- 1Department of Otolaryngology-Head and Neck Surgery, Loma Linda University Medical Center, Loma Linda, CA, United States
- 2Case Western Reserve University School of Medicine, Cleveland, OH, United States
Objectives: Recurrence and survival in early T-stage oral tongue squamous cell carcinoma (OTSCC) may be impacted by histopathologic risk factors. This study aims to examine which of these factors predict long-term outcomes of T1 and T2 OTSCC.
Methods: A retrospective review of T1 and T2 OTSCC patients treated with surgery at a single tertiary care center was conducted. Multivariate regression and Kaplan-Meier survival plots were used to identify predictors of recurrence and compare disease-free survival respectively.
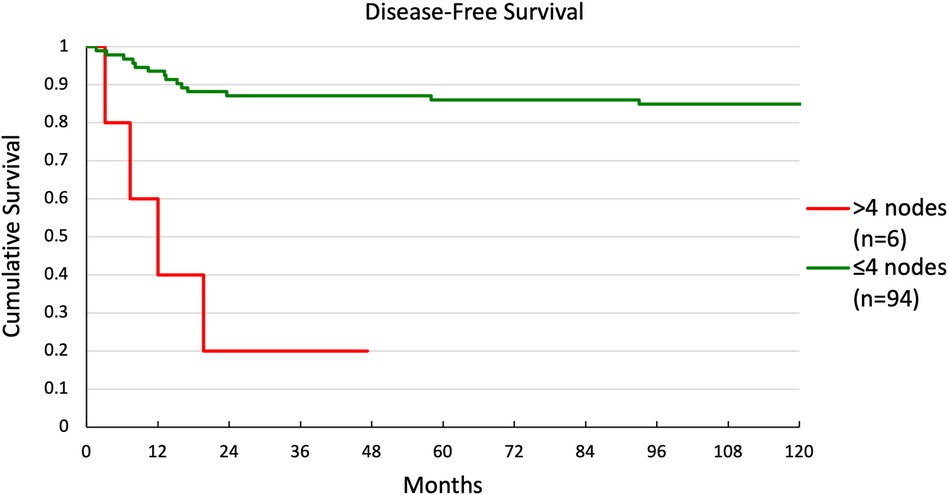
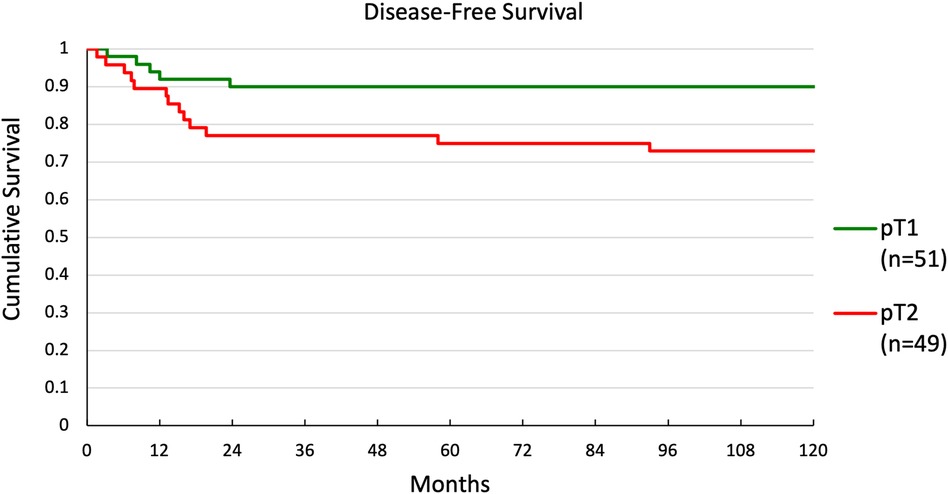
Results: 100 consecutive patients were studied. Of these, 51 were staged pT1, 49 pT2, 69 pN0, 10 pN1, and 21 pN2. Multivariate regression analysis revealed that >4 nodes was the strongest predictor of overall recurrence [odds ratio 1.68 (1.23–2.28), p = 0.001], while >4 nodes [odds ratio 1.14 (1.09–1.85), p = 0.008] and pT2 [odds ratio 1.15 (1.01–1.30), p = 0.033] were predictors of local recurrence (R2 = 0.112). Five-year disease-free survival was not significantly impacted by any risk factors except for the number of positive nodes—86% for ≤4 nodes vs. 20% for >4 nodes (p < 0.001)—and pathologic T-stage—90% for pT1 vs. 75% for pT2 (p = 0.035) regardless of adjuvant radiation and/or chemotherapy use.
