furthermore, the reactive species produced by CAP are energized and move at a high speed, so they can more easily enter complex and thin root canals to kill bacteria. Compared to traditional disinfection methods, CAP treatment demonstrated variable results. Some studies reported higher efficacy compared to CHX (50, 56) or NaOCl (61), while others observed lower efficacy relative to NaOCl (49, 60), although a reduction in CFU was consistently noted compared to the control. Habib et al. (57) observed that CAP treatment was as effective as NaOCl irrigation. CAP demonstrated a similar reduction in bacterial metabolic activity and viability compared to NaOCl treatment, with the potential for fewer side effects in patients.
The present systematic review has summarized data from in vitro and ex vivo studies with similar objectives for a better understanding of the possible CAP applications in endodontics. A great variability in CAP treatment parameters and CAP effects has emerged, claiming for standardization of protocols for a possible future clinical application of plasma therapy in endodontics. The literature data demonstrated that the antimicrobial effect of CAP depended on time and distance. The most used distances were between 0 and 15 mm whereas the time of application was in the range of 1–15 min; only in a few works, plasma was applied for seconds. Moreover, CAP efficacy was greater against planktonic than biofilm associated bacteria (53). Wen et al. (53) found that in order to obtain a similar CFU reduction, 1 min of treatment was necessary for the planktonic phenotype and at least 5 min for the biofilm phenotype. These results were not confirmed from the meta-analysis results of ex-vivo experiments, where effect size did not correlate with both time and distance. These results should be read with caution as numerical information. Indeed, most of the studies lacked detailed information about the effective output power and energy, which might help replicate the treatment protocol. Indeed, short exposure time in close distance might be similar in results to larger treatment time, but to an increased distance. While for in vitro study, reproducibility is easier to achieve, for ex vivo model more variables are taken into account, such as complex anatomy, bacterial species and biofilm formation, which represent more source of confounding effect.
The uncertainty of these results must be addressed in future studies, in which a better understanding of these protocol variables should be investigated under proper study designs.
Most of the selected ex vivo studies tested the efficacy of plasma treatment either alone or in combination with other chemical compounds (48–53, 61). CAP treatment alone had a similar or greater effect than chemical treatments. Indeed, in Wen et al. (53) CHX groups exhibited a lower antibacterial activity than CAP. Surprisingly, CAP had a better effect compared to CAP-CHX combination, since CHX might scavenge the reactive species generated by CAP. Similar results were obtained when comparing the efficacy of CAP vs. conventional chemical agents (50, 51, 61), indicating the possibility of using CAP in alternative to chemical treatments.
Kerlikowski et al. (61) demonstrated that plasma/O2 treatment for 6 and 12 min achieved the highest CFU reduction rates, when compared to conventional chemical treatments with NaOCl, octenidine (OCT), CHX and the negative control with NaCl. Furthermore, 12 min of plasma/O2 application elicited the highest reduction of CFU; this data was also supported by SEM. Plasma/O2 combination with NaOCl, OCT, or CHX did not show a synergistic effect with respect to the only plasma/O2 treatments. In this study there was a further confirmation of the time-dependent antimicrobial activity of plasma.
Li et al. (50) demonstrated the superiority of CAP treatment over other endodontic medicaments, namely Ca(OH)2 paste, 2% CHX gluconate gel and a combination of both. Specifically, plasma treatment for 12 min resulted in biofilm destruction and bacterial killing as demonstrated by SEM images and CFU count, respectively. CSLM 3D images of E. faecalis biofilm showed that almost all bacteria were dead (50).
Wang et al. (51) investigated the time-dependent effectiveness of plasma treatment in comparison to other intracanal medicaments. Plasma microjet (PMJ) treatment was able to inactivate E. faecalis after 8 min and was effective even against E. faecalis reinfection, given that the application of PMJ for 40 min presented no reinfection. In detail, PMJ for 40 min had the same efficacy as the drug formocresol and better efficacy than camphor phenol and Ca(OH)2.
Ureyen Kaya et al. (52) compared the efficacy of CAP vs. Ozone and NaOCl in biofilm disinfection and concluded that CAP treatment has a greater cleaning efficacy than ozone in bacteria killing but lower than 2.5% of NaOCl.
