- 1Center for Global Health Research, Saveetha Medical College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Chennai, India
- 2Department of Clinical Sciences, College of Dentistry, Centre of Medical and Bio-Allied Health Sciences and Research, Ajman University, Ajman, United Arab Emirates
Chronic periodontitis is a ubiquitous inflammatory disease in dental healthcare that is challenging to treat due to its impact on bone and tooth loss. Conventional mechanical debridement has been challenging in eliminating complex subgingival biofilms. Hence, adjunctive approaches like low-level laser antimicrobial photodynamic therapy (A-PDT) utilising methylene blue (MB) have been emerging approaches in recent times. This review evaluates the latest research on the use of MB-mediated A-PDT to decrease microbial count and enhance clinical results in chronic periodontitis. Studies have shown the interaction between laser light and MB generates a phototoxic effect thereby, eliminating pathogenic bacteria within periodontal pockets. Moreover, numerous clinical trials have shown that A-PDT using MB can reduce probing depths, improve clinical attachment levels, and decrease bleeding during probing in comparison to traditional treatment approaches. Notably, A-PDT shows superior antibiotic resistance compared to conventional antibiotic treatments. In conclusion, the A-PDT using MB shows promise as an adjunctive treatment for chronic periodontitis. Additional research is required to standardize treatment protocols and assess long-term outcomes of A-PDT with MB in the treatment of periodontitis.
Introduction
Chronic periodontitis, an endemic inflammatory condition of the gums observed within the field of dental healthcare; poses an alarming challenge due to intricate involvement in bone and tooth loss, thus causing several diseases such as pneumonia, cancer, cardiovascular diseases, autoimmune diseases, etc (1). Chronic periodontitis has a significant spread in the worldwide population, with estimates ranging from 20% to 50%, indicating its widespread prevalence as a disease (2). The primary cause is the accumulation of complex polymicrobial biofilms in the subgingival pockets surrounding the teeth. These plaque colonies adhere strongly and provoke a chronic immunoinflammatory response, leading to the elimination of the connective tissue, periodontal ligament cementum and alveolar bone (3). Conventional approaches have relied largely on mechanical disruption and physical removal of accessible biofilms through scaling, root planing and surgery. The presence of bacteria in biofilms poses significant challenges to completely eradicating them, thereby leading to disease recurrence in a significant number of patients (4).
However, there has been a considerable surge in exploring adjunctive treatments, and one such innovative method gaining traction is low-level laser-activated photodynamic therapy utilizing methylene blue (MB) (5). Low-level laser treatment promotes the proliferation of adenosine triphosphate at the mitochondrial level (6). A laser emits a specific wavelength of light to increase reactive oxygen species (ROS) production through photosensitizer (PS). This treatment is considered a safe and impressive therapy since it has no long-term side effects (7). The dominant used PS in dentistry is Photofrin, but in recent times, MB has been considerably utilized as PS. Antimicrobial photodynamic therapy (A-PDT) uses light to activate MB, which produces ROS and damages bacterial cells (8). This process oxidizes various cell components, resulting in microbicidal effects against periodontal pathogens. A-PDT has multiple advantages, including its ability to suppress bactericidal action against a wide range of bacteria, penetrate biofilms, promote microbial cytotoxicity, enhance wound healing, and induce tissue remodelling (9, 10). The wavelengths at which MB absorbs most intensely are approximately 660 nm, which is within the red laser emission range. In other words, cheap diode lasers can be used to activate it. Additional research is required to develop MB-mediated A-PDT protocols that specifically focus on effective treatment outcomes and managing infection in various oral conditions such as chronic periodontitis (11).
Periodontal diseases are typically triggered by anaerobic bacteria like Porphyromonas gingivalis (P.gingivalis), Actinobacillus actinomycetemcomitans (A. actinomycetemcomitans) and Tannerella forsythia (T. forsythia) P. gingivalis, which is widely recognized as the primary causative agent in chronic periodontitis among adult individuals. The A. actinomycetemcomitans is commonly considered the primary pathogen in cases of aggressive periodontitis (12). The robust in vitro findings emphasise MB-mediated laser-activated A-PDT potent bactericidal efficacy against these anaerobic gram-negative bacteria even at short exposure times. Unlike antibodies, MB-mediated A-PDT has the competence to eliminate bacterial cells within intact biofilms. Hence, A-PDT techniques exhibit the promise of eliminating residual subgingival infection unreachable by mechanical instrumentation alone (13). Beyond bacterial cytotoxicity, another research on the anti-inflammatory effects of MB photoactivation suppresses inflammatory markers and mediators—including cytokines, chemokines, TNF-α, IL-1β, PGE2, and nitric oxide (14). Such immuno-modulatory properties can facilitate the resolution of inflammation in diseased periodontal tissues. Furthermore, studies have also emphasized A-PDT ability to improve clinical outcomes like reduced probing depths and bone loss in vivo (15). However, researchers have evaluated the clinical usefulness of MB as a complementary treatment to conventional mechanical debridement for managing chronic periodontitis in patients due to their accumulated understanding of MB multifactorial therapeutic effects. Initial systematic reviews of these clinical studies emphasize the significant potential benefits of reducing microbial load and improving clinical parameters over extended periods, with minimal adverse effects (16). Therefore, this review aims to analyze the existing evidence regarding laser-activated A-PDT using MB against periodontal pathogens, particularly those related to chronic periodontitis. It evaluates the mechanisms, existing research outcomes, and discusses key parameters to enhance the delivery of this emerging adjunct therapy, and its potential in combating chronic periodontitis.