Conclusions: Patients who underwent adjuvant radiation and/or chemotherapy had similar survival to those who did not despite having worse overall tumor prognostic factors. Adding adjuvant therapy may equalize some high-risk histopathologic factors. In the highest risk patients—specifically those with pathologic >4 nodes and pT2 staging—adjuvant therapy should be considered.
1 Introduction
Oral cavity cancer is among the most prevalent head and neck malignancies, with oral tongue squamous cell carcinoma (OTSCC) being the most common variant (1). Typically, OTSCC is treated with primary surgery followed by adjuvant radiation and/or chemotherapy depending on the disease stage and tumor histopathologic features. Regional cervical metastasis rates vary from 15% to 47% with occult rates reaching up to 42% (2–5). Achieving disease cure requires complete surgical resection and appropriate adjuvant treatment, selected based on histopathologic risk stratification. Some histologic tumor features have been shown to predict survival and recurrence, such as perineural invasion (PNI) and tumor budding (6, 7). However, uncertainty remains regarding the value of other tumor characteristics (e.g., lymphovascular invasion [LVI], tumor differentiation, extracapsular spread [ECS], and number of positive nodes) as risk factors for recurrence and prognosticators for survival (8, 9). Understanding how these histopathologic risk factors predict long-term outcomes is essential to identify patients that would benefit from adjuvant therapy. This is especially important in early T stage (T1 and T2) OTSCC, where primary tumor resection is achievable and need for additional treatment may be unclear. We examined long-term outcomes in a cohort of patients with T1 and T2 OTSCC treated with primary surgical resection, elective or therapeutic neck dissection, and adjuvant radiation and/or chemotherapy. We aimed to identify predictors of local and regional recurrence and examine differences in survival based on these predictors.
2 Methods
This study received ethical approval from Loma Linda University Medical Center IRB (Approval #5150330). This is an IRB-approved retrospective study, all patient information was deidentified and patient consent was not required. We performed a review of a cohort of newly diagnosed and previously untreated adult patients with T1 and T2 OTSCC treated at LLUMC over a twelve-year consecutive period with a minimum five-year follow-up. Patients were identified through a query of the institutional electronic medical records database for all oral tongue cancers by International Classification of Diseases, Ninth Revision (ICD-9) codes and through a query of the institutional tumor registry. Patients with non-squamous histology, previous treatment for any head and neck cancer, and incomplete medical records were excluded. All staging was based on the American Joint Committee on Cancer (AJCC) 7th edition using surgical pathology findings based on glossectomy and neck dissection. Pathologic findings were reviewed by a head and neck trained pathologist. Disease free survival (DFS)—the length of time after treatment that the patient survives without any signs or symptoms of recurrence—and overall survival—the length of time after treatment that the patient is still alive—were both recorded.
2.1 Statistics
The Student t-test and the Fisher exact test were used to compare continuous and categorical variables, respectively. A univariate Cox proportional hazards regression model was used to find predictors of overall, local, regional and distal recurrence. A multivariate Cox proportional hazards regression was performed in a step-forward manner to find predictors of recurrence, considering interactions between variables. Survival curves were constructed using the Kaplan-Meier method for significant predictors of recurrence. The log-rank test was used to compare survival curves. Means are reported as mean ± standard deviation (SD). Significance was established at the p < 0.05 level. Hazards ratios (HR) with 95% confidence intervals (CI) are reported as HR [lower 95% CI, upper 95% CI]. Adjusted correlation coefficients (R2) are reported for significant variables in regression analyses. All statistical analyses were preformed using SPSS (SPSS Inc., Chicago, IL).
