- 1Oral Maxillofacial Surgery Diagnostic Sciences Department, College of Medicine and Dentistry, Riyadh Elm University, Riyadh, Saudi Arabia
- 2College of Dentistry, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 3College of Dentistry, King Saud University, Riyadh, Saudi Arabia
- 4College of Dentistry, University of Hail, Hail, Saudi Arabia
- 5Ministry of Health, Riyadh, Saudi Arabia
Objectives: This systematic review aimed to assess the effectiveness of submucosal tramadol injections in post-operative pain management following third molar surgical extraction.
Materials and methods: Databases, such as PubMed, Scopus, ScienceDirect, and Cochrane Library, were systematically searched using relevant keywords. Randomized clinical trials that met the inclusion criteria were assessed to determine the effectiveness of tramadol in managing acute post-operative pain following third molar surgery.
Results: In total, seven studies with participants of 18 and over following randomized placebo-controlled trials were considered for the analysis. A submucosal injection of 2 ml (50–100 mg) of tramadol adjacent to the impacted mandibular third molar effectively controlled pain for up to 6–24 h following surgery. Non-serious adverse events, such as nausea, vomiting, and headache, were reported in two studies. Meta-analysis (subgroup analysis) revealed heterogeneity among the studies, demonstrating variability in the results across the included studies. In addition, tramadol demonstrated a significant decrease in post-operative pain.
Conclusion: Submucosal tramadol is an efficient, safe, and dependable method for reducing post-operative acute pain, particularly in the first 6 h following impacted third molar surgery. However, due to the observed heterogeneity in the research, there is need for cautious interpretation of the findings and potential limitations in the evidence base. To enhance the quality of evidence on this topic, we strongly recommend conducting new RCTs using established methodologies.
Clinical relevance: Post operative pain following third molar surgeries is one of the common complications. Submucosal tramadol injections were found to be successful in reducing post extraction pain as well as other morbidities.
Introduction
Post-operative pain is a major complication of third molar extractions, which are one of the most frequently performed dental procedures (1). Third molar extraction is increasingly prevalent in modern dentistry, which leads to pronounced post-operative pain that becomes more severe when the anesthetic agent dissolves. Various methods and medications for reducing post-operative pain following extraction are available (2). Post-surgical pain following third molar extraction can be managed using a combination of nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, and local anesthetics. Additionally, multimodal analgesia strategies, such as incorporating corticosteroids and patient education on post-operative care, are employed to optimize pain relief while minimizing opioid usage (3, 4). Some of the pain management methods are related to alternative and complementary medicine (5–8). Third molar surgery is a routine procedure performed by oral and maxillofacial surgeons. It offers benefits including pain relief, caries and periodontal disease prevention, facilitation of orthodontic treatment and orthognathic surgery, and protection against pathological conditions such as dentigerous cyst formation and external root resorption of the adjacent second molar (9).
Mandibular third molar extraction is one of the most commonly performed dental procedures. Since the majority of mandibular third molars tend to be partially impacted, their extraction can be challenging, leading to subsequent post-operative sequelae, such as pain, swelling, and trismus (10). Moreover, transalveolar extraction of impacted mandibular third molars is the most commonly performed oral and maxillofacial surgical procedure with varying post-extraction outcomes. A high incidence of impacted third molars has been reported in Riyadh, Saudi Arabia (11). The reported prevalence of impacted 3rd molar worldwide is around 24.4% (12).
Maxillofacial surgeons routinely perform impacted third molar extraction in private and hospital settings. Moreover, most individuals may need to undergo this procedure at some point. The extraction involves manipulation of the surrounding tissue with respect to the position of the impacted tooth. The extent of bone removal directly affects patient morbidity, with more bone removal resulting in greater discomfort. Moreover, surgical complications, such as swelling, trismus, and discomfort, could impact patients’ daily activities (13–15). Impacted third molars are usually asymptomatic and incidentally found on imaging. The recommendation for the removal of impacted third molars to prevent complications or disease-related issues is a subject of debate. Proponents argue that extraction can prevent potential problems such as impaction-related pain, infection, and damage to adjacent teeth. Once detected, their removal is recommended to prevent further complications or the development of disease-related issues (16, 17). Opponents, however, contend that not all impacted third molars lead to issues and advocate for a more conservative approach, considering the potential risks and benefits of surgery on a case-by-case basis (17, 18).
Post-operative complications of third molar extraction, including pain, trismus, and swelling, have a high occurrence rate and can intensify over time (19, 20). Pain, infection, nerve damage, bleeding, and dry socket are the most commonly reported post-operative complications following third molar extraction; while surrounding tissue damage and trismus occur less frequently (21). Postoperative Symptoms Severity Scale (PoSSe Scale) is frequently used to assess the severity of these sequelae after third molar surgeries. Recent studies have reported this as a valid and reliable method for evaluation (1, 7).
Alveolar osteitis is a condition where the normal healing after extraction does occur as expected. Furthermore, in some cases, the early clot formed in the socket may undergo premature clot necrosis or loss, leading to pain and fetor oris. In addition, alveolar osteitis, dry socket, alveolitis sicca dolorosa, localized alveolar osteitis, and fibrinolytic alveolitis are disturbances that may hinder the healing process occurring after the formation of a mature blood clot and before the replacement of the blood clot with granulation tissue (2).
Transalveolar extraction of third molars is mostly performed traumatically and can result in swelling, limited mouth opening, and moderate-to-severe pain. Severe complications, such as infections and long-term nerve injuries, have a low rate of occurrence. Third molar extractions are accompanied by sequelae of postoperative complications, such as trismus, pain, bleeding, and edema. All these complications are influenced by factors, such as surgical technique, surgeo's experience and skill, and severity of the impaction (2, 20).
The major complications associated with third molar extractions include nerve damage, alveolar osteitis, bacterial infection, bleeding, and pain. Less severe complications include difficulty in mouth opening, iatrogenic damage to the second molar, and iatrogenic fracture of the mandible (22). Effective pain management is the primary goal in oral surgery, especially in anxious patients. A plethora of methods have been used to decrease post-operative pain following third molar extractions. However, submucosal tramadol has demonstrated significant results in managing pain and swelling, as documented by many studies (22).
Pain relief, reduction of possible complications, and healing in a controlled manner should be ensured following the third molar extraction. Analgesics, especially those with an anti-inflammatory action, aid in reducing pain. Dental post-operative pain can be adequately controlled using a variety of non-steroidal anti-inflammatory drugs (NSAIDs). Pain management can play a major role in the recovery of post-operative oral function. Nonetheless, NSAIDs may be contraindicated in patients with peptic ulcers, bleeding disorders, those undergoing anticoagulant therapy, and those allergic to one or more of the ingredients of NSAIDs. Thus, tramadol can be used safely in such patients; moreover, it does not have adverse effects on the respiratory function and consciousness of patients (2).
Pain is the main symptom following third molar removal, and effective pain management is crucial for improving function and quality of life. Pain can be effectively managed using various analgesics, such as NSAIDs; however, NSAIDs are contraindicated in many patients, including those with a history of allergies or peptic ulcers, bleeding diseases, and anticoagulant or steroid use. Tramadol, which is an opioid agonist, has demonstrated effective management of moderate-to-severe pain in both inpatients and outpatients. Effective pain control following oral surgery results in less morbidity and better recovery.
Surgical pain can manifest in several ways, and one novel approach for pain management involves delivering a local anesthetic agent directly to the socket of the tooth instead of using a block or any analgesic agent (NSAIDs). This method employs consistent irrigation with the anesthetic agents. However, patients may experience a needle-prick sensation during the injection of the anesthetic. Moreover, it is associated with a risk of intravascular or intraneural injection of the anesthetic solution, making it essential to take precautions to control and prevent this risk (23). This systematic review and meta-analysis aimed to evaluate the efficacy of submucosal administration of tramadol on acute pain following third molar surgery. The specific aims of this systematic review and meta-analysis are:
1. To evaluate and synthesize existing evidence on the effectiveness of submucosal administration of tramadol injection in managing acute pain following third molar surgery.
2. To assess the overall impact of submucosal tramadol on post-operative recovery, considering factors such as pain intensity, duration, and the occurrence of adverse effects.
3. To provide a comprehensive analysis of the existing literature, identifying trends, variations, and potential biases in studies evaluating the efficacy of submucosal tramadol for pain management after third molar surgery.
4. To offer insights into the practical implications of submucosal tramadol administration, considering its potential benefits and limitations in comparison to other pain management strategies.