Only in Ballout et al. (48) both plasma jet and DBD plasma irradiation did not show any effect against E. faecalis biofilm, given that the CFU count was similar to the one of negative controls in both coronal and apical part of the tooth. On the contrary, the positive control with NaOCl caused E. faecalis biofilm eradication as demonstrated by the total absence of CFU and by SEM images. The reason why both plasma jet and DBD plasma treatment modalities were not effective in biofilm disinfection was probably due to the low time of application (60 s) and by plasma source used. In fact, in most of the cited papers, plasma treatment was effective in CFU reduction only when applied for more than one minute. Furthermore, the type of gas used could influence the biofilm disinfection. A gas with He could be more effective than Ar as observed by Zhou et al. (59) who observed a significant reduction of biofilm starting from 1 min., although in this case an indirect approach is applied.
In an in vitro study Charoux et al. (44) used PAW as an antiseptic, showing a reduction in E. faecalis CFU following in vitro treatment. However, the characteristics of this technique make it less effective than gas, which has better capabilities in penetrating tortuous root canals as observed by Arguello-Sanchez et al. (54) in ex vivo study.
In summary, this systematic review demonstrates that CAP is a promising method in endodontic therapy whatever the approach used (mediated or not by liquid) and configuration of plasma sources applied (plasma jet-brush or plume, planar DBD). Different CAP devices have a better or similar effect in decreasing bacterial biofilms than conventional root canal disinfection methods based on rinsing the site of interest by a disinfecting solution. Furthermore, in most of the cited studies (1, 47, 50, 51, 53, 56, 57), CAP is effective in preventing E. faecalis reinfection, a condition which often leads to surgical therapy, probably due to plasma-generated reactive species which can successfully reach areas that are difficult to reach by traditional antiseptics. However, due to the heterogeneity among studies final conclusions on the CAP application modality as well as on plasma settings to obtain a proper root canal disinfection could not be drawn. Indeed, the considerable variety of plasma sources, settings, bacterial and biofilm models claim for the urgent need of standardization as building foundation for the clinical translation of CAP treatment. Although currently no types of plasma that can be used intraorally in patients, these studies provide suggestions on which setup parameters, biofilm models and examination methods should be used for further studies, so that at some point therapeutic recommendations or prerequisites for intraorally applicable plasma sources can be provided.
5 Limitations and future perspectives
A limitation of this study is the variety of devices used to produce CAP; furthermore, in this work only in vitro and ex-vivo experiments were analyzed, but none of in vivo studies were present. Moreover, there are not enough studies to compare the efficacy of the different methods of plasma administration, because only a few have analyzed the indirect method, such as the delivery of plasma activated means. This currently makes it impossible currently to establish an appropriate clinical protocol. Despite the limitations, CAP has shown efficacy against endodontic microorganisms in both in vitro and in ex-vivo studies, with an action similar to that observed in conventional methods, without damaging hard tissues of teeth as observed by Wen et al. (53). Regarding the effect of CAP on the oral mucosa, Jablonowski et al. (65) observed how treatment with CAP is well tolerated in the short term experiments by the murine model, while Evert et al. (66) observed that repeated exposure to CAP of the oral mucosa over 12 months is not carcinogenic in mice, confirming the safety of the treatment also for long term exposure. The results reported by Jablonowski et al. (65) and Evert et al. (66) demonstrated the safety of these CAP devices, making them a promising technique for future use in patients undergoing root canal treatment. Consequently, the focus on the use of CAP and the encouraging results observed in numerous studies could drive the progress of technical research, allowing the creation of increasingly advanced devices to enable the future application of CAP for the treatment of root canals.