3 Results
3.1 Demographics and tumor characteristics
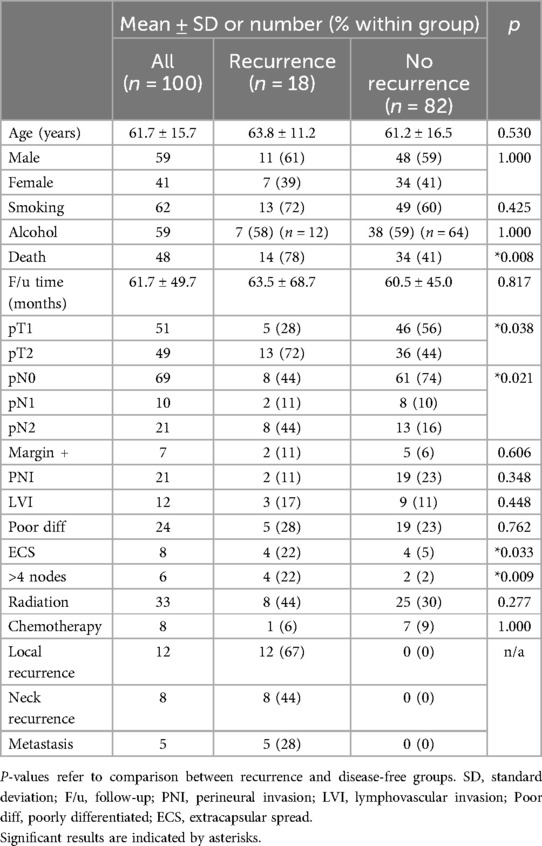
One hundred patients were included: 41 women and 59 men with a mean age of 61.7 ± 15.7 years (range 19–98 years). All patients underwent primary surgical therapy consisting of partial glossectomy and neck dissection. The mean follow-up time was 61.7 ± 49.7 months (range 1–261 months, median 45.8 months). Sixty-two patients were smokers and 59 were alcohol consumers. Fifty-one patients had pT1 disease, while 49 had pT2 disease. Sixty-nine were staged pN0, 10 patients were pN1, and 21 were pN2.
Overall, 18 patients developed recurrence, (12 local, 8 regional, 5 distant). Significantly fewer recurrences occurred in pT1 than pT2 patients (10% vs. 27%, p = 0.038), in pN0 than pN + patients (12% vs. 32%; p = 0.021), and in those without ECS (15% vs. 50%, p = 0.033). Of the pN + patients, those with ≤4 metastatic nodes developed significantly fewer recurrence (15%) vs. patients with >4 metastatic nodes (67%, p = 0.009). A summary of demographics and tumor characteristics is presented in Table 1.
3.2 Patients receiving radiation therapy and chemotherapy
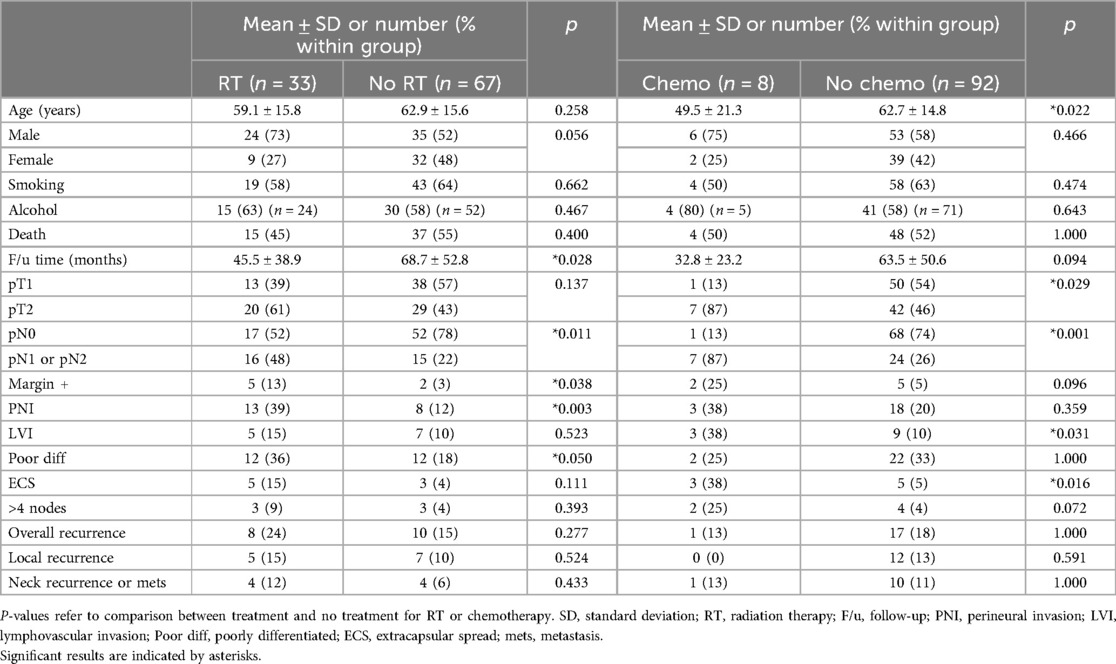
Of the 100 patients, 33 received adjuvant radiation therapy (RT) and 8 received both adjuvant RT and chemotherapy. Demographics and tumor characteristics of patients by adjuvant therapy are presented in Table 2.