5. To contribute evidence-based recommendations for clinicians and researchers regarding the use of submucosal tramadol as a viable option for acute pain control in the context of third-molar surgery.
Methodology
This systematic review and meta-analysis assess the efficacy of submucosal administration of tramadol injection in managing acute pain following third molar surgery. The study aims to provide a comprehensive evaluation of existing literature, adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, to contribute insights into the effectiveness of this pain management approach in the context of post-operative recovery after third molar surgery (24).
Inclusion criteria
Studies involving patients of any sex, aged >15 years, undergoing third molar extraction; those employing submucosal tramadol injections; and comparative studies (with and without injection) were included for the present meta-analysis. The primary outcome was the reduction in post-extraction pain. The secondary outcome was the reduction in trismus, swelling, and other complications. Additionally, studies conducted in dental clinics or hospitals, as well as randomized controlled trials (RCTs) or observational studies comparing submucosal injection of tramadol with other methods were included.
Exclusion criteria
Studies comprising patients <15 years or pregnant women; those with no comparative data; those with incomplete data or unclear description of the outcomes; those without controls; case reports, conference reports, animal studies, reviews, theses, and letters; and studies published in languages other than English were excluded. The search was conducted in October 2022.
Search strategy
Relevant information was collected from numerous databases, including PubMed, Web of Sciences, Scopus, ScienceDirect, Cochrane Library, and Google Scholar, using various keywords and MeSH (Medical Subject Headings) terms. Additionally, search terms such as “third molars,” “wisdom teeth,” “tramadol,” “pain,” and “morbidity” paired with Boolean operators such as “AND,” “NOT,” and “OR” were employed. The detailed keyword search strategy has been described below:
1. Google Scholar: allintitle: “molar*” AND Tramadol
2. Web of Sciences: (“Third molar” OR “Third molars” OR “3rd molars” OR “Wisdom tooth” OR “Wisdom teeth” OR “Third molar surgery”) AND (“Tramadol”) AND (“Pain” OR “Pain management” OR “Acute Pain” OR “Post extraction pain” OR “Trismus” OR “Edema” OR “Swelling” OR “Complications”)
Added suggested Keywords: should include “Dental Pain”, “third molar extraction” and “third molar surgery”. Must include “tramadol”
1. Cochrane Library Trials: all text (“Third molar” OR “Third molars” OR “3rd molars” OR “Wisdom tooth” OR “Wisdom teeth” OR “Third molar surgery”) AND (“Tramadol”) AND (“Pain” OR “Pain management” OR “Acute Pain” OR “Post extraction pain”); Plus all text (“Third molar”) AND (“Tramadol”) AND (“Trismus” OR “Edema” OR “Swelling”)
2. Scopus: (1) (“Third molar” OR “Third molars” OR “3rd molars” OR “Wisdom tooth” OR “Wisdom teeth” OR “Third molar surgery”) AND (“Tramadol”) AND (“Pain” OR “Pain management” OR “Acute Pain” OR “Post extraction pain”); Plus (2) (“Third molar” OR “Third molars” OR “3rd molars” OR “Wisdom tooth” OR “Wisdom teeth” OR “Third molar surgery”) AND (“Tramadol”) AND (“Trismus” OR “Edema” OR “Swelling” OR “Complications”) filter: RCT only and Articles only, within Article title, abstract and keywords
3. PubMed: (“Third molar*” OR “Wisdom teeth*” OR “3rd molar*” OR Molar* OR “tooth*”) AND (extraction* OR Surgery*) AND (“Tramadol”) AND (“Pain” OR “Trismus” OR “Swelling*” OR “Complication*” OR “infection*” OR “adverse effect*”)
4. ScienceDirect: (“Third molar” OR “3rd molar” OR “tooth”) AND (extraction OR Surgery) AND (“Tramadol”) AND (“Pain” OR “Trismus” OR “Swelling”)
Filters used: Research articles, Encyclopedia, Practice guidelines and English only.
Assessment
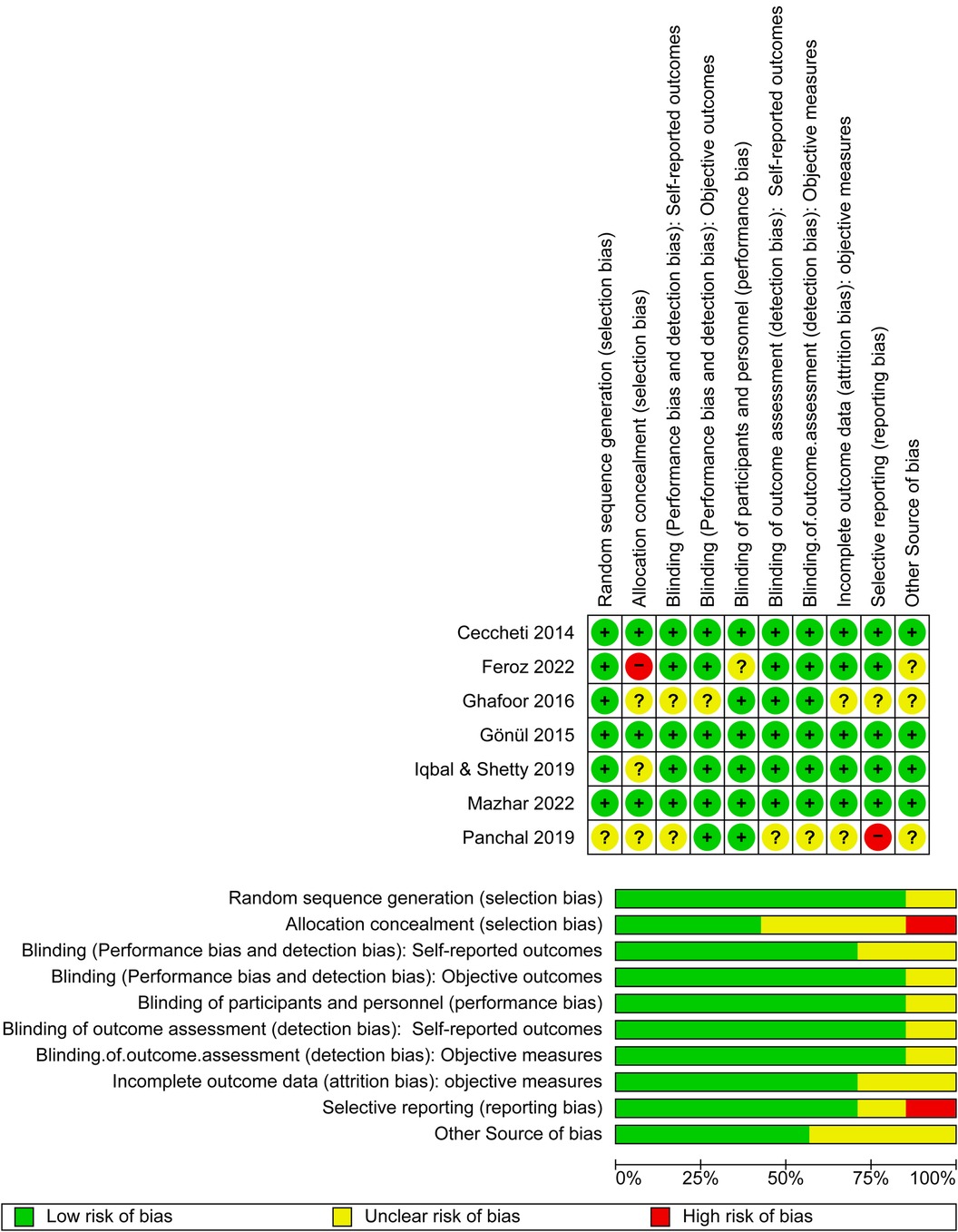
The primary articles were reviewed and the titles and abstracts of the studies were screened independently. Two researchers reviewed the articles during the initial search and later evaluated the full-text articles. Both researchers separately reviewed the methodology and were unaware of each othe's decisions. Any disagreement between the two researchers was discussed and resolved by consensus. If a conflict could not be resolved, a third researcher was consulted. The “Risk of Bias” table within RevMan 5.4 was used to systematically assess the risk of bias in individual studies included in this systematic review and meta-analysis. This table allowed reviewers to document their assessment of various domains of bias for each included study. The domains assessed included random sequence generation, allocation concealment, blinding of self-reported outcomes, blinding of objective outcomes, blinding of participants and personnel, blinding for outcome assessment for self-reported outcomes or objective outcomes, incomplete outcome data, selective reporting, and other biases.In the “Risk of Bias” table, each domain was evaluated and categorized as “low risk,” “high risk,” or “unclear risk” of bias, based on the information provided in the study report. Reviewers consider factors such as study design, methodology, and conduct to make judgments about the risk of bias in each domain.