Author contributions
LS: Conceptualization, Data curation, Formal Analysis, Investigation, Validation, Visualization, Writing – original draft. VaP: Data curation, Validation, Visualization, Writing – original draft. VC: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – original draft. MoP: Investigation, Methodology, Validation, Visualization, Writing – original draft. GB: Conceptualization, Data curation, Validation, Visualization, Writing – original draft. BF: Conceptualization, Data curation, Validation, Visualization, Writing – original draft. MiP: Data curation, Validation, Visualization, Writing – original draft. IC: Validation, Visualization, Writing – original draft. NK: Conceptualization, Validation, Visualization, Writing – original draft. EC: Methodology, Validation, Visualization, Writing – original draft. RG: Conceptualization, Validation, Visualization, Writing – original draft. ES: Conceptualization, Data curation, Validation, Visualization, Writing – original draft. ViP: Conceptualization, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financed by the European Union—NextGenerationEU through the Italian Ministry of University and Research under PNRR 2022 “Therapeutical effects of Cold atmospheric plasma in Head And Neck Cancer and oral hEalth preservation—C.H.A.N.C.E.”, CUP: D53D23013380006.
Acknowledgments
We thank COST Actions CA20114 (Therapeutical Applications of Cold Plasmas) for the stimulating environment provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2024.1483078/full#supplementary-material
References
1. Pan J, Sun K, Liang Y, Sun P, Yang X, Wang J, et al. Cold plasma therapy of a tooth root canal infected with enterococcus faecalis biofilms in vitro. J Endod. (2013) 39(1):105–10. doi: 10.1016/j.joen.2012.08.017
2. Seltzer S, Bender IB, Smith J, Freedman I, Nazimov H. Endodontic failures–an analysis based on clinical, roentgenographic, and histologic findings. II. Oral Surg Oral Med Oral Pathol. (1967) 23(4):517–30. doi: 10.1016/0030-4220(67)90547-6
3. Athanassiadis B, Abbott PV, Walsh LJ. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust Dent J. (2007) 52(1 Suppl):S64–82. doi: 10.1111/j.1834-7819.2007.tb00527.x
4. Siqueira JF J, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. (1999) 32(5):361–9. doi: 10.1046/j.1365-2591.1999.00275.x
5. Bastendorf KD, Strafela-Bastendorf N, Lussi A. Mechanical removal of the biofilm: is the curette still the gold standard? Monogr Oral Sci. (2021) 29:105–18. doi: 10.1159/000510187
6. Neelakantan P, Cheng CQ, Mohanraj R, Sriraman P, Subbarao C, Sharma S. Antibiofilm activity of three irrigation protocols activated by ultrasonic, diode laser or Er:YAG laser in vitro. Int Endod J. (2015) 48(6):602–10. doi: 10.1111/iej.12354
7. Xu J, Gao Y, Meng Y, Wu W, Tsauo C, Guo T, et al. Mechano-chemical coupling of irrigation enhances endodontic biofilm debridement. Biofouling. (2020) 36(7):792–9. doi: 10.1080/08927014.2020.1814753
8. Cai C, Chen X, Li Y, Jiang Q. Advances in the role of sodium hypochlorite irrigant in chemical preparation of root canal treatment. Biomed Res Int. (2023) 2023:8858283. doi: 10.1155/2023/8858283
9. Karamifar K, Tondari A, Saghiri MA. Endodontic periapical lesion: an overview on the etiology, diagnosis and current treatment modalities. Eur Endod J. (2020) 5(2):54–67. doi: 10.14744/eej.2020.42714
10. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1998) 85(1):86–93. doi: 10.1016/S1079-2104(98)90404-8