Patients with generally worse tumor characteristics were more likely to receive RT. These characteristics included significantly more pN + disease (p = 0.011), positive margins (p = 0.038), PNI (p = 0.003), and poorly differentiated cancer (p = 0.050). Similarly, patients who received chemotherapy had significantly more pT2 disease (p = 0.029), pN + disease (p = 0.001), LVI (p = 0.031), and ECS (p = 0.016).
Local, regional, and overall recurrence rates were similar when patients with poor histopathologic factors were selected to receive post-operative RT and chemotherapy, except in the groupings of pT2, ECS, pN+, and >4 LN + . In these groups, despite RT or chemotherapy, recurrence rates were still higher than their comparative group.
3.3 Recurrence
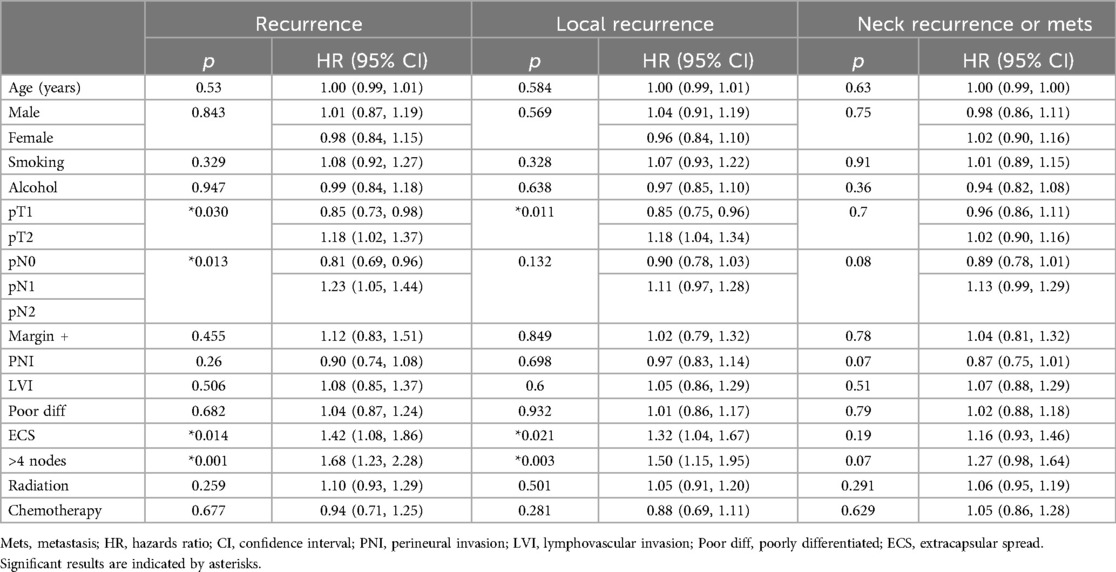
Univariate Cox regression showed that pT2, pN+, ECS, and >4 nodes were significant predictors of overall recurrence. For local recurrence, pT2, ECS, and >4 nodes were significant predictors. There were no significant predictors of neck recurrence or distant metastasis. P-values and hazards ratios for predictors of recurrence are presented in Table 3.
Multivariate Cox regression showed that >4 nodes was the only significant predictor of overall recurrence [HR 1.68 (1.23–2.28), p = 0.001]. The presence of >4 nodes [HR 1.14 (1.09, 1.85), p = 0.008] and pT2 [HR 1.15 (1.01, 1.30), p = 0.033] were the only significant predictors of local recurrence. Again, there were no significant predictors of neck recurrence or distant metastasis.
3.4 Survival
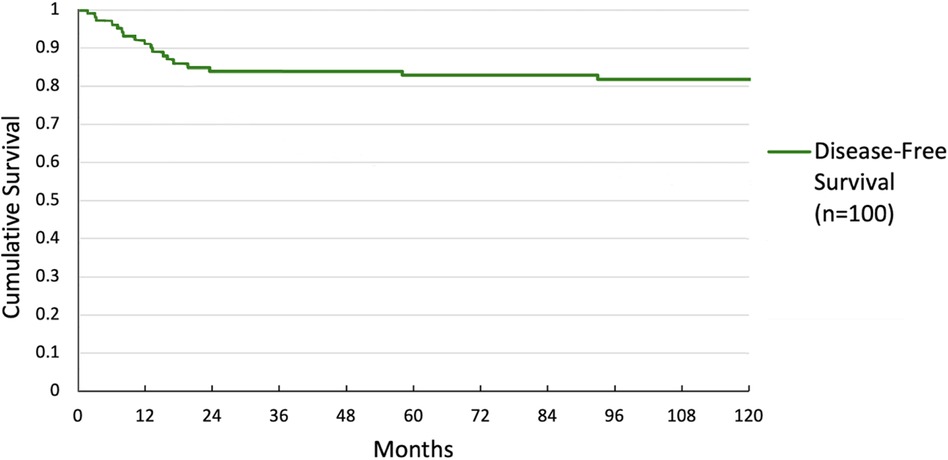
Overall survival was 59.6% at 5 years and 54.5% at 10 years. DFS was 82.8% at 5 years and 81.8% at 10 years. Overall and DFS are shown in Figures 1, 2.