Data extraction
The selected studies that satisfied the inclusion criteria underwent a quality assessment prior to data extraction. Screening was performed for the titles, abstracts, and full texts of the papers, and the extracted data were entered into a standardized data extraction form. The following information was extracted: study characteristics, including authors, year of study, affiliation, country of origin, and study design; participants’ characteristics, including sample size, age, sex, and other useful information; intervention; outcome measures; results; adverse events; materials and methods; tooth condition, evaluation method; follow-up; and conclusion. Reasons for exclusion were recorded and reported in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flowchart.
Data analysis
The articles included in the systematic review were summarized using qualitative analysis. For any outcomes that were consistent across the selected studies, RevMan 5.4 (25) was used to determine the Cochrane Q and I2 values, which measured the dispersion between trials. The significance threshold was set at 0.05, and a random-effects model was employed. For selected investigations, a critical evaluation of the results was also considered. Finally, the RCTs were evaluated to measure bias risk using the Cochrane technique (ROB 2.0) (26).
Heterogeneity assessment
Heterogeneity was evaluated using the I2 test. Interpretation of I2test was performed according to the Cochrane Handbook for Systematic Reviews of Interventions (25), as follows:
A) 0%–40%: indicates minimal or possibly not significant heterogeneity among the studies
B) 30%–60%: could indicate moderate heterogeneity
C) 50%–90%: may indicate significant heterogeneity
D) 75%–100%: significant heterogeneity
The significance of the observed I2 value may be influenced by the quantity, direction, and strength of evidence for heterogeneity (e.g., P-value from the chi-square test or a confidence interval for I2).
Additionally, a concise summary of the main outcomes of interest, along with the quality of evidence for each outcome was presented as a Summary of Findings (SoF) in a Table and a narrative description of the quality of evidence for each outcome was provided.
Results
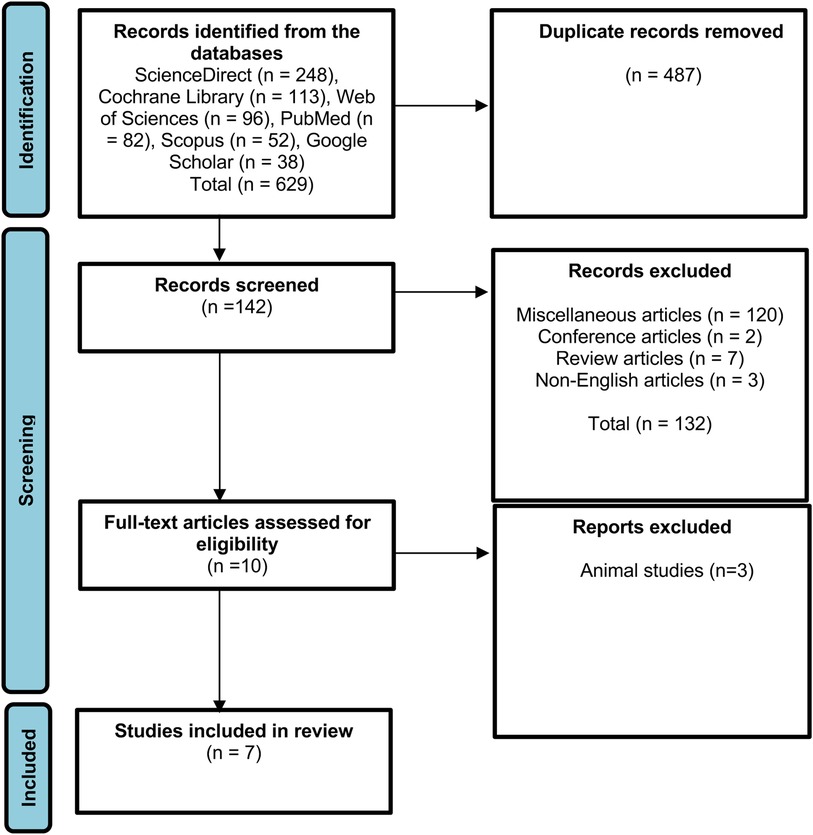
To identify relevant literature, several data sources were examined, and 629 articles were identified using Scopus, ScienceDirect, PubMed, Cochrane Library, Web of Sciences, and Google Scholar. In total, 487 duplicate articles were eliminated after initial screening, and the remaining 142 articles underwent additional screening according to the inclusion criteria. Additionally, 132 articles were excluded because they did not meet the inclusion criteria. Out of the 10 eligible studies selected for full-text assessment, 3 of them employed animals as test subjects. Finally, seven studies were selected for further investigation (Figure 1).
General characteristics of the included studies
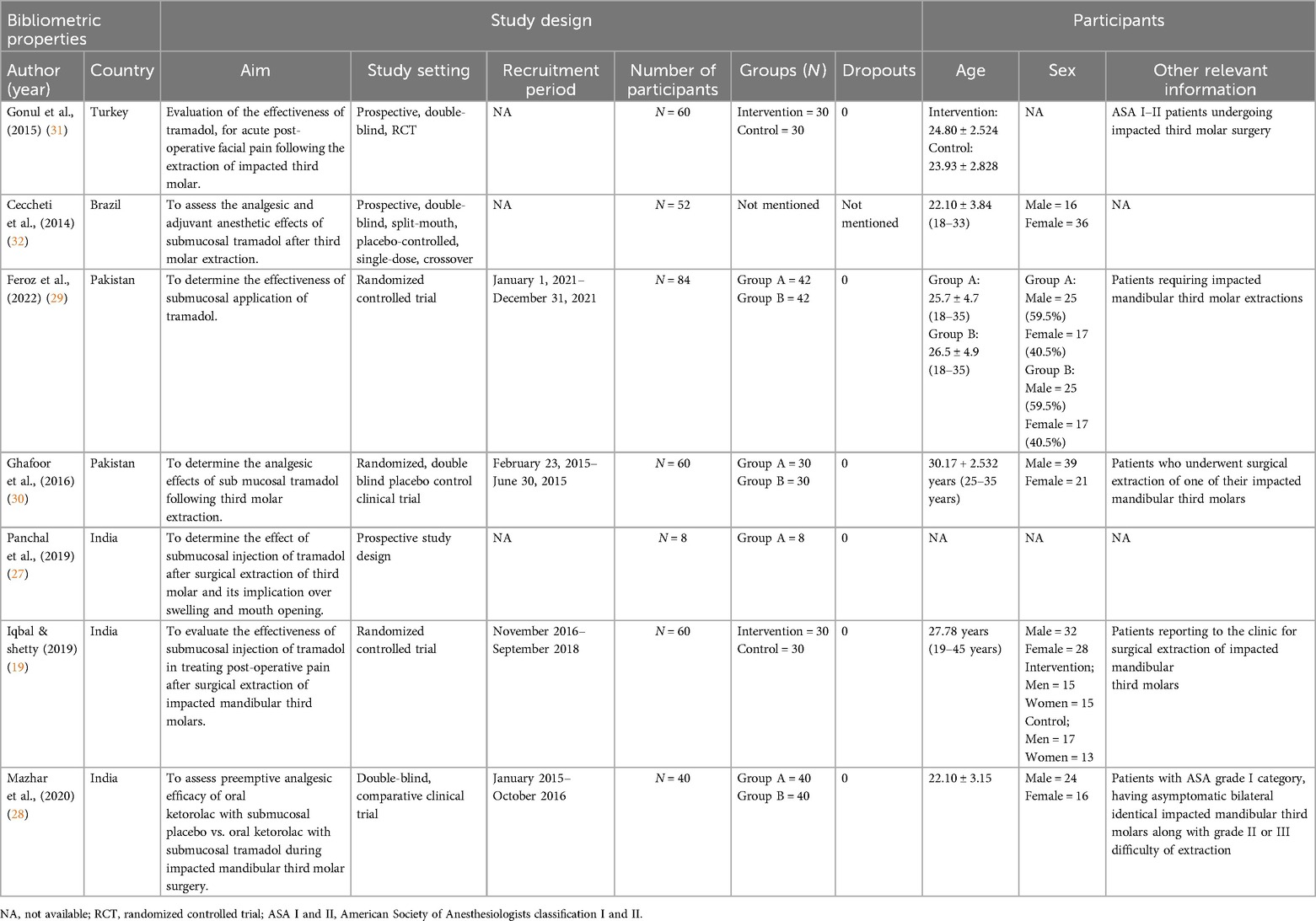
Most of the included studies were reported from India (19, 27, 28), whereas two studies from Pakistan (29, 30) and one study each from Turkey (31) and Brazil were reported (32). Six studies employed a blinded RCT study design (19, 28–32), whereas one study used a prospective study design (27) with the aim of evaluating the efficacy of submucosal application of tramadol injection following third molar extraction. The participants in the included studies had a mean age of 22–30 (range: 8–84) years. No dropouts were reported in any of the studies; five studies included both male and female participants (19, 28–30, 32), whereas two studies did not mention the sex of the participants (27, 31). Two studies reported that patients with an ASA I–III classification visited the clinic for third molar extraction (28, 31), whereas five studies reported normal third molar extraction (Table 1) (19, 27, 29, 30, 32).