11. Pereira RS, Rodrigues VAA, Furtado WT, Gueiros S, Pereira GS, Avila-Campos MJ. Microbial analysis of root canal and periradicular lesion associated to teeth with endodontic failure. Anaerobe. (2017) 48:12–8. doi: 10.1016/j.anaerobe.2017.06.016
12. Sabeti M, Chung YJ, Aghamohammadi N, Khansari A, Pakzad R, Azarpazhooh A. Outcome of contemporary nonsurgical endodontic retreatment: a systematic review of randomized controlled trials and cohort studies. J Endod. (2024) 50(4):414–33. doi: 10.1016/j.joen.2024.01.013
13. Stueland H, Ørstavik D, Handal T. Treatment outcome of surgical and non-surgical endodontic retreatment of teeth with apical periodontitis. Int Endod J. (2023) 56(6):686–96. doi: 10.1111/iej.13914
14. Shah T, Ramesh S, Sugumaran S, Choudhari S. Endodontic retreatment efficacy with and without solvents: a systematic review. J Conserv Dent Endod. (2023) 26(6):610–5. doi: 10.4103/JCDE.JCDE_86_23
15. Iqbal A. The factors responsible for endodontic treatment failure in the permanent dentitions of the patients reported to the college of dentistry, the University of Aljouf, kingdom of Saudi Arabia. J Clin Diagn Res. (2016) 10(5):Zc146–8. doi: 10.7860/JCDR/2016/14272.7884
16. Saunders WP, Saunders EM. Coronal leakage as a cause of failure in root-canal therapy: a review. Endod Dent Traumatol. (1994) 10(3):105–8. doi: 10.1111/j.1600-9657.1994.tb00533.x
17. Siqueira JF J. Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. (2001) 34(1):1–10. doi: 10.1046/j.1365-2591.2001.00396.x
18. Tabassum S, Khan FR. Failure of endodontic treatment: the usual suspects. Eur J Dent. (2016) 10(1):144–7. doi: 10.4103/1305-7456.175682
19. Dioguardi M, Di Gioia G, Illuzzi G, Arena C, Caponio VCA, Caloro GA, et al. Inspection of the Microbiota in endodontic lesions. Dentistry Journal. (2019) 7(2):47. doi: 10.3390/dj7020047
20. Muniz AB, da Cruz Vegian MR, Pereira Leite LD, da Silva DM, Moreira Milhan NV, Kostov KG, et al. Non-thermal atmospheric pressure plasma application in endodontics. Biomedicines. (2023) 11(5):1401. doi: 10.3390/biomedicines11051401
21. Zhou W, Wang X, Huang X. Cold atmospheric pressure plasmas applications in dentistry. Plasma Processes Polym. (2022) 19(8):2200024. doi: 10.1002/ppap.202200024
22. Privat-Maldonado A, Schmidt A, Lin A, Weltmann KD, Wende K, Bogaerts A, et al. ROS From physical plasmas: redox chemistry for biomedical therapy. Oxid Med Cell Longev. (2019) 2019:9062098. doi: 10.1155/2019/9062098
23. Isbary G, Stolz W, Shimizu T, Monetti R, Bunk W, Schmidt HU, et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: results of an open retrospective randomized controlled study in vivo. Clin Plasma Med. (2013) 1(2):25–30. doi: 10.1016/j.cpme.2013.06.001
24. Hui WL, Ipe D, Perrotti V, Piattelli A, Fang Z, Ostrikov K, et al. Novel technique using cold atmospheric plasma coupled with air-polishing for the treatment of titanium discs grown with biofilm: an in vitro study. Dent Mater. (2021) 37(2):359–69. doi: 10.1016/j.dental.2020.11.027
25. Lima GMG, Borges AC, Nishime TMC, Santana-Melo GF, Kostov KG, Mayer MPA, et al. Cold atmospheric plasma jet as a possible adjuvant therapy for periodontal disease. Molecules (Basel, Switzerland). (2021) 26(18):5590. doi: 10.3390/molecules26185590
26. Hong Q, Dong X, Chen M, Sun H, Hong L, Wang Y, et al. An in vitro and in vivo study of plasma treatment effects on oral biofilms. J Oral Microbiol. (2019) 11(1):1603524. doi: 10.1080/20002297.2019.1603524
27. Nam SH, Ok SM, Kim GC. Tooth bleaching with low-temperature plasma lowers surface roughness and Streptococcus mutans adhesion. Int Endod J. (2018) 51(4):479–88. doi: 10.1111/iej.12860
28. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
29. Sarkis-Onofre R, Skupien JA, Cenci MS, Moraes RR, Pereira-Cenci T. The role of resin cement on bond strength of glass-fiber posts luted into root canals: a systematic review and meta-analysis of in vitro studies. Oper Dent. (2014) 39(1):E31–44. doi: 10.2341/13-070-LIT
30. Silva CF, Cabral LC, Navarro de Oliveira M, da Mota Martins V, Machado AC, Blumenberg C, et al. The influence of customization of glass fiber posts on fracture strength and failure pattern: a systematic review and meta-analysis of preclinical ex-vivo studies. Journal of the mechanical behavior of biomedical materials. J Mech Behav Biomed Mater. (2021) 118:104433. doi: 10.1016/j.jmbbm.2021.104433
31. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
32. Chang YT, Chen G. Oral bacterial inactivation using a novel low-temperature atmospheric-pressure plasma device. J Dent Sci. (2016) 11(1):65–71. doi: 10.1016/j.jds.2014.03.007
33. Cao Y, Yang P, Lu X, Xiong Z, Ye T, Xiong Q, et al. Efficacy of atmospheric pressure plasma as an antibacterial agent against Enterococcus Faecalis in vitro. Plasma Sci Technol. (2011) 13(1):98. doi: 10.1088/1009-0630/13/1/19
34. Zhou X, Xiong Z, Cao Y, Lu X, Liu D. The antimicrobial activity of an atmospheric-pressure room-temperature plasma in a simulated root-canal model infected with Enterococcus Faecalis. IEEE Transactions on Plasma Sci. (2010) 38(12):3374. doi: 10.1109/TPS.2010.2078522
35. Theinkom F, Singer L, Cieplik F, Cantzler S, Weilemann H, Cantzler M, et al. Antibacterial efficacy of cold atmospheric plasma against Enterococcus faecalis planktonic cultures and biofilms in vitro. PLoS One. (2019) 14(11):e0223925. doi: 10.1371/journal.pone.0223925
36. Hirano Y, Hayashi M, Tamura M, Yoshino F, Yoshida A, Masubuchi M, et al. Singlet oxygen generated by a new nonthermal atmospheric pressure air plasma device exerts a bactericidal effect on oral pathogens. J Oral Sci. (2019) 61(4):521–5. doi: 10.2334/josnusd.18-0455
37. Jablonowski L, Koban I, Berg MH, Kindel E, Duske K, Schröder K, et al. Elimination of E. Faecalis by a new non-thermal atmospheric pressure plasma handheld device for endodontic treatment. A preliminary investigation. Plasma Processes Polym. (2013) 10(6):499–505. doi: 10.1002/ppap.201200156
38. Zhu M, Dang J, Dong F, Zhong R, Zhang J, Pan J, et al. Antimicrobial and cleaning effects of ultrasonic-mediated plasma-loaded microbubbles on Enterococcus faecalis biofilm: an in vitro study. BMC Oral Health. (2023) 23(1):133. doi: 10.1186/s12903-023-02813-6
39. Avukat EN, Akay C, Topcu Ersöz MB, Mumcu E, Pat S, Erdönmez D. Could helium plasma treatment be a novel approach to prevent the biofilm formation of Candida albicans? Mycopathologia. (2023) 188(4):361–9. doi: 10.1007/s11046-023-00747-9
40. Maisch T, Shimizu T, Isbary G, Heinlin J, Karrer S, Klämpfl TG, et al. Contact-free inactivation of Candida albicans biofilms by cold atmospheric air plasma. Appl Environ Microbiol. (2012) 78(12):4242–7. doi: 10.1128/AEM.07235-11
41. Pereira Leite LD, Carvalho de Oliveira MA, da Cruz Vegian MR, Sampaio A, Nishime TMC, Kostov KG, et al. Effect of cold atmospheric plasma jet associated to polyene antifungals on Candida albicans biofilms. Molecules. (2021) 26(19):5815. doi: 10.3390/molecules26195815
42. Delben JA, Murata RM, Wei X, Castro ML, Assunção WG, da Silva NRFA, et al. Low-temperature plasma: an effective approach against Candida albicans biofilm. Plasma Med. (2014) 4(1-4):231–44. doi: 10.1615/PlasmaMed.2014012361