Multivariate and univariate Cox regression showed significant differences in DFS for two groups. Kaplan-Meier curves were generated for these groupings. DFS for patients with ≤4 nodes or >4 nodes is shown in Figure 3. There was a significant difference in survival between the 2 groups (p < 0.001), with 5-year survival at 86% and 20%, respectively. DFS for patients with pT1 or pT2 disease is shown in Figure 4. Again, there was a significant difference in survival between the 2 groups (p = 0.037), with 5-year survival at 90% and 75%, respectively.
4 Discussion
The incidence of oral tongue cancers has been increasing in the United States (10–13). Treatment typically consists of primary surgery followed by adjuvant radiation or chemotherapy (14). Early T stage OTSCC generally has worse overall survival compared to other early T stage head and neck cancers (15). These tumors may be difficult to cure due to uncertainty about which histopathologic features are relevant for risk stratifying patients and predicting recurrence (9, 16–19). Patients experience treatment failure when they develop metastasis, tumor recurrence, or disease persistence despite completing treatment (20). Accurately identifying aggressive tumors and appropriately selecting adjuvant therapy decreases treatment failures (21, 22).
In the literature, recurrence free survival for early T stage OTSCC ranges from 75% to 89% (22–25). In this study, the recurrence rates were similar, ranging from 75% to 90% disease free survival at 5-year follow-up. At 10-year follow-up DFS was maintained at 90% for patients with T1 disease but decreased to 72.9% in patients with T2 disease. To predict which patients with low stage disease should undergo adjuvant therapy, researchers have attempted to identify risk factors for recurrence (6, 7, 26, 27). Suggested tumor features such as ECS, nodal metastasis, tumor size, PNI, and LVI have been associated with recurrence (7, 24, 26, 28–37).
In contrast to prior studies, we found no association between recurrence and margin positivity, PNI, LVI, or poor differentiation (6, 38–41). This difference is likely due to confounding from the pattern of adjuvant treatment use in our cohort. Patients with high-risk features, including positive margins, PNI, LVI, and poor differentiation, were more likely to receive adjuvant therapy (Table 2) causing the appearance that these factors were not associated with disease recurrence. Despite possessing more high-risk features on average, patients who received adjuvant therapy had similar survival to those who did not, suggesting that multimodal treatments were able to minimize the adverse effects of these features.
Increased T stage, increased N stage, presence of >4 metastatic nodes, and ECS at the time of treatment were significantly associated with a higher risk for local recurrence by univariate regression (Table 3). Our Kaplan-Meier curves also showed significantly worse survival for T2 vs. T1–consistent with prior reports showing 5-year overall survival of 73% for T1% and 64% for T2–and for patients with >4 nodes vs. ≤4 nodes (42). Patients with ECS and T2 were more likely to receive chemotherapy than patients without these features, but they did not receive radiation at higher rates. Furthermore, the absolute number of patients receiving chemotherapy was low in both groups (3/8 for ECS and 7/49 for T2). Previous work has shown that radiation therapy can improve outcomes in patients with ECS (9). Since both these features were predictors of recurrence and T2 was also a predictor for survival, these groups may have benefitted from more aggressive adjuvant therapy. Similarly, while patients with higher N stage were more likely than those with N0 to receive radiation and chemotherapy, the absolute number treated was likely not high enough to significantly reduce recurrence in this group. Finally, patients with >4 positive nodes, which was the only feature predictive of recurrence on both univariate and multivariate analysis, were not more likely to receive either adjuvant therapy. While features such positive margins, PNI, and ECS are commonly considered when selecting patients for adjuvant therapy, our results suggest that the number of positive lymph nodes may be overlooked (6, 38–41). Given the strength of this feature to predict recurrence and survival, the presence of multiple positive lymph nodes alone may be important to consider when selecting patients for adjuvant therapy.