Intervention and outcomes
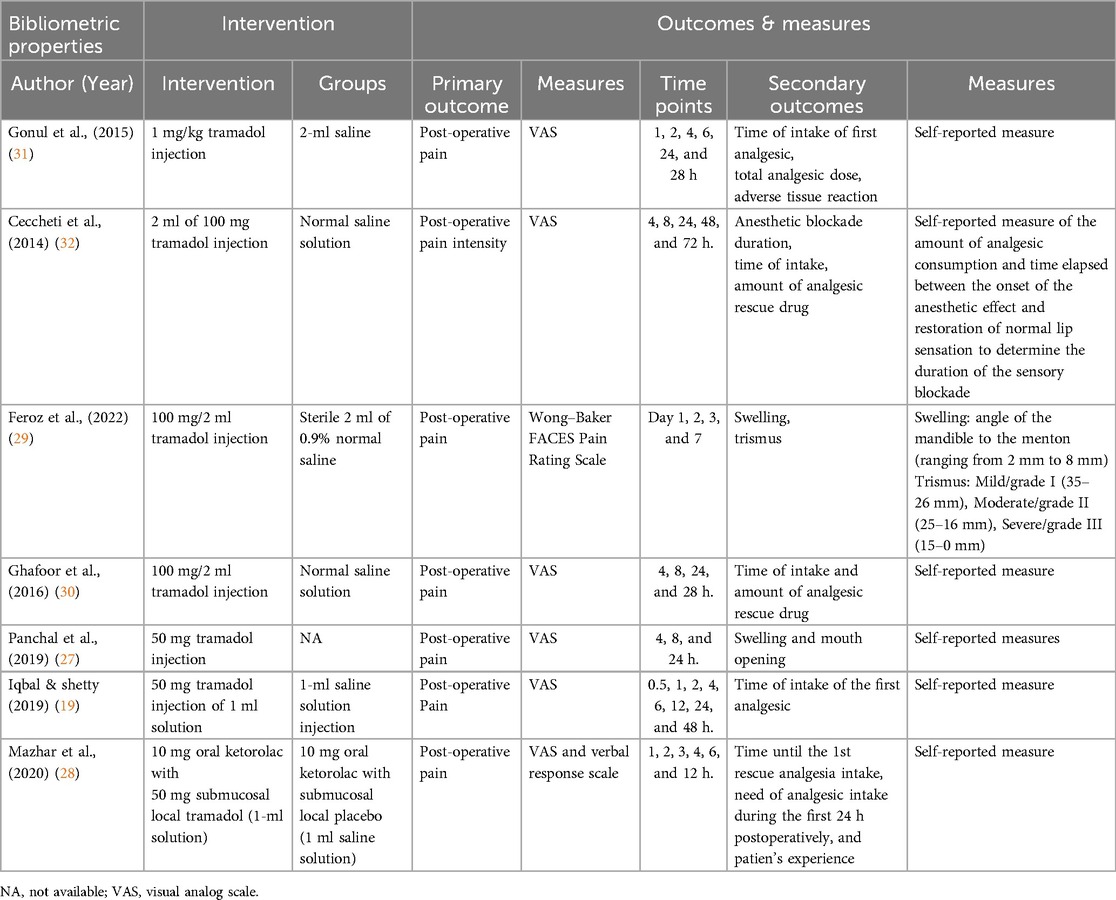
The summary of intervention and outcomes is presented in Table 2. In all the studies, tramadol injections of different doses were submucosally administered (19, 27–32); one study also used 10 mg ketorolac orally along with tramadol injection (28). Normal saline was used as the control. The primary outcome measured in all the studies was post-operative pain, whereas secondary outcomes included the first analgesic dose time, total dose, and adverse tissue reaction (19, 28, 30, 31). Time of ingestion, amount of analgesic rescue medication, and length of anesthetic blockade were reported in one study (32), whereas swelling and trimus were reported in two studies (27, 29). The visual analog scale (VAS) was employed in six studies to measure the level of pain following tramadol injection following extraction, whereas one study used the Wong–Baker FACES Pain Rating Scale (27, 29); measurements in all the studies were performed using a self-reported checklist following the time set by the researchers. The studies used different time points to measure the VAS scores, as shown in Table 2. There was a limited to two types of scales to assess Patient-Reported Outcomes (PROs). Assessing patient-reported outcomes (PROs) is crucial for evaluating the influence of interventions on patients’ quality of life and holistic well-being (33). The outcome measure tool for assessing oral health-related quality of life (OHRQoL) is a crucial component in evaluating the impact of oral health interventions on individuals’ overall well-being and satisfaction with their oral health status (33).However, none of the studies used any oral health-related Quality of Life (OHRQoL) Outcome measure tool.
Results and adverse events
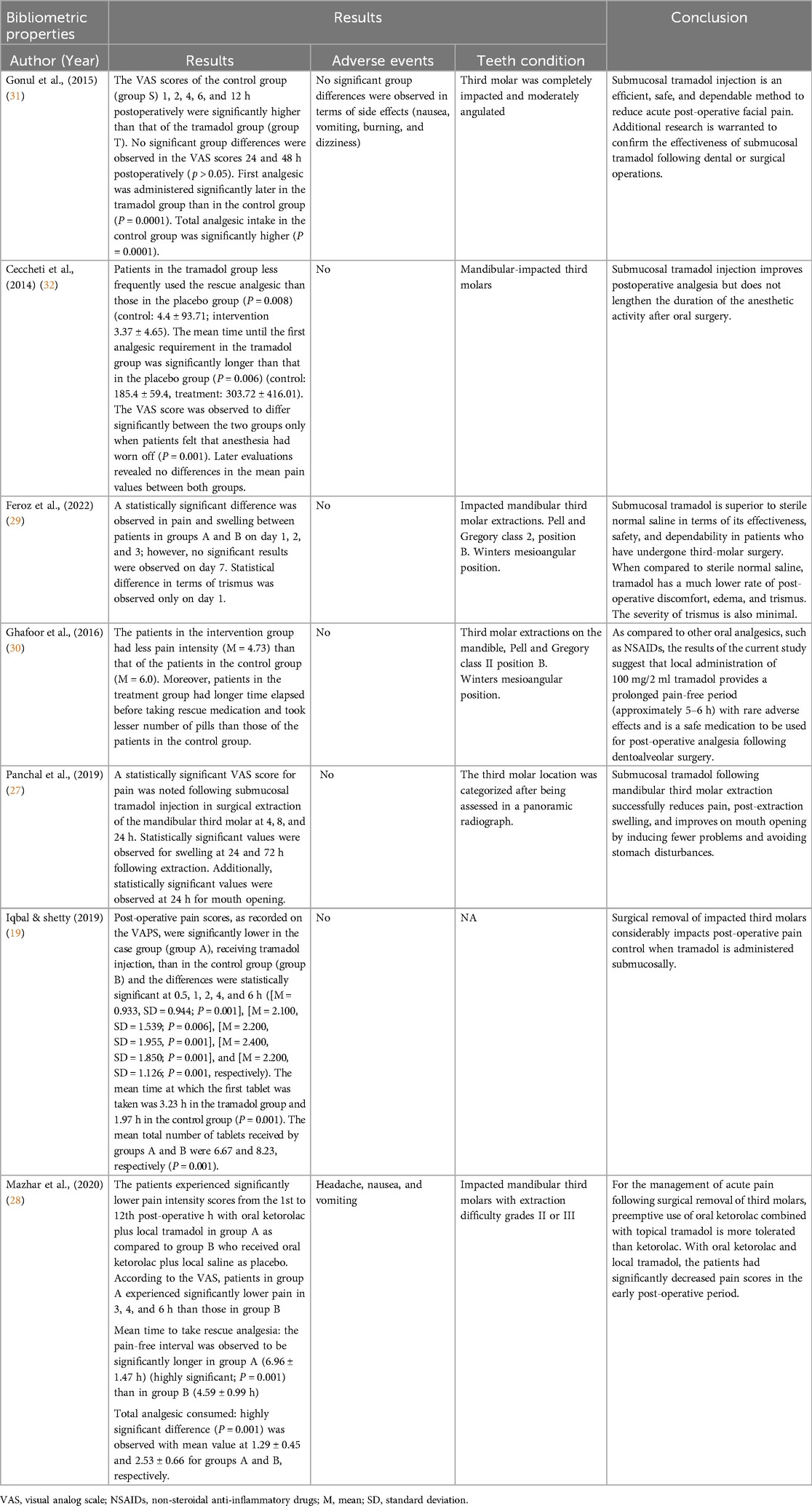
Based on the results of the included studies, the overall VAS scores were observed to be higher in the control group than in the tramadol group, especially during the first 24 h (P < 0.05) (19, 27–32); an insignificant (P > 0.05) difference was observed in the VAS scores after 24 h (31). Trismus-related statistical differences were only observed on day 1, whereas swelling demonstrated statistically significant values at 24 and 72 h following extraction. Additionally, statistically significant mouth opening was observed at every hour of the day (27, 29) as shown in Table 3. Five studies did not report any adverse events occurring during the treatment (19, 27, 29, 30, 32), whereas two studies reported adverse events including nausea, vomiting, and headache (Table 3) (28, 31). Before third molar extraction, the condition of the third molars were examined. Three of the studies reported completely impacted molar (27, 31, 32) and other three studies reported impacted molar teeth, classified as Pell and Gregory class 2 and 3, position B and Winters mesioangular position (28–30), whereas one study did not report the condition of the molars (19). Overall, all the studies demonstrated the effectiveness of tramadol in controlling post-operative pain, as shown in Table 3.