Fundamental mechanism underlying the photodynamic therapy
A PS, a light source, and oxygen in the tissue constitute the basic elements of PDT. PS is a nontoxic dye that can be selectively absorbed and retained in specific cells. The common PS used during PDT treatment are porphyrins, chlorins, dyes such as MB and toluidine. PDT is based on the activation of PS upon exposure to a specific wavelength of light. This activation involves transitioning the PS from a low-energy ground state (S0) to an excited singlet state (S1). The S1 state is transient and not very stable but can undergo intersystem crossing to become a more stable and longer-lasting excited triplet state. In order to retain stability, the S1 state can revert back to the ground state through fluorescence or internal conversion, which results in the loss of energy. Alternatively, it can also transform into an excited triplet state (S3) by altering the electron spin. The S3 state initiates two pathways influenced by oxygen. These pathways involve the interaction of the triplet state (T) with oxygen in the surrounding tissue, leading to the production of highly ROS like singlet oxygen and free radicals. The process is depicted in Figure 1 (17–19). Molecules in the T state have the capability of emitting light, which is known as phosphorescence. This occurs either by returning to ground states or by undergoing additional reactions through type 1 and type 2 pathways. Radical ions, such as superoxide, hydroxyl, and lipid-derived radicals, are produced through electron transfer from PS to substrate in the type 1 pathway. Furthermore, the type 2 pathways involve the transfer of energy from the PS triplet to the ground state, resulting in the production of singlet oxygen and the oxidation of various biomolecules such as proteins, nucleic acids, or lipids. This leads to the production of cytotoxic effects (20–22).
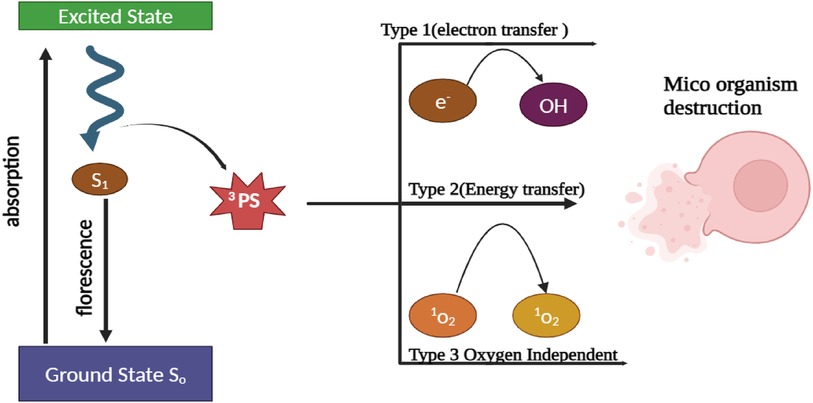
Figure 1. Schematic illustration depicting the mechanism of bacterial cell wall destruction through the use of A-PDT therapy.
Optimization of laser-activated A-PDT treatment
The key factor involved in determining the outcomes of A-PDT is defining optimal activation protocols that provide a targeted bactericidal effect while minimizing damage to surrounding tissues. Thus, the laser and PS have become significant factors for A-PDT therapy. In the case of MB-mediated A-PDT, the maximum adsorption peak was observed at 660–670 nm using low-level red lasers. Meanwhile, the diode lasers provide high-intensity monochromatic light with precision fibre-optic delivery; allowing controlled irradiation adapted to periodontal pocket topography (22). The diode family, Er,Cr:YSGG, and Nd:YAG lasers (635 nm – 2,780 nm) effectively remove debris and bacteria from root canals, using optical fibers (200 μm – 200 mm), power output (40 mW – 1.5 W), and irradiation duration (140 μs – 3 mins). For photodynamic therapy, methylene blue and toluidine blue (1–5 mins application) are utilized. Diode lasers (810 or 980 nm) with anti-bactericidal properties can reach the periapical region (23). Research on A-PDT utilizing EmunDo and diode laser against the dental pathogen Lactobacillus acidophilus, which affects the cavity. The results showed that by using the EmunDo and Diode laser systems, a considerable reduction in colonies was achieved (24). In addition to considering laser parameters, the A-PDT is also affected by other important factors including power density (irradiance), exposure time per site, and beam mode/diameter. These variables collectively contribute to the energy density (radiant exposure), which plays a crucial role in determining the phototoxic effect and overall treatment success. Toluidine blue O (TBO) activated by red LED light was found to inhibit cariogenic biofilms in an in vitro study. Biofilm analysis and cytotoxicity tests were used to identify the ideal parameters. It was found that while TBO at concentrations of 50 and 100 μg/ml preserved cell viability, higher concentrations prevented the formation of biofilms. The study stresses the importance of optimizing TBO concentration and light energy for better biofilm elimination (25). Likewise, the study involved 30 subjects: 15 patients received retreatment of the root canal in combination with 980 nm diode laser irradiation and 15 were given placebos. For each sample in a pulsing mode, the power output shall be 20 s at 1.5 W and 100 Hz. The diode laser significantly improved healing at 3 to 12 months follow-up (P < 0.05) and had 45% more healed cases than the placebo (26).