43. Matthes R, Jablonowski L, Koban I, Quade A, Hübner NO, Schlueter R, et al. In vitro treatment of Candida albicans biofilms on denture base material with volume dielectric barrier discharge plasma (VDBD) compared with common chemical antiseptics. Clin Oral Investig. (2015) 19(9):2319–26. doi: 10.1007/s00784-015-1463-y
44. Charoux CMG, Patange AD, Hinds LM, Simpson JC, O'Donnell CP, Tiwari BK. Antimicrobial effects of airborne acoustic ultrasound and plasma activated water from cold and thermal plasma systems on biofilms. Sci Rep. (2020) 10(1):17297. doi: 10.1038/s41598-020-74504-z
45. Liu T, Wu L, Babu JP, Hottel TL, Garcia-Godoy F, Hong L. Effects of atmospheric non-thermal argon/oxygen plasma on biofilm viability and hydrophobicity of oral bacteria. Am J Dent. (2017) 30(1):52–6.29178715
46. Yoo E-M, Choi Y-R, Kang M-K. Antimicrobial efficacy of nitrogen-based non-thermal atmospheric pressure plasma jet on dental biofilm. Iran J Sci Technol Trans A. (2020) 44(5):1547. doi: 10.1007/s40995-020-00943-y
47. Armand A, Khani M, Asnaashari M, AliAhmadi A, Shokri B. Comparison study of root canal disinfection by cold plasma jet and photodynamic therapy. Photodiagnosis Photodyn Ther. (2019) 26:327–33. doi: 10.1016/j.pdpdt.2019.04.023
48. Ballout H, Hertel M, Doehring J, Kostka E, Hartwig S, Paris S, et al. Effects of plasma jet, dielectric barrier discharge, photodynamic therapy and sodium hypochlorite on infected curved root canals. J Biophotonics. (2018) 11(3):e201700186. doi: 10.1002/jbio.201700186
49. Hufner A, Steffen H, Holtfreter B, Schlueter R, Duske K, Matthes R, et al. Effects of non-thermal atmospheric pressure plasma and sodium hypochlorite solution on Enterococcus faecalis biofilm: an investigation in extracted teeth. Plasma Process Polym. (2016) 14(3):1600064. doi: 10.1002/ppap.201600064
50. Li Y, Sun K, Ye G, Liang Y, Pan H, Wang G, et al. Evaluation of cold plasma treatment and safety in disinfecting 3-week root canal Enterococcus faecalis biofilm in vitro. J Endod. (2015) 41(8):1325–30. doi: 10.1016/j.joen.2014.10.020
51. Wang R, Zhou H, Sun P, Wu H, Pan J, Zhu W, et al. The effect of an atmospheric pressure, DC nonthermal plasma microjet on tooth root canal, dentinal tubules infection and reinfection prevention. Plasma Med. (2011) 1(2):143–55. doi: 10.1615/PlasmaMed.2011003259
52. Kaya BU, Kececi AD, Guldas HE, Cetin ES, Ozturk T, Oksuz L, et al. Efficacy of endodontic applications of ozone and low-temperature atmospheric pressure plasma on root canals infected with Enterococcus faecalis. Lett Appl Microbiol. (2014) 58(1):15. doi: 10.1111/lam.12148
53. Wen Y, Luo Y, Wei X, Tan H, Ai R, Xiong Z, et al. Antibacterial effects of liquid discharge cold plasma on Enterococcus faecalis planktonic cultures and biofilms: an in vitro study of root canal treatment. J Phys D: Appl Phys. (2022) 55(36):365204. doi: 10.1088/1361-6463/ac7423
54. Arguello-Sanchez R, Lopez-Callejas R, Rodriguez-Mendez BG, Scougall-Vilchis R, Velazquez-Enriquez U, Mercado-Cabrera A, et al. Innovative curved-tip reactor for non-thermal plasma and plasma-treated water generation: synergistic impact comparison with sodium hypochlorite in dental root canal disinfection. Materials (Basel). (2023) 16(22):7204. doi: 10.3390/ma16227204
55. Asnaashari M, Mehrabinia P, Yadegari Z, Hoseini H, Sadafi M, Shojaeian S. Evaluation of antibacterial effects of cold atmospheric plasma, calcium hydroxide, and triple antibiotic paste on Enterococcus faecalis biofilm in the root canal system: an in vitro study. J Lasers Med Sci. (2022) 13:e50. doi: 10.34172/jlms.2022.50