Meta-analysis
The VAS scores after 6, 12, 24, and 48 h were meta-analyzed to assess heterogeneity among the studies. After 6 h, the combined findings for pain intensity from the seven studies revealed a high degree of variability among them (I2 = 92%, P = 0.00001). The outcomes were combined using a random-effects model. As seen in Figure 2, a significant difference and correlation was observed between the tramadol injection and comparison groups (control or placebo) (RE 95% confidence interval [CI] = −0.36 [−1.39, 0.67], P = 0.00001). The pooled results for pain intensity after 12 h from the seven studies demonstrated significant inter-observer variation (I2 = 86%, P = 0.0009). The results were amalgamated using a random-effects model. The tramadol injection administration group differed significantly from the comparison groups (control or placebo) (RE [95% CI] = −0.01 [−0.60, 0.57], P = 0.0009), as shown in Figure 2. However, the diversity in the pooled results for pain intensity after 24 h from the seven studies was minimal (I2 = 0%, P = 0.49). The results were amalgamated using a random-effects model. The tramadol injection and comparison groups (control or placebo) did not differ significantly (RE [95% CI] = −0.13 [−0.26, 0.00], P = 0.49), as shown in Figure 2. The variability between them was still high (I2 = 80%, P = 0.03) in the pooled data for pain intensity after 48 h from the seven studies. A random-effects model was used to aggregate the results. Figure 2 shows a significant difference and association between the tramadol injection and control or placebo groups (RE [95% CI] = −0.89 [−1.70, −0.07], P = 0.03).
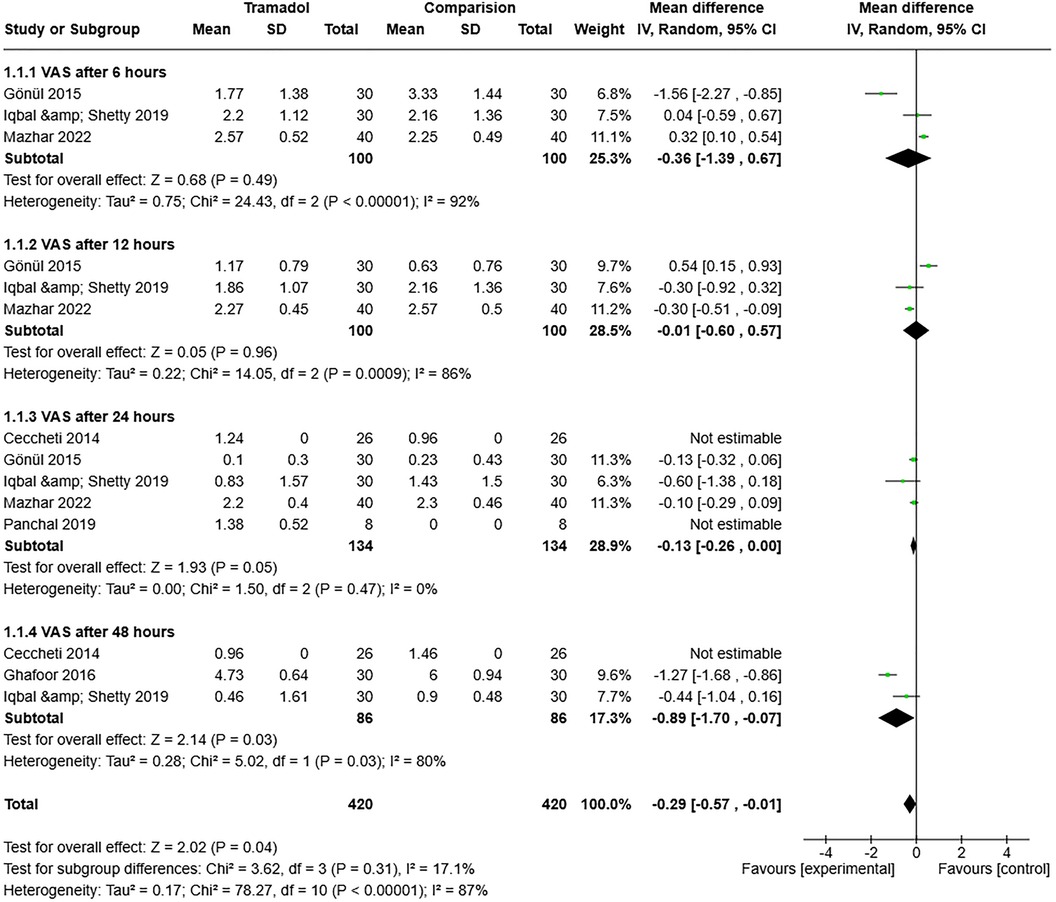
Figure 2. Forest plot of the pain intensity (after 6, 12, 24, and 48 h) in the tramadol injection and comparison (control or placebo) groups after impacted third molars surgery.
Overall, heterogeneity was observed among the included studies (I2 = 87% and P = 0.00001). However, the test for subgroup differences was non-significant (I2 = 17.1% and P = 0.31) (Figure 2).
Risk of bias
In RevMan 5.4, the “Risk of Bias” table was used to assess the quality of the included studies. A judgment (“low risk,” “high risk,” or “unclear risk” of bias) was applied to each input. Most of the studies exhibited a low risk of bias in terms of randomization, except for one study (27), which exhibited an unclear risk. In the allocation concealment, low, unclear, and high risk of bias was reported in three (28, 31, 32), three (19, 27, 30) and one study(s), respectively (29). In the blinding assessment, five studies exhibited a low risk (19, 28, 29, 31, 32) and two studies exhibited an unclear risk (27, 30); other domain assessments are shown in Figure 3. Overall, all the studies demonstrated good quality.
Discussion
The findings of this systematic review and meta-analysis provide valuable insights into the efficacy of submucosal tramadol administration for managing acute post-operative pain following third molar surgery. When interpreting these results, it is essential to consider them in the context of existing evidence and the broader landscape of pain management strategies in oral surgery. Submucosal tramadol administration has emerged as a promising approach for pain control in the immediate post-operative period, with significant reductions in pain intensity observed, particularly within the first 6 h after surgery. These findings align with previous research demonstrating the analgesic efficacy of tramadol in various surgical settings. However, it is essential to note that the heterogeneity observed among the included studies underscores the need for cautious interpretation of these results. Nonetheless, the overall trend toward improved pain management with submucosal tramadol is encouraging and warrants further investigation.