This study is limited by its retrospective nature and confounding from the use of adjuvant radiation and chemotherapy in patients with high-risk histopathologic features, possibly masking the effects of these features. Our long follow-up time also made it unfeasible to utilize the most up-to-date staging system. In the AJCC 8th edition staging system, depth of invasion (DOI) is now included in the T classification. Therefore, it is possibly that some of the tumors we assessed may have been upstaged to T3 or T4 using the 8th edition. The DOI data for the earliest patients in this cohort was not detailed enough to restage them based on the 8th edition, so the 7th edition was used to ensure internal validity. Despite these limitations, this study provides long-term outcomes for patients with T1 and T2 OTSCC and helps identify high-risk histopathologic features to guide adjuvant treatment plans. In many studies data for follow-up stops after 5 years, but this study presents data up to10 years following diagnosis. Future large, multi-institutional, randomized controlled trials would further elucidate the impact of certain risk factors on recurrence and survival, increasing clinicians’ ability to risk stratify patients.
5 Conclusions
Even in early T stage OTSCC, post-operative adjuvant treatment in patients with high-risk histopathologic features can allow for equivocal survival when compared to lower risk patients. Our study identified pN1 and pN2, pT2, ECS, and multiple nodes as univariate risk factors for recurrence and multiple nodes as the only significant risk factor on multivariate analysis. This suggests that these factors should be considered when selecting patients for adjuvant treatment. Furthermore, the number of involved nodes may serve as an independent indicator for adjuvant treatment.
Data availability statement
The datasets presented in this article are not readily available because the IRB protocols at our institution restrict sharing of patient data. Requests to access the datasets should be directed toamlubWFuQGxsdS5lZHU=.
Ethics statement
The studies involving humans were approved by Loma Linda University Medical Center IRB (Approval #5150330). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this study was retrospective. As a result, all included patients did not have modifications to their treatment, as the investigation was conducted from historical records. Furthermore, no patient identifiers are publicly available from this research.
Author contributions
BD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. NP: Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. YL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Writing – review & editing. JI: Conceptualization, Methodology, Project administration, Software, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74(3):229–63. doi: 10.3322/caac.21834
2. Rohan V SV. Cervical node metastasis in T1 squamous cell carcinoma of oral tongue- pattern and the predictive factors. Indian J Surg Oncol. (2014) 5(2):104–8. doi: 10.1007/s13193-014-0301-z
3. Nithya C, Pandey M, Naik B, Ahamed IM. Patterns of cervical metastasis from carcinoma of the oral tongue. World J Surg Oncol. (2003) 1(1):10. doi: 10.1186/1477-7819-1-10
4. Ho CM, Lam KH, Wei WI, Lau SK, Lam LK. Occult lymph node metastasis in small oral tongue cancers. Head Neck. (1992) 14(5):359–63. doi: 10.1002/hed.2880140504
5. Deo S, Singh V, Mokkapati PR, Shukla NK, Dwivedi SN, Sharma A, et al. Clinical spectrum, pattern, and level-wise nodal involvement among oral squamous cell carcinoma patients–audit of 945 oral cancer patient data. Indian J Surg Oncol. (2020) 11(1):86–91. doi: 10.1007/s13193-019-01011-7
6. Martínez-Flores R, Gómez-Soto B, Lozano-Burgos C, Niklander SE, Lopes MA, González-Arriagada WA. Perineural invasion predicts poor survival and cervical lymph node metastasis in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. (2023) 28(5):e496–503. doi: 10.4317/medoral.25916
7. Kligerman MP, Moon PK, Tusty M, Cloutier JM, Ma Y, Holsinger CF, et al. Impact of histologic risk factors on recurrence rates for oral cavity squamous cell carcinoma. Ann Otol Rhinol Laryngol. (2023) 132(7):731–7. doi: 10.1177/00034894221111223