Current and alternative treatments for chronic periodontitis
The conventional treatment for chronic periodontitis implies mechanical disruption and eliminating the bacterial biofilms, through scaling and root planing. This non-surgical treatment utilizes numerous specialized instruments to mechanically debride plaque and calculus deposits from the gum line. As an adjunct, antimicrobial mouth rinses may be prescribed. However, limitations in eliminating complex residual biofilms can drive recolonization (27). General antibiotics such as amoxicillin and metronidazole in combination with Scale root planing (SRP) have been extensively evaluated to improve microbial control; long-term usage of these antibiotics risks antibiotic resistance in non-target commensal bacteria. The potential adjuvant interventions like controlled-release vehicles, antibiotics embedded in bone grafts, PS dyes, host modulatory agents, probiotics and laser/phototherapy have been investigated for minimized spread of antimicrobial-resistant genes. Nevertheless, each approach has its inherent limitations and currently restricts widespread adoption (9).
Non-pharmacological adjuncts like ultrasonic devices, ozone therapy, and air-polishing with glycine powder show some improvement but lack strong clinical evidence (28). A study on the treatment of type 2 diabetes (T2D) and periodontitis discovered that a combination of antibiotics and SRP was successful in reducing HbA1c levels and decreasing probing pocket depth (PPD) after 3 months. However, after 6 months, only SRP was found to effectively lower HbA1c levels. Both SRP and antibiotics showed significant reductions in PPD. As a result, it is advisable to consistently utilize SRP for patients with T2D and periodontitis, as systemic antibiotics only provide temporary benefits (29). A study conducted on 58 patients with chronic periodontitis revealed that all groups, which underwent various treatments, demonstrated significant reductions in probing depth, clinical attachment loss, and bleeding on probing after 6 and 12 weeks. Furthermore, the addition of PDT treatment to scaling and root planing resulted in a significant decrease in bleeding in 5 mm probing depth pockets after 12 weeks (30). Similarly, another study was conducted to investigate the effectiveness of additional treatment methods for apical periodontitis in permanent teeth. The study evaluated several treatments, such as A-PDT, laser canal irradiation, ozone therapy, and ultrasonic irrigation. The results indicated that there was no statistically significant difference in healing rates or pain prevalence between the group receiving adjunctive treatment and the control group (31). In conclusion, the current guidelines still recommend SRP as the primary method of mechanical debridement. The use of additional measures, such as locally delivered antimicrobials, should only be considered for specific cases in which non-responsive sites pose significant treatment challenges. Therefore, there is a need to develop novel strategies that target biofilms specifically, offering improved and long-lasting effectiveness as well as safety advantages.
Non-invasive A-PDT therapy for the treatment of periodontitis: an overview
A-PDT utilizes lasers and PS agents to eliminate bacteria associated with periodontitis. It is extremely effective in eradicating bacteria, while also having minimal toxicity and being cost-effective. Various PS, including MB, toluidine blue, indocyanine green, malachite green, erythrosine dyes, rose bengal, the radiochlorine group, and curcumin, may be safely used with different light sources without causing any harm to the patient (32). A study was conducted to investigate the effects of A-PDT in patients who were diagnosed with T2D and periodontitis. The results, observed at 90 and 180 days, showed a significant reduction in pocket depth, bleeding on probing, and the number of remaining pockets (33). Researchers compared A-PDT and antibiotics with non-surgical treatment for aggressive periodontitis. The results indicate that the use of both A-PDT and antibiotics as adjuncts is effective. Both approaches also showed long-term improvements in periodontal parameters (34). The research was conducted to test the potency of A-PDT when combined with laser application against a specific periodontal pathogen, A. actinomycetemcomitans. The findings of the study indicated that using a wavelength of 940 nm, along with PS-dimethyl phenothiazine chloride, and irradiating with a diode laser for 30 s at a power of 5 W, resulted in a significant reduction of A. actinomycetemcomitans in vitro (35). Thirty-six patients with periodontal disease were randomly assigned to either the intervention or control group. Prior to treatment, all participants underwent scaling and root planning. Evaluations were conducted at three intervals, which included microbiological and clinical examinations. Laser therapy was administered using two PS solutions. The results of the study indicated that there were no significant differences between the groups in terms of reduction in bacteria levels and testing depths (36).