Systematic reviews and meta-analyses have become increasingly prevalent in the field of medicine (34), making the development of new clinical practice guidelines and future research more convenient, thereby benefitting both clinicians and researchers (35). These approaches allow for identifying, choosing, synthesizing, and evaluating high-quality research data to offer a high-level summary of a specific research or clinical questions (36). The present study aimed to provide an objective summary of the effectiveness of submucosal tramadol administration in managing acute post-operative dental pain following surgical extraction of third molar. Clinical techniques for submucosal tramadol administration include injection, infiltration, nerve block, topical application, and sustained-release formulations (32).Injection involves direct delivery of tramadol solution into submucosal tissue at the surgical site. Infiltration administers tramadol into surrounding submucosal tissue for localized analgesia. Nerve block targets specific nerves to block pain signals, commonly used in dental surgeries. Topical application applies tramadol gel or cream onto mucosal surfaces for transmucosal absorption. Sustained-release formulations offer prolonged analgesic effects postoperatively. Technique selection depends on clinical context, patient needs, and procedure nature. Consideration of appropriate technique is crucial for desired outcomes and patient characteristics. Submucosal tramadol administration provides effective pain management in various clinical scenarios. Tramadol administration for analgesia following surgery has been reviewed in numerous trials; however, few studies have examined its submucosal administration. Only one trial had documented the submucosal tramadol administration following pediatric tonsillectomy surgery and demonstrated a reduced need for post-surgical analgesia (37). The present study focused, exclusively on RCTs that included a control or placebo group for the analysis. RCTs were included because they provide an equal opportunity for selection of both male and female patients for either treatment or control groups, thereby reducing the chances of selection and randomization bias. Moreover, the study strictly adhered to the PRISMA statement (38), which ensured transparency and clarity in the conducted systematic review. Post-operative pain following third molar extraction is a common study model in efficacy trials because of the association of impacted third molars with caries, pericoronitis, periodontal abnormalities in the distal surface of second molars, odontogenic cysts, and dental crowding, necessitating extraction (39). Moreover, third molar surgery is one of the most common dental procedures, which could provide a sufficient number of patients to conduct a research (40). Tramadol, like lidocaine, has a blocking effect that reduces ectopic activities in hypersensitive neurons and suppresses neuronal transmission, making it suitable for reducing pain intensity. Both lidocaine and tramadol demonstrate usage-dependent blockade and have a high affinity for rapidly inactivated sodium ion channels compared to resting channels (41). The present study included both male and female patients of all age experiencing post-operative pain, as the literature has suggested that several variables, such as the complexity of the treatment, patien's age and sex, and the surgeo's experience, can influence post-operative pain (42).
Post-operative pain following the extraction of an impacted third molar is frequently used to assess the analgesic efficacy due to the consistency and intensity of the pain (43). The present study revealed that submucosal injection of 2 ml (50–100 mg) of tramadol adjacent to the impacted mandibular third molar was effective in reducing pain for up to 6 h following surgery, with positive effects continuing for up to 24 h. This finding is consistent with that of a previous systematic review, supporting the effectiveness of this approach. This procedure also aids in starting rescue analgesics sooner and using less rescue analgesics overall (44). In addition, the effects of tramadol administration, both systemically and locally (submucosally), on pain relief following the extraction of an impacted mandibular third molar were examined in another study. The findings revealed that tramadol (50 mg) injected locally into the surgical site greatly improves post-operative analgesia and significantly extends the duration of the anesthetic effect (45). In one study, a combination of tramadol with 10 mg ketorolac demonstrated a positive effect in reducing pain as a combination of analgesics can have a “sparing effect,” enabling pain relief with lower doses and fewer adverse effects. The World Health Organization recommends using a combination of drugs because of the presence of numerous nociception pathways n the human body (46). Similar findings were reported by Isiordia et al. and Kim et al. who reported that both medicines have substantial analgesic effects (47, 48). In the present review, only two studies reported adverse events in the form of nausea, vomiting, and headache. Although sleepiness is also a side effect of 75 mg tramadol, unpleasant reactions, including nausea and vomiting, are more common (49). Burning, discomfort, and localized pre-anesthetic erythema may occur even at the injectable dose of 50 mg tramadol (50). Tramadol was similarly or less tolerated by patients following submucosal injection than the control drug, indicating that the drug has a low incidence of side effects and the possibility of a nocebo effect (51). Therapeutic doses of tramadol, even when applied locally, do not impair the respiratory or circulatory systems (52). Tramadol showed lesser analgesic efficacy and a higher incidence of adverse effects compared to NSAIDs in a systematic review that evaluated oral and intramuscular routes of tramadol for third molar pain (53). Moreover, their findings indicated that a single dose of tramadol was not as effective or as safe as NSAIDs for the relief of pain following third molar operations.
Meanwhile, question can arise why injectable tramadol and possible explanation can be that injecting tramadol submucosally allows for a more rapid onset of action compared to oral administration, ensuring quicker pain relief during the critical postoperative period. Submucosal administration bypasses the gastrointestinal tract, avoiding potential delays associated with oral absorption, especially if the patient experiences nausea or has delayed gastric emptying after surgery (20). Moreover, submucosal injection allows for a more precise and controlled delivery of the drug directly to the site of action, maximizing its effectiveness.
A qualitative systematic review by Gounari et al. (54) assessed the impact of both parenteral and submucosal applications of tramadol on perioperative pain management of third molar extraction. The study showed submucosal infiltration of tramadol enhanced analgesic effects, particularly when used in conjunction with oral ketorolac, thus providing a viable alternative to conventional NSAID therapy. These findings underscore tramado's potential as a flexible analgesic that can be effectively tailored to individual patient needs, particularly for those who may not tolerate NSAIDs well (54).
The current meta-analysis revealed that tramadol administered submucosally 6 h after third molar surgery significant improved pain management compared to a placebo. This could be explained by the fact that tramadol has a 6-h plasma half-life, regardless of the method of administration, indicating that this medication has positive effects, particularly in early pain control. In contrast, placebo was more effective than submucosal tramadol in controlling pain 24 h after surgery. This was probably because the effect of tramadol was reduced by half, causing its analgesic effect to decrease over time. Additionally, the local application of the drug resulted in its gradual absorption into the systemic circulation. Another systematic review also reported a reduction in the analgesic effect of tramadol compared to a placebo, 12 h after surgery (44). Although heterogeneity was observed among the studies after 48 h of extraction, a positive effect of tramadol was still evident compared to the control or placebo. This may be attributed to the relatively small number of studies included in this review. Summary measurements of pain from 6 to 24 h after surgery revealed a significant difference in the VAS scores, favoring the tramadol group.
Limitations
The overall strength of evidence for the use of submucosal tramadol in managing postoperative pain following third molar surgery was rated as moderate. This means that the evidence is considered to be of reasonable quality and is likely to be reliable, but there are some limitations or uncertainties that may affect the confidence in the findings.
The main limitations of the evidence included the relatively small number of studies included in the analysis, the heterogeneity among the studies, and the potential risk of bias in some of the included studies. However, the consistency of the findings across multiple studies and the overall direction of the effect size provided some confidence in the results.
In our opinion, the evidence for clinical decision-making is constrained by the number of clinical trials that have been examined, lack of standardization in the classification system for third molar impaction, level of surgical difficulty, length of the procedure, and surgeo's experience. The relatively small number of research articles with a small sample size may also be a limitation of this study; thus, the findings may not be suitable in the decision-making process.