8. Mani C, Lakshminarayana G, Kurian A, Annapurneshwari A. Predictors of recurrence in early stage oral tongue squamous cell carcinoma. J Orofac Sci. (2015) 7:86–9. doi: 10.4103/0975-8844.169753
9. Thiagarajan S, Nair S, Nair D, Chaturvedi P, Kane SV, Agarwal JP, et al. Predictors of prognosis for squamous cell carcinoma of oral tongue. J Surg Oncol. (2014) 109(7):639–44. doi: 10.1002/jso.23583
10. Burus T, Damgacioglu H, Huang B, Christian WJ, Hull PC, Ellis AR, et al. Trends in oral tongue cancer incidence in the US. JAMA Otolaryngol Head Neck Surg. (2024) 150(5):436–43. doi: 10.1001/jamaoto.2024.0301
11. Tota JE, Anderson WF, Coffey C, Califano J, Cozen W, Ferris RL, et al. Rising incidence of oral tongue cancer among white men and women in the United States, 1973–2012. Oral Oncol. (2017) 67:146–52. doi: 10.1016/j.oraloncology.2017.02.019
12. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. (2016) 154(6):1034–40. doi: 10.1177/0194599816633690
13. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. (2016) 91(3):386–96. doi: 10.1016/j.mayocp.2015.12.017
14. Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2020) 18(7):873–98. doi: 10.6004/jnccn.2020.0031
15. Rusthoven K, Ballonoff A, Raben D, Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer. (2008) 112(2):345–51. doi: 10.1002/cncr.23183
16. Verde-Sánchez L, Capote AL, Sanz-García A, Brabyn P, Rodríguez-Campo FJ, Naval Gías L. Prognostic involvement of lymph node density in oral squamous cell carcinoma. A new predictive model. J Oral Maxillofac Surg. (2023) 81(3):358–69. doi: 10.1016/j.joms.2022.11.012
17. Subramaniam N, Balasubramanian D, Low T-HH, Murthy S, Clark JR, Thankappan K, et al. Factors affecting survival in surgically salvaged locoregional recurrences of squamous cell carcinoma of the tongue. J Oral Maxillofac Surg. (2018) 76(5):1133.e1–.e6. doi: 10.1016/j.joms.2017.12.029
18. Sinha N, Rigby MH, McNeil ML, Taylor SM, Trites JB, Hart RD, et al. The histologic risk model is a useful and inexpensive tool to assess risk of recurrence and death in stage I or II squamous cell carcinoma of tongue and floor of mouth. Mod Pathol. (2018) 31(5):772–9. doi: 10.1038/modpathol.2017.183
19. Almangush A, Heikkinen I, Mäkitie AA, Coletta RD, Läärä E, Leivo I, et al. Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer. (2017) 117(6):856–66. doi: 10.1038/bjc.2017.244
20. Hoşal AS, Unal OF, Ayhan A. Possible prognostic value of histopathologic parameters in patients with carcinoma of the oral tongue. Eur Arch Otorhinolaryngol. (1998) 255(4):216–9. doi: 10.1007/s004050050046
21. Chen TC, Wang CT, Ko JY, Lou PJ, Yang TL, Ting LL, et al. Postoperative radiotherapy for primary early oral tongue cancer with pathologic N1 neck. Head Neck. (2010) 32(5):555–61. doi: 10.1002/hed.21217
22. Garzino-Demo P, Zavattero E, Franco P, Fasolis M, Tanteri G, Mettus A, et al. Parameters and outcomes in 525 patients operated on for oral squamous cell carcinoma. J Craniomaxillofac Surg. (2016) 44(9):1414–21. doi: 10.1016/j.jcms.2016.06.007
23. Kamat M, Rai BD, Puranik RS, Datar UV. A comprehensive review of surgical margin in oral squamous cell carcinoma highlighting the significance of tumor-free surgical margins. J Cancer Res Ther. (2019) 15(3):449–54. doi: 10.4103/jcrt.JCRT_273_17
24. Ganly I, Goldstein D, Carlson DL, Patel SG, O'Sullivan B, Lee N, et al. Long-term regional control and survival in patients with low-risk, early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. (2013) 119(6):1168–76. doi: 10.1002/cncr.27872
25. Zelefsky MJ, Gaynor J, Kraus D, Strong EW, Shah JP, Harrison LB. Long-term subjective functional outcome of surgery plus postoperative radiotherapy for advanced stage oral cavity and oropharyngeal carcinoma. Am J Surg. (1996) 171(2):258–62. doi: 10.1016/s0002-9610(97)89563-3
26. Larson AR, Kemmer J, Formeister E, El-Sayed I, Ha P, George J, et al. Beyond depth of invasion: adverse pathologic tumor features in early oral tongue squamous cell carcinoma. Laryngoscope. (2020) 130(7):1715–20. doi: 10.1002/lary.28241
27. Yanamoto S, Yamada S, Takahashi H, Kawasaki G, Ikeda H, Shiraishi T, et al. Predictors of locoregional recurrence in T1-2N0 tongue cancer patients. Pathol Oncol Res. (2013) 19(4):795–803. doi: 10.1007/s12253-013-9646-9