Properties favouring methylene blue as an A-PDT photosensitizer
MB is a hydrophilic phenothiazinium cationic dye that has the ability to easily penetrate bacterial cell walls. MB-mediated antimicrobial A-PDT boosts neutrophil adhesion while inhibiting phagocytic activity (37). It possesses the ideal combination of a high quantum yield and excellent chemical stability necessary for effective photodynamic action. The positive charge of MB enhances its ability to adhere to negatively charged microbial cell surfaces and facilitates specific uptake and photosensitization (38). In addition to its direct antimicrobial properties, research indicates that the activation of MB via A-PDT can also trigger secondary local responses that are dependent on oxygen. These responses are beneficial for the healing of periodontal tissues and supporting the immune system (39). The absorption spectrum of MB exhibits a peak at approximately 660 nm, aligning with the emission range of affordable diode lasers. This alignment enables sufficient tissue penetration and photodynamic activation within periodontal pockets using cost-effective and small-sized light sources. Consequently, this ease of clinical implementation enables the effective utilization of this approach. Using these PS, A-PDT can reduce the number of harmful bacteria in periodontitis. It can also be combined with other treatments (40).
The various laser wavelengths and PS for A-PDT treatments are mentioned in Table 1. The effects of A-PDT therapy on patients with grade C periodontitis were examined in this study. Two groups of sixty-four teeth, with one receiving A-PDT therapy during SRP and the other receiving a placebo. In comparison to the control group, the three-month results for the A-PDT therapy group indicated a reduction in probing depth and an increase in clinical attachment. These results imply that A-PDT therapy might be helpful in the management of periodontitis (50). Similarly, A-PDT therapy improves clinical outcomes for people with T2D, according to an analysis of 11 studies. The study showed that when compared to receiving standard periodontal treatment, using A-PDT therapy led to more notable reductions in bleeding on probing and probing depth at 3 and 6 months (51).
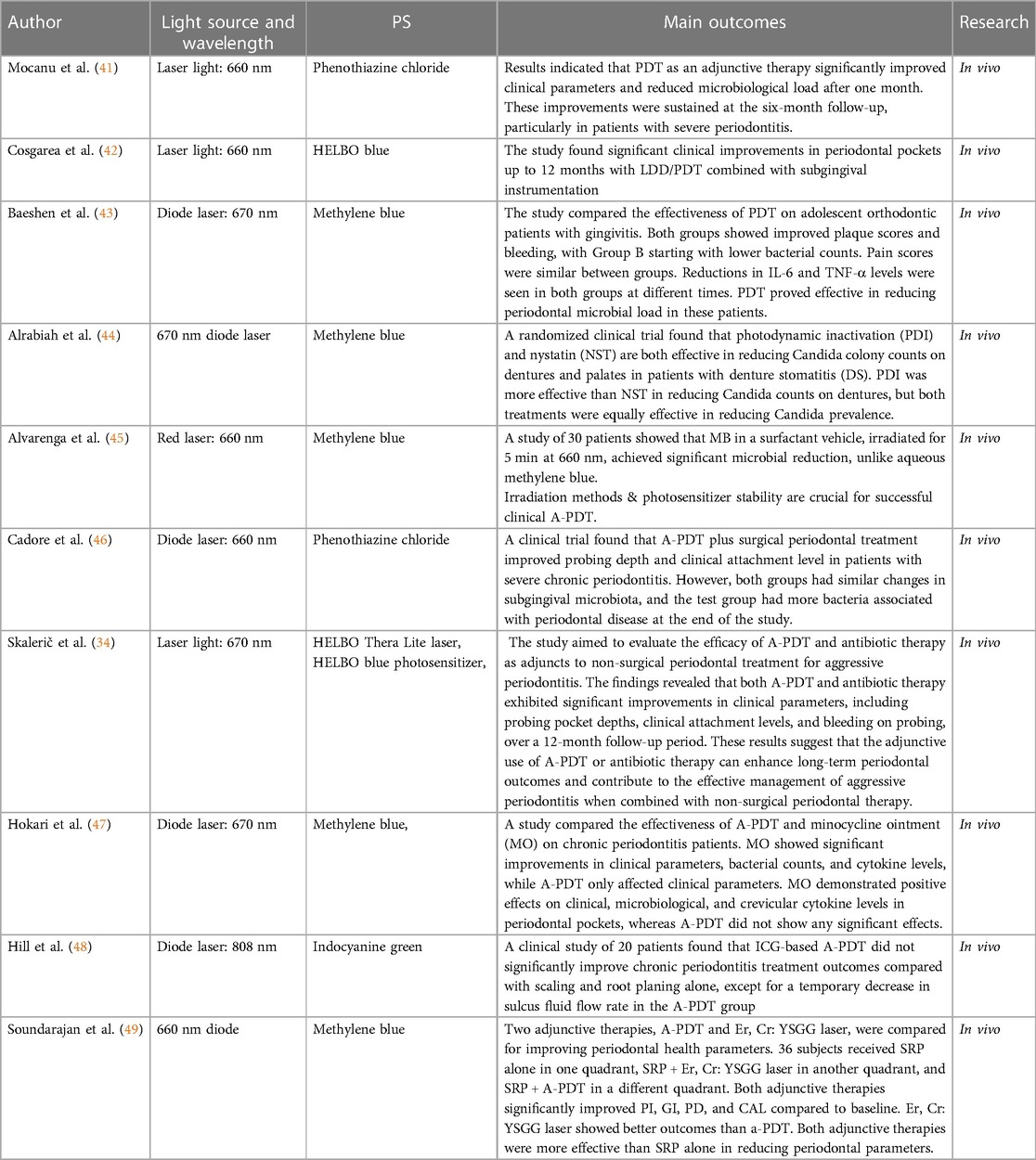
Table 1. The parameters of laser irradiation and the outcomes observed in antimicrobial photodynamic therapy (A-PDT).