Conclusion
Submucosal tramadol is an efficient, safe, and dependable method for reducing post-operative acute pain, particularly in the first 6 h following impacted third molar surgery. Additionally, submucosal tramadol decreased the need for rescue analgesics and combination therapies. Notably, no serious adverse events were reported, and summary VAS pain assessments from 6 to 24 h after surgery revealed a substantial difference in favor of the tramadol group. Based on the moderate strength of evidence, it can be concluded that submucosal tramadol is an effective and safe option for managing postoperative pain following third molar surgery. However, due to the observed heterogeneity in the research, caution must be exercised when interpreting the results of this study. To enhance the quality of evidence on this topic, we strongly recommend conducting new RCTs using established methodologies to address the limitations of the existing evidence, such as the heterogeneity among the studies and the potential risk of bias.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AHA: Conceptualization, Formal Analysis, Supervision, Writing – original draft, Writing – review & editing. EA: Conceptualization, Data curation, Methodology, Writing – review & editing. ABA: Data curation, Investigation, Methodology, Writing – original draft. BA: Conceptualization, Data curation, Methodology, Writing – original draft. YA: Conceptualization, Data curation, Methodology, Writing – original draft. RA: Conceptualization, Data curation, Methodology, Writing – review & editing. ALA: Conceptualization, Data curation, Methodology, Writing – original draft. ASA: Conceptualization, Data curation, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hassan B, Al-Khanati NM, Bahhah H. Effect of lingual-based flap design on postoperative pain of impacted mandibular third molar surgery: split-mouth randomized clinical trial. Med Oral Patol Oral Cir Bucal. (2020) 25(5):e660–7. doi: 10.4317/medoral.23666
2. Shipton EA, Roelofse JA, Blignaut RJ. An evaluation of analgesic efficacy and clinical acceptability of intravenous tramadol as an adjunct to propofol sedation for third molar surgery. Anesth Prog. (2003) 50(3):121–8. Available online at: https://pubmed.ncbi.nlm.nih.gov/14558587/14558587
3. Hersh EV, Moore PA, Ross GL, Gordon SM, Bassett DA, Karimjee OM, et al. Nonsteroidal anti-inflammatory drugs and opioids in postsurgical dental pain. J Dent Res. (2020) 99(7):777–86. doi: 10.1177/0022034520914254
4. Paladini A, Cipollone F, Manfredonia F, Gentili A, Santevecchi L, Di Filippo P, et al. Advances in the management of acute postsurgical pain: a review. Cureus. (2023) 15(8):e42974. doi: 10.7759/cureus.42974
5. Al-Khanati NM, Al-Moudallal Y. Effect of intrasocket application of Manuka honey on postsurgical pain of impacted mandibular third molars surgery: splitmouth randomized controlled trial. J Maxillofac Oral Surg. (2019) 18(1):147–52. doi: 10.1007/s12663-018-1142-z
6. Hamadi I, Al-Khanati NM, Kara Beit Z. Comparing the effect of facial compression bandage to that of systemic dexamethasone on postsurgical sequels after extraction of impacted mandibular third molars: a split-mouth randomized clinical trial. Open Access Maced J Med Sci. (2021) 9(D):160–5. doi: 10.3889/oamjms.2021.6659
7. Rokbah MQA, Al-Moudallal Y, Al-Khanati NM, Ali-Hsaian J, Kokash MB. Effects of German chamomile on symptoms and healing after mandibular third molar surgeries: a triple-blind split-mouth randomised controlled trial. Int J Surg Open. (2023) 56:100639. doi: 10.1016/j.ijso.2023.100639
8. Mawardi H, Ghazalh S, Shehatah A, Abdelwahid A, Aljohani A, Felemban O, et al. Systemic use of Arnica Montana for the reduction of postsurgical sequels following extraction of impacted mandibular third molars: a pilot study. Evid Based Complement Alternat Med. (2020) 2020:6725175. doi: 10.1155/2020/6725175
9. Lee CT, Wang HL, Patil S, Liou EJ, Santosh P, Rai A, et al. Patients’ satisfaction and prevalence of complications on surgical extraction of third molar. Patient Prefer Adherence. (2015) 9:257–63. doi: 10.2147/PPA.S76236
10. Mojsa IM, Pokrowiecki R, Lipczynski A, Wysluch A, Lipczynska-Lojek I, Kielbowicz Z, et al. Effect of submucosal dexamethasone injection on postoperative pain, oedema, and trismus following mandibular third molar surgery: a prospective, randomized, double-blind clinical trial. Int J Oral Maxillofac Surg. (2017) 46(4):524–30. doi: 10.1016/j.ijom.2016.11.006
11. Alfadil L, Almajed E. Prevalence of impacted third molars and the reason for extraction in Saudi Arabia. Saudi Dent J. (2020) 32(5):262–8. doi: 10.1016/j.sdentj.2020.01.002
12. Carter K, Worthington S. Predictors of third molar impaction: a systematic review and meta-analysis. J Dent Res. (2016) 95(3):267–76. doi: 10.1177/0022034515615857
13. de Santana-Santos T, de Oliveira E Souza P, de Andrade E Souza I, Dos Santos JN, Vajgel A, Silva EDO, et al. Prediction of postoperative facial swelling, pain and trismus following third molar surgery based on preoperative variables. Med Oral Patol Oral Cir Bucal. (2013) 18(1):70. doi: 10.4317/medoral.18039
14. Isolan C, Leivas TP, Begnini GC, Pacheco GD, Sousa PL, Quevedo LA, et al. Photobiomodulation therapy reduces postoperative pain after third molar extractions: a randomized clinical trial. Med Oral Patol Oral Cir Bucal. (2021) 26(3):8. doi: 10.4317/medoral.24228
15. Zandi M, Amini P, Keshavarz A. Effectiveness of cold therapy in reducing pain, trismus, and oedema after impacted mandibular third molar surgery: a randomized, self-controlled, observer-blind, split-mouth clinical trial. Int J Oral Maxillofac Surg. (2016) 45(1):118–23. doi: 10.1016/j.ijom.2015.10.021
16. Almidaj MA, Al Shaikh NA, Al Ageeli G, Al Harbi AF, Al Omari AF, Al Ghamdi SA, et al. Prevalence of impacted third molars in AZ-Zulfi region of Saudi Arabia: a cross-sectional study. Majmaah J Health Sci. (2021) 9(2):52–61. doi: 10.5455/mjhs.2021.02.006
17. Pitros P, Mitros A, Papadopoulou S, Xirou E, Christodoulou P, Marangos K, et al. A systematic review of the complications of high-risk third molar removal and coronectomy: development of a decision tree model and preliminary health economic analysis to assist in treatment planning. Br J Oral Maxillofac Surg. (2020) 58(9):24. doi: 10.1016/j.bjoms.2020.07.015
18. Friedman JW. The prophylactic extraction of third molars: a public health hazard. Am J Public Health. (2007) 97(9):1554–9. doi: 10.2105/AJPH.2006.100271
19. Iqbal AM, Shetty P. Effect of submucosal injection of tramadol on postoperative pain after third molar surgery. J Oral Maxillofac Surg. (2019) 77(9):1752–9. doi: 10.1016/j.joms.2019.03.029
20. Broome IJ, Turner ND, Zhang M, Alvi F, MacGregor C, Garton E, et al. The use of tramadol following day–case oral surgery. Anaesthesia. (1999) 54(3):289–92. doi: 10.1046/j.1365-2044.1999.00714.x
21. Agrawal A, Thakur S, Sen H, Kumar A, Singh P, Patil V, et al. Wisdom tooth–complications in extraction. J Contemp Dent Pract. (2014) 15(1):34–6. doi: 10.5005/jp-journals-10024-1484
22. Contar CM, De Oliveira S, Kanegusuku K, Berticelli RD, Azevedo-Alanis LR, Machado MA, et al. Complications in third molar removal: a retrospective study of 588 patients. Med Oral Patol Oral Cir Bucal. (2010) 15(1):8. doi: 10.4317/medoral.15.e74
23. Khorshidi Khiavi R, Tehranchi A, Yousefi P, Vaziri S, Salimi S, Ghaderi F, et al. Pain control following impacted third molar surgery with bupivacaine irrigation of tooth socket: a prospective study. J Dent Res Dent Clin Dent Prospects. (2010) 4(4):105–9. doi: 10.5681/joddd.2010.027
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021):372. doi: 10.1136/bmj.n71
25. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob Health. (2019) 4(Suppl 1):e000858. doi: 10.1136/bmjgh-2018-000858
26. Jørgensen L, Paludan-Müller AS, Laursen DR, Villumsen M, Fischer G, Wicherts JM, et al. Evaluation of the cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in cochrane and non-cochrane reviews. Syst Rev. (2016) 5:80. doi: 10.1186/s13643-016-0259-8