28. Yang X, Tian X, Wu K, Liu W, Li S, Zhang Z, et al. Prognostic impact of perineural invasion in early stage oral tongue squamous cell carcinoma: results from a prospective randomized trial. Surg Oncol. (2018) 27(2):123–8. doi: 10.1016/j.suronc.2018.02.005
29. Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. (2005) 29(2):167–78. doi: 10.1097/01.pas.0000149687.90710.21
30. Dik EA, Willems SM, Ipenburg NA, Adriaansens SO, Rosenber AJWP, van Es RJJ. Resection of early oral squamous cell carcinoma with positive or close margins: relevance of adjuvant treatment in relation to local recurrence: margins of 3 mm as safe as 5 mm. Oral Oncol. (2014) 50(6):611–5. doi: 10.1016/j.oraloncology.2014.02.014
31. Dan TD, Raben D, Schneider CJ, Hockstein NG, Witt RL, Dzeda M, et al. Freedom from local and regional failure of contralateral neck with ipsilateral neck radiotherapy for node-positive tonsil cancer: updated results of an institutional clinical management approach. Oral Oncol. (2015) 51(6):616–21. doi: 10.1016/j.oraloncology.2015.03.013
32. Ogawa T, Matsuura K, Shiga K, Tateda M, Katagiri K, Kato K, et al. Surgical treatment is recommended for advanced oral squamous cell carcinoma. Tohoku J Exp Med. (2011) 223(1):17–25. doi: 10.1620/tjem.223.17
33. Arduino PG, Carrozzo M, Chiecchio A, Broccoletti R, Trione F, Borra E, et al. Clinical and histopathologic independent prognostic factors in oral squamous cell carcinoma: a retrospective study of 334 cases. J Oral Maxillofac Surg. (2008) 66(8):1570–9. doi: 10.1016/j.joms.2007.12.024
34. Lim SC, Zhang S, Ishii G, Endoh Y, Kodama K, Miyamoto S, et al. Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin Cancer Res. (2004) 10(1 Pt 1):166–72. doi: 10.1158/1078-0432.ccr-0533-3
35. El-Mofty SK. Histopathologic risk factors in oral and oropharyngeal squamous cell carcinoma variants: an update with special reference to HPV-related carcinomas. Med Oral Patol Oral Cir Bucal. (2014) 19(4):e377–85. doi: 10.4317/medoral.20184
36. Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (II). Oral Oncol. (2010) 46(9):636–43. doi: 10.1016/j.oraloncology.2010.06.008
37. Troeltzsch M, Knösel T, Eichinger C, Probst F, Troeltzsch M, Woodlock T, et al. Clinicopathologic features of oral squamous cell carcinoma: do they vary in different age groups? J Oral Maxillofac Surg. (2014) 72(7):1291–300. doi: 10.1016/j.joms.2014.01.009
38. Zhang L, Judd RT, Zhao S, Rygalski C, Li M, Briody A, et al. Immediate resection of positive margins improves local control in oral tongue cancer. Oral Oncol. (2023) 141:106402. doi: 10.1016/j.oraloncology.2023.106402
39. Matsuo K, Akiba J, Kusukawa J, Yano H. Squamous cell carcinoma of the tongue: subtypes and morphological features affecting prognosis. Am J Physiol Cell Physiol. (2022) 323(6):C1611–23. doi: 10.1152/ajpcell.00098.2022
40. Comer JC, Harris AB, Hess AO, Hitchcock KE, Mendenhall WM, Bates JE, et al. Does lymphovascular invasion predict survival in oral cancer? A population-based analysis. Oral Oncol. (2023) 140:106387. doi: 10.1016/j.oraloncology.2023.106387
41. Mathur R, Malik A, Mishra A, Mair M, Singh AG, Singhavi HR, et al. Prognostic impact of poor differentiation of squamous cell carcinoma in treatment naïve node-negative early oral cancers. Indian J Surg Oncol. (2023) 14(2):524–30. doi: 10.1007/s13193-023-01712-0
Keywords: head and neck, recurrence, squamous cell carcinoma, radiotherapy, cancer
Citation: Damazo BJ, Punjabi NA, Liu YF and Inman JC (2024) Histopathologic predictors of recurrence and survival in early T stage oral tongue squamous cell carcinoma. Front. Oral. Health 5:1426709. doi: 10.3389/froh.2024.1426709
Received: 2 May 2024; Accepted: 25 July 2024;
Published: 6 August 2024.
Edited by:
Sven Eric Niklander, Universidad Andrés Bello, ChileReviewed by:
Emil Dediol, University of Zagreb, CroatiaRené Andrés Martinez-Flores, Universidad Andrés Bello, Chile
© 2024 Damazo, Punjabi, Liu and Inman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jared C. Inman, amlubWFuQGxsdS5lZHU=
 Benjamin J. Damazo1
Benjamin J. Damazo1 Nihal A. Punjabi
Nihal A. Punjabi