Conclusion
Chronic periodontitis poses a major threat to global health due to its involvement in systemic diseases and tooth loss pathogenesis. Complex residual biofilms are beyond the scope of conventional mechanical debridement techniques. This has led to the investigation of novel adjunctive strategies, including A-PDT, which uses MB as a PS. To evaluate the bactericidal efficacy of MB-mediated laser-activated A-PDT against important anaerobic periodontal pathogens. Additionally, research has revealed that when MB is activated, cytotoxic ROS are produced. These species enter biofilms and destroy bacteria, as well as trigger favourable immunomodulatory and wound-healing reactions. Based on clinical trials, this adjunctive approach offers numerous advantages including reduced bleeding and probing depths, as well as increased levels of attachment. A-PDT is a potential intervention with multiple benefits including its low cost, safety profile, and ease of use when combined with inexpensive red lasers. Moreover, future research should aim to refine treatment protocols, investigate complementary therapies, and conduct randomized controlled trials to confirm the effectiveness of MB-mediated A-PDT therapy for chronic periodontitis.
Author contributions
MK: Writing – original draft. RR: Conceptualization, Writing – review & editing. PM: Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank the Center of Medical and Bio-allied Health Sciences and Research, Ajman University, Ajman, UAE.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kavarthapu A, Gurumoorthy K. Linking chronic periodontitis and oral cancer: a review. Oral Oncol. (2021) 121:105375. doi: 10.1016/j.oraloncology.2021.105375
2. Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. (2017) 11:72–80. 28539867, PMCID: PMC5426403
3. Gasner NS, Schure RS. Periodontal disease. In: Cieplik F, editor. StatPearls. Treasure Island, FL: StatPearls Publishing (2023). p. 554590. Available online from: http://www.ncbi.nlm.nih.gov/books/NBK554590/ (Accessed January 20, 2024).
4. Li X, Liu Y, Yang X, Li C, Song Z. The oral microbiota: community composition, influencing factors, pathogenesis, and interventions. Front Microbiol. (2022) 13:895537. doi: 10.3389/fmicb.2022.895537
5. Mendes RJS, de Sousa NM, Furtado GS, Paschoal MAB, Lago ADN. Association of papacarie duo® and low-level laser in antimicrobial photodynamic therapy (PDT). Lasers Med Sci. (2024) 39:25. doi: 10.1007/s10103-024-03981-9
6. Tionardus M, Setiawatie EM. Photosensitizer in periodontal antimicrobial photodynamic therapy: a literature review. AIP Conf Proc. (2020) 2314:060017. doi: 10.1063/5.0034788
7. Stájer A, Kajári S, Gajdács M, Musah-Eroje A, Baráth Z. Utility of photodynamic therapy in dentistry: current concepts. Dent J. (2020) 8:43. doi: 10.3390/dj8020043
8. Lima E, Reis LV. Photodynamic therapy: from the basics to the current progress of N-heterocyclic-bearing dyes as effective photosensitizers. Molecules. (2023) 28:5092. doi: 10.3390/molecules28135092
9. Kumar V, Yasmeen N, Pandey A, Ahmad Chaudhary A, Alawam AS, Ahmad Rudayni H, et al. Antibiotic adjuvants: synergistic tool to combat multi-drug resistant pathogens. Front Cell Infect Microbiol. (2023) 13:1293633. doi: 10.3389/fcimb.2023.1293633
10. Pancu DF, Scurtu A, Macasoi IG, Marti D, Mioc M, Soica C, et al. Antibiotics: conventional therapy and natural compounds with antibacterial activity—a pharmaco-toxicological screening. Antibiotics. (2021) 10:401. doi: 10.3390/antibiotics10040401
11. Zhang H, Xu L, Gu X, Yu D, Li S. Amphiphilic di-cationic methylene blue for improving antibacterial photodynamic efficiency through high accumulation and low aggregation on bacterial cell surfaces. RSC Adv. (2023) 13:239–50. doi: 10.1039/D2RA06484G