27. Panchal KV, Verma AK, Bhateja S, Sharma Y, Bajpai D, Sinha A, et al. Evaluation of efficacy of submucosal tramadol after mandibular third molar surgery: a prospective pilot study. Int J Res Med Sci. (2019) 7(11):10–24. doi: 10.18203/2320-6012.ijrms20195009
28. Mazhar H, Alvi KA, Zaidi I, Qureshi R, Ali H, Rehman A, et al. Preemptive oral ketorolac with local tramadol versus oral ketorolac in third molar surgery: a comparative clinical trial. J Maxillofac Oral Surg. (2020) 21:1–8. doi: 10.1007/s12663-020-01400-4
29. Feroz R, Hassan M, Sadiq Z, Alam S, Kazmi N, Bashir M, et al. Effect of submucosal injection of tramadol on postoperative complications after third molar surgery. Pak J Med Health Sci. (2022) 16(03):479–81. doi: 10.53350/pjmhs22163479
30. Ghafoor MW, Ali SM, Janjua OS, Malik S, Zahid F, Noor S, et al. Role of submucosal tramadol in pain control after mandibular third molar surgery. Pak Armed Forces Med J. (2016) 66(3):346–50. Available online at: https://pafmj.org/PAFMJ/article/view/1300
31. Gönül O, Altun E, Kaya GS, Topuz Ö, Gökbulut T, Özkan B, et al. Effect of submucosal application of tramadol on postoperative pain after third molar surgery. Head Face Med. (2015) 11:35. doi: 10.1186/s13005-015-0090-9
32. Ceccheti MM, de Arruda JA, de Arruda MD, Marins M, da Costa SS, Salles MG, et al. Analgesic and adjuvant anesthetic effect of submucosal tramadol after mandibular third molar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2014) 117(3):54. doi: 10.1016/j.oooo.2012.05.015
33. Parhizkar P, Rashad A, Chamari K, Andersen LL, Martinez-Ramos J, Casals M, et al. Can adjunctive corticosteroid therapy improve patient-centered outcomes following third molar surgery? A systematic review. Med Oral Patol Oral Cir Bucal. (2022) 27(5):8. doi: 10.4317/medoral.25177
34. Moher D, Liberati A, Tetzlaff J, Altman DG, Mulrow C, Gotzsche PC, et al. Epidemiology and reporting characteristics of systematic reviews. PLoS Med. (2007) 4(3):e78. doi: 10.1371/journal.pmed.0040078
35. Stevens A, Shamseer L, Weinstein E, Yazdi F, Turner L, Thielman J, et al. Relation of completeness of reporting of health research to journals’ endorsement of reporting guidelines: systematic review. Br Med J. (2014) 348:g3804. doi: 10.1136/bmj.g3804
36. Rosenthal R. What has the Cochrane Archive of Systematic Reviews Added to the Medical Literature?. Chapel Hill, NC: University of North Carolina (2019). Available online at: https://cdr.lib.unc.edu/concern/masters_papers/cj82kc02b
37. Atef A, Fawaz AA. Peritonsillar infiltration with tramadol improves pediatric tonsillectomy pain. Eur Arch Otorhinol. (2008) 265(5):571–4. doi: 10.1007/s00405-007-0479-6
38. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62(10):34. doi: 10.1016/j.jclinepi.2009.06.006
39. Normando D. Third molars: to extract or not to extract? Dental Press J Orthod. (2015) 20(4):17–8. doi: 10.1590/2176-9451.20.4.017-018.edt
40. Barden J, Edwards JE, McQuay HJ, Andrew Moore R. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain. (2004) 107(1-2):86–90. doi: 10.1016/j.pain.2003.09.021
41. Barakat A. Revisiting tramadol: a multi-modal agent for pain management. CNS Drugs. (2019) 33(5):481–501. doi: 10.1007/s40263-019-00623-5
42. Jakse N, Bankaoglu V, Wissbuam T, Ivan S, Osman G, Steidler L, et al. Primary wound healing after lower third molar surgery: evaluation of 2 different flap designs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2002) 93(1):7–12. doi: 10.1067/moe.2002.119519
43. Medeiros-Albuquerque A-F, Oliveira CP, Santos MJ, Freitas PH, Batista LA, Melo LG, et al. Preemptive analgesia-related gene and protein expression in third molar surgeries under nonsteroidal anti-inflammatory drug protocols: a PROSPERO-registered systematic review of clinical studies. Med Oral Patol Oral Cir Bucal. (2018) 23(6):e723. doi: 10.4317/medoral.22576
44. Gonçalves KK, Pires T, Mendes BF, Reis Junior JA, Reis AS, Lemos TG, et al. Is the injection of tramadol effective at controlling pain after impacted mandibular third molar extractions? A systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. (2022) 27(6):e1–9. doi: 10.4317/medoral.25498
45. Pozos AJ, Garcia-Carillo R, Rodriquez-Fernandez P, Sanchez-Ledesma M, de la Rosa-Rivera MG, Gonzalez-Perez R, et al. Tramadol administered in a combination of routes for reducing pain after removal of an impacted mandibular third molar. J Oral Maxillofac Surg. (2007) 65(8):1633–9. doi: 10.1016/j.joms.2006.06.267
46. Trybek G, Kuropatwa S, Górski B, Młynarska-Bernacka D, Czeczotka A, Drozd M, et al. Effect of platelet-rich fibrin application on non-infectious complications after surgical extraction of impacted mandibular third molars. Int J Environ Res Public Health. (2021) 18(16):8249. doi: 10.3390/ijerph18168249
47. Kim K, Woo S, Jeong S, Park J, Lee D, Hwang K, et al. The use of corticosteroids and nonsteroidal anti-inflammatory medication for the management of pain and inflammation after third molar surgery: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2009) 107(5):630–40. doi: 10.1016/j.tripleo.2008.11.005
48. Isiordia Espinoza M, Sánchez-Prieto M, Reyes-Gasga J, González-Pérez B, Almanza Orozco S, Ríos Martínez K, et al. Preemptive analgesic effectiveness of oral ketorolac plus local tramadol after impacted mandibular third molar surgery. Med Oral Patol Oral Cir Bucal. (2011) 16(6):80. doi: 10.4317/medoral.16854
49. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. (2004) 43(13):879–923. doi: 10.2165/00003088-200443130-00004
50. Haeseler G, Maue D, Grosskreutz J, Bufler J, Nentwig B, Piepenbrock S, et al. Tramadol, fentanyl and sufentanil but not morphine block voltage-operated sodium channels. Pain. (2006) 126(1-3):234–44. doi: 10.1016/j.pain.2006.07.003
51. Jokinen V. Opioid analgesia: modulation by drug interactions and glial activation. In: Faculty of Medicine, Medicum, Pharmacology. Helsinki: University of Helsinki (2017).
52. Agrawal S, Patel PR, Gundloori RVN. Proteins as nanocarriers to regulate parenteral delivery of tramadol. ACS Omega. (2019) 4(4):6301–10. doi: 10.1021/acsomega.8b02060
53. Isiordia-Espinoza MA, de Jesus Pozos-Guillen A, Aragon-Martinez OH. Analgesic efficacy and safety of single-dose tramadol and non-steroidal anti-inflammatory drugs in operations on the third molars: a systematic review and meta-analysis. Br J Oral Maxillofac Surg. (2014) 52(9):775–83. doi: 10.1016/j.bjoms.2014.05.005
54. Gounari MM, Tsaousi G, Zouloumis L, Kouvelas D, Pourzitaki C. Efficacy and safety of parenteral and local application of tramadol in mandibular third molar extraction: a qualitative systematic review of current evidence. Oral Maxillofac Surg. (2024) 28(2):499–513. doi: 10.1007/s10006-023-01179-x
Keywords: analgesic, post-operative pain, opioids, impacted teeth, tramadol, submucosal
Citation: Assari AS, Algharbi EMA, Abuhabsha AM, Alshammry BB, Alanazi YA, Abuhaimed RA, Alzahrani AMA and Alduhaim AS (2024) Efficacy of submucosal administration of tramadol on acute pain following third molar surgery: a systematic review and meta-analysis. Front. Oral. Health 5:1360298. doi: 10.3389/froh.2024.1360298
Received: 22 December 2023; Accepted: 28 October 2024;
Published: 18 November 2024.
Edited by:
Rui Amaral Mendes, University of Porto, PortugalReviewed by:
Nuraldeen Maher Al-Khanati, Syrian Private University, SyriaAmaury Pozos, Autonomous University of San Luis Potosí, Mexico
Copyright: © 2024 Assari, Algharbi, Abuhabsha, Alshammry, Alanazi, Abuhaimed, Alzahrani and Alduhaim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad Salem Assari, YWFzc2FyaUBrZnNocmMuZWR1LnNh
†Present Address: Ahmad Salem Assari, Department of Dentistry – Oral & Maxillofacial Surgery Section, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
‡ORCID:
Ahmad Salem Assari
orcid.org/0000-0002-1940-7359
 Ahmad Salem Assari
Ahmad Salem Assari Elaf Mubarak Abdullah Algharbi
Elaf Mubarak Abdullah Algharbi Abdulmajeed Mohammed Abuhabsha
Abdulmajeed Mohammed Abuhabsha Basel Basheer Alshammry
Basel Basheer Alshammry Yosef Aeed Alanazi
Yosef Aeed Alanazi Reem Abdulaziz Abuhaimed
Reem Abdulaziz Abuhaimed Ali Mohammad Ali Alzahrani
Ali Mohammad Ali Alzahrani Abdulrahaman Saud Alduhaim
Abdulrahaman Saud Alduhaim