56. Du T, Ma J, Yang P, Xiong Z, Lu X, Cao Y. Evaluation of antibacterial effects by atmospheric pressure nonequilibrium plasmas against Enterococcus faecalis biofilms in vitro. J Endod. (2012) 38(4):545–9. doi: 10.1016/j.joen.2011.10.021
57. Habib M, Hottel TL, Hong L. Antimicrobial effects of non-thermal atmospheric plasma as a novel root canal disinfectant. Clin Plasma Med. (2014) 2(1):17–21. doi: 10.1016/j.cpme.2014.07.002
58. Kumar P, Soundharrajan P, Prakash R, Kombade SP, Yadav P, Chugh A, et al. An in vitro analysis to evaluate the disinfection effectiveness of cold atmospheric pressure (CAP) plasma jet in Enterococcus faecalis infected root canals. Biomater Investig Dent. (2023) 10(1):2193214. doi: 10.1080/26415275.2023.2193214
59. Zhou XC, Li YL, Liu DX, Cao YG, Lu XP. Bactericidal effect of plasma jet with helium flowing through 3% hydrogen peroxide against Enterococcus faecalis. Exp Ther Med. (2016) 12(5):3073–7. doi: 10.3892/etm.2016.3726
60. Schaudinn C, Jaramillo D, Freire MO, Sedghizadeh PP, Nguyen A, Webster P, et al. Evaluation of a nonthermal plasma needle to eliminate ex vivo biofilms in root canals of extracted human teeth. Int Endod J. (2013) 46(10):930–7. doi: 10.1111/iej.12083
61. Kerlikowski A, Matthes R, Pink C, Steffen H, Schlüter R, Holtfreter B, et al. Effects of cold atmospheric pressure plasma and disinfecting agents on Candida albicans in root canals of extracted human teeth. J Biophotonics. (2020) 13(12):e202000221. doi: 10.1002/jbio.202000221
62. Chávez de Paz LE. Development of a multispecies biofilm community by four root canal bacteria. J Endod. (2012) 38(3):318–23. doi: 10.1016/j.joen.2011.11.008
63. Madsen JS, Burmølle M, Hansen LH, Sørensen SJ. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol. (2012) 65(2):183–95. doi: 10.1111/j.1574-695X.2012.00960.x
64. Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. (2006) 32(2):93–8. doi: 10.1016/j.joen.2005.10.049
65. Jablonowski L, Kocher T, Schindler A, Müller K, Dombrowski F, von Woedtke T, et al. Side effects by oral application of atmospheric pressure plasma on the mucosa in mice. PloS One. (2019) 14(4):e0215099. doi: 10.1371/journal.pone.0215099
Keywords: biofilm, cold atmospheric plasma, oral microorganisms, plasma medicine, disinfection, dental root canals
Citation: Sanesi L, Puca V, Caponio VCA, Pinti M, Balice G, Femminella B, Paolantonio M, Cela I, Kaushik NK, Choi EH, Grande R, Sardella E and Perrotti V (2024) Disinfection of dental root canals by cold atmospheric plasma: a systematic review and meta-analysis of dental biofilm. Front. Oral. Health 5:1483078. doi: 10.3389/froh.2024.1483078
Received: 19 August 2024; Accepted: 9 October 2024;
Published: 3 December 2024.
Edited by:
Mariia Faustova, Poltava State Medical University, UkraineReviewed by:
Rutger Matthes, Universitätsmedizin Greifswald, GermanyNoala Milhan, São Paulo State University, Brazil
Copyright: © 2024 Sanesi, Puca, Caponio, Pinti, Balice, Femminella, Paolantonio, Cela, Kaushik, Choi, Grande, Sardella and Perrotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vittoria Perrotti, di5wZXJyb3R0aUB1bmljaC5pdA==
 Lorenzo Sanesi
Lorenzo Sanesi Valentina Puca
Valentina Puca Vito Carlo Alberto Caponio
Vito Carlo Alberto Caponio Morena Pinti2
Morena Pinti2 Ilaria Cela
Ilaria Cela Nagendra Kumar Kaushik
Nagendra Kumar Kaushik Eun Ha Choi
Eun Ha Choi Rossella Grande
Rossella Grande Eloisa Sardella
Eloisa Sardella Vittoria Perrotti
Vittoria Perrotti