12. Gholami L, Shahabi S, Jazaeri M, Hadilou M, Fekrazad R. Clinical applications of antimicrobial photodynamic therapy in dentistry. Front Microbiol. (2023) 13:1020995. doi: 10.3389/fmicb.2022.1020995
13. Glowacka-Sobotta A, Ziental D, Czarczynska-Goslinska B, Michalak M, Wysocki M, Güzel E, et al. Nanotechnology for dentistry: prospects and applications. Nanomaterials. (2023) 13:2130. doi: 10.3390/nano13142130
14. Hussein H, Kishen A. Local immunomodulatory effects of intracanal medications in apical periodontitis. J Endod. (2022) 48:430–56. doi: 10.1016/j.joen.2022.01.003
15. Haas AN, Furlaneto F, Gaio EJ, Gomes SC, Palioto DB, Castilho RM, et al. New tendencies in non-surgical periodontal therapy. Braz Oral Res. (2021) 35:e095. doi: 10.1590/1807-3107bor-2021.vol35.0095
16. Munasur SL, Turawa EB, Chikte UME, Musekiwa A. Mechanical debridement with antibiotics in the treatment of chronic periodontitis: effect on systemic biomarkers—a systematic review. Int J Environ Res Public Health. (2020) 17:5601. doi: 10.3390/ijerph17155601
17. Allison RR, Moghissi K. Photodynamic therapy (PDT): pDT mechanisms. Clin Endosc. (2013) 46:24–9. doi: 10.5946/ce.2013.46.1.24
18. Li Y, Sun G, Xie J, Xiao S, Lin C. Antimicrobial photodynamic therapy against oral biofilm: influencing factors, mechanisms, and combined actions with other strategies. Front Microbiol. (2023) 14:1192955. doi: 10.3389/fmicb.2023.1192955
19. Sharma D, Singh S, Kumar P, Jain GK, Aggarwal G, Almalki WH, Kesharwani P. 2—Mechanisms of photodynamic therapy. In: Kesharwani P, editor. Nanomaterials for Photodynamic Therapy. Woodhead Publishing Series in Biomaterials. Cambridge, MA: Woodhead Publishing (2023). p. 41–54. doi: 10.1016/B978-0-323-85595-2.00017-7
20. Wilson BC, Bown SG. Photodynamic therapy. Handbook of Laser Technology and Applications. Boca Raton: CRC Press (2021). p. 249–60.
21. Gunaydin G, Gedik ME, Ayan S. Photodynamic therapy—current limitations and novel approaches. Front Chem. (2021) 9:691697. doi: 10.3389/fchem.2021.691697
22. Algorri JF, López-Higuera JM, Rodríguez-Cobo L, Cobo A. Advanced light source technologies for photodynamic therapy of skin cancer lesions. Pharmaceutics. (2023) 15:2075. doi: 10.3390/pharmaceutics15082075
23. Bordea IR, Hanna R, Chiniforush N, Grădinaru E, Câmpian RS, Sîrbu A, et al. Evaluation of the outcome of various laser therapy applications in root canal disinfection: a systematic review. Photodiagnosis Photodyn Ther. (2020) 29:101611. doi: 10.1016/j.pdpdt.2019.101611
24. Ahrari F, Shahabi M, Fekrazad R, Eslami N, Mazhari F, Ghazvini K, et al. Antimicrobial photodynamic therapy of lactobacillus acidophilus by indocyanine green and 810-nm diode laser. Photodiagnosis Photodyn Ther. (2018) 24:145–9. doi: 10.1016/j.pdpdt.2018.08.013
25. Balhaddad AA, AlQranei MS, Ibrahim MS, Weir MD, Martinho FC, Xu HHK, et al. Light energy dose and photosensitizer concentration are determinants of effective photo-killing against caries-related biofilms. Int J Mol Sci. (2020) 21:7612. doi: 10.3390/ijms21207612
26. Pelozo LL, Silva-Neto RD, Salvador SL, Sousa-Neto MD, Souza-Gabriel AE. Adjuvant therapy with a 980-nm diode laser in root canal retreatment: randomized clinical trial with 1-year follow-up. Lasers Med Sci. (2023) 38:77. doi: 10.1007/s10103-022-03659-0
27. Kohli R. Scaling and root planing. In: Dibart S, Dietrich T, editors. Practical Periodontal Diagnosis and Treatment Planning. Hoboken, New Jersey city: John Wiley & Sons, Ltd (2023). p. 53–63 doi: 10.1002/9781119830344.ch4
28. Zhu M, Zhao M, Hu B, Wang Y, Li Y, Song J. Efficacy of glycine powder air-polishing in supportive periodontal therapy: a systematic review and meta-analysis. J Periodontal Implant Sci. (2021) 51:147–62. doi: 10.5051/jpis.1902340117
29. Wu S-Y, Wu C-Y, Lin L-Y, Chen Y, Huang H-Y, Lai Y-L, et al. Systemic antibiotics adjuvants to scaling and root planing in type 2 diabetic and periodontitis individuals: systematic review with network meta-analysis. Jpn Dent Sci Rev. (2023) 59:167–78. doi: 10.1016/j.jdsr.2023.06.001
30. Ge L, Shu R, Li Y, Li C, Luo L, Song Z, et al. Adjunctive effect of photodynamic therapy to scaling and root planing in the treatment of chronic periodontitis. Photomed Laser Surg. (2011) 29:33–7. doi: 10.1089/pho.2009.2727
31. Meire MA, Bronzato JD, Bomfim RA, Gomes BPFA. Effectiveness of adjunct therapy for the treatment of apical periodontitis: a systematic review and meta-analysis. Int Endod J. (2023) 56:455–74. doi: 10.1111/iej.13838
32. Balhaddad AA, Garcia IM, Ibrahim MS, Rolim JPML, Gomes EAB, Martinho FC, et al. Prospects on nano-based platforms for antimicrobial photodynamic therapy against oral biofilms. Photobiomodulation Photomed Laser Surg. (2020) 38:481–96. doi: 10.1089/photob.2020.4815
33. Cláudio MM, Nuernberg MAA, Rodrigues JVS, Belizário LCG, Batista JA, Duque C, et al. Effects of multiple sessions of antimicrobial photodynamic therapy (aPDT) in the treatment of periodontitis in patients with uncompensated type 2 diabetes: a randomized controlled clinical study. Photodiagnosis Photodyn Ther. (2021) 35:102451. doi: 10.1016/j.pdpdt.2021.102451
34. Skalerič E, Petelin M, Gašpirc B. Antimicrobial photodynamic therapy in treatment of aggressive periodontitis (stage III, grade C periodontitis): a comparison between photodynamic therapy and antibiotic therapy as an adjunct to non-surgical periodontal treatment. Photodiagnosis Photodyn Ther. (2023) 41:103251. doi: 10.1016/j.pdpdt.2022.103251
35. Martu M-A, Luchian I, Mares M, Solomon S, Ciurcanu O, Danila V, et al. The effectiveness of Laser applications and photodynamic therapy on relevant periodontal pathogens (Aggregatibacter actinomycetemcomitans) associated with immunomodulating anti-rheumatic drugs. Bioengineering. (2023) 10:61. doi: 10.3390/bioengineering10010061
36. Kassa CT, Salviatto LTC, Tortamano ACAC, Rost-Lima KS, Damante CA, Pavani C, et al. Antimicrobial photodynamic therapy mediated by methylene blue in surfactant vehicle as adjuvant to periodontal treatment. Randomized, controlled, double-blind clinical trial. Photodiagnosis Photodyn Ther. (2023) 41:103194. doi: 10.1016/j.pdpdt.2022.103194
37. Oniszczuk A, Wojtunik-Kulesza KA, Oniszczuk T, Kasprzak K. The potential of photodynamic therapy (PDT)—experimental investigations and clinical use. Biomed Pharmacother. (2016) 83:912–29. doi: 10.1016/j.biopha.2016.07.058
38. Ghorbani J, Rahban D, Aghamiri S, Teymouri A, Bahador A. Photosensitizers in antibacterial photodynamic therapy: an overview. Laser Ther. (2018) 27:293–302. doi: 10.5978/islsm.27_18-RA-01
39. Trevisan E, Menegazzi R, Zabucchi G, Troian B, Prato S, Vita F, et al. Effect of methylene blue photodynamic therapy on human neutrophil functional responses. J Photochem Photobiol B. (2019) 199:111605. doi: 10.1016/j.jphotobiol.2019.111605
40. Bourbour S, Darbandi A, Bostanghadiri N, Ghanavati R, Taheri B, Bahador A. Effects of antimicrobial photosensitizers of photodynamic therapy (PDT) to treat periodontitis. Curr Pharm Biotechnol. (2024) 25(10):1209–29. doi: 10.2174/1389201024666230720104516
41. Mocanu RC, Martu M-A, Luchian I, Sufaru IG, Maftei GA, Ioanid N, et al. Microbiologic profiles of patients with dental prosthetic treatment and periodontitis before and after photoactivation therapy—randomized clinical trial. Microorganisms. (2021) 9:713. doi: 10.3390/microorganisms9040713
42. Cosgarea R, Ramseier CA, Jepsen S, Arweiler NB, Jervøe-Storm PM, Batori-Andronescu I, et al. One-year clinical, microbiological and immunological results of local doxycycline or antimicrobial photodynamic therapy for recurrent/persisting periodontal pockets: a randomized clinical trial. Antibiotics. (2022) 11:738. doi: 10.3390/antibiotics11060738
43. Baeshen HA, Alshahrani A, Kamran MA, Alnazeh AA, Alhaizaey A, Alshahrani I. Effectiveness of antimicrobial photodynamic therapy in restoring clinical, microbial, proinflammatory cytokines and pain scores in adolescent patients having generalized gingivitis and undergoing fixed orthodontic treatment. Photodiagnosis Photodyn Ther. (2020) 32:101998. doi: 10.1016/j.pdpdt.2020.101998
44. Alrabiah M, Alsahhaf A, Alofi RS, Al-Aali KA, Abduljabbar T, Vohra F. Efficacy of photodynamic therapy versus local nystatin in the treatment of denture stomatitis: a randomized clinical study. Photodiagnosis Photodyn Ther. (2019) 28:98–101. doi: 10.1016/j.pdpdt.2019.08.028
45. Alvarenga LH, Gomes AC, Carribeiro P, Godoy-Miranda B, Noschese G, Simões Ribeiro M, et al. Parameters for antimicrobial photodynamic therapy on periodontal pocket—randomized clinical trial. Photodiagnosis Photodyn Ther. (2019) 27:132–6. doi: 10.1016/j.pdpdt.2019.05.035
46. Cadore UB, Reis MBL, Martins SHL, Invernici MM, Novaes AB Jr, Taba M Jr, et al. Multiple sessions of antimicrobial photodynamic therapy associated with surgical periodontal treatment in patients with chronic periodontitis. J Periodontol. (2019) 90:339–49. doi: 10.1002/JPER.18-0373
47. Hokari T, Morozumi T, Komatsu Y, Shimizu T, Yoshino T, Tanaka M, et al. Effects of antimicrobial photodynamic therapy and local administration of minocycline on clinical, microbiological, and inflammatory markers of periodontal pockets: a pilot study. Int J Dent. (2018) 2018:e1748584. doi: 10.1155/2018/1748584
48. Hill G, Dehn C, Hinze AV, Frentzen M, Meister J. Indocyanine green-based adjunctive antimicrobial photodynamic therapy for treating chronic periodontitis: a randomized clinical trial. Photodiagnosis Photodyn Ther. (2019) 26:29–35. doi: 10.1016/j.pdpdt.2019.02.019
49. Soundarajan S, Rajasekar A. Comparative evaluation of combined efficacy of methylene blue mediated antimicrobial photodynamic therapy (a-PDT) using 660 nm diode laser versus erbium-chromium-yttrium-scandium-gallium-garnet (er, cr: ySGG) laser as an adjunct to scaling and root planing on clinical parameters in supportive periodontal therapy: a randomized split-mouth trial. Photodiagnosis Photodyn Ther. (2022) 39:102971. doi: 10.1016/j.pdpdt.2022.102971
50. Rodrigues RD, Araujo NS, Filho JMP, Vieira CLZ, Ribeiro DA, dos Santos JN, et al. Photodynamic therapy as adjunctive treatment of single-rooted teeth in patients with grade C periodontitis: a randomized controlled clinical trial. Photodiagnosis Photodyn Ther. (2023) 44:103776. doi: 10.1016/j.pdpdt.2023.103776
51. da Silva-Junior PGB, Abreu LG, Costa FO, Cota LOM, Esteves-Lima RP. The effect of antimicrobial photodynamic therapy adjunct to non-surgical periodontal therapy on the treatment of periodontitis in individuals with type 2 diabetes mellitus: a systematic review and meta-analysis. Photodiagnosis Photodyn Ther. (2023) 42:103573. doi: 10.1016/j.pdpdt.2023.103573
Keywords: chronic periodontitis, antimicrobial photodynamic therapy, low-level laser therapy, methylene blue, periodontal pathogens
Citation: Karuppan Perumal MK, Rajan Renuka R and Manickam Natarajan P (2024) Evaluating the potency of laser-activated antimicrobial photodynamic therapy utilizing methylene blue as a treatment approach for chronic periodontitis. Front. Oral. Health 5:1407201. doi: 10.3389/froh.2024.1407201
Received: 26 March 2024; Accepted: 15 May 2024;
Published: 30 May 2024.
Edited by:
Rolando Vernal, University of Chile, ChileReviewed by:
Abdulrahman A. Balhaddad, Imam Abdulrahman Bin Faisal University, Saudi Arabia© 2024 Karuppan Perumal, Rajan Renuka and Manickam Natarajan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Remya Rajan Renuka, cmVteWEucHJhdmVlbjVAZ21haWwuY29t
Prabhu Manickam Natarajan, cHJhYmh1cGVyaW9AZ21haWwuY29t
 Manoj Kumar Karuppan Perumal1
Manoj Kumar Karuppan Perumal1 Remya Rajan Renuka
Remya Rajan Renuka Prabhu Manickam Natarajan
Prabhu Manickam Natarajan